- 1Department of General Practice, People’s Hospital of Leshan, Leshan, China
- 2Department of Medical Laboratory, People’s Hospital of Leshan, Leshan, China
Background: Increased PD-1 expression on CD8+ T cells is considered as a hallmark for T-cell exhaustion, and is thought to be related to the prognosis of cancer patients. However, discrepant results have made it difficult to apply PD-1+CD8+T cells and tumor prognosis to clinical practice. Therefore, we conducted a meta-analysis to evaluate its prognostic value in human cancers.
Methods: PRISMA reporting guidelines were strictly followed for conducting the current meta-analysis. The PubMed, Web of Science, Embase databases were searched from inception to November 2024. The pooled Hazard Ratio (HR) along with 95% confidence intervals (CIs) of each article were combined for the associations of PD-1+CD8+ T cells with overall survival (OS), progression- free survival (PFS) and disease-free survival(DFS). Subgroup analyses were performed for area, specimen type, cancer type, treatment, detected method and cancer stage.
Results: A total of 20 studies (23 cohorts, 3086 cancer patients) were included in our study. The expression PD-1+CD8+ T cells in cancer patients tended to predict poor overall survival (OS) (HR: 1.379, 95%CI: 1.084-1.753, p= 0.009), and unfavorable disease-free survival(DFS) (HR: 1.468, 95%CI: 0.931-2.316, p=0.099), though it did not reach statistical significance. Begg’s and Egger’s test demonstrated that no obvious publication bias was exist.
Conclusions: High PD-1 expression on CD8+ T cells is associated with worse survival outcomes, which can be potentially used as a prognostic marker of malignant tumor.
1 Introduction
Immune cells are known to be determinants of tumorigenesis, progression and metastasis, which play an important role in tumor elimination, surveillance and escape (1). Among these immune cells, cytotoxic CD8 + T cells represent the main anti-tumor TIL population and are considered as a positive prognostic factor in the majority of tumors. However, some studies found that the successful clearance of cancer cells by tumor-infiltrating lymphocytes (TILs) is impeded by a series of immune inhibitory mechanisms that are active in the tumor microenvironment (2–4), including the upregulation of immune checkpoint proteins such as PD-1, CTLA-4, Tim-3 and Lag-3 on TILs (5).
Programmed death-1 (PD-1) is a surface receptor expressed by lymphocytes that suppresses its proliferation and effector function by binding to PD-1 ligands such as B7-H1 (also known as PD-L1) and B7-DC (also known as PD-L2) expressed on other cells (6). Recently, some studies have showed T cells expressing these checkpoint molecules are considered to be characterized by a state of dysfunction, accompanied with loss the ability of cytokine production (IL-2, TNF-α and IFN-γ) and killing capacity, termed T cell exhaustion (7–9). A study pointed out that the tumor-infiltrating effector CD8+ T cells showed a drastic increase in PD-1 expression, and PD-1 upregulation promoted CD8+ T-cell apoptosis and postoperative recurrence in hepatocellular carcinoma patients (10). Although PD-1 can be expressed on any T cell during activation, it is frequently linked to the exhaustion of CD8 + T cells (11). Therefore, increasing researchers focused their attention on the role and activation status of infiltrating CD8+ T-cells including PD-1 expression in these immune cells.
Whether the expression of PD-1 on CD8+T cells can be used as a prognostic indicator has been explored. Hsu and his colleagues (12) found that increasing of programmed death-1-expressing intratumoral CD8+ T cells predicted a poor prognosis for nasopharyngeal carcinoma. In addition, according to Ma’s research, CD8 + T cells were classified intoPD1 Hi, PD1 Int and PD1-, PD1 Hi CD8 + T cells highly expressed exhaustion-related inhibitory receptors (TIM3, CTLA-4, etc.) and transcription factors (Eomes, BATF, etc.), and PD1 Hi CD8 + T cells were significantly correlated with poor prognosis (13). While some objections have been raised, they suggested that high proportion of PD-1 in CD8+ tumor-infiltrating T-cells improved survival outcomes in some cancers. Pokrývková et (14) indicated that increased expression of PD-1 in CD8+ T cells conferred improved survival outcomes, and PD1 + CD8 + Cells are an independent prognostic marker in patients with head and neck cancer. These results indicated a controversial prognostic value of PD-1+CD8+ T cells in human cancers.
The inconsistent results may be due to the different area, specimen type, cancer type, anti- cancer therapy, detected method and cancer stage. Hence, we conducted a meta-analysis to systematically assess the prognostic role of PD-1+CD8+ T cells in various cancers.
2 Materials and methods
2.1 Literature search and search strategy
The preferred reporting items of the Systematic Review and Meta-Analysis (PRISMA) Reporting Guidelines are strictly followed to document current meta-analyses. To verify compliance with established guidelines, the PRISMA checklist has been incorporated into Supplementary Table S1 as a key tool for determining compliance. The protocol for the meta-analysis was registered at the International Platform of Registered Systematic Review and Meta-analysis Protocols (INPLASY) and assigned the registration number INPLASY2024110075. We systematically searched the PubMed, Web of Science, Embase databases prior to November 2024. The search key words were as follows: (((((((cancer[Title/Abstract]) OR (carcinoma[Title/Abstract])) OR (neoplasm[Title/Abstract])) OR (tumor[Title/Abstract])) OR (tumor[Title/Abstract])) AND ((pd-1[Title/Abstract]) OR (programmed cell death 1[Title/Abstract]))) AND (CD8+ T cell[Title/Abstract])) AND (prognosis[Title/Abstract]). Studies investigating the association between PD-1 expression on CD8+ T cells and cancer patient survival is currently a candidate for our meta-analysis. Besides, manual review was conducted on the references of relevant articles for additional candidate studies. The search procedures and related keyword combinations are documented in Supplementary Table S2.
The PICOS framework was as follows:
Population: This study involved patients who were diagnosed with cancer.
Intervention: The intervention under consideration was the presence of programmed cell death 1 expression on CD8+ T cells.
Comparison: The comparison was conducted to assess the presence of programmed cell death 1 expression on CD8+ T cells on survival.
Outcomes: OS and/or PFS and/or DFS in the presence of programmed cell death 1 expression on CD8+ T cells.
Study design: Case−control.
2.2 Study selection and inclusion-exclusion criteria
In order to search for relevant articles, two independent reviewers first screened the title and abstract of the article, and then conducted further access to the entire article. Differences among reviewers are resolved through discussion or negotiation with a third researcher until consensus is reached. Articles were included if they met the following inclusion criteria: (1) the researcher population must be diagnosed cancer patients; (2) at least one of these patient groups must detect PD-1 expression on CD8+T cells; (3) the study population should include the hazard ratio (HR) and 95% confidence interval (95% CI) of PD-1+CD8+T cells associated with OS and/or PFS and/or DFS, or provide sufficient data to calculate HR and 95% CI. Besides, the exclusion criteria were as follows: (1) reviews, letters or case reports; (2) animal or cell-line articles; (3) accompanied by other detection markers; (4) inefficient in providing data for calculate HR and 95% CI.
2.3 Data retrieval and quality assessment
Two researchers extracted information from eligible studies, and the information was as follows: first author, publication year, country, number of samples, specimen type, anti-cancer therapy, cancer stage, HR, and 95% CI of survival outcomes. In addition, to evaluate the quality of the included articles, the Newcastle–Ottawa scale (NOS), which was used to assess the quality of cohort studies and case–control studies. The highest score is 9 points, and studies with a score of six or more are considered to be high-quality (15). Quality assessment was presented in Supplementary Table S3.
2.4 Statistical analysis
Stata 12.0 was adopted for the meta-analysis, and p<0.05 was considered statistically significant. HR for OS, PFS and DFS and 95% CIs were pooled to measure the relationship between time and events. Heterogeneity was assessed by Cochran’s Q and I-squared (I2) tests. I2>50% with p value of Q test <0.1 indicated significant heterogeneity, and then a random- effect model was applied to combine HR and 95% CI of survival outcomes. Otherwise, a fixed-effect model was used. In addition, to find the source of significant heterogeneity, sensitivity analysis and subgroup analysis were performed. Begg’s and Egger’s test were used to assess publication bias.
3 Results
3.1 Literature retrieval and selection
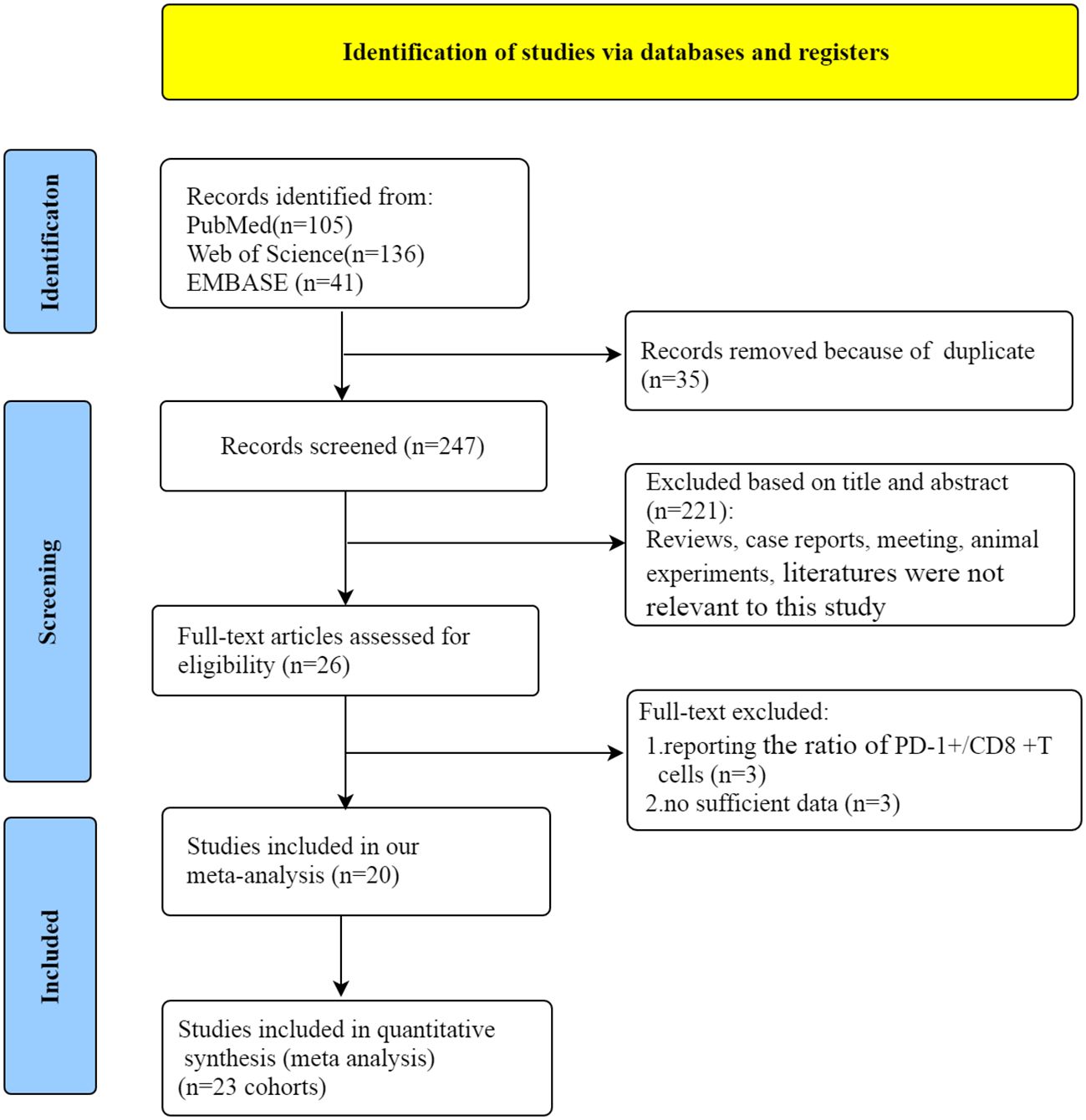
Initially, 282 articles were obtained through literature screening in PubMed (n=105), Web of Science (n=136), and Embase (n=41) databases. Of these, 35 articles were excluded due to duplication. After filtering through titles and abstracts, 224 articles were deleted for the following reasons: reviews, case reports, meeting, animal or cell-line articles and literatures were not relevant to our study. Then, a review of the entire article was conducted. Owing to report the ratio of PD-1+/CD8+ T cells and insufficient data, 6 articles were eliminated. Finally, 20 eligible studies (12, 13, 16–33) were included in our meta-analysis. The flow chart of the whole selection process is shown in Figure 1.
3.2 Study characteristics
A total of 20 studies with 3086 patients were included in our meta-analysis. They mainly come from China, Japan, South Korea, Singapore, Italy, the United States, and Switzerland, Greece. These studies included a variety of tumor types such as gastric cancer, non-small cell lung cancer, triple-negative breast cancer, pancreatic cancer, acute myeloid leukemia, hepatocellular carcinoma, head and neck cancer, mesothelioma. The specimen type of 14 articles was tissue, while 6 articles was peripheral blood. Five articles received immune checkpoint inhibitor therapy, 9 articles adopted other therapies(including surgical resection, chemotherapy, curative surgery+chemotherapy, and allo-SCT and so on) and 6 articles did not receive treatment. PD-1+CD8+ T cells was detected for protein expression by dual immunohistochemistry in 14 studies, and for flow cytometry in 6 studies. Specifically, three studies (13, 19, 20) each had two cohorts of cancer patients and then included each cohort in the quantitative analysis as an individual study. The characteristics of 20 studies are summarized in Table 1.
Seventeen studies comprising 2849 patients evaluated the association of PD-1+CD8+ T cells with OS. An obvious heterogeneity (I2 = 113.51%, p < 0.001) was observed, so a random-effect model was applied. Patients with high PD-1+CD8+ T cells had significantly worse OS (HR = 1.379, 95% CI 1.084-1.753, p = 0.009, Figure 2A). The association between PD-1+CD8+ T cells and PFS was evaluated in 5 studies comprising 306 patients. Pooled analysis using a random-effect model demonstrated that patients with elevated PD-1+CD8+ T cells were not associated with PFS (HR = 1.006, 95% CI 0.417-2.430, p = 0.989, Figure 2B). The association between PD-1+CD8+ T cells and DFS was evaluated in 8 studies comprising 1359 patients. A random-effect model indicated that patients with elevated PD-1+CD8+ T cells had a borderline association with DFS (HR = 1.468, 95% CI 0.931-2.316, p = 0.099, Figure 2C).
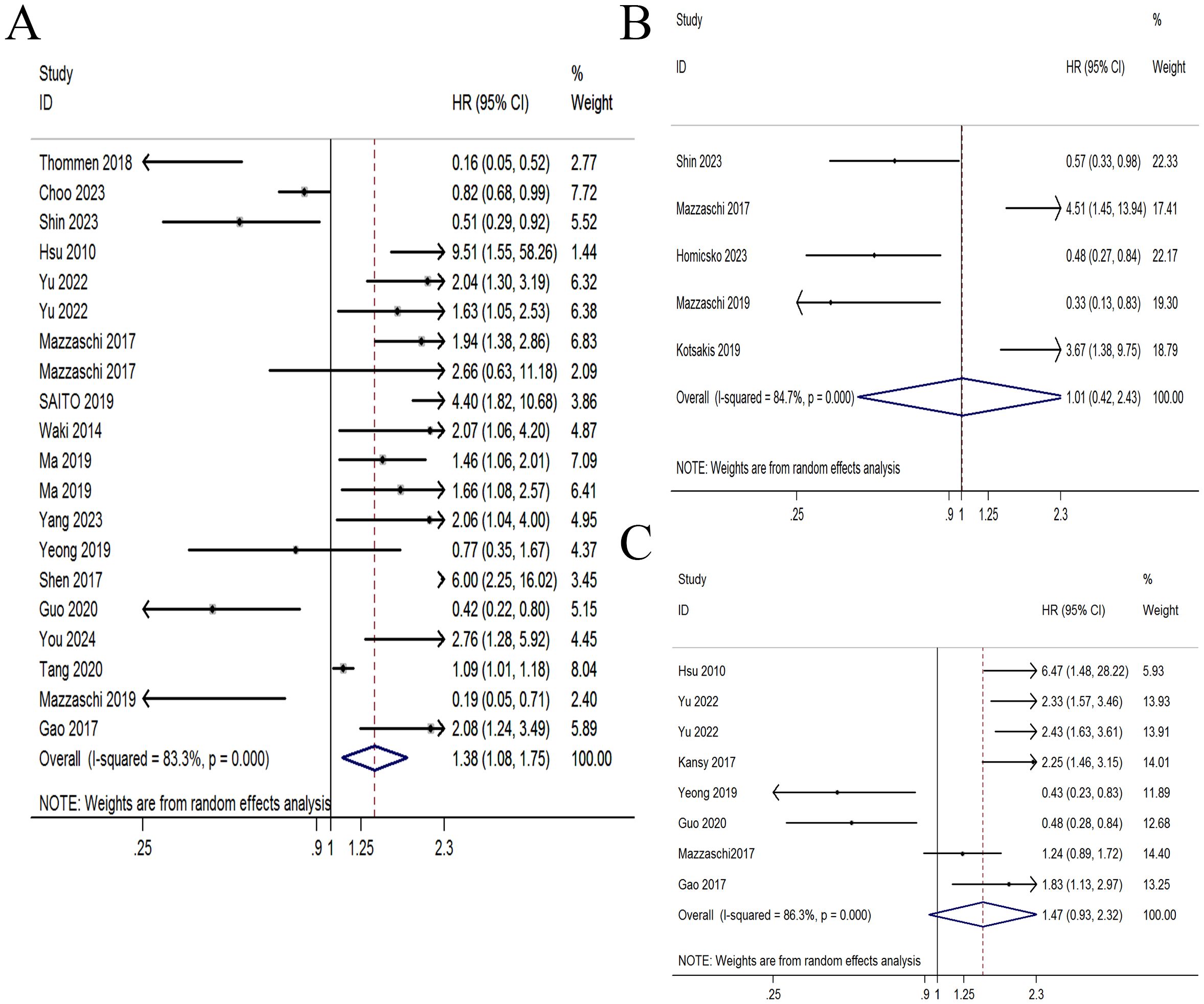
Figure 2. Forest plot of PD-1+CD8+ T cells with survival time. (A) Forest plot of PD-1+CD8+ T cells with overall survival. (B) Forest plot of PD-1+CD8+ T cells with progression-free survival. (C) Forest plot of PD-1+CD8+ T cells with disease-free survival.
3.3 Subgroup analyses of PD-1+CD8+ T cells in association with survival
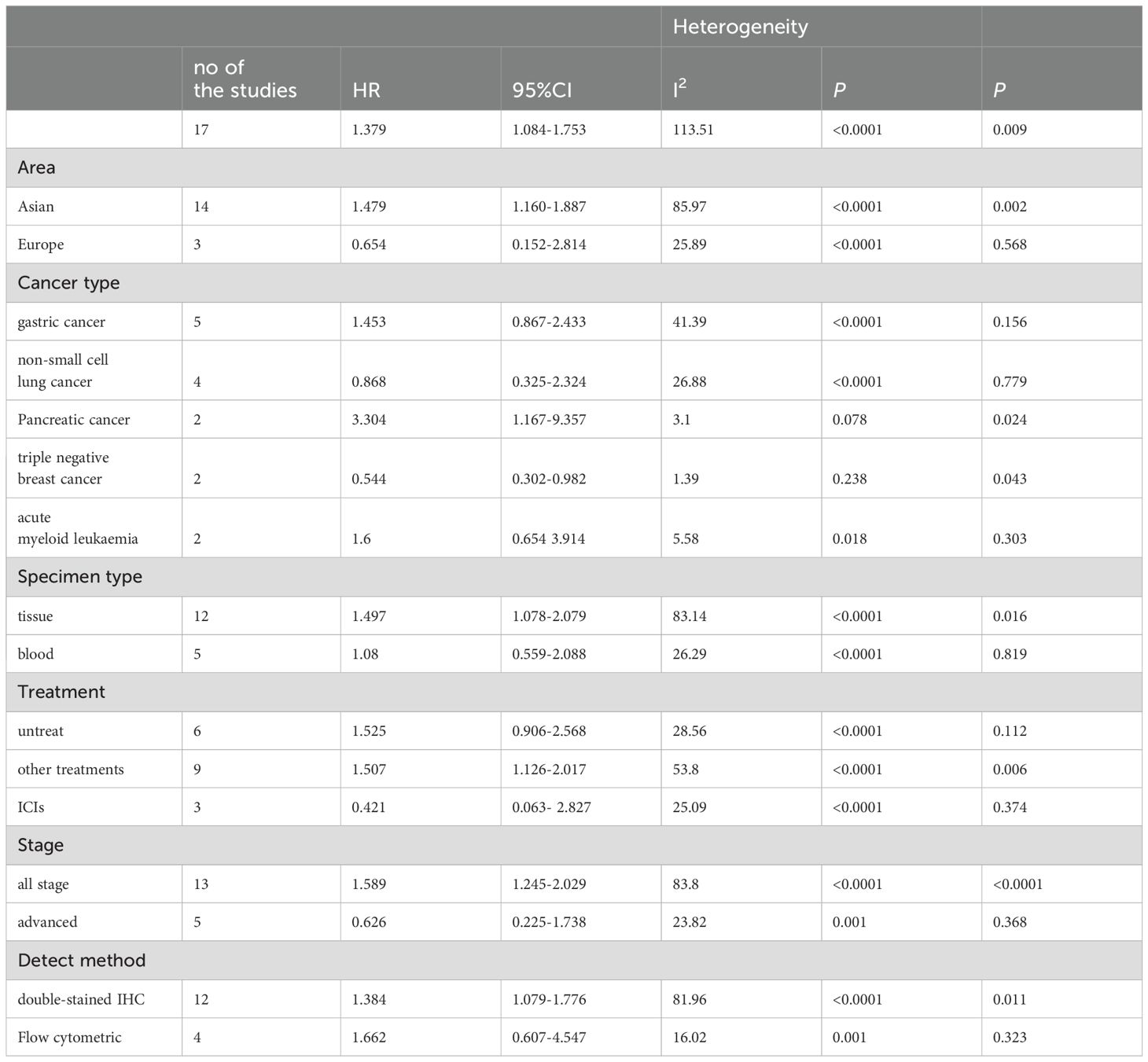
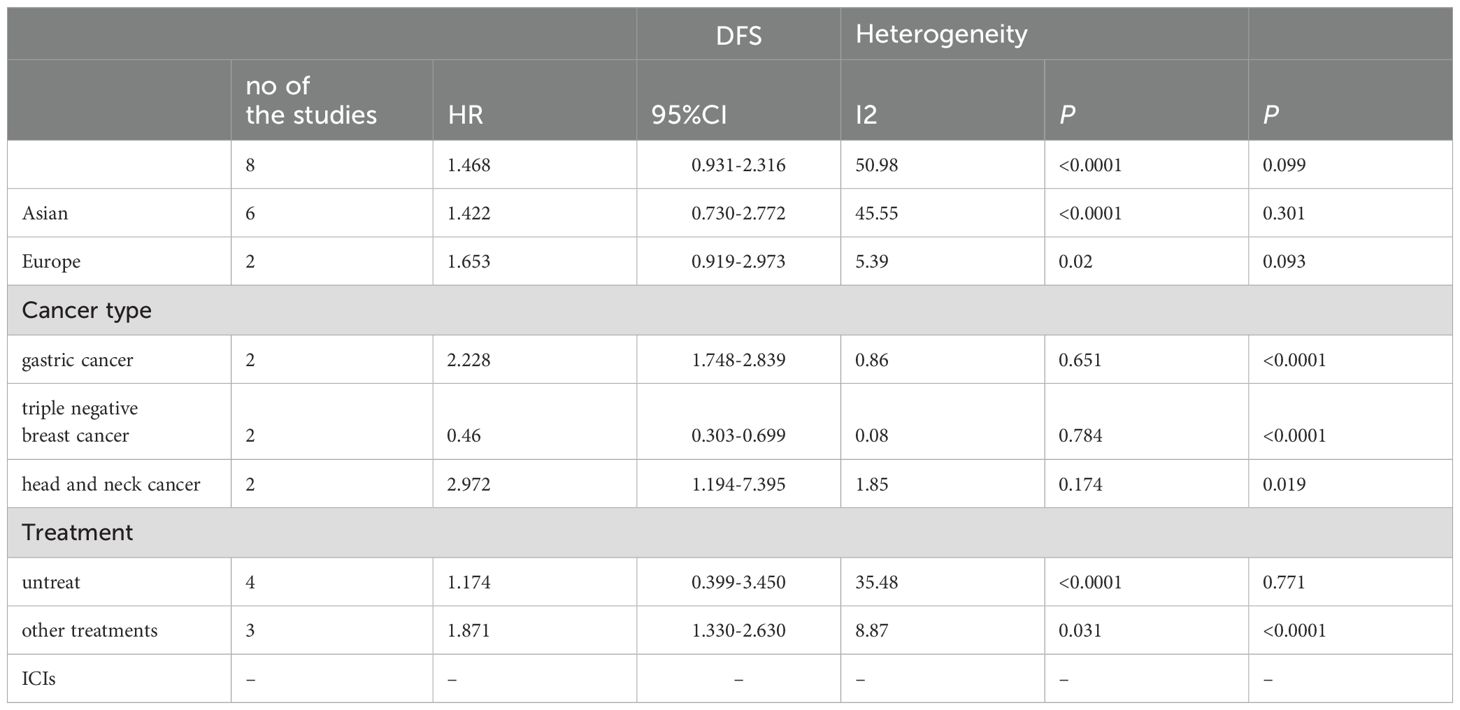
Due to significant heterogeneity in the pooled HRs of OS in cancer patients and moderate heterogeneity in the pooled HRs of DFS in cancer patients, we conducted subgroup analysis on the selected literature to find out the sources of heterogeneity. Therefore, a subgroup analysis based on study region, cancer type, specimen type, tumor treatment, stage and detect method was carried out. The results of subgroup analyses for OS and DFS are shown in Tables 2, 3, respectively.
There was heterogeneity between research in different regions. The pooled HRs for OS in the Asian subgroup were 1.479 (95%CI: 1.160-1.887, p= 0.002), the pooled HRs for OS in the Europe subgroup were 0.654 (95%CI: 0.152-2.814, p=0.568). The pooled HRs for DFS in the Asian subgroup were 1.422 (95%CI: 0.730-2.772, p=0.301), the pooled HRs for DFS in the Europe subgroup were 1.653 (95%CI: 0.919-2.973, p=0.093). Subgroup analysis based on sample types showed that tumor tissues as sample types had poor OS (HR = 1.497, 95% CI 1.078-2.079, p = 0.016), while peripheral blood were not associated with OS (HR = 1.08, 95% CI 0.559-2.088, p = 0.819).
When the included studies were analyzed by subgroups based on cancer type. PD-1+CD8+T cells predict significantly worse OS in pancreatic cancer (HR=3.304, 95%CI 1.167-9.357, p=0.024), and significant worsening of DFS in gastric cancer and head and neck cancer (HR = 2.228, 95% CI 1.748-2.839 p<0.0001; HR = 2.972, 95% CI 1.194-7.395, p=0.019, respectively), but was associated with improved OS and DFS in triple negative breast cancer (OS: HR = 0.544, 95% CI 0.302-0.982, p= 0.043; DFS: HR =0.46, 95% CI 0.303-0.699, p<0.0001). No association was found between PD- L1 + CTCs and survival in other cancers. Due to the small number of studies, further studies were needed to confirm our results in the future.
When stratified for treatment, PD-1+CD8+ T cells were significantly associated with worse OS and DFS for other therapies (OS: HR = 1.507, 95% CI 1.126-2.017, p =0.006; DFS: HR=1.871,95%CI 1.330-2.630, p<0.0001, respectively). Surprisingly, PD-1+CD8+ T cells seemed to predict a better OS (HR = 0.421, 95% CI 0.063- 2.827, p = 0.374) for ICI treatment, though not reach statistically significant.
PD-1+CD8+ T cells predicted poor OS in double-stained IHC (HR = 1.384, 95% CI 1.079-1.776, p = 0.011). but it was not found to be related to the patient’s prognosis in flow cytometric. It is possible that there are too few studies using the same method. What’s more, patients with all stage had a worse OS(HR = 1.589, 95% CI 1.245-2.029, p <0.0001), while were not associated with OS in advanced patients (HR = 0.626, 95% CI 0.225-1.738, p =0.368).
3.4 Sensitivity analyses
Due to significant heterogeneity was observed. In order to find the potential source of this heterogeneity, we carried out sensitivity analysis. Sensitivity analysis indicated that the results of our meta- analysis were robust and not significantly influenced by any single study (Figure 3).
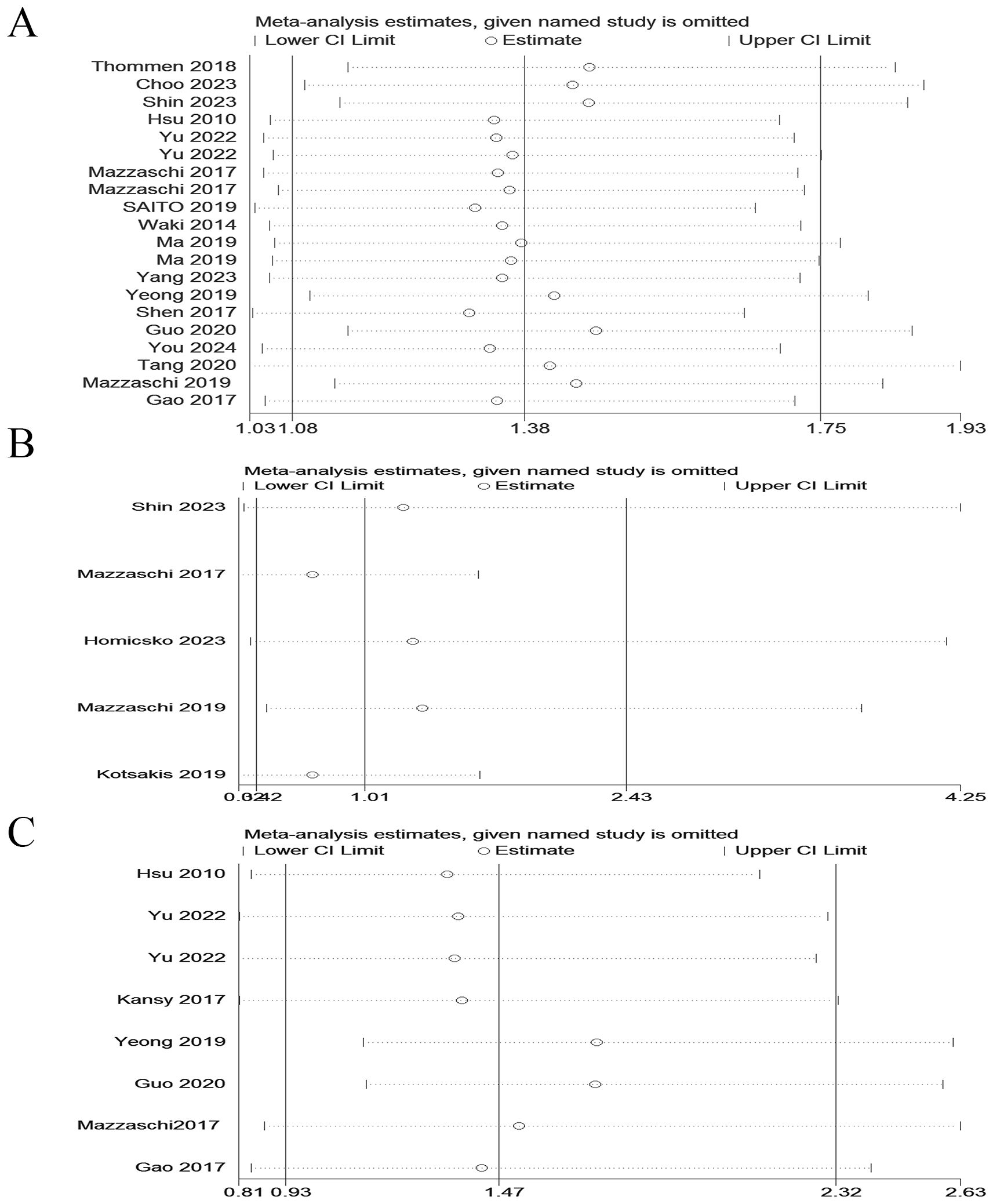
Figure 3. Sensitivity analyses of the pooled HRs for OS, PFS and DFS of cancer patients with PD-1+CD8+ T cells. (A) Sensitivity analyses of the pooled HRs for OS. (B) Sensitivity analyses of the pooled HRs for PFS. (C) Sensitivity analyses of the pooled HRs for DFS.
3.5 Publication bias
Publication bias is considered the main factor affecting predictive value, so we conducted Egger and Begg tests to assess the presence of publication bias and the funnel plot symmetry was examined. Based on the shape of the funnel plot and no significant publication bias was not observed and P values in Egger and Begg tests. (p > 0.05) (Figure 4).
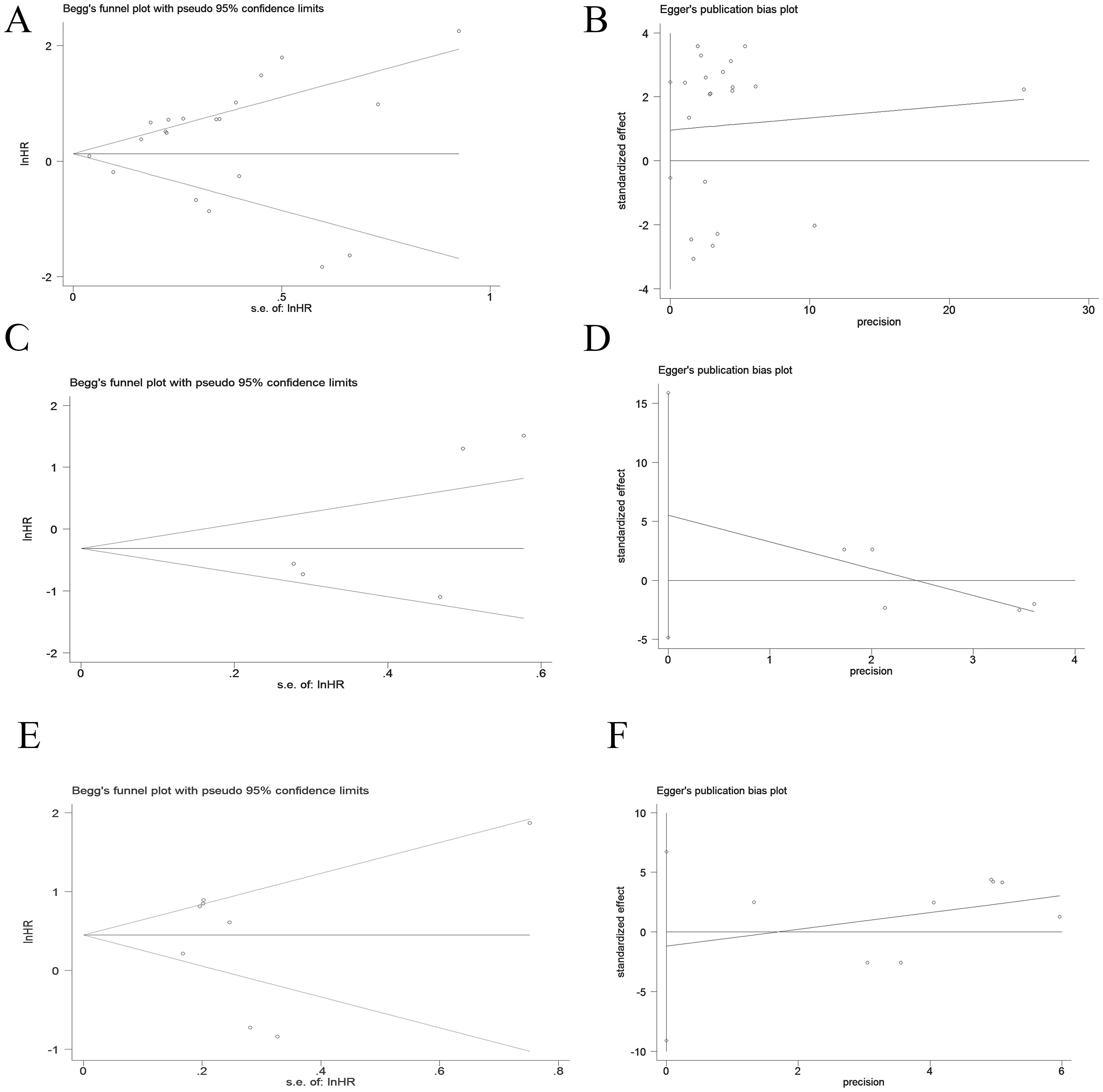
Figure 4. Publication bias of OS, PFS and DFS in PD-1+CD8+T cell cancer patients by Begg and Egger scatter plots. (A, B) Publication bias for OS. (C, D) Publication bias for PFS. (E, F) Publication bias for DFS.
4 Discussion
We conducted the first meta- analysis to evaluate the clinical application of PD-1 expression on CD8+ T cells in predicting the survivals of cancer patients and to identify factors that modulate prognostic value. Overall, high PD-1 expression on CD8+ T cells is associated with worse survival outcomes, which can be potentially used as a prognostic marker of malignant tumor.
CD8+T cells that express PD-1 thought to be characterized by a state of T cell exhaustion, which is accompanied with loss the ability of cytokine production (IL-2, TNF-α and IFN-γ) and killing capacity (13, 34, 35). Recent years, researchers have studied on the relationship between the expression of PD-1 on CD8+ Tcells and the prognosis of cancer patients. Lim et al (36) indicated that higher ratio of PD-1 +/CD8 + TILs was associated with poorer overall survival, relapse-free survival and distant metastasis-free survival, besides, the ratio of PD-1 +/CD8 + TILs was the independent prognostic factor in OS, RFS and DMFS after adjusting for other significant clinicopathologic variables. However, conflicting views have been raised and no consensus has been reached. Shen et al (37) showed that PD-1 + CD8 + T cells showed equivalent function to their PD-1 - CD8 +T cells counterparts and they did not predict tumor progression in gastric cancer. Due to the discrepant results among these studies, it is difficult to apply PD-1 + CD8 + T cells to clinical applicability. Therefore, our work emphasizes the predictive value of PD-1 expression on CD8+ T cells for cancer prognosis. We reviewed 20 studies of PD-1 + CD8 + T cells, and performed a systematic meta-analysis to evaluate the expression of PD-1 on CD8+ T cells in predicting the survivals of cancer patients.
Our study indicates elevating PD-1+CD8+ T cells may predict worse survival time for cancer patients. Consistent with previous studies, researchers indicated that no matter inhibitory receptors, transcription factors or functional molecules, PD1-Hi-CD8+TIL was a exhausted T cell, thus promoting tumor growth, avoiding immune surveillance and promoting immune escape. However, significant heterogeneity was observed in our study. To find the source of heterogeneity, we analyzed the factors that may affect heterogeneity. we stratified the meta-analysis by cancer type and found PD-1+CD8+ T cells predicted significantly worse OS in pancreatic cancer and poor DFS in gastric cancer and head and neck cancer while it was associated with improved OS and DFS in triple negative breast cancer. This may be due to the heterogeneity of PD-1 CD8+ T cells in their roles in different tumors. Odorizzi et al. (38) demonstrated that T cells can be differentiated to reach terminal exhaustion in the genetic absence of PD-1. Moreover, a recent breast cancer study also revealed that there is no significant reduction in cytokine production in PD-1 + T cells compared with PD-1- T cells (39). The immune microenvironment in TNBC may not be as suppressed as in other tumors. However, the exact mechanism of PD-1 CD8+ T cells in triple negative cancer requires further research.
PD-1 is a cytotoxic T cell immune checkpoint receptor that has inhibitory effects when bound to its ligand(PD-L1). The use of checkpoint blocking antibodies to block PD-1 immunotherapy can restore and enhance the response of cytotoxic T cells to chemotherapy resistant tumors, resulting in sustained response and tolerable toxicity, and prolonging overall survival. Studies have shown that PD-L1 expression responds better to ICI treatment and have a longer survival period in patients receiving immune checkpoint inhibitor therapy (40, 41). Due to this mechanism, the PD-L1/PD-1 axis has been found to be an crucial mechanism by which tumor cells evade T cell immunity, PD-1 inhibitors revitalize CD8+T cells by blocking the PD-1/PD-L1 pathway, promoting their proliferation and functional recovery (42–44). In addition, PD-1/PD-L1 axis inhibitors disrupt the interaction between PD-1 and PD-L1 and subsequently restore the immune response to tumor cells, ultimately improving survival outcomes for cancer patients (45). Homicsko et al (31) pointed out that PD-1 expression of CD8 T cells is an independent predictive factor for the clinical benefits of PD-1 inhibition in patients with advanced mesothelioma. In advanced NSCLC treated with anti-PD1 therapy, Mazzaschi et al (26) found high circulating PD-1+CD8+ cells provided a significantly prolonged progression-free survival. While some studies did not find a significant association between PD-1+CD8+ and survival for ICI treatment (20). Subgroup meta-analysis that gathered these studies showed that patients with high levels of PD-1+CD8+T cells and treated with PD- 1/PD- L1 inhibitors may have prolonged OS, though it not reach the significance. Nevertheless, the expression of PD-1+ CD8+ T cells is a potential prognostic marker for ICI treatment, which needs to be verified by larger studies in the future. More importantly, compared to ICI treatment, our meta-analysis showed a significant association between PD-1+ CD8+T cells and survival in patients receiving non-ICI treatment, with significantly shorter overall survival in patients with PD-1+ CD8+T cells.
According to the above analysis, high PD-1 expression on CD8+ T cells had a adverse survival time, which can be potentially used as a prognostic marker of malignant tumor. However, some limitations are exist in our study. First, although we tried to collect all the articles, some data were lost because our study was restricted to articles published only in English or Chinese, and it is hoped that future research efforts will include publications in other languages to ensure a more comprehensive analysis. Second, there was obvious heterogeneity in our study, which may be caused by the study region, cancer type, treatments, specimen type and detect method. Third, most of the included studies had very small sample sizes. More large-scale studies with PD-1expression on CD8+ T cells are needed in the future to validate the findings of our meta-analysis.
5 Conclusion
Our analysis demonstrated that high PD-1 expression on CD8+ T cells had a adverse survival time, which could be potentially used as a prognostic marker of malignant tumor.
Author contributions
ZW: Writing – original draft, Writing – review & editing. MC: Writing – original draft, Writing – review & editing, Funding acquisition. JY: Data curation, Writing – review & editing. DL: Data curation, Writing – review & editing. JC: Data curation, Writing – review & editing. FL: Supervision, Writing – review & editing. YX: Supervision, Writing – review & editing. ZC: Supervision, Writing – review & editing. YY: Visualization, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Leshan City science and technology plan project (21SZD151).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2024.1531219/full#supplementary-material
References
1. Mittal D, Gubin MM, Schreiber RD, Smyth MJ. New insights into cancer immunoediting and its three component phases–elimination, equilibrium and escape. Curr Opin Immunol. (2014) 27:16–25. doi: 10.1016/j.coi.2014.01.004
2. Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature. (2011) 480:480–9. doi: 10.1038/nature10673
3. Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science. (2011) 331:1565–70. doi: 10.1126/science.1203486
4. Schietinger A, Greenberg PD. Tolerance and exhaustion: defining mechanisms of T cell dysfunction. Trends Immunol. (2014) 35:51–60. doi: 10.1016/j.it.2013.10.001
5. Baitsch L, Baumgaertner P, Devevre E, Raghav SK, Legat A, Barba L, et al. Exhaustion of tumor-specific Cd8(+) T cells in metastases from melanoma patients. J Clin Invest. (2011) 121:2350–60. doi: 10.1172/JCI46102
6. Keir ME, Butte MJ, Freeman GJ, Sharpe AH. Pd-1 and its ligands in tolerance and immunity. Annu Rev Immunol. (2008) 26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331
7. Day CL, Kaufmann DE, Kiepiela P, Brown JA, Moodley ES, Reddy S, et al. Pd-1 expression on Hiv-specific T cells is associated with T-cell exhaustion and disease progression. Nature. (2006) 443:350–4. doi: 10.1038/nature05115
8. Thommen DS, Schreiner J, Muller P, Herzig P, Roller A, Belousov A, et al. Progression of lung cancer is associated with increased dysfunction of T cells defined by coexpression of multiple inhibitory receptors. Cancer Immunol Res. (2015) 3:1344–55. doi: 10.1158/2326-6066.CIR-15-0097
9. Schreiner J, Thommen DS, Herzig P, Bacac M, Klein C, Roller A, et al. Expression of inhibitory receptors on intratumoral T cells modulates the activity of a T cell-bispecific antibody targeting folate receptor. Oncoimmunology. (2016) 5:e1062969. doi: 10.1080/2162402X.2015.1062969
10. Shi F, Shi M, Zeng Z, Qi RZ, Liu ZW, Zhang JY, et al. Pd-1 and Pd-L1 upregulation promotes Cd8(+) T-cell apoptosis and postoperative recurrence in hepatocellular carcinoma patients. Int J Cancer. (2011) 128:887–96. doi: 10.1002/ijc.25397
11. Ahmadzadeh M, Johnson LA, Heemskerk B, Wunderlich JR, Dudley ME, White DE, et al. Tumor antigen-specific Cd8 T cells infiltrating the tumor express high levels of Pd-1 and are functionally impaired. Blood. (2009) 114:1537–44. doi: 10.1182/blood-2008-12-195792
12. Hsu MC, Hsiao JR, Chang KC, Wu YH, Su IJ, Jin YT, et al. Increase of programmed death-1-expressing intratumoral Cd8 T cells predicts a poor prognosis for nasopharyngeal carcinoma. Modern Pathol. (2010) 23:1393–403. doi: 10.1038/modpathol.2010.130
13. Ma J, Zheng B, Goswami S, Meng L, Zhang D, Cao C, et al. Pd1(Hi) Cd8(+) T cells correlate with exhausted signature and poor clinical outcome in hepatocellular carcinoma. J immunotherapy Cancer. (2019) 7:331. doi: 10.1186/s40425-019-0814-7
14. Pokryvkova B, Grega M, Klozar J, Vencalek O, Nunvar J, Tachezy R. Pd1(+)Cd8(+) cells are an independent prognostic marker in patients with head and neck cancer. Biomedicines. (2022) 10(11). doi: 10.3390/biomedicines10112794
15. Stang A. Critical evaluation of the newcastle-ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. (2010) 25:603–5. doi: 10.1007/s10654-010-9491-z
16. Thommen DS, Koelzer VH, Herzig P, Roller A, Trefny M, Dimeloe S, et al. A transcriptionally and functionally distinct Pd-1(+) Cd8(+) T cell pool with predictive potential in non-small-cell lung cancer treated with Pd-1 blockade. Nat Med. (2018) 24:994–1004. doi: 10.1038/s41591-018-0057-z
17. Choo J, Kua LF, Soe MY, Asuncion BR, Tan BKJ, Teo CB, et al. Clinical relevance of Pd-1 positive Cd8 T-cells in gastric cancer. Gastric Cancer. (2023) 26:393–404. doi: 10.1007/s10120-023-01364-7
18. Shin K, Kim J, Park SJ, Kim H, Lee MA, Kim O, et al. Early increase in circulating Pd-1(+)Cd8(+) T cells predicts favorable survival in patients with advanced gastric cancer receiving chemotherapy. Cancers. (2023) 15(15). doi: 10.3390/cancers15153955
19. Yu K, Gu Y, Zhang P, Fang H, Cao Y, Wang J, et al. Intratumoral Pd-1(+)Cd8(+) T cells associate poor clinical outcomes and adjuvant chemotherapeutic benefit in gastric cancer. Br J Cancer. (2022) 127:1709–17. doi: 10.1038/s41416-022-01939-8
20. Mazzaschi G, Madeddu D, Falco A, Bocchialini G, Goldoni M, Sogni F, et al. Low Pd-1 expression in cytotoxic Cd8(+) tumor-infiltrating lymphocytes confers an immune-privileged tissue microenvironment in Nsclc with a prognostic and predictive value. Clin Cancer Res. (2018) 24(2):407–19. doi: 10.1158/1078-0432.CCR-17-2156
21. Saito H, Shimizu S, Kono Y, Murakami Y, Shishido Y, Miyatani K, et al. Pd-1 expression on circulating Cd8(+) T-cells as a prognostic marker for patients with gastric cancer. Anticancer Res. (2019) 39:443–8. doi: 10.21873/anticanres.13132
22. Waki K, Yamada T, Yoshiyama K, Terazaki Y, Sakamoto S, Matsueda S, et al. Pd-1 expression on peripheral blood T-cell subsets correlates with prognosis in non-small cell lung cancer. Cancer Sci. (2014) 105:1229–35. doi: 10.1111/cas.12502
23. Kansy BA, Concha-Benavente F, Srivastava RM, Jie HB, Shayan G, Lei Y, et al. Pd-1 status in Cd8(+) T cells associates with survival and anti-Pd-1 therapeutic outcomes in head and neck cancer. Cancer Res. (2017) 77:6353–64. doi: 10.1158/0008-5472.CAN-16-3167
24. You E, Park CJ, Cho YU, Jang S, Lee MY, Kim H, et al. Increased Pd-1 expression of bone marrow T-cells in acute myeloid leukaemia patients after stem cell transplantation, and its association with overall survival. Ann Clin Biochem. (2024) 61:79–89. doi: 10.1177/00045632231184716
25. Tang L, Wu J, Li CG, Jiang HW, Xu M, Du M, et al. Characterization of immune dysfunction and identification of prognostic immune-related risk factors in acute myeloid leukemia. Clin Cancer Res. (2020) 26:1763–72. doi: 10.1158/1078-0432.CCR-19-3003
26. Mazzaschi G, Facchinetti F, Missale G, Canetti D, Madeddu D, Zecca A, et al. The circulating pool of functionally competent Nk and Cd8+ Cells predicts the outcome of anti-Pd1 treatment in advanced Nsclc. Lung Cancer. (2019) 127:153–63. doi: 10.1016/j.lungcan.2018.11.038
27. Guo L, Cao C, Goswami S, Huang X, Ma L, Guo Y, et al. Tumoral Pd-1hicd8+ T cells are partially exhausted and predict favorable outcome in triple-negative breast cancer. Clin Sci. (2020) 134:711–26. doi: 10.1042/CS20191261
28. Gao Y, Li S, Xu D, Chen S, Cai Y, Jiang W, et al. Prognostic value of programmed death-1, programmed death-ligand 1, programmed death-ligand 2 expression, and Cd8(+) T cell density in primary tumors and metastatic lymph nodes from patients with stage T1-4n+M0 gastric adenocarcinoma. Chin J Cancer. (2017) 36:61. doi: 10.1186/s40880-017-0226-3
29. Shen T, Zhou L, Shen H, Shi C, Jia S, Ding GP, et al. Prognostic value of programmed cell death protein 1 expression on Cd8+ T lymphocytes in pancreatic cancer. Sci Rep. (2017) 7:7848. doi: 10.1038/s41598-017-08479-9
30. Yeong J, Lim JCT, Lee B, Li H, Ong CCH, Thike AA, et al. Prognostic value of Cd8 + Pd-1+ Immune infiltrates and Pdcd1 gene expression in triple negative breast cancer. J immunotherapy Cancer. (2019) 7:34. doi: 10.1186/s40425-019-0499-y
31. Homicsko K, Zygoura P, Norkin M, Tissot S, Shakarishvili N, Popat S, et al. Pd-1-expressing macrophages and Cd8 T cells are independent predictors of clinical benefit from Pd-1 inhibition in advanced mesothelioma. J immunotherapy Cancer. (2023) 11(10). doi: 10.1136/jitc-2023-007585
32. Yang X, Wang G, Song Y, Zhuang T, Li Y, Xie Y, et al. Pd-1(+)Cd8(+) T cells proximal to Pd-L1(+)Cd68(+) macrophages are associated with poor prognosis in pancreatic ductal adenocarcinoma patients. Cancers. (2023) 15(5). doi: 10.3390/cancers15051389
33. Kotsakis A, Kallergi G, Aggouraki D, Lyristi Z, Koinis F, Lagoudaki E, et al. Cd8(+) Pd-1(+) T-cells and Pd-L1(+) circulating tumor cells in chemotherapy-naive non-small cell lung cancer: towards their clinical relevance? Ther Adv Med Oncol. (2019) 11:1758835919853193. doi: 10.1177/1758835919853193
34. Varki V, Ioffe OB, Bentzen SM, Heath J, Cellini A, Feliciano J, et al. Pd-L1, B7-H3, and Pd-1 expression in immunocompetent vs. Immunosuppressed patients with cutaneous squamous cell carcinoma. Cancer immunology immunotherapy: CII. (2018) 67:805–14. doi: 10.1007/s00262-018-2138-8
35. Lu X, Yang L, Yao D, Wu X, Li J, Liu X, et al. Tumor antigen-specific Cd8(+) T cells are negatively regulated by Pd-1 and Tim-3 in human gastric cancer. Cell Immunol. (2017) 313:43–51. doi: 10.1016/j.cellimm.2017.01.001
36. Lim YJ, Koh J, Kim K, Chie EK, Kim B, Lee KB, et al. High ratio of programmed cell death protein 1 (Pd-1)(+)/Cd8(+) tumor-infiltrating lymphocytes identifies a poor prognostic subset of extrahepatic bile duct cancer undergoing surgery plus adjuvant chemoradiotherapy. Radiotherapy Oncol. (2015) 117:165–70. doi: 10.1016/j.radonc.2015.07.003
37. Shen Y, Teng Y, Lv Y, Zhao Y, Qiu Y, Chen W, et al. Pd-1 does not mark tumor-infiltrating Cd8+ T cell dysfunction in human gastric cancer. J immunotherapy Cancer. (2020) 8(2). doi: 10.1136/jitc-2019-000422
38. Qin T, Zeng YD, Qin G, Xu F, Lu JB, Fang WF, et al. High Pd-L1 expression was associated with poor prognosis in 870 Chinese patients with breast cancer. Oncotarget. (2015) 6:33972–81. doi: 10.18632/oncotarget.5583
39. Egelston CA, Avalos C, Tu TY, Simons DL, Jimenez G, Jung JY, et al. Human breast tumor-infiltrating Cd8(+) T cells retain polyfunctionality despite Pd-1 expression. Nat Commun. (2018) 9:4297. doi: 10.1038/s41467-018-06653-9
40. Reck M, Rodriguez-Abreu D, Robinson AG, Hui R, Csoszi T, Fulop A, et al. Pembrolizumab versus chemotherapy for Pd-L1-positive non-small-cell lung cancer. New Engl J Med. (2016) 375:1823–33. doi: 10.1056/NEJMoa1606774
41. Schmid P, Adams S, Rugo HS, Schneeweiss A, Barrios CH, Iwata H, et al. Atezolizumab and Nab-paclitaxel in advanced triple-negative breast cancer. New Engl J Med. (2018) 379:2108–21. doi: 10.1056/NEJMoa1809615
42. Zhu Q, Qiao G, Huang L, Xu C, Guo D, Wang S, et al. Restored Cd8(+)Pd-1(+) T cells facilitate the response to anti-Pd-1 for patients with pancreatic ductal adenocarcinoma. Front Oncol. (2022) 12:837560. doi: 10.3389/fonc.2022.837560
43. Kamphorst AO, Pillai RN, Yang S, Nasti TH, Akondy RS, Wieland A, et al. Proliferation of Pd-1+ Cd8 T cells in peripheral blood after Pd-1-targeted therapy in lung cancer patients. Proc Natl Acad Sci U S A. (2017) 114:4993–8. doi: 10.1073/pnas.1705327114
44. Kumagai S, Togashi Y, Kamada T, Sugiyama E, Nishinakamura H, Takeuchi Y, et al. The Pd-1 expression balance between effector and regulatory T cells predicts the clinical efficacy of Pd-1 blockade therapies. Nat Immunol. (2020) 21:1346–58. doi: 10.1038/s41590-020-0769-3
Keywords: PD-1+CD8+ T cells, overall survival, progression-free survival, disease-free survival, cancer
Citation: Wan Z, Cui M, Yang J, Liao D, Chen J, Li F, Xiang Y, Cui Z and Yang Y (2025) Prognostic significance of programmed cell death 1 expression on CD8+T cells in various cancers: a systematic review and meta-analysis. Front. Oncol. 14:1531219. doi: 10.3389/fonc.2024.1531219
Received: 20 November 2024; Accepted: 20 December 2024;
Published: 14 January 2025.
Edited by:
Yu’e Liu, Boston Children’s Hospital and Harvard Medical School, United StatesReviewed by:
Jin-Ming Zhang, University of Texas Health Science Center at Houston, United StatesXutong Xue, Boston Children’s Hospital and Harvard Medical School, United States
Copyright © 2025 Wan, Cui, Yang, Liao, Chen, Li, Xiang, Cui and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yang Yang, NjQ0MjM4MTU0QHFxLmNvbQ==
†These authors have contributed equally to this work
 Zhiyong Wan
Zhiyong Wan Meng Cui
Meng Cui Jia Yang2
Jia Yang2