- 1P. Hertsen Moscow Oncology Research Institute – Branch of the National Medical Research Radiological Centre of the Ministry of Health of the Russian Federation, Moscow, Russia
- 2Peoples’ Friendship University of Russia, Moscow, Russia
The 5-year overall survival rate for stage IV gastric cancer is lower than 10%, despite the development of systemic therapy. Conversion surgery has shown to improve survival outcomes in patients with durable clinical response on chemotherapy. We report a clinical case of a patient, who underwent conversion surgery after pembrolizumab in the third-line setting for stage IV gastric cancer. The patient did not have recurrence for 22 months after conversion surgery.
1 Introduction
An estimated 26,500 cases of gastric cancer (GC) were diagnosed in the United States in 2023 (1). The incidence of GC has decreased in the Russian Federation, where it ranks fifth most commonly diagnosed cancer among the male population and ninth among the female population in 2022. However, GC still has a high mortality rate, ranking second in terms of mortality. The average age of patients with a first-time diagnosis of GC is 67.7 years. In addition, gastric and gastroesophageal junction adenocarcinoma demonstrates a high frequency of mortality: 5-year overall survival (OS) rate for patients with distant metastases accounts for 6%, according to the American Cancer Society (2, 3). Platinum-based chemotherapy is a gold standard of first-line treatment for metastatic GC with negative human epidermal growth factor receptor 2 (Her2-neu) expression and microsatellite stable subtype (MSS) (4–7). Real-world data show that 25% of patients with advanced gastric or GEJ cancer do not receive any treatment (8). Of those patients who are treated, 42% receive second-line therapy and only 19% have third-line therapy (8, 9). In the context of third-line therapy for GC, the KEYNOTE-012 trial assessed the efficacy of immune checkpoint inhibitors (ICIs), particularly pembrolizumab, in patients with PD-L1-positive recurrent or metastatic GC or GEJ cancer (GEJC). The results demonstrated significant anti-tumor activity and a manageable safety profile for pembrolizumab in patients with high PD-L1 expression (TPS ≥ 1). Following this, the KEYNOTE-059 trial further explored pembrolizumab as a third-line treatment for GC or GEJC patients, reporting an overall response rate (ORR) of 22.7% in patients with a combined positive score (CPS) ≥ 1, compared to 8.6% in those with PD-L1-negative tumors. Based on the favorable outcomes from KEYNOTE-059, the FDA granted accelerated approval for pembrolizumab for patients with recurrent, locally advanced, or metastatic GC or GEJC with CPS ≥ 1.
Conversion surgery is a preferred treatment option for patients with major clinical responses after first-line chemotherapy (10–13). Studies show that such patients can achieve significant improvement in survival outcomes; however, there has been a paucity of literature regarding the role of conversion surgery after second- or third-line systemic treatment for advanced GC. Furthermore, there is no standard approach after curative gastrectomy for M1 patients with a complete response of metastases after induction therapy.
We describe a patient with metastatic GC who underwent curative gastrectomy after a complete response of liver metastases on third-line pembrolizumab with no evidence of the disease for 22 months after surgery.
2 Case report
A 67-year-old man was admitted to the local hospital with abdominal pain in December 2019. Esophagogastroduodenoscopy (EGD) revealed infiltration of ulcerative changes in the antrum of the stomach. The pathological examination of the biopsied specimen indicated a poorly differentiated adenocarcinoma. Immunohistochemistry was negative for Her2neu, positive for programmed cell death ligand one expression (PD-L1) combined positive score=1% by clone 22C3, MSS. Computed tomography (CT) scan showed tumor invasion into the pancreas, multiple altered lymph nodes of the small omentum, gastrointestinal ligament, and carcinomatosis. The clinical diagnosis was cT4aN2M1 (per), Stage IV.
The patient received first-line chemotherapy with XELOX between February 2020 and May 2020 (Figure 1). CT scan showed a slight reduction in tumor size, and in June 2020, the patient had the first session of pressurized intraperitoneal aerosol chemotherapy (PIPAC). The peritoneal cancer index (PCI) score was 1. Then two cycles of XELOX were administered, followed by the second course of PIPAC in August 2020. PCI score remained 1. After 1 month, the patient underwent a gastroenterostomy for pyloric stenosis.
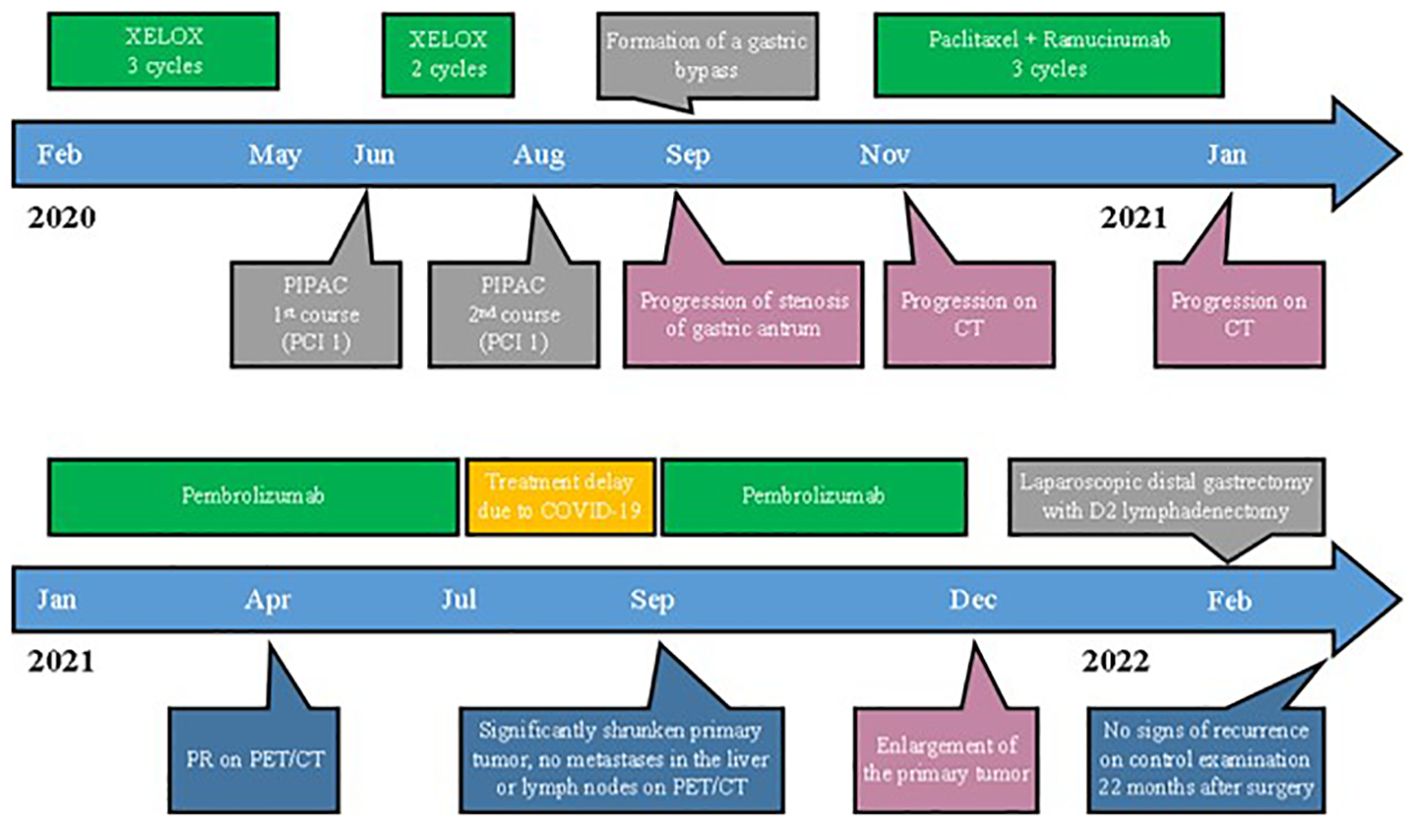
Figure 1. Timeline of the treatment and disease characteristics. PIPAC, pressurized intraperitoneal aerosol chemotherapy; PCI, peritoneal cancer index; CT, computed tomography.
In November of 2020, a CT scan of the abdomen showed progression of infiltrative changes in the stomach and enlarged perigastric and peripancreatic lymph nodes, and two metastases appeared in the liver.
Therefore, the systemic therapy was switched to a combination of paclitaxel and ramucirumab, which is the standard second-line treatment for metastatic GC. After three cycles, a CT scan showed growth of existing metastases and the appearance of new lesions (Figure 2).
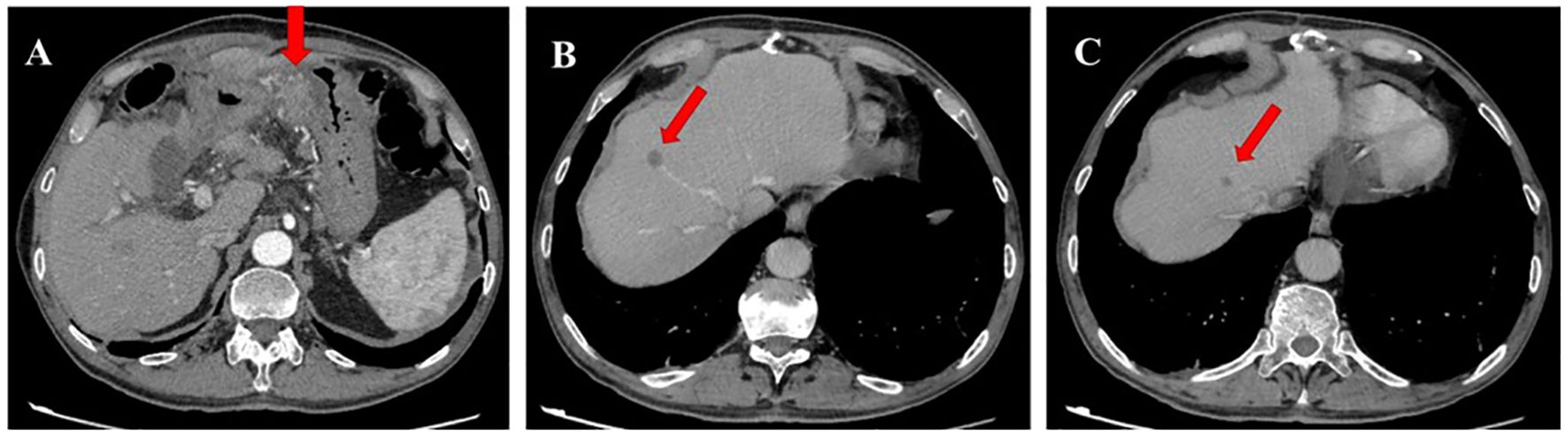
Figure 2. Contrast-enhanced computed tomography (CT) scan image in January 2021. (A) CT scan shows progression of infiltrative changes in the stomach. (B, C) CT scan shows new metastatic foces in the liver.
Hence, in January 2021, the patient started the third-line pembrolizumab due to positive CPS (1%).
Positron emission tomography (PET) in April 2021 showed a decrease in the size of infiltration in the distal part of the stomach from 73 mm × 104 mm to 39 mm × 28 mm and perigastric and peripancreatic lymph nodes from 20 mm to 7 mm. One metastatic lesion in the liver disappeared, and another decreased from 11 mm to 8 mm with no abnormal uptake (Figures 3A, B).
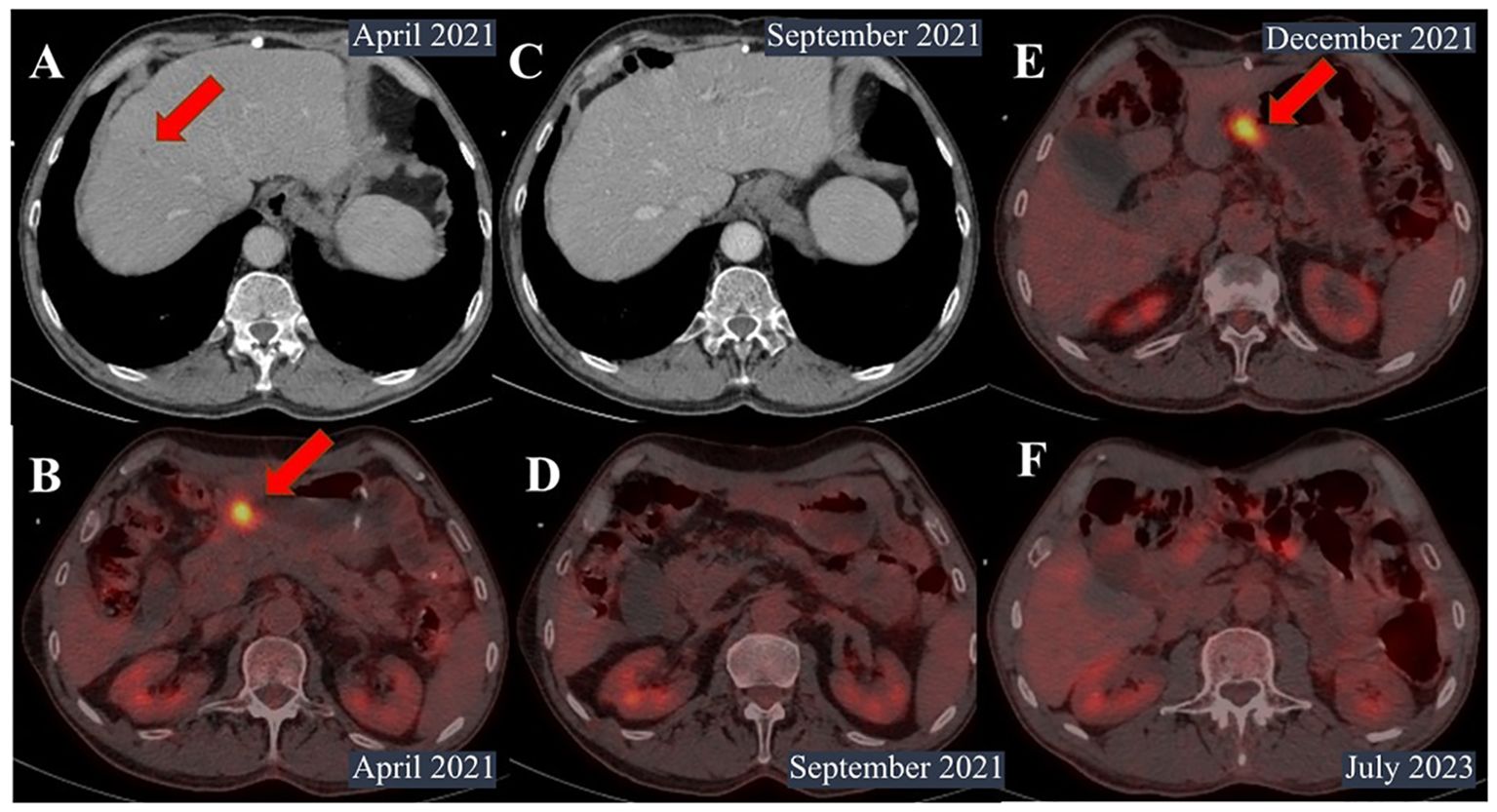
Figure 3. Contrast-enhanced computed tomography (CT) and PET scan images. (A) CT scan in April 2021. The size of metastatic foci in the S8 segment of the liver decreased from 11 mm to 8 mm. (B) Positron emission tomography (PET) in April 2021 showed a decrease in the size of infiltration in the distal part of the stomach from 73 mm × 104 mm to 39 mm × 28 mm. (C, D) PET scan in September 2021. No metastases in the liver or lymph nodes were identified. (E) PET scan in December 2021. The primary tumor was noted in the small curvature of the pylorus up to 18 mm × 24 mm with hypermetabolism of fluorodeoxyglucose (SUVmax=11.29). (F) PET scan in July 2023. No signs of recurrence were noted.
However, the patient was infected with coronavirus disease and had a break in treatment for 2 months between July and September 2021. PET scan showed a significantly decrease in primary tumor, with no metastasis in the liver or lymph nodes (Figures 3C, D).
The patient continued pembrolizumab until December 2021, when enlargement of the primary tumor and the appearance of hypermetabolism of fluorodeoxyglucose were noted (Figure 3Е).
Due to the absence of distant metastases, the patient underwent conversion surgery with laparoscopic distal gastrectomy with D2 lymphadenectomy (previously done staging laparoscopy indicated no peritoneal dissemination and negative peritoneal cytology). The postoperative histology showed well-differentiated adenocarcinoma, 25 mm tumor node with muscle layer invasion, and clear lymph nodes.
Since then, the patient has not received any treatment and showed no signs of recurrence on follow-up examinations with PET and EGD every 3 months for 22 months after surgery (Figure 3F).
3 Discussion
Advanced GC with peritoneal or distant metastases has a poor prognosis, despite the development of targeted drugs or immune checkpoint inhibitors; the median overall survival (OS) does not reach 1 year (8, 14–17). Although stage IV GC can present in various tumor characteristics and biology (18–20), in patients with major clinical response to systemic therapy, CS could provide a promising option and prolong their survival outcomes (21–23). It is important to convert the patient in time because systemic treatment could result in chemoresistance or cause severe adverse effects.
However, there is necessity to determine prognostic markers of patients, who benefit from CS.
The multicenter retrospective study by Kano et al. analyzed four cohorts of patients (n=79). Patients from the first cohort had resectable GC if they had positive peritoneal cytology, para-aortic lymph node metastases, or solitary liver metastasis minor 5 cm, and patients from other cohorts had unresectable disease (24, 25). Resectable GC (HR, 0.378; 95% CI, 0.173–0.824; p = 0.014) and R0 resection (HR, 0.439; 95% CI, 0.227–0.847; p = 0.014) of all metastatic sites were significant prognostic markers of favorable OS by multivariate Cox regression analysis.
In addition, Lin Ni et al. showed that the prognostic marker GPR176 correlates with sensitivity to drug therapy in GC (25). They found that high expression of GPR176 in tumors is associated with poor prognosis. Moreover, both CTLA4- and PD-1 positive and negative gastric adenocarcinomas with low expression of GPR176 had better responses to ICIs.
We report a clinical case of a patient, who progressed on two lines of systemic therapy; however, he had a durable response on pembrolizumab with MSS and low PD-L1 status, and subsequent conversion surgery. The described above clinical case is the fifth case report of CS after the third-line immunotherapy, and in the majority of these reports, no adjuvant therapy was administered. In addition, in the present clinical case, the patient did not receive adjuvant therapy, as there is currently limited evidence supporting its use. For instance, a retrospective study conducted at the National Cancer Center in China evaluated 122 patients who underwent conversion surgery (26). Of these, only 80 patients (65.6%) received postoperative adjuvant chemotherapy—either S-1 alone (n=36) or S-1 combined with platinum (n=28). The treatment was administered for a median of three cycles. Regarding OS, no significant difference was observed between the adjuvant therapy group and the observation group (63.9 months vs. 50.5 months, p=0.72). However, the adjuvant group did show a survival benefit in progression-free survival (PFS), with a median of 29.7 months compared to 14.6 months in the observation group (p=0.009). In a clinical case reported by Kosuke Fukuda et al., a patient underwent conversion surgery after second-line treatment with paclitaxel in combination with ramucirumab, followed by postoperative adjuvant chemotherapy with S-1 for 6 months (27). The patient survived without recurrence for 42 months after conversion surgery. In another case by Ryu Matsumoto et al., involving conversion surgery after third-line treatment with nivolumab, despite achieving a complete pathological response, the patient continued adjuvant therapy with nivolumab (28). Given the radical R0 surgical resection, the well-differentiated adenocarcinoma of the primary tumor, and the fact that the role of adjuvant immunotherapy remains unclear with limited supporting evidence, the decision in our case was made to proceed with active surveillance.
In the present case, given that primary tumor enlarged, but disappearance of distant metastases was shown after 11 months of immunotherapy, conversion surgery was performed. No peritoneal dissemination or positive peritoneal cytology was observed via staging laparoscopy.
This patient had favorable prognostic factors, such as a durable response on treatment, no peritoneal dissemination or positive peritoneal cytology observed via staging laparoscopy, and R0 resection of the primary tumor. Obviously, CS for stage IV GC is not routinely performed due to low objective responses on chemotherapy, especially after third-line treatment, because of tumor aggressiveness, performance status of the patient, and unresectable metastases. Considering that CS can improve survival outcomes, it is crucial to select patients based on favorable prognostic factors, such as a durable response to systemic treatment, limited extent of metastatic disease, negative peritoneal metastasis and negative peritoneal cytology findings, good performance status, and the potential for achieving complete resection. A multidisciplinary approach is essential for careful patient selection, maximizing the likelihood of successful outcomes and improving overall survival.
4 Conclusion
This case report illustrates the long-term survival for 22 months of the patient after CS for advanced GC without adjuvant therapy. Conversion surgery could be a treatment option after third-line immunotherapy; however, there is no standard treatment approach for postoperative treatment after conversion surgery for stage IV GC. Further research is needed to identify predictive biomarkers of response to immune checkpoint inhibitors and determine a cohort of patients, who will benefit from subsequent surgery.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
SE: Writing – original draft. ALK: Writing – review & editing. LB: Supervision, Writing – review & editing. IK: Methodology, Writing – review & editing. AF: Writing – review & editing. ADK: Writing – original draft.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. (2023) 73:17–48. doi: 10.3322/caac.21763
2. Stomach (Gastric) Cancer Survival Rates . Available online at: https://www.cancer.org/cancer/stomach-cancer/detection-diagnosis-staging/survival-rates.html (Accessed April 25, 2023).
3. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2018) 68:394–424. doi: 10.3322/caac.21492
4. Enzinger PC, Burtness BA, Niedzwiecki D, Ye X, Douglas K, Ilson DH, et al. CALGB 80403 (Alliance)/E1206: A randomized phase II study of three chemotherapy regimens plus cetuximab in metastatic esophageal and gastroesophageal junction cancers. J Clin Oncol. (2016) 34:2736–42. doi: 10.1200/JCO.2015.65.5092
5. Luo H, Xu R, Wang F, Qiu M, Li Y, Li F, et al. Phase II trial of XELOX as first-line treatment for patients with advanced gastric cancer. Chemotherapy. (2010) 56:94–100. doi: 10.1159/000305256
6. Al-Batran S-E, Hartmann JT, Probst S, Schmalenberg H, Hollerbach S, Hofheinz R, et al. Phase III trial in metastatic gastroesophageal adenocarcinoma with fluorouracil, leucovorin plus either oxaliplatin or cisplatin: A study of the arbeitsgemeinschaft internistische onkologie. J Clin Oncol. (2008) 26:1435–42. doi: 10.1200/JCO.2007.13.9378
7. Kim GM, Jeung H-C, Rha SY, Kim HS, Jung I, Nam BH, et al. A randomized phase II trial of S-1-oxaliplatin versus capecitabine–oxaliplatin in advanced gastric cancer. Eur J Cancer. (2012) 48:518–26. doi: 10.1016/j.ejca.2011.12.017
8. Le DT, Ott PA, Korytowsky B, Le H, Le TK, Zhang Y, et al. Real-world treatment patterns and clinical outcomes across lines of therapy in patients with advanced/metastatic gastric or gastroesophageal junction cancer. Clin Colorectal Cancer. (2020) 19:32–38.e3. doi: 10.1016/j.clcc.2019.09.001
9. Chan W, Lam K, So T, Lee VH, Kwong LD. Third-line systemic treatment in advanced/metastatic gastric cancer: a comprehensive review. Ther Adv Med Oncol. (2019) 11:1758835919859990. doi: 10.1177/1758835919859990
10. Fukuchi M, Ishiguro T, Ogata K, Suzuki O, Kumagai Y, Ishibashi K, et al. Prognostic role of conversion surgery for unresectable gastric cancer. Ann Surg Oncol. (2015) 22:3618–24. doi: 10.1245/s10434-015-4422-6
11. Yoshida K, Yamaguchi K, Okumura N, Tanahashi T, Kodera Y. Is conversion therapy possible in stage IV gastric cancer: the proposal of new biological categories of classification. Gastric Cancer. (2016) 19:329–38. doi: 10.1007/s10120-015-0575-z
12. Zurleni T, Gjoni E, Altomare M, Rausei S. Conversion surgery for gastric cancer patients: A review. World J Gastrointest Oncol. (2018) 10:398–409. doi: 10.4251/wjgo.v10.i11.398
13. Shin M-K, Choi M-G, Kim S-T, Kang W-K, Sohn T-S, An J-Y, et al. The clinical implication of conversion surgery in patients with stage IV gastric cancer who received systemic chemotherapy. Biomedicines. (2023) 11:3097. doi: 10.3390/biomedicines11113097
14. Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer. (2017) 20:1–19. doi: 10.1007/s10120-016-0622-4
15. Wagner AD, Unverzagt S, Grothe W, Kleber G, Grothey A, Haerting J, et al. Chemotherapy for advanced gastric cancer. In: Cochrane Database of Systematic Reviews. John Wiley & Sons, Ltd, Chichester, UK (2010). p. CD004064. doi: 10.1002/14651858.CD004064.pub3
16. Guimbaud R, Louvet C, Ries P, Ychou M, Maillard E, André T, et al. Prospective, Randomized, Multicenter, Phase III Study of Fluorouracil, Leucovorin, and Irinotecan Versus Epirubicin, Cisplatin, and Capecitabine in Advanced Gastric Adenocarcinoma: A French Intergroup (Fédération Francophone de Cancérologie Digestive, Fédération Nationale des Centres de Lutte Contre le Cancer, and Groupe Coopérateur Multidisciplinaire en Oncologie) Study. J Clin Oncol. (2014) 32:3520–6. doi: 10.1200/JCO.2013.54.1011
17. Hu H-M, Tsai H-J, Ku H-Y, Lo S-S, Shan Y-S, Chang H-C, et al. Survival outcomes of management in metastatic gastric adenocarcinoma patients. Sci Rep. (2021) 11:23142. doi: 10.1038/s41598-021-02391-z
18. Thomassen I, van Gestel YR, van Ramshorst B, Luyer MD, Bosscha K, Nienhuijs SW, et al. Peritoneal carcinomatosis of gastric origin: A population-based study on incidence, survival and risk factors. Int J Cancer. (2014) 134:622–8. doi: 10.1002/ijc.28373
19. Chan DYS, Syn NL-X, Yap R, Phua JNS, Soh TIP, Chee CE, et al. Conversion surgery post-intraperitoneal paclitaxel and systemic chemotherapy for gastric cancer carcinomatosis peritonei. Are We Ready? J Gastrointest Surg. (2017) 21:425–33. doi: 10.1007/s11605-016-3336-3
20. Kim SW. The result of conversion surgery in gastric cancer patients with peritoneal seeding. J Gastric Cancer. (2014) 14:266–70. doi: 10.5230/jgc.2014.14.4.266
21. Yamaguchi K, Yoshida K, Tanahashi T, Takahashi T, Matsuhashi N, Tanaka Y, et al. The long-term survival of stage IV gastric cancer patients with conversion therapy. Gastric Cancer. (2018) 21:315–23. doi: 10.1007/s10120-017-0738-1
22. Solaini L, Ministrini S, Bencivenga M, D’Ignazio A, Marino E, Cipollari C, et al. Conversion gastrectomy for stage IV unresectable gastric cancer: a GIRCG retrospective cohort study. Gastric Cancer. (2019) 22:1285–93. doi: 10.1007/s10120-019-00968-2
23. Ito S, Oki E, Nakashima Y, Ando K, Hiyoshi Y, Ohgaki K, et al. Clinical significance of adjuvant surgery following chemotherapy for patients with initially unresectable stage IV gastric cancer. Anticancer Res. (2015) 35(1):401–6.
24. Kano Y, Ichikawa H, Hanyu T, Muneoka Y, Ishikawa T, Aizawa M, et al. Conversion surgery for stage IV gastric cancer: a multicenter retrospective study. BMC Surg. (2022) 22:428. doi: 10.1186/s12893-022-01874-8
25. Ni L, Chen S, Liu J, Li H, Zhao H, Zheng C, et al. GPR176 is a biomarker for predicting prognosis and immune infiltration in stomach adenocarcinoma. Mediators Inflammation. (2023) 2023:7123568. doi: 10.1155/2023/7123568
26. Wang T, Wang N, Ren H, Zhou H, Zhou A, Jin J, et al. Long-term results of conversion therapy for initially unresectable gastric cancer: analysis of 122 patients at the national cancer center in China. J Cancer. (2019) 10:5975–85. doi: 10.7150/jca.35527
27. Fukuda K, Arigami T, Tokuda K, Yanagita S, Matsushita D, Kawasaki Y, et al. Successful conversion surgery for stage IV gastric cancer with liver metastases after second-line chemotherapy with ramucirumab and paclitaxel: a case report. Surg Case Rep. (2022) 8:58. doi: 10.1186/s40792-022-01412-x
Keywords: gastric cancer (GC), gastroesophageal junction (GEJ), immunotherapy, conversion surgery, pembrolizumab, case report
Citation: Evdokimova SF, Kornietskaya AL, Bolotina LV, Kolobayev IV, Fedenko AA and Kaprin AD (2024) Conversion surgery for stage IV gastric cancer after third-line immunotherapy: a case report. Front. Oncol. 14:1494669. doi: 10.3389/fonc.2024.1494669
Received: 11 September 2024; Accepted: 11 November 2024;
Published: 06 December 2024.
Edited by:
Hongwei Cheng, University of Macau, ChinaReviewed by:
Keren Jia, Peking University, ChinaXiaoDong Chen, First Affiliated Hospital of Wenzhou Medical University, China
Copyright © 2024 Evdokimova, Kornietskaya, Bolotina, Kolobayev, Fedenko and Kaprin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sevindzh F. Evdokimova, ZXZkb2tpbW92YS5zZXZpbmR6aEBnbWFpbC5jb20=
 Sevindzh F. Evdokimova
Sevindzh F. Evdokimova Anna L. Kornietskaya
Anna L. Kornietskaya Larisa V. Bolotina
Larisa V. Bolotina Iliya V. Kolobayev1
Iliya V. Kolobayev1