
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol., 25 November 2024
Sec. Gastrointestinal Cancers: Hepato Pancreatic Biliary Cancers
Volume 14 - 2024 | https://doi.org/10.3389/fonc.2024.1511583
 Jun Dong1,2
Jun Dong1,2 Zhengqiu Zhu2*
Zhengqiu Zhu2*Purpose: To investigate the effectiveness of neoadjuvant therapy and lymph node dissection(LND) on overall survival (OS) in patients with stage III/IV gallbladder cancer without distant metastases.
Methods: Data from 101 patients who received neoadjuvant therapy followed by surgery combined with adjuvant chemotherapy, and 1412 patients who received direct surgical treatment followed by adjuvant chemotherapy, were collected from the SEER database from 2004 to 2020. Patients were divided into group A (neoadjuvant therapy) and group B (direct surgery) based on the treatment modality. A total of 202 cases were obtained after propensity score matching, with 101 cases in each group (A and B). Cox unifactorial and multifactorial analyses were performed to identify independent risk factors for patients with advanced cholecystic carcinoma, and the Kaplan-Meier method was used to analyze overall survival (OS). The Cox proportional hazards model was used to investigate the effect of different subgroups on OS in both patient groups. Further survival analyses were conducted to determine whether lymph node dissection(LND) was beneficial for patients receiving neoadjuvant therapy for gallbladder cancer.
Results: Cox univariate analysis showed that marital status, AJCC stage, number of LND, tumor size, and treatment modality were associated with OS (P<0.05). Cox multifactorial regression analysis indicated that AJCC stage, LND, tumor size, and treatment modality were independent risk factors for OS in patients with non-metastatic advanced gallbladder cancer (P<0.05). Survival curves demonstrated that the OS in group A was longer than in group B (median OS: 30 months vs. 14 months, P<0.001). Subgroup analysis indicated that neoadjuvant therapy had a consistent effect on the OS of patients with advanced gallbladder cancer, improving both survival time and outcomes. Survival curves indicated that lymph node dissection was not significant in group A patients (p>0.05) but was significant in group B (p<0.05).
Conclusion: Neoadjuvant therapy can improve the OS of patients with non-metastatic stage III/IV gallbladder cancer and is an independent risk factor affecting prognosis; however, the significance of lymph node dissection in these patients still needs further study.
Gallbladder cancer is an aggressive malignant tumor, and due to its unique anatomical structure, subtle symptoms, and susceptibility to lymph node metastasis, most patients are already in stage III/IV at the time of consultation (1). Radical surgical resection is considered the only possible cure for gallbladder cancer (2, 3). However, for patients with stage III gallbladder cancer, radical resection is often not feasible due to the tumor’s invasive location, the patient’s systemic condition, and the inability to achieve R0 resection (4). No consensus exists on the further treatment of such patients. The role of radical surgical resection for patients with stage IV gallbladder cancer without distant metastases remains controversial (5). Neoadjuvant therapy aims to reduce tumor volume and achieve tumor downstaging through preoperative systemic therapy, thereby improving the success rate of R0 resection (6, 7). Neoadjuvant therapy is currently widely used in breast (8), ovarian (9), gastric (10), and colorectal cancers (11). Although neoadjuvant therapies have been shown to enhance overall survival in some malignancies, no completed large phase III clinical trial has conclusively demonstrated their therapeutic benefits in advanced gallbladder cancer. In this study, a propensity score matching (PSM) analysis was conducted using the SEER database to investigate the effectiveness of neoadjuvant therapy on the OS of patients with stage III/IV gallbladder cancer without distant metastases.
Data were from SEER*Stat 8.4.3 software and included patient demographics, clinicopathological, and treatment information. The inclusion criteria: (1) Primary tumor located in the gallbladder (anatomical code: C23.9); (2) Year of diagnosis from 2004 to 2020; (3) International Classification of Diseases for Oncology ICDO-3 code [adenocarcinoma (8140–8389), other]; (4) Patients with non-distant metastases (Stage III/IV), classified as cT1-cT4, cN0-2, and cM0, were re-staged according to the 8th edition of the AJCC staging system; (5) Use of ‘RX Summ - Systemic/Sur Seq’, “RX Summ - Surg Prim Site (1998+)”, ‘Chemotherapy recode,’ and ‘RX Summ–Surg/Rad Seq’ fields to screen patients who received neoadjuvant therapy followed by surgery combined with chemotherapy or direct surgical treatment followed by adjuvant chemotherapy; (6) All patients underwent radical surgical resection. The exclusion criteria: (1) Distant metastases; (2) Unknown surgical information or no surgical treatment; (3) Incomplete prognostic or clinical information.1513 patients were finally screened for the study (Figure 1). Patients were divided into group A (neoadjuvant therapy) and group B (direct surgery) based on the treatment modality.
The data extracted from the SEER database comprised variables such as gender, age at diagnosis, race, marital, histology, grade, the clinical T-stage, the clinical N-stage, AJCC, surgical treatment, radiotherapy, chemotherapy, survival time, and survival status.
Relevant data were analyzed and visualized utilizing SPSS 26 and R 4.3.3. Categorical variables were compared using the chi-square test or Fisher’s exact test. To minimize bias between group A and group B, propensity score matching (1:1) was performed with a caliper value of 0.02. Independent risk factors for OS were identified through Cox univariate and multivariate analyses. Survival curves were generated utilizing the ‘survival’ and ‘survminer’ packages, and the log-rank test was utilized to assess the predictive value of efficacy between groups. Forest plots for subgroups were created using the ‘forestploter’ package.
Statistically meaningful differences were found between the two groups before PSM, with 101 people in A and 1412 people in B. Significant differences were found in grade classification, T stage, AJCC stage, lymph node clearance, and tumor size. After PSM, the baseline data of the two groups were balanced, resulting in 101 matched pairs in group B for group A; see Table 1 for details.
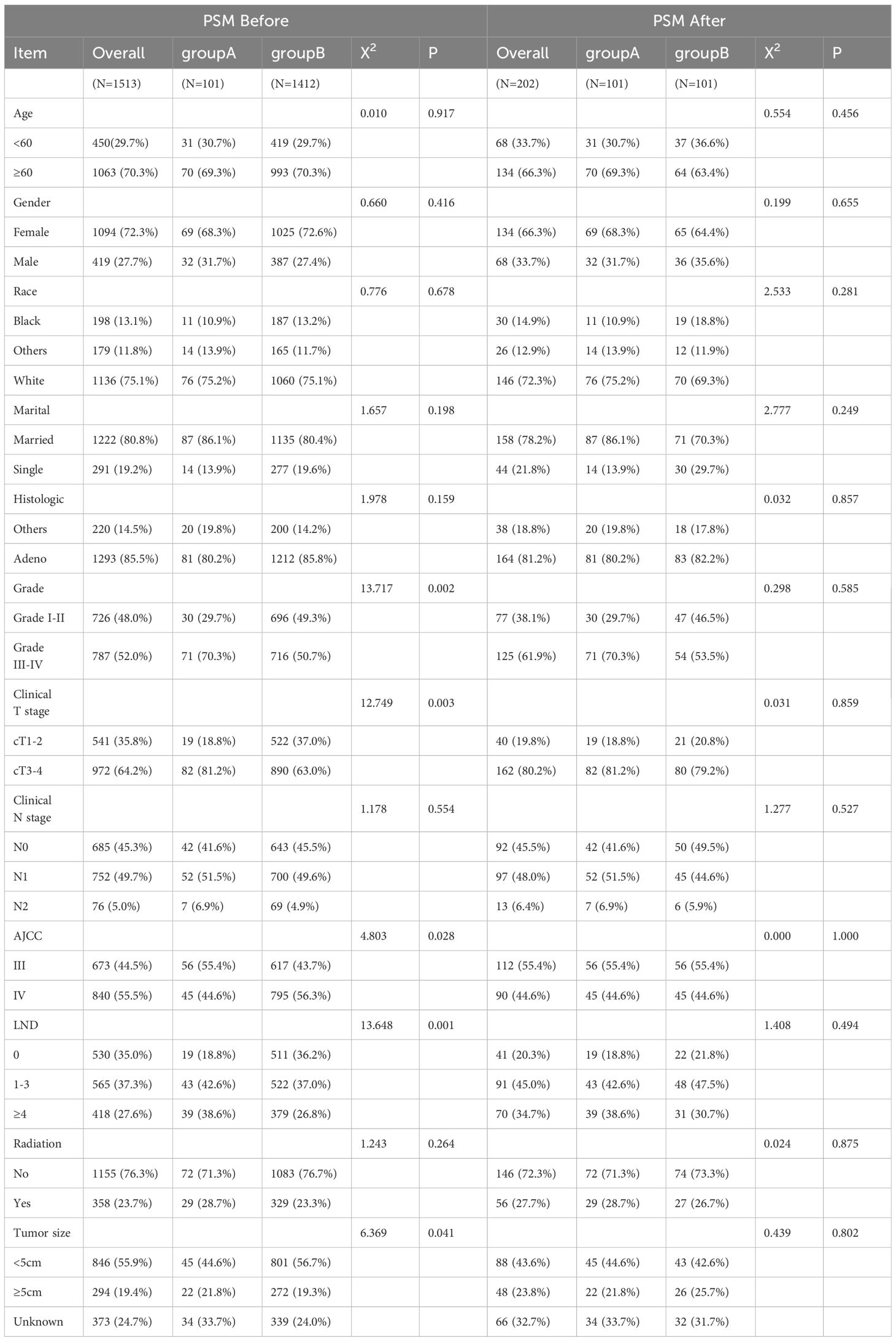
Table 1. Baseline data for patients with stage III/IV gallbladder cancer without distant metastasis.
On Cox univariate analysis, marital status, AJCC stage, LND, tumor size, and treatment modality were meaningfully associated with OS. On Cox multivariate analysis, AJCC stage, LND, tumor size, and treatment modality were independent risk factors. Stage IV people had a worse prognosis than stage III patients (HR: 1.758, 95% CI: 1.311-2.358, P < 0.001). Patients with 1-3 lymph nodes resected and those with ≥ 4 lymph nodes resected had a better prognosis (HR: 0.494, 95% CI: 0.331-0.739, P < 0.001) than patients with no lymph nodes removed (HR: 0.453, 95% CI: 0.295-0.695, P < 0.001). Additionally, patients with tumor size (<5cm) had a better prognosis. Compared with patients receiving neoadjuvant therapy, those undergoing direct surgery had a worse prognosis (HR: 2.322, 95% CI: 1.681-3.209, P < 0.001). For details, see Table 2.
Neoadjuvant therapy has been found to have a remarkable impact on people’s OS. The survival curves indicated that the OS in A was longer than in B (median OS: 30 months vs. 14 months, P<0.001). Further analysis was conducted to assess the effect of neoadjuvant therapy on OS across various patient subgroups, based on independent risk factors identified from a multifactorial Cox proportional hazards model. The results demonstrated that neoadjuvant therapy resulted in a longer median survival time compared to group B across different stages: stage III (median OS: 31 months vs. 14 months, P<0.001) and stage IV (median OS: 25 months vs. 10 months, P<0.001). For lymph node dissection (LND), the median OS was: LND=0 (16 months vs. 7 months, P<0.001), LND=1-3 (30 months vs. 15 months, P<0.001), and LND≥4 (21 months vs. 25 months, P=0.21). Regarding tumor size, <5 cm (38 months vs. 17 months, P<0.001), ≥5 cm (22 months vs. 10 months, P<0.001), and unknown tumor size (19 months vs. 21 months, P=0.28).
Overall, the OS in group A was longer than in group B across different stages (stage III/IV), lymph node dissection (LND=0/1-3), and tumor sizes (<5 cm/≥5 cm). No significant difference in OS was observed for LND≥4 and unknown tumor size (P>0.05), as illustrated in Figure 2.
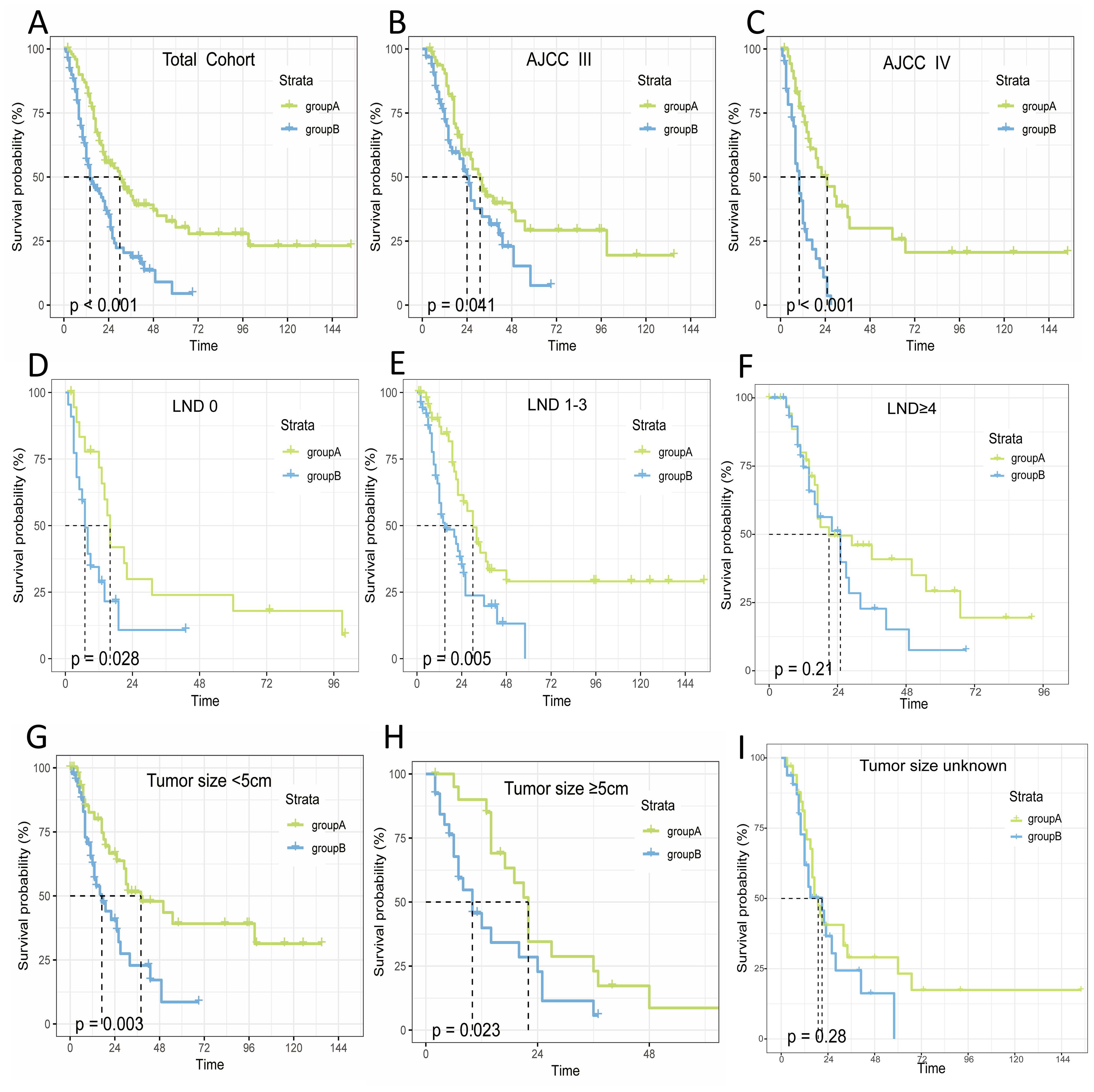
Figure 2. Relationship between OS and treatment modalities in patients with non-metastatic advanced gallbladder cancer. (A) Total; (B) StageIII; (C) StageIV; (D) LND=0; (E) LND=1-3; (F) LND≥4; (G) Tumor size<5cm; (H) Tumor size≥5cm; (I) Tumor size unknown.
To illustrate the interaction between other risk factors and treatment modalities, we performed 12 subgroup analyses on the total cohort. Figure 2 shows that neoadjuvant therapy was significantly associated with improved OS prognosis. The forest plot for the subgroup analyses (Figure 3) showed consistent proportional effects in the OS analyses, with no heterogeneity found in all 12 prespecified subgroups (P > 0.05 for all interactions).
Lymph node dissection (LND) is an independent risk factor for patients with advanced non-metastatic gallbladder cancer. However, our COX regression analyses of different treatment subgroups revealed that LND was an independent prognostic factor for patients in the direct surgery group (P<0.05), but not for those in the neoadjuvant group (P>0.05), Supplementary Tables 1, 2. Kaplan-Meier survival curves indicated that lymph node dissection (LND) provided a survival benefit in the direct surgery group (P=0.001), while no significant survival benefit was observed in the neoadjuvant therapy group (P>0.05). We found that in N0 patients receiving neoadjuvant therapy, the median OS for the LND=0, LND=1-3, and LND≥4 groups was 15 months, 34 months, and 21 months, respectively, with no statistically significant difference (P>0.05). In N1/N2 patients receiving neoadjuvant therapy, the median OS for the LND=0, LND=1-3, and LND≥4 groups was 18 months, 25 months, and 23.5 months, respectively, with no statistically significant difference (P>0.05). However, among patients without neoadjuvant therapy, the median OS for the three groups of N0 patients was 8, 20, and 25 months, respectively, with a statistically significant difference (P=0.045). The median OS for the three groups of N1 patients was 4, 14, and 17 months, with a remarkably meaningful difference (P<0.001), as shown in Figure 4.
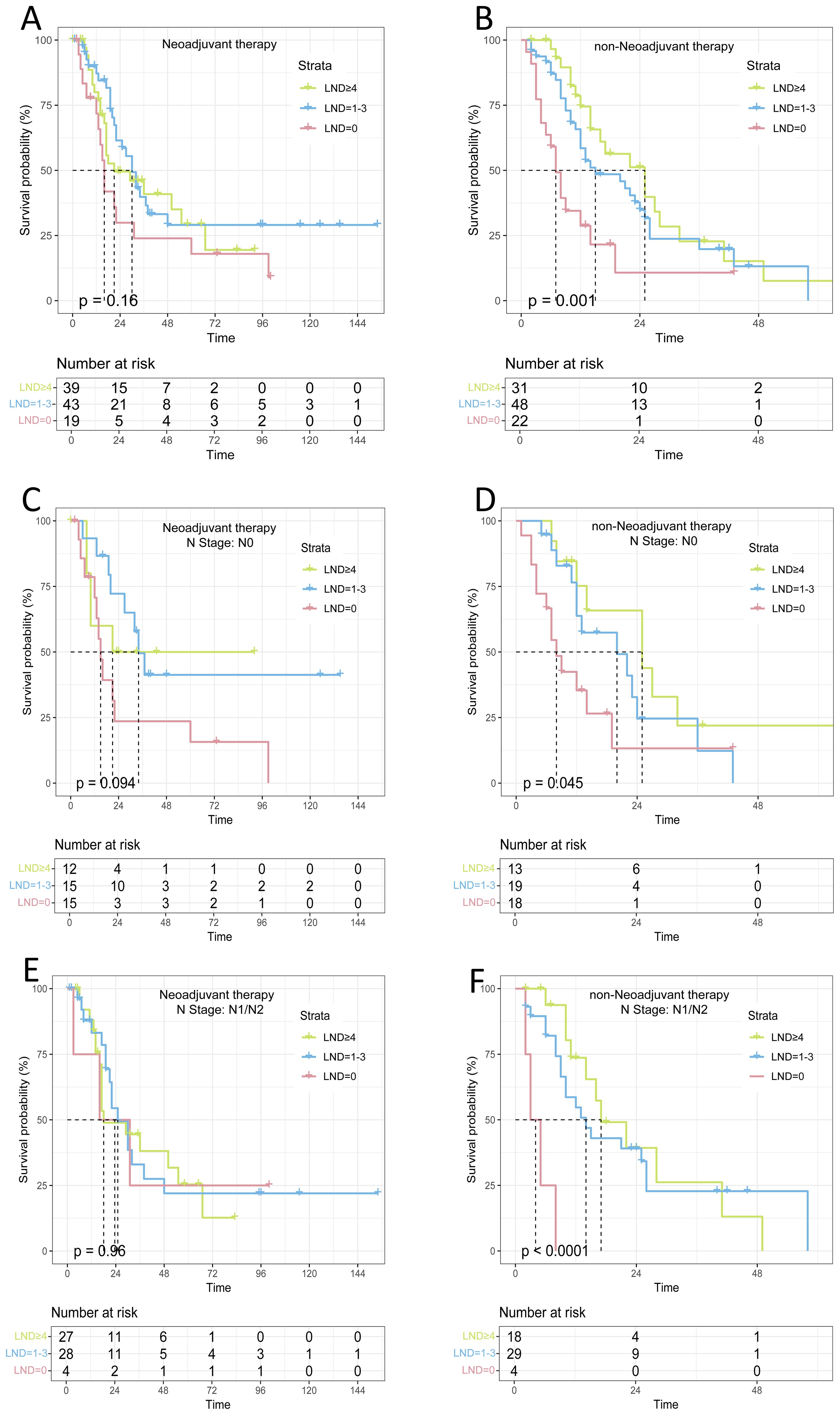
Figure 4. Survival analysis of lymph node dissection with different treatment modalities and lymph node dissection (A) Neoadjuvant therapy; (B) non-Neoadjuvant therapy; (C) Neoadjuvant therapy(N0); (D) non-Neoadjuvant therapy(N0); (E) Neoadjuvant therapy(N1/N2); (F) non-Neoadjuvant therapy(N1/N2);.
Radical surgical resection is an important therapy for advanced gallbladder cancer; however, only 10% of patients have the opportunity for surgical treatment (4). Additionally, direct surgical treatment of advanced patients without distant metastases has a low R0 resection rate and is prone to high postoperative recurrence. Gong Wei’s team conducted a study of gallbladder cancer in China, involving 6159 cases. The study showed that 34.26% of patients had lost the opportunity for surgery at the time of their initial consultation. Among those who underwent surgical treatment, 58.89% achieved R0 resection, leaving a high percentage of patients who did not achieve radical resection (12). Therefore, direct surgical resection is not an appropriate option for people.
With the increasing adoption of neoadjuvant therapy across various malignancies, it is also becoming a viable treatment option for people with advanced gallbladder cancer. A single-center retrospective research at the University of Montreal, Canada, showed that only 28.6% of patients with T3 GBC achieved R0 resection (13). An international multicenter study (14) initiated by the Anderson Cancer Centre showed that T3 to T4 stage was an independent factor in the recurrence of GBC, with a recurrence rate of up to 40% of patients at 1 year after surgery. Creasy et al. (15) found that 45% of patients could undergo radical resection following neoadjuvant therapy. The OS was different between the R0 resection group and others, with the former achieving 51 months compared to 11 months in the latter. Our study yielded comparable results, showing that the OS for patients in A was longer than that for patients in B (median OS: 25 months vs. 14 months, P<0.001). Multivariate analysis further identified neoadjuvant treatment as an independent prognostic factor in advanced gallbladder cancer (P<0.001). This indicates that neoadjuvant therapy enhances OS for people with gallbladder cancer without distant metastases. However, while a similar benefit was observed for OS in patients with locally advanced gallbladder cancer, the results did not reach statistical meaning (P>0.05) (16). Fareed et al. (17) conducted retrospective research on the OS of people with non-metastatic gallbladder cancer who received neoadjuvant radiotherapy and found no benefit in subgroup analysis. Consequently, the efficacy of neoadjuvant therapy in patients with advanced gallbladder cancer remains a topic of debate. The GAIN trial will confirm the good points of neoadjuvant therapy in gallbladder cancer. The results, expected to be published this year, will provide a higher level of evidence-based support for the use of neoadjuvant therapy in advanced gallbladder cancer (18).
Additionally, this study identified lymph node dissection as an independent prognostic factor for patients who did not receive neoadjuvant therapy, as determined by Cox regression analysis. Maegawa et al. (19) conducted a prognostic analysis of gallbladder cancer and reported that the OS rate of patients who did not undergo lymphatic clearance was lower than that of patients with positive lymph node metastases after clearance (HR=1.11, 95% CI 1.01-1.22). Tumor size and AJCC stage were also independent risk factors for patients, consistent with previous studies (20, 21). However, there are fewer studies on whether LND prolongs OS in patients who have undergone surgical resection after neoadjuvant therapy. In the present study, we analyzed the OS of patients who received neoadjuvant therapy and found no meaningful difference between patients with and without lymph node metastasis (P>0.05). However, there was a meaningful difference in survival among patients without neoadjuvant therapy based on lymph node metastasis status (P<0.05). Ito et al. (22), by retrospectively analyzing the data of 122 patients with gallbladder cancer, concluded that intraoperative clearance of ≥6 lymph nodes significantly improved patients’ prognosis, a conclusion shared by other scholars (23). Widmann et al. (24), through a literature review, found that LND improved the OS of patients with gallbladder cancer and that clearing at least 6 lymph nodes significantly improved patients’ prognosis. However, these studies were conducted in patients with gallbladder cancer who did not receive neoadjuvant therapy, and no large studies have been conducted in patients who received neoadjuvant therapy. We hypothesize that the differences in lymph node dissection outcomes between different treatment modalities may be due to the number of LND in this study being classified as ≥4 based on SEER database data, which may differ from previous classifications. Secondly, the lymph node status of a patient may change after receiving neoadjuvant therapy. Additionally, the small sample size of this study may have introduced a larger bias. Therefore, future multicenter, large-scale prospective researches are needed to investigate the significance of LND and the numbers of LND in the prognosis of patients undergoing radical resection after neoadjuvant therapy. There is an urgent need to explore prognostic biomarkers for patients undergoing neoadjuvant therapy. Currently, circulating tumor DNA and solid tumor microscopic residual disease (MRD) has shown significant value in lung, breast, and colon cancers (25). Other biomarkers, such as TTK (26), IL-22 (27), and the oncogenic Neuregulin 1 gene (NRG1) (28), have also demonstrated better predictive value in various solid tumors (29, 30). However, more research is required to determine their effectiveness in patients with advanced gallbladder cancer receiving neoadjuvant therapy.
Currently, there is no preferred neoadjuvant treatment option for gallbladder cancer (GBC). The gemcitabine + cisplatin (GC) regimen has been recommended as the first-line standard chemotherapy for biliary tract cancers (BTC) since the results of the ABC-02 trial (31). The 2023 National Comprehensive Cancer Network (NCCN) guidelines (32) recommend a gemcitabine-based combination chemotherapy regimen for gallbladder cancer. The combination regimens include GC, 5-fluorouracil (5-FU) + oxaliplatin + calcium folinate (FOLFOX), capecitabine + oxaliplatin, gemcitabine + capecitabine, durvalumab + gemcitabine + cisplatin, and gemcitabine + cisplatin + albumin paclitaxel. Gong Wei’s team is conducting a phase II clinical study on the combination of gemcitabine and albumin-paclitaxel for the treatment of progressive gallbladder cancer, with preliminary results showing an ORR of 48% (12). Radiotherapy in the neoadjuvant treatment of gallbladder cancer is often combined with chemotherapy. Engineer et al. (33) reported that people with T3 and T4 gallbladder cancer had the opportunity for R0 resection after gemcitabine monotherapy combined with high-dose radiotherapy, achieving a significant survival benefit. Fareed et al. (17) retrospectively analyzed patients with non-metastatic gallbladder and bile duct cancers who had undergone radical surgery at a single center and did not observe a significant survival benefit from neoadjuvant radiotherapy in gallbladder cancer patients. The POLCAGB study (34) is an ongoing phase III randomized clinical trial comparing neoadjuvant chemotherapy to neoadjuvant radiotherapy for the treatment of T3 and T4 gallbladder cancers. This study is expected to provide high-level evidence-based guidance for the use of neoadjuvant radiotherapy in gallbladder cancer and inform clinical practice.
Numerous limitations were identified in this study. Firstly, data were unavailable on patients who underwent neoadjuvant therapy and then experienced disease progression or were otherwise unable to undergo surgery, and data on postoperative R0 resection rates and changes in lymph node status after neoadjuvant therapy were difficult to obtain. Secondly, the stability of the conclusions of this study was affected by the small sample size. Thirdly, the study data were derived from the SEER database, which did not allow access to all relevant variables, resulting in selection bias. Fourthly, as a retrospective study, data collection and analyses were subject to bias. Finally, information on specific protocols for neoadjuvant therapy was lacking, which is crucial for clinical practice.
In conclusion, the results of this study indicated that the neoadjuvant therapy group exhibited a better prognosis than the direct surgery group. Future research should investigate in greater depth the impact of different neoadjuvant treatment regimens on the survival of patients with stage III/IV gallbladder cancer who have not developed distant metastases, to determine the treatment regimen with the optimal outcome. Secondly, no survival benefit of lymph node dissection was found in patients receiving neoadjuvant therapy, necessitating large-scale studies to confirm the impact of lymph node dissection on survival in neoadjuvant-treated patients, as well as multicenter trials to assess the generalizability of the results to different populations, and long-term follow-up studies to assess the durability of the survival benefit. Finally, the cost-effectiveness of neoadjuvant therapy and lymph node dissection needs further evaluation.
Neoadjuvant therapy can improve the OS of patients with non-metastatic stage III/IV gallbladder cancer and is an independent risk factor; however, the significance of lymph node dissection in these patients still needs further study.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethical approval was not required for the study involving humans in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and the institutional requirements.
JD: Writing – original draft, Writing – review & editing. ZZ: Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Each of the listed authors has provided explicit, intellectual, and significant input that has been incorporated into the work before its publication.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2024.1511583/full#supplementary-material
1. Rawla P, Sunkara T, Thandra KC, Barsouk A. Epidemiology of gallbladder cancer. ceh. (2019) 5:93–102. doi: 10.5114/ceh.2019.85166
2. Hickman L, Contreras C. Gallbladder cancer. Surg Oncol Clin N. (2019) 99:337–55. doi: 10.1016/j.suc.2018.12.008
3. Krell RW, Wei AC. Gallbladder cancer: surgical management. Chin Clin Oncol. (2019) 8:36–6. doi: 10.21037/cco.2019.06.06
4. Dasari BVM, Ionescu MI, Pawlik TM, Hodson J, Sutcliffe RP, Roberts KJ, et al. Outcomes of surgical resection of gallbladder cancer in patients presenting with jaundice: A systematic review and meta-analysis. J Surg Oncol. (2018) 118:477–85. doi: 10.1002/jso.25186
5. Alexakis N, Connor S. Meta-analysis of one- vs. two-stage laparoscopic/endoscopic management of common bile duct stones. HPB. (2012) 14:254–9. doi: 10.1111/j.1477-2574.2012.00439.x
6. Medin CR, Maithel SK. Neoadjuvant therapy trials in biliary tract malignancies. J Surg Oncol. (2021) 125:84–8. doi: 10.1002/jso.26714
7. Zhou Y, Yuan K, Yang Y, Ji Z, Zhou D, Ouyang J, et al. Gallbladder cancer: current and future treatment options. Front Pharmacol. (2023) 14:1183619. doi: 10.3389/fphar.2023.1183619
8. Ward ZJ, Atun R, Hricak H, Asante K, McGinty G, Sutton EJ, et al. The impact of scaling up access to treatment and imaging modalities on global disparities in breast cancer survival: a simulation-based analysis. Lancet Oncol. (2021) 22:1301–11. doi: 10.1016/S1470-2045(21)00403-4
9. Vergote I, Coens C, Nankivell M, Kristensen GB, Parmar MKB, Ehlen T, et al. Neoadjuvant chemotherapy versus debulking surgery in advanced tubo-ovarian cancers: pooled analysis of individual patient data from the EORTC 55971 and CHORUS trials. Lancet Oncol. (2018) 19:1680–7. doi: 10.1097/01.ogx.0000554460.78765.59
10. Li Z, Shan F, Wang Y, Zhang Y, Zhang L, Li S, et al. Correlation of pathological complete response with survival after neoadjuvant chemotherapy in gastric or gastroesophageal junction cancer treated with radical surgery: A meta-analysis. PloS One. (2018) 13:e0189294. doi: 10.1371/journal.pone.0189294
11. He F, Ju H-Q, Ding Y, Jiang Z, Li Z, Huang B, et al. Association between adjuvant chemotherapy and survival in patients with rectal cancer and pathological complete response after neoadjuvant chemoradiotherapy and resection. Br J Cancer. (2020) 123:1244–52. doi: 10.1038/s41416-020-0989-1
12. Yang Z, Wu Z, Xiong Y, Liu S, Cai C, Shao Z, et al. Successful conversion surgery for locally advanced gallbladder cancer after gemcitabine and nab-paclitaxel chemotherapy. Front Oncol. (2022) 12. doi: 10.3389/fonc.2022.977963
13. D’Hondt M, Lapointe R, Benamira Z, Pottel H, Plasse M, Letourneau R, et al. Carcinoma of the gallbladder: Patterns of presentation, prognostic factors and survival rate. An 11-year single centre experience. Eur J Surg Oncol. (2013) 39:548–53. doi: 10.1016/j.ejso.2013.02.010
14. Vega EA, Newhook TE, Kawaguchi Y, Qiao W, De Bellis M, Okuno M, et al. Conditional recurrence-free survival after oncologic extended resection for gallbladder cancer: an international multicenter analysis. Ann Surg Oncol. (2021) 28:2675–82. doi: 10.1245/s10434-021-09626-3
15. Creasy JM, Goldman DA, Dudeja V, Lowery MA, Cercek A, Balachandran VP, et al. Systemic chemotherapy combined with resection for locally advanced gallbladder carcinoma: surgical and survival outcomes. J Am Coll Surg. (2017) 224:906–16. doi: 10.1016/j.jamcollsurg.2016.12.058
16. Ozer M, Goksu SY, Sanford NN, Porembka M, Khurshid H, Ahn C, et al. A propensity score analysis of chemotherapy use in patients with resectable gallbladder cancer. JAMA Network Open. (2022) 5:e2146912. doi: 10.1001/jamanetworkopen.2021.46912
17. Fareed MM, DeMora L, Esnaola NF, Denlinger CS, Karachristos A, Ross EE, et al. Concurrent chemoradiation for resected gall bladder cancers and cholangiocarcinomas. J Gastrointest Oncol. (2018) 9:762–8. doi: 10.21037/jgo.2018.05.09
18. Goetze TO, Bechstein WO, Bankstahl US, Keck T, Königsrainer A, Lang SA, et al. Neoadjuvant chemotherapy with gemcitabine plus cisplatin followed by radical liver resection versus immediate radical liver resection alone with or without adjuvant chemotherapy in incidentally detected gallbladder carcinoma after simple cholecystectomy or in front of radical resection of BTC (ICC/ECC) – a phase III study of the German registry of incidental gallbladder carcinoma platform (GR)– the AIO/CALGP/ACO- GAIN-trial –. BMC Cancer. (2020) 20:122. doi: 10.1186/s12885-020-6610-4
19. Maegawa FB, Ashouri Y, Hamidi M, Hsu C-H, Riall TS. Gallbladder cancer surgery in the United States: lymphadenectomy trends and impact on survival. J Surg Res. (2020) 258:54–63. doi: 10.1016/j.jss.2020.08.041
20. Roa JC, García P, Kapoor VK, Maithel SK, Javle M, Koshiol J. Gallbladder cancer. Nat Rev Dis. (2022) 8:1–22. doi: 10.1038/s41572-022-00398-y
21. Vega EA, Newhook TE, Mellado S, Ruzzenente A, Okuno M, De Bellis M, et al. Benchmarks and geographic differences in gallbladder cancer surgery: an international multicenter study. Ann Surg Oncol. (2023) 30:4904–11. doi: 10.1245/s10434-023-13531-2
22. Negi SS, Singh A, Chaudhary A. Lymph nodal involvement as prognostic factor in gallbladder cancer: location, count or ratio? J Gastrointest Surg. (2011) 15:1017–25. doi: 10.1007/s11605-011-1528-4
23. Wu B, Shen Y, Chen X, Wang X, Zhong Z. Effect of lymphadenectomy on the prognosis for N0 gallbladder carcinoma patients: A study based on SEER database. Cancer Medicine. (2021) 10:7136–43. doi: 10.1002/cam4.4250
24. Widmann B, Warschkow R, Beutner U, Weitzendorfer M, Ukegjini K, Schmied BM, et al. Effect of lymphadenectomy in curative gallbladder cancer treatment: a systematic review and meta-analysis. Langenbecks Arch Surg. (2020) 405:573–84. doi: 10.1007/s00423-020-01878-z
25. Peng Y. Circulating tumor DNA and minimal residual disease (MRD) in solid tumors: current horizons and future perspectives. Front Oncol. (2021) 11. doi: 10.3389/fonc.2021.763790
26. Zhang L, Jiang B, Zhu N, Tao M, Jun Y, Chen X, et al. Mitotic checkpoint kinase Mps1/TTK predicts prognosis of colon cancer patients and regulates tumor proliferation and differentiation via PKCα/ERK1/2 and PI3K/Akt pathway. Med Oncol. (2020) 37:5. doi: 10.1007/s12032-019-1320-y
27. Xu L, Cai H, Zhu N, Zheng B. Interleukin-22 derived from cervical cancer-associated fibroblasts accelerates senescence of normal fibroblasts and promotes expression of tumorigenesis-related factors in HeLa cells. Eur J Gynaecol Oncol. (2020) 41:192–9. doi: 10.31083/j.ejgo.2020.02.4970
28. Zhang C, Mei W, Zeng C. Oncogenic Neuregulin 1 gene (NRG1) fusions in cancer: A potential new therapeutic opportunities. Biochim Biophys Acta (BBA) - Rev Cancer. (2022) 1877:188707. doi: 10.1016/j.bbcan.2022.188707
29. Li S, Li X, Yang X, Lei Y, He M, Xiang X, et al. Corilagin enhances the anti-tumor activity of 5-FU by downregulating the expression of GRP 78. Sci Rep. (2023) 13:22661. doi: 10.1038/s41598-023-49604-1
30. Dong S, Liu B, Hu S, Guo F, Zhong Y, Cai Q, et al. A novel oncolytic virus induces a regional cytokine storm and safely eliminates Malignant ascites of colon cancer. Cancer Med. (2022) 11:4297–309. doi: 10.1002/cam4.4772
31. Weigt J, Malfertheiner P. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. Expert Rev Gastroenterol Hepatol. (2010) 4:395–7. doi: 10.1586/egh.10.45
32. Benson AB, D’Angelica MI, Abrams T, Abbott DE, Ahmed A, Anaya DA, et al. NCCN guidelines® Insights: biliary tract cancers, version 2.2023: featured updates to the NCCN guidelines. J Natl Compr Cancer Netw. (2023) 21:694–704. doi: 10.6004/jnccn.2023.0035
33. Engineer R, Goel M, Chopra S, Patil P, Purandare N, Rangarajan V, et al. Neoadjuvant chemoradiation followed by surgery for locally advanced gallbladder cancers: A new paradigm. Ann Surg Oncol. (2016) 23:3009–15. doi: 10.1245/s10434-016-5197-0
34. Engineer R, Patkar S, Lewis SC, Sharma AD, Shetty N, Ostwal V, et al. A phase III randomised clinical trial of perioperative therapy (neoadjuvant chemotherapy versus chemoradiotherapy) in locally advanced gallbladder cancers (POLCAGB): study protocol. BMJ Open. (2019) 9:e028147. doi: 10.1136/bmjopen-2018-028147
Keywords: neoadjuvant therapy, radical resection, stage III/IV gallbladder cancer, lymph node dissection, survival analysis, SEER database
Citation: Dong J and Zhu Z (2024) Efficacy of neoadjuvant therapy and lymph node dissection in advanced gallbladder cancer without distant metastases: a SEER database analysis. Front. Oncol. 14:1511583. doi: 10.3389/fonc.2024.1511583
Received: 15 October 2024; Accepted: 06 November 2024;
Published: 25 November 2024.
Edited by:
Abdullah Esmail, Houston Methodist Hospital, United StatesReviewed by:
Qi Zhang, Huazhong University of Science and Technology, ChinaCopyright © 2024 Dong and Zhu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhengqiu Zhu, emhlbmdxaXV6aHUyMDE3QDE2My5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.