- 1Department of Gastroenterology, Jining First People’s Hospital, Jining, China
- 2Department of Pathology, Jining First People’s Hospital, Jining, China
Esophageal leiomyoma is the most common benign intramural tumor of the esophagus. Despite being the most common benign tumor in its category, esophageal leiomyomas constitute only 1.2% of all esophageal tumors. While esophageal leiomyoma itself is uncommon, the occurrence of multiple esophageal leiomyomas is even rarer, and the coexistence of high-grade squamous intraepithelial neoplasia (HGIN), involving both epithelial and mesenchymal tissues, is exceedingly rare. This case report describes a patient with multiple esophageal leiomyomas and localized HGIN, diagnosed using endoscopic ultrasonography, which identified a submucosal lesion within the muscularis mucosae. The lesion was successfully treated using endoscopic high-frequency electrocoagulation resection. This minimally invasive approach proved to be precise, safe, and effective, offering therapeutic outcomes comparable to those of traditional surgical resection, as confirmed by postoperative pathological analysis. As a primary goal, the abstract should render the general significance and conceptual advance of the work clearly accessible to a broad readership. References should not be cited in the abstract. Leave the Abstract empty if your article does not require one – please see the “Article types” on every Frontiers journal page for full details.
1 Introduction
Esophageal leiomyoma is a rare benign tumor that arises from the smooth muscle cells of the esophageal wall (1, 2). The incidence of benign esophageal tumors ranges from 0.005% to 7.9%, with leiomyomas accounting for 70-80% of these cases (3). It is often asymptomatic and discovered incidentally during imaging studies. Most patients with esophageal leiomyomas do not exhibit significant clinical symptoms; however, larger tumors (>5 cm) may cause dysphagia or chest pain (4, 5). These tumors are most commonly observed in individuals between 20 and 50 years of age, with a slight male predominance. While the exact etiology remains unclear, they are believed to result from localized smooth muscle hyperplasia or genetic mutations (6).
Although surgical resection has traditionally been the standard treatment for symptomatic esophageal leiomyomas, recent advances in endoscopic techniques, such as endoscopic ultrasonography (EUS) and high-resolution magnification endoscopy, have provided safer, minimally invasive alternatives for both diagnosis and treatment (6–8). However, the management of esophageal leiomyomas with concomitant early superficial lesions presents a challenge.
This case report discusses a rare instance of multiple esophageal leiomyomas in an elderly patient, complicated by localized high-grade squamous intraepithelial neoplasia (HGIN). The lesion was diagnosed through the use of EUS, chest imaging, and endoscopic pathology. High-frequency electrocoagulation resection was successfully performed via endoscopy to treat the lesion. The aim of this report is to delve into the role of advanced endoscopic techniques in the diagnosis and treatment of esophageal leiomyomas, especially in cases involving concurrent superficial lesions, and to review current management strategies for this condition.
2 Case report
A 72-year-old female was admitted for persistent abdominal pain lasting 4 months. She had no prior history of tumors or related diseases. On admission, chest and abdominal CT scans revealed no tumor lesions. There was no obvious thickening of the esophagus, and no enlarged lymph nodes around the esophagus were observed. Endoscopy showed a smooth-surfaced lesion on the anterior wall of the esophagus, approximately 1.2×0.6 cm in size, located 17 cm from the incisors. Another submucosal protrusion, approximately 1.2×0.6 cm in size, was found on the anterior esophageal wall, 20 cm from the incisors, partially covered by a white plaque (Figure 1). EUS of both lesions revealed clear and intact layers 1, 3, and 4, with a uniform hypoechoic area observed in the second layer, suggesting that the lesions originated from the mucosal muscularis (Figure 2). Both lesions were excised using high-frequency electrocoagulation, with no bleeding at the resection margins. Four hemostatic clips were used to close the wound bed.
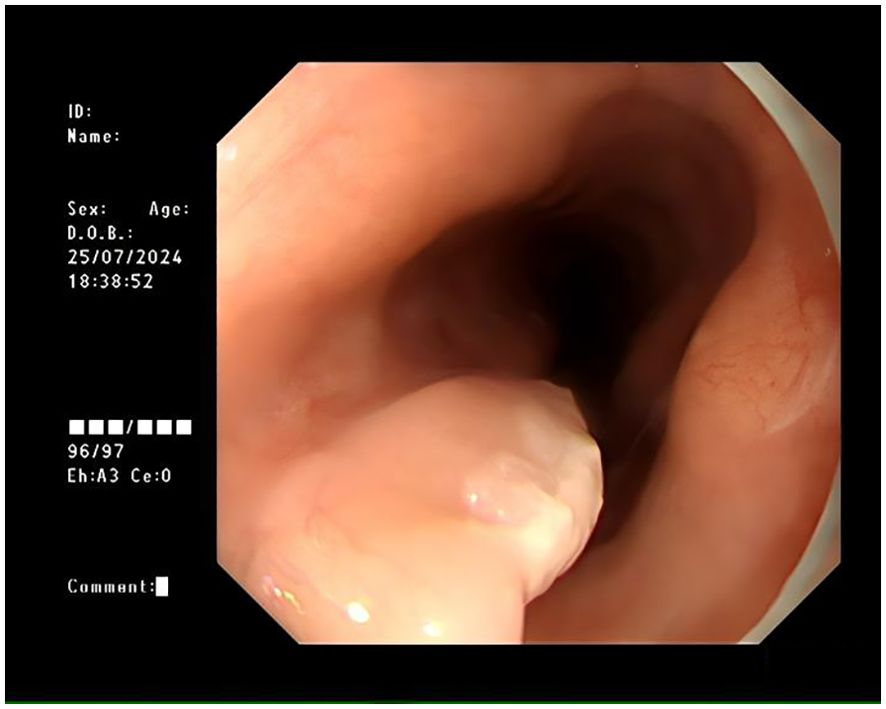
Figure 1. Submucosal protrusion in the esophagus with a smooth surface, partially covered by white patches, as observed under white light endoscopy.
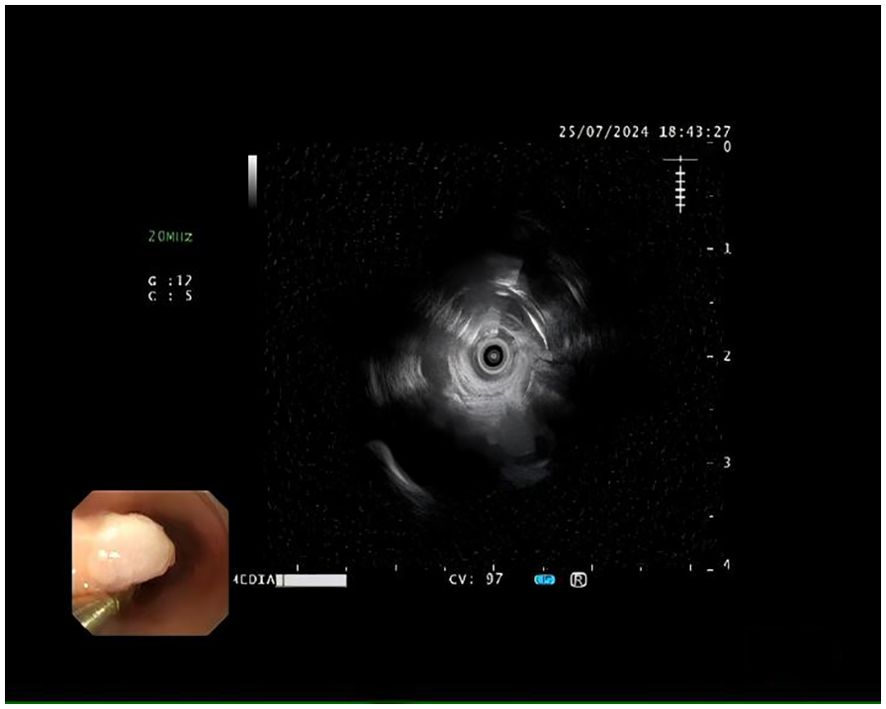
Figure 2. Hypoechoic tumor measuring approximately 12 mm in diameter, confined to the muscularis mucosae, with a smooth contour and well-defined borders, as seen on endoscopic ultrasonography.
Postoperative pathology revealed esophageal leiomyoma (17 cm from the incisors) and esophageal leiomyoma with high-grade squamous intraepithelial neoplasia (20 cm from the incisors). Figure 3A shows that after HE staining at ×40 magnification, spindle-shaped cells grow in bundles, with the surface covered by atypical squamous epithelium. Figure 3B shows that after HE staining at ×100 magnification, the squamous epithelial cell nuclei are enlarged, atypical, and deeply stained, with the lesion area involving more than half of the affected area. The diagnosis is high-grade squamous intraepithelial lesion (HGIN) of the esophagus.
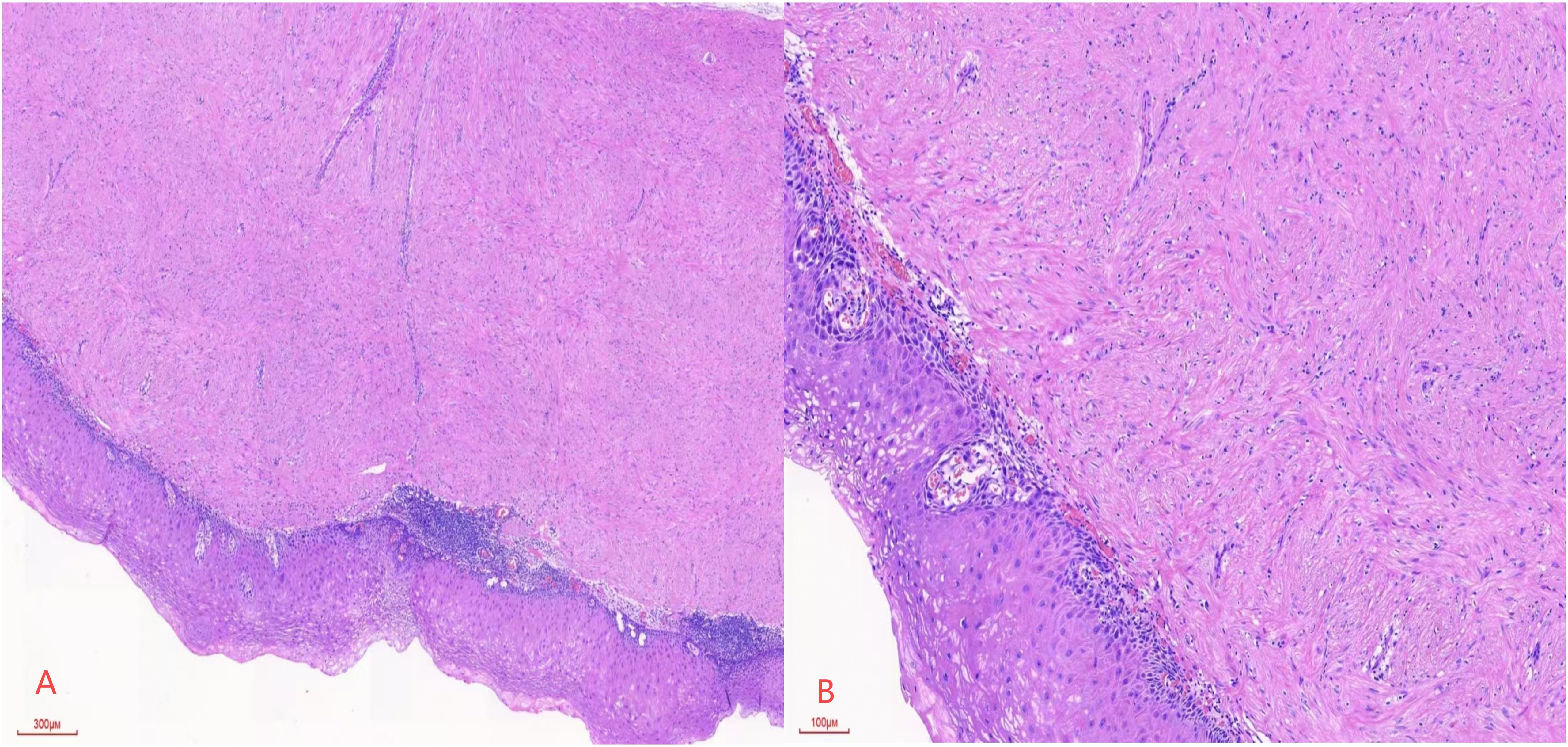
Figure 3. (A) Spindle cells are arranged in a fascicular pattern, with the surface covered by atypical squamous epithelium, at a magnification of HE×40. (B) Squamous epithelial cells exhibit enlarged, atypical, and hyperchromatic nuclei, with the lesion extending beyond half of the epithelial layer, at a magnification of HE×100.
Submucosal protrusions with white plaques in the esophagus represent a pathological condition that includes both smooth muscle tumors and squamous intraepithelial lesions. In pathological diagnosis, immunohistochemical markers such as Ki67, p53, SMA (smooth muscle actin), and Desmin play important roles in distinguishing smooth muscle tumors from squamous lesions. Ki-67 expression in tumor tissue can serve as a non-invasive biological marker for predicting tumor prognosis (9). In high-grade intraepithelial lesion areas, Ki67 expression is usually high and can assist in evaluating disease progression. p53 is a tumor suppressor protein, and studies have shown that using immunohistochemical detection of p53 mutations can diagnose high-grade atypical squamous epithelial hyperplasia in the esophagus (10). Mutations and overexpression of p53 may be associated with the malignant potential of smooth muscle tumors. SMA (smooth muscle antibody) (97.2%) and Desmin (94.5%) are positive in most smooth muscle tumor patients, serving as markers of smooth muscle cells and being helpful in confirming myogenic tumors (11). In squamous intraepithelial lesions, SMA and Desmin are usually negative. Ki67 and p53 help assess tumor proliferative activity and the risk of malignant transformation, while SMA and Desmin confirm the smooth muscle origin of the tumor. The expression patterns of these markers are useful for distinguishing smooth muscle tumors from squamous lesions. In pathological diagnosis, a comprehensive analysis of the expression of these markers along with other clinical and pathological features can provide more accurate diagnosis and treatment strategies for the patient.
In this case, immunohistochemical results showed localized high expression of Ki67, p53 mutation-positive staining, and positive expression of SMA and Desmin, which strongly supports the diagnosis of esophageal smooth muscle tumor with high-grade intraepithelial lesion. Combined with EUS examination, the lesion was located in the mucosal muscularis. Endoscopic high-frequency electrocoagulation was used to remove the esophageal lesion. Postoperative pathology remains the gold standard for diagnosis.
3 Conclusion
This case report presents a rare coexistence of esophageal leiomyoma and HGIN, accompanied by local white plaque on the surface of a submucosal protrusion. By combining computed tomography (CT), EUS, and EUS-guided biopsy, we were able to determine the depth of the lesion and the nature of the surface white plaques, providing guidance for our clinical treatment and offering a new perspective for our clinical diagnosis. Through high-frequency electrocautery endoscopic resection, we successfully treated the esophageal leiomyoma and removed the lesion with the associated white plaque. The results demonstrated that this treatment method is precise, minimally invasive, quick to recover, safe, and low-risk, achieving comparable outcomes to traditional surgical resection. In rare cases of concurrent esophageal leiomyoma and high-grade squamous intraepithelial neoplasia, particularly when white plaque suggests high-grade intraepithelial neoplasia, the combination of EUS, narrow-band imaging (NBI), and magnified endoscopy for evaluating lesion depth and plaque characteristics provides reliable clinical guidance, allowing the patient to achieve optimal treatment outcomes while avoiding radical esophageal resection.
4 Discussion
Rare Disease Coexistence: Esophageal leiomyoma is a rare benign tumor, typically characterized by the proliferation of smooth muscle cells within the esophageal wall, leading to localized thickening (5). Most esophageal leiomyomas are solitary lesions involving only smooth muscle cells (12). However, the simultaneous occurrence of esophageal leiomyoma and high-grade squamous intraepithelial neoplasia (HGIN) is exceedingly rare. It involves both abnormal proliferation of esophageal epithelial cells and changes in the mesenchymal cell components. High-grade squamous intraepithelial neoplasia is characterized by abnormal epithelial cell proliferation, accompanied by poor differentiation and increased nuclear atypia, and is generally considered a precancerous lesion that significantly increases the risk of developing esophageal squamous cell carcinoma (ESCC) (4, 13, 14). Therefore, early evaluation and intervention of epithelial changes are crucial to prevent malignant transformation. In addition, the proliferation of mesenchymal cells forms the main part of the tumor in esophageal leiomyoma (15). This proliferation may alter the normal structure and function of the esophagus, affecting its physiological state. Notably, In complex lesions involving esophageal leiomyoma and high-grade squamous intraepithelial neoplasia (HGIN), mesenchymal cells play crucial roles across various aspects of tumor biology, including the tumor microenvironment, immune regulation, and epithelial-mesenchymal interactions. These cells provide essential structural support within the tumor microenvironment, with smooth muscle cells in esophageal leiomyoma forming a framework that facilitates tumor growth and development. By regulating the extracellular matrix, mesenchymal cells promote tumor cell proliferation and migration, offering both physical space and biochemical support for tumor progression (1). Furthermore, mesenchymal cells actively engage in epithelial-mesenchymal interactions by secreting cytokines, chemokines, and growth factors, such as TGF-β and HGF. These factors encourage the proliferation, migration, and malignant transformation of epithelial cells, accelerating tumor progression through localized signaling networks, especially in the presence of HGIN (16, 17). Mesenchymal cells also contribute to immune regulation within the tumor microenvironment by secreting immune-suppressive factors like IL-10 and TGF-β, which help the tumor evade immune surveillance. This immune evasion mechanism enhances the tumor’s ability to develop undetected, thereby increasing its invasiveness (18). Additionally, mesenchymal cells promote fibrosis and tissue remodeling, which can worsen tumor progression. In esophageal leiomyoma, fibrosis is linked to the activation and proliferation of smooth muscle cells, which alter the tissue architecture and create a microenvironment conducive to continuous tumor growth. This fibrotic process, particularly in the context of coexisting HGIN, further increases the risk of tumor progression, as fibrosis often involves matrix hardening and immune escape (19, 20). Moreover, mesenchymal cells can drive epithelial-mesenchymal transition (EMT), a key process in tumor progression where epithelial cells lose polarity and cell junctions, acquiring a mesenchymal-like phenotype that enhances invasiveness. EMT activation in the context of HGIN may be influenced by signals from surrounding mesenchymal cells, promoting tumor cell migration, invasion, and metastasis (21, 22). Ultimately, mesenchymal cells, through their multifaceted roles in modulating the tumor microenvironment, immune response, fibrosis, and EMT, serve as a central driving force in the progression of esophageal leiomyoma and high-grade squamous intraepithelial neoplasia.
Innovative Perspective in Clinical Diagnosis: In this case, multiple esophageal leiomyomas was initially diagnosed by EUS, with the lesion presenting as a well-defined, smooth-edged mass, suggesting a benign tumor. The lesion was located in the mucosal muscularis and showed characteristic features of a typical esophageal leiomyoma: the tumor was sessile, with well-defined borders composed of smooth muscle bundles and a fibrous capsule. The lesion was small, multiple, eccentric, firm, and round in shape, with easy separability. The tumor was successfully removed by endoscopic high-frequency electrocautery, and histopathological examination confirmed the diagnosis. Research has shown that esophageal leiomyomas may have a protective role by preventing the spread of overlying squamous cell carcinoma and preventing deeper infiltration (12, 23). In this case report, the presence of high-grade squamous intraepithelial neoplasia in conjunction with the leiomyoma suggested that the lesion was at an early superficial stage. Although such cases are rare, esophageal submucosal tumors can sometimes be associated with squamous cell carcinoma, making it essential to accurately assess the presence and depth of squamous cell carcinoma. This requires further diagnostic investigations such as chest and abdominal CT scans to evaluate potential distant metastasis or abdominal lymph node involvement. Esophageal squamous cell carcinoma is the most common epithelial malignancy of the esophagus (24). For differential diagnosis of esophageal leiomyoma with leukoplakia, it is recommended to perform multiple endoscopic biopsies to obtain more accurate pathological information. Chest and abdominal CT scans are also crucial for assessing the size and extent of leukoplakia. NBI endoscopy enhances tissue contrast and scattering effects, helping to better identify lesions, especially when combined with magnifying endoscopy for histological analysis and lesion assessment, which has significant clinical value. Therefore, by combining CT, EUS and EUS-guided biopsy, we were able to accurately assess the depth and extent of the leukoplakia lesion, leading to a precise diagnosis. Further evaluation using narrow-band imaging (NBI) and magnification endoscopy provided a new perspective on the lesion depth, offering enhanced clinical diagnostic insight and ensuring both accuracy and comprehensiveness in the diagnosis.
Innovative Treatment Approach: This case utilized high-frequency electrocoagulation for endoscopic resection of the multiple esophageal leiomyomas, successfully removing the lesion accompanied by local leukoplakia. The results indicate that this treatment method is precise, minimally invasive, offers rapid recovery, is safe, and carries low risks, achieving results comparable to traditional surgical resection. In the rare case of coexisting multiple esophageal leiomyomas and HSIL, especially when leukoplakia suggests high-grade intraepithelial neoplasia, the complexity of diagnosis and treatment increases and should not be overlooked. The use of EUS, NBI, and magnification endoscopy to assess lesion depth and the nature of the leukoplakia provided reliable guidance for clinical decision-making, allowing the patient to achieve optimal treatment outcomes while avoiding the need for radical esophagectomy.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Research Ethics Committee of the First People’s Hospital of Jining City. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the participant/patient(s) for the publication of this case report.
Author contributions
LP: Writing – original draft, Writing – review & editing, Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization. CZ: Conceptualization, Data curation, Resources, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. RM: Conceptualization, Investigation, Methodology, Resources, Data curation, Formal analysis, Funding acquisition, Project administration, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. LF: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Freedman ND, Abnet CC, Leitzmann MF, Mouw T, Subar AF, Hollenbeck AR, et al. A prospective study of tobacco, alcohol, and the risk of esophageal and gastric cancer subtypes. Am J Epidemiol. (2007) 165:1424–33. doi: 10.1093/aje/kwm051
2. Rijcken E, Kersting CM, Senninger N, Bruewer M. Esophageal resection for giant leiomyoma: report of two cases and a review of the literature. Langenbecks Arch Surg. (2009) 394:623–9. doi: 10.1007/s00423-008-0365-8
3. Xu H, Li Y, Wang F, Wang W, Zhang L. Video-assisted thoracoscopic surgery for esophageal leiomyoma: A ten-year single-institution experience. J Laparoendosc Adv Surg Tech A. (2018) 28:1105–8. doi: 10.1089/lap.2018.0412
4. Chen L, Hussain K, Su Y, Gu Z, Ji C, Fang W. A novel hybrid approach for enucleation of esophageal leiomyoma. J Thorac Dis. (2019) 11:2576–80. doi: 10.21037/jtd.2019.06.23
5. Sun X, Wang J, Yang G. Surgical treatment of esophageal leiomyoma larger than 5 cm in diameter: A case report and review of the literature. J Thorac Dis. (2012) 4:323–6. doi: 10.3978/j.issn.2072-1439.2011.11.02
6. Shang QX, Yang YS, Wang WP, Hu WP, Chen LQ. Missed diagnosis of esophageal leiomyoma leading to esophagectomy: A case report and review of literatures. J Thorac Dis. (2018) 10:E65–E9. doi: 10.21037/jtd.2017.12.05
7. Jiang W, Rice TW, Goldblum JR. Esophageal leiomyoma: experience from a single institution. Dis Esophagus. (2013) 26:167–74. doi: 10.1111/j.1442-2050.2012.01345.x
8. Chen X, Xi Y, Wang H, Tan L. Minimally invasive surgery for giant esophageal leiomyoma: A case report & Review of the literatures. J Thorac Dis. (2017) 9:E26–31. doi: 10.21037/jtd.2017.01.34
9. Wang Y, Bai G, Zhang X, Shan W, Xu L, Chen W. Correlation analysis of apparent diffusion coefficient value and P53 and ki-67 expression in esophageal squamous cell carcinoma. Magn Reson Imaging. (2020) 68:183–9. doi: 10.1016/j.mri.2020.01.011
10. Xu YJ, Li R, Chen JM, Zhuang XY, Lin N, Wang LP, et al. Use of immunohistochemical P53 mutant-phenotype in diagnosis of high-grade dysplasia of esophageal squamous epithelia. Dig Dis. (2023) 41:685–94. doi: 10.1159/000531331
11. Sun LJ, Chen X, Dai YN, Xu CF, Ji F, Chen LH, et al. Endoscopic ultrasonography in the diagnosis and treatment strategy choice of esophageal leiomyoma. Clinics (Sao Paulo). (2017) 72:197–201. doi: 10.6061/clinics/2017(04)01
12. Iwaya T, Maesawa C, Uesugi N, Kimura T, Ikeda K, Kimura Y, et al. Coexistence of esophageal superficial carcinoma and multiple leiomyomas: A case report. World J Gastroenterol. (2006) 12:4588–92. doi: 10.3748/wjg.v12.i28.4588
13. Liu X, Zhao S, Wang K, Zhou L, Jiang M, Gao Y, et al. Spatial transcriptomics analysis of esophageal squamous precancerous lesions and their progression to esophageal cancer. Nat Commun. (2023) 14:4779. doi: 10.1038/s41467-023-40343-5
14. Liao G, Dai N, Xiong T, Wang L, Diao X, Xu Z, et al. Single-cell transcriptomics provides insights into the origin and microenvironment of human oesophageal high-grade intraepithelial neoplasia. Clin Transl Med. (2022) 12:e874. doi: 10.1002/ctm2.874
15. Orciani M, Caffarini M, Biagini A, Lucarini G, Delli Carpini G, Berretta A, et al. Chronic inflammation may enhance leiomyoma development by the involvement of progenitor cells. Stem Cells Int. (2018) 2018:1716246. doi: 10.1155/2018/1716246
16. Rhee KJ, Lee JI, Eom YW. Mesenchymal stem cell-mediated effects of tumor support or suppression. Int J Mol Sci. (2015) 16:30015–33. doi: 10.3390/ijms161226215
17. Sanchez LR, Borriello L, Entenberg D, Condeelis JS, Oktay MH, Karagiannis GS. The emerging roles of macrophages in cancer metastasis and response to chemotherapy. J Leukoc Biol. (2019) 106:259–74. doi: 10.1002/JLB.MR0218-056RR
18. Poggi A, Varesano S, Zocchi MR. How to hit mesenchymal stromal cells and make the tumor microenvironment immunostimulant rather than immunosuppressive. Front Immunol. (2018) 9:262. doi: 10.3389/fimmu.2018.00262
19. Zeltz C, Primac I, Erusappan P, Alam J, Noel A, Gullberg D. Cancer-associated fibroblasts in desmoplastic tumors: emerging role of integrins. Semin Cancer Biol. (2020) 62:166–81. doi: 10.1016/j.semcancer.2019.08.004
20. Ridge SM, Sullivan FJ, Glynn SA. Mesenchymal stem cells: key players in cancer progression. Mol Cancer. (2017) 16:31. doi: 10.1186/s12943-017-0597-8
21. Hong D, Liu T, Huang W, Liao Y, Wang L, Zhang Z, et al. Gremlin1 delivered by mesenchymal stromal cells promoted epithelial-mesenchymal transition in human esophageal squamous cell carcinoma. Cell Physiol Biochem. (2018) 47:1785–99. doi: 10.1159/000491060
22. Zhang LN, Kong CF, Zhao D, Cong XL, Wang SS, Ma L, et al. Fusion with mesenchymal stem cells differentially affects tumorigenic and metastatic abilities of lung cancer cells. J Cell Physiol. (2019) 234:3570–82. doi: 10.1002/jcp.27011
23. Mizobuchi S, Kuge K, Matsumoto Y, Yokoyama Y, Ookawauchi K, Tamura S, et al. Co-existence of early esophageal carcinoma and leiomyoma: A case report. Jpn J Clin Oncol. (2004) 34:751–4. doi: 10.1093/jjco/hyh133
Keywords: esophageal leiomyoma, esophageal bulge, high-grade intraepithelial neoplasia, leukoesophageal plaques, endoscopic resection
Citation: Pan L, Zhang C, Ma R and Fan L (2024) Case report: Esophageal bulge with white patch: endoscopic removal of leiomyoma and high-grade intraepithelial neoplasia. Front. Oncol. 14:1515288. doi: 10.3389/fonc.2024.1515288
Received: 22 October 2024; Accepted: 18 November 2024;
Published: 06 December 2024.
Edited by:
Babak Pakbin, Texas A and M University, United StatesReviewed by:
Zahra Hosseinkhani, Qazvin University of Medical Sciences, IranArash Shahsavari, Texas A and M University, United States
Yousef Khazaei Moonfaced, Harvard Medical School, United States
Copyright © 2024 Pan, Zhang, Ma and Fan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lijuan Fan, ZmFubGlqdWFuNTIxQDEyNi5jb20=; Ran Ma, Mjk2NDA5MTZAcXEuY29t
†These authors have contributed equally to this work
 Lili Pan
Lili Pan Chong Zhang2
Chong Zhang2