
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
BRIEF RESEARCH REPORT article
Front. Oncol., 03 December 2024
Sec. Pediatric Oncology
Volume 14 - 2024 | https://doi.org/10.3389/fonc.2024.1501977
This article is part of the Research TopicCritical Complications In Pediatric Oncology and Hematopoietic Cell Transplant - Volume IIIView all 3 articles
 Kyle B. Lenz1*
Kyle B. Lenz1* R. Scott Watson1,2
R. Scott Watson1,2 Jennifer J. Wilkes3
Jennifer J. Wilkes3 Matthew R. Keller4
Matthew R. Keller4 Mary E. Hartman5
Mary E. Hartman5 Elizabeth Y. Killien1,2
Elizabeth Y. Killien1,2Children with cancer or hematopoietic cell transplant (HCT) frequently require ICU care. We conducted a retrospective cohort study using Healthcare Cost and Utilization Project’s State Inpatient Databases from 21 U.S. states from 2001-2019. We included children <18 years with oncologic or HCT diagnosis and used ICD-9-CM and ICD-10-CM codes to identify diagnoses, comorbidities, and organ failures. We used generalized linear Poisson regression and Cuzick’s test of trend to evaluate changes from 2001-2019. Among 2,157,991 total pediatric inpatient admissions, 3.9% (n=82,988) were among oncology patients and 0.3% (n=7,381) were among HCT patients. ICU admission prevalence rose from 13.6% in 2001 to 14.4% in 2019 for oncology admissions and declined from 23.9% to 19.5%, for HCT admissions. Between 2001-2019, the prevalence of chronic non-oncologic comorbidities among ICU patients rose from 44.3% to 69.1% for oncology patients (RR 1.60 [95% CI 1.46-1.66]) and from 41.4% to 81.5% (RR 1.94 [95% CI 1.61-2.34]) for HCT patients. The risk of Multiple Organ Dysfunction Syndrome more than tripled for oncology (9.5% to 33.3%; RR 3.52 [95% CI 2.97-4.18]) and HCT (12.4% to 39.7%; RR 3.20 [95% CI 2.09-4.89]) patients. Mortality decreased most for ICU patients with acute myeloid leukemia (AML) (14.6% to 8.5%) and oncology-related HCTs (15.5% to 9.2%). Critically ill pediatric oncology and HCT patients are increasingly medically complex with greater prevalence of chronic comorbidities and organ failure, but mortality did not increase. Pediatric ICUs may require increased financial and staffing support to care for these patients in the future.
Children with cancer frequently require pediatric intensive care unit (PICU) admission during their treatment course with PICU admission prevalence as high as 46% for some diagnoses (1–3). In some cohorts, the percentage of oncology patients admitted to a PICU has increased in recent years (4, 5). Children who undergo hemopoietic stem cell transplant (HCT) also have a high PICU admission prevalence, ranging from 15-39% (6, 7). Pediatric oncology patients admitted to the PICU have high rates of organ dysfunction and support needs (8–10), and HCT patients may experience additional complications including sinusoidal obstructive syndrome, graft versus host disease, transplant-associated thrombotic microangiopathy, and transplant-related respiratory failure (11–14). In addition to high morbidity rates, mortality compared to the general PICU population is three-fold higher for oncology patients (1, 9, 15) and eight-fold higher for HCT patients (16, 17).
Little is known about how patient characteristics, organ support, and outcomes have changed over time across the entire spectrum of ICUs that care for children with cancer or HCTs. One study in 36 children’s hospitals demonstrated that pediatric oncology patients admitted to PICUs received increased organ support between 2012-2021 (5), but no studies have included care occurring outside of academic PICUs. It is unknown how commonly children with oncologic conditions or HCTs are receiving care outside of children’s hospitals or academic PICUs. Additionally, no studies have compared ICU admissions, organ support, or outcomes between types of oncologic diagnoses. Better understanding of these facets of critically ill pediatric oncology and HCT populations could inform health care delivery and resource allocation by adjusting surveillance for high-risk groups and directing future research priorities.
We analyzed a nationally-representative dataset including all hospital admissions from 21 U.S. states to assess how ICU admission frequency, patient and hospital characteristics, organ dysfunction and support, and outcomes have changed for pediatric oncology and HCT patients from 2001 to 2019, and compared these factors across oncologic diagnoses. We hypothesized that, consistent with general PICU trends (18), ICU admission frequency, prevalence of chronic comorbid conditions, and organ dysfunction have all increased among pediatric oncology and HCT patients over the past two decades.
We conducted a retrospective population-based cohort study using the Healthcare Cost and Utilization Project’s (HCUP) State Inpatient Databases (SIDs) from 21 geographically disperse U.S. states in 2001, 2004, 2010, 2016, and 2019 (Supplementary Table 1). The SIDs include inpatient records for all discharges from non-federal acute care hospitals within each state. We included states that submitted SIDs to HCUP with revenue codes allowing identification of ICU care, and we followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline (19). The study was determined to be exempt from human subject review by the Seattle Children’s Hospital Institutional Review Board.
We included all children aged 0-17 years, excluding those in major diagnostic categories 14 (delivering a baby) and 15 (newborns and other neonates) and those admitted to rehabilitation and psychiatric hospitals. We identified ICU care using revenue codes and included admissions to any non-neonatal ICU in the analyses.
To evaluate representation compared to the general ICU population, we obtained patient characteristics including age, sex, race, ethnicity, and insurance status as reported by individual hospitals. We used International Classification of Diseases, Ninth and Tenth Revision (ICD-9 and ICD-10) codes to identify diagnoses, comorbid conditions, organ failures, and procedures (Supplementary Table 2). We categorized admissions into those involving hematologic malignancies, solid malignancies, and HCTs, as well as specific oncologic diagnoses and oncologic versus non-oncologic indications for HCT. We classified comorbidities using the Pediatric Complex Chronic Conditions classification system (20), excluding codes for hematologic or oncologic comorbidities. Technology dependence per the classification system includes presence of devices such as tracheostomies, gastrostomies, colostomies, ventricular shunts, dialysis access, and pacemakers. Metabolic comorbidities included endocrine, amino acid metabolism, lipid metabolism, and storage disorders. We identified organ failures using ICD-9 and ICD-10 codes per previously published algorithms (21, 22) and considered patients to have multiple organ dysfunction syndrome (MODS) if they had two or more dysfunctional organ systems. We estimated total hospital costs using hospital-specific cost-to-charge ratios adjusted to 2019 dollars using the Consumer Price Index (23).
We summarized categorical variables using percentages and continuous variables using medians and interquartile ranges (IQR). We determined the population-based rate of inpatient and ICU admissions per 1000 children using U.S. census data from each included year and calculated incidence rate ratios (IRR) for each year relative to 2001. We used generalized linear Poisson regression to estimate the relative risk (RR) of each categorical variable in each year relative to 2001 and linear regression to estimate mean change in each year relative to 2001 for continuous variables. We used Cuzick’s test of trend to determine overall trends from 2001-2019. We conducted all analyses using Stata version 17 (StataCorp LLC, College Station, TX).
In all study years combined, there were 2,157,991 pediatric hospital admissions, of which 12.8% (n=275,656) included ICU care. There were 82,988 admissions for children with oncologic diagnoses (3.8% of hospital admissions) and 7,381 admissions for children who had received an HCT (0.3% of hospital admissions) (Table 1). A total of 13.9% (n=11,517) of oncologic admissions and 23.7% (n=1,749) of HCT admissions included ICU admission.
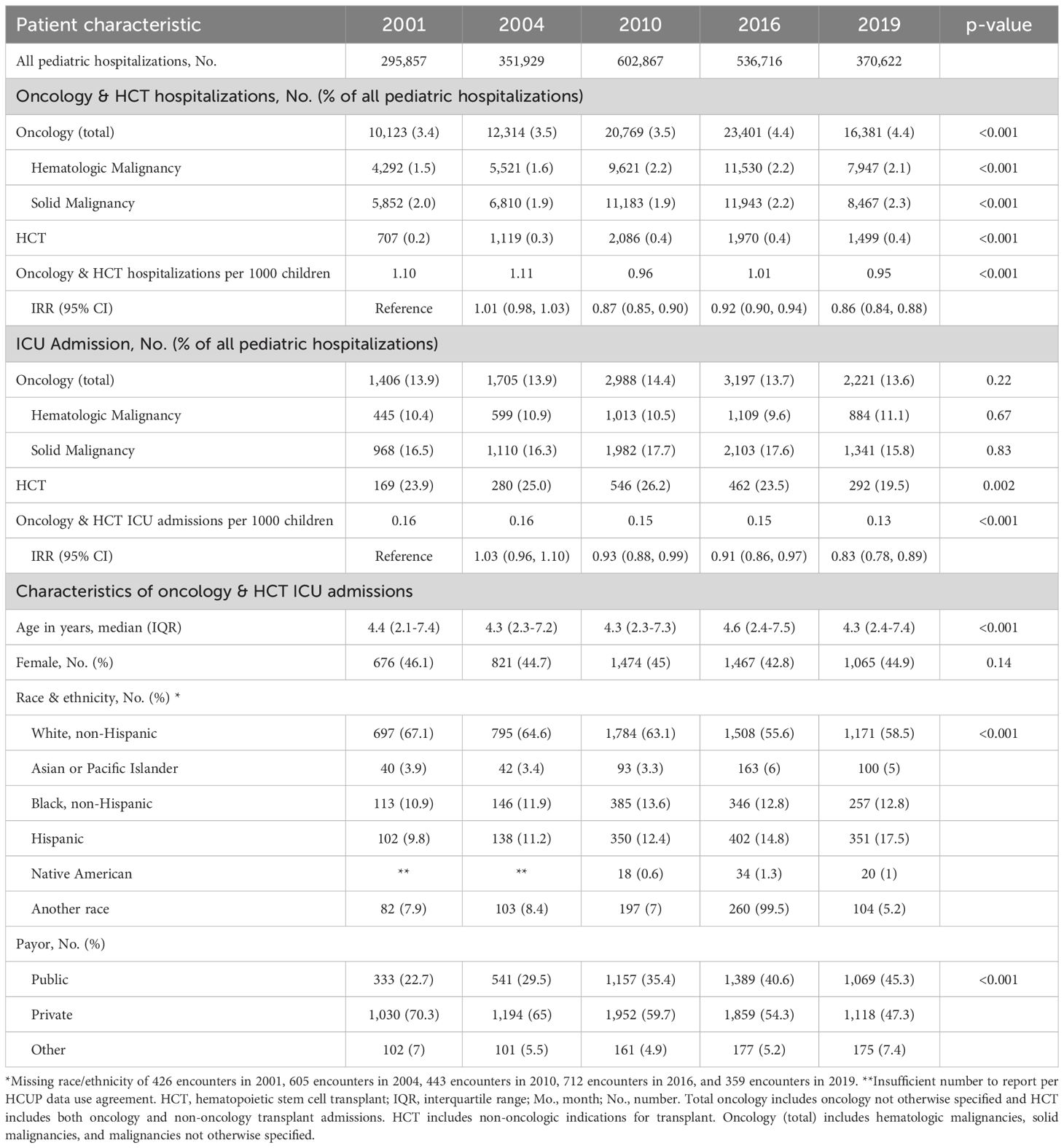
Table 1. Demographic traits of pediatric oncology and hematopoietic cell transplant patients admitted to U.S. intensive care units.
Among all oncology and HCT patients, age did not change substantially over time with a median age of 4.3 years (IQR 2.4-7.4) in 2019 (Table 1). The percentage of non-Hispanic White patients decreased from 67.1% in 2001 to 58.5% in 2019, while the percentage of Hispanic patients increased from 9.8% in 2001 to 17.5% in 2019. In contrast, Hispanic patients in the non-oncology ICU population only increased from 12.5% to 16.1%. The use of public insurance among oncology and HCT patients admitted to an ICU doubled from 22.7% in 2001 to 45.5% in 2019 (RR 1.99, 95% CI 1.79-2.21) (Table 1).
From 2001 to 2019, children with oncologic diagnoses comprised an increasing percentage of hospital admissions, rising from 3.4% in 2001 to 4.4% in 2019, and the percentage of HCT admissions increased from 0.2% in 2001 to 0.4% in 2019. (Table 1). ICU admission prevalence among hospitalized patients remained similar for patients with either hematologic or solid malignancies, while it decreased for HCT admissions from a peak of 26.2% in 2010 to 19.5% in 2019 (p=0.002).
In contrast, the population-based rate of combined oncology and HCT admissions decreased from 1.10 to 0.95 hospitalizations per 1000 children per year during that same period (IRR 0.86 [95% CI 0.84-0.88]). The population-based rate of ICU admissions for oncology and HCT patients decreased from 0.16 to 0.13 ICU admissions per 1000 children per year from 2001-2019 (IRR 0.83 [95% CI 0.78-0.89]) (Table 1).
Of 520 hospitals in the dataset that admitted children with oncologic conditions or HCTs across the five sample years, only 75 (14.4%) were dedicated children’s hospitals. Of the 180 hospitals that admitted pediatric oncology and HCT patients to the ICU, only 66 (36.7%) were children’s hospitals. There were 55 ICUs that provided care to >25 oncology or HCT patients in any given year, and of those, 38 (69.1%) were in a children’s hospital.
Patients were increasingly admitted to ICUs in children’s hospitals over the course of the study period, rising from 62.5% to 92.9% of oncology admissions (p<0.001) and from 71.0% to 88.8% of HCT admissions from 2001-2019 (p<0.001) (Supplementary Table 3). The prevalence of ICU admission for oncology patients declined from 14.1% in 2001 to 13.3% in 2019 (p=0.003) in children’s hospitals and increased from 13.6% to 14.5% in general hospitals. Among HCT patients, the prevalence of ICU admissions decreased from 33.7% in 2001 to 18.1% in 2019 in children’s hospitals and increased from 14.0% to 40.5% over the same period in general hospitals. Oncology and HCT patients treated in children’s hospitals were of similar age to patients treated in general hospitals but had higher prevalence of non-oncologic and non-hematologic comorbid conditions with higher in-hospital mortality and hospitalization costs (Supplementary Table 3).
The prevalence of chronic non-oncologic and non-hematologic comorbidities among children admitted for an oncologic diagnosis increased from 44.3% in 2001 to 69.1% in 2019 (RR 1.56 [95% CI 1.46-1.66]) (Figure 1). Children with HCTs had a greater increase in prevalence of chronic comorbidities from 41.4% to 80.5% (RR 1.94 [95% CI 1.61-2.34]) (Table 2). In 2019, the most common types of comorbidities among patients with hematologic malignancies were metabolic (35.5%), renal (21.7%), and cardiovascular (15.4%), while patients with solid malignancies most commonly had neuromuscular comorbidities (44.4%). The most common comorbidities among patients with HCTs were metabolic (33.2%) and gastrointestinal (31.9%). Each group had high rates of technology dependence, including 29.8% of patients with hematologic malignancies, 41.5% of patients with solid malignancies, and 52.1% of HCT patients (Supplementary Table 4).
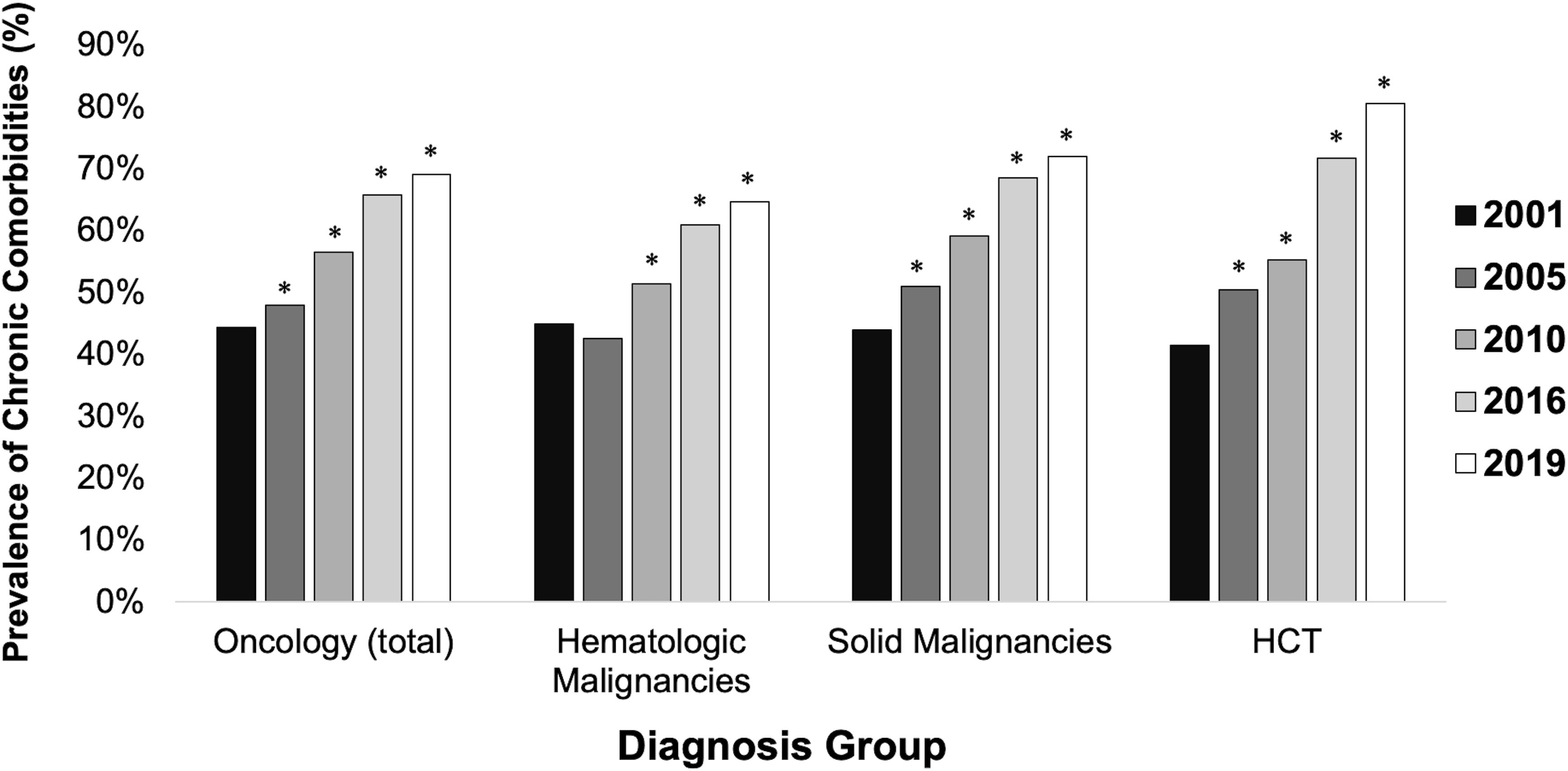
Figure 1. Prevalence of chronic comorbidities among intensive care unit pediatric patients by diagnosis group. * indicates statistically significant relative risk compared to referent group (2001). HCT = hematopoietic stem cell transplant. Comorbidities excluded oncologic and hematologic categories.
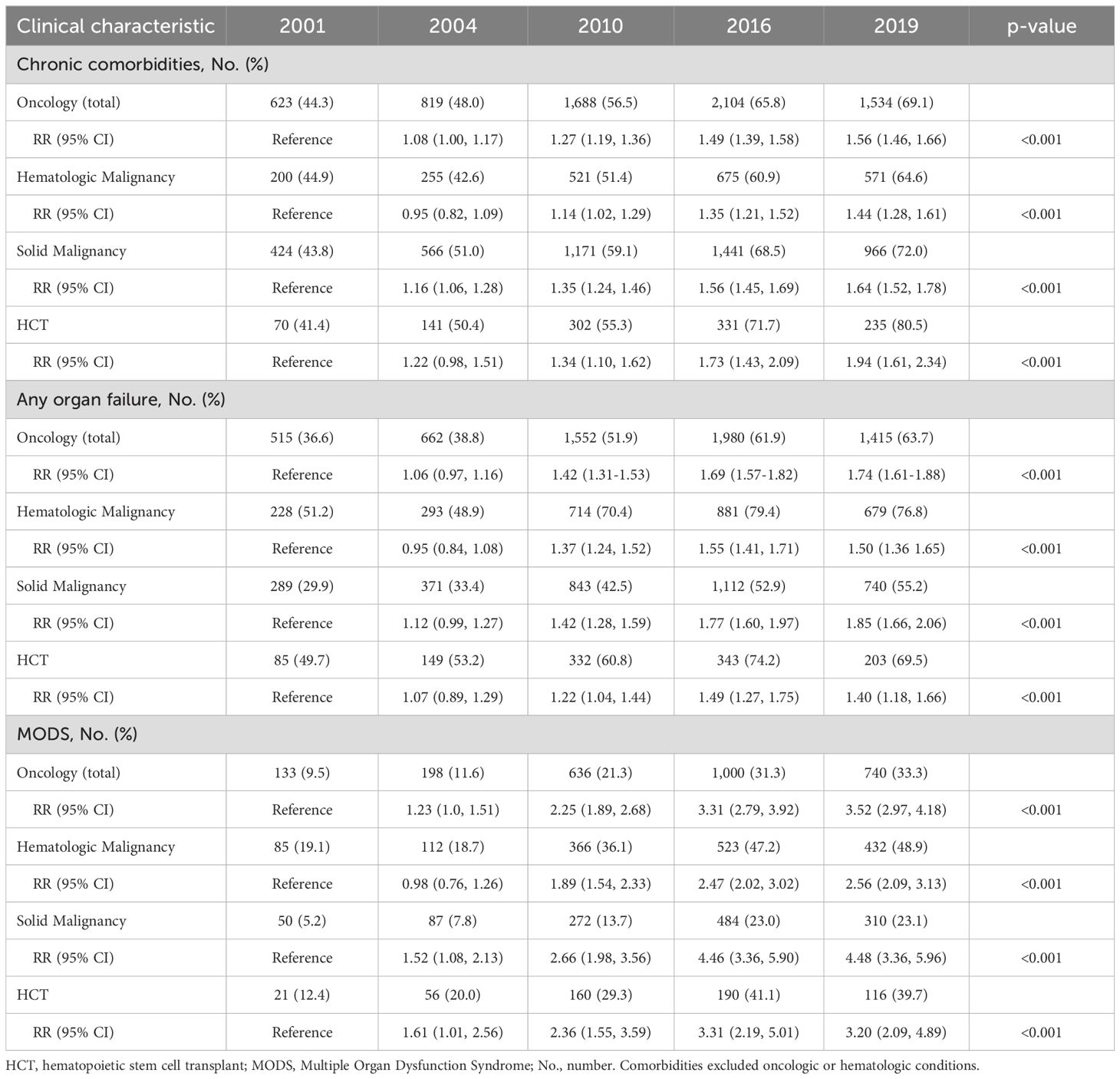
Table 2. Clinical traits of pediatric oncology and hematopoietic cell transplant patients admitted to U.S. intensive care units.
By 2019, 63.7% of oncology patients admitted to an ICU had at least one organ failure, an increase from 36.6% in 2001 (RR 1.74 [95% CI 1.61-1.88]) (Table 2). Patients with hematologic malignancies had the highest prevalence of organ failure at 76.8% in 2019, while patients with solid malignancies had the greatest rise over time from 29.9% in 2001 to 55.2% in 2019 (RR 1.85 [95% CI 1.66-2.06]). Prevalence of organ failure among HCT admissions increased from 49.7% to 69.5% (RR 1.40 [95% CI 1.18-1.66]). Neurologic failure was the most common organ failure for all subgroups and was highest among patients with hematologic malignancies (41.7%). Cardiovascular failure was the second most common organ failure among patients with hematologic malignancies, doubling from 18% to 36.5% from 2001-2019, while respiratory failure was the second most common organ failure among patients with solid malignancies or HCTs (Supplementary Table 5).
From 2001-2019, the percentage of admissions with MODS increased from 9.5% to 33.3% for oncology patients (RR 3.52, 95% CI 2.97-4.18) and from 12.4% to 39.7% for HCT patients (RR 3.20, 95% CI 2.09-4.89). MODS prevalence was highest among patients with hematologic malignancies at 48.9% in 2019 (Table 2). Use of mechanical ventilation did not change over time, while both hematologic malignancy and HCT admissions had increasing prevalence of dialysis use, peaking at 7.1% and 7.9% respectively in 2019. The prevalence of extracorporeal membrane oxygenation support (ECMO)was highest among patients with hematologic malignancies, increasing from 0.3% in 2001 to 1.2% in 2019 (Supplementary Table 6). ECMO use ranged from 0% to 1%, depending on the year, in patients with HCT.
The median hospital LOS remained unchanged for all groups between 2001 and 2019, with the longest LOS among patients with hematologic malignancies. Despite stable LOS, hospitalization costs increased in each group. Hematologic malignancy admissions had the highest median cost, reaching $51,895 in 2019, and HCT admissions had the largest increase from $23,622 in 2001 to $40,958 per admission in 2019 (p<0.001) (Table 3).
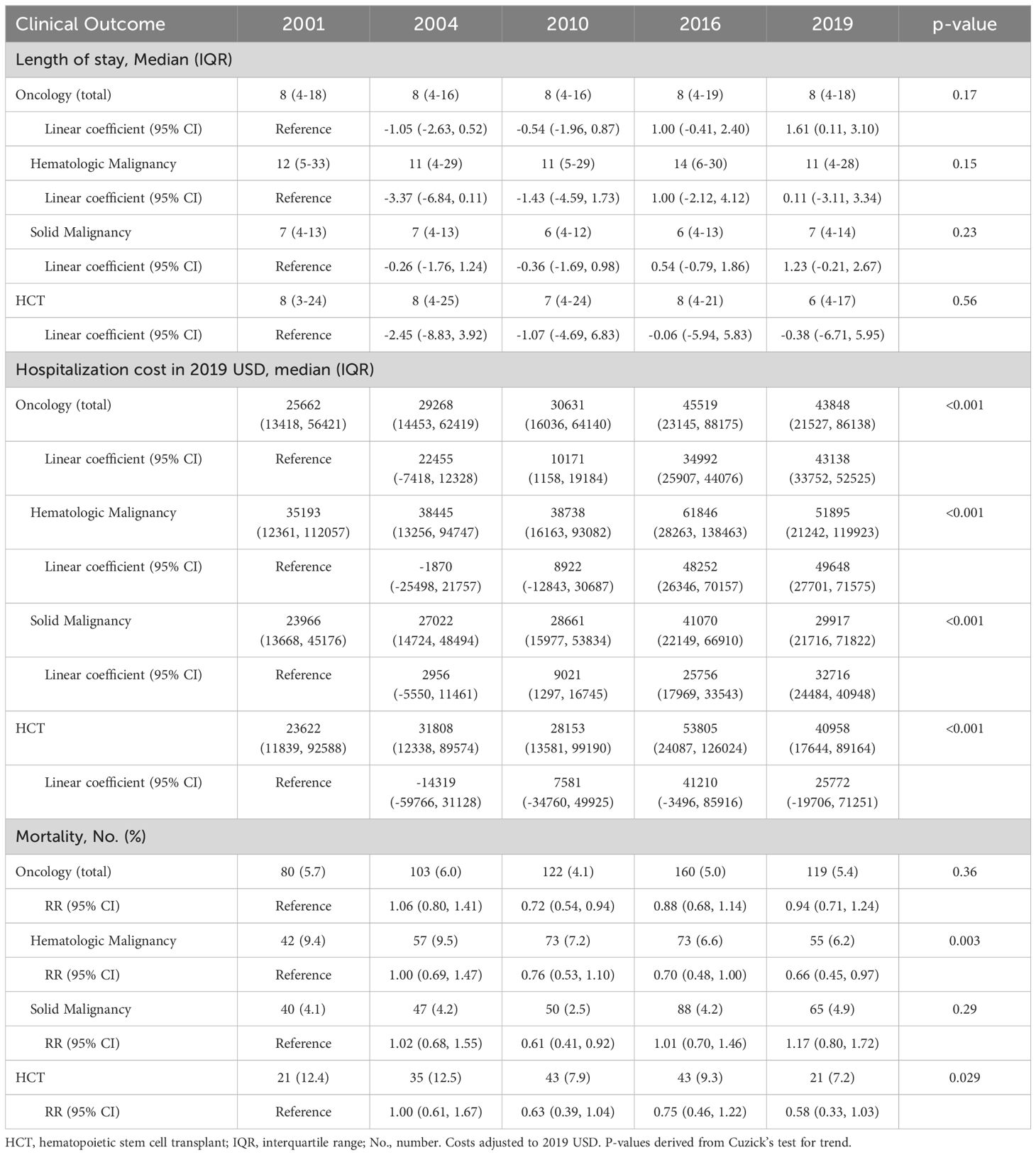
Table 3. Outcomes of pediatric oncology and hematopoietic cell transplant patients admitted to U.S. intensive care units.
Hospital mortality for oncology admissions overall ranged from 4.1-5.7% in each included year, with no change from 2001 to 2019. Mortality declined for admissions with hematologic malignancies from 9.4% in 2001 to 6.2% in 2019 (RR 0.66 [95% CI 0.45-0.97]) while mortality for solid malignancy admissions remained unchanged, ranging from 2.5-4.9% in each year. Mortality for HCT admissions declined from 12.4% in 2001 to 7.2% in 2019 (RR 0.58 [95% CI 0.33-1.03]) (Table 3).
Among hematologic malignancies, hospitalized patients with acute lymphoblastic leukemia (ALL) and lymphoma had an increasing ICU admission prevalence, rising from 8.8% to 10.4% of admissions for patients with ALL and from 8.5% to 10.2% of admissions for patients with lymphoma from 2001-2019. Among solid tumors, brain tumors had the largest increase in ICU admission prevalence from 28.9% to 35.3% of admissions from 2001-2019, while the prevalence of ICU admissions for patients with neuroblastomas decreased from 19.4% to 13.0%. Among HCT patients, ICU admission prevalence was higher for patients with non-oncologic HCTs than oncologic HCTs. ICU admission prevalence declined over time for patients with non-oncologic HCTs from 27.4% in 2001 to 22.5% in 2019 and remained similar for oncology-related HCTs (Supplementary Table 7).
The prevalence of MODS increased from 2001-2019 across all oncologic diagnoses evaluated, with the highest prevalence of MODS observed among patients with acute myeloid leukemia (AML), with an increase from 24.4% to 63.3%, and oncologic HCTs, with an increase from 13.6% to 44.0% (Supplementary Figure 1). MODS prevalence was lowest among patients with nerve tumors (9.5% in 2019) and brain tumors (19.6%).
Despite the increase in MODS, mortality decreased for patients with AML from 14.6% of ICU admissions in 2001 to 8.5% in 2019, while mortality remained similar from 2001-2019 for patients with other oncologic diagnoses including ALL (7.6% to 7.1%), brain tumors (5.1% to 5.0%), and neuroblastoma (5.9% to 3.9%). Among patients with HCTs, mortality was lower for non-oncologic HCT patients than oncologic HCT patients throughout the time period, but with a greater relative decline for oncologic HCT patients from 15.5% in 2001 to 9.2% in 2019 (Supplementary Table 7).
In the largest study to date of longitudinal trends in the critically ill pediatric oncology and HCT population in the U.S., we found that oncology and HCT patients requiring ICU care are becoming increasingly medically complex with rising prevalence of MODS. Organ dysfunction was most prevalent among patients with hematologic malignancies, particularly AML. Despite this, mortality remained similar or improved across all oncology and HCT subtypes. By using a large, population-based dataset including patients from 21 states and 520 general and children’s hospitals, we have for the first time provided an assessment of ICU admission trends and outcomes across the range of facilities caring for children with oncologic conditions and HCTs. We have augmented previous studies by examining mortality and morbidity trends in unique oncologic and HCT subgroups. With this new information, clinicians can target specific patient populations for further research and intervention.
Previous studies have found an increase in the ICU admission rate for oncology patients (4, 5, 24) yet our study did not find a universal trend across groups, with ICU admission prevalence among hospitalized patients rising for patients with ALL, lymphoma, and brain tumors but remaining similar or decreasing in other groups. These trends may be in part due to novel therapeutics, such as chimeric antigen receptor T cells (CAR-T), being more readily available for certain types of diagnoses and not others. Although, generally well-tolerated, CAR-T therapy has known side effects such as Cytokine Release Syndrome (CRS) or Immune effector cell-Associated Neurotoxicity Syndrome (ICANS) that frequently result in ICU-level of care (25–27). Other immunotherapies have expanded in their application, as is the case of bispecific T cell engagers like blinatumomab. These therapies, used both in primary and relapsed leukemias, can be accompanied fever, hypotension, or encephalopathy (28). Declines in ICU admission prevalence over time among patients with neuroblastoma and both oncologic and non-oncologic HCTs may be due to changes in treatment regimens and improved management of therapeutic toxicities (29). To our knowledge, no other study investigated ICU admissions by both broad and specific oncologic diagnoses; by examining both of these categories, our study provides insight into which diagnoses may be driving overall trends.
We found that pediatric oncology and HCT patients are being admitted to the ICU with greater underlying and acute complexity: a higher prevalence of chronic comorbid conditions, at least one organ failure, and MODS. Oncology patients frequently require interventions such as mechanical ventilation, inotropic support, or central vascular access due to acute illness severity (8), and together with HCT patients have higher illness severity scores and mortality than the general pediatric ICU population (9, 13, 17, 30). We found that neurologic failure was the most frequent organ failure in all groups. Neurologic complications in pediatric oncology patients are common, and there are many potential etiologies including effects of chemotherapy, radiation, infection, procedures, surgery, and the neoplasm itself (31). The diversity of etiologies may partially explain why patients experienced neurologic failure at higher rates than other organ systems. Another common organ failure was cardiovascular compromise. Sepsis may play a role in this increased risk of cardiovascular failure, but our dataset lacks the granular clinical data necessary to make an association between the two. This increase in cardiovascular failure may be multifactorial and related to cardiovascular risk factors, treatment side effects, or an increase in chronic cardiovascular comorbidities. Similarly, the increased prevalence of chronic comorbidities in patients may predispose them to the increased observed prevalence of organ failure and MODS. Patients with relapsed leukemic disease also may experience new baseline organ dysfunction as a consequence of their disease and treatment requirements (32). Their disease may be salvageable but necessitate more frequent ICU care. Our findings of increased organ failures over time suggest that illness severity in these populations continues to rise and that clinicians will be increasingly managing multi-organ failure in these vulnerable patients. In the context of existing immense variability in resource use (33), hospitals and ICUs may need to dedicate additional ICU staff and resources to care for children with oncologic conditions and HCTs given the escalating proportion of admissions with severe illness and high resource needs.
Despite changes in illness severity, we did not find any change in LOS across the groups. We did, however, identify an increase in hospital costs, which may be related to increased illness severity and ICU interventions and costs of new cancer therapies. We noted a trend of increased ECMO use in oncologic diagnoses, which is consistent with recent studies (34, 35), and may contribute to the rising costs. Access to specialized cancer therapies and associated clinical trials may also be contributing to the increasing prevalence of oncology and HCT care occurring in specialized pediatric hospitals. Increasing regionalization of care for critically ill pediatric oncology and HCT patients may contribute to improved outcomes (36), though may also contribute to delays in care for patients living in geographically disperse areas (37). Some evidence suggests that increased geographical distance to treatment centers portends worse mortality trends in ALL (38). However, one study conducted in rural Virginia found comparable survival outcomes in adults with AML (39). While further studies are needed to determine the extent of travel that patients require to receive specialized care and how it impacts severity of illness and outcomes, investment in supplementary efforts such as telehealth, education, and collaboration with local healthcare providers may reduce undesirable outcomes. Importantly, we found that many general hospitals continue to admit children with cancer and HCTs to both the inpatient ward and ICUs, suggesting the need to include both children’s and non-children’s hospitals in efforts to improve processes of care and outcomes for pediatric oncology and HCT populations.
There were several limitations in our study. First, the included states varied each year, and only four states were included in all 5 years of data analyzed. Second, all counts refer to admissions rather than patients so we cannot determine the extent to which encounters were representative of individual patients or readmissions of the same patient. Third, we excluded patients 18 years or older despite many pediatric hospitals treating young adult patients with oncologic diagnoses, and exclusion of these patients limits our ability to understand their contribution to trends and findings. Fourth, diagnoses, comorbid conditions, and organ failures were determined by ICD codes, and changes in prevalence may reflect changes between ICD-9 and ICD-10 coding, which has imperfect sensitivity and specificity. Changes in coding practices over time may have particularly influenced the frequency with which comorbid conditions and organ failures were recorded. Finally, we were unable to identify the timing of events such as development of organ failures or mortality and thus cannot determine whether they occurred during the ICU stay.
Pediatric oncology and HCT patients are increasingly medically complex, with increasing prevalence of baseline comorbid conditions and increasing illness severity if admitted to an ICU. Despite this, mortality has improved for critically ill oncology and HCT patients. While care is becoming increasingly regionalized to children’s hospitals, many general hospitals continue to admit pediatric oncology and HCT patients, and ICU admission prevalence varies widely based on the type of hospital in which they receive care. These facilities will increasingly need to devote ICU resources to care for these children, especially with the ongoing development of novel immunotherapies, and further support for both children’s and general hospitals may be needed to continue to improve outcomes.
The data analyzed in this study is subject to the following licenses/restrictions: Cost, Data Use Agreement. Requests to access these datasets should be directed to https://hcup-us.ahrq.gov/sidoverview.jsp.
KL: Conceptualization, Data curation, Formal analysis, Methodology, Validation, Visualization, Writing – original draft, Writing – review & editing. RW: Conceptualization, Formal analysis, Methodology, Supervision, Writing – original draft, Writing – review & editing. JW: Formal analysis, Methodology, Writing – original draft, Writing – review & editing. MK: Formal analysis, Writing – review & editing. MH: Conceptualization, Formal analysis, Writing – review & editing. EK: Data curation, Formal analysis, Methodology, Resources, Supervision, Writing – original draft, Writing – review & editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development grant K23HD100566 (Killien), the University of Washington’s Institute of Translational Health Sciences which is supported in part by the National Center for Advancing Translational Sciences (NCATS) of the NIH under Award Number UL1 TR002319, and the Washington University Center for Administrative Data Research which is supported in part by the Washington University Institute of Clinical and Translational Sciences grant UL1 TR002345 from the NCATS of the NIH.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2024.1501977/full#supplementary-material
1. Wösten-van Asperen RM, van Gestel JPJ, van Grotel M, Tschiedel E, Dohna-Schwake C, Valla FV, et al. PICU mortality of children with cancer admitted to pediatric intensive care unit a systematic review and meta-analysis. Crit Rev Oncol Hematol. (2019) 142:153–63. doi: 10.1016/j.critrevonc.2019.07.014
2. Ranta S, Broman LM, Abrahamsson J, Karlsson L, Noren-Nystrom U, Palle J, et al. High need for intensive care in paediatric acute myeloid leukaemia: A population-based study. Acta Paediatr. (2022) 111:2235–41. doi: 10.1111/apa.v111.11
3. Rosenman MB, Vik T, Hui SL, Breitfeld PP. Hospital resource utilization in childhood cancer. J Pediatr Hematol Oncol. (2005) 27:295–300. doi: 10.1097/01.mph.0000168724.19025.a4
4. Wraight TI, Namachivayam SP, Maiden MJ, Erickson SJ, Oberender F, Singh P, et al. Trends in childhood oncology admissions to ICUs in Australia and New Zealand. Pediatr Crit Care Med. (2023) 24(10):e487–97. doi: 10.1097/PCC.0000000000003268
5. Rogerson CM, Rowan CM. Critical care utilization in children with cancer: U.S. Pediatric health information system database cohort 2012-2021. Pediatr Crit Care Med. (2024) 25:e52–e8. doi: 10.1097/PCC.0000000000003380
6. Zinter MS, Brazauskas R, Strom J, Chen S, Bo-Subait S, Sharma A, et al. Intensive care risk and long-term outcomes in pediatric allogeneic hematopoietic cell transplant recipients. Blood Adv. (2024) 8:1002–17. doi: 10.1182/bloodadvances.2023011002
7. Johnson AK, Cornea S, Goldfarb S, Cao Q, Heneghan JA, Gupta AO. Risk factors predicting need for the pediatric intensive care unit (PICU) post-hematopoietic cell transplant, PICU utilization, and outcomes following HCT: a single center retrospective analysis. Front Pediatr. (2024) 12:1385153. doi: 10.3389/fped.2024.1385153
8. Caballero M, Faura A, Margarit A, Bobillo-Perez S, Català A, Alonso-Saladrigues A, et al. Outcomes for paediatric acute leukaemia patients admitted to the paediatric intensive care unit. Eur J Pediatr. (2022) 181:1037–45. doi: 10.1007/s00431-021-04292-9
9. Zinter MS, DuBois SG, Spicer A, Matthay K, Sapru A. Pediatric cancer type predicts infection rate, need for critical care intervention, and mortality in the pediatric intensive care unit. Intensive Care Med. (2014) 40:1536–44. doi: 10.1007/s00134-014-3389-2
10. Gaugler M, Swinger N, Rahrig AL, Skiles J, Rowan CM. Multiple organ dysfunction and critically ill children with acute myeloid leukemia: single-center retrospective cohort study. Pediatr Crit Care Med. (2023) 24:e170–e8. doi: 10.1097/PCC.0000000000003153
11. Lindell RB, Gertz SJ, Rowan CM, McArthur J, Beske F, Plunkett A, et al. High levels of morbidity and mortality among pediatric hematopoietic cell transplant recipients with severe sepsis: insights from the sepsis PRevalence, OUtcomes, and therapies international point prevalence study. Pediatr Crit Care Med. (2017) 18:1114–25. doi: 10.1097/PCC.0000000000001338
12. Rowan CM, Gertz SJ, McArthur J, Fitzgerald JC, Nitu ME, Loomis A, et al. Invasive mechanical ventilation and mortality in pediatric hematopoietic stem cell transplantation: A multicenter study. Pediatr Crit Care Med. (2016) 17:294–302. doi: 10.1097/PCC.0000000000000673
13. Lenz KB, Nishisaki A, Lindell RB, Yehya N, Laverriere EK, Bruins BB, et al. Peri-intubation adverse events in the critically ill child after hematopoietic cell transplant. Pediatr Crit Care Med. (2023) 24:584–93. doi: 10.1097/PCC.0000000000003243
14. Lindell RB, Fitzgerald JC, Rowan CM, Flori HR, Di Nardo M, Napolitano N, et al. The use and duration of preintubation respiratory support is associated with increased mortality in immunocompromised children with acute respiratory failure. Crit Care Med. (2022) 50:1127–37. doi: 10.1097/CCM.0000000000005535
15. Maude SL, Fitzgerald JC, Fisher BT, Li Y, Huang YS, Torp K, et al. Outcome of pediatric acute myeloid leukemia patients receiving intensive care in the United States. Pediatr Crit Care Med. (2014) 15:112–20. doi: 10.1097/PCC.0000000000000042
16. Balit CR, Horan R, Dorofaeff T, Frndova H, Doyle J, Cox PN. Pediatric hematopoietic stem cell transplant and intensive care: have things changed? Pediatr Crit Care Med. (2016) 17:e109–16. doi: 10.1097/PCC.0000000000000607
17. Zinter MS, Dvorak CC, Spicer A, Cowan MJ, Sapru A. New insights into multicenter PICU mortality among pediatric hematopoietic stem cell transplant patients. Crit Care Med. (2015) 43:1986–94. doi: 10.1097/CCM.0000000000001085
18. Killien EY, Keller MR, Watson RS, Hartman ME. Epidemiology of intensive care admissions for children in the US from 2001 to 2019. JAMA Pediatr. (2023) 177:506–15. doi: 10.1001/jamapediatrics.2023.0184
19. Vandenbroucke JP, von Elm E, Altman DG, Gotzsche PC, Mulrow CD, Pocock SJ, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. PloS Med. (2007) 4:e297. doi: 10.1097/EDE.0b013e3181577511
20. Feudtner C, Feinstein JA, Zhong W, Hall M, Dai D. Pediatric complex chronic conditions classification system version 2: updated for ICD-10 and complex medical technology dependence and transplantation. BMC Pediatr. (2014) 14:199. doi: 10.1186/1471-2431-14-199
21. Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. (2003) 348:1546–54. doi: 10.1056/NEJMoa022139
22. Johnston JA, Yi MS, Britto MT, Mrus JM. Importance of organ dysfunction in determining hospital outcomes in children. J Pediatr. (2004) 144:595–601. doi: 10.1016/j.jpeds.2004.01.045
24. Pechlaner A, Kropshofer G, Crazzolara R, Hetzer B, Pechlaner R, Cortina G. Mortality of hemato-oncologic patients admitted to a pediatric intensive care unit: A single-center experience. Front Pediatr. (2022) 10:795158. doi: 10.3389/fped.2022.795158
25. Logan GE, Miller K, Kohler ME, Loi M, Maddux AB. Outcomes of critically ill children with acute lymphoblastic leukemia and cytokine release syndrome due to chimeric antigen receptor T cell therapy: US, multicenter PICU, cohort database study. Pediatr Crit Care Med. (2022) 23:e595–600. doi: 10.1097/PCC.0000000000003079
26. Hucks G, Rheingold SR. The journey to CAR T cell therapy: the pediatric and young adult experience with relapsed or refractory B-ALL. Blood Cancer J. (2019) 9:10. doi: 10.1038/s41408-018-0164-6
27. Sheth VS, Gauthier J. Taming the beast: CRS and ICANS after CAR T-cell therapy for ALL. Bone Marrow Transplant. (2021) 56:552–66. doi: 10.1038/s41409-020-01134-4
28. Marrapodi MM, Mascolo A, di Mauro G, Mondillo G, Pota E, Rossi F. The safety of blinatumomab in pediatric patients with acute lymphoblastic leukemia: A systematic review and meta-analysis. Front Pediatr. (2022) 10:929122. doi: 10.3389/fped.2022.929122
29. Barone G, Barry A, Bautista F, Brichard B, Defachelles AS, Herd F, et al. Managing adverse events associated with dinutuximab beta treatment in patients with high-risk neuroblastoma: practical guidance. Paediatr Drugs. (2021) 23:537–48. doi: 10.1007/s40272-021-00469-9
30. Rowan CM, Fitzgerald JC, Agulnik A, Zinter MS, Sharron MP, Slaven JE, et al. Risk factors for noninvasive ventilation failure in children post-hematopoietic cell transplant. Front Oncol. (2021) 11:653607. doi: 10.3389/fonc.2021.653607
31. Sun LR, Cooper S. Neurological complications of the treatment of pediatric neoplastic disorders. Pediatr Neurol. (2018) 85:33–42. doi: 10.1016/j.pediatrneurol.2018.05.011
32. Sun W, Malvar J, Sposto R, Verma A, Wilkes JJ, Dennis R, et al. Outcome of children with multiply relapsed B-cell acute lymphoblastic leukemia: a therapeutic advances in childhood leukemia & lymphoma study. Leukemia. (2018) 32:2316–25. doi: 10.1038/s41375-018-0094-0
33. Fitzgerald JC, Li Y, Fisher BT, Huang YS, Miller TP, Bagatell R, et al. Hospital variation in intensive care resource utilization and mortality in newly diagnosed pediatric leukemia. Pediatr Crit Care Med. (2018) 19:e312–e20. doi: 10.1097/PCC.0000000000001525
34. Suzuki Y, Cass SH, Kugelmann A, Mobli K, Taylor WP, Radhakrishnan RS. Outcome of extracorporeal membrane oxygenation for pediatric patients with neoplasm: an extracorporeal life support organization database study (2000-2019). Pediatr Crit Care Med. (2022) 23:e240–e8. doi: 10.1097/PCC.0000000000002915
35. Mowrer MC, Lima L, Nair R, Li X, Sandhu H, Bridges B, et al. Pediatric hematology and oncology patients on extracorporeal membrane oxygenation: outcomes in a multicenter, retrospective cohort 2009-2021. Pediatr Crit Care Med. (2024) 25(11):1026–34. doi: 10.1097/PCC.0000000000003584
36. Gupta P, Rettiganti M, Fisher PL, Chang AC, Rice TB, Wetzel RC. Association of freestanding children’s hospitals with outcomes in children with critical illness. Crit Care Med. (2016) 44:2131–8. doi: 10.1097/CCM.0000000000001961
37. Cushing AM, Bucholz E, Michelson KA. Trends in regionalization of emergency care for common pediatric conditions. Pediatrics. (2020) 145:4. doi: 10.1542/peds.2019-2989
38. Rotz SJ, Wei W, Thomas SM, Hanna R. Distance to treatment center is associated with survival in children and young adults with acute lymphoblastic leukemia. Cancer. (2020) 126:5319–27. doi: 10.1002/cncr.v126.24
Keywords: pediatric, critical care, hematopoietic stem cell transplant, oncology, epidemiology
Citation: Lenz KB, Watson RS, Wilkes JJ, Keller MR, Hartman ME and Killien EY (2024) The epidemiology of pediatric oncology and hematopoietic cell transplant admissions to U.S. intensive care units from 2001-2019. Front. Oncol. 14:1501977. doi: 10.3389/fonc.2024.1501977
Received: 26 September 2024; Accepted: 18 November 2024;
Published: 03 December 2024.
Edited by:
Jennifer Ann McArthur, St. Jude Children’s Research Hospital, United StatesReviewed by:
Saad Ghafoor, St. Jude Children’s Research Hospital, United StatesCopyright © 2024 Lenz, Watson, Wilkes, Keller, Hartman and Killien. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kyle B. Lenz, a3lsZS5sZW56QHNlYXR0bGVjaGlsZHJlbnMub3Jn
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.