- 1School of Clinical Medicine, Shandong Second Medical University, Weifang, Shandong, China
- 2Department of Neurosurgery, Zibo Central Hospital, Zibo, China
Meningiomas are some of the most prevalent primary brain tumors in adults, and are typically non-neuroglial in nature. A variety of symptoms may be observed, including headaches, fluctuations in mental status, ataxia, muscle weakness, nausea and vomiting, seizures, visual changes, speech disorders, and sensory abnormalities. The World Health Organization (WHO) has a grading system for meningiomas based on histological criteria, which is as follows: Grade 1 meningiomas are considered benign; Grade 2 meningiomas have a moderately aggressive nature and usually present with histological atypia; and Grade 3 meningiomas exhibit aggressive malignant behavior. Grade 3 meningiomas are distinguished by aberrant and accelerated cellular proliferation, which increases the probability of invasion and recurrence within the central nervous system relative to the other grades. Malignant meningiomas are further classified by tumor size. For example, WHO grade 3 meningiomas with diameter >5 cm are designated giant meningiomas. Giant meningiomas are complicated by their potential for compression of the brain tissue, which can lead to increased intracranial pressure and hemodynamic changes. In many cases, these changes induce vasogenic edema in the adjacent brain tissue. This article details a rare case of rapidly growing atypical giant meningioma that progressed to an anterior-posterior diameter of 13 cm within 3 years, occupying the majority of the left hemisphere of the brain and encroaching upon the right intracranial structures. Through recent advances in medical diagnostics and heightened public awareness of health issues, cases with such large meningiomas have become exceedingly rare. Fortunately, the tumor in the present case was successfully resected using advanced surgical techniques that employed microscopy in conjunction with sodium fluorescein, resulting in complete removal of the tumor and restoration of the patient’s muscle strength postoperatively. The value of fluorescence-guided surgery in this type of procedure is support in the present case report.
Introduction
Meningiomas are the most prevalent intracranial neoplasms, accounting for approximately 15% to 20% of all such cases (1, 2). They are relatively slow-growing extra-axial tumors (3, 4), most frequently observed in the convex, parasagittal, or sickle regions of the skull, the pterygoid wings, the saddle nodes, and the posterior cranial fossa. Based on their slow growth rate (5, 6), meningiomas frequently remain undetected until they have reached a size that causes clinical symptoms, particularly when they are located in the “silent areas” of the brain. Meningiomas may be of considerable size (>3 cm) or very large (>5 cm) at the time of diagnosis (7, 8). Giant meningiomas can be distinguished from other types of meningiomas by three key characteristics: their large size, the fact that they can cause increased intracranial pressure, and their proximity to critical anatomical structures. These tumors are exceedingly rare and their characteristics frequently make them challenging for surgeons to excise completely (9). In the present case, the patient developed a large meningioma in the left hemisphere that grew to a diameter of 13 cm during a 3-year period. By employing sodium fluorescein and microscope-assisted techniques, we successfully achieved complete resection of the tumor. In the postoperative period, the patient’s muscle strength in the right upper extremity improved from grade 0 to grade III following a cerebral infarction, while the muscle strength in the right lower extremity improved from grade 0 to grade IV.
Case presentation
A 77-year-old female patient was admitted to our hospital on 19 March 2024 with chief complaints of dysphoria and unfavorable speech that had been present for more than 1 month. Although the symptoms were intermittent, they were persistently worsening and accompanied by occasional headaches. The patient had a history of a cerebral infarction. A cranial MRI conducted in 2021 (Figure 1) did not reveal any obvious signs of an intracranial tumor. On admission, a physical examination revealed Glasgow Coma Scale (GCS) score of 11, incomplete motor aphasia, bilateral ocular collapse, unresisted neck, voluntary movement of the left limbs, and decreased muscle tone in the right limbs, with a muscle strength of grade 0. Laboratory tests, including electrocardiogram and routine blood and urine tests, yielded results within the normal ranges. A cranial MRI (Figure 2A) revealed a large occupying lesion (12.9×9.1×6.1 cm3) in the left cerebral hemisphere, invading the superior sagittal sinus in the right intracranial area. Multiple tortuous and increased vascular shadows were also observed in the vicinity of the lesion. A diffusion tensor imaging examination revealed that the projecting nerve bundles in the left frontal-parietal lobe and corpus callosum area showed partial atrophy compared with those on the contralateral side, with local irregularities suggestive of damage caused by tumor infiltration. The preoperative examination revealed a robust blood supply to the tumor, accompanied by diminished visualization of the left internal carotid artery system. Cerebral angiography was planned to identify any cerebral vascular lesions and the blood supply to the tumor. This was scheduled to be followed by a craniotomy at a later stage. The patient underwent the cerebral angiography under general anesthesia with embolization of the artery supplying blood to the tumor. Postoperative medications were administered to prevent complications, such as reduced intracranial pressure, and provide neuroprotection and symptomatic support. On postoperative day 6, the patient underwent fluorescein sodium labeling microscopy under general anesthesia to evaluate the extent of the resection in the left frontoparietal lobe. During this procedure, the tumor was visualized by sodium fluorescein via the frontotemporal-parietal approach over the midline (Figure 3A). A tumor measuring approximately 13×11×6 cm3 was successfully resected (Figure 3B), with the bilateral anterior cerebral arteries being well-protected and uninjured. Intraoperative dynamic monitoring of blood gas analysis revealed transfusion requirements for 11 units, 500 mL of plasma, and 10 units of cold precipitation. The patient’s intraoperative blood pressure and heart rate remained stable, the anesthesia was effective, and the patient was transferred back to the neurosurgical intensive care unit under anesthesia. The postoperative pathological examination (Figure 3C) revealed a giant atypical meningioma (WHO grade 2). A review of the postoperative cranial MRI revealed complete resection of the tumor (Figure 2B). On postoperative day 3, the patient had a GCS score of 9 and was able to perform simple verbal communication, with intermittent handshake movements in the left upper limb, grade III muscle strength in the left lower limb, and stimulation of the right lower limb with slight flexion. On postoperative day 7, the patient developed a fever that reached 39°C. A lumbar puncture was performed, and the cerebrospinal fluid culture revealed staphylococcus capitatus, indicating an intracranial infection. Meanwhile, the lung infection showed signs of worsening, and the patient was initiated on an escalating antibiotic regimen, comprising intravenous vancomycin 1 g every 12 hours and meropenem 0.5 g every 8 hours. Subsequently, the patient exhibited a rash, prompting the cessation of vancomycin and initiation of oral linezolid therapy. On postoperative day 12, the patient’s condition was characterized by severe intermittent fever, lethargy, and grade IV muscle strength in the left limbs. The left upper limb exhibited partial compliance with movement. The muscle strength of the right upper limb was classified as grade I, while that of the right lower limb was classified as grade III. After receiving the family’s consent, lumbar large-pool tube drainage was initiated. By postoperative day 20, the patient’s condition had stabilized, with no further fever and a smooth course of lumbar large-pool drainage. By postoperative day 48, the patient had made a full recovery. Her mental status was clear, her spirits were high, and her GCS score was 15. The muscle strength of the left limbs was classified as grade V-, the muscle strength of the right upper limb was classified as grade III (her muscle strength after the previous cerebral infarction was classified as grade III), and the muscle strength of the right lower limb was classified as grade IV. The patient had a right Babinski sign of (±). The patient (Figure 4) was discharged with instructions to continue rehabilitation, supplemented by radiation therapy.
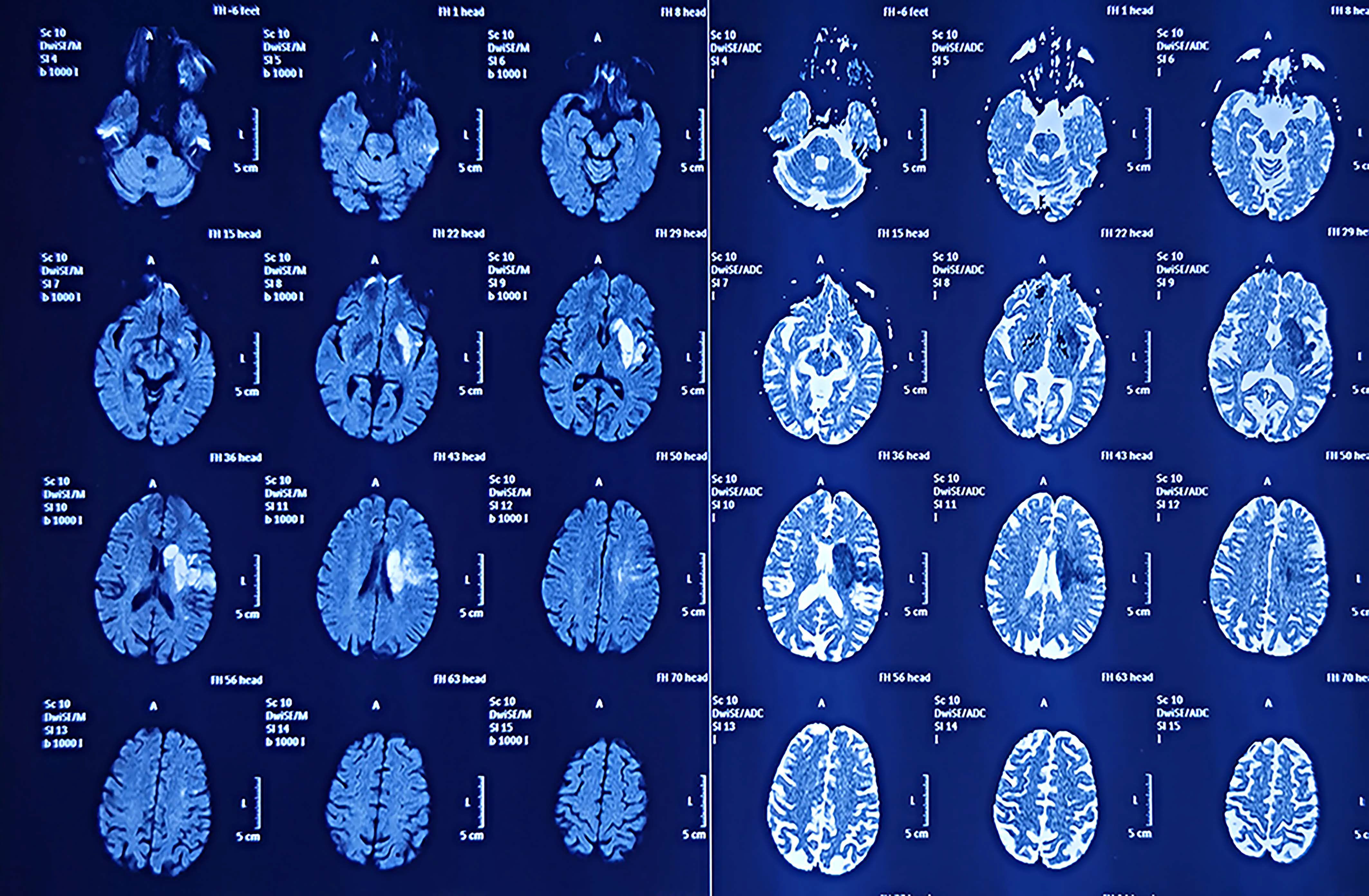
Figure 1. The patient’s MRI examination in 2021 showed a cerebral infarct but did not reveal the presence of a tumor.
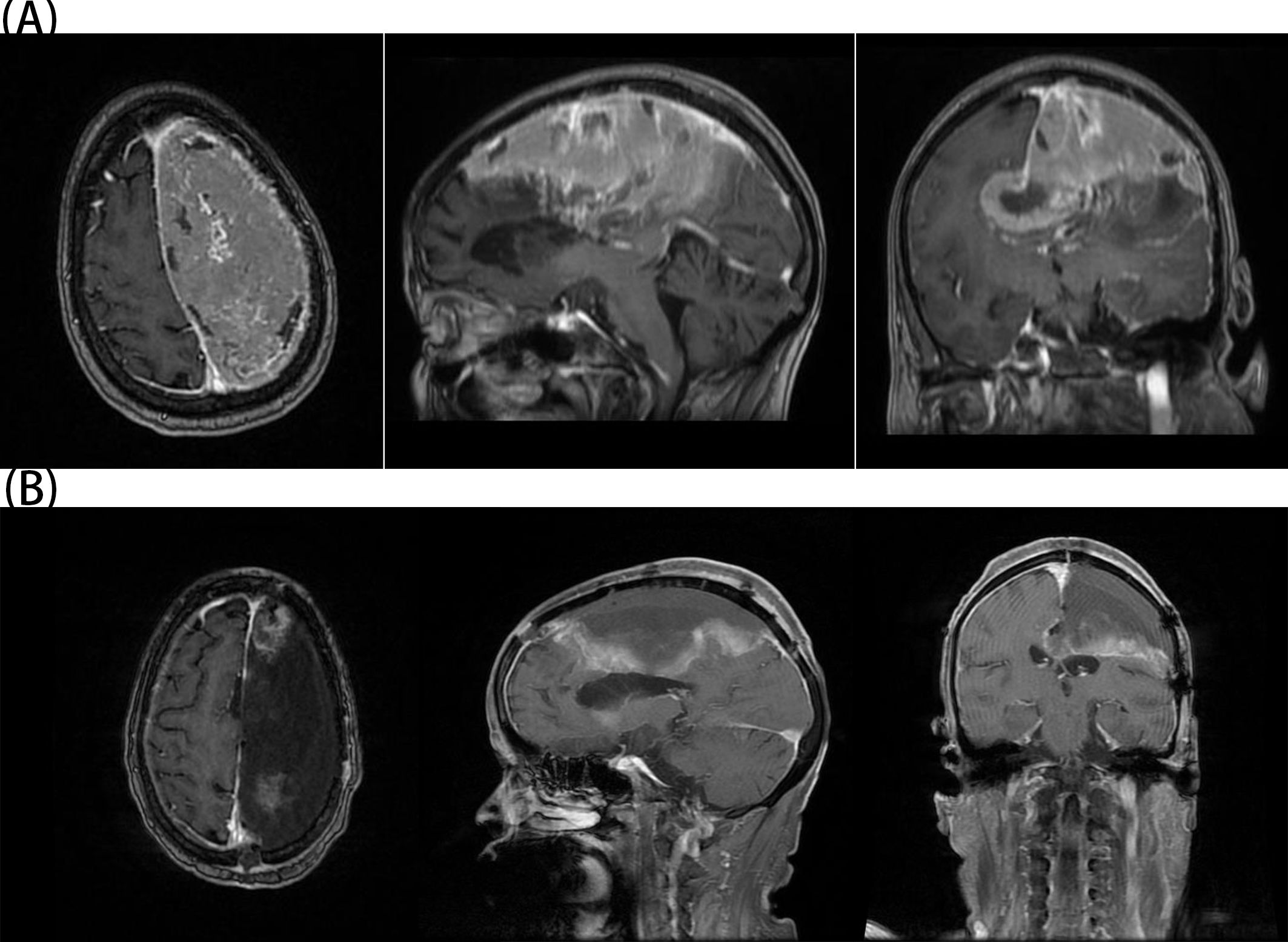
Figure 2. (A) The patient’s preoperative MRI showed a giant meningioma on the left side. (B) The patient’s postoperative MRI confirmed complete resection of the tumor.
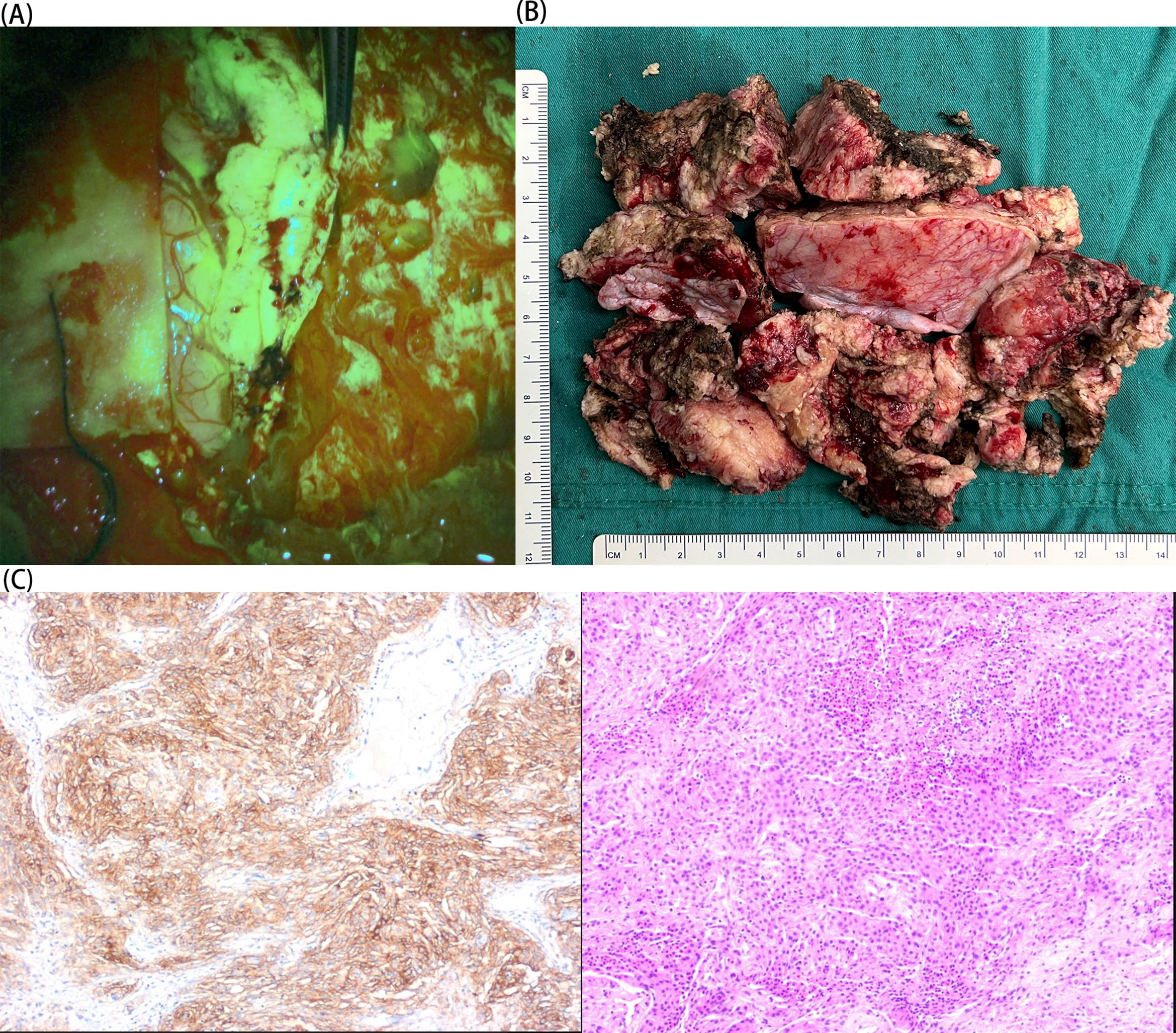
Figure 3. (A) Intraoperative sodium fluorescein visualization of the tumor. (B) Postoperative tumor specimen. (C) The patient’s histological findings (immunohistochemistry and HE: 10×magnification). The immunohistochemistry findings were:SSTR2,(+); vimentin, (+); S-100, (+); EMA, partial (+); GFAP, (−); ER, (−); PR, (−); AR, (−); SMA, (−); desmin, (−); CD10, (−); CD34, (−); Olig-2, (−); IDH-1, scattered (+); CKAE1/AE3, (−); CK8/18, (−); CK5/6, (−); P53, (−); Ki-67, (+) S accounted for 5%–20%.
Discussion
Meningiomas comprise 15% to 20% of intracranial tumors (1, 2). The majority of these tumors are slow-growing and benign, while the remainder are aggressive or truly malignant. The tumors are typically situated in the subdural space and represent the most prevalent non-glial primary tumors within the skull. It is crucial to acknowledge that all brain tumors, irrespective of their pathological classification, have the potential to elicit severe or even fatal symptoms due to their mass effect, a phenomenon exemplified by meningiomas. Meningiomas typically grow relatively slowly, with an average growth rate of approximately 2.41 mm per year (5, 6). Consequently, meningiomas rarely cause clinical symptoms in their early stages. However, as time progresses, these tumors increase in size and begin to cause a range of different symptoms. A standard definition for the diameter indicating a giant meningioma remains to be established within the academic community. Definitions vary, with some defining diameters exceeding 4.5 cm, others defining diameters exceeding 5 cm, 6 cm, or 7 cm (7, 8, 10–14), and the majority of the literature defining diameters exceeding 5 cm as giant meningiomas (9). In 1950, White et al. (15) reported a meningioma weighing 1,353 g. In 1971, Rao et al. (16) reported a meningioma weighing 1,890 g. In 1982, Cech et al. (17) reported a meningioma with a maximum diameter of 22 cm. Finally, in 2002, Gutteridge and Wallace (18) reported a meningioma with a maximum diameter of >10 cm. A review of the literature reveals that meningiomas with very large diameters or large weights have been observed in conjunction with cranial lesions or significant extracranial masses. In the present case, the meningioma was entirely confined to the skull, resulting in significant compression of the brain tissue and a grade 0 muscle strength rating for the right limbs. Furthermore, the patient’s meningioma, which was absent on the previous MRI, developed and grew to a diameter of 13 cm in only 3 years, inconsistent with the conventional notion of a slow-growing meningioma. Nevertheless, the precise biological mechanism by which meningiomas attain such enormous sizes remains unclear. Ultimately, the tumor in the present case was completely resected using microscopy in conjunction with sodium fluorescein. Following the procedure, the patient’s muscle strength in the right upper limb was restored from grade 0 to grade III, while that in the right lower limb was restored from grade 0 to grade IV, indicating significant improvement. In the context of meningioma resection, identifying the caudal border between the meningioma and the surrounding dura mater represents a significant challenge. Sodium fluorescein, a fluorescent agent with an analogous mechanism of action to gadolinium, a contrast-enhancing substance utilized in magnetic resonance imaging, is capable of accumulating in regions where the blood–brain barrier is compromised, particularly in the area surrounding a tumor (19). By precisely controlling the injection time, it is possible to ensure that sufficient quantities of sodium fluorescein are flushed out of healthy areas while being retained in areas with an altered blood–brain barrier (20). This provides real-time fluorescence contrast during surgery, enabling neurosurgeons to identify tumor areas with greater clarity. The technique can also mitigate the shortcomings of conventional neuronavigation techniques, such as brain displacement or localization inaccuracies, and allow visualization of the contrast-enhanced tumor regions in real time (21–25). In a study involving 30 patients with newly diagnosed or recurrent meningiomas, 88% of the tumors exhibited homogeneous diffuse enhancement with sodium fluorescein, and the resection rate was 87%. The present findings also indicate that fluorescence-guided neurosurgery may be a promising technique for extending the resection of brain tumors. The use of sodium fluorescein as an alternative to 5-aminolevulinic acid addresses some of the limitations associated with that reagent (26). Studies have demonstrated that sodium fluorescein can effectively delineate adjacent vascular and neural structures during meningioma surgery, facilitating separation of the tumor from the brain tissue. The technique enhances surgical safety, facilitates the resection of complex vascularized meningiomas, and provides unique advantages for the visualization of hidden vascular structures (27, 28). In cases where meningiomas have a large blood supply, preoperative embolization of the dural arteries may facilitate surgical resection and reduce blood loss and complications (29–36). Nevertheless, preoperative embolization remains a topic of contention, because it has the potential to cause complications such as edema, hemorrhage, stroke, and cerebral nerve palsy (37–39). These risks are more prevalent in cases with large, highly vascularized meningiomas (40, 41), where embolization remains a viable option because the supplying artery is challenging to access. Therefore, the elevated risk of cerebral edema, hemorrhage, and suboptimal discharge outcomes is not unexpected. It has been reported (42) that embolization may have a negative impact on WHO grade 2/3 tumors, although the findings may have been affected by bias stemming from the fact that these tumors are large, have abundant blood flow, are challenging to resect, and are more likely to undergo embolization. Conversely, it has been proposed that embolization may diminish the likelihood of tumor recurrence and could be a valuable alternative for patients at elevated risk for surgical intervention (33, 40, 43–45). Furthermore, this fluorescence-guided surgical technique is particularly beneficial for patients whose tumors are located in nonverbal, sensory, motor, and cognitive regions (e.g., temporal and occipital lobes) and does not increase the incidence of postoperative complications. Moreover, the technique can minimize the probability of postoperative recurrence and does not impose an additional financial burden on the patient. Neurosurgery central nervous system infections are a group of infections that occur within the skull and spinal canal with an incidence of 4.6%–25% (46). The pathogenic organisms include gram-negative bacteria, gram-positive bacteria, and fungi, with the former two being predominant (47). In the event of a suspected central nervous system infection, it is imperative that samples such as cerebrospinal fluid are collected for testing prior to the administration of any antimicrobial agents. Furthermore, empirical antimicrobial therapy should be initiated without delay (48, 49). Antimicrobials are the preferred treatment option over fungicides that can readily cross the blood–brain barrier, such as ceftriaxone, cefotaxime, meropenem, and vancomycin. Infections caused by methicillin-susceptible Staphylococcus aureus can be treated with ampicillin/sulbactam. Despite its unfavorable pharmacokinetic and pharmacodynamic profile, vancomycin is currently recommended as a first-line agent for methicillin-resistant S. aureus infections. For the treatment of third-generation cephalosporin-susceptible Gram-negative bacillus infections, ceftriaxone or cefotaxime is recommended; for Pseudomonas spp. strains, cefepime, ceftazidime, or meropenem is recommended. It is further recommended that preoperative antimicrobial prophylaxis should target the bacteria most likely to cause an infection, rather than killing all organisms (50). It is also important to note that routine and continuous prophylactic use of antimicrobials does not reduce the incidence of intracranial infections; rather, it increases the risk of drug-resistant strains of bacteria (51).
Conclusion
The advent of advanced medical imaging techniques has enabled early diagnosis of meningiomas, prior to the onset of symptoms. Nevertheless, some patients may not be diagnosed until their tumor is at an advanced stage, by which time their symptoms may have persisted for years or have been previously misdiagnosed as other conditions. The risk of complete resection is elevated for giant meningiomas, because they frequently infiltrate crucial regions of the brain and are intricately linked to vital neurovascular structures. The surgical procedures employed to remove these tumors are particularly challenging for several reasons, including limited visual field, increased brain edema, high tumor vascularization, and potential need for extensive craniotomies. Furthermore, patients with larger meningiomas exhibit a higher incidence of peritumoral edema than patients with smaller tumors. The utilization of fluorescence-guided surgery in meningioma surgery can facilitate dissection of the tumor interface through clear visualization of adjacent vascular and neural structures. In critical areas of the brain, where cerebral edema is exacerbated and tumor necrosis is poorly demarcated from the cortex, the tumor can be resected as much as possible while protecting the blood vessels and nerves. The resection can also be combined with preoperative embolization in patients with large tumors or meningiomas with abundant vascularization. The present case corroborates the value of fluorescence-guided surgery in such procedures, illustrating its benefits in maximizing tumor resection while safeguarding the normal brain tissue.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Medical Ethics Expert Committee of Zibo Central Hospital Zibo Central Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. The animal study was approved by Medical Ethics Expert Committee of Zibo Central Hospital Zibo Central Hospital. The study was conducted in accordance with the local legislation and institutional requirements. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
JC: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Writing – original draft, Writing – review & editing. HS: Data curation, Formal analysis, Investigation, Methodology, Writing – original draft. SS: Data curation, Methodology, Software, Writing – review & editing. HZ: Data curation, Methodology, Writing – review & editing. YG: Data curation, Formal analysis, Investigation, Resources, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
The author(s) acknowledge support from the Department of Imaging, Zibo Central Hospital and the Department of Neurosurgery.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI disclosure
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Sawaya R, Y G. Intracranial osteolytic Malignant meningiomas appearing as extracranial soft-tissue masses. Neurosurgery. (1992) 30(6):932–5. doi: 10.1227/00006123-199206000-00022
2. Sasaki K, Saito A, Nishijima Y, Inoue T, Suzuki S, Ezura M, et al. Giant intraosseous meningioma associated with calvarial hyperostosis and subcutaneous invasion: Case reports and literature review. Asian J Neurosurg. (2022) 16:589–94. doi: 10.4103/ajns.AJNS_534_20
3. Buerki RA, Horbinski CM, Kruser T, Horowitz PM, James ea CD. An overview of meningiomas. Future Oncol. (2018) 14(21):2161–77. doi: 10.2217/fon-2018-0006
4. Wiemels J, Wrensch M, Claus EB. Epidemiology and etiology of meningioma. J Neuro-Oncology. (2010) 99:307–14. doi: 10.1007/s11060-010-0386-3
5. Kurokawa Y, Ishiguro M, Kurokawa TA. Giant true ossified meningioma removed with surgical ultrasonic aspirator with shear wave technology. Clin Surg. (2017) 2:1829.
6. Ushio Y, K J, K M. Incidence and clinical features of asymptomatic meningiomas. J Neurosurg. (2000) 92(5):766–70. doi: 10.3171/jns.2000.92.5.0766
7. Behari S, Das K, Kumar A, Mehrotra A, Srivastava A, Sahu R, et al. Large/giant meningiomas of posterior third ventricular region: Falcotentorial or velum interpositum? Neurol India. (2014) 62(3):290–5. doi: 10.4103/0028-3886.136934
8. da Silva C, de Freitas PP. Large and giant skull base meningiomas: The role of radical surgical removal. Surg Neurol Int. (2015) 6:113. doi: 10.4103/2152-7806.159489
9. Yasar S, Kirik A. Surgical management of giant intracranial meningiomas. Eurasian J Med. (2021) 53:73–8. doi: 10.5152/eurasianjmed.2021.20155
10. Narayan V, Bir SC, Mohammed N, Savardekar AR, Patra DP, Nanda A. Surgical management of giant intracranial meningioma: operative nuances, challenges, and outcome. World Neurosurg. (2018) 110:e32–41. doi: 10.1016/j.wneu.2017.09.184
11. Antunes C, Ramos R, MaChado MJ, Filipe MA. Giant posterior fossa meningioma: the importance of early diagnosis and challenges concerning treatment. BMJ Case Rep. (2019) 12(3):e228454. doi: 10.1136/bcr-2018-228454
12. Tuna M, Göçer AI, Gezercan Y, Vural A, Ildan F, Haciyakupoğlu ea S. Huge meningiomas: A review of 93 cases. Skull Base. (1999) 9(3):227–38. doi: 10.1055/s-2008-1058151
13. Güzel A, Ö, K M, Ö Aİ, A T, A A, et al. Intracranial benign giant meningiomas: A clinical analysis of 56 cases. Neurosurg Q. (2013) 23:27–32. doi: 10.1097/WNQ.0b013e318266c501
14. Sanai N, McDermott MW. A modified far-lateral approach for large or giant meningiomas of the posterior fossa. J Neurosurg. (2010) 112:907–12. doi: 10.3171/2009.6.Jns09120
15. Kubik CS, W JC, B R. Meningioma of record size with unusual features. J Neurosurg. (1950) 7(5):455–60. doi: 10.3171/jns.1950.7.5.0455
16. Rao SB, D I, Rao KS. Giant intracranial epidural meningioma. Case report. J Neurosurg. (1971) 35(6):748–50. doi: 10.3171/jns.1971.35.6.0748
17. Larson DL, C DA, L M. Giant intracranial and extracranial meningioma: case report and review of the literature. Neurosurgery. (1982) 11(5):694–7. doi: 10.1227/00006123-198211000-00015
18. Wallace D, G IF. Giant intracranial meningioma without hemianopic visual field loss. Clin Exp Optometry. (2002) 85(2):101–6. doi: 10.1111/j.1444-0938.2002.tb03016.x
19. Acerbi F, Broggi M, Schebesch K-M, Höhne J, Cavallo C, De Laurentis C, et al. Fluorescein-guided surgery for resection of high-grade gliomas: A multicentric prospective phase II study (FLUOGLIO). Clin Cancer Res. (2018) 24:52–61. doi: 10.1158/1078-0432.Ccr-17-1184
20. Schupper AJ, Rao M, Mohammadi N, Baron R, Lee JYK, Acerbi F, et al. Fluorescence-guided surgery: A review on timing and use in brain tumor surgery. Front Neurol. (2021) 12:682151. doi: 10.3389/fneur.2021.682151
21. Biana CB, Cecagno D, Porto AR, Cecagno S, Marques V, Soares MC. Non-pharmacological therapies applied in pregnancy and labor: an integrative review. Rev da Escola Enfermagem da USP. (2021) 9(3):227–38. doi: 10.1590/s1980-220x2019019703681
22. Wang LM, Banu MA, Canoll P, Bruce JN. Rationale and clinical implications of fluorescein-guided supramarginal resection in newly diagnosed high-grade glioma. Front Oncol. (2021) 11:666734. doi: 10.3389/fonc.2021.666734
23. Diaz RJ, Dios RR, Hattab EM, Burrell K, Rakopoulos P, Sabha N, et al. Study of the biodistribution of fluorescein in glioma-infiltrated mouse brain and histopathological correlation of intraoperative findings in high-grade gliomas resected under fluorescein fluorescence guidance. J Neurosurg. (2015) 122:1360–9. doi: 10.3171/2015.2.Jns132507
24. Acerbi F, Cavallo C, Broggi M, Cordella R, Anghileri E, Eoli M, et al. Fluorescein-guided surgery for Malignant gliomas: a review. Neurosurgical Rev. (2014) 37:547–57. doi: 10.1007/s10143-014-0546-6
25. Schebesch K-m, Brawanski A, Hohenberger C, Hohne J. Fluorescein sodium-guided surgery of Malignant brain tumors: history, current concepts, and future projects. Turkish Neurosurg. (2016) 26(2):185–94. doi: 10.5137/1019-5149.Jtn.16952-16.0
26. Akcakaya MO, Goker B, Kasimcan MO, Hamamcioglu MK, Kiris T. Use of sodium fluorescein in meningioma surgery performed under the YELLOW-560 nm surgical microscope filter: feasibility and preliminary results. World Neurosurg. (2017) 107:966–73. doi: 10.1016/j.wneu.2017.07.103
27. da Silva CE, da Silva VD, da Silva JL. Sodium fluorescein in skull base meningiomas: a technical note. Clin Neurol Neurosurg. (2014) 120:32–5. doi: 10.1016/j.clineuro.2014.02.015
28. Schebesch KM, Brawanski A, Hohne J. Fluorescein sodium in intracranial meningioma surgery. World Neurosurg. (2017) 108:967. doi: 10.1016/j.wneu.2017.08.046
29. Haider AS, Rana H, Lee LK, Shail MS, Leonard D, Khan U, et al. Large transcalvarial meningioma: surgical resection aided by preoperative embolization. Cureus. (2017) 9:e1229. doi: 10.7759/cureus.1229
30. Borg A, Ekanayake J, Mair R, Smedley T, Brew S, Kitchen N, et al. Preoperative particle and glue embolization of meningiomas: indications, results, and lessons learned from 117 consecutive patients. Neurosurgery. (2013) 73:ons244–51; discussion ons52. doi: 10.1227/NEU.0000000000000187
31. Dubel GJ, Ahn SH, Soares GM. Contemporary endovascular embolotherapy for meningioma. Semin Intervent Radiol. (2013) 30:263–77. doi: 10.1055/s-0033-1353479
32. Fujii K, O H, K A, K N, S H, K I, et al. Preoperative superselective embolization of skull-base meningiomas: Indications and limitations. J Neuro-Oncology. (1998) 40:67–71. doi: 10.1023/a:1006196420398
33. Macpherson P. The value of pre-operative embolisation of meningioma estimated subjectively and objectively. Neuroradiology. (1991) 33:334–7. doi: 10.1007/BF00587818
34. McDermott MW, QH A, K T, C K, S N, P A, et al. Pre-operative factors affecting resectability of giant intracranial meningiomas. Can J Neurological Sci / J Canadien Des Sci Neurologiques. (2009) 36(5):623–30. doi: 10.1017/s0317167100008143
35. Richling B, G A, K M, M P, B G. Preoperative embolization of intracranial meningiomas:A 17-years single center experience. min - Minimally Invasive Neurosurg. (2000) 43(1):18–29. doi: 10.1055/s-2000-8812
36. Spetzler RF, D BL, F RA, W RC, K MH, O NA, et al. Efficacy of endovascular treatment of meningiomas: evaluation with matched samples. AJNR Am J neuroradiol. (1994) 15(9):1675–80.
37. Dion J, K DF, E AJ, K GJ, M JM, J JA. Hemorrhagic complications in embolization of a meningioma: Case report and review of the literature. Neuroradiology. (1997) 39(12):877–80. doi: 10.1007/s002340050526
38. Schachenmayr W, R H. Preoperative embolization of intracranial meningiomas. Neurosurgery. (1983) 13(3):261–8. doi: 10.1227/00006123-198309000-00008
39. Teitelbaum G, L M, G SL, H G, H R, H V, et al. Embolization of neurosurgical lesions involving the ophthalmic artery. Neurosurgery. (1998) 43(6):1298–303. doi: 10.1097/00006123-199812000-00016
40. Houdart R, D R, C J, T J, M J. Embolization by superselective arteriography from the femoral route in neuroradiology review of 60 cases. Neuroradiology. (1973) 6(1):20–6. doi: 10.1007/BF00338854
41. Raper DM, Starke RM, Henderson F Jr., Ding D, Simon S, Evans AJ, et al. Preoperative embolization of intracranial meningiomas: efficacy, technical considerations, and complications. AJNR Am J Neuroradiol. (2014) 35:1798–804. doi: 10.3174/ajnr.A3919
42. Wirsching HG, Richter JK, Sahm F, Morel C, Krayenbuehl N, Rushing EJ, et al. Post-operative cardiovascular complications and time to recurrence in meningioma patients treated with versus without pre-operative embolization: a retrospective cohort study of 741 patients. J Neurooncol. (2018) 140:659–67. doi: 10.1007/s11060-018-2996-0
43. Michelsen JW, H SK. Therapeutic percutaneous embolization for extra-axial vascular lesions of the head, neck, and spine. J Neurosurg. (1975) 43(3):275–87. doi: 10.3171/jns.1975.43.3.0275
44. Richling B, G A, B G, K M. Preoperative embolization of hypervascular skull base tumors. min - Minimally Invasive Neurosurg. (2000) 43(2):62–71. doi: 10.1055/s-2000-8321
45. Ruscalleda J, M C, L P. Preoperative embolization of intracranial meningiomas. AJNR Am J neuroradiol. (1986) 7(5):963–72.
46. Hernandez Ortiz OH, Garcia Garcia HI, Munoz Ramirez F, Cardona Florez JS, Gil Valencia BA, Medina Mantilla SE, et al. Development of a prediction rule for diagnosing postoperative meningitis: a cross-sectional study. J Neurosurg. (2018) 128:262–71. doi: 10.3171/2016.10.JNS16379
47. Brouwer MC, van de Beek D. Management of bacterial central nervous system infections. Handb Clin Neurol. (2017) 140:349–64. doi: 10.1016/B978-0-444-63600-3.00019-2
48. van de Beek D, Brouwer MC, Thwaites GE, Tunkel AR. Advances in treatment of bacterial meningitis. Lancet. (2012) 380:1693–702. doi: 10.1016/S0140-6736(12)61186-6
49. Auburtin M, Wolff M, Charpentier J, Varon E, Le Tulzo Y, Girault C, et al. Detrimental role of delayed antibiotic administration and penicillin-nonsusceptible strains in adult intensive care unit patients with pneumococcal meningitis: the PNEUMOREA prospective multicenter study. Crit Care Med. (2006) 34:2758–65. doi: 10.1097/01.CCM.0000239434.26669.65
50. Vallejo JG, Cain AN, Mason EO, Kaplan SL, Hulten KG. Staphylococcus aureus central nervous system infections in children. Pediatr Infect Dis J. (2017) 36:947–51. doi: 10.1097/INF.0000000000001603
Keywords: meningioma, giant meningioma, sodium fluorescein, surgical operation, functional area
Citation: Cui J, Sun H, Sun S, Zhao H and Gu Y (2024) Case report: Giant meningioma of the left hemisphere. Front. Oncol. 14:1506297. doi: 10.3389/fonc.2024.1506297
Received: 04 October 2024; Accepted: 20 November 2024;
Published: 06 December 2024.
Edited by:
Ismail Zaed, Neurocenter of Southern Switzerland, SwitzerlandReviewed by:
Jacopo Falco, IRCCS Carlo Besta Neurological Institute Foundation, ItalyYavor Enchev, Medical University of Varna, Bulgaria
Copyright © 2024 Cui, Sun, Sun, Zhao and Gu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yinghao Gu, Z3V5aW5naGFvMTc4N0AxNjMuY29t
 Junxiang Cui
Junxiang Cui Hu Sun
Hu Sun Shuo Sun
Shuo Sun Hao Zhao
Hao Zhao Yinghao Gu
Yinghao Gu