
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Oncol. , 27 January 2025
Sec. Surgical Oncology
Volume 14 - 2024 | https://doi.org/10.3389/fonc.2024.1521253
Background: Perivascular epithelioid cell tumours (PEComas) occurring in the uterus are rare, with surgery being the most recommended primary treatment for malignant cases. This study aims to provide clinical guidance on the clinicopathological features and appropriate treatment options for patients with uterine PEComas of uncertain malignant potential.
Cases: This case series summarises the clinical courses of 13 patients diagnosed with uterine PEComas of uncertain malignant potential, including clinical and pathological data as well as their outcomes. We identified one case at our hospital, and data for the other 12 cases were extracted from the PubMed database. The 13 patients were aged 9–75 years, with tumour sizes ranging from 1 to 21 cm, and follow-up times ranging from 2 to 71 months. The most common signs and symptoms included abnormal uterine bleeding (AUB) and abdominal pain. Most of the patients (11/13) were managed surgically without any chemotherapy or radiation therapy. Except for the patients who were lost to follow-up, 11 patients were free of any recurrence or metastasis at their last follow-up. Patients with group A tumours (abundant HMB45 expression) had a longer disease-free survival than those with group B tumours.
Conclusions: Surgery alone may be appropriate for uterine PEComas of uncertain malignant potential. Surgical treatment plans should consider the patient’s age, fertility requirements, and personal preferences. Mass resection is a viable treatment option for fertility preservation in reproductive-age patients.
Perivascular epithelioid cell tumours (PEComas) are very rare mesenchymal tumours characterised by the expression of both melanocytic and myogenic markers (1). The World Health Organisation (WHO) previously defined PEComas as mesenchymal tumours composed of histologically and immunohistochemically distinctive perivascular epithelioid cells (2). PEComas are known to occur at various anatomical locations, including the lung, kidney, bladder, and prostate. Nearly a quarter of PEComas occur in the female genital tract (3), and uterine PEComas were described initially by Bonetti (4).
The clinical behaviour of uterine PEComas is usually benign; these cases display benign features and show no evidence of recurrence or metastasis after surgery alone (5). However, the malignant potential of uterine PEComas is variable, and a proportion of cases may recur and metastasise. Bennett suggested that all gynaecological PEComas should be classified as either malignant or of uncertain malignant potential (6). In such cases, complete surgical resection and chemotherapy are crucial for preventing clinical recurrence (7). Therefore, PEComas with uncertain malignant potential present great difficulties in medical decision-making. However, the criteria for PEComas of uncertain malignant potential have not been established by the WHO due to their rarity. Based on the criteria proposed by Folpe et al. (8), a PEComa of “uncertain malignant potential” is defined as having only a single high-risk histological feature, such as nuclear pleomorphism or multinucleated giant cells, or a size > 5 cm. Owing to the paucity of cases, there are controversies over the management of uterine PEComas of uncertain malignant potential, especially in reproductive-age patients. This study summarises the clinical course of uterine PEComas of uncertain malignant potential and explores the possibility of preserving fertility in these patients.
A 35-year-old woman was referred to our hospital presented with a 3-day history of lower abdominal pain. Ultrasound revealed a solid mass measuring 10.9 cm × 9.3 cm × 9.3 cm on the left side of the uterus (Supplementary Figure S1A). Pelvic magnetic resonance imaging (MRI) later showed a solid mass in the left pelvis, measuring 10.5 cm × 8.2 cm × 9.5 cm with uneven enhancement (Supplementary Figures S1B, C). A metastatic workup, including whole-body computed tomography (CT) and serum tumour marker detection, was negative for disease. Therefore, subserosal uterine fibroids with degeneration were considered, although ovarian cyst torsion could not be ruled out. After obtaining informed consent, a mass resection was performed. Macroscopically, the mass was located in the left broad ligament, with a maximum diameter of 11 cm. It appeared dark purple, had an uneven surface, and displayed a beef-like soft texture. A malignant tumour was not considered based on intraoperative frozen pathology. To maintain the patient’s fertility, no additional operations were performed. Histological analysis revealed that the tumour consisted of round and spindle cells with an eosinophilic granular cytoplasm. The tumour cells presented slight to moderate nuclear atypia, with nuclear enlargement and distinct nucleolus. No mitotic figures, vascular invasion, haemorrhage, or necrosis were observed (Supplementary Figure S2A). The immunohistochemical results were as follows: positive staining for SMA (Supplementary Figure S2B), desmin (Supplementary Figure S2C), melan A (Supplementary Figure S2D), Ki-67 (20%), and TFE3 (Supplementary Figure S2E). Negative staining was observed for HMB45, CK(Pan), S-100, CD117, CD34, MyoD1, SDHB, H-caldesmon, and SOX-10. In addition, fluorescence in situ hybridisation (FISH) did not detect TFE3 gene rearrangement (Supplementary Figure S2F). A uterine PEComa of uncertain malignant potential was diagnosed based on the above findings. The patient has been undergoing regular physical examinations and remained free of disease at 8 months after surgery.
Previous cases were extracted from the PubMed database and analysed to provide additional information. The final search was conducted in May 2024. Two researchers independently screened eligible publications based on titles and abstracts using the following search query: (perivascular epithelioid cell neoplasm) OR (perivascular epithelioid cell tumours) OR (PEComa*). All English-language articles reporting uterine PEComas of uncertain malignant potential with available full texts were included. The selection flowchart is presented in Figure 1. As of 2024, 12 cases were extracted from the PubMed database (5, 9–17). The clinicopathological features of the patients, including their clinical manifestations, pathology, treatment, and outcomes, are summarised in Table 1. The 13 patients were aged 9–75 years (mean: 36.5 years), with tumour sizes ranging from 1 to 21 cm (mean: 8.5 cm) and follow-up durations ranging from 2 to 71 months (mean: 17.5 months). The most common signs and symptoms included abnormal uterine bleeding (AUB) and abdominal pain.

Table 1. Clinicopathologic features of patients with uterine PEComa of uncertain malignant potential.
The immunohistochemical profiles of the 13 patients are presented in Supplementary Table S1. We further separated these patients into two groups based on the immunohistochemical expression as follows: group A tumours typically exhibited high HMB-45 expression but low muscle marker expression, whereas group B tumours exhibited the opposite pattern (5). In this study, six patients had group A tumours. The mean age of the patients was 33 years. Three patients presented with AUB, while others presented with abdominal pain. Four patients underwent hysterectomy, with one of them receiving chemotherapy and radiation therapy (17). The other two patients were treated via mass resection. Clinical follow-up data were available for five patients, with follow-up times ranging from 7 to 71 months (mean: 27.6 months). Conversely, seven patients had group B tumours, with a mean age of 39 years. These patients exhibited diverse symptoms: two presented with AUB, two with abdominal pain, one with haemoperitoneum, and two reported no apparent discomfort. Four patients underwent hysterectomy, while the remaining patients underwent mass resection. Clinical follow-up data were available for six patients, with durations ranging from 2 to 24 months (mean: 10 months) (Table 2). Excluding patients lost to follow-up, all 11 remaining patients were free of recurrence or metastasis at their last follow-up. Patients with group A tumours demonstrated longer disease-free survival compared to those with group B tumours (Figure 2A).
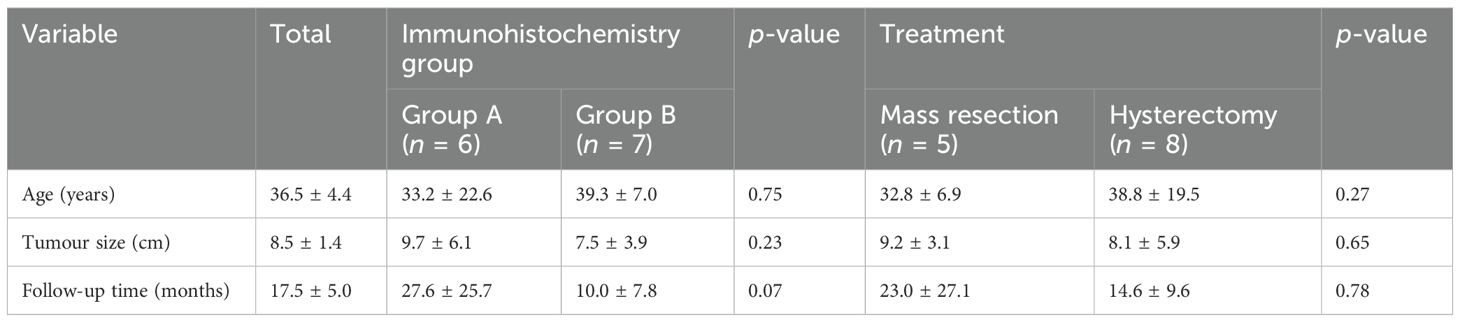
Table 2. Clinical features of patients with uterine PEComas of uncertain malignant potential, categorised by subgroups.
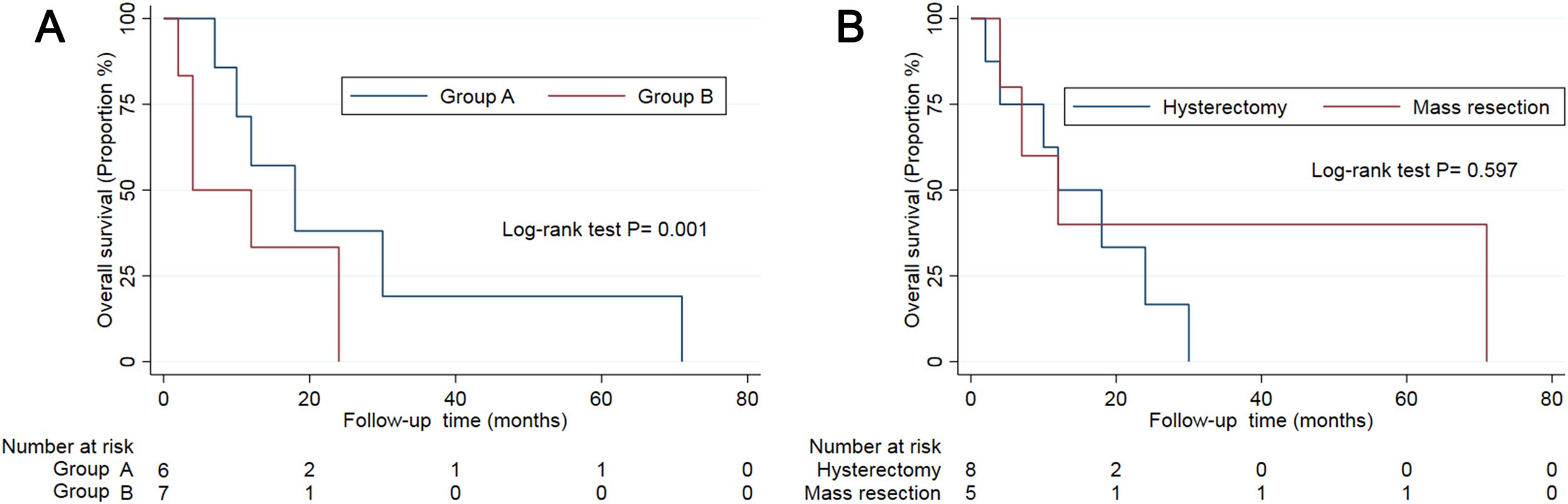
Figure 2. The survival curve of patients in subgroups by immunohistochemistry (A) and treatment (B).
To date, therapeutic protocols for uterine PEComas of uncertain malignant potential have not been established, with treatment based on protocols for managing malignant PEComas. In this study, all 13 patients underwent surgical excision, including eight who underwent hysterectomy and five who underwent mass resection. Most of the patients (11/13) were treated surgically without receiving chemotherapy or radiation therapy. Disease-free survival did not differ significantly between patients who underwent hysterectomy and those who underwent mass resection for uterine PEComas of uncertain malignant potential (Table 2, Figure 2B). Due to the paucity of cases, the role of chemotherapy and radiation therapy remains unclear.
PEComas are rare mesenchymal tumours that can occur in various anatomical locations. Nearly a quarter of PEComas are found in the female genital tract, most frequently arising in the uterus. In this study, we report a case of uterine PEComas in a 35-year-old woman who presented with sudden lower abdominal pain but no other specific symptoms. Ultrasound and MRI imaging revealed a palpable mass, though typical presentations were absent. Consequently, the serum tumour markers in this patient were negative. Therefore, the nonspecific symptoms and imaging findings posed challenges in making a preoperative diagnosis of uterine PEComas.
In addition, defining the behaviour and prognosis of uterine PEComas is challenging due to their rarity. Folpe et al. (8) proposed risk stratification criteria based on five high-risk histopathological features: a large tumour size of more than 5 cm, high nuclear grade and cellularity, a mitotic rate more than 1/50 HPF, an infiltrative growth pattern, and the presence of necrosis and vascular invasion. In particular, uterine PEComas of uncertain malignant potential had a tumour size larger than 5 cm or exhibited only nuclear pleomorphism or multinucleated giant cells. The differential diagnosis of uterine PEComas included leiomyoma, uterine sarcoma, ovarian tumour, and adenocarcinoma (Table 1). Notably, the uterine PEComas in the 13 patients shared clinical features similar to those of leiomyoma, including an abdominal mass, AUB, and abdominal pain. Only two cases were diagnosed with uterine PEComas before surgery. Therefore, uterine PEComas of uncertain malignant potential are often misdiagnosed as uterine leiomyoma. A distinctive feature is that melanocytic markers are positive in PEComas but negative in leiomyomas.
All 13 patients were diagnosed after surgery through pathology and immunohistochemicals. In most cases, the uterine PEComas had exhibited characteristic immunohistochemical features, including immunoreactivity for HMB45 and negativity for the S-100 protein. However, case 1 was negative for HMB45 expression (Supplementary Table S1). Based on the immunohistochemical expression of HMB45 and muscle markers, we separated uterine PEComas into two groups. Group A tumours typically presented abundant HMB45 expression but scant muscle marker expression and appeared to be associated with longer disease-free survival than did the group B tumours. Due to the variable malignant potential of PEComas, 50% of gynaecological PEComas are considered to have malignant potential (18). Although the optimal management of uterine PEComas is still controversial, surgical excision remains the preferred treatment, followed by adjuvant chemotherapy and radiotherapy (1). Our study revealed that surgery alone appeared to be appropriate for uterine PEComas of uncertain malignant potential, particularly for those with a tumour size > 5 cm.
However, the diagnoses were made based on pathological reports combined with immunohistochemical staining, which has inevitable hysteresis. Therefore, uterine PEComas of uncertain malignant potential present significant challenges in making decision-making during a second operation, especially for patients who have undergone only mass resection. Shan et al. suggested that mass resection is sufficient if the uterine PEComas tend are pathologically benign, but long-term follow-up is still necessary (11). As a typical example, case 4 involved a 23-year-old unmarried woman diagnosed with uterine PEComas. Given the consideration for fertility preservation, she underwent laparoscopic mass resection and continued with long-term follow-up. The patient subsequently became pregnant spontaneously and gave birth to a healthy boy via caesarean section 5 years after the surgery. Moreover, she had the longest disease-free survival period among the 13 cases. Therefore, the patient’s age, fertility requirements, and personal preferences should be considered when developing surgical treatment plans.
This study represents the largest case series of uterine PEComas with uncertain malignant potential, including one case from our institution and 12 additional cases from a literature database. Although the case series is small, this limitation is inherent to any rare condition. In addition, some patients only had short-term follow-ups or were even lost to follow-up, highlighting the importance of long-term monitoring to gain a deeper understanding of this condition.
PEComas occurring in the uterus are very rare. Surgery alone may be suitable for uterine PEComas with uncertain malignant potential. Surgical treatment plans should consider the patient’s age, fertility requirements, and personal preferences. Mass resection is a potential treatment option for fertility preservation in reproductive-age patients.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
The studies involving humans were approved by Ethical Review Committee of the Second Affiliated Hospital of Guangxi Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin. Written informed consent was obtained from the individual(s), and minor(s)’ legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article. Written informed consent was obtained from the participant/patient(s) for the publication of this case report.
HS: Data curation, Investigation, Methodology, Writing – original draft. YY: Formal analysis, Methodology, Writing – review & editing. SL: Software, Visualization, Writing – original draft. CL: Software, Visualization, Writing – original draft. YH: Methodology, Validation, Writing – review & editing. LZ: Conceptualization, Supervision, Validation, Writing – review & editing. BL: Conceptualization, Resources, Supervision, Validation, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. Funding for this work was supported by the self-financing project of the Guangxi Zhuang Autonomous Region Health and Family Planning Commission (Z-A20220655).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2024.1521253/full#supplementary-material
Supplementary Figure 1 | Transvaginal ultrasound (A) and Magnetic resonance imaging (BC) of case 1
Supplementary Figure 2 | Histopathologic view of uterine PEComas of Case 1. (A) Hematoxylin-eosin staining, (B) Immunohistochemical staining for SMA. (C) Immunohistochemical staining for Desmin. (D) Immunohistochemical staining for melan A. (E) Immunohistochemical staining for TFE3. (F) Fluorescence in situ hybridization for TFE3. (Supplementary Figure S2D), Ki-67 (20%) and (Supplementary Figure S2E).
1. Musella A, De Felice F, Kyriacou AK, Barletta F, Di Matteo FM, Marchetti C, et al. Perivascular epithelioid cell neoplasm (PEComa) of the uterus: A systematic review. Int J Surg. (2015) 19:1–5. doi: 10.1016/j.ijsu.2015.05.002
2. Doyle LA. Sarcoma classification: an update based on the 2013 World Health Organization Classification of tumours of Soft Tissue and Bone. Cancer. (2014) 120:1763–74. doi: 10.1002/cncr.28657
3. Gadducci A, Zannoni GF. Perivascular epithelioid cell tumours (PEComa) of the female genital tract: A challenging question for gynaecologic oncologist and pathologist. Gynecol Oncol Rep. (2020) 33:100603. doi: 10.1016/j.gore.2020.100603
4. Bonetti F, Martignoni G, Colato C, Manfrin E, Gambacorta M, Faleri M, et al. Abdominopelvic sarcoma of perivascular epithelioid cells. Report of four cases in young women, one with tuberous sclerosis. Mod Pathol. (2001) 14:563–8. doi: 10.1038/modpathol.3880351
5. Vang R, Kempson RL. Perivascular epithelioid cell tumour ('PEComa') of the uterus: a subset of HMB-45-positive epithelioid mesenchymal neoplasms with an uncertain relationship to pure smooth muscle tumours. Am J Surg Pathol. (2002) 26:1–13. doi: 10.1097/00000478-200201000-00001
6. Bennett JA, Braga AC, Pinto A, Van de Vijver K, Cornejo K, Pesci A, et al. Uterine PEComas: A morphologic, immunohistochemical, and molecular analysis of 32 tumours. Am J Surg Pathol. (2018) 42:1370–83. doi: 10.1097/pas.0000000000001119
7. Schoolmeester JK, Howitt BE, Hirsch MS, Dal Cin P, Quade BJ, Nucci MR. Perivascular epithelioid cell neoplasm (PEComa) of the gynecologic tract: clinicopathologic and immunohistochemical characterization of 16 cases. Am J Surg Pathol. (2014) 38:176–88. doi: 10.1097/pas.0000000000000133
8. Folpe AL, Mentzel T, Lehr HA, Fisher C, Balzer BL, Weiss SW. Perivascular epithelioid cell neoplasms of soft tissue and gynecologic origin: a clinicopathologic study of 26 cases and review of the literature. Am J Surg Pathol. (2005) 29:1558–75. doi: 10.1097/01.pas.0000173232.22117.37
9. Kundu R, Saha PK, Dey P. Sclerosing perivascular epithelioid cell tumour of the uterus: A rare entity posing diagnostic challenge. J Midlife Health. (2022) 13:328–30. doi: 10.4103/jmh.jmh_120_22
10. Xholli A, Kratochwila C, Vellone VG, Schiaffino MG. Acute and repeated haemoperitoneum: a challenging case of lymphangioleiomyomatosis with uterine PEComa. BMJ Case Rep. (2021) 14. doi: 10.1136/bcr-2021-244257
11. Shan W, Shi Y, Zhu Q, Yang B, Xie L, Li B, et al. Five cases of uterine perivascular epithelioid cell tumours (PEComas) and review of literature. Arch Gynecol Obstet. (2019) 299:185–90. doi: 10.1007/s00404-018-4920-4
12. Han Y, Liu TT, Qiu XS, Li QC, Zhao Y, Pang XY, et al. PEComa of the uterus with coexistence of situs inversus totalis, a case report and literature review. Diagn Pathol. (2015) 10:142. doi: 10.1186/s13000-015-0351-8
13. Poomtavorn Y, Warnnissorn N, Warnnissorn M, Boonyarangkul A. Caesarean section unmasking perivascular epithelioid cell tumour of the uterus. J Obstet Gynaecol. (2014) 34:441–2. doi: 10.3109/01443615.2014.901305
14. Yamada Y, Yamamoto H, Ohishi Y, Nishiyama K, Fukuhara M, Saitou T, et al. Sclerosing variant of perivascular epithelioid cell tumour in the female genital organs. Pathol Int. (2011) 61:768–72. doi: 10.1111/j.1440-1827.2011.02737.x
15. Sharma S, Kotru M, Gupta R. PEComata: highly melanotic multiple perivascular epithelioid cell tumours (PEComa) of the uterus. Apmis. (2008) 116:1000–3. doi: 10.1111/j.1600-0463.2008.01034.x
16. Azad NS, Aziz AB, Pervez S, Kayani N. Uterine perivascular epithelioid cell tumour presenting as a cervical mass. J Pak Med Assoc. (2006) 56:83–4.
17. Jeon IS, Lee SM. Multimodal treatment using surgery, radiotherapy, and chemotherapy in a patient with a perivascular epithelioid cell tumour of the uterus. J Pediatr Hematol Oncol. (2005) 27:681–4. doi: 10.1097/01.mph.0000193475.06870.d5
Keywords: uterine PEComas, perivascular epithelioid cell, uncertain malignant potential, fertility preservation, case report
Citation: Shi H, Yin Y, Liang S, Liu C, Huang Y, Lu B and Zhang L (2025) Mass resection as a candidate treatment for uterine PEComas of uncertain malignant potential: a case report and literature review. Front. Oncol. 14:1521253. doi: 10.3389/fonc.2024.1521253
Received: 01 November 2024; Accepted: 30 December 2024;
Published: 27 January 2025.
Edited by:
Ulrich Ronellenfitsch, Medical Faculty of the Martin-Luther-University Halle-Wittenberg, GermanyReviewed by:
Giuseppe Angelico, Agostino Gemelli University Polyclinic (IRCCS), ItalyCopyright © 2025 Shi, Yin, Liang, Liu, Huang, Lu and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Liying Zhang, emhhbmdsaXlpbmdAZ3htdS5lZHUuY24=; Bingfeng Lu, MTQzNTU4ODk1MUBxcS5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.