- 1Department of Hematology, Linyi People’s Hospital, Linyi, Shandong, China
- 2School of Clinical Medicine, Shandong Second Medical University, Weifang, Shandong, China
- 3Spine surgery, Linyi People’s Hospital, Shandong University, Linyi, Shandong, China
Background: Primary cardiac lymphoma (PCL) is an exceedingly uncommon type of lymphoma that primarily affects the heart and/or pericardium, or manifests through cardiac symptoms due to myocardial infiltration. The infrequency of PCL, coupled with its non-specific clinical presentations, often complicates early diagnosis. This study aims to fill the existing gap in clinical knowledge regarding PCL by detailing a case of PCL and examining its clinical features, auxiliary examinations, treatment approaches, and prognostic outcomes, thereby facilitating early detection and enhancing patient care.
Methods: A thorough search of the PubMed and Chinese National Knowledge Infrastructure (CNKI) database was performed using keywords “heart” and “lymphoma” or “primary cardiac lymphoma”. This search encompassed publications from January 1, 2014, to November 1, 2024.
Results: The review included 121 cases. These cases usually present with atypical symptoms, mainly circulatory and respiratory, including chest tightness, dyspnea, and edema, along with occasional neurological and gastrointestinal symptoms. Echocardiography served as the primary diagnostic method in 92.6% of cases, while a definitive diagnosis was achieved through pathological examination in all cases (100%). Treatment strategies predominantly included surgical intervention (44.6%) and chemotherapy (76.0%). Although surgery did not have a significant effect on survival rates, chemotherapy proved to be critical in improving patient survival.
Conclusions: PCL, which arises in the cardiac or pericardial areas, is generally associated with a poor prognosis. It is essential for clinicians to develop a greater awareness and understanding of the characteristics of PCL to enhance early diagnosis. The timely initiation of chemotherapy is vital for improving survival rates and the overall quality of life for patients with PCL.
1 Introduction
Primary cardiac lymphoma (PCL) is a very rare lymphoma that primarily affects the heart and/or pericardium and causes cardiac symptoms due to myocardial infiltration. Early clinical diagnosis is challenging due to the lack of specificity in early clinical symptoms and ancillary testing, and the survival of PCL patients is restricted to a few months if not treated early and effectively. It is therefore critical to investigate the clinical aspects of PCL patients and develop more effective auxiliary diagnostics. This research presents one case of PCL and compares our findings to those described in the literature. To characterize the clinicopathological characteristics, management, and outcome of PCL patients at home and abroad, data from 121 affected individuals were analyzed retrospectively.
2 Materials and methods
2.1 Data retrieval and methodology
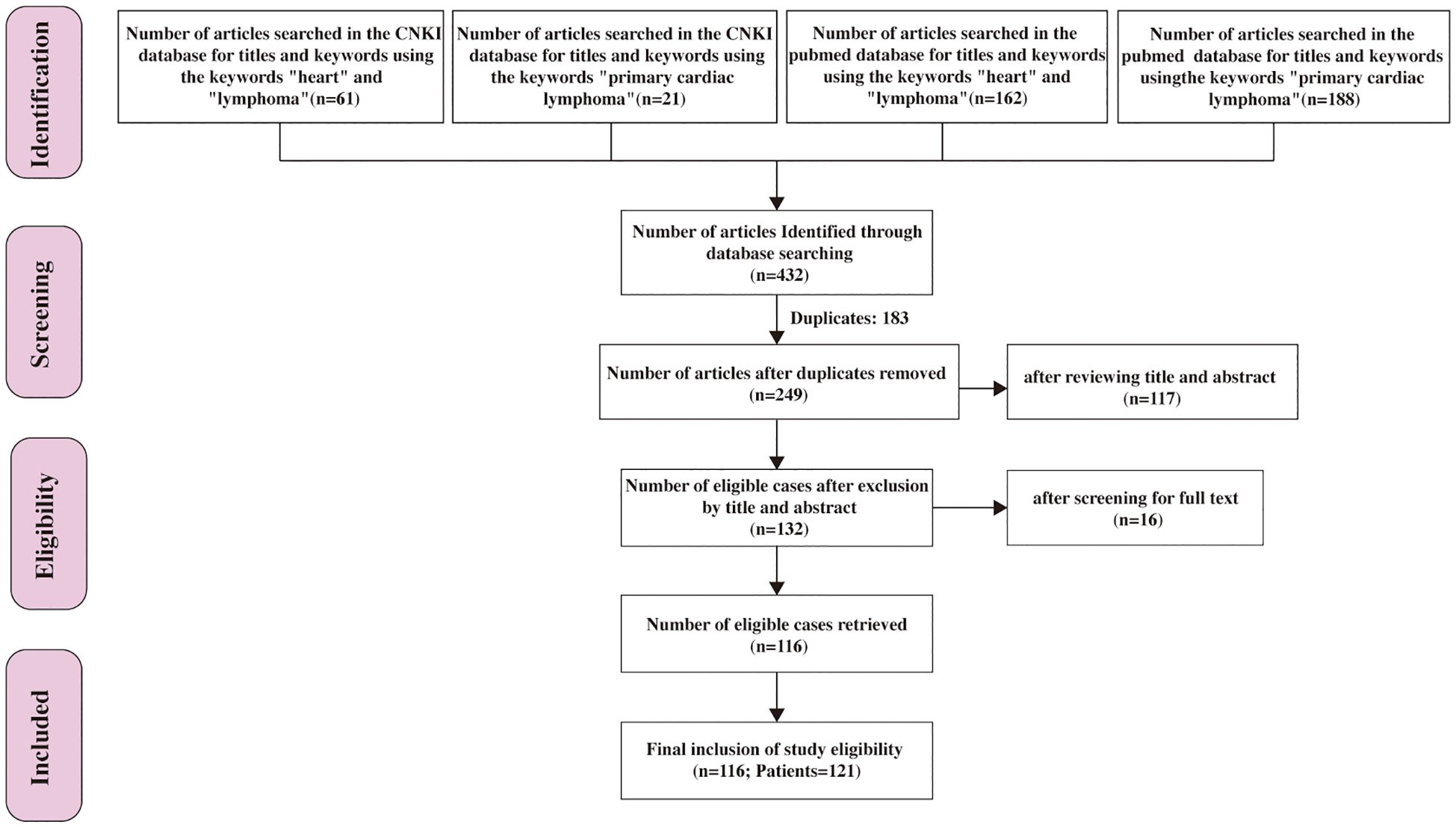
432 papers were retrieved through a search of the PubMed and Chinese National Knowledge Infrastructure (CNKI) database using the keywords “heart” and “lymphoma” or “primary cardiac lymphoma”. After excluding 183 duplicate entries, 249 articles published between January 1, 2014, and November 1, 2024, were identified. Following a title and abstract screening, 132 papers underwent full-text review. Ultimately, 116 papers were included in this systematic review, consisting of 121 PCL patients, as illustrated in Figure 1.
The inclusion criteria were as follows: (1) Diagnosis of PCL established according to the 2015 WHO Classification of Cardiac and Pericardial Tumors (1) under any of the following conditions: i) lymphoma originating in the heart or pericardium; ii) lymphoma presenting with cardiac-related symptoms at initial presentation; or iii) lymphoma primarily manifesting as a cardiac mass. The literature also defines PCL as lymphoma diagnosed in the heart and/or pericardium at initial diagnosis or presenting with cardiac symptoms due to myocardial infiltration by lymphoma, potentially with metastatic manifestations (2–4); (2) No age or gender restrictions were applied; (3) Absence of other serious comorbidities leading to event termination; and (4) In cases of repeated reports, only the earliest published cases were included. Based on the Lugano 2014 criteria for evaluating the efficacy of lymphoma treatment, therapeutic efficacy is assessed using specific standards for patients undergoing positron emission tomography-computed tomography (PET-CT) scans and those who do not. For patients undergoing PET-CT scans, the evaluation is based on the Deauville scoring system. A complete response (CR) is defined as a Deauville score of “1 to 3,” with or without a residual mass, while a partial response (PR) is characterized by a Deauville score of “4 or 5,” accompanied by a reduction in 18F-fluorodeoxyglucose (18F-FDG) uptake compared to baseline, with residual lesions of any size. The Deauville scoring criteria further specify that a score of 1 indicates complete disappearance of tumors, a score of 2 reflects 18F-FDG uptake in the lesion that is less than or equal to the mediastinal blood pool, and a score of 3 indicates 18F-FDG uptake in the lesion that is greater than the mediastinal blood pool but less than or equal to the liver blood pool. A score of 4 represents 18F-FDG uptake in any lesion that is mildly or moderately increased compared to the liver blood pool, while a score of 5 signifies 18F-FDG uptake in any lesion that is significantly increased compared to the liver blood pool (SUVmax > 2 times the liver blood pool) or the appearance of new lesions. For patients who do not undergo PET-CT scans, therapeutic efficacy is deemed effective if there is a reduction or disappearance of target lesions, alleviation of clinical symptoms, or echocardiographic evidence showing that patients with prior significant pericardial effusion exhibit no notable fluid accumulation following puncture and drainage. This comprehensive framework provides a standardized approach to evaluating therapeutic responses in lymphoma patients, ensuring consistency and objectivity in clinical assessments.
2.2 Statistical analysis
Statistical analysis was performed using SPSS version 27.0. Quantitative data were expressed as mean ± standard deviation or median ± interquartile range, while qualitative data were presented as case numbers and percentages.
3 Results
3.1 General information
A total of 121cases met the inclusion criteria. The study included 72 males (59.5%) and 49 females (40.5%), with a male-to-female ratio of 1.435:1. The ages of the patients ranged from 11 to 92 years, with a median age of 62.
3.2 Site of onset and clinical manifestations
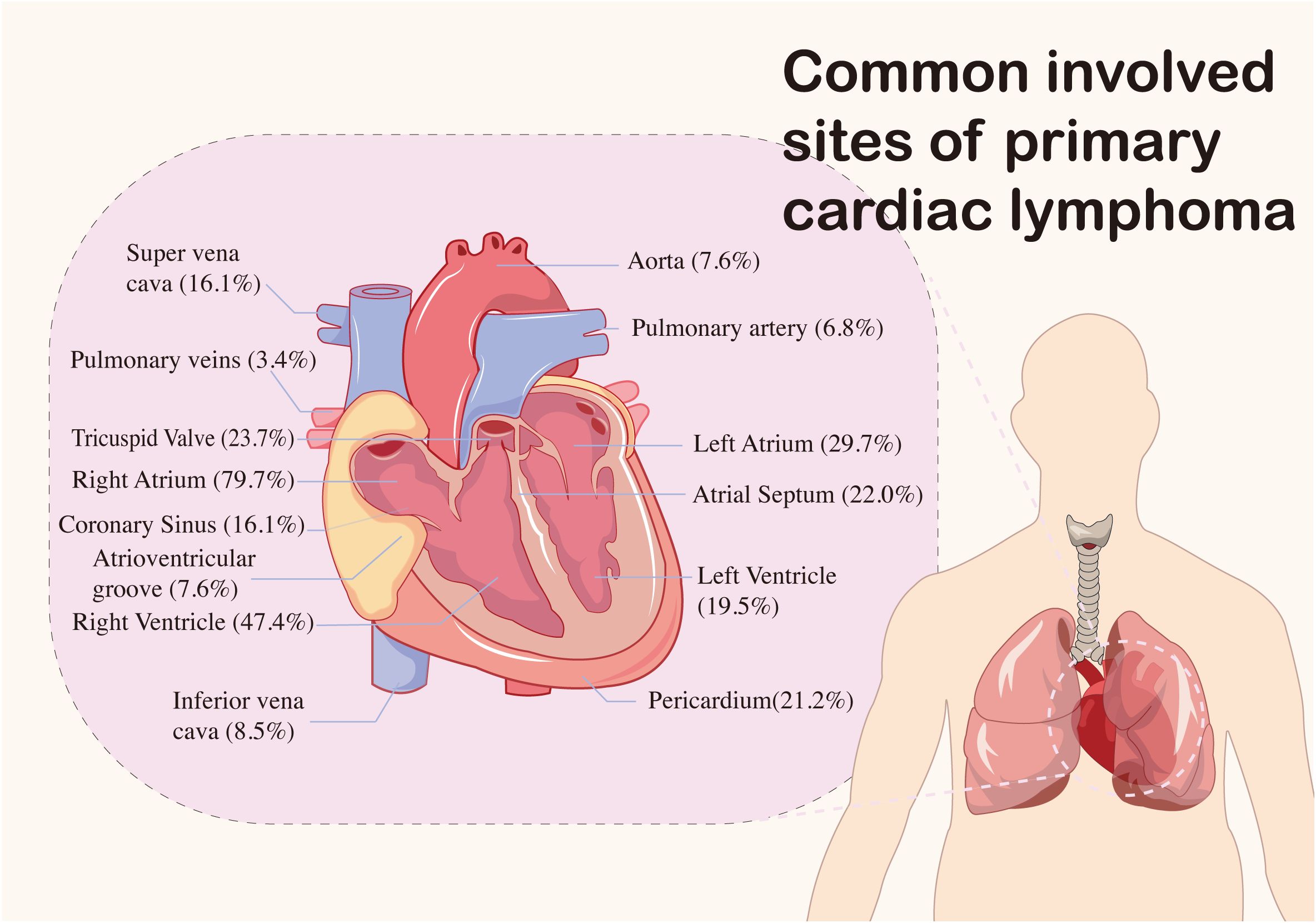
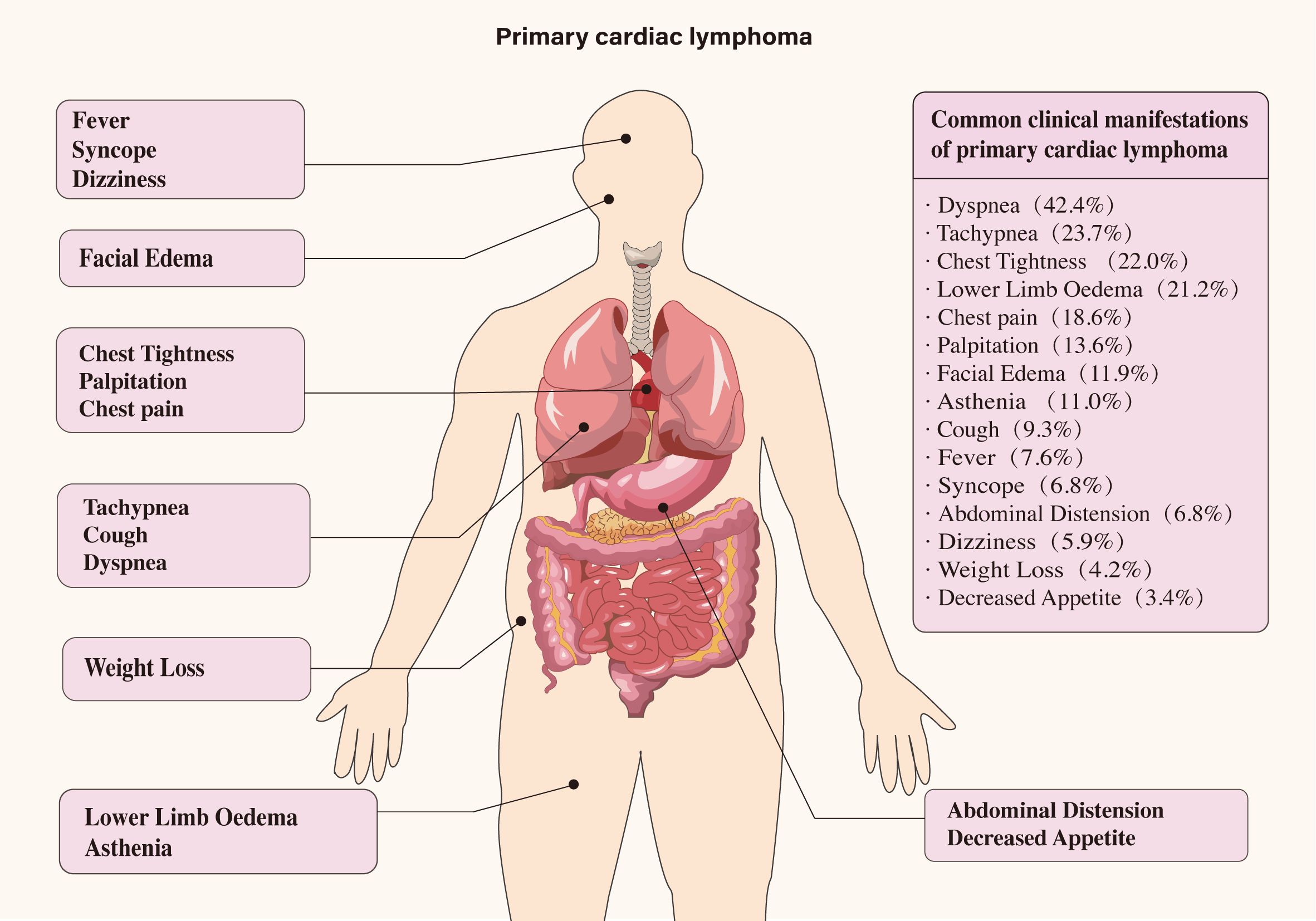
The primary sites of mass involvement were predominantly the right atrium and ventricle. Other affected areas included the left ventricle, left atrium, and pericardium (Figure 2). Clinical symptoms were non-specific and primarily circulatory and respiratory in nature, including dyspnea, chest tightness, chest pain, and tachypnea. Neurological and gastrointestinal symptoms were also observed (details provided in Figure 3). Pericardial effusion was noted in 52 patients (43.0%), while pleural effusion was observed in 10 patients (8.2%). Mass sizes varied, with the largest measuring 9.0 × 16.0 × 6.0 cm.
3.3 Ancillary tests and type of pathology
Imaging examinations, particularly cardiac color ultrasound, are important in the diagnosis and monitoring of disease progression. Cardiac ultrasound was conducted in 112 out of 121 cases (92.6%), supplemented by computed tomography (CT), magnetic resonance imaging (MRI), and PET-CT, among other modalities. Electrocardiograms and cytological analyses of pericardial and pleural effusions are also instrumental in the diagnostic process; however, pathological confirmation remains essential for the definitive diagnosis of cardiac lymphoma. Of the 112 ultrasound cases, 100 (89.3%) successfully identified the mass, with 37 (33.3%) reporting its size. Others can also clarify the size of the mass through chest CT scans and cardiac MRI. Pericardial effusion was detected in 17 cases (48.6%). Additional ultrasound findings included delineation of mass boundaries, mobility, extent of encroachment, and potential obstruction of valve and vena cava orifices.
Electrocardiographic abnormalities were observed in 44 patients, including atrioventricular block in 19 patients (50%), atrial flutter or atrial fibrillation in 13 patients (29.5%), and complete right bundle branch block in 3 patients (6.8%). Other notable findings included atrial tachycardia, Partial ST-T changes, and escape rhythm.
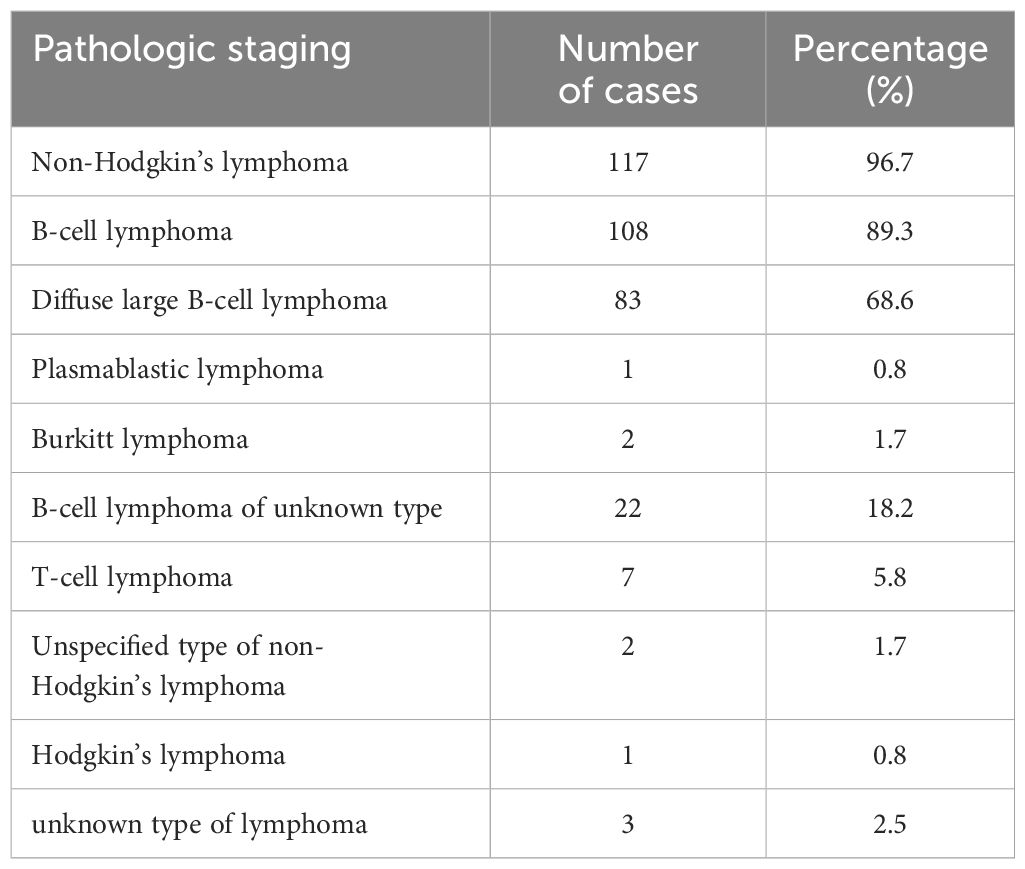
Pathology confirmed the diagnosis in all 121 patients. 54 cases (44.6%) underwent open chest tumor resection biopsy; 42 cases (34.7%) underwent percutaneous biopsy, including punctures of the mass, lymph node, endocardium, and myocardium. Notably, 6 cases (5.0%) were definitively diagnosed through autopsy; 4 cases (3.4%) were diagnosed via biopsy of metastatic lesions; and 9 cases (7.4%) were assessed diagnostically through pericardial effusion. Pathologically, all 117 cases were classified as non-Hodgkin’s lymphoma; of these, 108 cases (92.3%) were identified as B-cell lymphoma. This category included 83 cases of diffuse large B-cell lymphoma (DLBCL), 2 cases of Burkitt’s lymphoma, a case of plasmablastic lymphoma and 22 cases of unspecified B-cell lymphoma type (Table 1).
3.4 Treatment and prognosis
In terms of treatment and prognosis, 54 patients (44.6%) underwent cardiotomy under general anesthesia, while 91 patients (75.2%) received chemotherapy. Among those who received chemotherapy, 78 patients (85.7%) were treated with R-CHOP, E-CHOP, CHOP, R-COP, COP or HOP regimens (R: Rituximab, C: Cyclophosphamide, H: Doxorubicin, O: Vincristine P: Prednisone, E: Etoposide); the specific regimen for 12 patients was not reported. Of the patients who underwent chemotherapy, 74 (80.4%) exhibited effective responses, while 6 (6.5%) had poor outcomes, and 11 (12.1%) outcome was not specified. Additional treatments included heart transplantation in situ, radiotherapy, pleural adhesion release, pacemaker implantation, superior vena cava evacuation, pericardial drainage, blood transfusion, anti-infection measures, and other symptomatic interventions (Table 2).
Regarding patient prognosis, only patients with available prognostic information (excluding those who received ASCT) were included in this analysis, totaling 59 cases. A total of 8 patients (13.3%) did not undergo any treatment, with a median survival of only 1 week; 5 patients (8.3%) received tumor resection only, of whom 2 died within 30 days, with a 30-day mortality rate of 40%; 22 patients (36.7%) received chemotherapy only, of whom 4 (18.2%) died, with a median survival time of 6 months; 18 patients survived, with a median follow-up time of 7 months by the date of follow-up; 24 patients (40.0%) received both tumor resection and chemotherapy, of whom 7 (29.2%) died, with a median survival time of 1 month; 17 patients survived, with a median follow-up time of 11 months by the date of follow-up.
4 Case report
A 73-year-old female patient was admitted to the hospital on November 6, 2020, reporting chest tightness after physical activity, which had persisted for over a month. The patient had a four-year history of thrombocytosis, which was managed with oral hydroxyurea. Upon examination, her vital signs were as follows: temperature 36.1°C, heart rate 110 beats/min, respiratory rate 12 breaths/min, blood pressure 94/60 mmHg, peripheral capillary oxygen saturation (SpO2) 98%. The patient was conscious and exhibited steady breathing and responsiveness, and had an Eastern Collaborative Oncology Group performance status of 1. The physical examination showed no significant abnormalities, except for coarse breath sounds detected in both lungs.
Initial laboratory tests indicated leukocyte levels at 11.90×109/L, hemoglobin at 135.0 g/L, neutrophils at 7.78×109/L, platelets at 265×109/L, and a monocyte percentage of 18.4%. C-reactive protein was measured at 18.3 mg/L, lactate dehydrogenase at 360.0 U/L, while liver and kidney function tests, coagulation parameters, and electrolyte levels remained within normal ranges. Cardiac color ultrasound revealed enlargement of both the right atrium and ventricle, with the right ventricular end-diastolic transverse diameter measuring 42 mm. A hyperechoic mass, approximately 56 mm × 53 mm, was identified in the right atrium, indicating a possible mucinous tumor. This mass partially shifted toward the right ventricular side through the tricuspid valve during diastole. This movement resulted in accelerated blood flow at the tricuspid opening. Additionally, a small amount of pericardial effusion was observed (Figure 4A). A follow-up ultrasound on November 11, 2020, demonstrated an increase in the size of the hypoechoic mass in the right atrium, which now measured approximately 75 mm × 50 mm (Figure 4B). This mass, attached to the atrial septum, exhibited increased mobility and intermittently obstructed the tricuspid valve orifice during cardiac cycles. The patient’s family, considering their financial situation, did not proceed with PET-CT for tumor staging assessment.
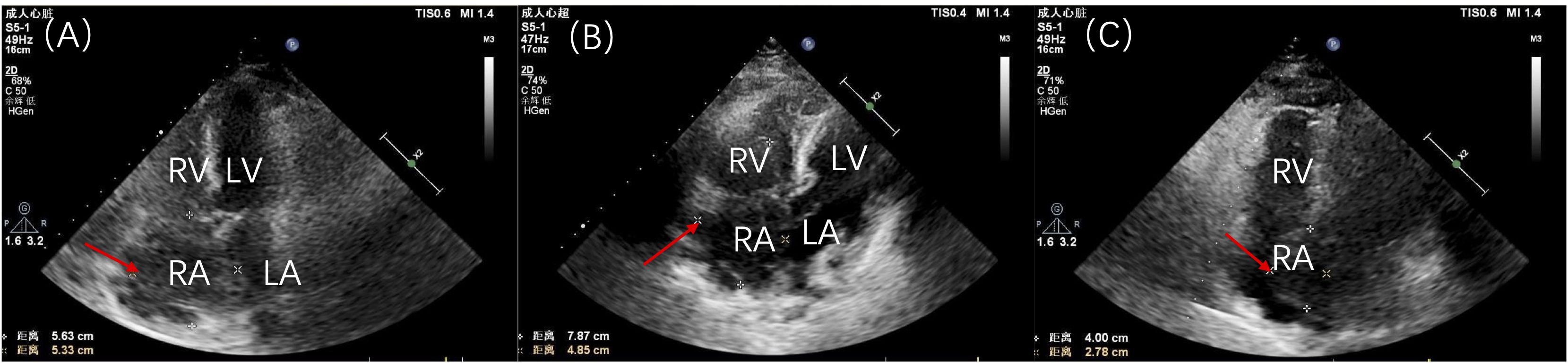
Figure 4. Patient’s cardiac ultrasound findings. (A) Initial cardiac ultrasound demonstrating a large tumor occupying nearly the entire right atrium. (B) Preoperative cardiac ultrasound review indicating an increase in size of the hypoechoic mass in the right atrium, now measuring approximately 75 mm × 50 mm and attached to the atrial septum. (C) Postoperative cardiac ultrasound revealing a hypoechoic mass in the right atrium, measuring approximately 40 mm × 28 mm. RV, right ventricle; RA, right atrium; LV, left ventricle; LA, left atrium.
The patient underwent resection of a cardiac tumor under general anesthesia on November 16. Intraoperative findings revealed multiple cauliflower-shaped masses in the right atrium, varying in size, with the largest measuring approximately 8 cm × 4.5 cm, extending to the tricuspid valve and interatrial septum. A palliative resection of the tumor was performed. Postoperative pathology identified the masses as diffuse large B-cell lymphoma (non-germinal center origin), confirmed through immunohistochemical profiling: CD3 (-), CD20 (+), CD21 (-), Ki67-MIB1 (70%), CD30 (few +), Bcl-2 (+), CK (-), CD10 (-), Bcl-6 (+), MUM-1 (+), c-myc (50%), P53 (few +), Pax-5 (+), Cyclin D1 (-), CD5 (-), and negative EBER in situ hybridization (Figure 5).
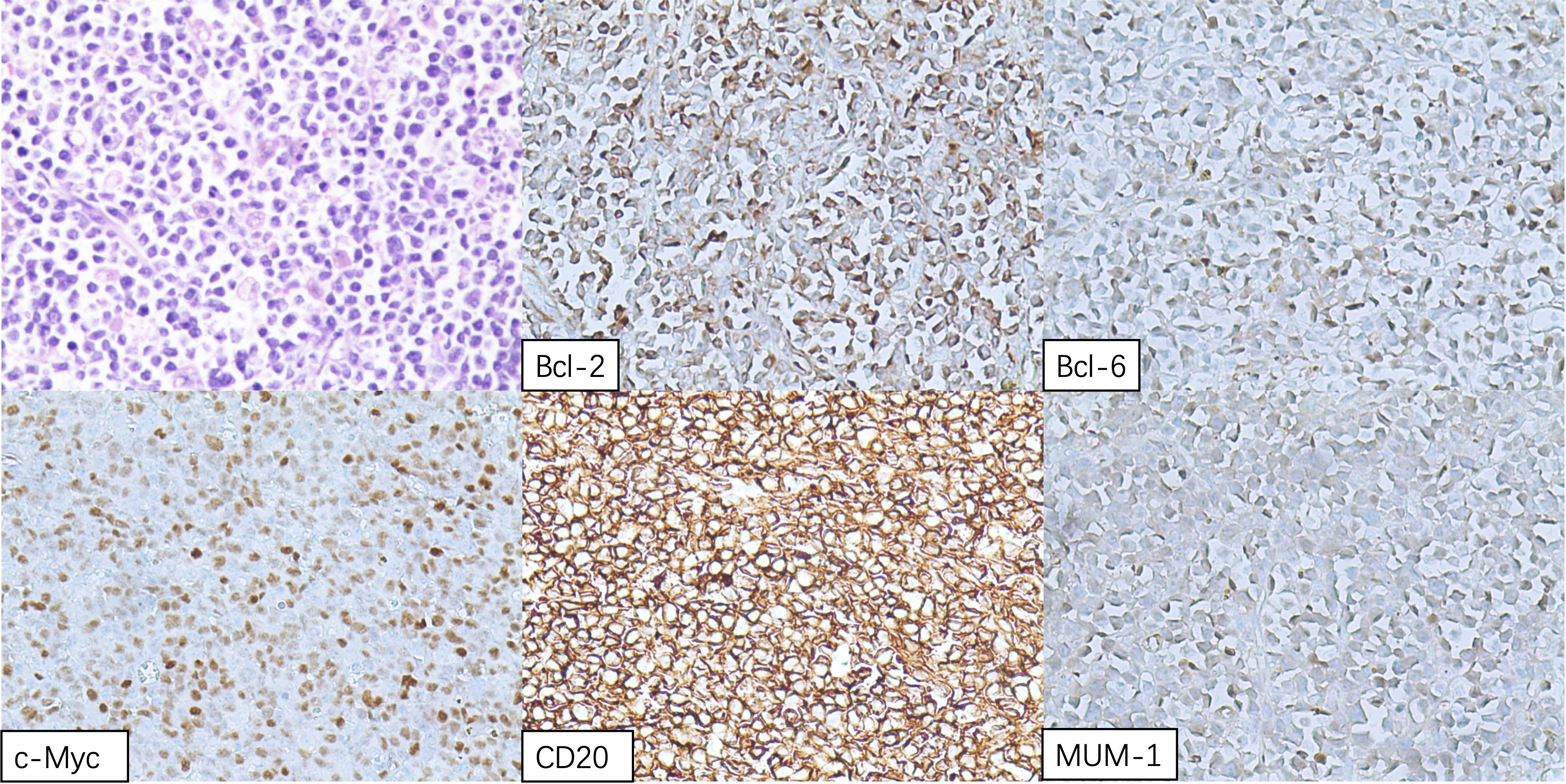
Figure 5. Hematoxylin and eosin (H&E) staining of the right atrial mass displaying sheets of lymphomatous large cells.
The final diagnosis was cardiac diffuse large B-cell lymphoma (non-germinal center origin), according to the 2015 World Health Organization (WHO) Classification of Cardiac and Pericardial Tumors (1). Considering the patient’s age, the hematology department recommended low-dose chemotherapy. However, her family, taking into account the patient’s age and their own financial situation, decided to refuse chemotherapy. The postoperative cardiac ultrasound revealed findings consistent with changes typically observed in right and left atrial occupying lesions. A hypoechoic mass, measuring approximately 40 mm × 28 mm, was present in the right atrium, extending from the base to the upper part of the interatrial septum, with a protrusion into the left atrium measuring approximately 22 mm × 16 mm (Figure 4C). After recovery, the patient was discharged on November 20, 2020. Unfortunately, she passed away on April 20, 2021, without seeking further medical consultations during this period.
This case aligns with several characteristics of PCL described in the existing literature, including its demographic predilections, tumor size, common sites of involvement, and histological subtypes. The patient’s presenting symptoms of chest discomfort and exertional dyspnea are consistent with the commonly reported symptoms of PCL, such as chest pain and dyspnea. Moreover, the brief interval between surgical intervention and the patient’s demise highlights the highly aggressive nature, rapid progression, and high mortality rate associated with PCL. This also underscores the limitations of palliative surgical resection as a standalone treatment strategy. However, this case exhibits unique clinical features that may be influenced by individual factors and disparities in access to medical resources. Notably, the patient had a four-year history of primary thrombocythemia and was undergoing treatment with hydroxyurea, an unusual finding among PCL patients. Additionally, the patient demonstrated an exceptionally rapid tumor growth rate, as evidenced by a marked increase in tumor volume between the initial echocardiogram and subsequent follow-up examinations. This suggests a more aggressive disease course than what is typically observed in PCL cases. These distinct features warrant further investigation to better understand their implications for disease progression and management.
5 Discussion
PCL is an exceedingly rare malignancy, predominantly presenting as cardiac and/or pericardial tumor tissue or as myocardial infiltration of lymphomas, which results in cardiac symptoms. Representing only 1% of primary cardiac malignancies and 0.5% of all extranodal lymphomas (121). This article summarizes the clinical presentation, ancillary investigations, diagnosis, treatment and prognosis from a decade of case reports and describes a particular PCL patient.
As the second most prevalent primary cardiac malignancy, PCL has a dire prognosis without treatment, often limited to just a few months. Therefore, prompt and accurate diagnosis and treatment are crucial. However, the non-specific clinical manifestations and auxiliary examinations present challenges for early diagnosis. Clinically, the approach to pathological biopsy should evolve from non-invasive to invasive methods to achieve efficient and cost-effective diagnosis.
The incidence of B symptoms in patients with PCL is notably low. Instead, the condition commonly presents with symptoms such as shortness of breath, chest tightness, and lower limb edema. These may occasionally be accompanied by additional symptoms, including loss of appetite, anxiety, and chest pain, further contributing to the clinical complexity of PCL. Besides, it can cause a variety of arrhythmias, with atrial fibrillation, atrial flutter, and third-degree atrioventricular block being the most common. Lesions are predominantly located in the cardiac and pericardial regions, with the right atrium being the most commonly involved site. This finding aligns with our study, where 86.1% of cases involved the right atrium. Notably, 8 patients (6.6%) presented to the hospital with syncope, which was associated with a poor prognosis. Among these, 2 patients experienced disease progression, and 3 succumbed to the condition within 4 months. These findings underscore the importance of considering this disease in the differential diagnosis when evaluating comatose patients. Clinicians are advised to maintain a high index of suspicion to ensure timely identification and management. Overall, the symptoms of PCL are subtle at first, becoming more pronounced as the lymphoma progresses, often leading to late-stage diagnoses.
The need for new diagnostic methods is paramount, even as the accuracy of existing imaging examinations improves. Commonly utilized clinical auxiliary examinations include electrocardiography (ECG), transthoracic echocardiography (TTE), transesophageal echocardiography (TEE), cardiac computed tomography (CT), cardiac magnetic resonance (CMR) and FDG-PET. Echocardiography, as an adjunctive test for the initial diagnosis of cardiac tumors, is able to dynamically observe the location, size, morphology, mobility of cardiac tumors and their relationship with the surrounding tissues, to find the presence of hypoechoic masses and to detect associated pericardial effusions. TTE is not as sensitive as TEE in identifying cardiac tumors, and a retrospective study by Ceresoli (2) et al. demonstrated that TTE detected a cardiac tumors, whereas TEE provides better visualization of cardiac structures, especially those away from the chest wall (63). Therefore, we prefer TEE for the initial diagnosis of cardiac tumors. The use of CT and CMR imaging in the diagnosis of PCL has been increasing steadily in recent years. Asadian (122) et al. have highlighted that these imaging modalities, with their ability to employ various parameter settings and provide excellent soft tissue contrast, are valuable tools for characterizing PCL. They allow for detailed assessment of tumor characteristics, differentiation between benign and malignant lesions, and evaluation with or without contrast enhancement. This capability is particularly beneficial in distinguishing PCL from cardiac thrombus, thereby aiding in the differential diagnosis. Furthermore, PET-CT offers a non-invasive approach to assess the metabolic activity of tumors using fluorodeoxyglucose (FDG). Studies have demonstrated that PCL exhibits higher standardized uptake values (SUVs) and larger metabolic tumor volumes on PET imaging compared to primary cardiac sarcoma (PCS) (123). Additionally, PET-CT provides a clearer delineation of tumor invasion, facilitating safer biopsy procedures and guiding subsequent surgical interventions.
The treatment options for PCL include surgical resection, chemotherapy, radiotherapy, and hematopoietic stem cell transplantation, etc. Chemotherapy plays a central role in the treatment of PCL, especially the R-CHOP regimen, which has been widely utilized since 2010. This regimen has notably enhanced the progression-free survival of patients with non-Hodgkin’s B-cell lymphoma (the most common type of PCL). In our study, 44.6% of patients underwent tumor removal surgery, while 76.0% opted for chemotherapy, and only 32.2% combined these approaches. An analysis conducted by Yin et al. (124) using the SEER database revealed that surgical intervention did not improve survival outcomes in patients with PCL, with chemotherapy identified as the sole effective treatment modality (65, 125, 126). However, our study demonstrated that patients who underwent combined surgical resection and chemotherapy exhibited superior survival outcomes compared to those who received surgery or chemotherapy alone. Moreover, for hemodynamically unstable patients, surgical intervention remains a critical and urgent treatment to stabilize their condition (127). Emerging therapeutic options, such as autologous hematopoietic stem cell transplantation (auto-HSCT), allogeneic hematopoietic stem cell transplantation (allo-HSCT), and molecularly targeted therapeutic agents, are showing promise in improving survival rates and prognosis for PCL patients. In parallel, supportive care plays a vital role in the comprehensive management of PCL. This includes symptomatic treatment, nutritional support, palliative care, and psychological counseling. For instance, while the Fontan procedure does not directly treat PCL, it enhances cardiac function, improves the patient’s quality of life, and increases the likelihood of long-term survival, thereby facilitating opportunities for subsequent follow-up treatments.
The prognosis for PCL is generally unfavorable. According to statistics from the SEER database, which included 184 cases of PCL, the 1-year, 3-year, and 5-year survival rates were 59%, 41%, and 34% (128), respectively. More than half of the patients had an overall survival (OS) of less than 3 years, or even shorter. Although there is no uniform conclusion on the treatment of PCL at home and abroad, the R-CHOP regimen is still the most important treatment for PCL because of its remarkable efficacy in B-cell lymphoma. In the case presented, a large, mobile PCL mass that obstructed the tricuspid valve and extended into the left atrium was surgically removed. However, the absence of postoperative chemotherapy led to a poor prognosis, and the patient unfortunately passed away within two months after being discharged from the hospital. This underscores the significance of chemotherapy in the treatment process.
In conclusion, it is crucial to consider cardiac lymphoma as a differential diagnosis when patients present with unexplained cardiac abnormalities, such as heart failure, atrial fibrillation, pericardial effusion, or superior vena cava syndrome, particularly if cardiac ultrasound identifies an intracardiac mass accompanied by unexplained fever. Advancements in imaging modalities, including cardiac CT, CMR, and PET-CT, play a pivotal role in characterizing the mass, assessing its benign or malignant nature, and determining the disease stage. These tools not only guide subsequent biopsy and potential surgical intervention but also enable dynamic adjustments to diagnostic and therapeutic strategies, thereby minimizing the risk of misdiagnosis or delayed diagnosis. Once a diagnosis of cardiac lymphoma is confirmed, individualized clinical judgment is essential to evaluate the need for surgical tumor resection to alleviate cardiac dysfunction. This approach, combined with chemotherapy, can significantly enhance treatment efficacy and improve patient outcomes.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Ethics Review Committee of biomedical research of Linyi People’s Hospital (No. 202410-H-007). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
SZ: Conceptualization, Validation, Visualization, Writing – original draft, Writing – review & editing. LC: Formal analysis, Investigation, Writing – original draft, Writing – review & editing. XF: Data curation, Methodology, Writing – original draft. WH: Data curation, Methodology, Writing – original draft. ZY: Resources, Writing – review & editing. YZ: Funding acquisition, Project administration, Resources, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Shandong Provincial Postdoctoral Innovation Project (201903077); Xuzhou Medical University Affiliated Hospital Development Fund Project (XYFM20200031); Shandong Provincial Natural Science Foundation (ZR2018PH014); Linyi People’s Hospital Doctoral Fund Project (2016LYBS13); Shandong Key Laboratory on Hematoimmunology Open Project (2019XYKF009).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Burke A, Tavora F. The 2015 WHO classification of tumors of the heart and pericardium. J Thorac Oncol. (2016) 11:441–52. doi: 10.1016/j.jtho.2015.11.009
2. Ceresoli GL, Ferreri AJ, Bucci E, Ripa C, Ponzoni M, Villa E. Primary cardiac lymphoma in immunocompetent patients: diagnostic and therapeutic management. Cancer. (1997) 80:1497–506. doi: 10.1002/(sici)1097-0142(19971015)80:8<1497::aid-cncr18>3.0.co;2-0
3. Gelman KM, Ben-Ezra JM, Steinschneider M, Dutcher JP, Keefe DL, Factor SM. Lymphoma with primary cardiac manifestations. Am Heart J. (1986) 111:808–11. doi: 10.1016/0002-8703(86)90129-8
4. Somers K, Lothe F. Primary lymphosarcoma of the heart.Review of the literature and report of 3 cases. Cancer. (1960) 13:449–57. doi: 10.1002/1097-0142(196005/06)13:3<449::AID-CNCR2820130306>3.0.CO;2-V
5. Zhang Y, Yang QQ, Yong WW, Du HP. Primary cardiac diffuse large B lymphoma: a case report and review of the literature. Modern Pract Med. (2024) 36:266–7. doi: 10.3969/j.issn.1671-0800.2024.02.036
6. Liu T, Tian PY, Tang LK, Xiang YL, Liu SY, Peng Y, et al. Primary cardiac lymphoma involving brain tissue: a case report and review of the literature. Sichuan Med J. (2024) 45:688–90. doi: 10.16252/j.cnki.issn1004-0501-2024.06.023
7. Wu XQ, Feng YL, Zang TY, Li CP, Li R. Diffuse large B-cell lymphoma of the heart:one case report. J Med Imaging. (2023) 33:1937–8.
8. Li J, Sun JH. Echocardiographic manifestations of diffuse large B-cell lymphoma with cardiac involvement:a case report. J Clin Ultrasound Med. (2023) 25:77–8. doi: 10.16245/j.cnki.issn1008-6978.2023.01.008
9. Feng X, He P, Chen J, Feng GL, Li B, Du Y. A case of primary cardiac lymphoma with extracardiac mass type MRI presentation. Chin J Med Imaging. (2023) 31:34–5. doi: 10.3969/j.issn.1005-5185.2023.01.009
10. Sun Y, Xu H, Guo ZQ, Ren XC, Li XD, Zhao HG. PRIMARY PERICARDIAL LYMPHOMA:A CASE REPORT AND LITERATURE REVIEW. J Qingdao University(Medical Sciences). (2022) 58:150–2. doi: 10.11712/jms.2096-5532.2022.58.021
11. Zhong ZQ, Guo FF, Lan QH, Liu X, Zhan T, Tang LM, et al. A case of primary cardiac lymphoma. J Modern Oncol. (2022) 30:701–3. doi: 10.3969/j.issn.1672-4992.2022.04.029
12. Zhai XJ, Gao YL, Zhang H. Imaging showing of primary cardiac lymphoma in one case. Chin J Evidence-Based Cardiovasc Med. (2021) 13:1395. doi: 10.3969/j.issn.1674-4055.2021.11.30
13. Yu XX, Chen Z, Song LJ. Primary cardiac B cell lymphoma:Case report. Chin J Med Imaging Technology. (2021) 37:1149. doi: 10.13929/j.issn.1003-3289.2021.08.007
14. Huang L, Teng Y, Jiang XM, Li WY. Comprehensive diagnosis and treatment of lymphoma with cardiac neoplasm and massive ascites. J Evidence-Based Med. (2021) 21:189–92. doi: 10.12019/j.issn.1671-5144.2021.03.011
15. Li L, Wang Z, You CY. The relapse of primary cardiac high-grade B-cell lymphoma after remission: a case report. China Oncol. (2021) 31:156–60. doi: 10.19401/j.cnki.1007-3639.2021.02.011
16. Yuan FJ, Wang XW, Ding WW, Liu Q, Meng H, Wu N. A case of diffuse large B-cell lymphoma of the heart. Chin J Clin Exp Pathology. (2020) 36:1500–1. doi: 10.13315/j.cnki.cjcep.2020.12.036
17. Guo HL, Xi X, Zhou H. A case of primary cardiac lymphoma combined with third degree atrioventricular block. J Pract Electrocardiology. (2019) 28:214–6. doi: 10.13308/j.issn.2095-9354.2019.03.014
18. Wang LQ, Ma YQ, Li J, Sun L, Song X, Guo AT. Clinicopathological analysis of four cases of cardiac lymphoma. Chin J Diagn Pathology. (2019) 26:793–6. doi: 10.3969/j.issn.1007-8096.2019.12.001
19. Li YT, Zhang R, Sun DM, Ma L. A case of primary cardiac lymphoma encroaching on both atria diagnosed by echocardiography. Chin J Med Imaging. (2018) 26:596. doi: 10.3969/j.issn.1005-5185.2018.08.009
20. Wang YB, Shi CY, Li YH. One case of primary cardiac lymphoma and literature review. J Clin Hematology. (2017) 30:881–3. doi: 10.13201/j.issn.1004-2806.2017.11.018
21. Wu XQ, Wei WL. Primary cardiac lymphoma:a case report and review of the literature. Chin J Cardiovasc Res. (2017) 15:93–4. doi: 10.3969/j.issn.1672-5301.2017.01.026
22. Yang GX, Peng GH. Ultrasonographic features of primary cardiac lymphoma:Case report. Chin J Med Imaging Technology. (2017) 33:123. doi: 10.13929/j.1003-3289.201607127
23. Meng ML, Zhang JD, He CM, Yuan DM, Zhong MX. A case of cardiac lymphoma with literature review. Pract J Cancer. (2016) 31:2103–4. doi: 10.3969/j.issn.1001-5930.2016.12.060
24. Zhu Q, He CY, Chen D, Wu Y, Fang W, Shang JF, et al. A case of primary cardiac lymphoma and review of the literature. J Cardiovasc Pulmonary Diseases. (2016) 35:890–1. doi: 10.3969/j.issn.1007-5062.2016.11.010
25. Yin J, Hua SH, Li LJ. Primary cardiac lymphoma:Case report. Chin J Med Imaging Technology. (2016) 32:880. doi: 10.13929/j.1003-3289.2016.06.016
26. Wang LL, Hu HX, Zhao MZ, Luo C, He F, Li G, et al. A case of primary cardiac diffuse large B-cell lymphoma. In: Compilation of Papers from the Summit forum on Hematopoietic Stem Cell Transplantation and Cellular Immunotherapy, the 2016 Zhejiang Provincial Hematology Annual Conference and the Progress Study Class on Diagnosis and Treatment of Hematological Diseases. Zhejiang, China: Hematology Branch of Zhejiang Medical Association (2016). p 1.
27. Jiang WJ, Liu L, Zhang F. A case of primary cardiac lymphoma misdiagnosed as mucinous tumor. Chin J Ultrasound Med. (2016) 32:258–9. doi: 10.3969/j.issn.1002-0101.2016.03.023
28. Feng ZW, Jiang GJ, Feng JC. A case of primary cardiac lymphoma. J Pract Med. (2015) 31:2234. doi: 10.3969/j.issn.1006-5725.2015.13.056
30. Zhan BC, Liu J. A case of primary cardiac lymphoma. Chin J Clin Thorac Cardiovasc Surgery. (2014) 21:806. doi: 10.7507/1007-4848.20140231
31. Cao GP, Deng XY, Wang HS. primary cardiac lymphoma:a case report. J Clin Radiology. (2014) 33:152–3. doi: 10.13437/j.cnki.jcr.2014.01.040
32. Rezvani A, Shah S. Treatment of primary cardiac diffuse large B-cell lymphoma involving the coronary sinus with R-EPOCH: a case report and literature review. Ann Hematol. (2024) 103:2557–60. doi: 10.1007/s00277-024-05793-x
33. Drewniowska J, Cugier O, Garus M, Sokolska J, Garus B, Sokolski M. The role of multimodal imaging in diffuse large B-cell lymphoma with primary cardiac involvement. Pol Arch Intern Med. (2024) 134:16796. doi: 10.20452/pamw.16796
34. Martinho M, BroChado L, Ferreira BM, Guimarães S, Almeida AR, Pereira H. Primary diffuse large B-cell lymphoma of the heart: A rare case of heart failure. Cardiovasc Pathol. (2024) 72:107664. doi: 10.1016/j.carpath.2024.107664
35. Alattar K, Dodhia S, Huang CY, Neves JB, Haris A. Intracardiac diffuse large B-cell lymphoma: an unexpected diagnosis. BMJ Case Rep. (2024) 17:e259242. doi: 10.1136/bcr-2023-259242
36. Farhoud N, Farhoud H, Deutsch JM. Primary cardiac lymphoma in a young and immunocompetent patient diagnosed by percutaneous transvenous biopsy. Am J Cardiol. (2023) 201:139–41. doi: 10.1016/j.amjcard.2023.05.067
37. Arun Kumar S, Mishra R, Malempati SC, Bindal P. Primary cardiac large B cell lymphoma. BMJ Case Rep. (2023) 16:e256167. doi: 10.1136/bcr-2023-256167
38. Vogl TJ, Martin SS, Koch V, Scholtz JE, Booz C, Leistner DM, et al. Letter to the editor: CT guided biopsy of a right ventricle primary cardiac lymphoma-A case report. Cardiovasc Intervent Radiol. (2023) 46:970–2. doi: 10.1007/s00270-023-03482-2
39. Salas-Llamas J, Mendez-Ruiz A, Jimenez-Jimenez M, Fuentes-Mendez I, Ramirez-Godinez F. Primary cardiac triple-hit lymphoma. Int Heart J. (2022) 63:411–5. doi: 10.1536/ihj.21-513
40. Nakata A, Takamatsu H, Eguchi Y, Izumida T, Aburadani I, Hirota S, et al. Primary cardiac lymphoma arising from both sides of the heart. Intern Med. (2022) 61:3377–81. doi: 10.2169/internalmedicine.8936-21
41. Chen P, Hao Y, Qiu X, Xiao X, Zhu W, Xu Y, et al. Case report: primary cardiac T-cell lymphoma with complete atrio-ventricular block diagnosed by endomyocardial biopsy. Front Immunol. (2022) 13:890059. doi: 10.3389/fimmu.2022.890059
42. Aksu U, Korucu C, Atilgan K, Aybek T, Gökaslan ÇÖ, Aksu D, et al. A rare cause of dyspnea: Primary large B cell lymphoma causes giant cardiac mass. Echocardiography. (2022) 39:1370–2. doi: 10.1111/echo.15459
43. Amin A, Chitsazan M, Ghavidel AA, Pouraliakbar H, Parsaee M, Khesali H. A rare cause of complete heart block in an adult: Primary cardiac lymphoma. Asian Cardiovasc Thorac Ann. (2022) 30:321–4. doi: 10.1177/02184923211001697
44. Chen K, Chang L, Chen JZ, Wei X, Guo GJ, Lu JR, et al. A case of primary cardiac lymphoma diagnosed by intravenous right atrial catheter forceps biopsy. Zhonghua Xin Xue Guan Bing Za Zhi. (2022) 50:1105–7. doi: 10.3760/cma.j.cn112148-20220303-00143
45. Rector G, Koh SJ, Tabbaa R. A case of isolated cardiac burkitt lymphoma causing right-sided heart failure. Tex Heart Inst J. (2022) 49:e217575. doi: 10.14503/THIJ-21-7575
46. Hu B, Zhao J, Liang X, Ren C, Li N, Liang C. A case of complete atrioventricular block associated with primary cardiac lymphoma reversed without cardiac pacemaker implantation. J Int Med Res. (2022) 50:3000605221089780. doi: 10.1177/03000605221089780
47. Fukui T, Ogasawara N, Hasegawa S. Unique autopsy case of primary cardiac lymphoma. BMJ Case Rep. (2021) 14:e242174. doi: 10.1136/bcr-2021-242174
48. Taguchi T, Fukushima S, Yajima S, Saito T, Kawamoto N, Tadokoro N, et al. Successful surgical resection and reconstruction for a huge primary cardiac lymphoma filling the right heart. J Card Surg. (2021) 36:342–4. doi: 10.1111/jocs.15152
49. Rubalcava Lara LF, Aviles-Salas A, Candelaria M. Primary myocardial diffuse large B cell lymphoma. Report of one case. Rev Med Chil. (2021) 149:1231–5. doi: 10.4067/s0034-98872021000801231
50. Miyawaki M, Aoyama R, Ishikawa J, Harada K. Primary cardiac B cell lymphoma in an immunocompetent patient. BMJ Case Rep. (2021) 14:e243068. doi: 10.1136/bcr-2021-243068
51. Wang L, Cai L, Chen X, Zheng Z. Paroxysmal supraventricular tachycardia as a major clinical presentation of the primary coronary sinus lymphoma: A case report. Med (Baltimore). (2021) 100:e24225. doi: 10.1097/MD.0000000000024225
52. Maeda D, Kanzaki Y, Sohmiya K, Hoshiga M. A case of primary cardiac lymphoma: Difficult to diagnose. J Nucl Cardiol. (2021) 28:1186–8. doi: 10.1007/s12350-020-02089-1
53. Kil WJ. Shape of the heart: A 68-year-old man with a primary cardiac non-hodgkin lymphoma. Int J Radiat Oncol Biol Phys. (2020) 106:460–1. doi: 10.1016/j.ijrobp.2018.12.024
54. Sromicki J, Rodriguez H, Reser D, Van Hemelrijck M, Maisano F, Mestres CA. Primary cardiac lymphomas may present under different phenotypes. Asian Cardiovasc Thorac Ann. (2020) 28:168–71. doi: 10.1177/0218492319900597
55. Fukuzaki H, Fukuda K, Sato A, Shiozaki M, Kubota N, Tamura H, et al. Primary cardiac diffuse large B-cell lymphoma promptly and safely diagnosed with pericardial effusion cytology. Intern Med. (2020) 59:1997–2001. doi: 10.2169/internalmedicine.4716-20
56. Matsunaga K, Kobayashi T, Takahashi M, Gohra H. Huge primary cardiac Malignant lymphoma in the left ventricle. Ann Thorac Surg. (2020) 110:e115–8. doi: 10.1016/j.athoracsur.2019.12.052
57. Zoma JM, Aggarwal S, Gabali A, Kobayashi D. A rare case of primary cardiac Hodgkin lymphoma in a child. Cardiol Young. (2020) 30:866–8. doi: 10.1017/S1047951120001183
58. Cichowska-Cwalińska N, Dutka M, Klapkowski A, Pęksa R, Maciej Zaucha J, Zaucha R. The role of radiotherapy in the management of primary cardiac lymphoma a case report and the literature review. Leuk Lymphoma. (2019) 60:812–6. doi: 10.1080/10428194.2018.1509321
59. Liu S, Ren C, Wang Y. Primary lymphoma of the heart: A case report of surgical treatment and review of the literature. Heart Surg Forum. (2019) 22:E225–8. doi: 10.1532/hsf.2217
60. Rozas Fernández P, López Caleya JF, García Campos A. Primary lymphoma of the heart. Med Clin (Barc). (2019) 153:e13–4. doi: 10.1016/j.medcli.2018.10.033
61. Farfán-Leal F, Esteban A, Hinojar R, García-Cosío M, Contreras F. Primary cardiac natural killer/T-cell lymphoma, a very rare form of lymphoma. Asian Cardiovasc Thorac Ann. (2019) 27:210–2. doi: 10.1177/0218492318798230
62. Li Y, Zhou Z, Xin F, Zhang C, Zhang R, Sun D, et al. Primary cardiac lymphoma in both atria: A case report. J Clin Ultrasound. (2019) 47:561–3. doi: 10.1002/jcu.22738
63. Chia AXF, Zhao Z, Lim SL. Primary cardiac lymphoma. BMJ Case Rep. (2019) 12:e230468. doi: 10.1136/bcr-2019-230468
64. Roncon L, Marco Z, Aggio S, Rigatelli G, Rinuncini M. Multimodality imaging for early diagnosis and treatment of primary cardiac diffuse “double-expressor” lymphoma. J Cardiovasc Med (Hagerstown). (2019) 20:851–2. doi: 10.2459/JCM.0000000000000847
65. Bonou M, Kapelios CJ, Marinakos A, Adamopoulos S, Diamantopoulos P, Foukas PG, et al. Diagnosis and treatment complications of primary cardiac lymphoma in an immunocompetent 28-year old man: a case report. BMC Cancer. (2019) 19:191. doi: 10.1186/s12885-019-5405-y
66. Zhang F, Luo DL, Chen Y, Liu J, Luo LQ, He J, et al. Clinicopathological features of primary cardiac CD5-positive and bcl-2 and C-MYC double expression diffuse large B-cell lymphoma. Zhonghua Bing Li Xue Za Zhi. (2019) 48:951–4. doi: 10.3760/cma.j.issn.0529-5807.2019.12.007
67. Wei X, Li X, Tang H, Chen M. ALK-negative primary cardiac T-cell lymphoma coexpressing CD3 and CD30 in an immunocompetent adult. Eur Heart J. (2019) 40:3804. doi: 10.1093/eurheartj/ehz479
68. Chen FM, Zhang L, Ni JM, Cao JN, You CY. A case report of tricuspid stenosis, right atrium and pulmonary artery thrombosis caused by primary cardiac Burkitt lymphoma. Zhonghua Xin Xue Guan Bing Za Zhi. (2019) 47:921–2. doi: 10.3760/cma.j.issn.0253-3758.2019.11.014
69. Endo Y, Nakamura Y, Kuroda M, Nakanishi Y, Ito Y, Hori T, et al. Treatment of Malignant primary cardiac lymphoma with tumor resection using minimally invasive cardiac surgery. J Cardiothorac Surg. (2018) 13:97. doi: 10.1186/s13019-018-0778-6
70. Ömeroğlu SN, Balkanay OO, Göksedef D, Öz B, İpek G. The surgical treatment of primary cardiac B-cell lymphoma of clinically unstable patient. Ann Thorac Surg. (2018) 105:e215–7. doi: 10.1016/j.athoracsur.2017.11.056
71. Tikka J, Vaittinen S, Pakanen L, Lunetta P. Sudden unexpected death from unusually large primary cardiac B-cell lymphoma. Am J Forensic Med Pathol. (2018) 39:161–3. doi: 10.1097/PAF.0000000000000379
72. Perrone MA, Intorcia A, Morgagni R, Marchei M, Sergi D, Pugliese L, et al. Primary cardiac lymphoma: the role of multimodality imaging. J Cardiovasc Med (Hagerstown). (2018) 19:455–8. doi: 10.2459/JCM.0000000000000668
73. Ito I, Nakaoka Y, Kubokawa SI, Sugane H, Kusume T, Matsuda H, et al. Primary cardiac lymphoma: A lesson learned from an unsuccessful experience. Intern Med. (2018) 57:3569–74. doi: 10.2169/internalmedicine.0594-17
74. Yoshihara S, Matsunaga M, Tanioka F, Naito M. Primary cardiac diffuse large B-cell lymphoma. Circ J. (2018) 82:2919–20. doi: 10.1253/circj.CJ-18-0202
75. Taylor JE, Harless A, Shah S, Huang L, Gilliland YE, Qamruddin S. Large primary cardiac lymphoma causing functional tricuspid valve stenosis. Tex Heart Inst J. (2018) 45:275–6. doi: 10.14503/THIJ-17-6413
76. Coulier B, Colin GC, Tourmous H, Floris N, Van Eeckhout P, Scavée C. Imaging features of primary cardiac lymphoma. Diagn Interv Imaging. (2018) 99:115–7. doi: 10.1016/j.diii.2017.05.013
77. Ospina-García N, Román GC, Pascual B, Schwartz MR, Preti HA. Hypothalamic relapse of a cardiac large B-cell lymphoma presenting with memory loss, confabulation, alexia-agraphia, apathy, hypersomnia, appetite disturbances and diabetes insipidus. BMJ Case Rep. (2018) 2018:bcr2016217700. doi: 10.1136/bcr-2016-217700
78. Cheng JF, Lee SH, Hsu RB, Yu SC, Shun CT, Huang PS, et al. Fulminant primary cardiac lymphoma with sudden cardiac death: A case report and brief review. J Formos Med Assoc. (2018) 117:939–43. doi: 10.1016/j.jfma.2018.03.011
79. Inoue R, Nakamura B, Inagaki M, Fujii T, Hirano K, Maze Y, et al. Successful surgical treatment of primary cardiac lymphoma detected with the onset of acute heart failure;Report of a case. Kyobu Geka. (2017) 70:1021–4.
80. Özcem B, Soner Kemal H, Balcıoğlu Ö, Özkayalar H, Sanisoğlu İ. Successful resection and reconstruction of primary cardiac lymphoma. Turk Kardiyol Dern Ars. (2017) 45:755–7. doi: 10.5543/tkda.2017.65745
81. Pirzada A, Connors S, Harris S, Adams C. Primary cardiac T cell lymphoma mimicking ST-elevation myocardial infarction. Cardiology. (2017) 138:259–63. doi: 10.1159/000479676
82. Parato VM, Muscente F, Scarano M. Primary cardiac lymphoma: a case report. G Ital Cardiol (Rome). (2017) 18:11–3. doi: 10.1714/2628.27022
83. Perna GP, Gini G, Brambatti M, Battistoni I, Marini M, Angelini L, et al. Primary cardiac lymphoma in an immunocompetent young adult: outcome with chemotherapy. G Ital Cardiol (Rome). (2017) 18:7–10. doi: 10.1714/2628.27021
84. Wan Y, He D, Ye Y, Zhang W, Zhao S, Long Y, et al. Primary cardiac diffuse large B-cell lymphoma with concurrent high MYC and BCL2 expression in an immunocompetent Chinese elderly woman. Cardiovasc Pathol. (2017) 31:54–6. doi: 10.1016/j.carpath.2017.07.006
85. Sarr SA, Gaye AM, Aw F, de Dieu Nzambaza J, Bodian M, Babaka K, et al. Obstructive primary cardiac T-cell lymphoma: A case report from Senegal. Am J Case Rep. (2017) 18:281–5. doi: 10.12659/ajcr.901455
86. Hu S, Zhu LL, Ke J, Sun Y, Hu CH. Imaging features of primary cardiac lymphoma. Chin Med J (Engl). (2017) 130:2123–5. doi: 10.4103/0366-6999.213425
87. Sherkat R, Sabri MR, Dehghan B, Bigdelian H, Reisi N, Afsharmoghadam N, et al. EBV lymphoproliferative-associated disease and primary cardiac T-cell lymphoma in a STK4 deficient patient: A case report. Med (Baltimore). (2017) 96:e8852. doi: 10.1097/MD.0000000000008852
88. Xiao Y, Cai Y, Tang H, Xiao X. De novo CD5-positive primary cardiac diffuse large B-cell lymphoma coexpressing C-myc and BCL2 in an immunocompetent adult. Eur Heart J. (2017) 38:1937. doi: 10.1093/eurheartj/ehw523
89. Moss E, Goldstein DA, Bradley KT, Flowers CR, Murphy DA. Successful robotic excision and early chemotherapy for primary cardiac lymphoma. Ann Thorac Surg. (2016) 102:304–5. doi: 10.1016/j.athoracsur.2015.08.036
90. Hishikari K, Kuwahara T, Kimura S, Hikita H, Takahashi A, Isobe M. Reversible atrial fibrillation with bradycardia associated with primary cardiac B-cell lymphoma. Intern Med. (2016) 55:635–8. doi: 10.2169/internalmedicine.55.5851
91. Liu Y, Xu M, Fan H, Chen M, Wang J, Zhang L, et al. Primary cardiac lymphoma: Two rare cases. Int J Cardiol. (2016) 203:763–5. doi: 10.1016/j.ijcard.2015.11.043
92. Pagé M, Grasso AE, Carpenter JP, Sheppard MN, Karwatowski SP, Mohiaddin RH. Primary cardiac lymphoma: diagnosis and the impact of chemotherapy on cardiac structure and function. Can J Cardiol. (2016) 32:931.e1–3. doi: 10.1016/j.cjca.2015.09.002
93. Campos-Quintero A, Patiño-Bahena E, Sánchez-Flores A, Benita-Bordes A, Aranda-Fraustro A, Buendía-Hernández A. Primary cardiac lymphoma, a extremely rare presentation. Arch Cardiol Mex. (2016) 86:94–6. doi: 10.1016/j.acmx.2015.06.005
94. Benzerdjeb N, Ameur F, Ikoli JF, Sevestre H. Primary cardiac B cell lymphoma: Manifestation of Felty’s syndrome or TNFα antagonist. Pathol Res Pract. (2016) 212:1191–3. doi: 10.1016/j.prp.2016.10.004
95. Sinha A, Davies T, Saif A, Apps A. Pericarditis with anaemia as a herald syndrome in a fatal presentation of cardiac lymphoma. BMJ Case Rep. (2016) 2016:bcr2015212810. doi: 10.1136/bcr-2015-212810
96. Santhosh S, Bahl A, Saikia UN, Lad D, Mittal BR, Malhotra P, et al. FDG PET/CT in the staging and follow-up of primary cardiac “T” cell lymphoma presenting as hypertrophic cardiomyopathy. J Nucl Cardiol. (2016) 23:581–4. doi: 10.1007/s12350-015-0238-9
97. Hong TH, Jeong DS. Successful management of primary cardiac lymphoma with minimal debulking surgery combined with adjuvant chemotherapy. Heart Surg Forum. (2015) 18:E242–244. doi: 10.1532/hsf.1317
98. Jandali A, Kabach A, Al Halabi S, Alraies MC. Rare cause of a common symptom: primary cardiac lymphoma. Am J Emerg Med. (2015) 33:1849.e5–6. doi: 10.1016/j.ajem.2015.04.068
99. Zhu J, Li Q, Zeng W, Wu Z, Fan L, Xu W, et al. Primary cardiac plasmablastic lymphoma: report of a case and literature review. Zhonghua Xue Ye Xue Za Zhi. (2015) 36:862–5. doi: 10.3760/cma.j.issn.0253-2727.2015.10.012
100. Gyoten T, Doi T, Nagura S, Yamashita A, Fukahara K, Kotoh K, et al. Primary cardiac Malignant lymphoma: survival for 13 years after surgical resection and adjuvant chemotherapy. Ann Thorac Surg. (2015) 99:1060–2. doi: 10.1016/j.athoracsur.2014.05.074
101. Jonavicius K, Salcius K, Meskauskas R, Valeviciene N, Tarutis V, Sirvydis V. Primary cardiac lymphoma: two cases and a review of literature. J Cardiothorac Surg. (2015) 10:138. doi: 10.1186/s13019-015-0348-0
102. Montanaro C, Loiacono F, Fragasso G, De Cobelli F, Foppoli M, Margonato A. Primary cardiac lymphoma in an immunocompetent 71-year-old man. Tex Heart Inst J. (2015) 42:561–4. doi: 10.14503/THIJ-14-4269
103. Allain G, Hajj-Chahine J, Lacroix C, Jayle C. Primary cardiac lymphoma complicated by cardiogenic shock: successful treatment with chemotherapy delivered under extracorporeal membrane oxygenation support. Eur J Cardiothorac Surg. (2015) 48:968–70. doi: 10.1093/ejcts/ezv031
104. Pires Ferreira Filho LI, Ribeiro Junior HL, Pinheiro Junior ED, Pinheiro RF. Primary cardiac lymphoblastic B-cell lymphoma: Should we treat more intensively? J Cancer Res Ther. (2015) 11:1034. doi: 10.4103/0973-1482.154063
105. Pistritto AM, Pavo N, Maurer G, Binder T, Goliasch G. Multimodality imaging of a primary cardiac diffuse large B-cell lymphoma. Eur Heart J Cardiovasc Imaging. (2015) 16:909. doi: 10.1093/ehjci/jev099
106. Riccioli V, Privitera G, Luca T, Passanisi R, Loreto C, Musumeci G, et al. Collateral circulation resulting from obstruction due to cardiac lymphoma of right atrium. Clin Lymphoma Myeloma Leuk. (2015) 15:e173–176. doi: 10.1016/j.clml.2015.07.648
107. Kato Y, Kawata M, Yumura M, Suehiro H, Takada H, Matsuura T, et al. Case Report; A case of primary cardiac diffuse large B cell lymphoma with heart failure. Nihon Naika Gakkai Zasshi. (2015) 104:99–102. doi: 10.2169/naika.104.99
108. Tomikawa T, Tabayashi T, Tokuhira M, Watanabe R, Sagawa M, Nemoto T, et al. Cardiac and breast diffuse large B-cell lymphoma with pericardial effusion and AV-block. Rinsho Ketsueki. (2015) 56:9–15. doi: 10.11406/rinketsu.56.9
109. Sayal K, Ali T, Tasker A, Carroll N. Broadening the scope of thoracic oncological intervention: a novel minimally invasive method for the diagnosis of primary cardiac lymphoma. BMJ Case Rep. (2015) 2015:bcr2014208632. doi: 10.1136/bcr-2014-208632
110. Khan-Kheil AM, Mustafa HM, Anand DV, Banerjee P. A rare case of primary cardiac lymphoma. BMJ Case Rep. (2015) 2015:bcr2015211208. doi: 10.1136/bcr-2015-211208
111. Nagatomo D, Oyama J, Yoshihara M, Node K. Successful treatment of primary cardiac lymphoma causing ST-elevation myocardial infarction by percutaneous coronary intervention combined with chemotherapy. BMJ Case Rep. (2014) 2014:bcr2014207267. doi: 10.1136/bcr-2014-207267
112. Kim DH, Kim YH, Song WH, Ahn JC. Primary cardiac lymphoma presenting as an atypical type of hypertrophic cardiomyopathy. Echocardiography. (2014) 31:E115–119. doi: 10.1111/echo.12477
113. Groebner M, Südhoff T, Doering M, Kirmayer M, Nitsch T, Prügl L, et al. Fever, atrial fibrillation, and angina pectoris in a 58-year-old man. Internist (Berl). (2014) 55:595–600. doi: 10.1007/s00108-014-3477-y
114. Cioc AM, Jessurun J, Vercellotti GM, Pambuccian SE. De novo CD5-positive primary cardiac diffuse large B-cell lymphoma diagnosed by pleural fluid cytology. Diagn Cytopathol. (2014) 42:259–67. doi: 10.1002/dc.22918
115. Chattranukulchai P, Puwanant S, Rungpradubvong V, Singhatanadgige S, Boonyaratavej S. Combined mechanism of refractory shock in primary cardiac lymphoma: a rare dilemma. Heart Lung Circ. (2014) 23:e160–163. doi: 10.1016/j.hlc.2014.03.004
116. Nijjar PS, Masri SC, Tamene A, Kassahun H, Liao K, Valeti U. Benefits and limitations of multimodality imaging in the diagnosis of a primary cardiac lymphoma. Tex Heart Inst J. (2014) 41:657–9. doi: 10.14503/THIJ-13-3595
117. Matos AP, Palas J, Doulaptsis C, Ramalho M, Duarte S, Bogaert J. B-cell lymphoma of the heart: A rare diagnosis. Rev Port Cardiol. (2014) 33:803.e1–3. doi: 10.1016/j.repc.2014.07.004
118. Llitjos JF, Redheuil A, Puymirat E, Vedrenne G, Danchin N. AIDS-related primary cardiac lymphoma with right-sided heart failure and high-grade AV block: insights from magnetic resonance imaging. Ann Cardiol Angeiol (Paris). (2014) 63:99–101. doi: 10.1016/j.ancard.2013.03.004
119. Habertheuer A, Ehrlich M, Wiedemann D, Mora B, Rath C, Kocher A. A rare case of primary cardiac B cell lymphoma. J Cardiothorac Surg. (2014) 9:14. doi: 10.1186/1749-8090-9-14
120. Jung YH, Woo IS, Ko YJ, Lee JH, Lim JW, Han CW. A case of primary cardiac lymphoma showing isolated central nervous system relapse. Clin Lymphoma Myeloma Leuk. (2014) 14:e31–33. doi: 10.1016/j.clml.2013.09.003
121. Petrich A, Cho SI, Billett H. Primary cardiac lymphoma: An analysis of presentation, treatment, and outcome patterns. Cancer. (2011) 117:581–9. doi: 10.1002/cncr.25444
122. Asadian S, Rezaeian N, Hosseini L, Toloueitabar Y, Hemmati Komasi MM. The role of cardiac CT and MRI in the diagnosis and management of primary cardiac lymphoma: A comprehensive review. Trends Cardiovasc Med. (2022) 32:408–20. doi: 10.1016/j.tcm.2021.08.010
123. Calabretta R, Hacker M. A PET-derived tumor expansion pattern to differentiate between primary cardiac lymphoma from primary cardiac sarcoma. J Nucl Cardiol. (2022) 29:2878–80. doi: 10.1007/s12350-022-03097-z
124. Yin K, Brydges H, Lawrence KW, Wei Y, Karlson KJ, McAneny DB, et al. Primary cardiac lymphoma. J Thorac Cardiovasc Surg. (2022) 164:573–580.e1. doi: 10.1016/j.jtcvs.2020.09.102
125. Suen HC, Hsin MKY. Commentary: Surgical resection has limited role in primary cardiac lymphoma. J Thorac Cardiovasc Surg. (2022) 164:581–2. doi: 10.1016/j.jtcvs.2020.09.025
126. Miguel CE, Bestetti RB. Primary cardiac lymphoma. Int J Cardiol. (2011) 149:358–63. doi: 10.1016/j.ijcard.2010.02.016
127. Johri A, Baetz T, Isotalo PA, Nolan RL, Sanfilippo AJ, Ropchan G. Primary cardiac diffuse large B cell lymphoma presenting with superior vena cava syndrome. Can J Cardiol. (2009) 25:e210–212. doi: 10.1016/s0828-282x(09)70110-2
Keywords: primary cardiac lymphoma, diffuse large B-cell lymphoma, diagnosis, treatment, prognosis
Citation: Zhuang S, Chang L, Feng X, Hu W, Yang Z and Zhang Y (2025) Primary cardiac lymphoma: a clinicopathological study of 121 cases. Front. Oncol. 14:1509100. doi: 10.3389/fonc.2024.1509100
Received: 10 October 2024; Accepted: 10 December 2024;
Published: 07 January 2025.
Edited by:
Carmelo Caldarella, Fondazione Policlinico Universitario A. Gemelli IRCCS, Roma, ItalyReviewed by:
Giulia Iannaccone, Catholic University of the Sacred Heart, Rome, ItalyStefano Poletto, University of Turin, Italy
Copyright © 2025 Zhuang, Chang, Feng, Hu, Yang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuanyuan Zhang, ZG9jdG9yemhhbmd5eUAxNjMuY29t; Zhaobo Yang, eWFuZ3poYW9ibzIwMDhAMTI2LmNvbQ==
†These authors have contributed equally to this work and share first authorship
‡ORCID: Yuanyuan Zhang, orcid.org/0009-0007-7675-5863
Yuanyuan Zhang, orcid.org/0000-0002-1498-304X
 Shuhui Zhuang
Shuhui Zhuang Liudi Chang
Liudi Chang Xiaoxi Feng1,2
Xiaoxi Feng1,2 Weiwen Hu
Weiwen Hu Yuanyuan Zhang
Yuanyuan Zhang