
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Oncol. , 21 January 2025
Sec. Cardio-Oncology
Volume 14 - 2024 | https://doi.org/10.3389/fonc.2024.1530403
This article is part of the Research Topic Case Reports in Cardio-Oncology: 2024 View all 11 articles
In this study, we present the case of a 38-year-old woman who was diagnosed with primary cardiac undifferentiated sarcoma after hospital admission. Following postoperative treatment that included radiotherapy and immunotherapy, the patient developed esophagus stenosis.
Primary cardiac tumors are rare with an incidence between 0.0017% and 0.28% (1). Twenty-five percent of cardiac tumors are malignant, and nearly 20% of these are sarcomas. Among these, Cardiac angiosarcomas constitute 37% of cardiac sarcomas, while undifferentiated sarcomas account for less than 24%, leiomyosarcomas constitute 8% to 9% of cardiac sarcomas, and rhabdomyosarcomas account for 4% to 7%. In addition, Osteosarcoma, fibrosarcoma, liposarcoma, and synovial sarcoma represent a small proportion of cardiac sarcomas (2, 3). Cardiac sarcomas are often asymptomatic until advanced, and even then produce non-specific symptoms such as palpitations, shortness of breath, dyspnea, chest pain, syncope, anemia, and weight loss (4–6). As a result, they can easily be misdiagnosed as other conditions, such as pneumonia or pericarditis (4). The dismal prognosis results from extensive local invasion or distant metastases at presentation. The initial diagnosis of cardiac sarcoma primarily relies on imaging techniques such as echocardiography, MRI and PET-CT, although a definitive histopathological diagnosis is essential (7, 8). In this report, we described a case that underwent initial cardiac tumor resection, and was confirmed to be undifferentiated sarcoma based on pathological findings. Following postoperative treatment that included radiotherapy and immunotherapy, the patient developed esophagus stenosis. To the best of our knowledge, this is the first case report detailing a primary cardiac undifferentiated sarcoma complicated by esophagus stenosis.
A 38-year-old female patient was admitted to the hospital on December 9, 2023 with complaints of “palpitations for 1 week.” She did not report any significant chest tightness, wheezing, or other noteworthy symptoms. She was healthy in the past and had no family history of hereditary disease. An outpatient echocardiogram revealed a large left atrium with a medium to strong echo mass (69 mm x 41 mm) attached to the roof of the left atrium. The mass had an irregular shape with uneven internal echogenicity and was partially protruding into the left ventricle near the mitral valve (Figure 1A). Cardiac mass resection was performed on December 12, 2023. The right atrium and interatrial septum were incised, revealing a large tumor (7 cm x 5 cm x 5 cm), and the tumor is somewhat resilient in texture (such as palpating the nose tip), that occupied the left atrium and extended into the openings of the left and right pulmonary veins. The tumor was completely excised along its attachment to the surrounding tissues. The postoperative immunohistochemical analysis revealed the following results: CK (focal +), SMA (focal +), Desmin (focal +), S-100 (–), SOX 10 (–), CD34 (vascular +), Ki67-MIB1 (50%), Vimentin (+), MyoD1 (–), Myogenin (–), CD68 (+). (Figures 2A–G), confirmed that the tumor was consistent with cardiac sarcoma, undifferentiated sarcoma. The patient’s recovery following surgery was uneventful. However, on January 25, 2024, cardiac MRI revealed abnormal signals in the posterior wall of the left atrium, raising concerns of tumor recurrence (Figure 1B). Radiotherapy was started in February, 2024, (18 times, Radiation dose: 3x18Gy) and was completed on March 1, 2024. In accordance with the guidelines from the Chinese Society of Clinical Oncology for the Diagnosis and Treatment of Soft Tissue Sarcoma, the patient began oral anlotinib (10 mg QD) during radiotherapy. Regular immunotherapy with cindarizumab (administered once every 21 days) commenced on February 28, 2024. In April 2024, the patient was readmitted due to dysphagia. Gastroscopy indicated that the distance between the esophagus and the incisor was approximately 28 cm, revealing an esophagus ulcer covered with white exudate, narrowing of the lumen, and obstruction preventing endoscopic passage (Figure 3A). Mediastinal CT showed that after left atrial surgery, the posterior wall of the left atrium and the posterior esophagus were thickened (Figures 1C, D). PET-CT revealed thickening of the left atrial wall and the esophagus wall, with increased FDG metabolism (Figures 4A, B). On August 8, 2024, follow-up gastroscopy demonstrated an esophagus ulcer located about 30 cm from the incisor, consistent with an inability to pass the endoscope (Figure 3B). The comprehensive analysis considered the patient’s recurrence of cardiac undifferentiated sarcoma and invasion of the esophagus, resulting in esophagus stenosis. After consulting with the cardiovascular surgeon, the patient was informed about the necessity and risks of undergoing another surgical procedure. However, the patient and his family ultimately refused further surgical treatment. For treatment, a multidisciplinary team, including oncologists and gastroenterologists, recommended esophagus stent implantation, which was successfully performed on August 28, 2024 (Figures 3C, D). The procedure was uneventful, and the patient’s swallowing capability was deemed acceptable. As of the time of writing, the patient remains alive and independent.
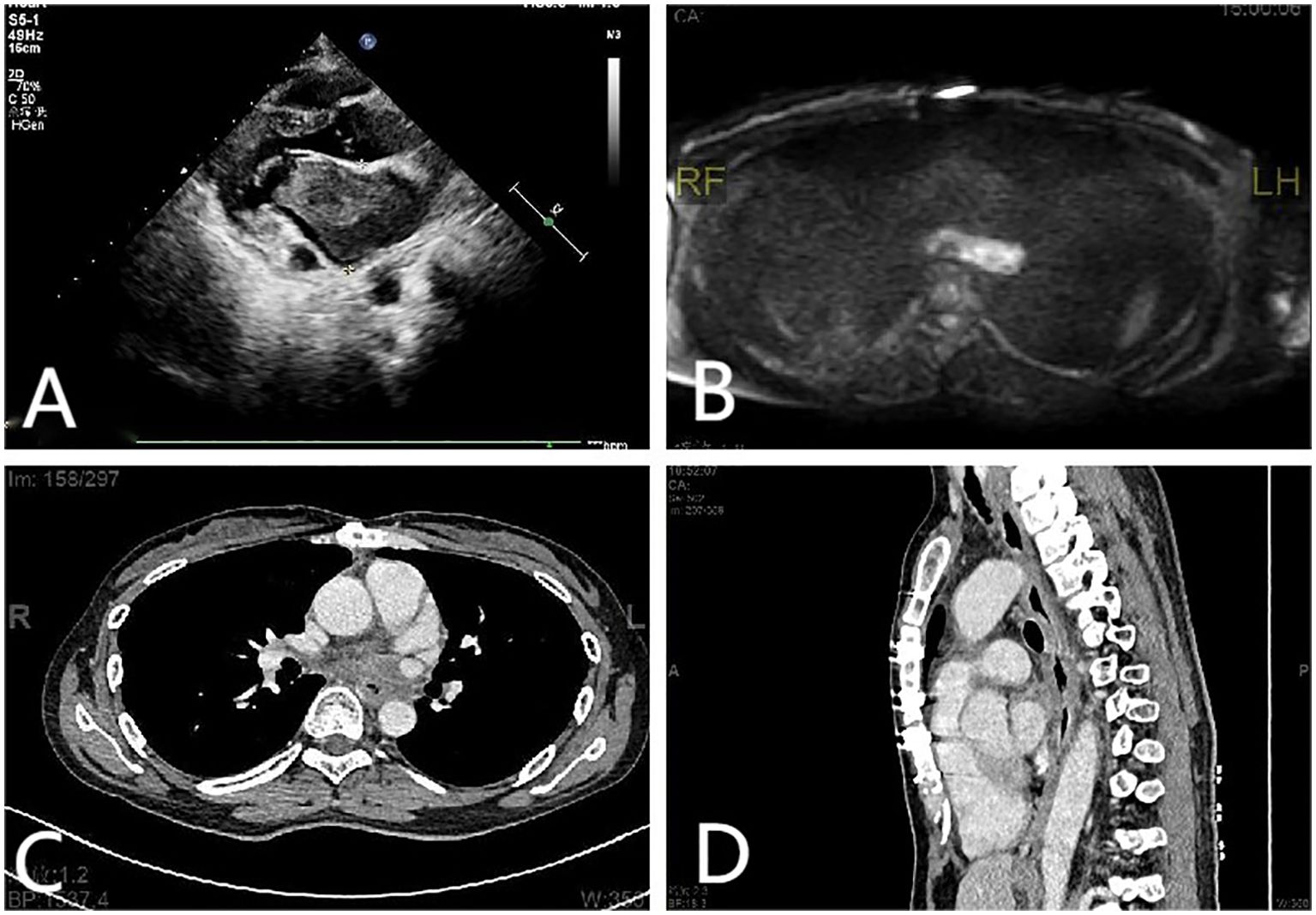
Figure 1. (A) Echocardiographic findings: A mass measuring approximately 69 mm × 41 mm was identified in the anterior wall of the left atrium. (B) Cardiac MRI scan findings: abnormal signals in the posterior wall of the left atrium. (C, D) Mediastinal CT scan findings: the posterior wall of the left atrium and the posterior esophagus were thickened. The gap between the esophagus and the left atrium disappeared, and tumor cell infiltration of the esophagus was considered.
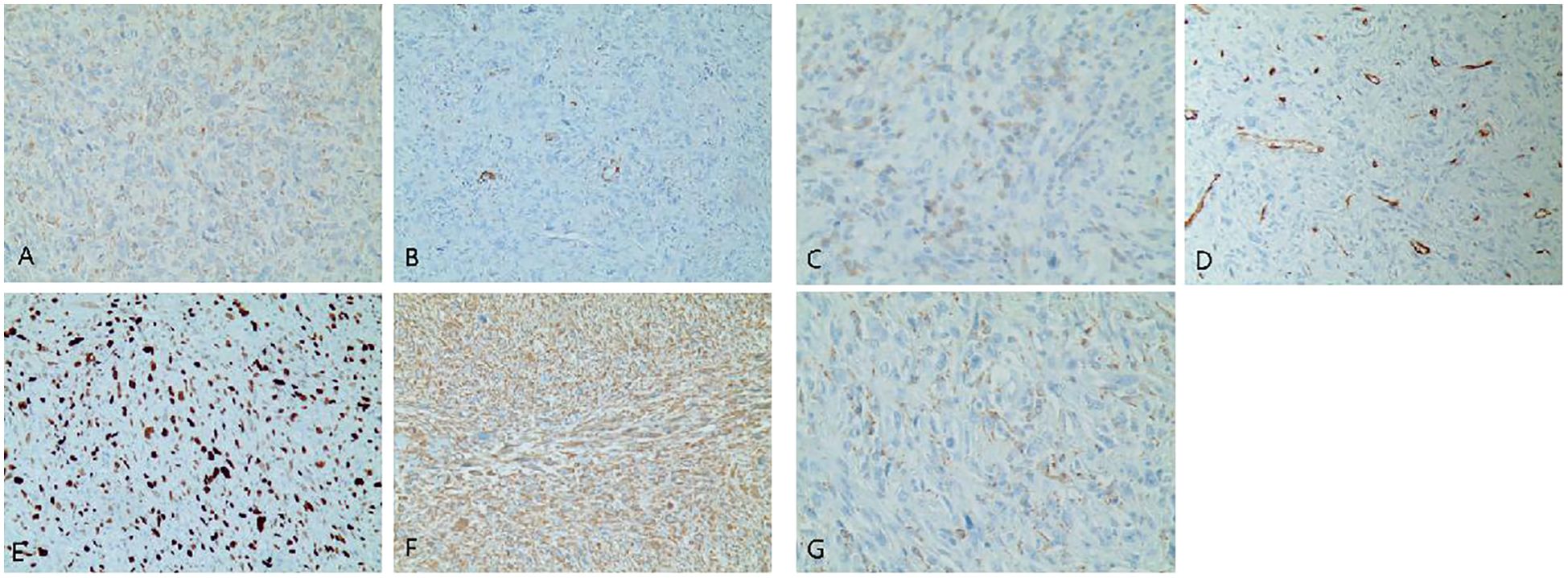
Figure 2. (A–G) Immunohistochemistry of the left atrium mass. (A) CK (focal +). (B) SMA (focal +). (C) Desmin (focal +). (D) CD34 (vascular +). (E) Ki67-MIB1 (50%). (F) Vimentin (+). (G) CD68 (+).
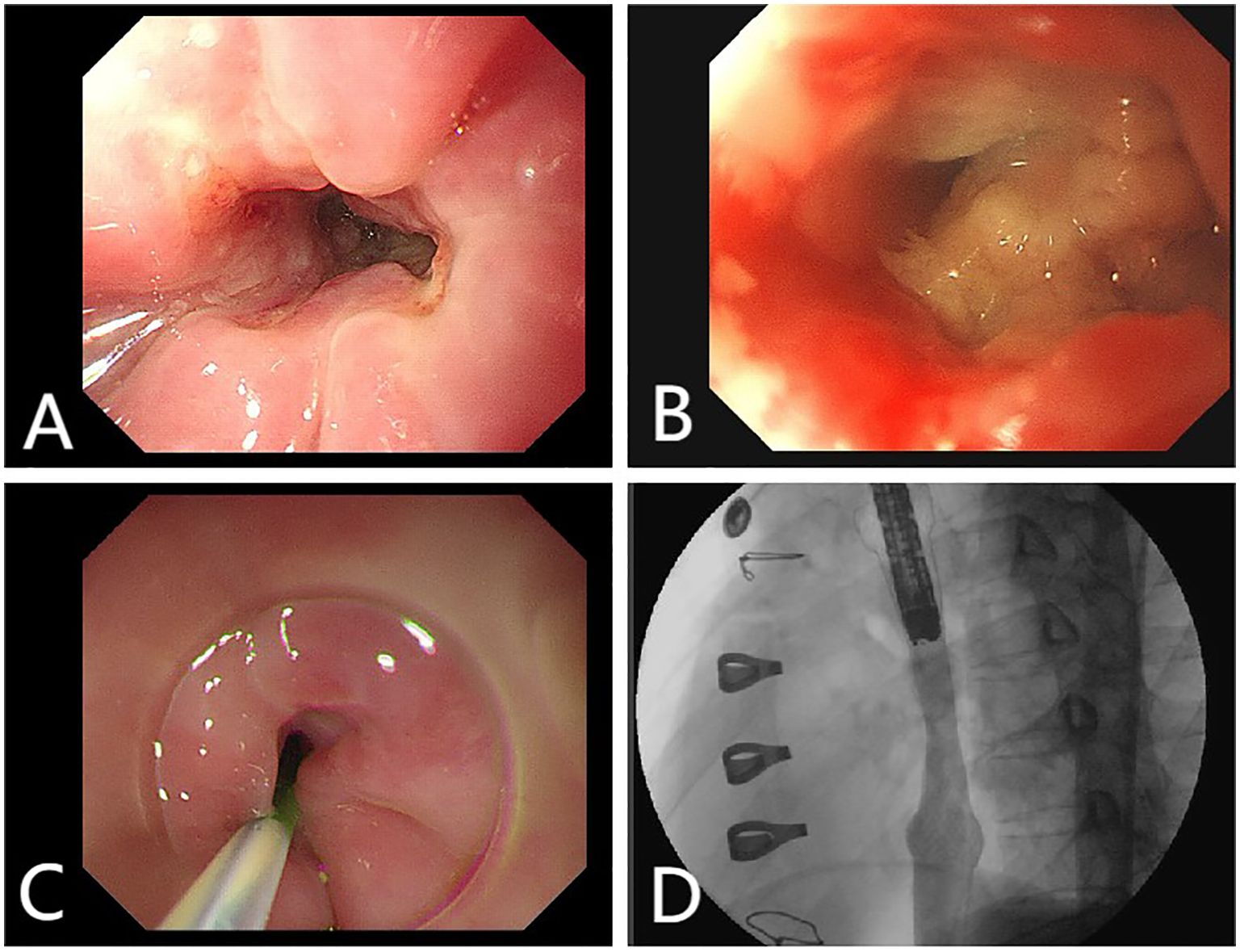
Figure 3. (A) Endoscopy reveals an esophagus ulcer covered with white exudate, narrowing of the lumen. (B) Endoscopy reveals an esophagus ulcer. (C, D) Esophagus stent implantation.
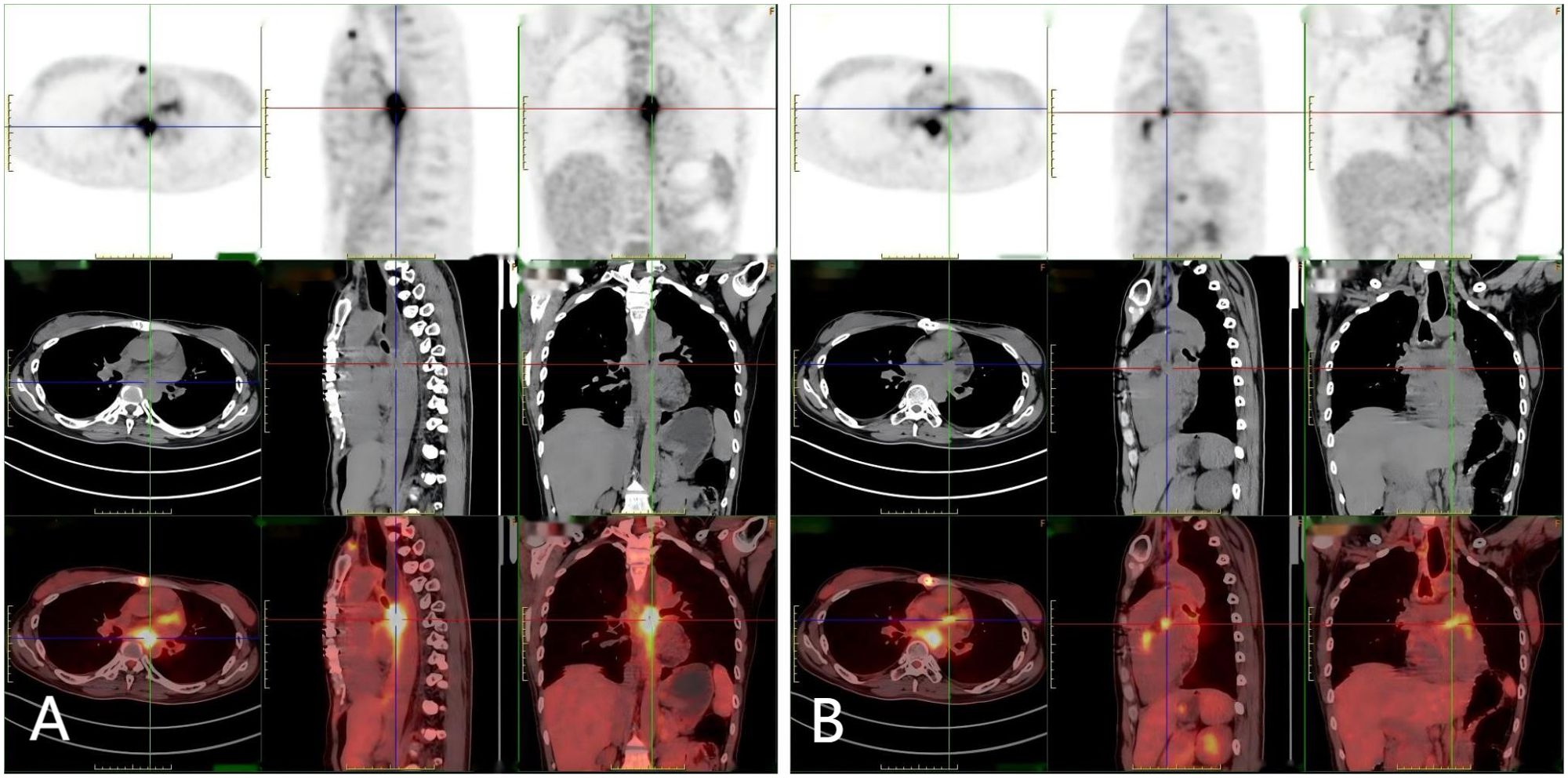
Figure 4. (A, B) A 18F-FDG-PET-CT scan: Hypermetabolic lesion in the left atrium wall and esophagus.
The incidence of primary cardiac tumors ranges from 0.0017% to 0.28% (1). These sarcomas typically have a poor prognosis, as they are often widely disseminated and prone to hematogenous metastasis to the lungs or liver by the time of diagnosis (9, 10). Undifferentiated sarcoma, the most aggressive type of cardiac malignant tumor, accounts for approximately 12% of all primary cardiac sarcomas and is associated with an extremely poor prognosis (11). Due to their local aggressiveness, complete resection of undifferentiated sarcomas is often not feasible, with only about 12% of patients achieving R0 resection (complete response) (12). Despite aggressive treatment, the median overall survival for patients with cardiac sarcomas is low, typically ranging from 6 to 12 months (13). Notably, patients who receive multimodal therapy may experience better survival outcomes compared to those who undergo surgery alone (14). The treatment of undifferentiated sarcoma necessitates a multidisciplinary approach, often derived from case reports and series, due to a lack of robust data, randomized clinical trials, or standardized treatment protocols (15, 16). In this case, the patient was pathologically confirmed as undifferentiated sarcoma after cardiac mass resection, and active intervention was immediately taken, including radiotherapy, immunization and targeted therapy. Currently, the patient is experiencing esophagus stenosis, 18FDG-PET-CT showed a hypermetabolic lesion in the left atrium wall and esophagus, so we considered the possibility of recurrence and invasion of cardiac malignant tumors (17). However, pathological results did not confirm any malignancy, which may be attributed to the effects of prior radiotherapy. Research indicates that the incidence of esophagus stenosis is 1%-2% when the radiation dose is less than 60 Gy, increasing to 5%-6% when the radiation dose exceeds 60 Gy (18). Studies have found that there is a significant correlation between radiotherapy dose and the occurrence of esophagus stenosis. As the radiation dose increases, the degree of fibrosis in the esophageal muscle layer and submucosa progressively worsens, thereby elevating the risk of esophageal stenosis (19, 20). Therefore, the specific causes need to be further examined. Finally, the patient underwent esophagus stent implantation. The procedure was successful, and the patient reported improved swallowing function postoperatively. From the review of literature, we conclude that although surgical intervention was necessary for resection of the tumor, it is important to identify malignant tumors early, in order to enable patients to undergo radiotherapy or chemotherapy as soon as possible. In addition, We believe that cardiac MRI examinations should be enhanced prior to surgery to provide better guidance during the procedure. In this case, the cardiac MRI was not adequately improved before the operation, which reflects our oversight. In the future, we should adopt a more rigorous approach to diagnosis and treatment, ensuring that preoperative examination and evaluation are thoroughly optimized. The treatment of undifferentiated cardiac sarcoma requires a multidisciplinary approach. Advances in anti-tumor immunotherapy, targeted therapy, and chemotherapy offer new options for managing cardiac sarcomas. Moving forward, more basic and clinical research is needed to develop additional treatment strategies for this disease, ultimately aiming to extend patient survival.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
YG: Conceptualization, Writing – original draft, Writing – review & editing. XLv: Data curation, Writing – original draft. RZ: Formal analysis, Writing – original draft. DH: Visualization, Writing – original draft. GS: Supervision, Validation, Writing – original draft. YL: Software, Writing – original draft. XY: Supervision, Writing – review & editing. XLi: Supervision, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by grants from the Linyi People’s Hospital Innovation Team Development Project (LYSRMYY-KCTD-008).
We express our gratitude to the patients and their families for providing informed consent to use their personal data.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Morphet JA. A 30-year analysis of cardiac neoplasms at autopsy. Can J Cardiol. (2006) 22:80. doi: 10.1016/S0828-282X(06)70247-1
2. Hoffmeier A, Sindermann JR, Scheld HH, Martens S. Cardiac tumors–diagnosis and surgical treatment. Dtsch Arztebl Int. (2014) 111:205–11. doi: 10.3238/arztebl.2014.0205
3. Tyebally S, Chen D, Bhattacharyya S, Mughrabi A, Hussain Z, Manisty C, et al. Cardiac tumors: JACC cardioOncology state-of-the-art review. JACC CardioOncol. (2020) 2:293–311. doi: 10.1016/j.jaccao.2020.05.009
4. Dulal Karki S, Westhoff M, Maschek H, Augustyniak J, Gupta V, Welter S. A rare diagnostic challenge in a female patient with a rapid recurrent pleural effusion: Autopsy revealed cardiac angiosarcoma with bilateral pleural and pulmonary metastases. A Case Rep Int J Surg Case Rep. (2021) 78:278–83. doi: 10.1016/j.ijscr.2020.12.034
5. Alizadehasl A, Hakimian H, Dokhani N, Pouraliakbar H, Firoozbakhsh P. Early presentation of undifferentiated pleomorphic cardiac sarcoma. Radiol Case Rep. (2024) 19:4308–11. doi: 10.1016/j.radcr.2024.06.088
6. Guerra-Raygada M, Saavedra-Sanchez AJ, Hidalgo-Avendaño D, Bermudez-Pelaez MF, Guevara-Lazo D, Nombera-Aznaran N. From dyspnea to diagnosis, unmasking undifferentiated cardiac sarcoma: a case report. Egypt Heart J. (2024) 76:86. doi: 10.1186/s43044-024-00520-3
7. Maraj S, Pressman GS, Figueredo VM. Primary cardiac tumors. Int J Cardiol. (2009) 133:152–6. doi: 10.1016/j.ijcard.2008.11.103
8. Oh SJ, Yeom SY, Kim KH. Clinical implication of surgical resection for the rare cardiac tumors involving heart and great vessels. J Korean Med Sci. (2013) 28:717–24. doi: 10.3346/jkms.2013.28.5.717
9. Ramlawi B, Leja MJ, Abu Saleh WK, Al Jabbari O, Benjamin R, Ravi V, et al. Surgical treatment of primary cardiac sarcomas: review of a single-institution experience. Ann Thorac Surg. (2016) 101:698–702. doi: 10.1016/j.athoracsur.2015.07.087
10. Centofanti P, Di Rosa E, Deorsola L, Dato GM, Patanè F, La Torre M, et al. Primary cardiac tumors: early and late results of surgical treatment in 91 patients. Ann Thorac Surg. (1999) 68:1236–41. doi: 10.1016/S0003-4975(99)00700-6
11. Randhawa JS, Budd GT, Randhawa M, Ahluwalia M, Jia X, Daw H, et al. Primary cardiac sarcoma: 25-year cleveland clinic experience. Am J Clin Oncol. (2016) 39:593–9. doi: 10.1097/COC.0000000000000106
12. Siontis BL, Zhao L, Leja M, McHugh JB, Shango MM, Baker LH, et al. Primary cardiac sarcoma: A rare, aggressive Malignancy with a high propensity for brain metastases. Sarcoma. (2019) 2019:1960593. doi: 10.1155/2019/1960593
13. Truong PT, Jones SO, Martens B, Alexander C, Paquette M, Joe H, et al. Treatment and outcomes in adult patients with primary cardiac sarcoma: the British Columbia Cancer Agency experience. Ann Surg Oncol. (2009) 16:3358–65. doi: 10.1245/s10434-009-0734-8
14. Nakamura-Horigome M, Koyama J, Eizawa T, Kasai H, Kumazaki S, Tsutsui H, et al. Successful treatment of primary cardiac angiosarcoma with docetaxel and radiotherapy. Angiology. (2008) 59:368–71. doi: 10.1177/0003319707308212
15. Abbas HA, Amini B, Wang WL, Ravi V. Maneuvering the management of a rare case of primary undifferentiated cardiac sarcoma. Am J Case Rep. (2020) 21:e918878. doi: 10.12659/AJCR.918878
16. Sun YP, Wang X, Gao YS, Zhao S, Bai Y. Primary cardiac sarcoma complicated with cerebral infarction and brain metastasis: A case report and literature review. Cancer biomark. (2017) 21:247–50. doi: 10.3233/CBM-170448
17. Utsunomiya Y, Miyake KK, Fukushima S, Kinoshita H, Ikeda Y, Matsumoto M, et al. (18)F-FDG PET/CT in left atrial undifferentiated pleomorphic sarcoma with osteosarcomatous differentiation. J Cardiol Cases. (2024) 29:30–4. doi: 10.1016/j.jccase.2023.09.005
18. Morichau-Beauchant M, Touchard G, Battandier D, Maire P, Fontanel JP, Daban A, et al. Chronic radiation-induced esophagitis after treatment of oropharyngolaryngeal cancer: a little-known anatomo-clinical entity. Gastroenterol Clin Biol. (1983) 7:843–50.
19. Roeder F, Nicolay NH, Nguyen T, Saleh-Ebrahimi L, Askoxylakis V, Bostel T, et al. Intensity modulated radiotherapy (IMRT) with concurrent chemotherapy as definitive treatment of locally advanced esophageal cancer. Radiat Oncol. (2014) 9:191. doi: 10.1186/1748-717X-9-191
Keywords: cardiac undifferentiated sarcoma, endoscopy, esophagus stenosis, immunohistochemistry, pathology
Citation: Ge Y, Lv X, Zhang R, Hao D, Si G, Li Y, Yuan X and Li X (2025) Case report: Primary cardiac undifferentiated sarcoma complicated by esophagus stenosis. Front. Oncol. 14:1530403. doi: 10.3389/fonc.2024.1530403
Received: 18 November 2024; Accepted: 30 December 2024;
Published: 21 January 2025.
Edited by:
Reto Asmis, Wake Forest University, United StatesCopyright © 2025 Ge, Lv, Zhang, Hao, Si, Li, Yuan and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xuemin Yuan, bHl4aHl4bUAxNjMuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.