- 1Department of Gastroenterology, Shengli Oilfield Central Hospital, Dongying, China
- 2Shengli Oilfield Central Hospital, Affiliated Binzhou Medical University, Dongying, Shandong, China
- 3Department of pathology, Shengli Oilfield Central Hospital, Dongying, China
Introduction: Gastric glomus tumor is a rare submucosal mesenchymal tumor with no distinct features on endoscopy. In clinical practice, it is often treated with laparoscopic partial gastrectomy. Here, we report a case of gastric glomus tumor successfully resected using a combination of gastroscopy and laparoscopy.
Case description: The patient was an elderly male who underwent gastroscopy, which revealed a submucosal mass in the gastric antrum. The lesion was suspected to be a stromal tumor. Further evaluation with computed tomography (CT) imaging indicated a space-occupying lesion in the gastric antrum, with the possibility of benign pathology. Endoscopic ultrasonography revealed that the tumor originated from the muscular layer and was approximately 28.8 mm ×22.5 mm. Blood flow was detected behind the lesion, suggesting the possibility of a gastric glomus tumor. The tumor was removed using a combination of gastroscopy and laparoscopy. Postoperative pathology suggested that it was a benign gastric glomus tumor. The patient recovered uneventfully and was discharged 7 days later.
Conclusion: Gastric glomus tumor is a rare submucosal tumor that should be included in the differential diagnosis of gastric submucosal tumors. A combined approach using gastroscopy and laparoscopy offers a minimally invasive and effective treatment option.
Introduction
A glomus tumor is a rare mesenchymal neoplasm originating from the neuromuscular arterial canal or glomus body. While common in the extremities, it is rarely found in internal organs. However, tumors are reported in the mediastinum, trachea, kidneys, uterus, vagina, and stomach (1–3). Gastric glomus tumors (GGT) typically occur in the antrum or pylorus, involving the muscularis propria or submucosa. GGT accounts for only 1% of stromal tumors in the stomach (4). GGT can manifest as indigestion, upper abdominal pain, nausea, vomiting, hematemesis, or melena in clinical practice. However, they lack specific clinical manifestations, and some cases are asymptomatic (5). Owing to the lack of specific clinical and endoscopic features, GGT is often misdiagnosed as more common gastrointestinal stromal tumors (6). Here, we report a case of a GGT resected using a combination of endoscopy and laparoscopy.
Case description
The patient was an elderly male without any underlying diseases. A routine health checkup revealed a submucosal bulge in the gastric antrum during gastroscopy (Figure 1A). We conducted tests on blood routine, liver and kidney function, electrolytes, and gastrointestinal tumor markers, all of which showed no significant abnormalities. Further improvement of the CT scan indicated an occupied lesion in the gastric antrum, which was considered benign (Figure 1B). Endoscopic ultrasonography (EUS) revealed that the tumor originated from the muscle layer and measured approximately 28.8 × 22.5 mm. A small amount of blood flow was observed within the tumor, and blood vessels behind it were also visualized (Figure 1C). The tumor exhibited mixed echogenicity, with a clear boundary and grew inside and outside the gastric cavity. We considered the tumor to be more of a gastric stromal tumor; however, glomus tumors and schwannomas could not be excluded. To ensure accurate removal, laparoscopy combined with gastroscopy was performed. First, an endoscopic gastric tumor resection was conducted under general anesthesia with tracheal intubation. During the procedure, a 30 mm bulge was observed on the greater curvature of the gastric antrum. Using a Dual knife, we incised the mucosa down to the muscularis propria, exposed the tumor, gradually dissected it to reveal most of its structure, and then proceeded with laparoscopic resection. Under the laparoscope, a 5 mm perforation was observed approximately 30 mm from the anterior wall of the gastric antrum, surrounded by a small amount of bloody effusion. An ultrasonic scalpel was used to carefully dissect the edge of the tumor and the perforation site while ensuring the gastric wall was protected. After surgery, the size of the tumor was approximately 30 mm × 30 mm, and the capsule remained intact. Histopathological analysis revealed that the tumor was clearly confined to the submucosa. Microscopic examination revealed that it consisted of clusters of round and spindle-shaped cells surrounded by capillaries (Figure 1D). Immunohistochemistry revealed positive expression of smooth muscle actin and vimentin (Figures 1E, F) but negative expression of synaptophysin (Syn) and C-kit (Figures 1G, H). Based on these findings, the tumor was diagnosed as a GGT. After surgery, patients were restricted from eating, and a gastric tube was used for drainage and decompression. Nutritional and electrolyte support was administered intravenously to meet their basic physiological needs. After 3 days, the patient transitioned to a liquid diet and was discharged after 7 days. The patient was followed up at our hospital 1year after the operation. No abdominal pain, abdominal distension, or other discomfort were observed. Gastroscopy was performed again, and the analysis revealed that the surgical wound was perfectly healed (Figure 1I).
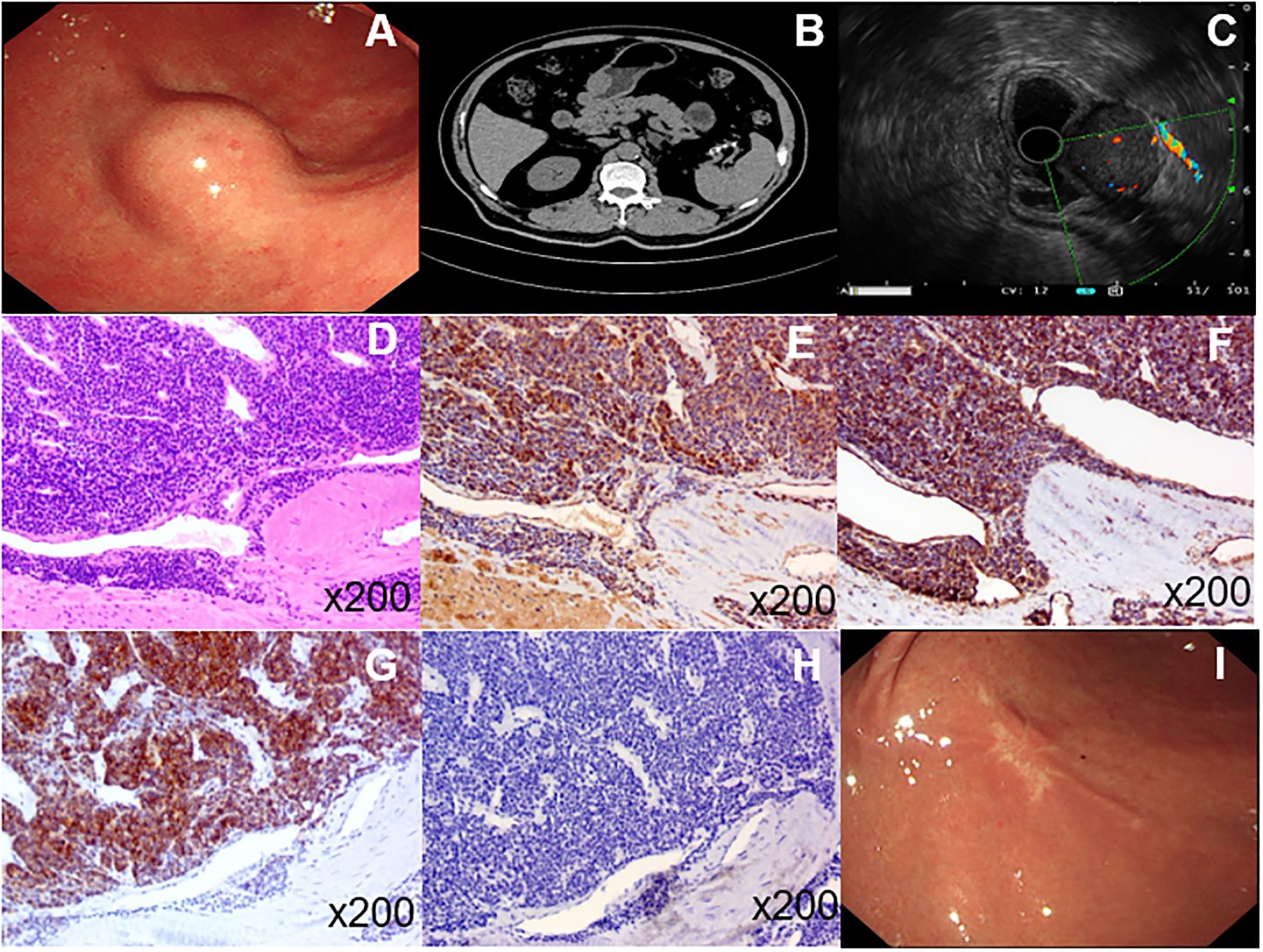
Figure 1. (A), Endoscopy of the upper digestive tract revealed a submucosal tumor in the gastric antrum. (B), CT imaging identified a space-occupying lesion in the gastric antrum. (C), Endoscopic ultrasonography showed that the tumor originated from the muscular layer with minimal blood flow inside and detectable blood vessels behind it. Under the microscope, the tumor appeared to be composed of aggregated round and spindle-shaped cells surrounded by capillaries (D). Immunohistochemistry revealed positive expression of smooth muscle actin and vimentin (E, F); however, no expression of syn and C-kit (G, H). The patient was re-examined by gastroscopy 1 year after the operation, and the wound healed well (I).
Glomus tumor is a rare mesenchymal tumor originating from the neuromuscular arterial canal or glomus. Gastrointestinal glomus tumors are rare within the stomach, particularly the gastric antrum, being the most common site of involvement. These tumors typically range in size from 8–110 mm and are often misdiagnosed as the more common gastrointestinal stromal tumors. It is often difficult to diagnose gastric glomus tumors using conventional imaging examinations, such as computed tomography and magnetic resonance imaging (7). EUS clearly identifies the origin of the lesion, reveals the stratification of the digestive tract wall, and provides information on echo intensity, echo uniformity, and the boundary of the lesion. Despite these advantages, the endoscopic ultrasound appearance of glomus tumor has similar manifestations with other submucosal tumors, which are often difficult to identify (Table 1) (2, 8). In color Doppler images, blood flow within the glomus tumor is sometimes visible. However, when the blood flow velocity is low, or the acoustic angle is suboptimal, blood flow is not well displayed or entirely absent. Endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA) is a reliable method for obtaining pathological specimens from gastric submucosal tumors. However, for tumors exhibiting malignant characteristics (e.g., necrotic changes and rapid growth), EUS-FNA should be performed cautiously to minimize the risk of tumor cell metastasis through the needle tract (9, 10). In this case report, the tumor of the patient was large. A high-frequency-probe was used during EUS to repeatedly adjust the observation settings. The tumor appeared as a low-echo mass originating from the third or fourth submucosal layer, with a clear boundary; however, uneven internal echo. A dot-like strong echo was observed within the tumor, although no significant blood flow signals were detected. We recommended that the patient undergo EUS-FNA for further examination; however, the patient declined. The tumor was suspected to be a stromal tumor; however, the possibility of a glomus tumor could not be ruled out.
The National Comprehensive Cancer Network guidelines recommend EUS-guided fine-needle aspiration or core needle biopsy for undiagnosed gastric submucosal tumors < 2 mm diameter (11, 12). Surgical resection is recommended if high-risk features are present —such as tumor size (> 30 mm), irregular margins, regional lymph node involvement, and tumor rupture as identified by EUS—surgical resection is advised (13). Surgical approaches vary, ranging from traditional complex organ and gastric wedge resections to advanced techniques such as endoscopic full-thickness resections, endoscopic submucosal dissections, and combined endoscopic/laparoscopic procedures (14, 15). Currently, an increasing number of clinicians and patients prefer endoscopic surgery owing to its minimal trauma and faster recovery (16, 17). The exact nature of the gastric antrum tumor in this patient remains unclear. We consider the prospect of a stromal tumor to be high; however, the possibility of a glomus tumor cannot be excluded. Based on enhanced CT and EUS, the tumor was observed to grow inside and outside the gastric cavity, with a relatively large size and a rich blood supply. We identified three major problems in managing the tumor through simple gastroscopic procedures. First, the significant growth of the tumor outside the cavity obscures the visualization of external blood vessels, creating a poor field of view and making bleeding difficult to control if it occurs. Second, the large size of the tumor and adherence to the muscular layer made perforation inevitable during the dissection process. Moreover, the perforation area was extensive, making it difficult to seal effectively using endoscopic techniques. Third, if the tumor margin were positive, additional surgery would be necessary. Therefore, we opted for a combined gastroscopy and laparoscopy approach to ensure complete resect tumor removal. First, the intracavitary portion of the tumor was dissected endoscopically, followed by laparoscopic removal of the extracavitary growth. This method allowed full visualization of the intracavitary and extracavitary components, minimized accidental injury and unnecessary resection, and enabled precise excision. Pathological tests confirmed the tumor as a benign gastric glomus tumor with negative surgical margins. After surgery, the patient underwent acid suppression, rehydration, fasting, nutritional support, and other treatments. On the first postoperative day, the patient was mobilized out of bed. After 3 days, oral intake was gradually resumed. The patient showed steady recovery and was discharged after 1 week.
Conclusion
GGT are rare submucosal lesions that should be considered in the differential diagnosis of gastric submucosal tumors. While EUS offers valuable insights for the diagnosis of submucosal tumors, immunohistochemical analysis remains essential. Gastroscopy combined with laparoscopic resection is an accurate, effective, and safe treatment option for glomus tumors.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author/s.
Ethics statement
Written informed consent was obtained from the participant/patient(s) for the publication of this case report.
Author contributions
XuY: Writing – original draft. YG: Writing – original draft. XiY: Writing – original draft. BX: Writing – original draft. ZC: Writing – original draft. ZG: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Singh S, Kumar A, Singh V. Gastric glomus tumor. Niger J Surg. (2020) 26:162–5. doi: 10.4103/njs.NJS_8_19
2. Malik A, Yousaf MN, Samiullah S, Tahan V. Gastric glomus tumors: the roles of endoscopic ultrasound and shared decision-making. Case Rep Gastroenterol. (2023) 17:356–61. doi: 10.1159/000534643
3. Zhang Y, Yang YF, Wang JP. A case of gastric glomus tumor. Zhonghua Nei Ke Za Zhi. (2023) 62:1476–8.
4. Pizzillo IA, Fang C, Sun W, Brandler TC. Gastric glomus tumor diagnosed by fine needle aspiration of the stomach: A report of two cases and review of the literature. Diagn Cytopathol. (2022) 50:E100–6. doi: 10.1002/dc.24914
5. Vyawahare MA, Musthyala BN, Tayade RT. Gastric glomus tumor: A rare etiology of upper gastrointestinal bleed. Indian J Pathol Microbiol. (2021) 64:795–8. doi: 10.4103/IJPM.IJPM_470_20
6. Stahl C, Wong WG, Fanburg-Smith JC, Vining CC. Unsuspected gastric glomus tumor. BMJ Case Rep. (2023) 16. doi: 10.1136/bcr-2022-253020
7. Xing JJ, Huang WP, Wang F, Chai YR, Gao JB. Computed tomography features and clinicopathological characteristics of gastric glomus tumor. BMC Gastroenterol. (2022) 22:174. doi: 10.1186/s12876-022-02241-w
8. Desai N, Monsrud A, Willingham FF. Gastric submucosal mass lesions. Curr Opin Gastroenterol. (2022) 38:581–7. doi: 10.1097/MOG.0000000000000877
9. Aryal SC, Husain S, Zhang Z, Yuan L. Gastric glomus tumor on EUS-FNA-based cytology: clinicopathologic and immunohistochemical features of 4 cases, including 1 case with associated MIR143HG-NOTCH2 fusion gene. J Am Soc Cytopathol. (2023) 12:296–306. doi: 10.1016/j.jasc.2023.03.004
10. Kato S, Kikuchi K, Chinen K, Murakami T, Kunishima F. Diagnostic utility of endoscopic ultrasound-guided fine-needle aspiration biopsy for glomus tumor of the stomach. World J Gastroenterol. (2015) 21:7052–8. doi: 10.3748/wjg.v21.i22.7052
11. Hirata I, Miyahara K, Nakagawa M. A rapidly growing gastric submucosal tumor. Intern Med. (2021) 60:2503–4. doi: 10.2169/internalmedicine.6725-20
12. von Mehren M, Kane JM, Riedel RF, Sicklick JK, Pollack SM, Agulnik M, et al. NCCN guidelines(R) insights: gastrointestinal stromal tumors, version 2.2022. J Natl Compr Canc Netw. (2022) 20:1204–14.
13. Shah P, Gao F, Edmundowicz SA, Azar RR, Early DS. Predicting Malignant potential of gastrointestinal stromal tumors using endoscopic ultrasound. Dig Dis Sci. (2009) 54:1265–9. doi: 10.1007/s10620-008-0484-7
14. Alkhatib AA, Faigel DO. Endoscopic ultrasonography-guided diagnosis of subepithelial tumors. Gastrointest Endosc Clin N Am. (2012) 22:187–205. doi: 10.1016/j.giec.2012.04.006
15. Willingham FF, Reynolds P, Lewis M, Ross A, Maithel SK, Rocha FG. Hybrid push-pull endoscopic and laparoscopic full thickness resection for the minimally invasive management of gastrointestinal stromal tumors: a pilot clinical study. Gastroenterol Res Pract. (2015) 2015:618756. doi: 10.1155/2015/618756
16. Wang WH, Shen TT, Gao ZX, Zhang X, Zhai ZH, Li YL. Combined laparoscopic-endoscopic approach for gastric glomus tumor: A case report. World J Clin cases. (2021) 9:7181–8. doi: 10.12998/wjcc.v9.i24.7181
Keywords: gastric glomus tumor, submucosal tumor, gastroscopy, laparoscopy, male
Citation: Yang X, Guo Y, Yan X, Xu B, Cui Z and Guo Z (2025) Case report: One case of precise resection of gastric glomus tumor by gastroscopy combined with laparoscopy. Front. Oncol. 14:1501442. doi: 10.3389/fonc.2024.1501442
Received: 25 September 2024; Accepted: 11 December 2024;
Published: 07 January 2025.
Edited by:
Andras Papp, University of Pecs, HungaryCopyright © 2025 Yang, Guo, Yan, Xu, Cui and Guo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhuang Guo, c2x5dGd6QDE2My5jb20=
 Xuan Yang
Xuan Yang Yunping Guo1
Yunping Guo1 Zhenqin Cui
Zhenqin Cui