
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Oncol. , 24 January 2025
Sec. Gastrointestinal Cancers: Gastric and Esophageal Cancers
Volume 15 - 2025 | https://doi.org/10.3389/fonc.2025.1404695
This article is part of the Research Topic Immune Checkpoint Inhibitors in Advanced Gastric and Esophageal Cancers View all 12 articles
Objective: We carried out the meta-analysis to determine the predictive value of baseline neutrophil to lymphocyte ratio (NLR) and derived neutrophil to lymphocyte ratio (dNLR) levels in patients with gastroesophageal junction or gastric cancer (GJGC) who underwent immune checkpoint inhibitor (ICI) treatment.
Methods: Eligible articles were obtained through PubMed, the Cochrane Library, EMBASE, and Google Scholar, until April 15, 2023. The clinical outcomes evaluated in this study encompassed overall survival (OS), progression-free survival (PFS), objective response rate (ORR), and disease control rate (DCR)
Results: A total of 24 articles with 2221 patients were included in this meta-analysis. The pooled results demonstrated that patients with high NLR levels had significantly poorer OS (HR: 1.860, 95% CI: 1.564-2.213, p < 0.001) and PFS (HR: 1.678, 95% CI: 1.354-2.079, p < 0.001), and lower ORR (OR: 0.754, 95% CI: 0.621-0.915, p = 0.004) and DCR (OR: 0.391, 95% CI: 0.262-0.582, p < 0.001). Besides, we also found that high dNLR levels were significantly associated with shorter OS (HR: 2.117, 95% CI: 1.590-2.820, p < 0.001) and PFS (HR: 1.803, 95% CI: 1.415-2.297, p < 0.001).
Conclusion: Low baseline (Derived) NLR has the potential to predict the good efficacy of ICIs and survival outcomes in patients with GJGC. (Derived) NLR could be useful in determining the optimal treatment strategies for these patients.
Gastroesophageal junction or gastric cancer (GJGC) is one of the major causes of cancer-related mortality globally (1). Fluoropyrimidine plus a platinum agent is the most commonly used first-line therapy for patients with unresectable advanced or metastatic GJGC (2–4). Second-line chemotherapy, such as taxane with or without ramucirumab or irinotecan (2–4), is recommended for those who are refractory to such treatment. Despite significant advancements in chemotherapy and targeted therapies over the past few decades, the prognosis for patients with advanced GJGC remains unfavorable (5, 6). However, the emergence of immune checkpoint inhibitors (ICIs) has ushered in a new era of GJGC treatment. The monoclonal antibody against programmed death 1 (PD-1) has become a widely accepted standard of treatment for patients who have not responded to second-line or subsequent systemic therapies (7, 8). Nevertheless, due to limited efficacy and the possibility of severe toxicities, not all advanced GJGC patients are eligible for ICIs. Thus, the identification of predictive biomarkers that can distinguish advanced GJGC patients likely to have prolonged survival and tumor response to ICI therapy is crucial.
Numerous molecular and genomic biomarkers have been identified as predictive indicators for ICI therapy. These biomarkers include programmed death-ligand 1 (PD-L1) expression, tumor mutational burden, microsatellite instability status, and specific gene mutations (9–12). However, their clinical application is limited due to requirements for adequate tumor tissue and DNA sequencing and the absence of standardized quantitative scoring methods for PD-L1 immunohistochemistry (13, 14). This presents a need for easily accessible and cost-effective biomarkers that can be used in diverse settings, such as resource-poor areas, without relying on advanced genomic technologies or specialized expertise. Factors in the peripheral blood are potential candidates for such biomarkers.
The main focus of studies exploring immune-related markers in peripheral blood with respect to cancer has been on the neutrophil-to-lymphocyte ratio (NLR). NLR is calculated by dividing the absolute counts of neutrophils and lymphocytes and is thought to reflect the balance between the anti-tumoral immune response and the pro-tumoral inflammatory status. Numerous meta-analysis studies have demonstrated the value of NLR as a prognostic factor across a variety of cancers (15–17).
Nevertheless, there is disagreement regarding the predictive relationship between NLR levels and ICI-treated GJGC patients, and no pertinent meta-analysis has been carried out. Thus, in GJGC patients receiving ICI treatment, the prognostic value of NLR was comprehensively assessed in this investigation. This analysis could help in prognostication and treatment strategy development, leading to more precise, economical, and minimally harmful treatments.
The analysis conducted adhered to the PRISMA statement (18). On April 15, 2023, a thorough literature search was conducted on PubMed, EMBASE, and the Cochrane Library. A comprehensive range of search terms were used, including “Immune Checkpoint Inhibitors [Mesh]”, “Immune Checkpoint Blockers”, “Pembrolizumab”, “Nivolumab”, “Atezolizumab”, “Stomach Neoplasms [MeSH]”, “Stomach Cancer”, “Gastric Cancer”, “Neutrophil to Lymphocyte Ratio", “NLR”, “Derived Neutrophil to Lymphocyte Ratio", and “dNLR”. Supplementary Table S1 has a thorough description of the search tactics. In addition to the primary database search, we conducted a supplementary search on Google Scholar to identify grey literature. Furthermore, we manually reviewed the reference lists of relevant articles to identify additional studies that may have been missed in the initial electronic search.
Only research publications that met the following criteria were considered for inclusion in our study: patients who had been diagnosed with GJGC were treated with ICIs, and the prognostic importance of NLR, also known as derived Neutrophil to Lymphocyte Ratio (dNLR), was evaluated. Moreover, the articles reported on at least one of the following endpoints: overall survival (OS), progression-free survival (PFS), objective response rate (ORR), and disease control rate (DCR). Studies that were excluded from the analysis included conference abstracts, editorials, case reports, and comments. In cases where there were patients who were included in more than one study, we chose only those studies that had the most comprehensive data and methodologies that were manually extracted (19).
We systematically extracted the following data from the eligible studies (1): Study characteristics: author(s), year of publication, study period, and region of study; (2) Participant characteristics: sample size, mean age, gender distribution; (3) Intervention details: therapeutic drugs used, cancer type, and cut-off values for NLR/dNLR; (4) Outcome measures: reported endpoints (OS, PFS, ORR, DCR), including hazard ratios (HR), odds ratios (OR), and confidence intervals (CI). For studies that included both univariate and multivariate analyses, we prioritized the extraction of multivariate data (HR and OR) as these analyses adjust for potential confounders and provide more reliable estimates. The quality of each observational study was assessed using the Newcastle-Ottawa Scale (NOS), with studies receiving a score of 6 or higher considered to be of excellent quality (20). This was calculated by examining three factors: method of patient selection, comparability of the study groups and number of outcomes reported. Two reviewers conducted literature search, literature screening, data extraction and literature quality assessment independently, and any disagreements were resolved by re-examining the relevant papers until a consensus was reached.
This analysis was conducted using Stata 15.0. The chi-squared test was used to assess heterogeneity. A random effects model was employed when the p-value was less than 0.1 or the I2 statistic exceeded 50%; otherwise, a fixed effects model was used (21). The estimation of publication bias was conducted using the Egger and Begg tests. If a publishing bias exists, the "trim and fill" method was additionally employed to evaluate the influence on the combined outcomes. In addition, a sensitivity analysis was performed by systematically omitting each study to evaluate the strength and reliability of the findings.
After eliminating duplicates and carefully reviewing titles and abstracts, 32 articles were subjected to full-text examination, out of which 24 articles with a total of 2221 patients were selected for analysis (22–45). Figure 1 shows the selection process using a PRISMA flow diagram. Table 1 summarizes the main features of the included studies.
Of the 24 studies, 20 were retrospective studies and 4 were prospective studies. Seventeen included patients with GJGC, while seven included patients with gastroesophageal junction or gastric cancer. The low risk of bias was indicated by the NOS ratings for 24 publications, which ranged from 6 to 8.
By examining the survival information from 20 trials with 1841 participants, we investigated the association between baseline NLR levels and OS in GJGC patients treated with ICIs. A random-effects model was utilized because of the significant heterogeneity among studies (I2 = 46.4%, p = 0.012). Our findings demonstrated that patients with high NLR levels had significantly worse OS (HR: 1.860, 95% CI: 1.564-2.213, p < 0.001) (Figure 2).
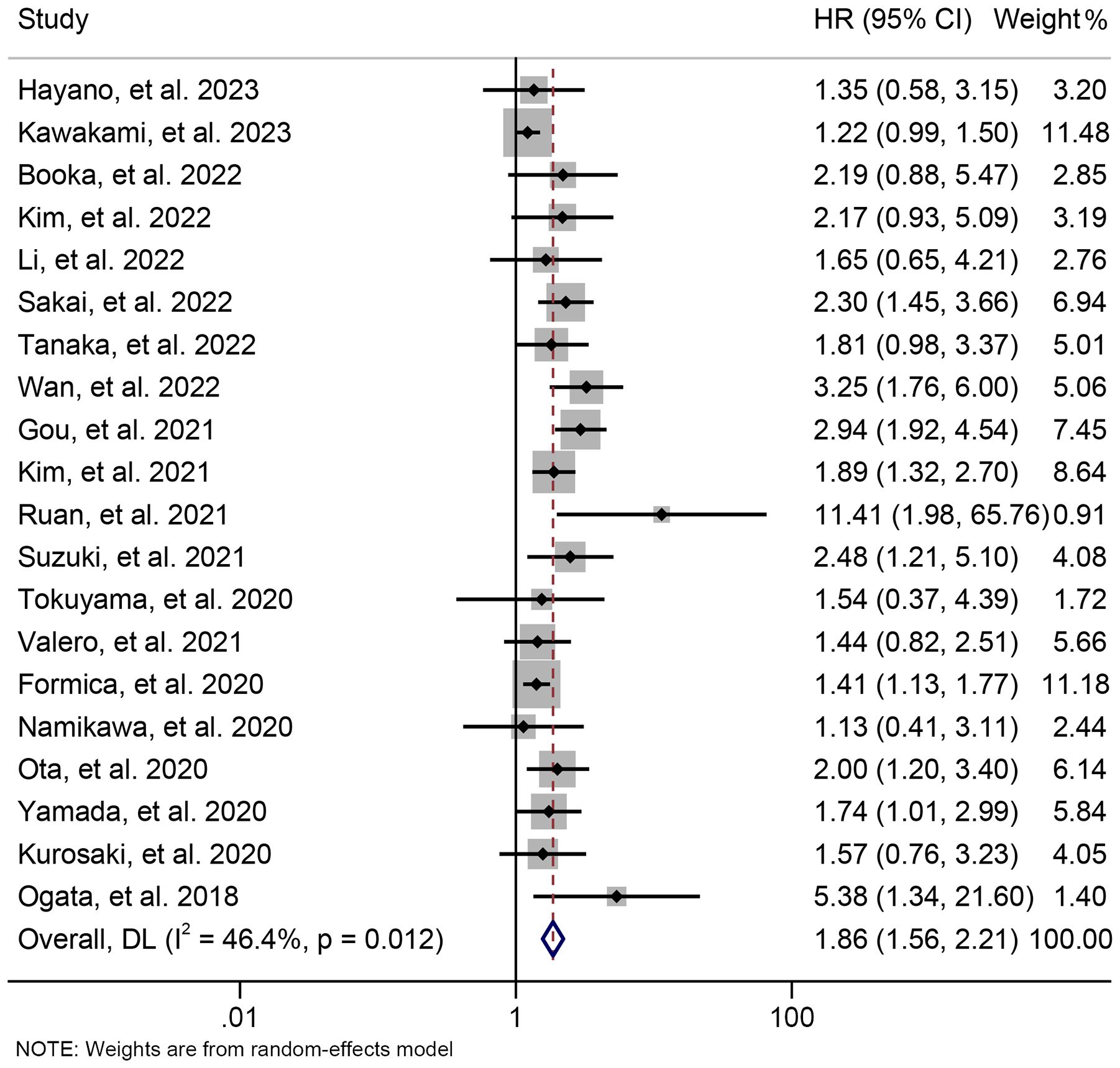
Figure 2. Forest plots of the relationship between neutrophil to lymphocyte ratio levels and overall survival. HR, hazard ratio; CL, confidence interval.
The subgroup analysis was conducted based on the cut-off values and the Cox model. The above findings were consistent in each subgroup, as demonstrated in Figure 3 and Supplementary Figure S1. Furthermore, a leave-one-out sensitivity analysis was carried out to evaluate the impact of each study on the overall results. The results indicated that the exclusion of any individual study did not significantly alter the pooled HR for OS, which ranged from 1.763 (95% CI: 1.496-2.078) after excluding Gou et al., 2021 to 1.935 (95% CI: 1.597-2.344) after excluding Formica et al., 2020 (Figure 4).
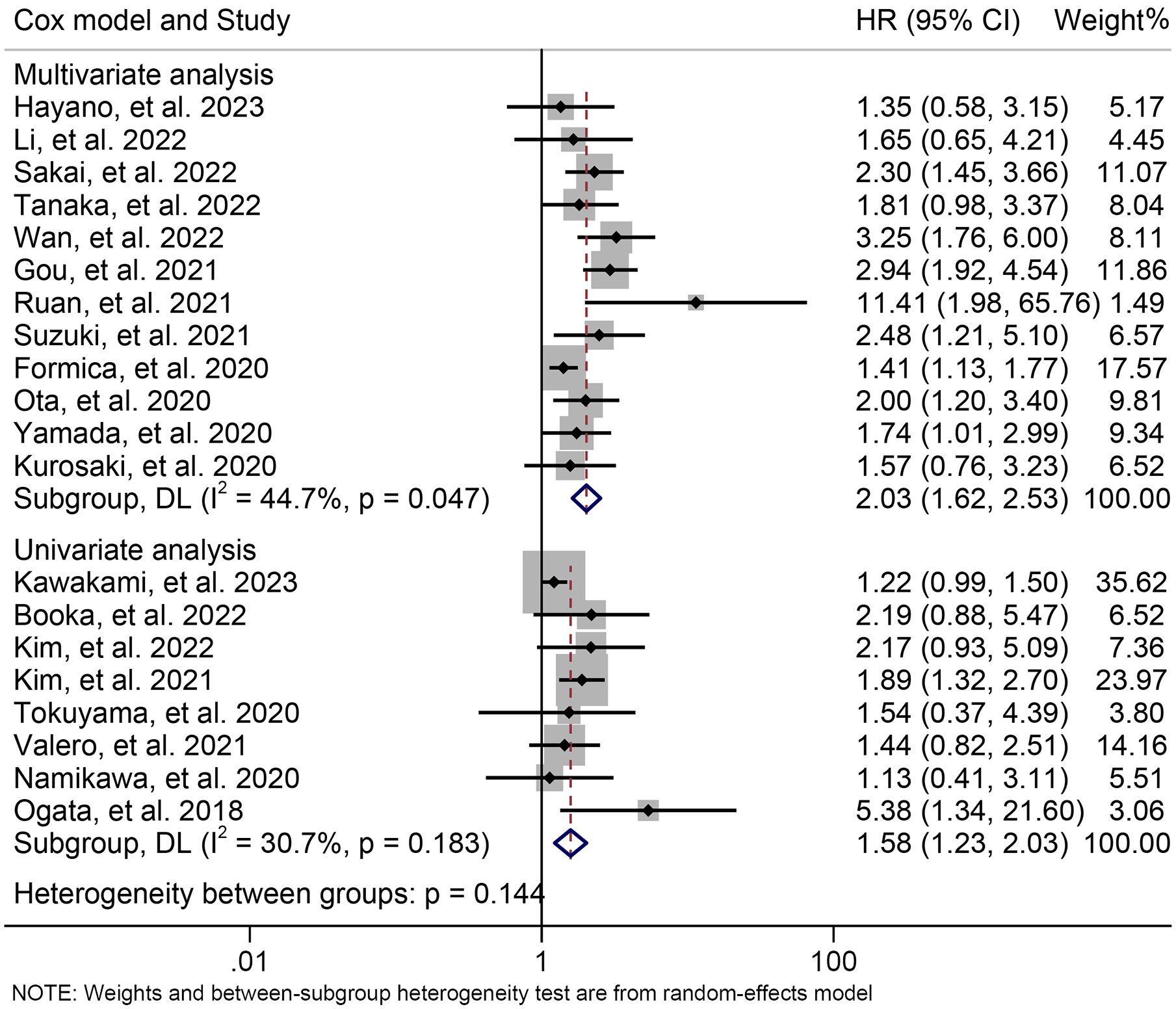
Figure 3. Subgroup analysis of the relationship between neutrophil to lymphocyte ratio levels and overall survival based on the Cox model. HR, hazard ratio; CL, confidence interval.
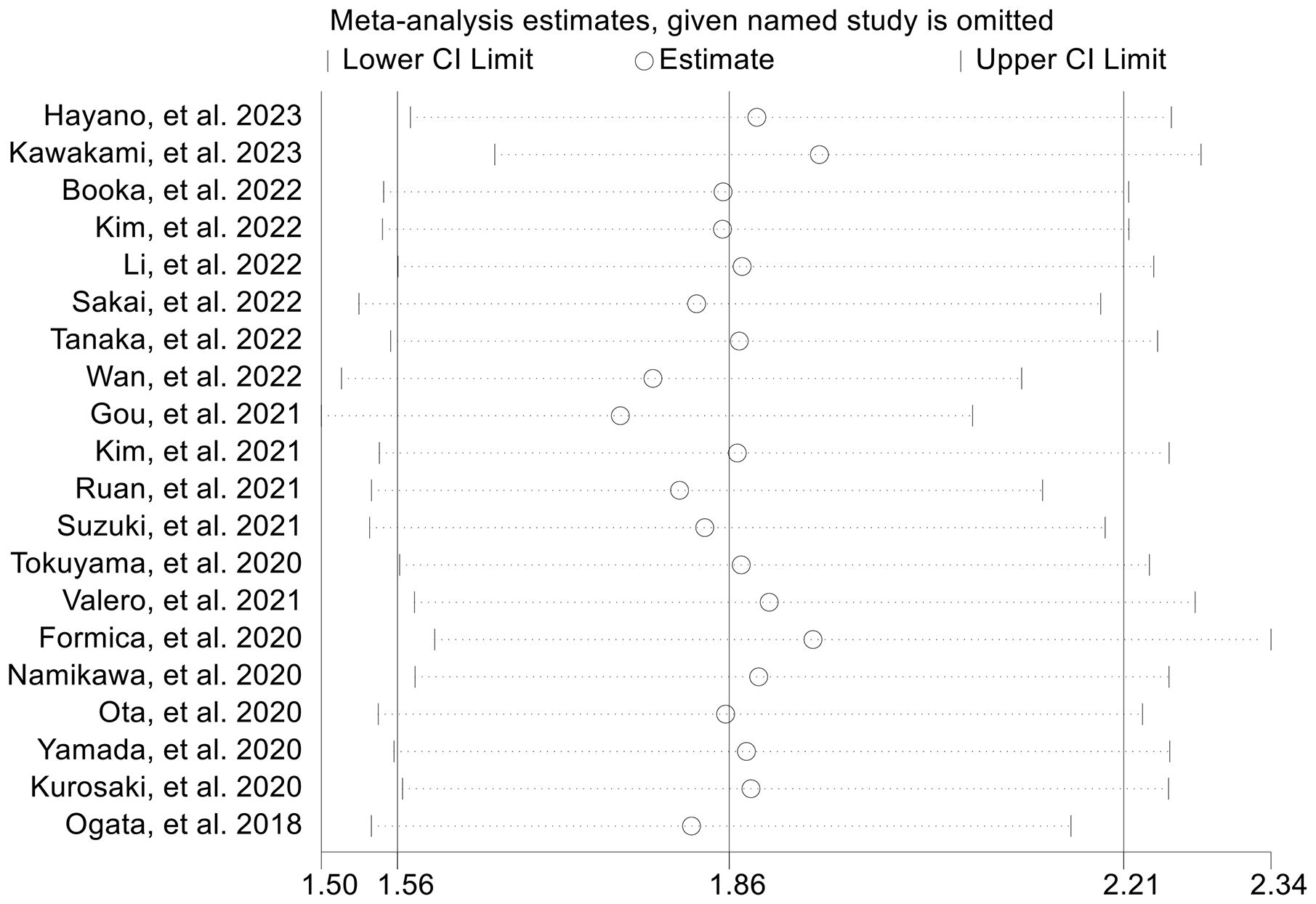
Figure 4. Sensitivity analysis of the association between baseline neutrophil to lymphocyte ratio levels and overall survival. CL, confidence interval.
Using data from 14 studies involving 1300 patients, we examined the relationship between baseline NLR levels and PFS in ICI-treated GJGC patients. Our analysis revealed that high NLR levels were related to a 67.8% increase in the risk of progression (HR: 1.678, 95% CI: 1.354-2.079, p < 0.001, Figure 5). Due to significant heterogeneity (I2 = 59.5%, p = 0.002), we employed a random-effects model.
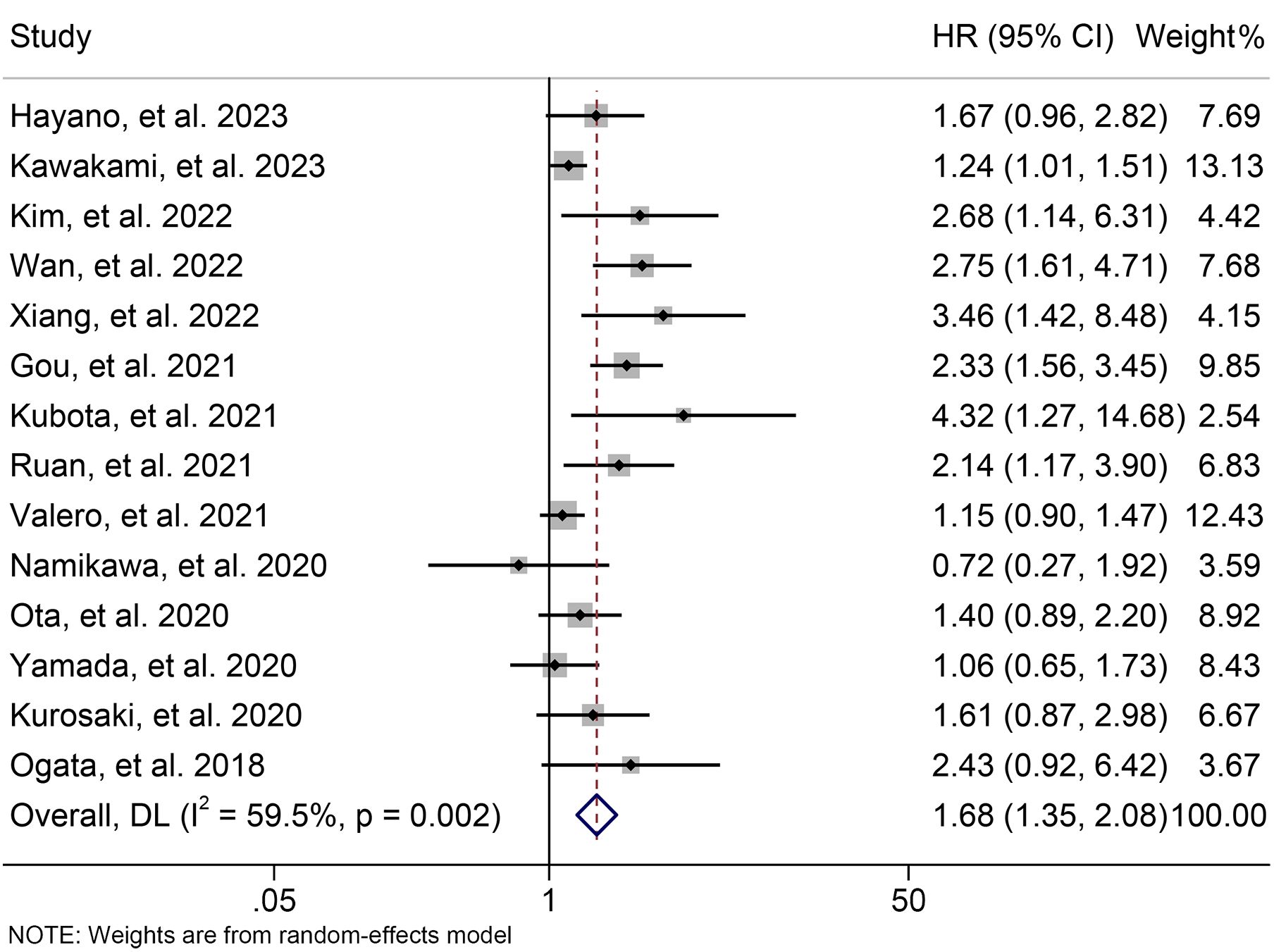
Figure 5. Forest plots of the relationship between neutrophil to lymphocyte ratio levels and progression-free survival. HR, hazard ratio; CL, confidence interval.
The subgroup analysis demonstrated that the above findings hold for both multivariate and univariate analyses (Figure 6). Further subgroup analysis based on the cutoff value showed that high NLR levels were significantly linked to a shorter PFS when the cutoff value exceeded 2.5 (Supplementary Figure S2). However, we found no significant association between NLR levels and PFS when the cutoff value was ≤ 2.5 (Supplementary Figure S2). Moreover, the sensitivity analysis revealed that the exclusion of any single study did not result in a significant alteration of the pooled HR for PFS. The range of HR values was from 1.604 (95% CI: 1.294- 1.989) when removing Gou, et al., 2021 to 1.771 (95% CI: 1.387-2.261) when removing Kawakami, et al., 2023 (Figure 7).
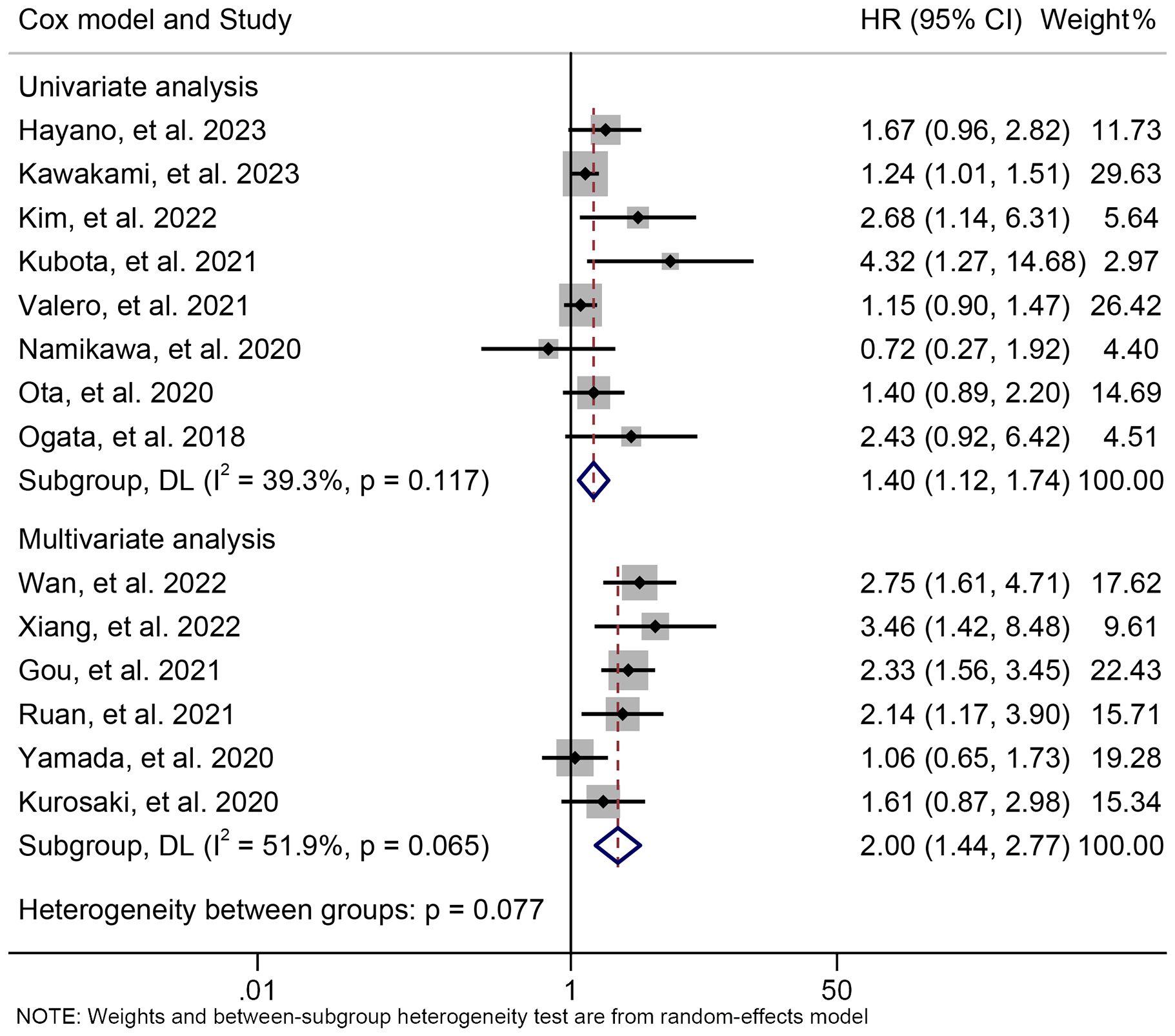
Figure 6. Subgroup analysis of the relationship between neutrophil to lymphocyte ratio levels and progression-free survival based on the Cox model. HR, hazard ratio; CL, confidence interval.
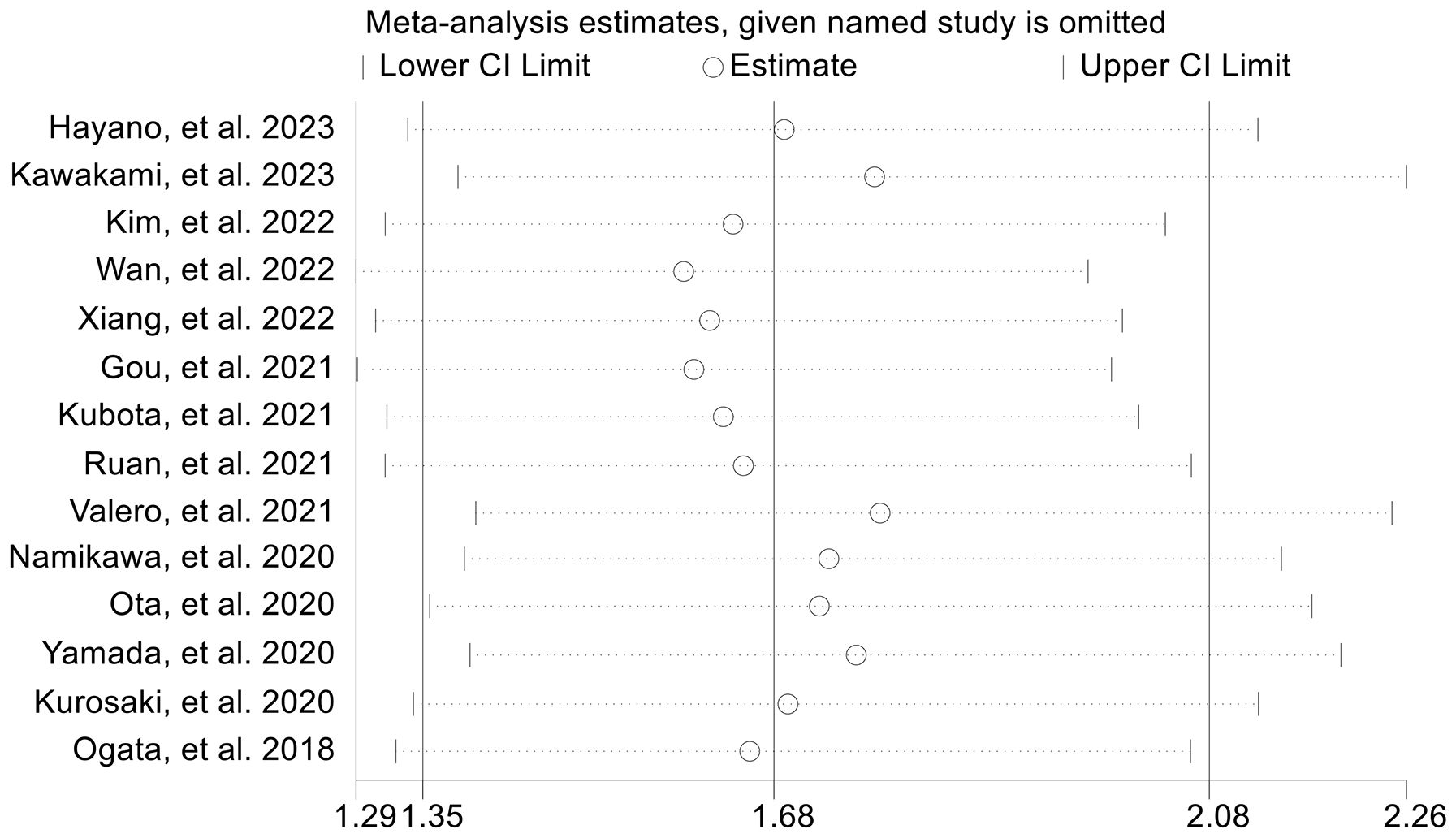
Figure 7. Sensitivity analysis of the association between baseline neutrophil to lymphocyte ratio levels and progression-free survival. CL, confidence interval.
Next, we evaluated the relationship between NLR levels and response to ICI treatment in GJGC patients. As shown in Figures 8A, B, no significant heterogeneity was observed, so a fixed-effect model was used. We found that the ORR (8 studies with 694 patients, OR: 0.754, 95% CI: 0.621-0.915, p = 0.004, Figure 8A) and DCR (8 studies with 694 patients, OR: 0.391, 95% CI: 0.262-0.582, p < 0.001, Figure 8B) were lower in GJGC patients with high NLR levels. Besides, the pooled HRs for ORR (Figure 9A) and DCR (Figure 9B) were not significantly different in the sensitivity analysis.
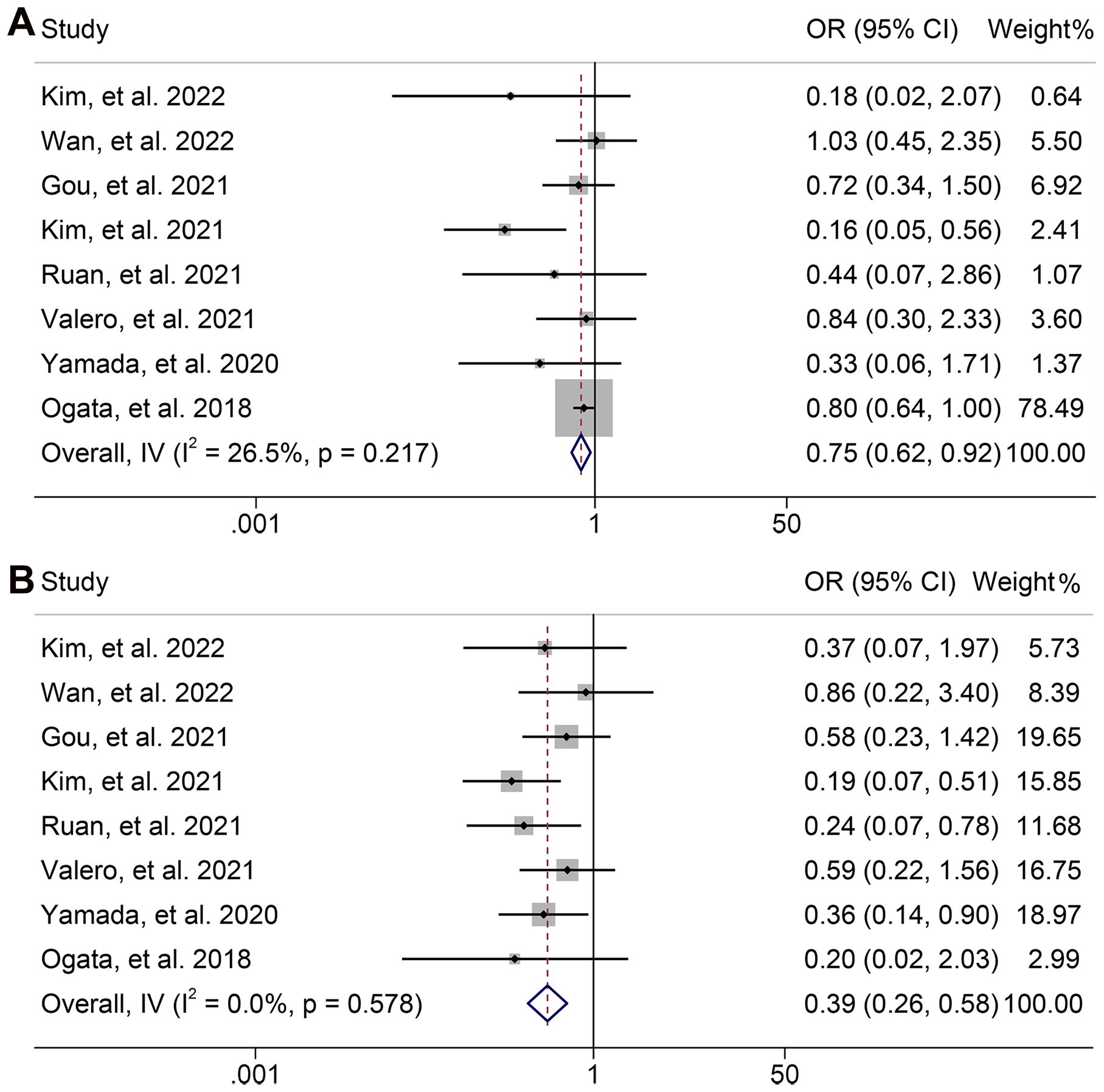
Figure 8. Forest plots of the relationship between neutrophil to lymphocyte ratio levels and objective response rate (A) and disease control rate (B). OR, odds ratio; CL, confidence interval.
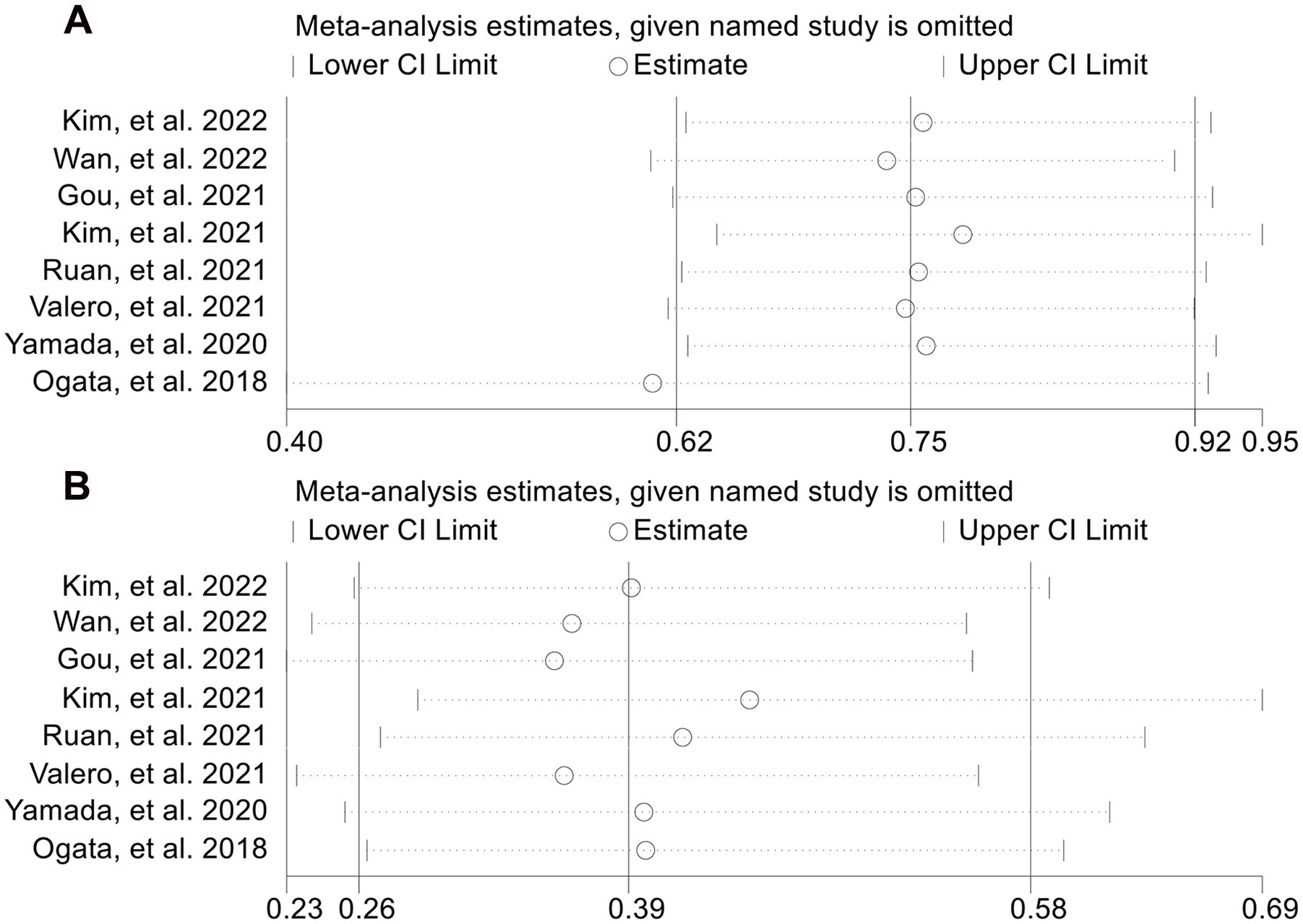
Figure 9. Sensitivity analysis of the association between baseline neutrophil to lymphocyte ratio levels and objective response rate (A) and disease control rate (B). CL, confidence interval.
A total of three articles comprising 405 patients and two studies involving 338 participants were included in the analysis to investigate the relationship between dNLR levels and OS and PFS in GJGC patients receiving ICI treatment, respectively. The pooled results revealed that high dNLR levels were significantly associated with shorter OS (HR: 2.117, 95% CI: 1.590-2.820, p < 0.001; I2 = 47.6%, p = 0.149, Figure 10A) and PFS (HR: 1.803, 95% CI: 1.415-2.297, p < 0.001; I2 = 0.0%, P = 0.984, Figure 10B).
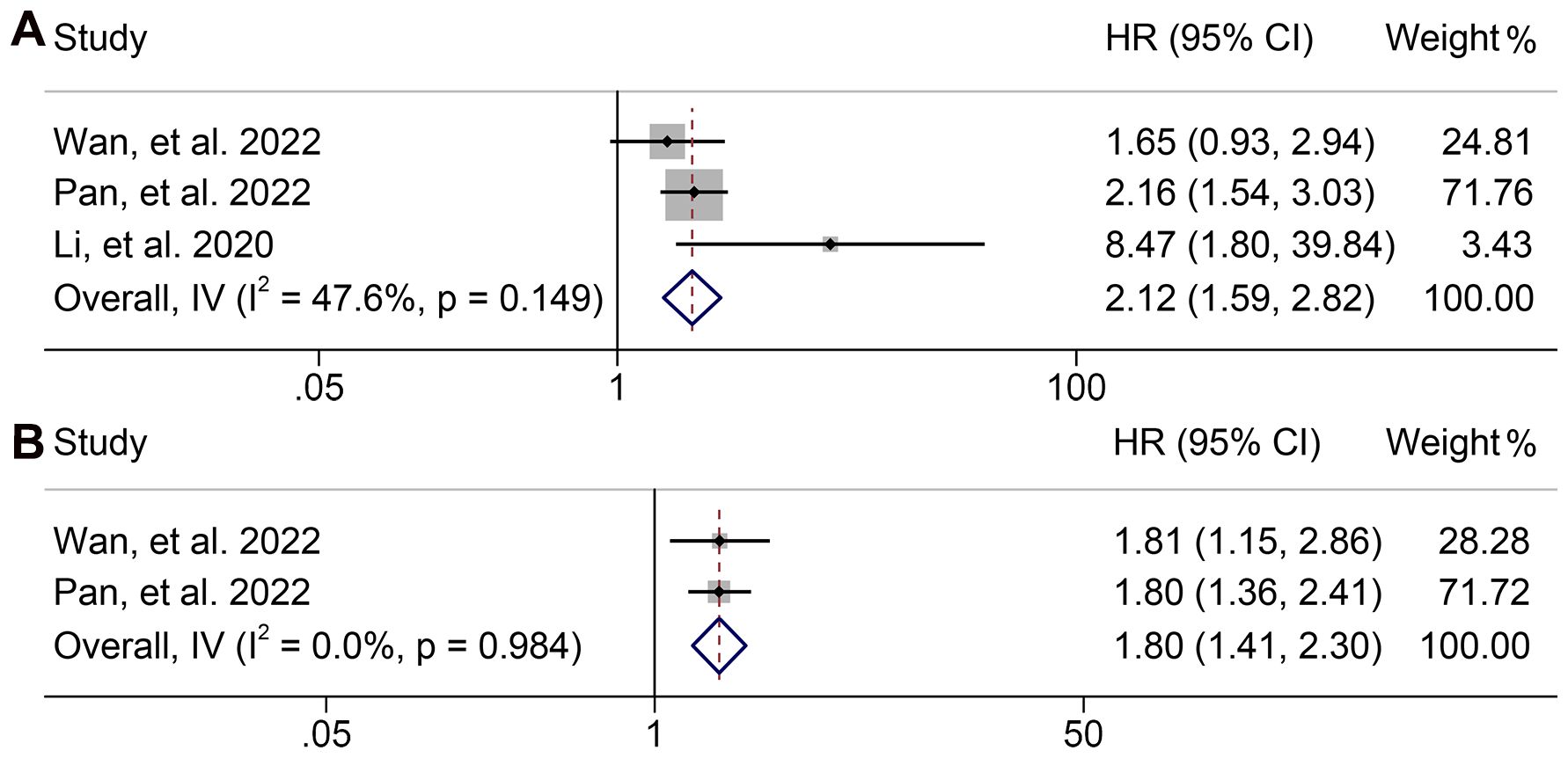
Figure 10. Forest plots of the relationship between derived neutrophil to lymphocyte ratio levels and overall survival (A) and progression-free survival (B). HR, hazard ratio; CL, confidence interval.
We conducted Begg's and Egger's tests to evaluate potential publication bias in our analysis of NLR. The findings indicated the absence of significant publication bias concerning the ORR (Egger: p = 0.095, Begg: p = 0.073) and DCR (Egger: p = 0.727, Begg: p = 0.711). Nevertheless, in the assessment of OS (Egger: p = 0.006, Begg: p = 0.820) and PFS (Egger: p = 0.019, Begg: p = 0.228) using Egger's test, we identified evidence of publication bias.
To mitigate this concern, we applied the trim and fill method to estimate the potential number of omitted studies in OS and PFS. Following the incorporation of hypothetical missing studies, we recalculated the combined HR for OS (HR: 1.494, 95% CI: 1.237-1.805, p < 0.001) and PFS (HR: 1.393, 95% CI: 1.117-1.738, p < 0.001). Notably, this re-estimated value exhibited no significant deviation from the original result (see Supplementary Figure S3).
Our study sought to examine the prognostic relevance of NLR in GJGC patients undergoing ICI treatment. Our meta-analysis confirmed a robust correlation between high NLR and inferior OS and PFS and lower ORR and DCR outcomes. Subgroup analysis revealed that NLR cutoff values greater than 2.5 had stronger predictive efficacy. In addition, we also confirmed that high dNLR levels were significantly related to shorter OS and PFS. Our study provide a comprehensive analysis of the influence of NLR or dNLR on the prognosis of ICI-treated GJGC patients. Given that NLR is a routine clinical parameter, evaluating its levels before ICI therapy could assist clinicians in predicting clinical outcomes more precisely and efficiently. This knowledge can facilitate timely adjustments to treatment plans and potentially enhance therapeutic benefits.
Kotecha et al. revealed an association between NLR and lymph node positive status in gastric cancer patients, which has important implications for staging and preoperative personalized treatment (46). Mellor et al. confirmed that NLR is an important prognostic indicator of OS and DFS after R0 resection of gastric cancer (47). Our study looked at ICI-treated patients with GJGC and confirmed the predictive value of NLR in such patients.
Cancer growth and progression are known to be characterized by ongoing inflammation and immune system evasion (48). The NLR has been suggested as a surrogate indicator of both inflammatory status and adaptive immune surveillance, reflecting the equilibrium between these two forces. The relationship between NLR and ICI outcomes may be explained by the association between circulating neutrophils and tumor microenvironment neutrophils (49–51).
Reactive oxygen species, matrix metalloproteinase 9, and vascular endothelial growth factor are examples of angiogenic and immunosuppressive mediators that can be produced by neutrophil infiltration and contribute to the development of a pro-tumor microenvironment (52–54). According to a study by Hiramatsu et al., there is a positive association between the NLR and the number of infiltrating tumor-associated neutrophils in the GJGC (55). Besides, Choi et al. confirmed that an elevated NLR is related to a reduced density of CD4+ lymphocytes in the tumor microenvironment (56). Furthermore, higher levels of myeloid cells, including neutrophils, may further suppress the T-cell response (57, 58). All of these elements combine to build an immunosuppressive tumor environment, which may lessen the likelihood that ICIs will work. Interestingly, recent studies suggest that neutrophils in the peripheral blood can interact with circulating tumor cells and enhance their metastatic potential by promoting cell cycle progression and accelerating metastasis seeding (59–62). These theories significantly support our finding that NLR levels can be a valid indicator of response to ICI in GJGC patients.
Proctor et al. have introduced the dNLR, which is calculated as the ratio of the neutrophil count to the difference between the white cell count and the neutrophil count (63). We also found that high dNLR levels also predicted a poor prognosis for ICI-treated GJGC patients. Then, the number of included studies was too small, and the finding needs to be further confirmed.
Our study provides preliminary evidence supporting the prognostic value of NLR and dNLR in predicting outcomes for ICI-treated GJGC patients. However, our findings also highlight areas where additional research is needed. First, as our results are largely based on data from retrospective studies and predominantly sourced from East Asian populations, it is crucial to conduct multicenter, prospective studies across diverse geographic and ethnic populations. This would help to validate our findings and improve their generalizability, ensuring they are applicable to a broader patient population. Additionally, future research should aim to identify the optimal NLR and dNLR cutoff values for clinical use, as these values may vary between different cancer subtypes and treatment regimens. Establishing standardized cutoff thresholds would facilitate the integration of NLR and dNLR measurements into routine clinical practice, providing a more consistent basis for treatment decision-making. Furthermore, mechanistic studies exploring the precise role of neutrophils and lymphocytes in the tumor microenvironment are warranted to elucidate the underlying biological pathways that link elevated NLR and dNLR with poorer ICI outcomes. Such insights could lead to targeted interventions aimed at modulating inflammatory and immune responses in the tumor microenvironment, potentially enhancing the efficacy of ICIs in patients with high NLR or dNLR.
In conclusion, our study underscores the prognostic relevance of NLR and dNLR in GJGC patients receiving ICI therapy, while also pointing toward critical avenues for future investigation. There is some heterogeneity in some results, so caution should be exercised in interpreting the conclusions. By addressing these areas, upcoming research can build on our findings, ultimately contributing to more precise and effective clinical management strategies for GJGC.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
CY: Conceptualization, Investigation, Methodology, Supervision, Writing – original draft, Writing – review & editing. HJ: Conceptualization, Data curation, Methodology, Writing – original draft. LW: Methodology, Writing – original draft. ZJ: Conceptualization, Methodology, Writing – original draft. CJ: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2025.1404695/full#supplementary-material
Supplementary Table S1 | The detailed search strategies for Pubmed.
Supplementary Figure S1 | Subgroup analysis of the relationship between neutrophil to lymphocyte ratio levels and overall survival based on cut-off. HR, hazard ratio; CL, confidence interval.
Supplementary Figure S2 | Subgroup analysis of the relationship between neutrophil to lymphocyte ratio levels and progression-free survival based on cut-off. HR, hazard ratio; CL, confidence interval.
Supplementary Figure S3 | The picture of the trim-and-fill method for OS (A) and PFS (B).
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2018) 68:394–424. doi: 10.3322/caac.21492
2. Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer. (2017) 20:1–19. doi: 10.1007/s10120-016-0622-4
3. Ajani JA, D'Amico TA, Almhanna K, Bentrem DJ, Chao J, Das P, et al. Gastric cancer, version 3.2016, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. (2016) 14:1286–312. doi: 10.6004/jnccn.2016.0137
4. Smyth EC, Verheij M, Allum W, Cunningham D, Cervantes A, Arnold D. Gastric cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. (2016) 27:v38–49. doi: 10.1093/annonc/mdw350
5. Ding P, Yang P, Yang L, Sun C, Chen S, Li M, et al. Impact of skeletal muscle loss during conversion therapy on clinical outcomes in lavage cytology positive patients with gastric cancer. Front Oncol. (2022) 12:949511. doi: 10.3389/fonc.2022.949511
6. Ding P, Lv J, Sun C, Chen S, Yang P, Tian Y, et al. Combined systemic inflammatory immunity index and prognostic nutritional index scores as a screening marker for sarcopenia in patients with locally advanced gastric cancer. Front Nutr. (2022) 9:981533. doi: 10.3389/fnut.2022.981533
7. Kang YK, Boku N, Satoh T, Ryu MH, Chao Y, Kato K, et al. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. (2017) 390:2461–71. doi: 10.1016/S0140-6736(17)31827-5
8. Janjigian YY, Bendell J, Calvo E, Kim JW, Ascierto PA, Sharma P, et al. CheckMate-032 study: efficacy and safety of nivolumab and nivolumab plus ipilimumab in patients with metastatic esophagogastric cancer. J Clin Oncol. (2018) 36:2836–44. doi: 10.1200/JCO.2017.76.6212
9. Samstein RM, Lee CH, Shoushtari AN, Hellmann MD, Shen R, Janjigian YY, et al. Tumor mutational load predicts survival after immunotherapy across multiple cancer types. Nat Genet. (2019) 51:202–6. doi: 10.1038/s41588-018-0312-8
10. Chowell D, Morris LGT, Grigg CM, Weber JK, Samstein RM, Makarov V, et al. Patient HLA class I genotype influences cancer response to checkpoint blockade immunotherapy. Science. (2018) 359:582–7. doi: 10.1126/science.aao4572
11. Le DT, Durham JN, Smith KN, Wang H, Bartlett BR, Aulakh LK, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. (2017) 357:409–13. doi: 10.1126/science.aan6733
12. Havel JJ, Chowell D, Chan TA. The evolving landscape of biomarkers for checkpoint inhibitor immunotherapy. Nat Rev Cancer. (2019) 19:133–50. doi: 10.1038/s41568-019-0116-x
13. Anagnostou V, Niknafs N, Marrone K, Bruhm DC, White JR, Naidoo J, et al. Multimodal genomic features predict outcome of immune checkpoint blockade in non-small-cell lung cancer. Nat Cancer. (2020) 1:99–111. doi: 10.1038/s43018-019-0008-8
14. Savic Prince S, Bubendorf L. Predictive potential and need for standardization of PD-L1 immunohistochemistry. Virchows Arch. (2019) 474:475–84. doi: 10.1007/s00428-018-2445-7
15. Templeton AJ, McNamara MG, Šeruga B, Vera-Badillo FE, Aneja P, Ocaña A, et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst. (2014) 106:dju124. doi: 10.1093/jnci/dju124
16. Leng J, Wu F, Zhang L. Prognostic significance of pretreatment neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, or monocyte-to-lymphocyte ratio in endometrial neoplasms: A systematic review and meta-analysis. Front Oncol. (2022) 12:734948. doi: 10.3389/fonc.2022.734948
17. Zhang L, Feng J, Kuang T, Chai D, Qiu Z, Deng W, et al. Blood biomarkers predict outcomes in patients with hepatocellular carcinoma treated with immune checkpoint Inhibitors: A pooled analysis of 44 retrospective sudies. Int Immunopharmacol. (2023) 118:110019. doi: 10.1016/j.intimp.2023.110019
18. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. (2009) 6:e1000100. doi: 10.7326/0003-4819-151-4-200908180-00136
19. Zhang L, Kuang T, Chai D, Deng W, Wang P, Wang W. The use of antibiotics during immune checkpoint inhibitor treatment is associated with lower survival in advanced esophagogastric cancer. Int Immunopharmacol. (2023) 119:110200. doi: 10.1016/j.intimp.2023.110200
20. Zhang L, Chen C, Chai D, Li C, Guan Y, Liu L, et al. The association between antibiotic use and outcomes of HCC patients treated with immune checkpoint inhibitors. Front Immunol. (2022) 13:956533. doi: 10.3389/fimmu.2022.956533
21. Zhang L, Chen C, Chai D, Li C, Kuang T, Liu L, et al. Effects of PPIs use on clinical outcomes of urothelial cancer patients receiving immune checkpoint inhibitor therapy. Front Pharmacol. (2022) 13:1018411. doi: 10.3389/fphar.2022.1018411
22. Ogata T, Satake H, Ogata M, Hatachi Y, Inoue K, Hamada M, et al. Neutrophil-to-lymphocyte ratio as a predictive or prognostic factor for gastric cancer treated with nivolumab: a multicenter retrospective study. Oncotarget. (2018) 9:34520–7. doi: 10.18632/oncotarget.26145
23. Formica V, Morelli C, Patrikidou A, Murias C, Butt S, Nardecchia A, et al. Gastric inflammatory prognostic index (GIPI) in patients with metastatic gastro-esophageal junction/gastric cancer treated with PD-1/PD-L1 immune checkpoint inhibitors. Target Oncol. (2020) 15:327–36. doi: 10.1007/s11523-020-00723-z
24. Li S, Zou J, Liu C, Jiao X, Gong J, Li J, et al. Baseline derived neutrophil-to-lymphocyte ratio as a prognostic biomarker for non-colorectal gastrointestinal cancer patients treated with immune checkpoint blockade. Clin Immunol. (2020) 212:108345. doi: 10.1016/j.clim.2020.108345
25. Namikawa T, Yokota K, Tanioka N, Fukudome I, Iwabu J, Munekage M, et al. Systemic inflammatory response and nutritional biomarkers as predictors of nivolumab efficacy for gastric cancer. Surg Today. (2020) 50:1486–95. doi: 10.1007/s00595-020-02048-w
26. Ota Y, Takahari D, Suzuki T, Osumi H, Nakayama I, Oki A, et al. Changes in the neutrophil-to-lymphocyte ratio during nivolumab monotherapy are associated with gastric cancer survival. Cancer Chemother Pharmacol. (2020) 85:265–72. doi: 10.1007/s00280-019-04023-w
27. Yamada T, Hayashi T, Inokuchi Y, Hayashi K, Watanabe H, Komori K, et al. Impact of the neutrophil-to-lymphocyte ratio on the survival of patients with gastric cancer treated with nivolumab monotherapy. Target Oncol. (2020) 15:317–25. doi: 10.1007/s11523-020-00716-y
28. Gou M, Qu T, Wang Z, Yan H, Si Y, Zhang Y, et al. Neutrophil-to-lymphocyte ratio (NLR) predicts PD-1 inhibitor survival in patients with metastatic gastric cancer. J Immunol Res. (2021) 2021:2549295. doi: 10.1155/2021/2549295
29. Kim N, Yu JI, Lim DH, Lee J, Kim ST, Hong JY, et al. Prognostic impact of sarcopenia and radiotherapy in patients with advanced gastric cancer treated with anti-PD-1 antibody. Front Immunol. (2021) 12:701668. doi: 10.3389/fimmu.2021.701668
30. Kubota Y, Yoshimura K, Hamada K, Hirasawa Y, Shida M, Taniguchi M, et al. Rare nivolumab-associated super hyper progressive disease in patients with advanced gastric cancer. In Vivo. (2021) 35:1865–75. doi: 10.21873/invivo.12449
31. Pan Y, Si H, Deng G, Chen S, Zhang N, Zhou Q, et al. A composite biomarker of derived neutrophil-lymphocyte ratio and platelet-lymphocyte ratio correlates with outcomes in advanced gastric cancer patients treated with anti-PD-1 antibodies. Front Oncol. (2021) 11:798415. doi: 10.3389/fonc.2021.798415
32. Ruan DY, Chen YX, Wei XL, Wang YN, Wang ZX, Wu HX, et al. Elevated peripheral blood neutrophil-to-lymphocyte ratio is associated with an immunosuppressive tumour microenvironment and decreased benefit of PD-1 antibody in advanced gastric cancer. Gastroenterol Rep (Oxf). (2021) 9:560–70. doi: 10.1093/gastro/goab032
33. Suzuki H, Yamada T, Sugaya A, Ueyama S, Yamamoto Y, Moriwaki T, et al. Retrospective analysis for the efficacy and safety of nivolumab in advanced gastric cancer patients according to ascites burden. Int J Clin Oncol. (2021) 26:370–7. doi: 10.1007/s10147-020-01810-x
34. Valero C, Lee M, Hoen D, Weiss K, Kelly DW, Adusumilli PS, et al. Pretreatment neutrophil-to-lymphocyte ratio and mutational burden as biomarkers of tumor response to immune checkpoint inhibitors. Nat Commun. (2021) 12(1):729. doi: 10.1038/s41467-021-20935-9
35. Booka E, Kikuchi H, Haneda R, Soneda W, Kawata S, Murakami T, et al. Neutrophil-to-lymphocyte ratio to predict the efficacy of immune checkpoint inhibitor in upper gastrointestinal cancer. Anticancer Res. (2022) 42:2977–87. doi: 10.21873/anticanres.15781
36. Kim JH, Ryu MH, Park YS, Ma J, Lee SY, Kim D, et al. Predictive biomarkers for the efficacy of nivolumab as ≥ 3(rd)-line therapy in patients with advanced gastric cancer: a subset analysis of ATTRACTION-2 phase III trial. BMC Cancer. (2022) 22:378. doi: 10.1186/s12885-022-09488-2
37. Li T, Liu T, Zhao L, Liu L, Zheng X, Wang J, et al. Effectiveness and safety of anti-PD-1 monotherapy or combination therapy in Chinese advanced gastric cancer: A real-world study. Front Oncol. (2022) 12:976078. doi: 10.3389/fonc.2022.976078
38. Sakai D, Omori T, Fumita S, Fujita J, Kawabata R, Matsuyama J, et al. Real-world effectiveness of third- or later-line treatment in Japanese patients with HER2-positive, unresectable, recurrent or metastatic gastric cancer: a retrospective observational study. Int J Clin Oncol. (2022) 27:1154–63. doi: 10.1007/s10147-022-02162-4
39. Tanaka K, Tanabe H, Sato H, Ishikawa C, Goto M, Yanagida N, et al. Prognostic factors to predict the survival in patients with advanced gastric cancer who receive later-line nivolumab monotherapy-The Asahikawa Gastric Cancer Cohort Study (AGCC). Cancer Med. (2022) 11:406–16. doi: 10.1002/cam4.v11.2
40. Wan M, Ding Y, Mao C, Ma X, Li N, Xiao C, et al. Association of inflammatory markers with survival in patients with advanced gastric cancer treated with immune checkpoint inhibitors combined with chemotherapy as first line treatment. Front Oncol. (2022) 12:1029960. doi: 10.3389/fonc.2022.1029960
41. Xiang J, Gong W, Sun P, Wang X, Liu A. Efficacy and safety of camrelizumab plus chemotherapy versus chemotherapy alone in patients with untreated, HER2-negative, unresectable locally advanced, or metastatic gastric cancer or gastroesophageal junction cancer: a retrospective comparative cohort study. J Gastrointest Oncol. (2022) 13:2874–84. doi: 10.21037/jgo-22-1229
42. Hayano K, Ohira G, Kano M, Suito H, Matsumoto Y, Kurata Y, et al. Prognostic impact of hepatic steatosis evaluated by CT on immunotherapy for gastric cancer: associations with sarcopenia, systemic inflammation, and hormones. Oncology. (2023) 101:185–92. doi: 10.1159/000528005
43. Kawakami H, Sunakawa Y, Inoue E, Matoba R, Noda K, Sato T, et al. Soluble programmed cell death ligand 1 predicts prognosis for gastric cancer patients treated with nivolumab: Blood-based biomarker analysis for the DELIVER trial. Eur J Cancer. (2023) 184:10–20. doi: 10.1016/j.ejca.2023.02.003
44. Tokuyama N, Takegawa N, Nishikawa M, Sakai A, Mimura T, Kushida S, et al. Pretreatment Glasgow prognostic score as a predictor of outcomes in nivolumab-treated patients with advanced gastric cancer. PLoS One. (2021) 16:e0247645. doi: 10.1371/journal.pone.0247645
45. Kurosaki T, Kawakami H, Mitani S, Kawabata R, Takahama T, Nonagase Y, et al. Glasgow prognostic score (GPS) and tumor response as biomarkers of nivolumab monotherapy in third- or later-line setting for advanced gastric cancer. In Vivo. (2020) 34:1921–9. doi: 10.21873/invivo.11989
46. Kotecha K, Singla A, Townend P, Merrett N. Association between neutrophil-lymphocyte ratio and lymph node metastasis in gastric cancer: A meta-analysis. Med (Baltimore). (2022) 101:e29300. doi: 10.1097/MD.0000000000029300
47. Mellor KL, Powell A, Lewis WG. Systematic review and meta-analysis of the prognostic significance of neutrophil-lymphocyte ratio (NLR) after R0 gastrectomy for cancer. J Gastrointest Cancer. (2018) 49:237–44. doi: 10.1007/s12029-018-0127-y
48. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. (2011) 144:646–74. doi: 10.1016/j.cell.2011.02.013
49. Takakura K, Ito Z, Suka M, Kanai T, Matsumoto Y, Odahara S, et al. Comprehensive assessment of the prognosis of pancreatic cancer: peripheral blood neutrophil-lymphocyte ratio and immunohistochemical analyses of the tumour site. Scand J Gastroenterol. (2016) 51:610–7. doi: 10.3109/00365521.2015.1121515
50. Ohki S, Shibata M, Gonda K, Machida T, Shimura T, Nakamura I, et al. Circulating myeloid-derived suppressor cells are increased and correlate to immune suppression, inflammation and hypoproteinemia in patients with cancer. Oncol Rep. (2012) 28:453–8. doi: 10.3892/or.2012.1812
51. Moses K, Brandau S. Human neutrophils: Their role in cancer and relation to myeloid-derived suppressor cells. Semin Immunol. (2016) 28:187–96. doi: 10.1016/j.smim.2016.03.018
52. Yuan Q, Deng D, Pan C, Ren J, Wei T, Wu Z, et al. Integration of transcriptomics, proteomics, and metabolomics data to reveal HER2-associated metabolic heterogeneity in gastric cancer with response to immunotherapy and neoadjuvant chemotherapy. Front Immunol. (2022) 13:951137. doi: 10.3389/fimmu.2022.951137
53. Piccard H, Muschel RJ, Opdenakker G. On the dual roles and polarized phenotypes of neutrophils in tumor development and progression. Crit Rev Oncol Hematol. (2012) 82:296–309. doi: 10.1016/j.critrevonc.2011.06.004
54. Nassar AH, Mouw KW, Jegede O, Shinagare AB, Kim J, Liu CJ, et al. A model combining clinical and genomic factors to predict response to PD-1/PD-L1 blockade in advanced urothelial carcinoma. Br J Cancer. (2020) 122:555–63. doi: 10.1038/s41416-019-0686-0
55. Hiramatsu S, Tanaka H, Nishimura J, Sakimura C, Tamura T, Toyokawa T, et al. Neutrophils in primary gastric tumors are correlated with neutrophil infiltration in tumor-draining lymph nodes and the systemic inflammatory response. BMC Immunol. (2018) 19:13. doi: 10.1186/s12865-018-0251-2
56. Choi Y, Kim JW, Nam KH, Han SH, Kim JW, Ahn SH, et al. Systemic inflammation is associated with the density of immune cells in the tumor microenvironment of gastric cancer. Gastric Cancer. (2017) 20:602–11. doi: 10.1007/s10120-016-0642-0
57. Zito Marino F, Ascierto PA, Rossi G, Staibano S, Montella M, Russo D, et al. Are tumor-infiltrating lymphocytes protagonists or background actors in patient selection for cancer immunotherapy? Expert Opin Biol Ther. (2017) 17:735–46. doi: 10.1080/14712598.2017.1309387
58. Gonzalez H, Hagerling C, Werb Z. Roles of the immune system in cancer: from tumor initiation to metastatic progression. Genes Dev. (2018) 32:1267–84. doi: 10.1101/gad.314617.118
59. Yuan Q, Zhang W, Shang W. Identification and validation of a prognostic risk-scoring model based on sphingolipid metabolism-associated cluster in colon adenocarcinoma. Front Endocrinol (Lausanne). (2022) 13:1045167. doi: 10.3389/fendo.2022.1045167
60. Sprouse ML, Welte T, Boral D, Liu HN, Yin W, Vishnoi M, et al. PMN-MDSCs Enhance CTC Metastatic Properties through Reciprocal Interactions via ROS/Notch/Nodal Signaling. Int J Mol Sci. (2019) 20(8):1916. doi: 10.3390/ijms20081916
61. Tüting T, de Visser KE. CANCER. How neutrophils promote metastasis. Science. (2016) 352:145–6. doi: 10.1126/science.aaf7300
62. Szczerba BM, Castro-Giner F, Vetter M, Krol I, Gkountela S, Landin J, et al. Neutrophils escort circulating tumour cells to enable cell cycle progression. Nature. (2019) 566:553–7. doi: 10.1038/s41586-019-0915-y
Keywords: immune checkpoint inhibitors, gastroesophageal junction or gastric cancer, neutrophil to lymphocyte ratio, derived neutrophil to lymphocyte ratio, prognosis
Citation: Yu C, Jiang H, Wang L, Jiang Z and Jin C (2025) Baseline (derived) neutrophil-lymphocyte ratio associated with survival in gastroesophageal junction or gastric cancer treated with ICIs. Front. Oncol. 15:1404695. doi: 10.3389/fonc.2025.1404695
Received: 21 March 2024; Accepted: 06 January 2025;
Published: 24 January 2025.
Edited by:
Alessandro Mangogna, University of Udine, ItalyReviewed by:
Hongwei Cheng, University of Macau, ChinaCopyright © 2025 Yu, Jiang, Wang, Jiang and Jin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chong Jin, MTM3NTc2ODkwNjVAMTYzLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.