
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Oncol. , 06 December 2024
Sec. Gastrointestinal Cancers: Hepato Pancreatic Biliary Cancers
Volume 14 - 2024 | https://doi.org/10.3389/fonc.2024.1495517
 Youfeng Xue1†
Youfeng Xue1† Baojie Shi2†
Baojie Shi2† Jianfa Zhong2†
Jianfa Zhong2† Guimei Wang2
Guimei Wang2 Jie Wang2
Jie Wang2 Wenbin An2
Wenbin An2 Yunyun Qian2
Yunyun Qian2 Zhaojie Su2
Zhaojie Su2 Zhihai Peng2*
Zhihai Peng2* Hao Li2*
Hao Li2*A 13-year-old boy was admitted to Xiang’an Hospital of Xiamen University due to HBV-related liver cancer. Intrahepatic metastasis was considered to occur by CT scan. A gastroscope revealed esophagogastric variceal bleeding, and later, the patient underwent a successful liver transplantation. Fourteen months posttransplant, chest CT indicated lung metastasis, and the patient underwent thoracoscopic radical resection of lung cancer. Twenty-one months posttransplant, gastroscopy revealed a gastric fundus tumor growing into the gastric cavity. Proximal gastrectomy was performed, and pathology indicated moderately to poorly differentiated carcinoma without invasion of serosa, suggesting the first study to report HCC metastasis to the stomach lumen without invasion of serosa after LT. Currently, the alpha-fetoprotein (AFP) level of the patient has dropped below normal.
In January 2021, a 13-year-old boy was enrolled in our center of liver transplantation at Xiang’an Hospital of Xiamen University because of HBV-related liver cancer.
The patient had been well until 2 weeks before this presentation, when diarrhea and bloody stool developed, which occurred 5-6 times/day and was accompanied by vomiting blood. After admission, a CT scan showed multiple intrahepatic masses (with the largest diameter of approximately 6.1cm, liver cancer with intrahepatic metastasis was considered), right portal vein cancer thrombus (not involving the main trunk of the portal vein, classified as Type I according to Chang’s classification), cirrhosis, splenomegaly, portal hypertension, and ascites (Figure 1A). Laboratory tests showed that AFP>1210 ng/ml, hemoglobin (HB) 98 g/L, and hepatitis B surface antigen were positive. Gastroscopy showed severe esophagogastric varices, and endoscopic variceal ligation was performed. The patient had been on oral antiviral therapy for HBV for more than ten years.
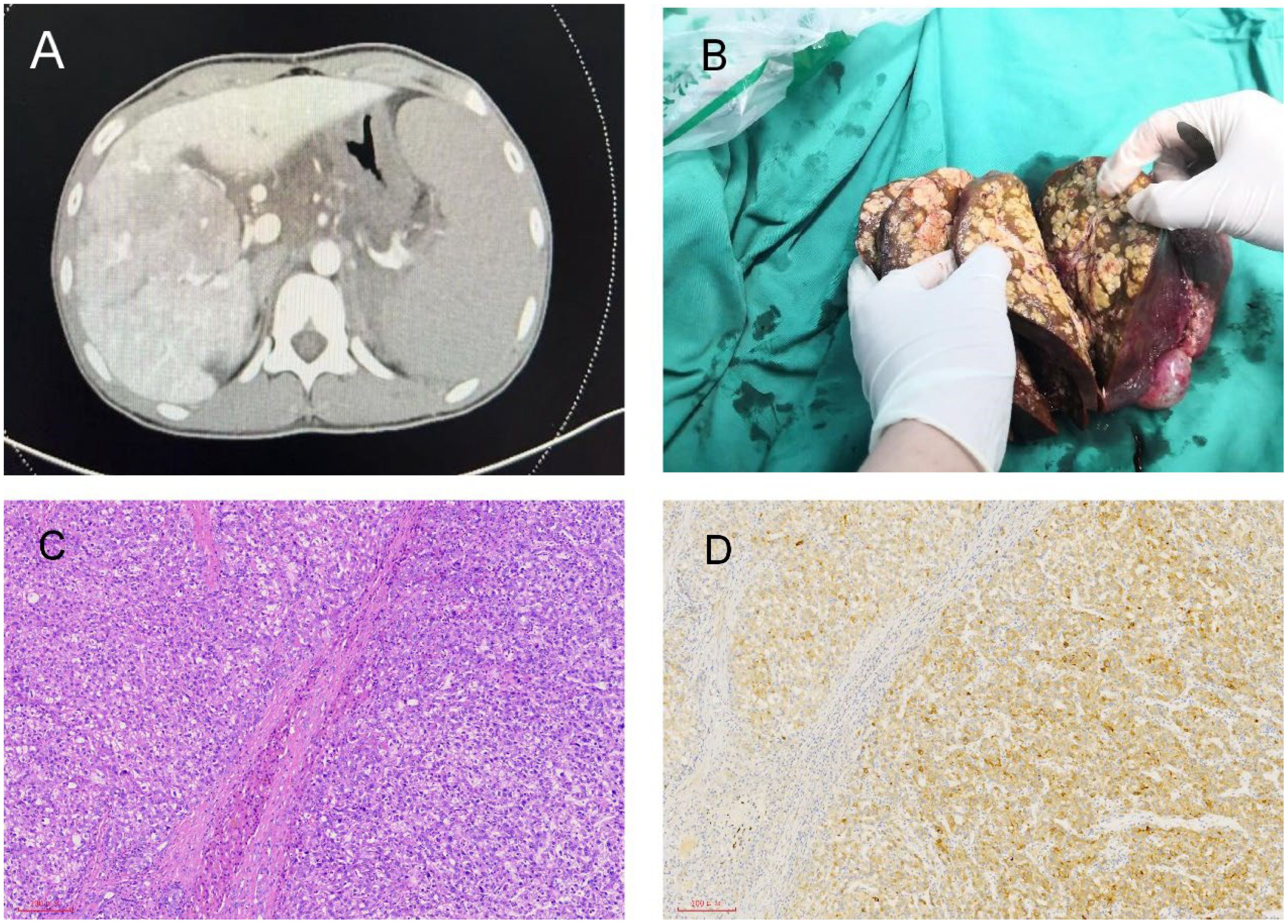
Figure 1. Radiological and pathological images of liver lesions. (A) Multiple intrahepatic lesions shown in CT. (B) Multiple nodules in the right lobe of the liver shown in the post-liver transplant specimen. (C) Liver H&E staining. (D) Liver immunohistochemistry: GPC3 (+).
After 30 days of admission, the patient received hepatic arterial infusion of raltitrexed plus epirubicin. There was no evidence of extrahepatic metastasis on PET-CT. Four days prior to liver transplantation, the patient suddenly exhibited confusion and unresponsiveness to verbal stimuli. Vital signs at that time included a temperature of 36.3°C, a pulse rate of 142 beats per minute, blood pressure readings of 65/35 mmHg, a respiratory rate of 31 breaths per minute, and an oxygen saturation of 100% while on supplemental oxygen. Following rapid fluid resuscitation, volume expansion, and pressor support, the patient’s consciousness was regained, and his blood pressure stabilized at 110/62 mmHg. After 45 days of admission, the patient successfully underwent orthotopic whole liver transplantation (LT). Postoperative specimens revealed multiple nodules in the right lobe of the liver, with tumor diameters ranging from 0.3 cm to 4.5 cm (Figure 1B). Multiple tumors showed vascular invasion, and there was also a portal vein tumor thrombus. However, there was no hilar lymph node metastasis. The postoperative pathological examination revealed moderately differentiated hepatocellular carcinoma (Figure 1C). Immunohistochemistry showed that the expression of GPC3 was positive (Figure 1D). The patient received standard immunosuppressive drugs (tacrolimus 1.5mg twice daily), antiviral agents, and hepatic arterial infusion chemotherapy. The AFP level was reduced to 5.57 ng/ml, and PIVKA-II was decreased to 3.76 mAU/mL.
In April 2022, the AFP level began to rise to 30 ng/ml. Chest CT detected new nodules in the right lungs, and lung metastases were considered (Figure 2A). After two months, the patient underwent thoracoscopic radical resection of lung cancer. The pathology showed a malignant tumor of the upper lobe nodule of the right lung, hepatocellular carcinoma-like differentiation (Figure 2B). Lenvatinib-targeted drugs were used, and AFP was regularly tested.
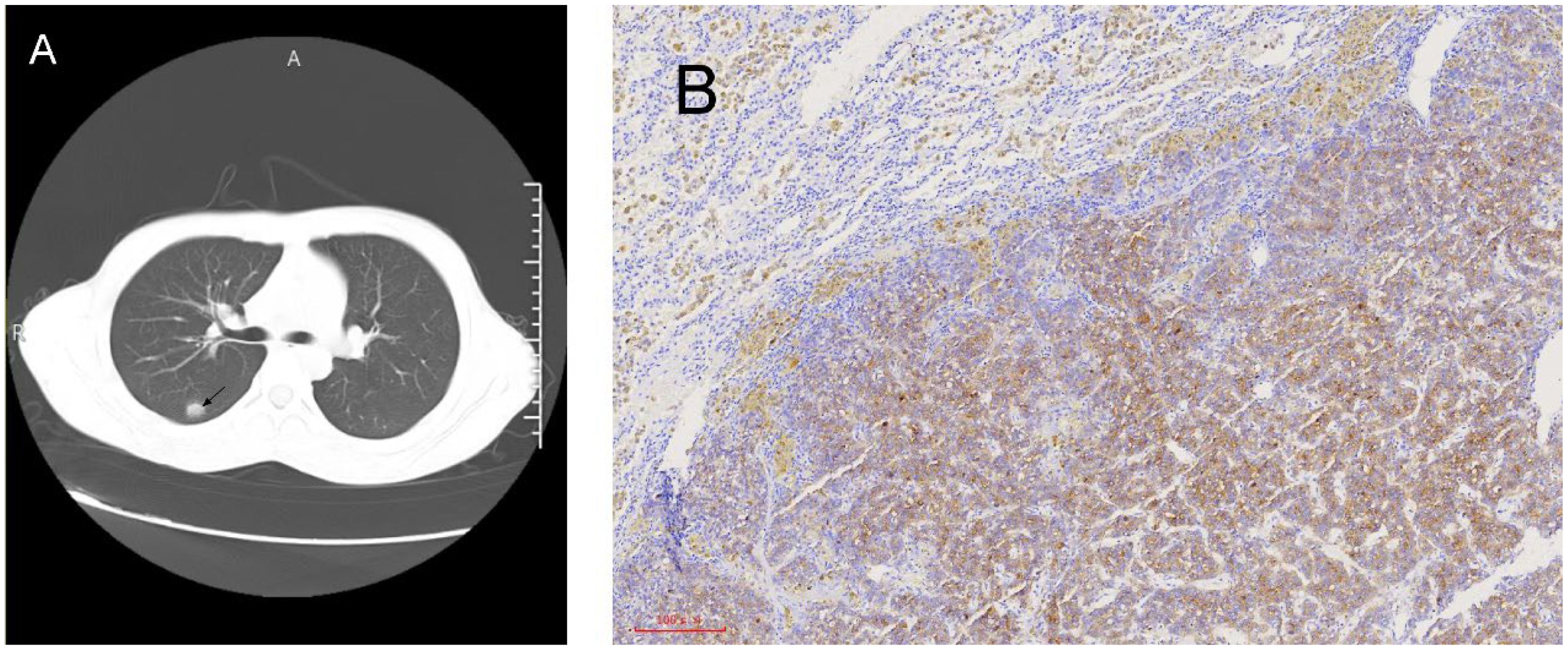
Figure 2. Radiological and pathological images of lung lesions. (A) Chest CT reveals right lung nodules. (B) Lung immunohistochemistry after thoracoscopic radical surgery for lung cancer: GPC3 (+).
In October 2022, the patient’s AFP level rose to 66.70 ng/ml. A PET-CT scan revealed multiple new masses, such as a mass in the left upper renal area (with a diameter of approximately 1.5cm), a mass in the left adrenal gland (measuring 1.3cm × 1.8cm), and a mass in the gastric cardia area (measuring 3.1cm × 2.3cm) (Figure 3A). The patient was given radiation therapy and continued Lenvatinib-targeted therapy. In January 2023, the patient underwent CyberKnife treatment for a left adrenal mass at an external hospital. After two months, the patient developed melena and hematemesis. Gastroscopy showed that the tumor was located in the fundus of the stomach and had grown into the lumen of the stomach, and stromal tumor rupture with bleeding was considered (Figure 3B). PET-CT showed that the mass of the fundus (4.1cm × 3.0cm) and the lateral side of the left upper renal pole (with a diameter of approximately 1.8cm) was larger than before, and the metabolism was increased. The left adrenal mass (1.3cm × 1.0cm) was smaller than before, and metabolism was reduced. The AFP level rose to 434 ng/ml. Due to repeated hematemesis and the fact that the bleeding could not be stopped by gastroscopy, the patient underwent radical gastric resection (proximal stomach) plus abdominal lymph node dissection. Pathology revealed a medium-poorly differentiated carcinoma. Immunohistochemistry showed Hepatocyte (+) (Figures 3C, D), in addition, GPC-3 and Arg-1 are also positive. Gastric metastasis of hepatocellular carcinoma was considered. Until now, the patient has been living and undergoing studies, and the AFP levels have returned to normal.
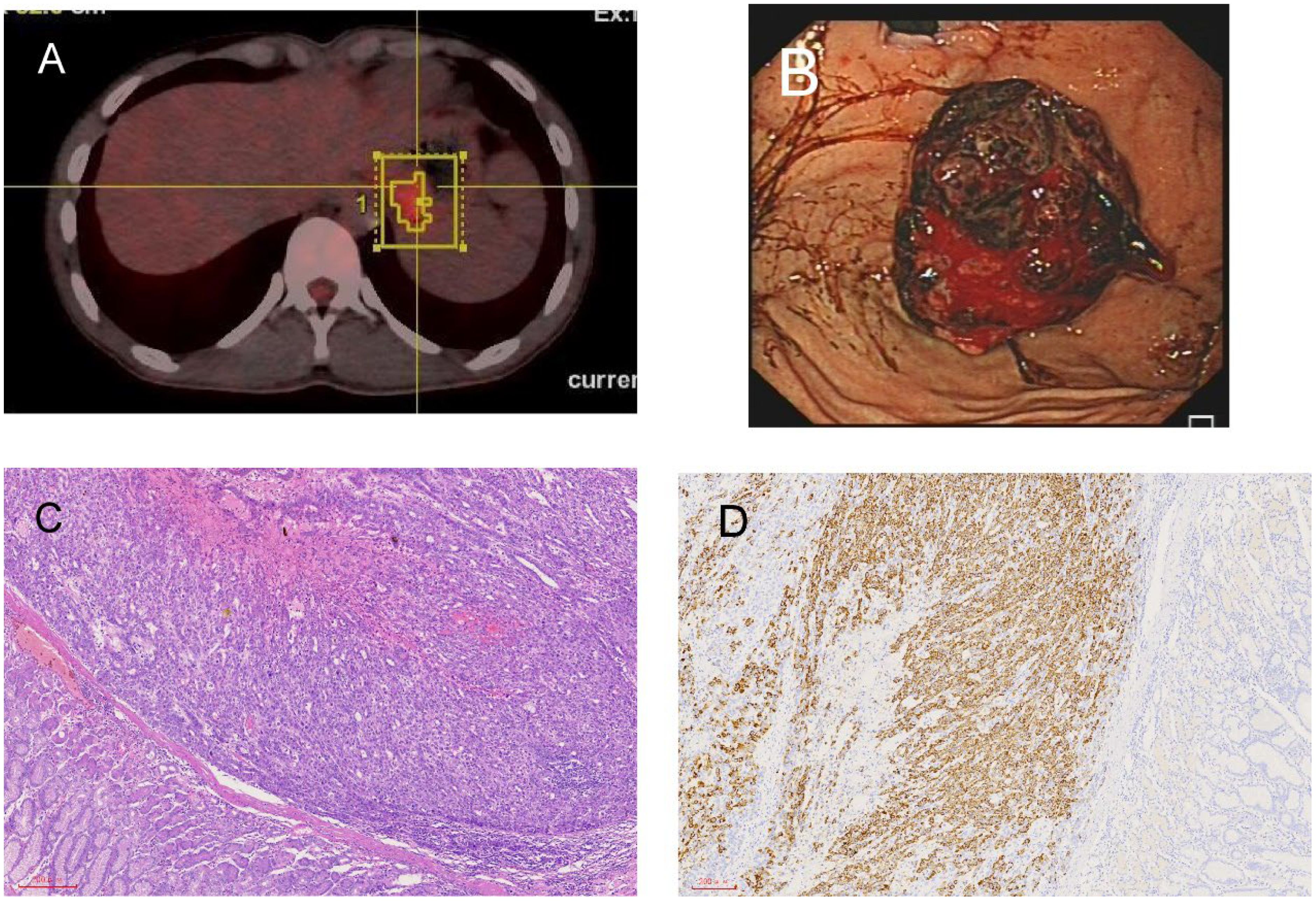
Figure 3. Radiological and pathological images of left adrenal gland and stomach lesions. (A) Newly detected tumor in the left adrenal gland shown in PET-CT. (B) The tumor grew from the fundus into the gastric cavity, and the gastric stromal tumor ruptured and bled. (C) Stomach H&E staining. (D) Stomach immunohistochemistry: hepatocyte (+).
Liver cancer is one of the most common malignancies worldwide, ranking sixth (1–3). It is also the fourth leading cause of cancer-related deaths (1, 4). Hepatocellular carcinoma (HCC) is the most common form of liver cancer and accounts for nearly 90% of cases (5–7), and it stands as the third leading cause of cancer-related deaths worldwide (8). The incidence of HCC is increasing and is the main event leading to death in patients with cirrhosis (9). Intrahepatic metastasis is the most common metastasis of HCC, followed by lung metastases (10–12), and lung metastases are among the leading causes of hepatocellular liver cancer-related mortality (13). Liver cancer can directly invade the stomach but not intragastrically. Intragastric metastasis of HCC is very rare (14).
Several treatment modalities are available for patients with HCC, and LT remains the optimal treatment strategy for patients (15). The Milan criteria are the universally applicable standard of LT for HCC based on their significantly good outcome (16). HCC patients who meet the Milan criteria (single tumor <5 cm or up to three tumors <3 cm with no macrovascular invasion) are the most suitable for liver transplantation, with 5-year overall survival (OS) reaching 70% for HCC patients (16–18). For patients who were beyond the Milan criteria, the OS was no more than 3 months. After LT, the OS of these patients is more than 2 years. These results showed that liver transplantation can significantly promote the survival of patients who are beyond the Milan criteria. There was a prospective, randomized, controlled trial exploring the benefit of LT in patients who achieved successful and sustained downstaging of HCC exceeding the Milan criteria. These results showed that LT can significantly prolong patient survival beyond the Milan criteria. At the same time, the patient is relatively young, as HCC is uncommon in the pediatric population. The prognosis of HCC in children after liver transplantation (LT) differs from that of adult patients, with unique considerations for growth, development, and the long-term complications of immunosuppression. The one-year survival rate after pediatric liver transplantation is typically between 80% to 90%, and the five-year survival rate is between 70% to 80%. Although downstaging of HCC was not achieved in our patient after hepatic arterial infusion chemotherapy, in this study, LT still significantly increased the survival time of patients with HCC.
HCC frequently exhibits extrahepatic metastasis, with the typical sequence being the lungs, bones, lymph nodes, and adrenal glands (19). Extrahepatic recurrence typically develops after transplantation and almost always carries a better prognosis than intrahepatic recurrence (20). In our center of liver transplantation, approximately 60% of patients with HCC after LT experience extrahepatic metastases. For these metastatic patients, due to normal liver function, systemic chemotherapy, targeted therapy, or local radiation therapy is often given. Liver transplantation is the best option to remove liver cancer (21, 22). Postoperative recurrence is mainly due to hematogenous metastasis of circulating tumor cells (23, 24). This can explain the cause of lung and bone metastases after LT. Intragastric metastasis of HCC was very rare, and hematogenous metastasis, lymph node metastases, and local infiltration of tumors were not explained by the reasons for metastasis. To our knowledge, this is the first study to report HCC metastasis to the stomach lumen without invasion of serosa after LT. This patient had a portal vein cancer thrombus, high portal vein pressure and gastric esophageal varices leading to bleeding. The above reasons cause cancer cells to colonize the bleeding site through the portal vein to the left gastric vein and then through the fundusophageal vein. After liver transplantation, the low immunity of patients and acidic environment of the stomach both easily promote tumor cell growth.
In our study, we reported a rare metastatic pathway, intragastric metastasis of HCC. Liver transplantation can significantly prolong patient survival beyond the Milan criteria by downstaging.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
The studies involving humans were approved by Medical Ethics Committee, Xiamen University School of Medicine. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
YX: Funding acquisition, Investigation, Methodology, Supervision, Writing – original draft. BS: Conceptualization, Data curation, Formal analysis, Writing – review & editing. JZ: Investigation, Methodology, Resources, Validation, Writing – original draft, Writing – review & editing. GW: Resources, Writing – review & editing. JW: Formal analysis, Writing – review & editing. WA: Supervision, Visualization, Writing – review & editing. YQ: Validation, Writing – review & editing. ZS: Investigation, Resources, Writing – review & editing. ZP: Data curation, Project administration, Resources, Writing – review & editing. HL: Data curation, Funding acquisition, Investigation, Methodology, Resources, Supervision, Validation, Writing – original draft, Writing – review & editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work received financial support from the Natural Science Fund for Distinguished Young Scholars of Fujian Province (2023J01310519).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Akinyemiju T, Abera S, Ahmed M, Alam N, Alemayohu MA, Allen C, et al. The burden of primary liver cancer and underlying etiologies from 1990 to 2015 at the global, regional, and national level: results from the global burden of disease study 2015. JAMA Oncol. (2017) 3:1683. doi: 10.1001/jamaoncol.2017.3055
2. Fitzmaurice C, Global Burden of Disease Cancer Collaboration. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 29 cancer groups, 2006 to 2016: A systematic analysis for the Global Burden of Disease study. JCO. (2018) 36:1568–8. doi: 10.1200/JCO.2018.36.15_suppl.1568
3. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer J Clin. (2018) 68:394–424. doi: 10.3322/caac.21492
4. Wang H, Naghavi M, Allen C, Barber R, Bhutta ZA, Carter A, et al. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. (2016) 388:1459–544. doi: 10.1016/S0140-6736(16)31012-1
5. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
6. Llovet JM, Kelley RK, Villanueva A, Singal AG, Pikarsky E, Roayaie S, et al. Hepatocellular carcinoma. Nat Rev Dis Primers. (2021) 7:6. doi: 10.1038/s41572-020-00240-3
7. Petrick JL, Braunlin M, Laversanne M, Valery PC, Bray F, McGlynn KA. International trends in liver cancer incidence, overall and by histologic subtype, 1978-2007: International trends in liver cancer incidence. Int J Cancer. (2016) 139:1534–45. doi: 10.1002/ijc.30211
8. Vogel A, Meyer T, Sapisochin G, Salem R, Saborowski A. Hepatocellular carcinoma. Lancet. (2022) 400:1345–62. doi: 10.1016/S0140-6736(22)01200-4
9. Fattovich G, Stroffolini T, Zagni I, Donato F. Hepatocellular carcinoma in cirrhosis: Incidence and risk factors. Gastroenterology. (2004) 127:S35–50. doi: 10.1053/j.gastro.2004.09.014
10. Yan X, Wang P, Zhu Z, Ning Z, Xu L, Zhuang L, et al. Site-specific metastases of intrahepatic cholangiocarcinoma and its impact on survival: a population-based study. Future Oncol. (2019) 15:2125–37. doi: 10.2217/fon-2018-0846
11. Lau H, Fan ST, Ng IOL, Wong J. Long term prognosis after hepatectomy for hepatocellular carcinoma: A survival analysis of 204 consecutive patients. Cancer. (1998) 83:2302–11. doi: 10.1002/(SICI)1097-0142(19981201)83:11<2302::AID-CNCR9>3.0.CO;2-1
12. Arii S, Teramoto K, Kawamura T, Okamoto H, Kaido T. Characteristics of recurrent hepatocellular carcinoma in Japan and our surgical experience. J Hepato-Biliary-Pancreatic Surgery. (2001) 8:397–403. doi: 10.1007/s005340100000
13. Yin Y, Dai H, Sun X, Xi Z, Zhang J, Pan Y, et al. HRG inhibits liver cancer lung metastasis by suppressing neutrophil extracellular trap formation. Clin Trans Med. (2023) 13:e1283. doi: 10.1002/ctm2.1283
14. Nakashima T, Okuda K, Kojiro M, Jimi A, Yamaguchi R, Sakamoto K, et al. Pathology of hepatocellular carcinoma in Japan: 232 consecutive cases autopsied in ten years. Cancer. (1983) 51:863–77. doi: 10.1002/1097-0142(19830301)51:5<863::AID-CNCR2820510520>3.0.CO;2-D
15. Mehta N, Bhangui P, Yao FY, Mazzaferro V, Toso C, Akamatsu N, et al. Liver transplantation for hepatocellular carcinoma. Working group report from the ILTS transplant oncology consensus conference. Transplantation. (2020) 104:1136–42. doi: 10.1097/TP.0000000000003174
16. Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. (1996) 334:693–700. doi: 10.1056/NEJM199603143341104
17. Sugawara Y, Hibi T. Surgical treatment of hepatocellular carcinoma. BST. (2021) 15:138–41. doi: 10.5582/bst.2021.01094
18. Pavel MC, Fuster J. Expansion of the hepatocellular carcinoma Milan criteria in liver transplantation: Future directions. WJG. (2018) 24:3626–36. doi: 10.3748/wjg.v24.i32.3626
19. Katyal S, Oliver JH, Peterson MS, Ferris JV, Carr BS, Baron RL. Extrahepatic metastases of hepatocellular carcinoma. Radiology. (2000) 216:698–703. doi: 10.1148/radiology.216.3.r00se24698
20. Roayaie S, Schwartz JD, Sung MW, Emre SH, Miller CM, Gondolesi GE, et al. Recurrence of hepatocellular carcinoma after liver transplant: Patterns and prognosis. Liver Transpl. (2004) 10:534–40. doi: 10.1002/lt.20128
21. Varol Fİ. Pediatric hepatocellular carcinoma. J Gastrointest Canc. (2020) 51:1169–75. doi: 10.1007/s12029-020-00494-w
22. Hackl C. Liver transplantation for Malignancy: Current treatment strategies and future perspectives. WJG. (2014) 20:5331. doi: 10.3748/wjg.v20.i18.5331
23. Cristofanilli M, Hayes DF, Budd GT, Ellis MJ, Stopeck A, Reuben JM, et al. Circulating tumor cells: A novel prognostic factor for newly diagnosed metastatic breast cancer. JCO. (2005) 23:1420–30. doi: 10.1200/JCO.2005.08.140
Keywords: hepatocellular carcinoma, intragastric metastasis, liver cancer, liver transplantation, pediatric liver transplantation
Citation: Xue Y, Shi B, Zhong J, Wang G, Wang J, An W, Qian Y, Su Z, Peng Z and Li H (2024) Case report: A rare case of intragastric metastasis after liver transplantation for liver cancer. Front. Oncol. 14:1495517. doi: 10.3389/fonc.2024.1495517
Received: 12 September 2024; Accepted: 19 November 2024;
Published: 06 December 2024.
Edited by:
Chao Li, Eastern Hepatobiliary Surgery Hospital, ChinaReviewed by:
Jun Zheng, Third Affiliated Hospital of Sun Yat-sen University, ChinaCopyright © 2024 Xue, Shi, Zhong, Wang, Wang, An, Qian, Su, Peng and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hao Li, bGloYW82NjU2QDE2My5jb20=; Zhihai Peng, MTUyMTY2ODkzOTZAMTYzLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.