
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 04 February 2025
Sec. Surgical Oncology
Volume 14 - 2024 | https://doi.org/10.3389/fonc.2024.1533800
This article is part of the Research Topic Exploring Robotic-Assisted Techniques in Urologic Oncology: Challenges and Future Directions View all 3 articles
 Carolin Siech1*
Carolin Siech1* Mike Wenzel1
Mike Wenzel1 Georgina Knoblich1
Georgina Knoblich1 Cristina Cano Garcia1
Cristina Cano Garcia1 Clara Humke1
Clara Humke1 Felix Preisser2
Felix Preisser2 Miriam Traumann1
Miriam Traumann1 Luis A. Kluth1
Luis A. Kluth1 Felix K. H. Chun1†
Felix K. H. Chun1† Philipp Mandel1,2†
Philipp Mandel1,2†Objective: To investigate the association between the interval from biopsy to radical prostatectomy (RP) and biochemical recurrence (BCR) in prostate cancer patients.
Methods: Within a tertiary-care database (01/2014 to 06/2023), D’Amico intermediate- and high-risk prostate cancer patients were stratified according to interval from biopsy to RP (≤3 vs. >3-≤6 months). Kaplan-Meier survival analyses and Cox regression models addressed BCR.
Results: Of 680 patients, 328 vs. 153 exhibited intermediate-risk prostate cancer and had interval from biopsy to RP ≤3 vs. >3-≤6 months. Similarly, 158 vs. 41 exhibited high-risk prostate cancer and had interval from biopsy to RP ≤3 vs. >3-≤6 months. Median interval from biopsy to RP was 59 vs. 113 days in intermediate- and 55 vs. 117 days in high-risk patients, respectively. In both intermediate- and high-risk patients, rates of adverse histopathological outcomes, namely pT3/pT4, pN1, and R1 status, did not differ according to interval from biopsy to RP. In survival analyses, three-year BCR-free survival rates were 82 vs. 88% in intermediate-risk (p=0.5) and 76 vs. 75% in high-risk patients (p=1). In multivariable Cox regression models, BCR did not significantly differ according to interval from biopsy to RP in intermediate- (hazard ratio 0.85, 95% confidence interval 0.49-1.46; p=0.5) and high-risk patients (hazard ratio 1.05, 95% confidence interval 0.50-2.22; p=0.9).
Conclusions: Both intermediate- and high-risk prostate cancer patients with an interval from biopsy to RP >3-≤6 months did not differ from those treated with RP ≤3 months after biopsy, regarding adverse histopathological outcomes and BCR rates. Therefore, it might be safe to postpone RP up to six months.
Radical prostatectomy (RP) represents a well-established curative treatment option in patients with non-metastatic prostate cancer (1, 2). Various factors can cause patients to postpone RP rather than undergo immediate surgery after being diagnosed with prostate cancer. These factors may include patient-related factors such as difficulty in decision making regarding curative treatment options due to the availability of alternative oncologically equivalent strategies as external beam radiotherapy (EBRT) (2). Some patients, especially those who are well-informed, ask for second or third opinions and need time to make their decision. Other patients may need further treatment to optimise comorbidities prior to RP (3). Further potential reasons for delayed treatment include limited resources of the health care system. Especially during the COVID-19 pandemic, but also afterward, surgical capacities have been limited due to staff shortages (4). These factors may lead to long waiting lists for elective urooncologic procedures, such as RP (4). The question for both, surgeons and patients, remains how long RP can be postponed safely.
In a preliminary study, we observed no differences between patients undergoing RP ≤3 months vs. >3 and ≤6 months after diagnosis for postoperative tumor characteristics, such as non-organ confined pathologic tumor stage, lymph node invasion, and positive surgical margins in patients with intermediate- and high-risk prostate cancer (5). Conversely, a Canadian multicenter study observed a higher risk of biochemical recurrence (BCR) following surgery in high-risk prostate cancer patients with time to RP ≥3 months (6), despite no differences in pathological outcomes (7).
We addressed this uncertainty and hypothesized that prostate cancer patients with an interval from biopsy to RP >3 and ≤6 months do not differ from those with an interval from biopsy to RP ≤3 months regarding histopathological outcomes at RP as well as BCR rates after RP. To address this hypothesis, we used a contemporary cohort of D’Amico intermediate- and high-risk prostate cancer patients treated with RP in a tertiary care referral center.
Relying on a prospectively maintained database of a tertiary-care referral center, we retrospectively identified D’Amico intermediate- and high-risk histologically confirmed prostate cancer patients who were treated with open retropubic or robotic-assisted RP between January 2014 and June 2023 (Figure 1). Starting in November 2017, RP was routinely performed using the intraoperative frozen section technique (NEUROSAFE) and preserving the full functional length of the prostatic urethra (FFLU), as previously described by Preisser et al. (8).
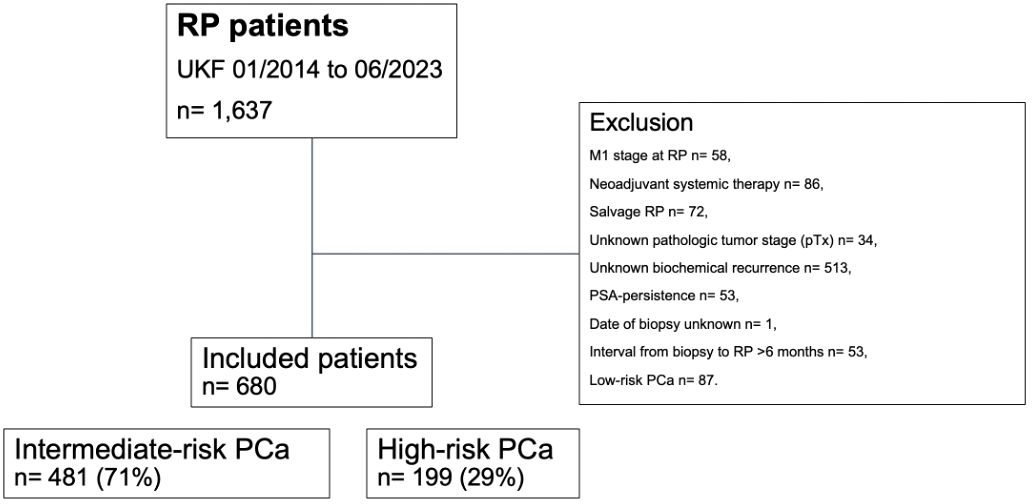
Figure 1. Consort diagram. PCa, prostate cancer; PSA, prostate-specific antigen; pT, pathologic tumor stage at surgery; RP, radical prostatectomy; UKF, University hospital Frankfurt.
Patients with D’Amico low-risk prostate cancer were not included in the study cohort, as they should be subjected to active surveillance in accordance with current guideline treatment recommendations (9–11). Inclusion criteria consisted of known follow-up regarding BCR and absence of prostate-specific antigen (PSA) persistence, defined as post-RP PSA of >0.1 ng/ml within six weeks after surgery (9–11). All patients with clinical suspicion of metastases at time of surgery (cM1), treatment with neoadjuvant systemic therapy (chemotherapy and/or hormonal therapy), previous radiation therapy of the prostate (salvage RP), unknown pathologic tumor stage (pTx), and unknown date of prostate biopsy were excluded. Due to limited sample size (n=30 for intermediate- and n=3 for high-risk prostate cancer), all patients with an interval from biopsy to RP >6 months were excluded from the study cohort.
Informed written consent to participate in this study was given by all patients. Prior to data collection, approval by the local ethics committee has been obtained. Reporting follows the precepts established by the Helsinki Declaration.
All included D’Amico intermediate- and high-risk prostate cancer patients were stratified according to interval from biopsy to RP ≤3 months (≤90 days) vs. >3 and ≤6 months (>90 and ≤180 days). BCR represented the primary endpoint of the study and was defined according to the European Association of Urology (EAU) guidelines valid at the timepoint of BCR and the American Urological Association (AUA) guidelines as an initial serum PSA-value of ≥0.2 ng/ml, with a second confirmatory level of >0.2 ng/ml derived from patients’ self-reports in follow-up after RP (9–11). Upstaging was defined as non-organ confined stage (pT3/pT4 and/or pN1) in RP specimen in patients with clinically organ-confined stage (cT1 or cT2). Upgrading was defined as an increase of one or more Gleason Grade group from biopsy to RP specimen (12).
Four analytical steps were completed. First, clinical characteristics as well as histopathological outcomes, rates of nerve sparing surgery, and adjuvant radiation therapy were tabulated according to interval from biopsy to RP (≤3 vs. >3-≤6 months). For continuously coded variables, medians and interquartile ranges (IQR) were reported and for categorical variables, frequencies and respective proportions were recorded. Wilcoxon rank sum test assessed the statistical significance of medians’ differences for continuously coded variables and Pearson’s Chi-squared test examined the statistical significance in proportions’ differences for categorical variables. Moreover, Fisher’s exact test was used to compute an exact p-value when expected counts were less than ten. Second, estimated annual percentage changes (EAPC) for the proportion of patients treated with RP ≤3 months were tested with the least squares linear regression. Third, Kaplan-Meier plots depicted BCR-free survival rates after stratification according to interval from biopsy to RP. Finally, univariable and multivariable Cox regression models addressed BCR according to interval from biopsy to RP. Adjustment variables represented PSA-value at initial diagnosis (continuously coded), adverse histopathological outcomes at RP, namely pathologic tumor stage (pTstage), Gleason Grade group in specimen (ISUP grade), pathologic lymph node stage (pNstage), and positive surgical margin, as well as adjuvant radiation therapy. All analyses were separately performed in D’Amico intermediate- and high-risk prostate cancer patients.
All statistical tests were two sided, with a level of significance set at p<0.05. R software environment was used for statistical computing and graphics (R version 4.3.2; R Foundation for Statistical Computing, Vienna, Austria) (13).
Relying on our institutional tertiary-care database of 1,637 prostate cancer patients treated with RP between 01/2014 and 06/2023, 680 (42%) patients met the above-described inclusion criteria (Figure 1). Of these, 481 (71%) harbored intermediate-risk and 199 (29%) harbored high-risk prostate cancer (Table 1). Among intermediate-risk prostate cancer patients, the interval from biopsy to RP ranged from 16 to 177 days. Specifically, 328 (68%) patients had an interval from biopsy to RP ≤3 months and 153 (32%) patients >3 and ≤6 months. Median interval from biopsy to RP was 59 (IQR 44-72) vs. 113 (IQR 99-133) days, respectively. Over time, the proportion of patients treated with RP ≤3 months per year ranged from 88% in 2014 to 6% in 2023 (EAPC: −6.7%; 95% confidence interval [CI] −12.5 to −1.1). Among high-risk prostate cancer patients, the interval from biopsy to RP ranged from 14 to 180 days. Specifically, 158 (79%) patients had an interval from biopsy to RP ≤3 months and 41 (21%) >3 and ≤6 months. Median interval from biopsy to RP was 55 (IQR 42-69) vs. 117 (IQR 98-127) days, respectively. Over the study period, the proportion of patients treated with RP ≤3 months per year decreased from 80% in 2014 to 50% in 2023 (EAPC: −3.9%; 95% CI −7.2 to −0.7). Further clinical characteristics of the study cohort are summarized in Table 1.

Table 1. Clinical characteristics of 481 D’Amico intermediate- and 199 high-risk prostate cancer patients treated with radical prostatectomy (RP) between 01/2014 and 06/2023.
In 481 intermediate-risk prostate cancer patients stratified according to interval from biopsy to RP (≤3 vs. >3 and ≤6 months), rates of non-organ confined tumor stage (pT3/pT4) were 40 vs. 35%, rates of high-risk Gleason Grade group in specimen (ISUP grade 4/5) were 7 vs. 5%, rates of lymph node invasion (pN1) were 2 vs. 2%, and rates of positive surgical margins (R1) were 28 vs. 22% (Table 2). Upstaging from clinically organ-confined (cT1 or cT2) to pathological non-organ confined stage (pT3/pT4 and/or pN1) was experienced by 40 vs. 35%. Comparing Gleason Grade group in biopsy with those in RP specimen, upgrading was evident in 20 vs. 22% and downgrading in 19 vs. 17%. Rates of nerve sparing surgery were 92 vs. 93% in intermediate-risk patients with an interval from biopsy to RP ≤3 months vs. >3 and ≤6 months. Adjuvant radiation therapy rates were 8 vs. 6%, respectively.
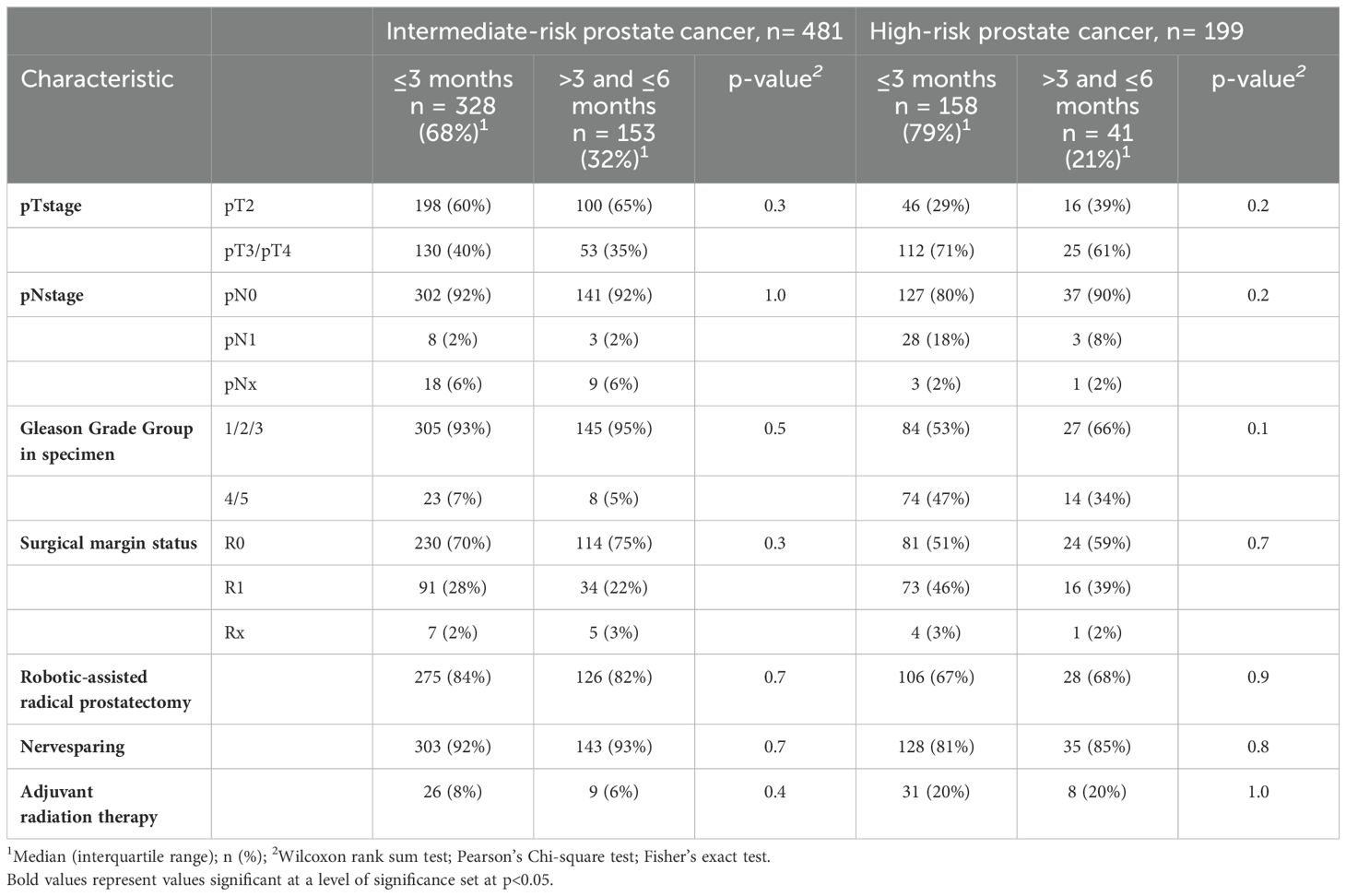
Table 2. Histopathological outcomes, proportion of nerve sparing surgery, and adjuvant radiation therapy of 481 D’Amico intermediate- and 199 high-risk prostate cancer patients treated with radical prostatectomy (RP) between 01/2014 and 06/2023.
In 199 high-risk prostate cancer patients stratified according to interval from biopsy to RP (≤3 vs. >3 and ≤6 months), rates of non-organ confined tumor stage (pT3/pT4) were 71 vs. 61%, rates of high-risk Gleason Grade group in specimen (ISUP grade 4/5) were 47 vs. 34%, rates of lymph node invasion (pN1) were 18 vs. 8%, and rates of positive surgical margins (R1) were 46 vs. 39% (Table 2). Upstaging from clinically organ-confined (cT1 or cT2) to pathological non-organ confined stage (pT3/pT4 and/or pN1) was experienced by 63 vs. 54%. Comparing Gleason Grade group in biopsy with those in RP specimen, upgrading was evident in 15 vs. 24% and downgrading in 44 vs. 46%. Rates of nerve sparing surgery were 81 vs. 85% in high-risk patients with an interval from biopsy to RP ≤3 months vs. >3 and ≤6 months. Adjuvant radiation therapy rates were 20 vs. 20%, respectively.
Of all 481 intermediate-risk prostate cancer patients, BCR was experienced by 51 of 328 (16%) patients with an interval from biopsy to RP ≤3 months and by 18 of 153 (12%) patients with an interval from biopsy to RP >3 and ≤6 months. This was reflected in three-year BCR-free survival rates of 82% in patients with an interval from biopsy to RP ≤3 months and 88% in patients with an interval from biopsy to RP >3 and ≤6 months (p=0.5; Figure 2A). These rates resulted in a univariable hazard ratio (HR) for BCR of 0.84 (95% CI 0.49-1.45; p=0.5; Table 3). After multivariable adjustment for preoperative PSA-value, pTstage, Gleason Grade group in RP specimen, pNstage, surgical margin status, and adjuvant radiation therapy, the multivariable HR for BCR remained at 0.85 (95% CI 0.49-1.46; p=0.5; Table 3).
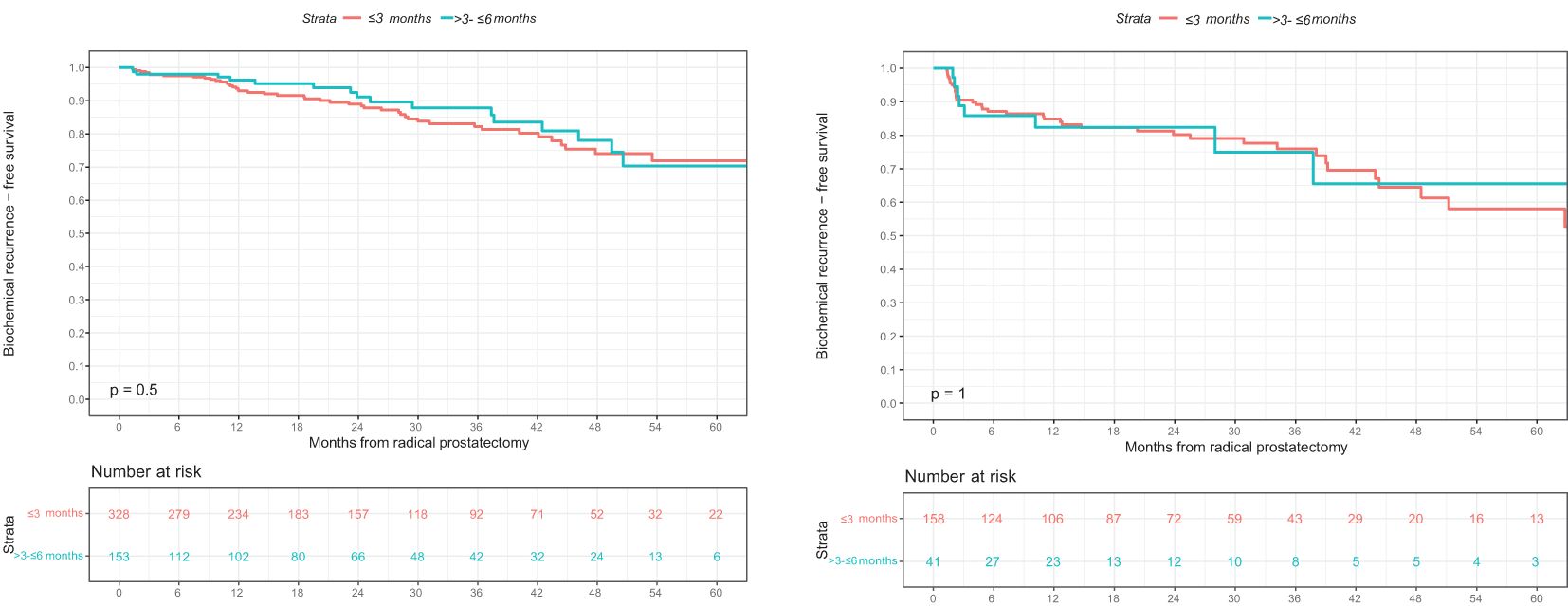
Figure 2. Kaplan-Meier survival analyses addressing biochemical recurrence (BCR)-free survival after radical prostatectomy (RP) according to interval from biopsy to RP in (A) D’Amico intermediate-risk and (B) high-risk prostate cancer patients. BCR, biochemical recurrence; BCRFS, biochemical recurrence-free survival; RP, radical prostatectomy.
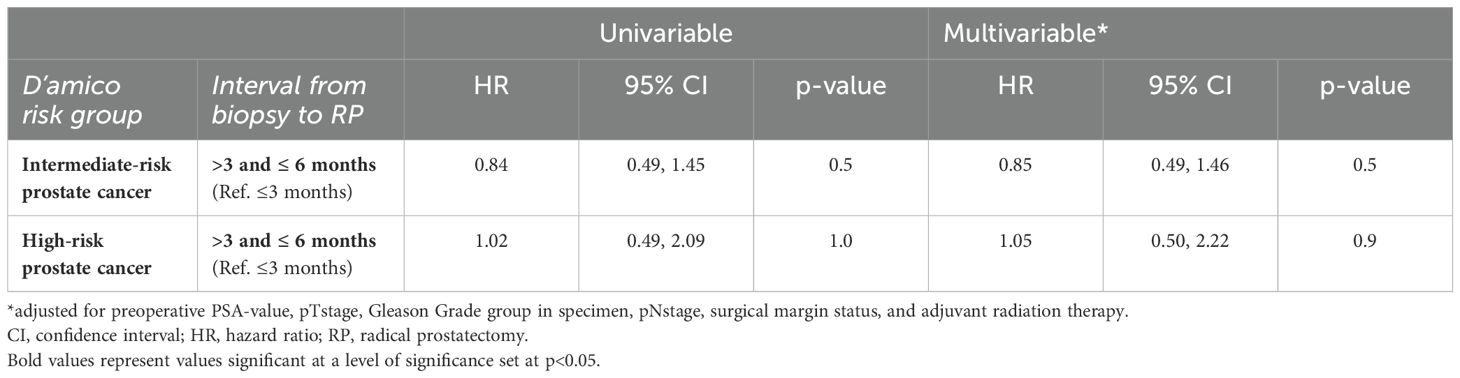
Table 3. Univariable and multivariable Cox regression models addressing rates of biochemical recurrence (BCR) after radical prostatectomy (RP), according to interval from biopsy to RP.
Of all 199 high-risk patients, BCR was experienced by 41 of 158 (26%) patients with an interval from biopsy to RP ≤3 months and by 9 of 41 (22%) patients with an interval from biopsy to RP >3 and ≤6 months. This was reflected in three-year BCR-free survival rates of 76% in patients with an interval from biopsy to RP ≤3 months and 75% in patients with an interval from biopsy to RP >3 and ≤6 months (p=1; Figure 2B). These rates resulted in a univariable HR for BCR of 1.02 (95% CI 0.49-2.09; p=1; Table 3). After multivariable adjustment, the multivariable HR for BCR remained at 1.05 (95% CI 0.50-2.22; p=0.9; Table 3).
Within the current study, we hypothesized that both D’Amico intermediate- as well as high-risk prostate cancer patients with interval from biopsy to RP ≤3 months compared to those with interval from biopsy to RP >3 and ≤6 months do not differ in BCR rates after RP. Relying on a contemporary cohort of RP-treated D’Amico intermediate- and high-risk prostate cancer patients at a tertiary care referral center between 01/2014 and 06/2023, we made several noteworthy observations.
First, among 481 D’Amico intermediate-risk prostate cancer patients, 328 (68%) patients had an interval from biopsy to RP ≤3 months and 153 (32%) patients had an interval from biopsy to RP >3 and ≤6 months. Similarly, among 199 high-risk prostate cancer patients, 158 (79%) patients had an interval from biopsy to RP ≤3 months and 41 (21%) patients had an interval from biopsy to RP >3 and ≤6 months. These distributions of intervals from biopsy to RP do not only validate the hypothesis that the majority of patients receives curative treatment within three months. Moreover, they are also consistent with the distributions of intervals from biopsy to RP reported by other prostate cancer centers in Europe (14) and North America (15–17).
Second, we identified no differences regarding rates of non-organ confined tumor stage (pT3/pT4), high-risk Gleason Grade group in RP specimen (ISUP grade 4/5), lymph node invasion (pN1), and positive surgical margins (R1). Moreover, no differences in the rates of upstaging from organ-confined to non-organ-confined stage were observed. Conversely, in high-risk but not in intermediate-risk prostate cancer patients, upgrading was more frequent in those with an interval from biopsy to RP of >3 and ≤6 months compared to those with an interval of ≤3 months. The higher upgrading rate in high-risk prostate cancer patients with an interval from biopsy to RP of >3 and ≤6 months (34 vs. 15%) may be attributed to the higher rate of Gleason Grade group 5 in biopsies in patients who underwent RP within ≤3 months (35 vs 15%). In consequence, the findings reported within the present study may suggest that a treatment delay of up to six months does not impair histopathologic outcomes at RP in both intermediate- as well as high-risk prostate cancer patients. Hereby, the current results confirm previous studies in which interval from biopsy to RP represented the variable of interest (5, 16, 18–22).
Third, the proportion of patients who received adjuvant radiation therapy was 8 vs. 6% in intermediate-risk (p=0.4) and 20 vs. 20% in high-risk prostate cancer patients who were treated with RP ≤3 vs. >3 and ≤6 months (p=1), respectively. The above findings demonstrate that not only histopathological outcomes at RP but also the rates of further treatments do not differ significantly between prostate cancer patients treated at ≤3 vs. >3 and ≤6 months after diagnosis.
Fourth, we observed no differences in BCR rates between prostate cancer patients who underwent RP ≤3 vs. >3 and ≤6 months after biopsy. Specifically, three-year BCR-free survival rates were 82 vs. 88% in patients with intermediate-risk prostate cancer (p=0.5) and 76 vs. 75% in patients with high-risk prostate cancer treated with RP ≤3 vs. >3 and ≤6 months after biopsy (p=1). Moreover, after multivariable adjustment for preoperative PSA-value, pTstage, Gleason Grade group in RP specimen, pNstage, surgical margin status, and adjuvant radiation therapy, interval from biopsy to RP did not receive independent predictor status for BCR after RP in both intermediate- and high-risk prostate cancer patients (p=0.5 and p=0.9). In intermediate- and high-risk prostate cancer patients treated with RP, a contemporary metanalysis by Laukhtina et al. included five studies which did not identify any significant association between treatment delay and BCR (23). Conversely, four included studies reported an unfavorable impact of treatment delay on BCR (23). However, the definitions of RP delay varied significantly between the included studies, ranging from continuously coded interval from biopsy to RP (6, 24) to cutoffs at 4-6 weeks (18–20) to >12 months (25). These differences render direct comparisons of such studies impossible.
Taken together, we identified no differences between immediate and delayed RP in either the intermediate- or high-risk groups regarding histopathological characteristics at RP, the proportion of patients receiving adjuvant radiation therapy, as well as BCR rates in patients treated with RP within six months. The observations recorded within the present study suggest that patients with intermediate- and high-risk prostate cancer may be reassured about waiting to pursue RP for up to 6 months after biopsy. Therefore, the above findings are of high clinical value in patient counselling and treatment decision making in times of limited surgical capacities and staff shortages.
Besides its strengths, the current study has limitations. First, due to its retrospective nature, a potential for residual selection biases between patients who underwent immediate compared to those who underwent delayed surgery remained, despite systematic adjustment for biases and confounders in multivariable models. Especially patients with very high-risk features might be allocated to the “early treatment” group (≤3 months). This might be indicated by the higher Gleason Grade group in biopsies in the “early treatment” groups. This limitation is applicable to all previous studies relying on a retrospective study design (5–7, 14–16, 18–22, 24–26). However, it is highly unlikely that a prospective trial randomizing patients to immediate vs. delayed RP will ever be initiated and completed. Second, our single-institutional database is limited by sample size. Therefore, the association between interval from biopsy to RP >6 months and adverse histopathological outcomes, adjuvant radiation therapy rates, as well as BCR rates could not be addressed in the current study. Moreover, time to event analyses focusing specifically on patients with very high-risk prostate cancer were not possible. Third, postoperative follow-up within our study cohort was also limited. In consequence, other study endpoints that could be equally as interesting as BCR, namely metastasis, cancer-specific, other-cause, or overall mortality could not be addressed within the current database.
Both intermediate- and high-risk prostate cancer patients with an interval from biopsy to RP >3 and ≤6 months did not differ from those treated within 3 months after biopsy, regarding adverse pathologic outcomes and BCR rates after RP. Therefore, it might be safe to postpone RP up to 6 months.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by Ethics Committee of the Faculty of Medicine and the University Hospital of the Goethe University Frankfurt. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
CS: Conceptualization, Methodology, Data curation, Writing – original draft. MW: Data curation, Formal analysis, Validation, Writing – review & editing. GK: Data curation, Writing – review & editing. CG: Data curation, Formal analysis, Validation, Writing – review & editing. CH: Data curation, Formal analysis, Validation, Writing – review & editing. FP: Data curation, Formal analysis, Validation, Writing – review & editing. MT: Data curation, Writing – review & editing. LK: Data curation, Formal analysis, Validation, Writing – review & editing. FC: Conceptualization, Data curation, Supervision, Validation, Writing – review & editing. PM: Conceptualization, Data curation, Supervision, Validation, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Williams IS, McVey A, Perera S, O’Brien JS, Kostos L, Chen K, et al. Modern paradigms for prostate cancer detection and management. Med J Aust. (2022) 217:424–33. doi: 10.5694/mja2.51722
2. Chierigo F, Wenzel M, Amling C, Flammia RS, Horlemann B, Zhe T, et al. Survival after Radical Prostatectomy versus Radiation Therapy in High-Risk and Very High-Risk Prostate Cancer. J Urol. (2022) 207:375–84. doi: 10.1097/JU.0000000000002250
3. Siech C, Gruber A, Wenzel M, Humke C, Karakiewicz PI, Kluth LA, et al. Cardiovascular disease and chronic pulmonary disease increase the risk of short-term major postoperative complications after robotic-assisted radical prostatectomy. Med (Mex). (2024) 60:1–10. doi: 10.3390/medicina60010173
4. Scheipner L, Jankovic D, Jasarevic S, Altziebler J, Simunovic I, Mischinger J, et al. Elective urological procedures in times of reduced operating room capacity. Dtsch Arztebl Int. (2024) 121:300–1. doi: 10.3238/arztebl.m2024.0025
5. Engl T, Mandel P, Hoeh B, Preisser F, Wenzel M, Humke C, et al. Impact of “Time-from-biopsy-to-prostatectomy” on adverse oncological results in patients with intermediate and high-risk prostate cancer. Front Surg. (2020) 7. doi: 10.3389/fsurg.2020.561853
6. Zanaty M, Alnazari M, Ajib K, Lawson K, Azizi M, Rajih E, et al. Does surgical delay for radical prostatectomy affect biochemical recurrence? A retrospective analysis from a Canadian cohort. World J Urol. (2018) 36:1–6. doi: 10.1007/s00345-017-2105-6
7. Zanaty M, Alnazari M, Lawson K, Azizi M, Rajih E, Alenizi A, et al. Does surgical delay for radical prostatectomy affect patient pathological outcome? A retrospective analysis from a Canadian cohort. Can Urol Assoc J. (2017) 11:265–9. doi: 10.5489/cuaj.4149
8. Preisser F, Theissen L, Wild P, Bartelt K, Kluth L, Köllermann J, et al. Implementation of intraoperative frozen section during radical prostatectomy: short-term results from a german tertiary-care center. Eur Urol Focus. (2021) 7:95–101. doi: 10.1016/j.euf.2019.03.007
9. Cookson Michael S, Gunnar A, Burnett Arthur L, Canby-Hagino Edith D, D’Amico Anthony V, Dmochowski Roger R, et al. Variation in the definition of biochemical recurrence in patients treated for localized prostate cancer: the american urological association prostate guidelines for localized prostate cancer update panel report and recommendations for a standard in the reporting of surgical outcomes. J Urol. (2007) 177:540–5. doi: 10.1016/j.juro.2006.10.097
10. Pisansky Thomas M, Thompson Ian M, Valicenti Richard K, D’Amico Anthony V. Selvarajah shalini. Adjuvant and salvage radiotherapy after prostatectomy: ASTRO/AUA guideline amendment 2018-2019. J Urol. (2019) 202:533–8. doi: 10.1097/JU.0000000000000295
11. EAU Guidelines Office. (2024). EAU guidelines on prostate cancer, in: the EAU Annual Congress Paris 2024. Available ohnline at: https://uroweb.org/guidelines/prostate-cancer/chapter/citation-information.
12. Di Mauro E, Di Bello F, Califano G, Morra S, Creta M, Celentano G, et al. Incidence and Predicting Factors of Histopathological Features at Robot-Assisted Radical Prostatectomy in the mpMRI Era: Results of a Single Tertiary Referral Center. Med (Mex). (2023) 59:1–15. doi: 10.3390/medicina59030625
13. R Core Team. R: A Language and Environment for Statistical Computing. R Lang Environ Stat Comput (2022). Available online at: https://www.R-project.org/ (Accessed August 27, 2023).
14. Aas K, Fosså SD, Kvåle R, Møller B, Myklebust TÅ, Vlatkovic L, et al. Is time from diagnosis to radical prostatectomy associated with oncological outcomes? World J Urol. (2019) 37:1571–80. doi: 10.1007/s00345-018-2570-6
15. Abern MR, Aronson WJ, Terris MK, Kane CJ, Presti JC Jr., Amling CL, et al. Delayed radical prostatectomy for intermediate-risk prostate cancer is associated with biochemical recurrence: Possible implications for active surveillance from the SEARCH database. Prostate. (2013) 73:409–17. doi: 10.1002/pros.22582
16. Gupta N, Bivalacqua TJ, Han M, Gorin MA, Challacombe BJ, Partin AW, et al. Evaluating the impact of length of time from diagnosis to surgery in patients with unfavourable intermediate-risk to very-high-risk clinically localised prostate cancer. BJU Int. (2019) 124:268–74. doi: 10.1111/bju.14659
17. Vickers AJ, Bianco FJ Jr., Boorjian S, Scardino PT, Eastham JA. Does a delay between diagnosis and radical prostatectomy increase the risk of disease recurrence? Cancer. (2006) 106:576–80. doi: 10.1002/cncr.21643
18. Qu LG, Jack G, Perera M, Evans M, Evans S, Bolton D, et al. Impact of delay from transperineal biopsy to radical prostatectomy upon objective measures of cancer control. Asian J Urol. (2022) 9:170–6. doi: 10.1016/j.ajur.2021.08.008
19. Korets R, Seager CM, Pitman MS, Hruby GW, Benson MC, McKiernan JM. Effect of delaying surgery on radical prostatectomy outcomes: a contemporary analysis. BJU Int. (2012) 110:211–6. doi: 10.1111/j.1464-410X.2011.10666.x
20. Westerman ME, Sharma V, Bailey GC, Boorjian SA, Frank I, Gettman MT, et al. Impact of time from biopsy to surgery on complications, functional and oncologic outcomes following radical prostatectomy. Int Braz J Urol. (2019) 45:468–77. doi: 10.1590/S1677-5538.IBJU.2018.0196
21. Ginsburg KB, Curtis GL, Timar RE, George AK, Cher ML. Delayed radical prostatectomy is not associated with adverse oncologic outcomes: implications for men experiencing surgical delay due to the COVID-19 pandemic. J Urol. (2020) 204:720–5. doi: 10.1097/JU.0000000000001089
22. Nesbitt AL, Smith PG, Antoniou S, Evans GA, Pridgeon SW. Delay to radical prostatectomy: Who, why and does it matter? J Clin Urol. (2021) 14:207–12. doi: 10.1177/2051415820945933
23. Laukhtina E, Sari Motlagh R, Mori K, Quhal F, Schuettfort VM, Mostafaei H, et al. Oncologic impact of delaying radical prostatectomy in men with intermediate- and high-risk prostate cancer: a systematic review. World J Urol. (2021) 39:4085–99. doi: 10.1007/s00345-021-03703-8
24. Fossati N, Rossi MS, Cucchiara V, Gandaglia G, Dell’Oglio P, Moschini M, et al. Evaluating the effect of time from prostate cancer diagnosis to radical prostatectomy on cancer control: Can surgery be postponed safely? Urol Oncol Semin Orig Investig. (2017) 35:150.e9–150.e15. doi: 10.1016/j.urolonc.2016.11.010
25. Morini MA, Muller RL, de Castro Junior PCB, de Souza RJ, Faria EF. Time between diagnosis and surgical treatment on pathological and clinical outcomes in prostate cancer: does it matter? World J Urol. (2018) 36:1225–31. doi: 10.1007/s00345-018-2251-5
26. Siech C, Hoeh B, Rohlfsen E, Cano Garcia C, Humke C, Köllermann J, et al. Organ-confined pT2 ISUP4/5 vs. nonorgan confined pT3/4 ISUP2 vs. ISUP3 prostate cancer: Differences in biochemical recurrence-free survival after radical prostatectomy. Urol Oncol Semin Orig Investig. (2024) 42(12):448.e1-448.e8. doi: 10.1016/j.urolonc.2024.07.008
Keywords: BCR, prostate cancer, prostate biopsy, radical prostatectomy, time to event
Citation: Siech C, Wenzel M, Knoblich G, Cano Garcia C, Humke C, Preisser F, Traumann M, Kluth LA, Chun FKH and Mandel P (2025) The association between the interval from biopsy to radical prostatectomy and biochemical recurrence in patients with intermediate- and high-risk prostate cancer. Front. Oncol. 14:1533800. doi: 10.3389/fonc.2024.1533800
Received: 24 November 2024; Accepted: 26 December 2024;
Published: 04 February 2025.
Edited by:
Claudia Collà Ruvolo, University of Naples Federico II, ItalyReviewed by:
Francesco Di Bello, University of Naples Federico II, ItalyCopyright © 2025 Siech, Wenzel, Knoblich, Cano Garcia, Humke, Preisser, Traumann, Kluth, Chun and Mandel. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Carolin Siech, U2llY2hAbWVkLnVuaS1mcmFua2Z1cnQuZGU=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.