- 1Department of Urology, Chengdu Second People’s Hospital, Chengdu, China
- 2Department of Urology, The General Hospital of Western Theater Command, Chengdu, China
- 3Department of Gynecology and Obstetrics, West China Second University Hospital, Sichuan University, Chengdu, China
- 4Department of Urology, Yongchuan Hospital of Chongqing Medical University, Chongqing, China
- 5Department of Neurosurgery, The Second Affiliated Hospital of Chongqing Medical University, Chongqing, China
- 6Department of Biotherapy, Cancer Center, West China Hospital, Sichuan University, Chengdu, China
Objective: To investigate the optimal cut-off value of immunohistochemical marker Ki67 as a prognostic factor to predict the recurrence of non-muscle invasive bladder urothelial carcinoma (NMIBUC).
Methods: A total of 331 patients diagnosed with NMIBUC who underwent surgery in the Yongchuan Hospital and the Second Affiliated Hospital of Chongqing Medical University from January 2012 to January 2020 were finally included in this study. The optimal cut-off value of Ki67 for predicting recurrence of NMIBUC was calculated by ROC curve and Youden index. According to the cut-off value, the patients were divided into high ratio group and low ratio group, and the clinicopathological data of the two groups were compared. Univariate and multivariate regression analysis were used to analyze the relationship between the expression of Ki67 and postoperative recurrence of NMIBUC. The Kaplan-Meier curve was used for survival analysis.
Results: 18% is the optimal cut-off value of Ki67 for predicting postoperative recurrence of NMIBUC. High Ki67 expression (Ki67>18%) was significantly correlated with tumor stage (P=0.001), tumor grade (P=0.014), immediate postoperative instillation (P=0.001), the expression of P53 (P=0.019) and CK20 (P=0.001). Ki67 expression greater than 18% was an independent risk factor for high recurrence rate of NMIBUC (P=0.001). Moreover, the 1-year and 3-year recurrence-free survival (RFS) of the high Ki67 group were 56.6% (95%CI 51.2%-62%) and 43.6% (95%CI 37.5%-49.7%) respectively, which were significantly lower than those in low Ki67 group which present as 92.9% (95%CI 89.0%-96.8%) and 88.3% (95%CI 82.4%-94.2%) respectively, and the difference was statistically significant (P<0.001).
Conclusions: 18% is the optimal cut-off value of Ki67 for predicting recurrence of NMIBUC. Ki67>18% is an independent risk factor for high recurrence rate of NMIBUC. This cut-off value can more accurately predict the risk of recurrence and has the potential clinical value for guiding the postoperative adjuvant treatment and follow-up strategy of NMIBUC.
1 Introduction
According to the latest statistics, bladder cancer (BCa) is one of the most common malignant tumors of the urinary system, with increasing mortality and morbidity (1). Of all types of BCa, non-muscular invasive bladder urothelial carcinoma (NMIBUC) is the most common pathological classification, accounting for 70% of BCa cases (2–4). Currently, transurethral resection of bladder tumor (TURBT) is the primary therapeutic approach for NMIBUC. Unfortunately, still approximately 60-70% of patients are reported to develop recurrence after surgery (5). Therefore, it is particularly critical to determine more indicators to accurately evaluate the postoperative prognosis of NMIBUC patients and guide management to reduce relapse.
With great advances in molecular biology and tumor biology, immunohistochemical markers have been considered for cancer prognostic assessments. Ki67, a nuclear antigen associated with the cell cycle, is significantly related to cell proliferation. It contributes to better estimation of the aggressive biological behavior of malignant tumor cells (6, 7). Many studies have found that the expression of the Ki67 protein is associated with tumor biological characteristics and prognosis (8, 9). In clinical practice, Ki67 is regarded as a clinical indicator to detect tumor cell proliferation activity and cancer prognosis because the expression of Ki67 can be easily detected by immunohistochemistry (7). For example, as reported in breast cancer, the high expression of Ki67 is helpful for identifying patients with poor prognosis (10). Similarly, Ki67, as a high-risk factor, is already used to assess the risk of endometrial cancer recurrence and guide the strategy effective adjuvant treatments (11). Additionally, some studies concluded that high-expression of Ki67 is associated with poorer prognostic indicators, including progression free survival (PFS), cancer specific survival (CSS), recurrence free survival (RFS), and overall survival (OS) in NMIBUC patients, which is particularly prominent in Europe and the United States; however, among Asian NMIBUC patients with recurrence and progression, the correlation between Ki67 expression and the above indicators is not obvious (12). In addition, many studies have shown that Ki67 may also be used as a significant predictor for postoperative recurrence of NMIBUC (13, 14). However, most clinicians only pay attention to the interpretation of positive or negative Ki67 expression (15). As a result, there is a lack of theoretical consensus on the criteria for defining positive or negative expression. Furthermore, there have been very few studies on the cut-off value of Ki67 to predict NMIBUC recurrence, which indicates that it is deficient to correctly assess the tumor consequence.
Therefore, this study aimed to evaluate the correlation between NMIBUC prognosis and Ki67 expression, as well as determine the optimal cut-off value of Ki67 to predict NMIBUC recrudescence, thus providing guidance for clinical treatments.
2 Materials and methods
2.1 Research population
The retrospective analysis has gathered medical information of the patients from January 2012 to January 2020 in the Yongchuan Hospital and the Second Affiliated Hospital of Chongqing Medical University. The pathological grading was evaluated by the WHO bladder cancer grading system (2004/2016): UCC-LG (short for low-grade urothelial carcinoma), UCC-HG (short for high-grade urothelial carcinoma), and PUNLMP (short for Papillary urothelial neoplasms of low malignant potential) (16). The inclusion criteria were as follows: (I) patients who primarily underwent the standard TURBT surgery; (II) the pathological type was NMIBUC (including the stage of Ta and T1) according to the Union for International Cancer Control (UICC); and (III) patients with sufficient medical information and requisite immunohistochemical markers, including CK20, P53, and Ki67. The cases that met the following criteria were excluded: (I) incomplete medical records; (II) patients failing to undergo TURBT surgery or receiving total or subtotal bladder resection; (II) the pathological type was nonurothelial tumor, including adenocarcinoma and squamous cell carcinoma; (III) bladder carcinoma in situ (stage Tis) (17): although this type belongs to NMIBC, it was also excluded due to its high risk of muscle invasion, poor differentiation, and high grade of aggression; (V) tumor metastasis; (VI;) no regular follow-up records: and (VII) Only a small number of patients in this study underwent BCG intravesical chemotherapy. These patients were excluded because they could not tolerate side effects such as cystitis, hematuria, etc. The procedures were conducted following the principles of the Declaration of Helsinki, and in accordance with the ethical standards of our hospital. All patients signed informed consent forms after the study protocol was fully explained.
2.2 Postoperative and follow-up
Approximately 60.4% (200/331) patients included in this study underwent bladder instillation chemotherapy. One of three drugs was selected: pirarubicin(40mg each time), epirubicin(50-80mg each time), or gemcitabine(1000mg each time). Due to some adverse effects of bacille Calmette-guerin vaccine such as hematuria, severe cystitis and etc., only a few patients were treated with BCG for bladder infusion chemotherapy, so they were excluded. Chemotherapy drugs are infused into the bladder through a urinary catheter and retained for 0.5-2 hours (according to the label). The participants were required to drain the bladder and drink less fluid before intravesical instillation. The appropriate solvent was selected according to the instructions of the drug. Standard TURBT surgery was performed in all participants. The standard strategy for follow-up was arranged from the operation date. The following subjects underwent foundational physical inspections, imaging and cystoscopy. The strategy adopted in this follow-up was: rechecking every three months within 1-2 years after surgery, rechecking every six months within 3-4 years after surgery, and rechecking every year after five years of surgery (18). The main imaging techniques for rechecking were urinary tract ultrasound and computed tomography (CT). During the follow-up, if the tests suggested the recurrence of BCa, further cystoscopy histopathological examination was needed (17); if clinical symptoms or imaging examination suggested no recurrence, cystoscopy was regularly conducted according to the established follow-up protocol. The deadline for follow-up was January 31, 2021.
2.3 Recurrence
During the follow-up, when clinical symptoms (such as hematuria) or imaging examination suggested BCa recurrence, secondary TURBT or cystoscopic biopsy was further performed to confirm tumor recurrence. The final result for recurrence should be identified by histopathological diagnosis (19). Recurrence is defined as any stage or grade of bladder urothelial carcinoma found in the bladder after surgery. Tumor progression is the occurrence of bladder wall myometrial invasion, regional lymph node invasion or distant metastasis during tumor recurrence. RFS was defined as the period from the initial surgical operation to recrudescence or the following deadline, and OS was defined as the time from the first operation to the last follow-up or death (20).
2.4 Immunohistochemistry analysis
All specimens were timely treated after an operation and were further processed following a standard and unified procedure in the pathology laboratory (21). Initially, specimens were fixed with formalin and further transformed into paraffin examples. Subsequently, according to eosin staining and hematoxylin, the lesion location was first identified. Next, CK20, P53 and Ki67 immunohistochemical analysis was conducted on an automatic immunostaining instrument (Leica Bond-Max, Milton Keynes, UK). Mouse monoclonal antibodies such as CK20 (clone Ks20.8), P53 (clone DO-7), and Ki-67 (clone 30-9) were used in immunohistochemistry. The tumor grade, lesion size, histological type, and infiltration depth were evaluated by our professional junior pathologists and then reviewed by our senior physician. The immunohistochemistry results were independently assessed by two expert pathologists. When the positive tumor cells were calculated the same, the evaluation was considered consistent; and when the initial assessment was different, the outcome was re-evaluated until reaching a consensus. The immunohistochemical parameters were as follows: CK20 cells without staining were negative (-); light yellow was weakly positive (1+); brownish yellow was moderately positive (2+), and brownish brown was strongly positive (3+) (18). The scoring system was as follows: < 5% P53 positive cells was scored as -; 5% ~ 25% was +; 26% ~ 49% was 2+; and ≥50% was 3+. Ki67 expression is shown by the percentage of positively stained cells (0% to 100%).
2.5 Statistical analysis
Data analysis was conducted using SPSS software (Version 26.0). Numeric variable was shown as the median and mean ± SD. The comparison was performed by t-test or rank-sum test. Categorical variables were shown as percentages and frequencies, and were compared by Chi-square test. The Youden index and receiver operating characteristic (ROC) curve were used to find the best cut-off Ki67 value. Multivariate and univariate Cox regression analyses were performed to verify the relationship between NMIBUC relapse and Ki67 expression. The survival analysis was shown in the Kaplan-Meier curve and Log-rank test. Using two-tailed tests, P<0.05 was considered statistically significant.
3 Results
3.1 Clinical and pathological features of patients and tumors
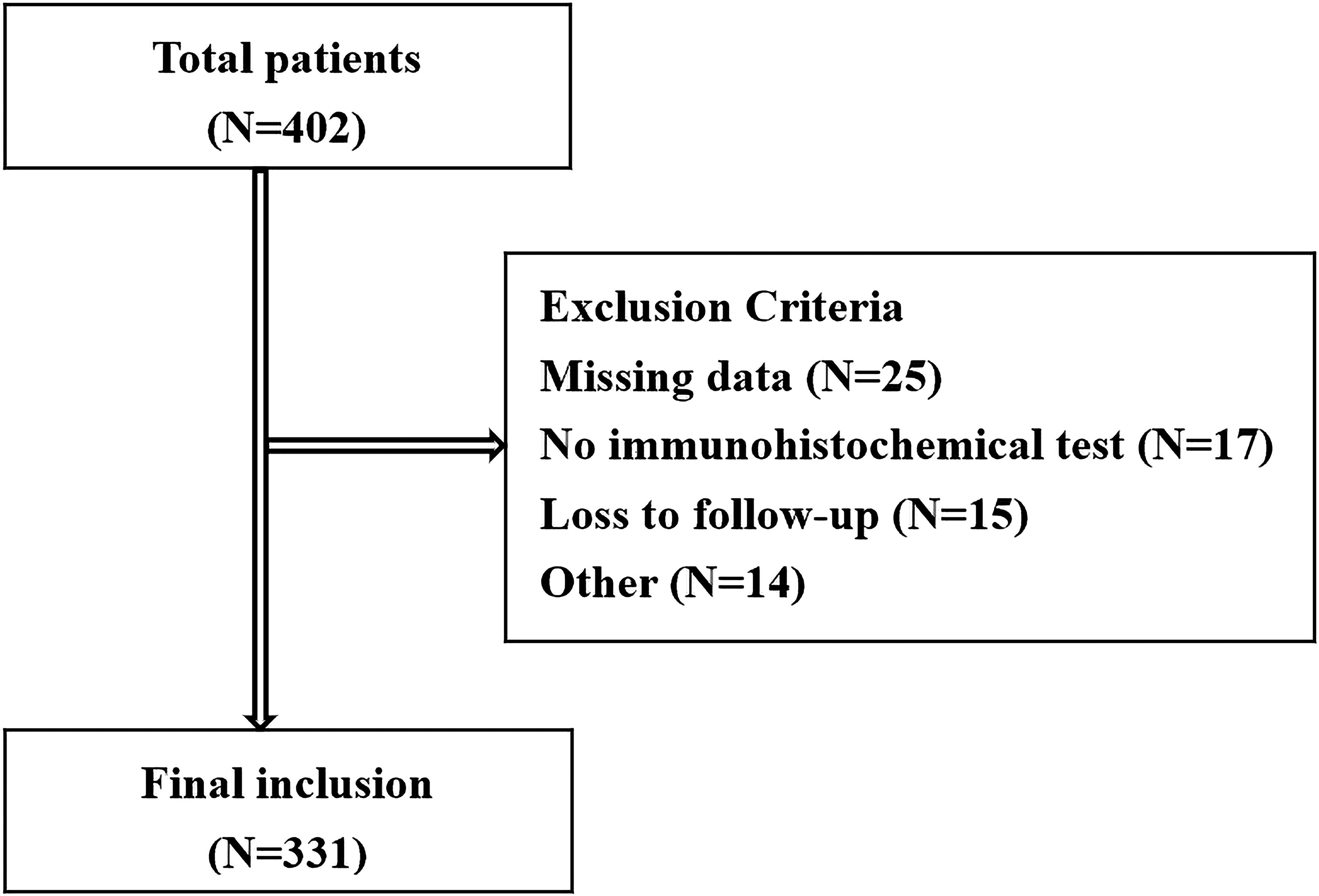
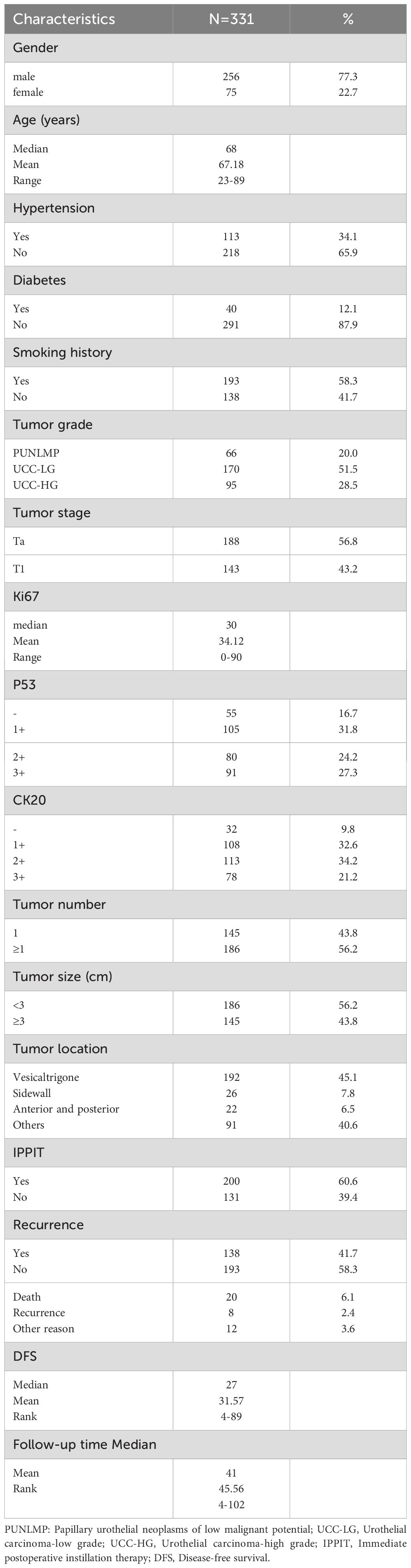
A total of 402 patients who were diagnosed with BCa receiving TURBT surgery were enrolled. According to the exclusion and inclusion criteria, 331 NMIBUC cases were included in the study Figure 1. Among these 331 patients, 138 (41.7%) reported recurrence, and 20 (6.1%) reported death. The Ki67 expression level ranged from 0% to 90% (median 30%). The median follow-up time and RFS were 41 months (4-102) and 27 months (4-89), respectively. Other clinicopathological data are shown in Table 1.
3.2 Optimal cut-off value of the Ki67 index
The correlation between postoperative NMIBUC recurrence and Ki67 expression was calculated by the ROC curve. Finally, the optimal Ki67 cut-off value expression level to forecast relapse was recognized to be 18% [N = 331, area under the curve = 0.802, specificity 50.0%, sensitivity 92.6%, P <0.001, 95% confidence interval (CI)=0.728-0.875] Figure 2.
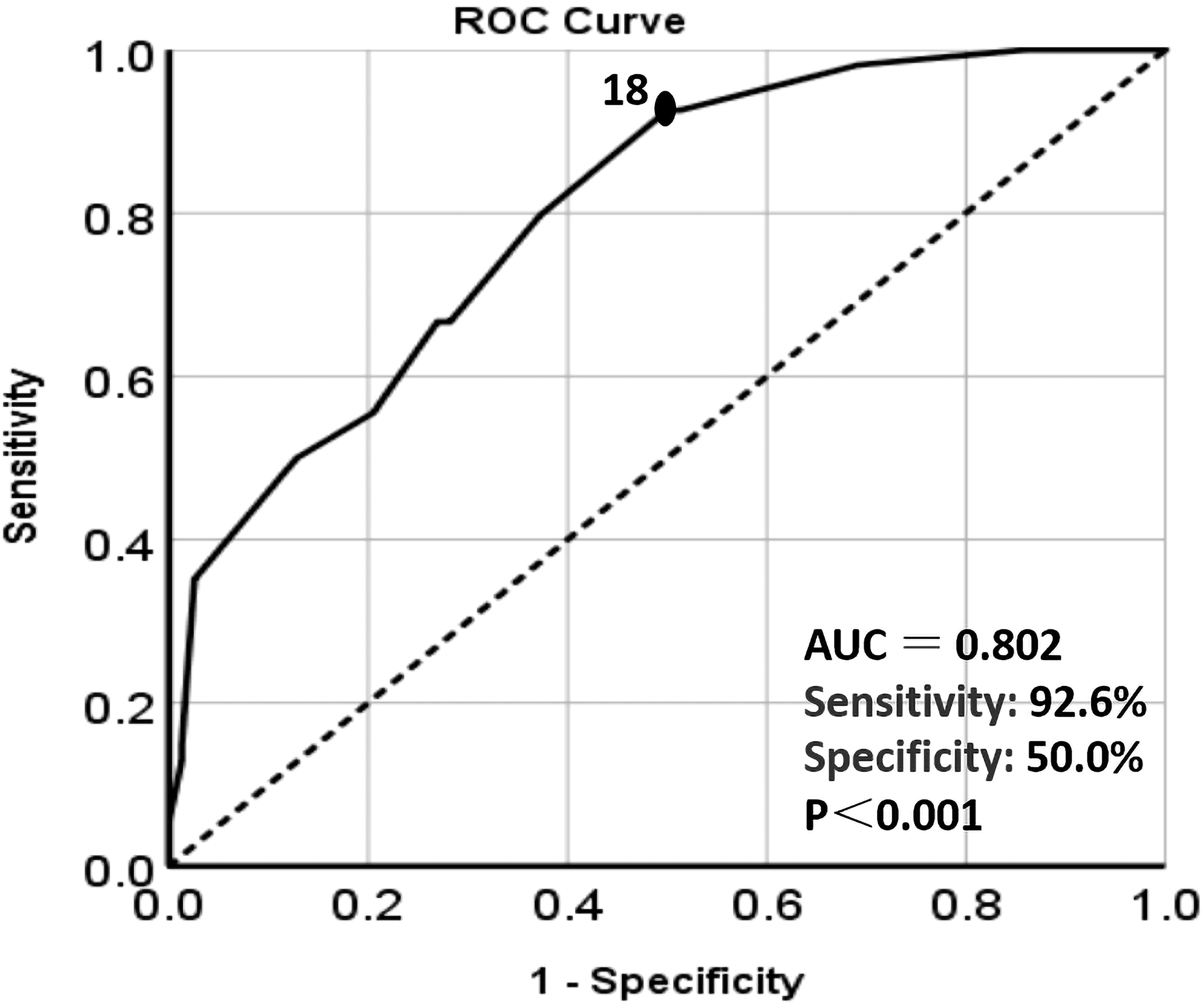
Figure 2. The ROC curve analysis of the relationship between Ki67 expression level and recurrence. The black dot: the area under the curve(AUC) at this point is the largest, which indicates the optimal cut-off value of Ki67. Dotted line: Reference line. Solid line: The ROC curve of Ki67.
3.3 Prognostic factors of recurrence based on univariate and multivariate analyses
The clinical parameters and immunohistochemical markers that might cause recurrence were included in the univariate analysis. Gender (P=0.091), diabetes (P=0.331), age (P=0.143), smoking (P=0.128) and hypertension (P=0.545) did not correlate significantly with RFS. Thus, the parameters with P<0.05, tumor size and number, tumor stage, grade, immediate postoperative adjuvant therapy, CK20, P53, and Ki67, were further included in the multivariate analysis. Tumor grade (P=0.033), immediate postoperative adjuvant therapy (P=0.031), Ki67 (P=0.030), P53 (P=0.048) and CK20 (P=0.044) were shown to be significantly independent risk indicators for postoperative recurrence of NMIBC by multivariate analysis Table 2.
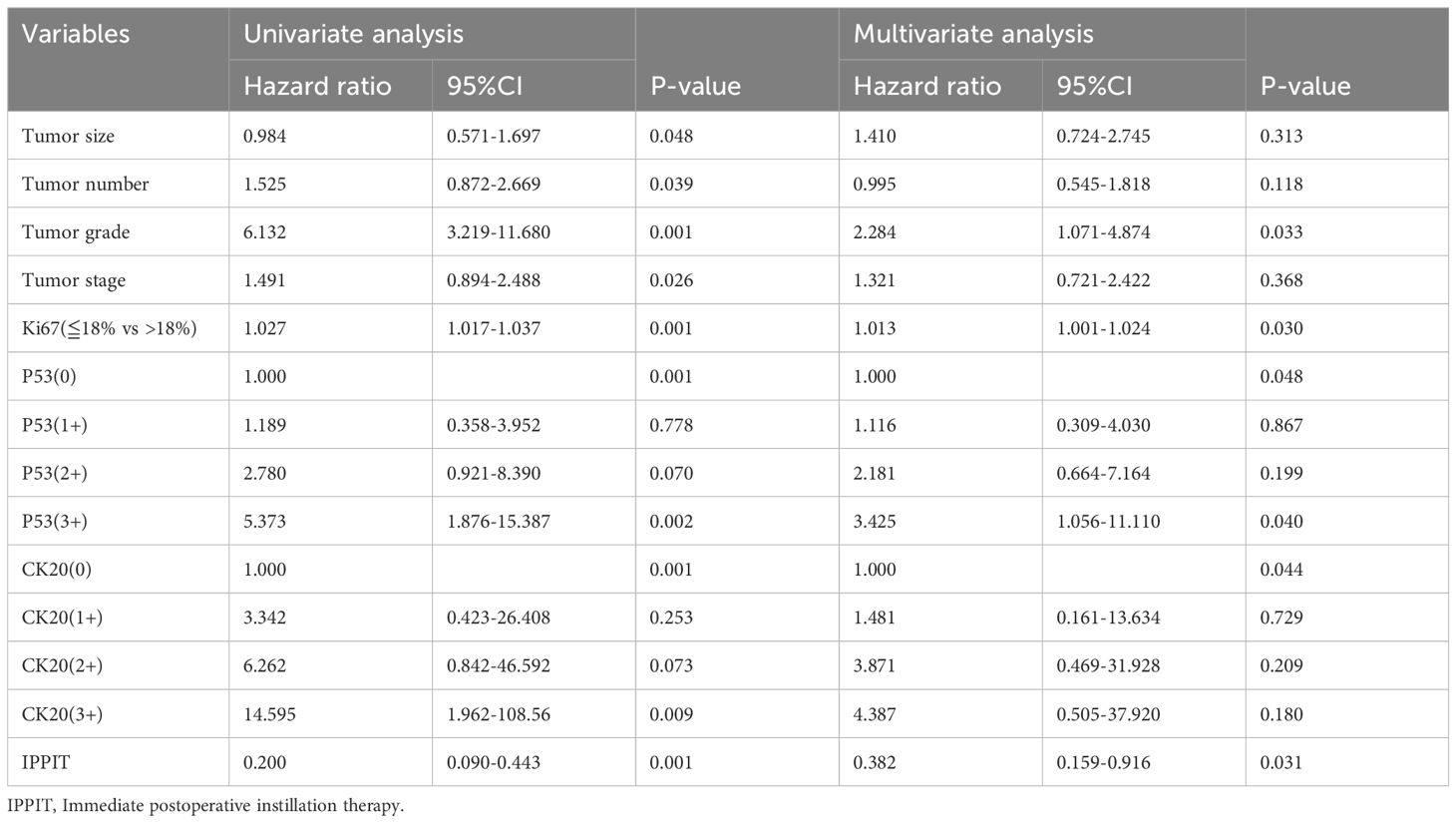
Table 2. The univariate and multivariate COX regression analysis of factors predicting non-muscle invasive bladder cancer recurrence.
3.4 Comparison of clinicopathological features between groups
According to the threshold of Ki67 (18%) concluded above, all patients were split into two groups: patients with Ki67>18% (high-Ki67 group) and patients with Ki67≦18% (low-Ki67 group). We found that the expression of high Ki67 was related to more aggressive characteristics: pathological grade (P=0.014), phase (P=0.001), the expression of P53 (P=0.019), immediate postoperative adjuvant therapy (P=0.001) and CK20 (P=0.001) Table 3. Furthermore, Kaplan-Meier analysis showed that the 3-year RFS was 43.6% (95% CI 37.5%-49.7%) in the high-Ki67 group and 88.3% (95% CI 82.4%-94.2%) in the low-Ki67 group, and the 5-year RFS were 30.0% (95% CI 22.7%-37.3%) and 88.3% (95% CI 82.4%-94.2%), respectively (P<0.001) Figure 3.

Table 3. Comparison of clinicopathological parameters of patients between low Ki67 group and high Ki67 group.
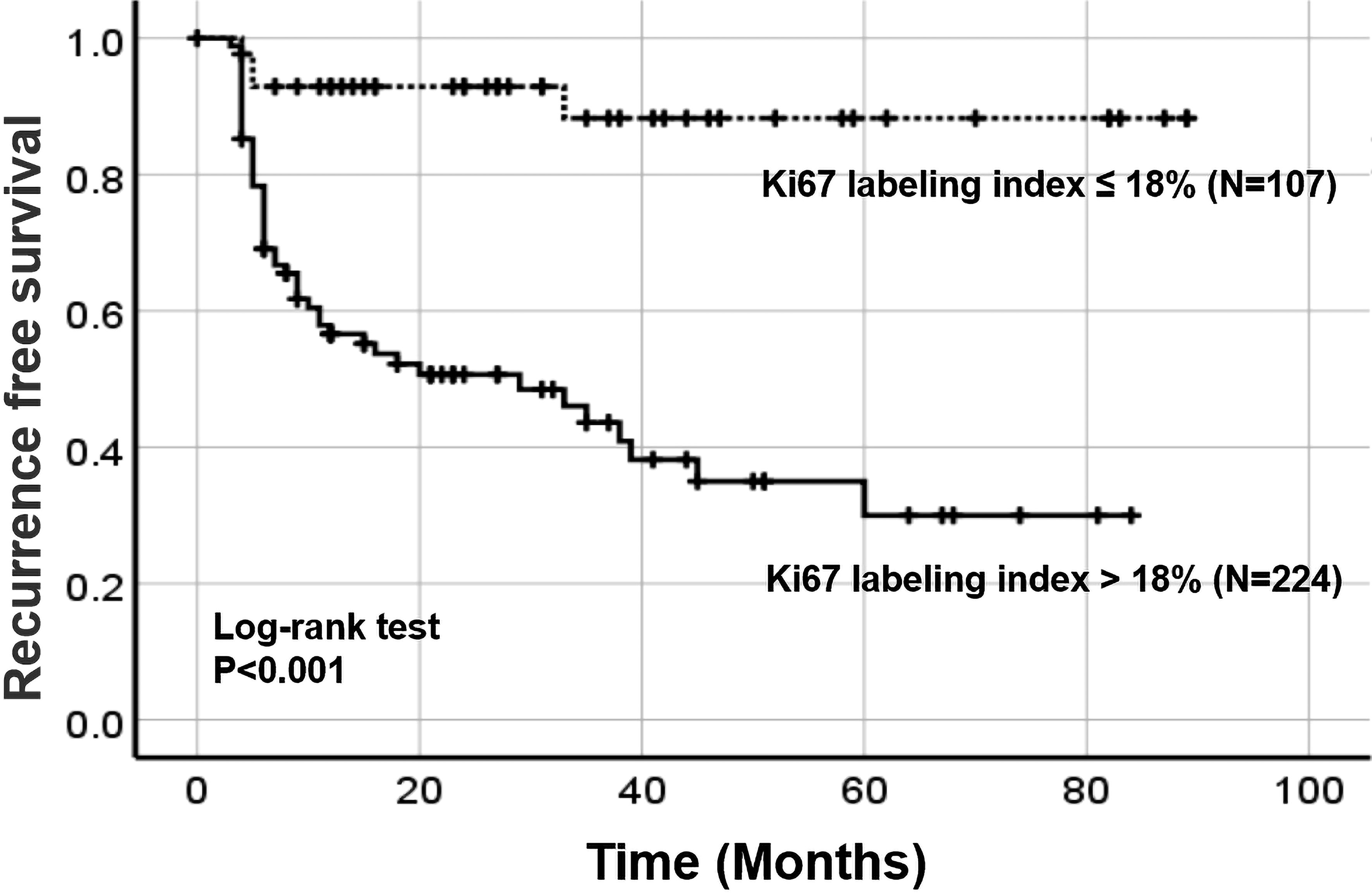
Figure 3. The recurrence-free survival of patients according to the optimal cut-off value of Ki67. Dotted line: The recurrence-free survival curve of the low Ki67 group. Solid line: The recurrence-free survival of curve the high Ki67 group.
4 Discussion
As an indicator of cell proliferation, Ki67 is associated with the progression of cancer cells and has been universally used as a prognostic predictor to guide therapeutic decisions (22, 23). In this study, 18% was validated as the optimal cut-off value of Ki67 to predict the relapse of NMIBUC Figure 4. Even though the specificity was only 50% when the sensitivity was 92.6%, the maximum value of the Youden index assures the greatest specificity as well as the highest sensitivity, instead of only concentrating on the specificity or sensitivity. In addition, Ki67 expression was Strongly associated with tumor grade and stage as well as CK20 and P53 expression. This is because Ki67 expression indicates the proliferation activity of tumors. When the tumor is accompanied by more aggressive parameters such as advanced stage, worse grade, and higher expression of P53 and CK20, the expression of Ki67 is universally higher, which is consistent with other clinical studies (24). Ki67 overexpression plays a leading role in the prediction of bladder cancer recurrence, which indicates that the optimal cut-off value has the potential value for assessing the outcome of NMIBUC. Moreover, in the multivariate analysis, it was further concluded that Ki67>18% was an independent risk factor for prognosis. In other words, if the Ki67 value is higher, the recurrence risk is suggested to be higher. Moreover, we found that the RFS of patients with Ki67>18% was significantly lower than that of patients with Ki67 ≤ 18%, which suggests that more concern should be paid when Ki67 is over this cut-off value.
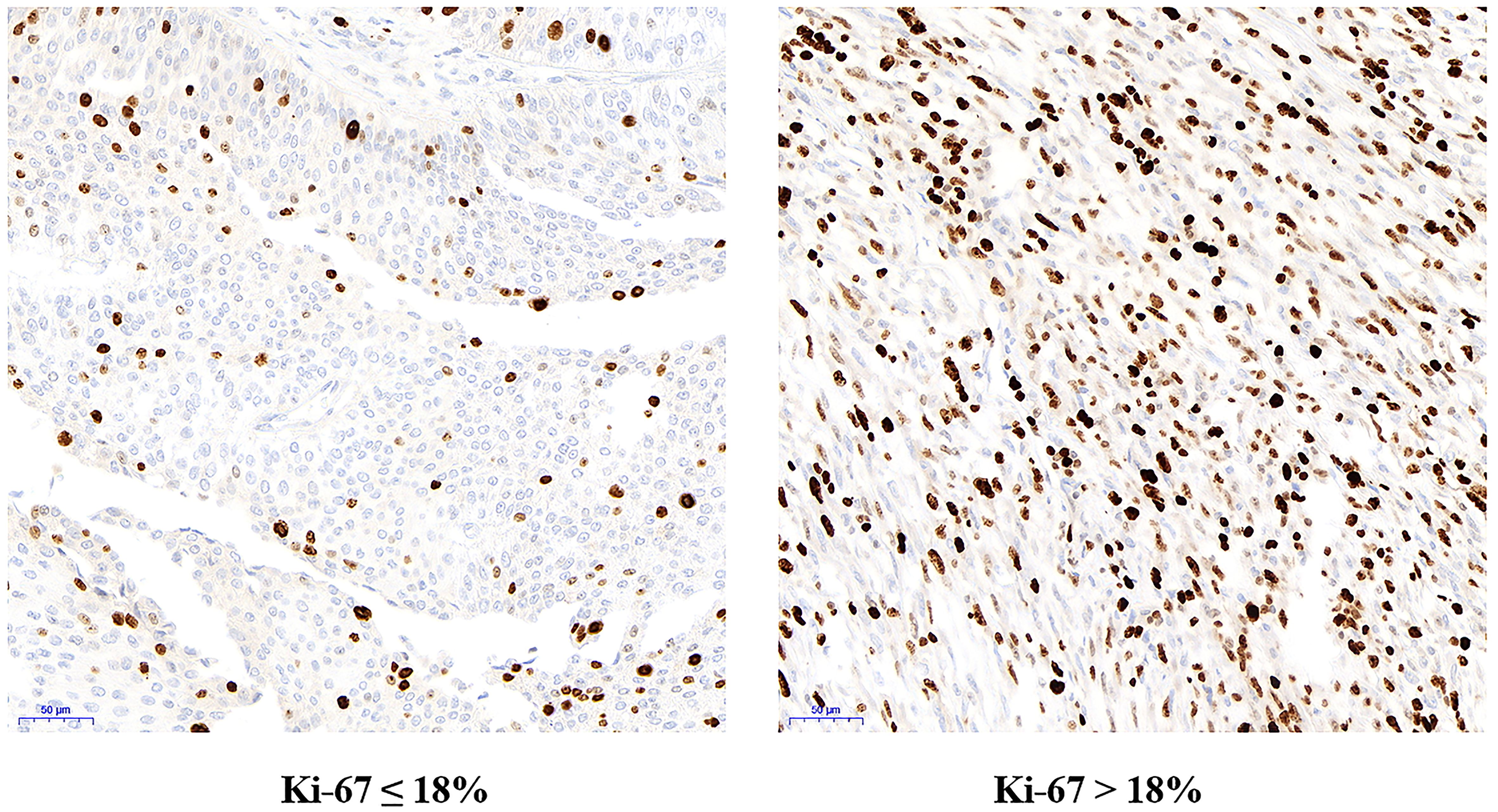
Figure 4. Immunohistochemical staining for Ki67 in low Ki67 group (Ki67 ≤ 18%) and high Ki67 group (Ki67 > 18%) under electron microscope.
In this study, all included patients were treated with postoperative bladder infusion chemotherapy. According to the guidelines, for patients with low-risk BCa recurrence, only immediate infusion chemotherapy within 24 hours after surgery and early infusion therapy 4-8 times (once a week for 4-8 weeks) after surgery are needed; for patients with middle- or high-risk BCa recurrence, except for immediate postoperative bladder chemotherapy and early infusion chemotherapy 6-12 times (once a month, a total of 6-12 months), continuous infusion chemotherapy should also be completed (19). To define low-, middle- and high-risk recurrence patients, apart from traditional clinical parameters such as tumor number, size, tumor stage, grade and whether it is carcinoma in situ, the immunohistochemical marker Ki-67 can also be used as an effective tool (25, 26). For patients with Ki67>18%, it is suggested that active and comprehensive postoperative management be carried out, such as adding cycles of bladder infusion chemotherapy, extending the time of chemotherapy and performing frequent follow-up. In contrast, patients with Ki67≦18% incorporate favorable clinical features. Thus, to improve their quality of life, bladder infusion chemotherapy is not routinely recommended (27, 28).
The standard treatment regimen for NMIBUC is TURBT followed by adjuvant bladder infusion chemotherapy. Among the specimens obtained from TURBT, the acquisition of detrusor muscle (DM) tissue is extremely important (29). Studies have reported that the recurrence rates in patients with middle- and high-risk BCa recurrence range from 24% to 61% after one to five years of surgery (30). Currently, related studies are mainly about the impact of DM on prognosis in patients with middle- and high-risk BCa recurrence. For those with DM tissue deficiency, there is a greater risk of tumor residual, which significantly increases the possibility of tumor recurrence or progression (31). Comparatively, the recurrence rates in patients with low-risk recurrence (including Ta low grade) range from 15% to 31% after one to five years of surgery (30). Since tumor infiltration can be negligible in submucosal tissue, DM tissue plays a minimal role in postoperative recurrence in patients with low-risk recurrence (32).
Among other scoring systems for predicting postoperative recurrence of NMIBUC, the most commonly used is EORTC risk scores, in which the number of tumors, tumor size, prior recurrence rate, T category, carcinoma in situ, and grade are independent risk factors for the prediction of recurrence (32). Although this common model has high accuracy, it is limited to traditional clinical parameters and does not include immunohistochemical markers. In this study, Ki67 was an independent risk factor for predicting postoperative recurrence of NMIBUC. By introducing Ki67 into the above prediction model, its accuracy could be increased. Furthermore, this study also found that immediate postoperative adjuvant therapy, tumor stage, and the expression of P53 and CK20 are independent risk factors that affect postoperative recurrence, which is also consistent with other studies (33, 34). Nevertheless, this study illustrated that NMIBUC patients’ age, gender, smoking, tumor grade, tumor number, and size were not significantly related to postoperative recurrence. However, it does not deny the importance of these factors in predicting relapse; in fact, many studies have shown that they are important factors in predicting the prognosis of NMIBUC (35, 36). Therefore, when making the decision of adjuvant treatment, it is necessary not only to accurately evaluate the tumor’s biological behavior, but also to consider the level of immunohistochemistry such as Ki67 or according to the patients’ other clinicopathological indicators to reduce the risk of postoperative recrudescence and enhance prognosis (37).
Our study has some limitations. First, this study is a retrospective study, but it cannot be guaranteed that all data were completely and accurately recorded through the medical record system. Second, the postoperative specimens failed to undergo a unified review, but all specimens were analyzed in a unified standard procedure in the same authoritative institution. Additionally, the detection of Ki67 expression still lacks consensus. At present, as the method “hottest” is generally adopted, this study also used this hotspot scoring method to prove the predictive significance of the Ki67 index. However, there is still a necessity to establish a unified standard method for Ki67 expression. As a kind of nuclear antigen, the immunohistochemical marker Ki67 is related to cell proliferation, so it is closely associated with tumor progression. Regrettably, because of the small number of tumor-progression cases in this study, most patients with recurrence could only be diagnosed as recurrence at the time of diagnosis; only a small number of recurrent cases were judged as progressive and the value of Ki67 was positively correlated with tumor progression. Therefore, our team will strengthen the analysis of cases with tumor progression in subsequent studies.
5 Conclusions
In conclusion, the study demonstrated that the best cut-off value of Ki67 expression to predict NMIBUC postoperative recurrence is 18%. According to the cut-off value, clinicians can effectively develop treatment and follow-up strategies, which is of great clinical significance for promoting patient health.
Data availability statement
The data analyzed in this study is subject to the following licenses/restrictions: Patient privacy concerns. Requests to access these datasets should be directed to cGlqaWFuZ2NodWFuQDE2My5jb20=.
Ethics statement
The studies involving humans were approved by the Ethics Committee of the Yongchuan Hospital of Chongqing Medical University (Science Ethics Review No. 105 2022). Additionally, each participating institutional ethical review committee obtained approval and permission to conduct the study. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
RP: Investigation, Methodology, Project administration, Resources, Writing – review & editing. MJ: Software, Supervision, Visualization, Writing – review & editing, Project administration. XPY: Software, Supervision, Writing – review & editing, Validation, Visualization. XYY: Supervision, Formal Analysis, Project administration, Software, Writing – review & editing. JP: Conceptualization, Funding acquisition, Supervision, Writing – original draft. WM: Conceptualization, Data curation, Formal Analysis, Visualization, Writing – original draft, Writing – review & editing. YZ: Methodology, Software, Validation, Writing – review & editing. SL: Conceptualization, Funding acquisition, Methodology, Validation, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Science and Education Commission of Yongchuan District, Chongqing (YJLC202032); and The General Hospital of Western Theater Command (2021-XZYG-A11).
Acknowledgments
We acknowledge the Pathology Experimental Center of Chongqing Medical University and Chengdu Second People’s Hospital for providing technological assistance.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. (2019) 69:7–34. doi: 10.3322/caac.21551
2. Tan WS, Rodney S, Lamb B, Feneley M, Kelly J. Management of non-muscle invasive bladder cancer: A comprehensive analysis of guidelines from the United States, Europe and Asia. Cancer Treat Rev. (2016) 47:22–31. doi: 10.1016/j.ctrv.2016.05.002
3. Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, et al. Global cancer statistics 2022: globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2024) 74:229–63. doi: 10.3322/caac.21834
4. Zhang Y, Li X, Li X, Zhao Y, Zhou T, Jiang X, et al. Comprehensive analysis of cuproptosis-related long noncoding rna immune infiltration and prediction of prognosis in patients with bladder cancer. Front Genet. (2022) 13:990326. doi: 10.3389/fgene.2022.990326
5. Lu M, Chen S, Zhou Q, Wang L, Peng T, Wang G. Predicting recurrence of nonmuscle-invasive bladder cancer (Ta-T1): A study based on 477 patients. Med (Baltimore). (2019) 98:e16426. doi: 10.1097/MD.0000000000016426
6. Miller I, Min M, Yang C, Tian C, Gookin S, Carter D, et al. Ki67 is a graded rather than a binary marker of proliferation versus quiescence. Cell Rep. (2018) 24:1105–12 e5. doi: 10.1016/j.celrep.2018.06.110
7. Inwald EC, Klinkhammer-Schalke M, Hofstadter F, Zeman F, Koller M, Gerstenhauer M, et al. Ki-67 is a prognostic parameter in breast cancer patients: results of a large population-based cohort of a cancer registry. Breast Cancer Res Treat. (2013) 139:539–52. doi: 10.1007/s10549-013-2560-8
8. Kloppel G, La Rosa S. Ki67 labeling index: assessment and prognostic role in gastroenteropancreatic neuroendocrine neoplasms. Virchows Arch. (2018) 472:341–9. doi: 10.1007/s00428-017-2258-0
9. Constantinou C, Papadopoulos S, Karyda E, Alexopoulos A, Agnanti N, Batistatou A, et al. Expression and clinical significance of claudin-7, pdl-1, pten, C-kit, C-met, C-myc, alk, ck5/6, ck17, P53, egfr, ki67, P63 in triple-negative breast cancer-a single centre prospective observational study. In Vivo. (2018) 32:303–11. doi: 10.21873/invivo.11238
10. Lashen AG, Toss MS, Ghannam SF, Makhlouf S, Green A, Mongan NP, et al. Expression, assessment and significance of ki67 expression in breast cancer: an update. J Clin Pathol. (2023) 76:357–64. doi: 10.1136/jcp-2022-208731
11. Jiang P, Jia M, Hu J, Huang Z, Deng Y, Lai L, et al. Prognostic value of ki67 in patients with stage 1-2 endometrial cancer: validation of the cut-off value of ki67 as a predictive factor. Onco Targets Ther. (2020) 13:10841–50. doi: 10.2147/OTT.S274420
12. Tian Y, Ma Z, Chen Z, Li M, Wu Z, Hong M, et al. Clinicopathological and prognostic value of ki-67 expression in bladder cancer: A systematic review and meta-analysis. PloS One. (2016) 11:e0158891. doi: 10.1371/journal.pone.0158891
13. He Y, Wang N, Zhou X, Wang J, Ding Z, Chen X, et al. Prognostic value of ki67 in bcg-treated non-muscle invasive bladder cancer: A meta-analysis and systematic review. BMJ Open. (2018) 8:e019635. doi: 10.1136/bmjopen-2017-019635
14. Breyer J, Wirtz RM, Laible M, Schlombs K, Erben P, Kriegmair MC, et al. Esr1, erbb2, and ki67 mrna expression predicts stage and grade of non-muscle-invasive bladder carcinoma (Nmibc). Virchows Arch. (2016) 469:547–52. doi: 10.1007/s00428-016-2002-1
15. Shi S, Ma HY, Sang YZ, Ju YB, Liu XY, Zhang ZG. Expression and clinical significance of cmtm6 and pd-L1 in triple-negative breast cancer. BioMed Res Int. (2022) 2022:8118909. doi: 10.1155/2022/8118909
16. Humphrey PA, Moch H, Cubilla AL, Ulbright TM, Reuter VE. The 2016 who classification of tumours of the urinary system and male genital organs-part B: prostate and bladder tumours. Eur Urol. (2016) 70:106–19. doi: 10.1016/j.eururo.2016.02.028
17. Cambier S, Sylvester RJ, Collette L, Gontero P, Brausi MA, van Andel G, et al. Eortc nomograms and risk groups for predicting recurrence, progression, and disease-specific and overall survival in non-muscle-invasive stage ta-T1 urothelial bladder cancer patients treated with 1-3 years of maintenance bacillus calmette-guérin. Eur Urol. (2016) 69:60–9. doi: 10.1016/j.eururo.2015.06.045
18. Pi J, Xiong Y, Liu C, Liao J, Liu J, Li C, et al. A nomogram model to predict recurrence of non-muscle invasive bladder urothelial carcinoma after resection based on clinical parameters and immunohistochemical markers. J Invest Surg. (2022) 35:1186–94. doi: 10.1080/08941939.2021.2017080
19. Babjuk M, Burger M, Compérat EM, Gontero P, Mostafid AH, Palou J, et al. European association of urology guidelines on non-muscle-invasive bladder cancer (Tat1 and carcinoma in situ) - 2019 update. Eur Urol. (2019) 76:639–57. doi: 10.1016/j.eururo.2019.08.016
20. Jiang P, Jia M, Hu J, Huang Z, Deng Y, Hu Z. A nomogram model involving immunohistochemical markers for predicting the recurrence of stage I-ii endometrial cancer. Front Oncol. (2020) 10:586081. doi: 10.3389/fonc.2020.586081
21. Yu X, Guo S, Song W, Xiang T, Yang C, Tao K, et al. Estrogen receptor alpha (Eralpha) status evaluation using rnascope in situ hybridization: A reliable and complementary method for ihc in breast cancer tissues. Hum Pathol. (2017) 61:121–9. doi: 10.1016/j.humpath.2016.12.005
22. Fasching PA, Gass P, Haberle L, Volz B, Hein A, Hack CC, et al. Prognostic effect of ki-67 in common clinical subgroups of patients with her2-negative, hormone receptor-positive early breast cancer. Breast Cancer Res Treat. (2019) 175:617–25. doi: 10.1007/s10549-019-05198-9
23. Liu J, Ma C, Li X, Li A, Wang Z. Circulating tumor cells correlating with ki-67 predicts the prognosis of bladder cancer patients. Int Urol Nephrol. (2023) 55:309–18. doi: 10.1007/s11255-022-03406-y
24. Ziaran S, Harsanyi S, Bevizova K, Varchulova Novakova Z, Trebaticky B, Bujdak P, et al. Expression of E-cadherin, ki-67, and P53 in urinary bladder cancer in relation to progression, survival, and recurrence. Eur J Histochem. (2020) 64:3098. doi: 10.4081/ejh.2020.3098
25. Ravvaz K, Walz ME, Weissert JA, Downs TM. Predicting nonmuscle invasive bladder cancer recurrence and progression in a United States population. J Urol. (2017) 198:824–31. doi: 10.1016/j.juro.2017.04.077
26. Straccia P, Fiorentino V, Martini M, Pierconti F. A systematic review and meta-analysis of ck20, cd44, ki67 and P53 as immunohistochemical markers in bladder carcinoma in situ. Actas Urol Esp (Engl Ed). (2022) 46:521–30. doi: 10.1016/j.acuroe.2022.08.013
27. Sylvester RJ, Oosterlinck W, Holmang S, Sydes MR, Birtle A, Gudjonsson S, et al. Systematic review and individual patient data meta-analysis of randomized trials comparing a single immediate instillation of chemotherapy after transurethral resection with transurethral resection alone in patients with stage pta-pt1 urothelial carcinoma of the bladder: which patients benefit from the instillation? Eur Urol. (2016) 69:231–44. doi: 10.1016/j.eururo.2015.05.050
28. Bosschieter J, van Moorselaar RJA, Vis AN, van Ginkel T, Lissenberg-Witte BI, Beckers GMA, et al. The effect of timing of an immediate instillation of mitomycin C after transurethral resection in 941 patients with non-muscle-invasive bladder cancer. BJU Int. (2018) 122:571–5. doi: 10.1111/bju.14124
29. Dalbagni G. Editorial comment on: detrusor muscle in the first, apparently complete transurethral resection of bladder tumour specimen is a surrogate marker of resection quality, predicts risk of early recurrence, and is dependent on operator experience. Eur Urol. (2010) 57:849. doi: 10.1016/j.eururo.2009.05.048
30. Mastroianni R, Brassetti A, Krajewski W, Zdrojowy R, Salhi YA, Anceschi U, et al. Assessing the impact of the absence of detrusor muscle in ta low-grade urothelial carcinoma of the bladder on recurrence-free survival. Eur Urol Focus. (2021) 7:1324–31. doi: 10.1016/j.euf.2020.08.007
31. Dadiani M, Bossel Ben-Moshe N, Paluch-Shimon S, Perry G, Balint N, Marin I, et al. Tumor evolution inferred by patterns of microrna expression through the course of disease, therapy, and recurrence in breast cancer. Clin Cancer Res. (2016) 22:3651–62. doi: 10.1158/1078-0432.CCR-15-2313
32. Sylvester RJ, van der Meijden AP, Oosterlinck W, Witjes JA, Bouffioux C, Denis L, et al. Predicting recurrence and progression in individual patients with stage ta T1 bladder cancer using eortc risk tables: A combined analysis of 2596 patients from seven eortc trials. Eur Urol. (2006) 49:466–5. doi: 10.1016/j.eururo.2005.12.031
33. Bertz S, Otto W, Denzinger S, Wieland WF, Burger M, Stohr R, et al. Combination of ck20 and ki-67 immunostaining analysis predicts recurrence, progression, and cancer-specific survival in pt1 urothelial bladder cancer. Eur Urol. (2014) 65:218–26. doi: 10.1016/j.eururo.2012.05.033
34. Poli G, Cochetti G, Boni A, Egidi MG, Brancorsini S, Mearini E. Characterization of inflammasome-related genes in urine sediments of patients receiving intravesical bcg therapy. Urol Oncol. (2017) 35:674 e19– e24. doi: 10.1016/j.urolonc.2017.08.004
35. Bosschieter J, Nieuwenhuijzen JA, van Ginkel T, Vis AN, Witte B, Newling D, et al. Value of an immediate intravesical instillation of mitomycin C in patients with non-muscle-invasive bladder cancer: A prospective multicentre randomised study in 2243 patients. Eur Urol. (2018) 73:226–32. doi: 10.1016/j.eururo.2017.06.038
36. Messing EM, Tangen CM, Lerner SP, Sahasrabudhe DM, Koppie TM, Wood DP Jr., et al. Effect of intravesical instillation of gemcitabine vs saline immediately following resection of suspected low-grade non-muscle-invasive bladder cancer on tumor recurrence: swog S0337 randomized clinical trial. JAMA. (2018) 319:1880–8. doi: 10.1001/jama.2018.4657
Keywords: immunohistochemical markers, ki67, non-muscular invasive bladder urothelial carcinoma, recurrence, prognosis, optimal cut-off value
Citation: Peng R, Zhang Y, Jia M, Yi X, Yi X, Li S, Pi J and Meng W (2025) Predicting recurrence of non-muscle invasive bladder urothelial carcinoma: predictive value of the optimal cut-off value of Ki67. Front. Oncol. 14:1522009. doi: 10.3389/fonc.2024.1522009
Received: 03 November 2024; Accepted: 26 December 2024;
Published: 16 January 2025.
Edited by:
Hongbing Zhang, Tianjin Medical University General Hospital, ChinaReviewed by:
Hong Ma, University of Massachusetts Medical School, United StatesYouqiang Ke, Royal Liverpool University Hospital, United Kingdom
Junyu Lai, Southwest Medical University, China
Copyright © 2025 Peng, Zhang, Jia, Yi, Yi, Li, Pi and Meng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shadan Li, bGlzaGFkYW4yMDA1QDEyNi5jb20=; Jiangchuan Pi, cGlqaWFuZ2NodWFuQDE2My5jb20=; Wenjun Meng, bXdqMTk5NUBmb3htYWlsLmNvbQ==
†ORCID: Wenjun Meng, orcid.org/0000-0002-6780-8720
 Rende Peng1
Rende Peng1 Yaoyu Zhang
Yaoyu Zhang Mingzhu Jia
Mingzhu Jia Xinping Yi
Xinping Yi Shadan Li
Shadan Li Jiangchuan Pi
Jiangchuan Pi Wenjun Meng
Wenjun Meng