- 1Department of Radiation Medicine, MedStar Georgetown University Hospital, Washington, DC, United States
- 2Department of Therapeutic Radiology, Yale University School of Medicine, New Haven, CT, United States
- 3University of South Florida (USF) Health Morsani College of Medicine, Tampa, FL, United States
- 4Biotechnology Research Institute, North Carolina Central University, Durham, NC, United States
- 5Department of Oncology, Lombardi Comprehensive Cancer Center, Georgetown University Medical Center, Washington, DC, United States
Introduction: Studies have demonstrated that injectable GnRH receptor agonists further suppress cancer progression when paired with radiotherapy (RT) in patients with intermediate- to high-risk prostate adenocarcinoma. Relugolix is a newly available oral GnRH receptor antagonist that achieves swift and profound castration (total testosterone <20 ng/dl) at high rates, which may shape patients’ health-related quality of life. The main objective of this prospective study was to explore the effects of neoadjuvant relugolix on health-related quality of life in prostate cancer patients immediately prior to stereotactic body radiation therapy (SBRT).
Methods: Patients treated at Georgetown between January 2021 and September 2023 with neoadjuvant relugolix per an institutional protocol were included in the study (IRB 12-1775). The five-item EQ-5D-3L, a well-established tool for quantifying patient-reported health status, was administered to each patient at baseline (prior to relugolix treatment) and again 1 h before the start of SBRT. Higher EQ Visual Analog Scale (VAS) overall scores reflected better quality of life (range 0 to 100). In line with the questionnaire framework, individual elements (mobility, self-care, usual activities, pain/discomfort, and anxiety/depression) were rated on a three-point scale from 1 (no problems) to 3 (severe problems). McNemar’s test and paired-sample t-test were performed to analyze changes pre- and post-relugolix treatment. Our investigation determined clinical significance based on minimally important difference (MID) calculated as 0.5 times the baseline standard deviation.
Results: Among the 87 patients, average age was 71 years, 42% were non-white, and 24% were considered obese (BMI ≥30 kg/m²). Relugolix was initiated a median of 4 months before SBRT initiation (IQR: 3.9–5.4), with 87% of patients reaching profound castration (<20 ng/dl). The VAS overall score was notably higher at baseline (mean ± SD: 82 ± 10) compared to the paired score before RT (79 ± 14, p = 0.02), although this difference was not clinically significant. No statistically or clinically significant changes were observed in any of the five individual items.
Conclusion: The use of neoadjuvant relugolix prior to prostate radiation therapy had no clinically significant impact on patient-reported health-related quality of life. Moreover, no statistically significant reductions were observed in any of the five individual health-related quality of life measures. As a key direction for future research, relugolix-associated changes to healthy-related quality of life should be contrasted to those brought about by injectable GnRH agonists.
1 Introduction
Prostate cancer continues to be one of the most prevalent malignancies affecting men worldwide, with localized cases often requiring a combination of therapeutic approaches to optimize outcomes. The National Comprehensive Cancer Network (NCCN) guidelines suggest that patients with unfavorable localized prostate cancer should undergo a combination of radiation therapy (RT) and androgen deprivation therapy (ADT) (1). ADT paired with conventionally fractionated RT substantially enhances metastases-free and overall survival (2). Emerging data also indicate that adding ADT to ultrahypofractionated RT, or stereotactic body radiation therapy (SBRT), for unfavorable prostate cancer may lower local disease persistence and biochemical recurrence comparable to SBRT alone (3, 4). Despite its effectiveness, ADT in combination with RT continues to be underutilized likely due to concerns over its bothersome side effects and the associated risks of exacerbating cardiovascular comorbidities (5, 6).
In 2020, the FDA granted approval for relugolix, an oral antagonist of gonadotropin-releasing hormone (GnRH) receptors. By directly inhibiting the release of luteinizing hormone (LH) and follicle-stimulating hormone (FSH), relugolix induces a swift reduction of testosterone levels to significant castration levels (total testosterone 0.7 nmol/L, <20 ng/dl) (7). The phase 3 HERO trial (NCT03085095) compared the effectiveness of this oral GnRH receptor antagonist versus GnRH agonist leuprolide and demonstrated that relugolix outperformed leuprolide in achieving and maintaining castration (total testosterone 1.73 nmol/L, <50 ng/dl) (8). By day 29 of treatment, 95% of patients on relugolix attained profound castrate levels compared to just 57% of those receiving leuprolide (8). Among patients taking relugolix, 96.7% maintained castration after 48 weeks versus 88.8% of patients on leuprolide (8). Notably, there were no statistical differences in rates of hormonal toxicities such as generalized fatigue, hot flashes, and musculoskeletal pain (8).
Neoadjuvant/adjuvant relugolix administered over 6 months has been examined in unfavorable localized prostate cancer when alongside conventionally fractionated RT (79.2 Gy in 44 fractions) (9). This combination resulted in 95% achieving castration and 87% reaching profound castration (9). However, the high incidence of rapid profound castration may influence health-related quality of life, particularly among minority and underserved populations, as these groups have previously been reported to exhibit greater rates of non-adherence to hormonal therapies (10). Furthermore, patient-specific characteristics, such as age or comorbidities, may amplify these effects. We conducted a prospective study to evaluate the influence of neoadjuvant relugolix on health-related quality of life (HRQoL) in intermediate- and high-risk prostate cancer patients prior to starting SBRT.
2 Materials and methods
Our team carried out a prospective study of subjects with unfavorable, localized prostate adenocarcinoma treated at Georgetown University Hospital (IRB 12-1175). As per institutional protocol, patients received a short-term treatment of relugolix for 6 months, along with SBRT. We reviewed patients’ medical records to gather demographic and oncologic information, including age, ethnicity, body mass index (BMI), prostate volume, pretreatment PSA levels, T stage, Gleason score, and treatment dosage. D’Amico criteria were utilized to categorize patient risk groups.
2.1 Pharmacologic treatment
Relugolix was started no less than 2 months before SBRT, beginning with a 360-mg loading dose on day 1, followed by an oral dose of 120 mg daily.
2.2 Follow-up and assessment
Total testosterone levels were measured concurrently with questionnaire administration. Serum testosterone under 50 ng/dl (<1.73 nmol/L) defined effective castration, while levels below 20 ng/dl (<0.7 nmol/L) defined profound castration (8). Each patient’s health status was assessed using the validated five-item EQ-5D-3L questionnaire collected at baseline (before initiating relugolix) and again 1 h prior to SBRT (11). The EQ VAS score spans from 0 to 100, with 0 reflecting the worst conceivable health and 100 reflecting the best conceivable health (11). The minimally important difference (MID) in the EQ VAS score was specified as a change of one-half standard deviation (SD) from the baseline (12). Individual items, including mobility, self-care, usual activities, pain/discomfort, and anxiety/depression, were scored on a three-point scale: 1 (no problems), 2 (some problems), and 3 (extreme problems) (11). For example, a patient who reports no problems in all five dimensions of EQ-5D-3L is described to have a health state of “11111” (11).
2.3 Statistical methods
Continuous variables were summarized by mean and standard deviation when normally distributed and compared using t-test. Non-normally distributed, continuous variables were calculated based on median and interquartile range and compared between groups with Wilcoxon rank-sum test. All categorical variables were summarized by frequencies and percentages and were compared using Pearson’s Chi-squared test and Fisher’s exact test. We assessed changes before and after relugolix treatment with McNemar’s test and paired-sample t-test for categorical and continuous variables, respectively. A p-value <0.05 determined statistical significance. Clinical significance was evaluated based on MID. All analyses were performed using R version 4.3.2 or a more recent version, as provided by The R Foundation of Statistical Computing (http://www.r-project.org/).
3 Results
3.1 Baseline demographic, clinical, and treatment characteristics
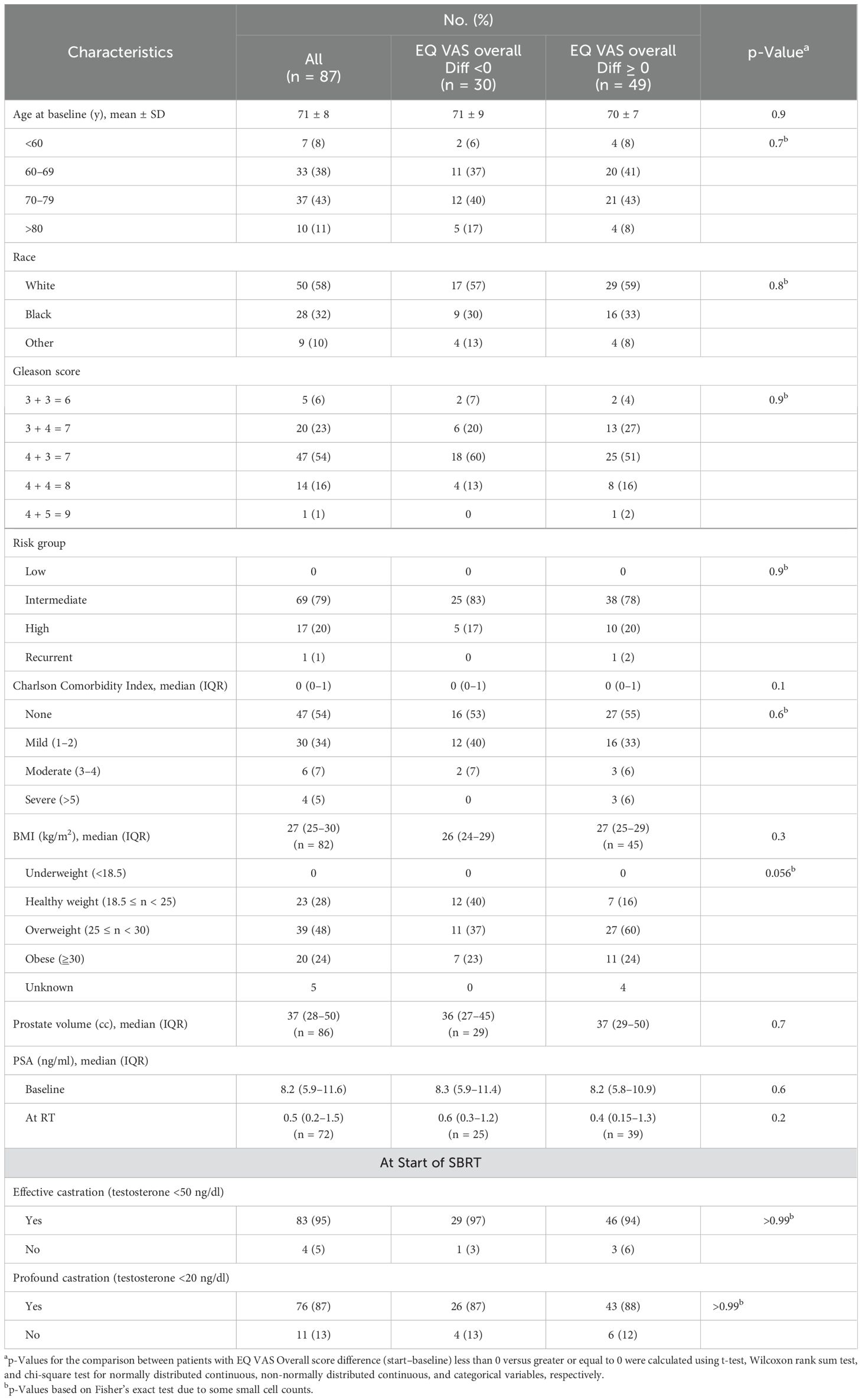
Table 1 outlines the demographic, treatment, and tumor characteristics. From January 2021 to September 2023, 87 patients with intermediate- and high-risk prostate cancer were treated at Georgetown University Hospital. Patient ages ranged from 49 to 87 years, with an average age of 71. Most patients identified as Caucasian (57%) or African American (32%). A significant portion of the participants were considered overweight (48%) or obese (24%) with a BMI of 25–29.9 and ≥30 kg/m², respectively. Before treatment, the median PSA was 8.2 ng/ml, with levels ranging from 2.3 to 40.0 ng/ml. Most patients (71%) were ≥Grade Group 3. In terms of risk stratification, 69 patients (79%) had intermediate-risk disease and 17 (20%) were considered high-risk. The majority of patients had none (54%) to mild (34%) Charlson Comorbidity Index. The median time from relugolix initiation to SBRT was 4 months (IQR: 3.9–5.4 months). By SBRT initiation, 95% of patients had achieved effective castration (testosterone ≤50 ng/dl), and 87% had reached profound castration (testosterone ≤20 ng/dl).
3.2 Problems reported on the EQ-5D-3L dimensions and EQ VAS score
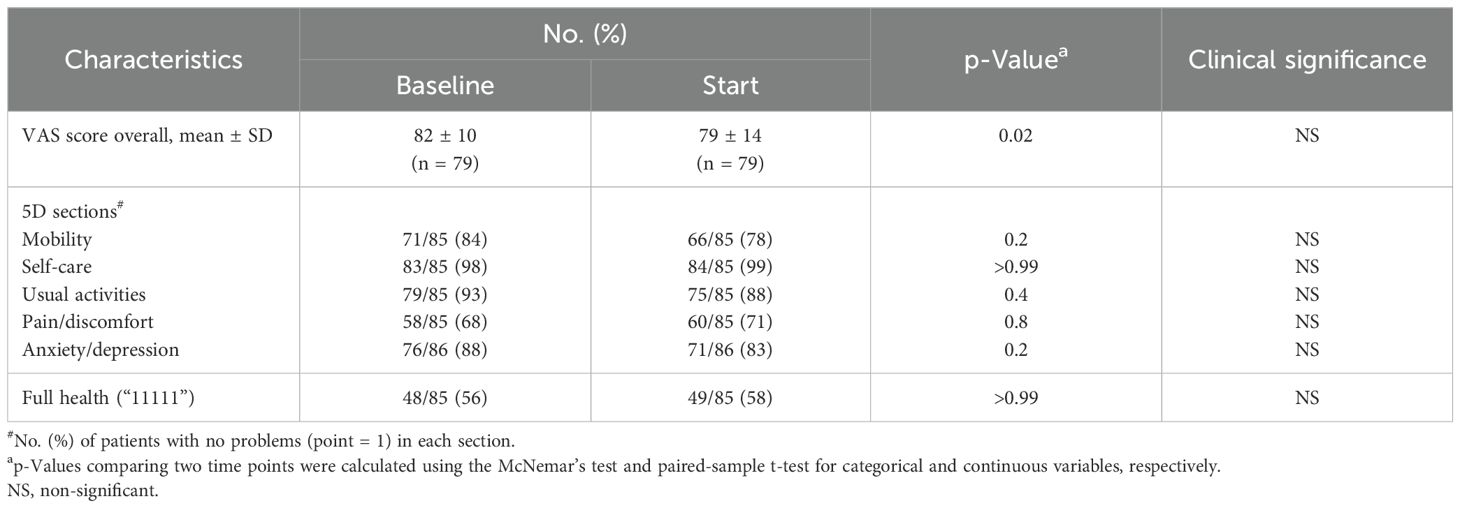
Patient-reported health status outcomes measured by EQ-5D-3L and EQ-VAS score are reported in Table 2. A total of 79 patients completed the VAS questionnaire, while 85 completed the EQ-5D-3L. Baseline health-related problems were relatively uncommon in our patients; the majority (56%) of them had a good health state, defined as “11111.” Prior to treatment, the pain/discomfort dimension (32%) had the highest proportion of reported problems (some or extreme), followed by mobility (16%), anxiety/depression (12%), usual activities (7%), and self-care (2%) (Figure 1).
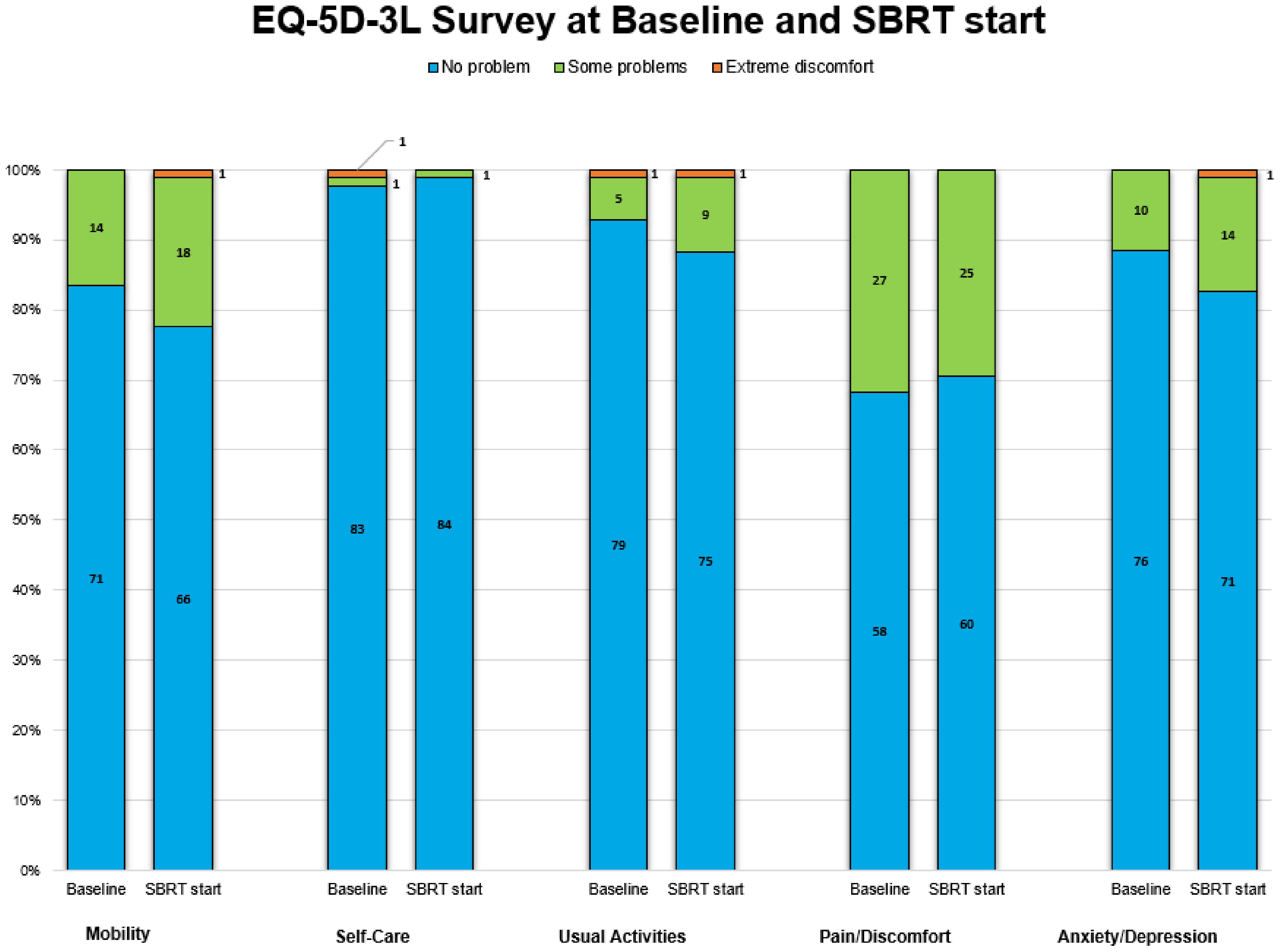
Figure 1. Proportions of three discomfort levels in each of five dimensions in stacked bar plots. The number in black on the bar plots denotes the number of patients.
Post hormonal therapy, a slightly higher number (58%) of patients were in good health state “11111.” The pain/discomfort dimension (29%) remained the highest proportion of five problems reported. The order remained the same as baseline: mobility (22%), anxiety/depression (17%), usual activities (12%), and self-care (1%) (Figure 1). While the proportions of reported problem slightly increased in mobility, anxiety/depression, usual activities and slightly decreased in pain/discomfort and self-care, no changes in any of the five individual items were statistically or clinically significant (Table 2, Figure 2).
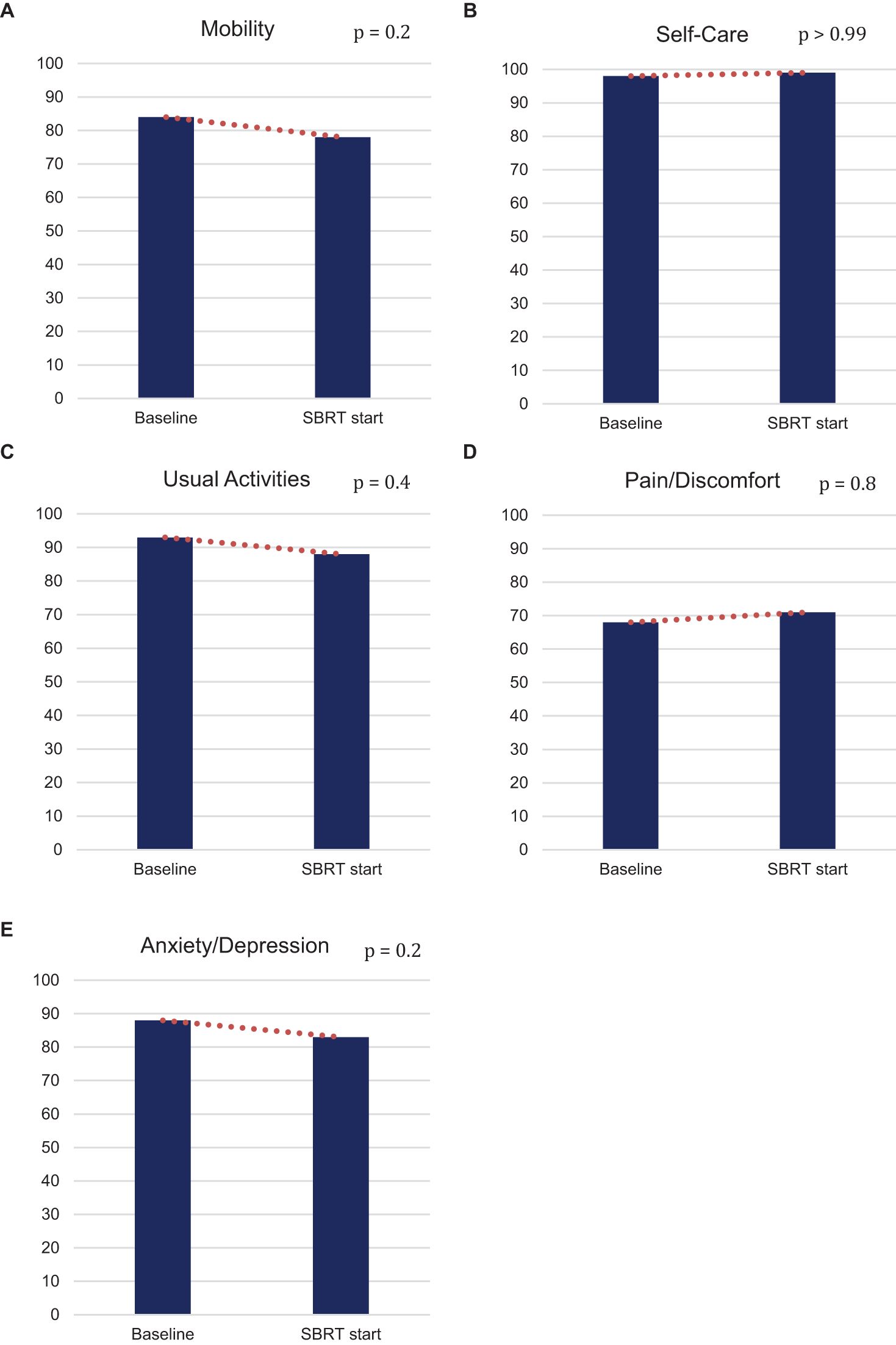
Figure 2. Percentage of patients who do not have any problem (EQ-5D-3L) in (A) mobility, (B) self-care, (C) usual activities, (D) pain/discomfort, and (E) anxiety/depression at baseline and at SBRT initiation.
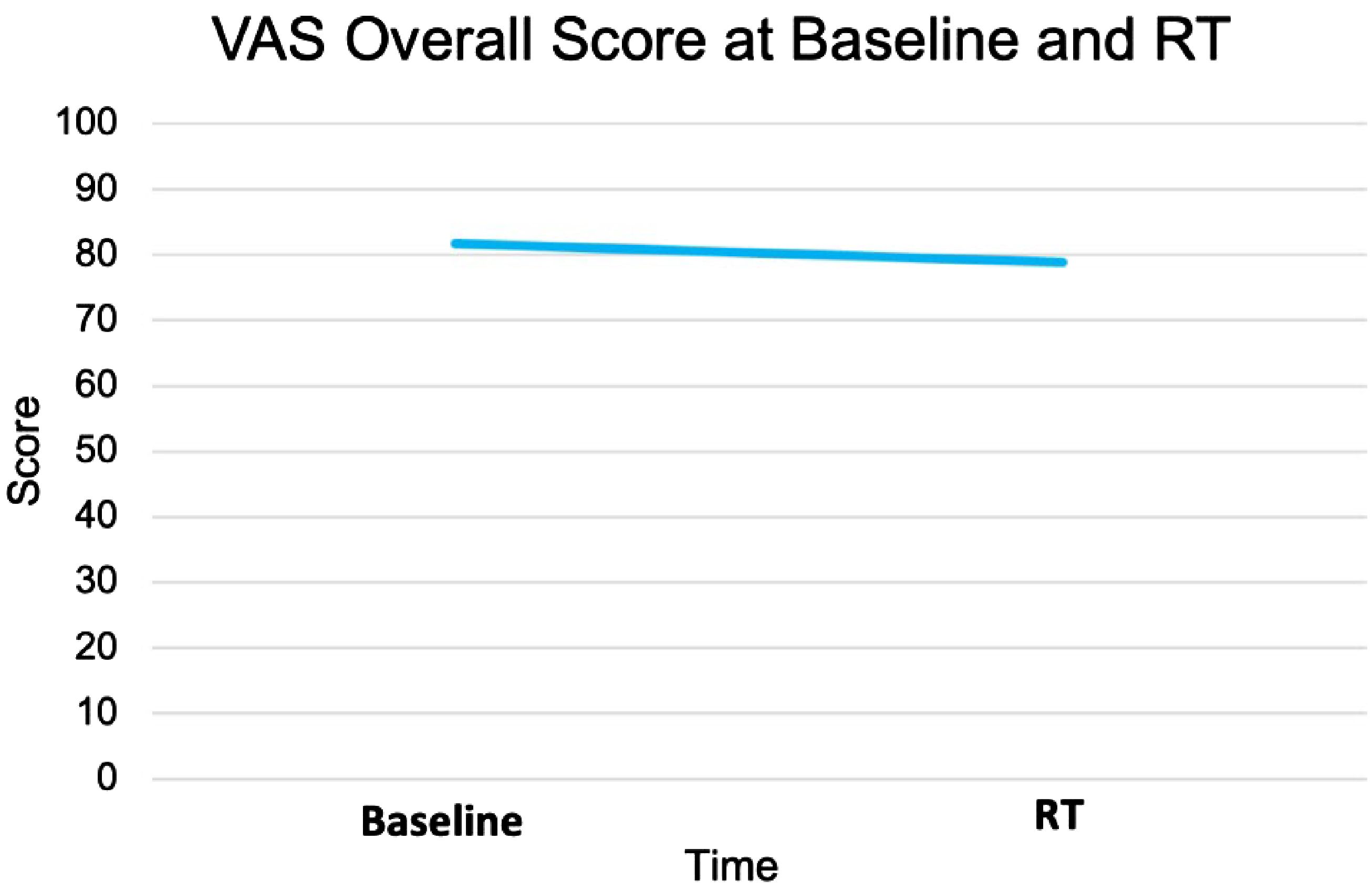
The EQ VAS score was statistically significantly higher at baseline (mean ± SD: 82 ± 10) than the paired score at the start of SBRT (79 ± 14, p = 0.02) (Table 2, Figure 3). This change was not clinically significant as evaluated by MID. The paired difference in VAS score overall between the two time points had a median of 0 (IQR: −5, 2.5) (Figure 4). Patients were divided into two groups, one group whose VAS score overall remained the same or increased (62%) versus the other whose score decreased (38%). These two groups had no statistically significant differences across demographic and clinical characteristics, as reported in Table 1.
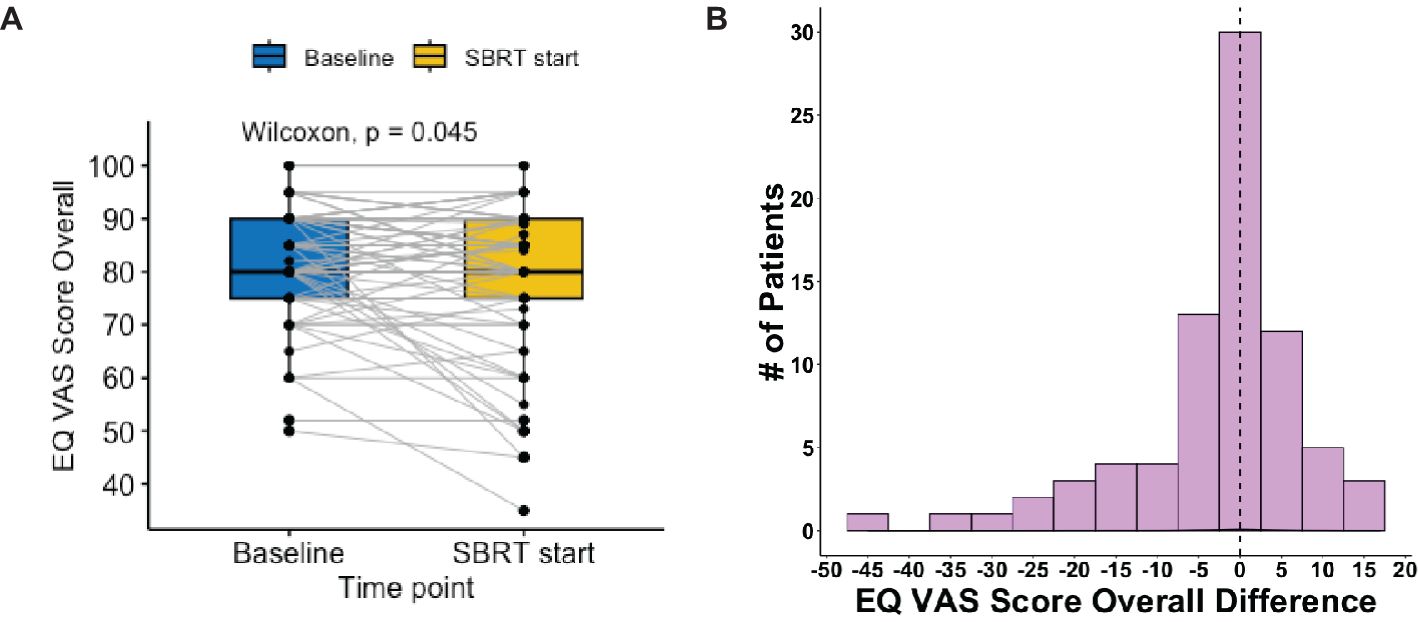
Figure 4. Distribution of EQ VAS score overall pre- vs. post-relugolix therapy. (A) Paired boxplot of EQ-VAS score overall, in which each box has horizontal lines indicating median values and extends from the 25th to the 75th percentile and in which each gray line connecting two boxes indicates individual patient. (B) Histogram of EQ-VAS score overall difference (start–baseline) and the vertical line at 0 indicates patient median.
4 Discussion
Growing evidence suggests that achieving profound castration (serum testosterone levels of <20 ng/dl) may yield better outcomes in specific clinical contexts, although current guidelines define effective castration as testosterone levels of <50 ng/dl (13). While the impact of testosterone levels of <20 ng/dl on HRQoL remains ambiguous, it is reassuring to note that 87% of our patient cohort achieved profound castration without any clinically meaningful deterioration in HRQoL. This result aligns with findings from the Radiation Therapy Oncology Group (RTOG) 0815 randomized trial, which investigated dose-escalated radiation with or without short-term androgen suppression in intermediate-risk prostate adenocarcinoma (5). In that study, short-term androgen suppression using injectable GnRH receptor agonists combined with oral antiandrogen therapy over a 6-month period demonstrated no effect on EQ-5D VAS scores up to 5 years following radiation therapy. These findings are particularly relevant for intermediate-risk patients considering the addition of relugolix to their SBRT regimen.
Similar to other SBRT cohorts, our patient population was older and presented with moderate reductions in baseline HRQoL before treatment. While androgen deprivation therapy is associated with musculoskeletal pain (30%) and fatigue (25%) (8), we did not see increased difficulties with self-care, usual activities, mobility, or pain/discomfort in patients receiving short-term relugolix. Notably, 41% of our study population was non-white, representing diverse socioeconomic and racial backgrounds (14). The absence of adverse HRQoL impacts in this socially diverse cohort further supports the safety of short-term relugolix use in these patients.
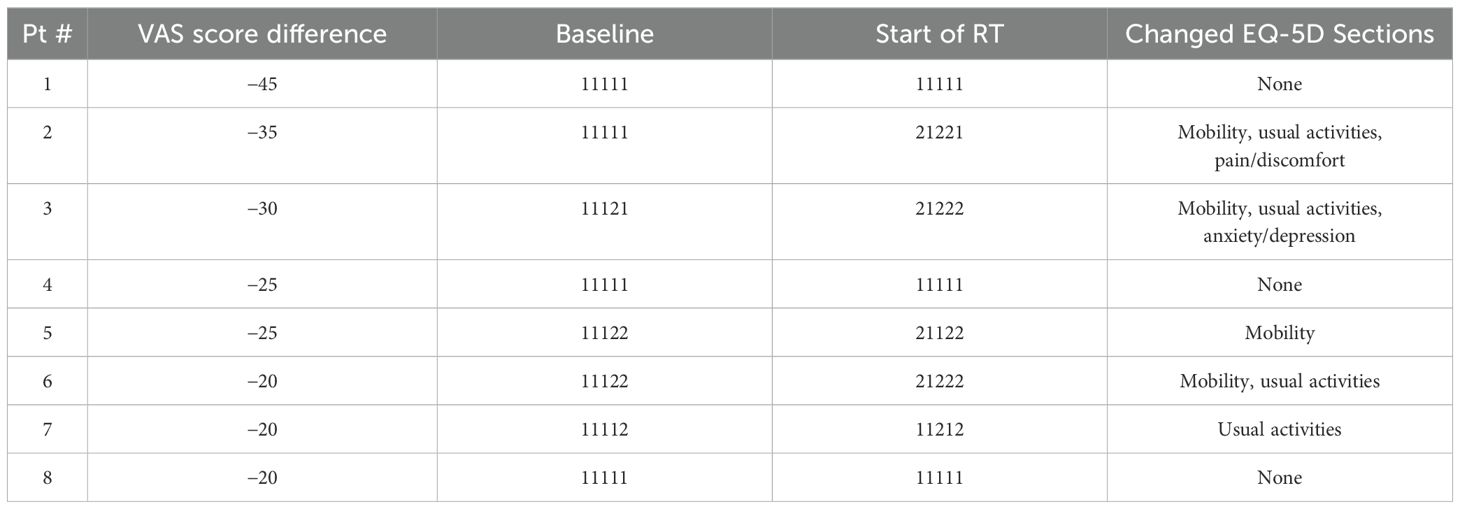
We also conducted a deeper investigation into a subset of eight patients who had “severely” negative effect from ADT defined as those whose EQ VAS score decreased by 20 or greater. An arbitrary cutoff point of 20 was decided because there were recent studies, which suggested more flexible criteria to define the clinical significance in differences as two or three times the conventional MID (0.5 of SD at baseline) (15) or as the standard error of the measure (SEM) represented by (16).
Interestingly, these eight patients had no significant differences in all of the reported baseline demographic and clinical characteristics compared to the rest of the cohort. Among these eight patients, the majority reported to have “some problems” from “no problem” in baseline in the mobility (n = 4) and usual activities (n = 4) sections (Table 3).
Also, the EQ VAS score decrease did not seem to reflect on the individual items, as three of these eight patients reported to have no change in all five sections and remained as good health state (“11111”), including the patient who reported to have the greatest decrease in the EQ VAS score of 45 points. This could suggest that these five dimensions may not have adequately represent the side effects the patients experienced from the hormonal therapy as represented in the EQ VAS score.
One possible missing component accounting for the observed discrepancy is patient-reported fatigue, a commonly reported bothersome effect of hormonal therapy; our previous study showed that while most patients had significantly increased patient-reported fatigue, they were not impacted in the self-care aspect (17). It is also worth noting that a recent study conducted by our team on the effects of relugolix on patient-reported sexual function found that, while neoadjuvant relugolix was linked to a significant decline in sexual function, the majority of patients did not express concern about this effect (18) Last, the major advantage of relugolix, particularly for patients experiencing significant side effects from hormonal therapy, is that the medication effects can be resolved quickly when stopped (8).
4.1 Strengths and limitations
The rigorous standard in overall survey methodology and quality of data collection were assured by experienced clinical research data managers at an identical hospital to that where the patients received clinical treatment. Patients in our cohort were treated in one clinical site, which ensures less heterogeneity of treatment. Although patient reported, this study’s outcomes are based on EQ-5D, a widely used and respected HRQoL survey globally (19). Selecting a generic measure, such as the EQ-5D, as the main outcome allowed this study to encompass a spectrum of HRQoL that might be overlooked in measures focused solely on specific physical, functional, or mental health facets.
The external validity of our investigation is constrained by the small number of participants and slight differences in the treatment schedule. Although we intended for all patients to receive relugolix for 4–6 months starting 2 months prior to SBRT, there were variations in the timing of therapy. We did not explore how scheduling divergences may have affected outcomes.
5 Conclusions
Future studies should focus on comparing it to GnRH agonist-induced change in quality of life. Neoadjuvant relugolix administered before prostate SBRT did not have a clinically meaningful impact on patient-reported health-related quality of life. There were no statistically significant reductions in any of the five individual items comprising EQ-5D-3L. Additional research should be directed toward comparing quality-of-life outcomes for patients on relugolix to those resulting from GnRH agonist treatment.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Georgetown-MedStar Institutional Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
MuK: Conceptualization, Data curation, Formal analysis, Software, Visualization, Writing – original draft, Writing – review & editing. MiK: Conceptualization, Data curation, Writing – original draft, Writing – review & editing, Formal analysis, Software, Visualization. JH: Data curation, Writing – original draft, Writing – review & editing. LG: Data curation, Writing – original draft, Writing – review & editing. MD: Writing – review & editing. AZ: Writing – review & editing. MA: Writing – review & editing. DK: Writing – review & editing. MC: Writing – review & editing. PL: Writing – review & editing. ND: Writing – review & editing. SS: Writing – review & editing. SC: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The Department of Radiation Medicine at Georgetown University Hospital receives a grant from Accuray to support a research coordinator. This work was supported by The James and Theodore Pedas Family Foundation. SC and DK acknowledge the grant R01MD012767 from the National Institute on Minority Health and Health Disparities.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. National Comprehensive Cancer Network. Prostate Cancer (2023). Available online at: https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf (Accessed September 1, 2024).
2. Kishan AU, Sun Y, Hartman H, Pisansky TM, Bolla M, Neven A, et al. Androgen deprivation therapy use and duration with definitive radiotherapy for localised prostate cancer: an individual patient data meta-analysis. Lancet Oncol. (2022) 23:304–16. doi: 10.1016/S1470-2045(21)00705-1
3. Gorovets D, Wibmer AG, Moore A, Lobaugh S, Zhang Z, Kollmeier M, et al. Local failure after prostate SBRT predominantly occurs in the PI-RADS 4 or 5 dominant intraprostatic lesion. Eur Urol Oncol. (2022) 6(3):275–81. doi: 10.1016/j.euo.2022.02.005
4. van Dams R, Jiang NY, Fuller DB, Loblaw A, Jiang T, Katz AJ, et al. Stereotactic Body Radiotherapy for High-Risk Localized Carcinoma of the Prostate (SHARP) Consortium: Analysis of 344 Prospectively Treated Patients. Int J Radiat Oncol Biol Phys. (2021) 110:731–7. doi: 10.1016/j.ijrobp.2021.01.016
5. Movsas B, Rodgers JP, Elshaikh MA, Martinez AA, Morton GC, Krauss DJ, et al. Dose-escalated radiation alone or in combination with short-term total androgen suppression for intermediate-risk prostate cancer: patient-reported outcomes from NRG/Radiation Therapy Oncology Group 0815 randomized trial. J Clin Oncol. (2023) 41:3217–24. doi: 10.1200/JCO.22.02389
6. Falchook AD, Basak R, Chen RC. Androgen deprivation therapy and dose-escalated radiotherapy for intermediate- and high-risk prostate cancer—reply. JAMA Oncol. (2017) 3:281. doi: 10.1001/jamaoncol.2016.3974
7. Markham A. Relugolix: first global approval. Drugs. (2019) 79:675–9. doi: 10.1007/s40265-019-01105-0
8. Shore ND, Saad F, Cookson MS, George DJ, Saltzstein DR, Tutrone R, et al. Oral relugolix for androgen-deprivation therapy in advanced prostate cancer. N Engl J Med. (2020) 382:2187–96. doi: 10.1056/NEJMoa2004325
9. Dearnaley DP, Saltzstein DR, Sylvester JE, Karsh L, Mehlhaff BA, Pieczonka C, et al. The oral gonadotropin-releasing hormone receptor antagonist relugolix as neoadjuvant/adjuvant androgen deprivation therapy to external beam radiotherapy in patients with localised intermediate-risk prostate cancer: a randomised, open-label, parallel-group phase 2 trial. Eur Urol. (2020) 78:184–92. doi: 10.1016/j.eururo.2020.03.001
10. Wassermann J, Gelber SI, Rosenberg SM, Ruddy KJ, Tamimi RM, Schapira L, et al. Nonadherent behaviors among young women on adjuvant endocrine therapy for breast cancer. Cancer. (2019) 125:3266–74. doi: 10.1002/cncr.32192
11. EuroQol Research Foundation. EQ-5D-3L User Guide (2018). Available online at: https://euroqol.org/publications/user-guides (Accessed September 1, 2024).
12. Norman GR, Sloan JA, Wyrwich KW. Interpretation of changes in health-related quality of life: the remarkable universality of half a standard deviation. Med Care. (2003) 41:582–92. doi: 10.1097/01.MLR.0000062554.74615.4C
13. Djavan B, Eastham J, Gomella L, Tombal B, Taneja S, Dianat SS, et al. Testosterone in prostate cancer: the Bethesda consensus: role of testosterone in prostate cancer. BJU Int. (2012) 110:344–52. doi: 10.1111/j.1464-410X.2011.10719.x
14. Sholklapper TN, Creswell ML, Payne AT, Markel M, Pepin A, Carrasquilla M, et al. Patient-reported financial burden following stereotactic body radiation therapy for localized prostate cancer. Front Oncol. (2022) 12:852844. doi: 10.3389/fonc.2022.852844
15. Ali FM, Salek MS, Finlay AY. Two minimal clinically important difference (2MCID): a new twist on an old concept. J Eur Acad Dermatol Venereol. (2018) 98(7):715–7. doi: 10.1111/jdv.14950
16. Pickard AS, Neary MP, Cella D. Estimation of minimally important differences in EQ-5D utility and VAS scores in cancer. Health Qual Life Outcomes. (2007) 5:70. doi: 10.1186/1477-7525-5-70
17. Hsueh JY, Gallagher L, Koh MJ, Shah S, Danner M, Zwart A, et al. The impact of neoadjuvant relugolix on multi-dimensional patient-reported fatigue. Front Oncol. (2024) 14:1412786. doi: 10.3389/fonc.2024.1412786
18. Hsueh JY, Gallagher L, Koh MJ, Eden S, Shah S, Wells M, et al. Impact of neoadjuvant relugolix on patient-reported sexual function and bother. Front Oncol. (2024) 14. doi: 10.3389/fonc.2024.1377103
Keywords: prostate cancer, androgen deprivation therapy, SBRT, health related quality of life, EQ-5D
Citation: Koh MJ, Koh MJ, Hsueh JY, Gallagher L, Danner M, Zwart A, Ayoob M, Kumar D, Carrasquilla M, Leger P, Dawson NA, Suy S and Collins SP (2025) Maintenance of patient-reported health-related quality of life post neoadjuvant relugolix prior to the initiation of prostate radiation therapy. Front. Oncol. 14:1496646. doi: 10.3389/fonc.2024.1496646
Received: 15 September 2024; Accepted: 28 November 2024;
Published: 17 January 2025.
Edited by:
Andrea Lancia, San Matteo Hospital Foundation (IRCCS), ItalyReviewed by:
Matteo Sepulcri, Veneto Institute of Oncology (IRCCS), ItalyFederica Ferrario, University of Milano Bicocca, Italy
Copyright © 2025 Koh, Koh, Hsueh, Gallagher, Danner, Zwart, Ayoob, Kumar, Carrasquilla, Leger, Dawson, Suy and Collins. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sean P. Collins, Y29sbGluc3MxOUB1c2YuZWR1
 Min Jung Koh
Min Jung Koh Min Ji Koh
Min Ji Koh Jessica Y. Hsueh
Jessica Y. Hsueh Lindsey Gallagher
Lindsey Gallagher Malika Danner
Malika Danner Alan Zwart1
Alan Zwart1 Deepak Kumar
Deepak Kumar Michael Carrasquilla
Michael Carrasquilla Paul Leger
Paul Leger Simeng Suy
Simeng Suy Sean P. Collins
Sean P. Collins