
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Oncol., 05 December 2024
Sec. Hematologic Malignancies
Volume 14 - 2024 | https://doi.org/10.3389/fonc.2024.1491100
This article is part of the Research TopicHost Features Affecting CAR T Cell Therapy of Hematological MalignanciesView all articles
 Danqing Kong1,2†
Danqing Kong1,2† Nana Ping1,2†
Nana Ping1,2† Qian Zhu1,2†
Qian Zhu1,2† Xiao Zhang1,2
Xiao Zhang1,2 Junhong Li1,2
Junhong Li1,2 Rui Zou1
Rui Zou1 Depei Wu1,2*‡
Depei Wu1,2*‡ Zhengming Jin1,2*‡
Zhengming Jin1,2*‡ Changju Qu1,2*‡
Changju Qu1,2*‡Chimeric antigen receptor T-cell (CAR-T) therapy has demonstrated both efficacy and safety in relapsed/refractory diffuse large B-cell lymphoma (DLBCL) patients infected with hepatitis B virus (HBV). However, its applicability in individuals with liver cirrhosis remains largely unexplored due to the potential for unpredictable complications. Here, we report three cases (P1, P2, and P3) of relapsed/refractory DLBCL with HBV-related cirrhosis treated with CAR-T cell infusion. P1 and P2 received CAR-T cell infusion following a conditioning regimen of fludarabine and cyclophosphamide (FC) for lymphodepletion, while P3 received the SEAM (semustine, etoposide, cytarabine, and melphalan) regimen and autologous stem cell transplantation bridging CAR-T cell infusion. P1 and P2 achieved rapid complete remission (CR), whereas P3 initially exhibited stable disease a month after CAR-T infusion and subsequently achieved CR after local radiation salvage therapy and lenalidomide maintenance. With a median follow-up of 42 months after CAR-T, the progression-free survival rate was 100%. Notably, during follow-up, these patients experienced complications associated with cirrhosis, including endoscopic variceal bleeding, HBV reactivation, or the diagnosis of hepatic malignancy. Our findings suggest that CAR-T therapy is applicable and effective for the treatment of DLBCL patients with HBV-related cirrhosis, albeit necessitating monitoring for potential hepatic complications.
Hepatitis B virus (HBV) infection represents a significant global public health concern, precipitating a cascade of hepatic complications including cirrhosis and hepatocellular carcinoma (HCC), often culminating in fatal outcomes in the absence of timely intervention. Concurrently, diffuse large B-cell lymphoma (DLBCL) stands as one of the predominant B-cell malignancies, characterized by its aggressive nature and challenging treatment landscape. The prevalence of HBV infection in DLBCL patients is higher than that in the general population and often leads to worse clinical outcomes (1, 2). However, there is limited information on treatment recommendations and a lack of appropriate clinical trials for DLBCL patients facing disease progression.
Chimeric antigen receptor T-cell (CAR-T) therapy has been reported as safe and effective in treating relapsed/refractory (R/R) DLBCL and approximately 40% even achieve sustained response (3, 4). Similarly, CAR-T therapy has brought ORR and CR rates of 74.5% and 39.2%, respectively, in our center for DLBCL patients who failed R-CHOP (rituximab, cyclophosphamide, vindesine, adriamycin, and dexamethasone regimen) and other salvage lines of immunochemotherapy (5). Because of the prolonged immunosuppressive state induced by CAR-T therapy, HBV reactivation is one big concern for patients with chronic HBV infection. The risk of HBV reactivation varies according to the patient’s HBV serologic status and treatment modality (6, 7). Recent reports have shown that HBV reactivation occurs in approximately 5.3%–20% of HBsAg-positive patients receiving antiviral prophylaxis after CAR-T therapy, mostly present with asymptomatic increase of HBV DNA copies, and the risk of hepatitis is approximately 5.3% (8–10). Nevertheless, it appears that HBV infection does not adversely affect CAR-T cell viability or efficacy of CAR-T therapy; meanwhile, the incidence and severity of cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity syndrome (ICANS) do not increase (5, 9, 10).
In 2021, the World Health Organization (WHO) disclosed that among individuals afflicted with chronic HBV infection, up to 40% may progress to cirrhosis if left untreated, with a subset of patients facing the dire consequences of liver malignancy, hepatic failure, or mortality. Liver cirrhosis was also an independent risk factor for HBV reactivation in hematological malignancies (11). Within the subset of patients diagnosed with DLBCL complicated by HBV-related cirrhosis, the utilization of CAR-T therapy had not been reported in the literature. The clinical efficacy and potential adverse events during and after infusion were to be detected. Herein, we presented a single-center case series of R/R DLBCL patients with cirrhosis, who underwent CAR-T cell infusion, delineating both the therapeutic efficacy and associated adverse outcomes.
We retrospectively reviewed R/R DLBCL patients who received CAR-T therapy at the First Affiliated Hospital of Soochow University from January 2018 to January 2023. Three DLBCL patients (P1, P2, and P3) complicated with hepatitis B-related cirrhosis were drawn out and analyzed. All patients provided written informed consent. The follow-up visit was conducted until the last visit, June 2024.
The diagnosis of DLBCL was made based on the fifth edition of WHO classification. Chronic HBV infection was defined by the detection of hepatitis B surface antigen (HBsAg) for more than 6 months.
The diagnosis of hepatitis B-related cirrhosis requires a comprehensive approach, involving (1) HBV infection medical history and symptoms such as fatigue or jaundice; (2) laboratory tests including HBV serological tests, HBV-DNA, and liver function; and (3) imaging studies by ultrasonography, computed tomography (CT), or magnetic resonance imaging (MRI) of the liver to detect the signs of cirrhosis and evaluate the complications. The Child–Pugh score system was used to assess the severity of liver disease and predict prognosis. HBV reactivation was defined as at least one of the following: (1) newly detectable HBV-DNA; (2) ≥2 log HBV-DNA increase compared to baseline; and (3) reappearance of HBsAg. The hepatitis flare was defined as alanine aminotransferase (ALT) or aspartate aminotransferase (AST) increased to ≥3 the upper limit of normal. The lower reference limit of HBV-DNA was 60 international units (IU)/milliliter (mL) based on our laboratory standards.
All three DLBCL patients had a known history of concomitant HBV infection for years characterized by positive results for HBsAg, but did not pay proper attention. HBV-related cirrhosis was diagnosed through a combination of clinical assessment, laboratory tests, and imaging studies by ultrasonography or CT (see details in Table 1). For P1 and P2, cirrhosis was diagnosed at the presentation of DLBCL. P3 was diagnosed with cirrhosis only when the disease relapsed. He was diagnosed with DLBCL and received six cycles of the CHOP regimen, achieving complete remission 5 years ago. At that time, he underwent a short course of antiviral treatment due to chronic HBV infection.
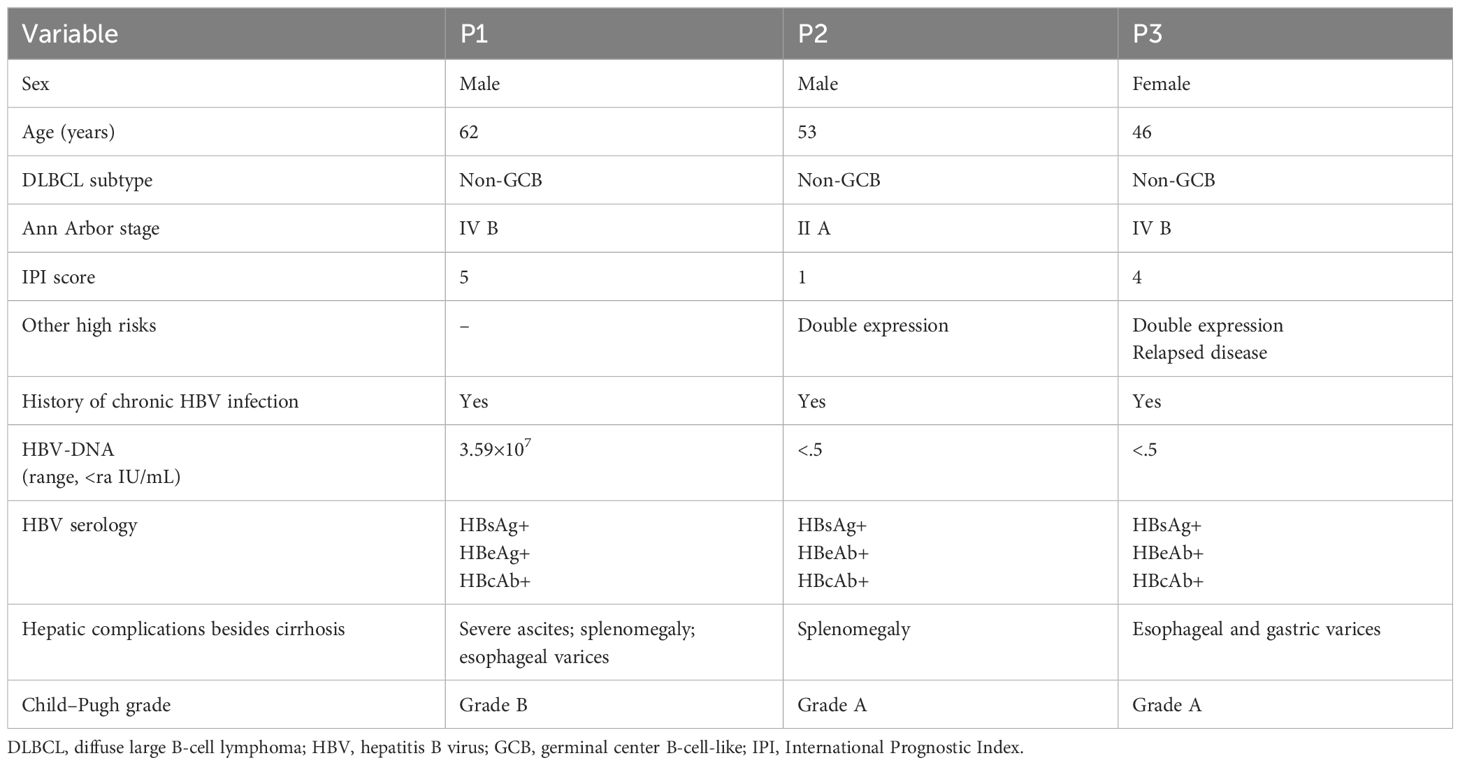
Table 1. Characteristics of the three patients at the diagnosis of DLBCL complicated with HBV-related cirrhosis.
They received antiviral treatment with entecavir at a daily dose of 0.5 mg along with supportive measures, and when HBV-DNA was undetectable and liver function improved to a Child–Pugh classification of A, they initiated standard immunochemotherapy without dose reduction, but with split dosing. However, the optimal therapeutic outcomes were not realized, prompting the recommendation for salvage treatment with CAR-T or ASCT bridging CAR-T therapy as salvage treatment. The treatment course and corresponding responses of the patients are summarized in Figure 1.
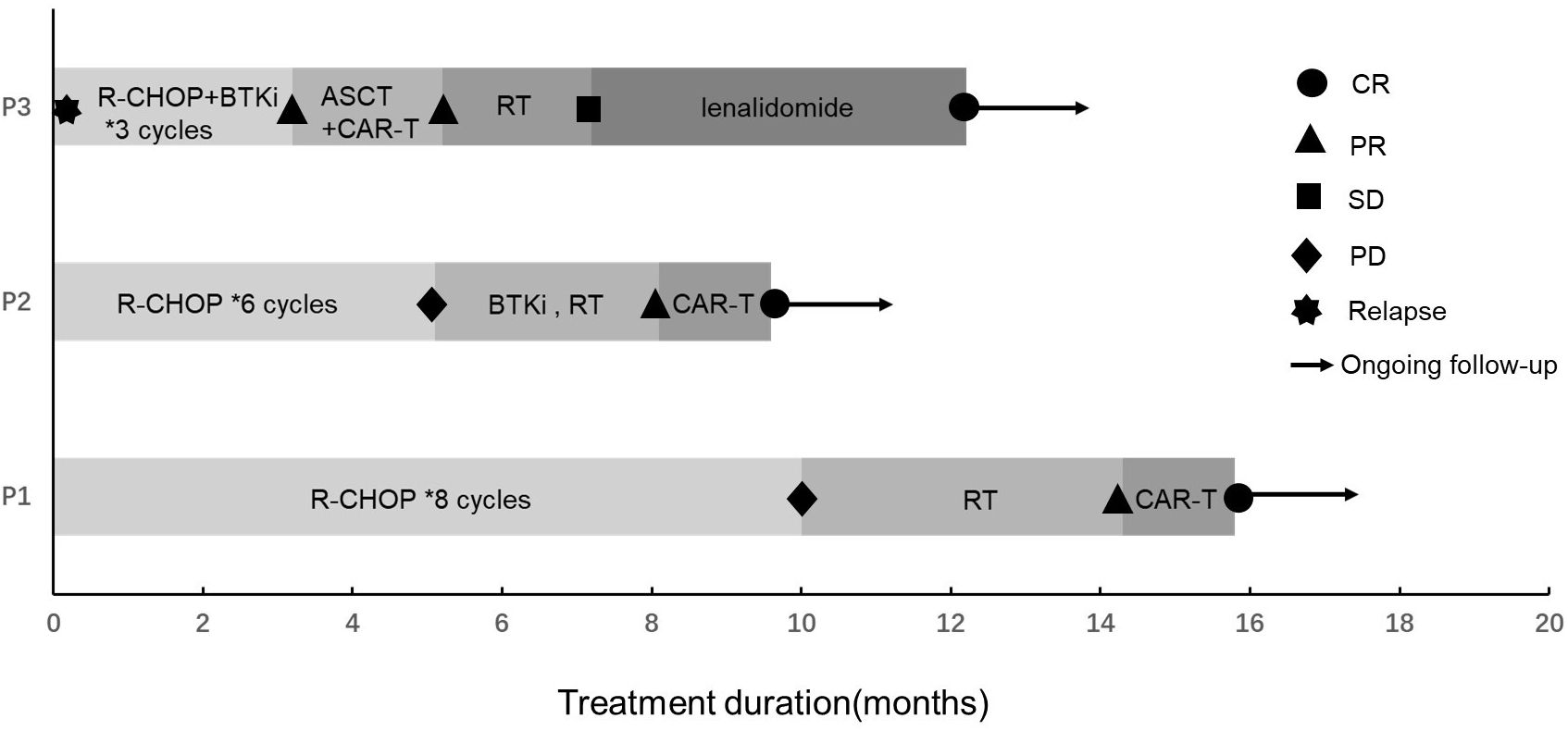
Figure 1. Treatment and response of three DLBCL patients with HBV-related cirrhosis. R-CHOP, rituximab + cyclophosphamide + vindesine + adriamycin + dexamethasone regimen; CAR-T, chimeric antigen receptor T cell; BTKi, Bruton’s tyrosine kinase inhibitor; ASCT, autologous stem cell transplantation; RT, radiotherapy; CR, complete remission; PR, partial remission; SD, stable disease; PD, progressive disease.
CAR-T products were provided by the Unicar-Therapy Bio-Medicine Technology Co. (Shanghai, China), and quality tests were performed before infusion to patients as previously described (12, 13); the detailed procedures are described in Supplementary Data S1.
The FC (fludarabine 30 mg/m2/d, day −5, −4, and −3; cyclophosphamide 300 mg/m2/d, day −5, −4, and −3) conditioning regimen was used for lymphodepletion prior to CAR-T infusion in both P1 and P2. Then, P1 received a scheduled infusion of anti-CD19 and anti-CD22 CAR-T cells at total doses of 1.5×107/kg and 2×107/kg, respectively; P2 received an infusion of tandem CD19/CD22 CAR-T cells at a total dose of 1×107/kg by dose escalation in 3 days (10%, 30%, and 60%). P3 underwent ASCT bridging tandem CD19/CD22 CAR-T therapy. Transplant conditioning used the SEAM (semustine 250 mg/m2/d, day −8; etoposide 200 mg/m2/d, day −7, −6, −5, and −4; cytarabine 400 mg/m2/d, day −7, −6, −5, and −4; melphalan, 140 mg/m2/d, day −3) regimen and then autologous stem cells were infused at day 0; the CD34+ cell count was 4.12×107/kg. CAR-T cells were infused from day 1 to day 3 at a total dose of 1×107/kg (10%, 30%, and 60%).
The evaluation included complete blood count (CBC), coagulation routine, organ function, expansion of CAR-T cells in peripheral blood, cytokines, and other inflammatory markers such as ferritin.
Response criteria were defined in accordance with the National Comprehensive Cancer Network (NCCN) guidelines. All adverse events were assessed according to the National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events (CTCAE, Version 4.0). CRS and ICANS were evaluated based on the widely accepted scoring system (14). Progression-free survival (PFS) was calculated from the day of CAR-T infusion to disease progression, relapse, death from any cause, or the last follow-up.
After CAR-T infusion, positron emission tomography–computed tomography (PET-CT) assessments confirmed complete remission (CR) in P1 and P2. Both individuals received comprehensive clinical oversight from hepatologists and hematologists for concurrent cirrhosis and DLBCL management, resulting in sustained DLBCL remission. P3 exhibited stable disease (SD) 1 month after ASCT bridging CAR-T, subsequently undergoing salvage local radiotherapy, but with minimal effect. Following this, lenalidomide maintenance therapy was initiated 6 months after ASCT bridging CAR-T, remarkably achieving complete disease control, and the patient finally achieved CR at 1 year after ASCT bridging CAR-T and maintained thereafter. Comprehensive radiological evaluations via PET-CT scans of three patients were detailed in Supplementary Data S2.
As of June 2024, these three patients had been followed up for a median of 42 months (range, 26.5 to 73 months) after CAR-T infusion; they all achieved sustained PFS. Patients expressed satisfaction with the effectiveness of the CAR-T cell therapy and had an acceptable view of the associated complications detailed below. After undergoing regular checkups and assessments, they were optimistic about the possibility of long-term survival.
The short-term toxicities associated with CAR-T infusion within a month were mild and reversible in three patients (detailed in Table 2). In particular, all showed grade 4 hematologic toxicity, but no CRS ≥grade 3 or ICANS was observed. CD19+ B-cell deletion persisted after CAR-T infusion for up to 6 months (range, 4–6 months).
All patients received indefinite antiviral treatment with entecavir. Laboratory tests indicated a persistent HBsAg-positive, Child–Pugh classification of A ever since induction therapy. They also maintained undetectable HBV-DNA throughout the whole episode except P1.
P1 was diagnosed as hepatic malignancy based on typical radiographic signs that both enhanced CT and MRI scans revealed newly detected nodules with a maximum diameter exceeding 2 cm 14 months after CAR-T therapy (Figure 2). Laboratory assessments indicated HBV reactivation, with an HBV-DNA level of 94.4 IU/mL and elevated serum bilirubin levels of 36.4 μmol/L, suggestive of liver function impairment. Serum alpha-fetoprotein (AFP), AST, ALT, platelet counts, and coagulation tests remained within normal range while Child–Pugh grade maintained an A classification. The patient refused surgical intervention or chemotherapy/radiotherapy options due to concerns about adverse reactions, and he opted for continuous antiviral therapy with entecavir and short-term adefovir, coupled with several sessions of transcatheter arterial chemoembolization (TACE), ablation therapy, and three cycles of anti-PD-1 inhibitor camrelizumab administration. Throughout his last visit, the patient remained alive with liver neoplasm under good control and undetectable HBV-DNA (the trend of HBV-DNA copies is shown in Supplementary Data S3).
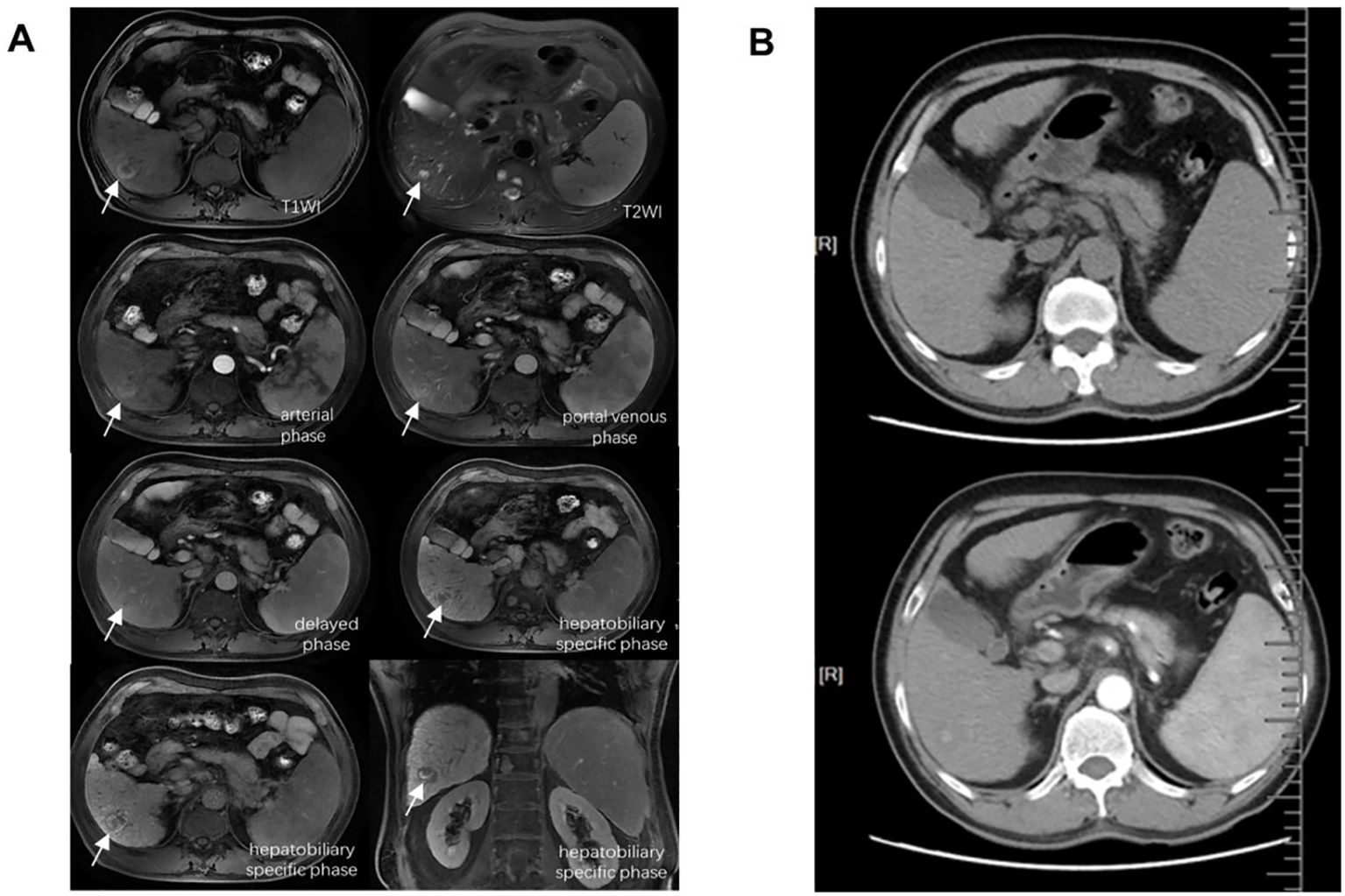
Figure 2. Major radiographic assessments of P1 for the diagnosis of liver malignant tumor. (A) Enhanced MRI by Gd-EOB-DTPA. (B) Enhanced CT scan. Both revealed newly detected nodules with a maximum diameter exceeding 2 cm. MRI, magnetic resonance imaging; CT, computed tomography.
P3 underwent indefinite lenalidomide maintenance therapy to manage DLBCL, maintaining platelet levels between 50 and 100×109/L. However, the patient experienced two episodes of esophagogastric variceal bleeding. She underwent endoscopic tissue glue injection and variceal ligation surgeries successively, additionally with supporting treatments such as fasting, proton pump inhibitors (PPIs), pancreatic secretion inhibition, and nutritional support. Her condition remained stable and under control until the last follow-up assessment.
DLBCL patients with chronic HBV infection revealed distinct clinical features of a younger age, more advanced disease stage, and even poorer outcomes (1, 2). The role of HBV in the mechanism of DLBCL had not been fully elucidated; one hypothesis was that, like hepatitis C, a protracted antigenic stimulation of B cells produced virus-specific antibodies; another hypothesis was based on genome-wide analysis, which revealed that enhanced gene mutation load possibly mediated by apolipoprotein B mRNA-editing enzyme catalytic polypeptide (APOBEC) enzyme activity and the activity of the B-cell-specific activation induced cytidine deaminase (AID) (1, 15).
Currently, there were no specific guidelines or sufficient clinical trials available for treating R/R DLBCL with HBV infection. HBV X protein (HBX) overexpressed in DLBCL with HBV infection and HBX expression potentiated resistance to S-phase arrest-inducing chemotherapeutics like methotrexate (MTX) and cytarabine (Ara-C) in vitro (16, 17), suggesting that neither MTX nor Ara-C was suitable as second-line treatments. CAR-T therapy had been attempted in this disease entity under prophylactic antiviral therapy (8–10). In a study of 15 R/R DLBCL patients with HBV infection, overall response (OR) and CR rate of CAR-T therapy was 66.7% and 60%, respectively, accompanying controllable CRS. HBV reactivation was seen in three patients (9).
However, DLBCL patients with HBV-related cirrhosis have received scant attention in medical literature. A retrospective case series of eight patients assessed the administration of rituximab in DLBCL patients with HBV-related cirrhosis, all of whom were undergoing standardized antiviral therapy. Among them, one patient experienced HBV reactivation, yet neither hepatitis flares nor abnormal liver function was observed. The median overall survival (OS) was 39 months (range, 7–82 months), indicating that rituximab was a safe option for these patients (18). In our study, the CR rate was 66.7%, and P3 did not achieve the expected efficacy despite receiving more intensive treatment with ASCT bridging CAR-T. HBV reactivation occurred in P1 despite whole-course combination of entecavir prophylaxis. The crucial risk factors for HBV reactivation can be broadly classified into three categories: host-related factors, virus-related factors, and immunosuppressive therapy. Immunosuppressive therapy is the major risk and quite common applied in DLBCL patients. In oncological practice, it is recommended to initiate antiviral prophylaxis with nucleoside analogs (NAs) before immunosuppressive therapies. Compared to the first approved NA lamivudine, the next-generation NA entecavir is less likely to cause drug resistance and is more effective in viral suppression and in preventing HBV reactivation among HBsAg-positive lymphoma patients undergoing chemotherapy (19). It is reported that tenofovir may be more effective than entecavir in HBeAg-positive patients (20). Entecavir and tenofovir are now recommended standard agents for the prevention and treatment of HBV reactivation in patients under immunosuppressive therapy. In the case of P1, entecavir was quite effective, but considering the transient detectable HBV-DNA presenting without drug withdrawal, entecavir was not a guarantee for avoiding HBV reactivation.
CRS is a major concern since chronic HBV infection may induce higher IL-6 production (21). Neither our cases nor other reported patients with HBV infection observed an increase in the frequency or severity of CRS or ICANS. However, owing to the constraint of a small sample size, the relationship between HBV infection and CRS warrants further investigation.
The clinical presentation of HBV reactivation or HBV-related mortality varied, ranging from unnoticed elevated HBV-DNA levels and/or ALT to life-threatening fulminant hepatitis, hepatic encephalopathy, and liver failure (9, 10, 22). However, the occurrence of newly developed hepatic malignancy was rare. In chronic viral hepatitis-related cirrhosis patients, the estimated cumulative incidence of HCC over a 10-year period was only approximately 4.0% (23). HBV plays a direct role in HCC transformation by triggering specific oncogenic pathways as well as stimulating host immune response and driving liver chronic necro-inflammation (24). Furthermore, complex immune imbalance may promote HCC transformation (25). In P1, despite the potential for CAR-T therapy to induce immune dysregulation characterized by cytokine elevation and B lymphocyte abnormalities, the likelihood of it significantly contributing to HCC transformation appears low. This conclusion is supported by the relatively modest and transient elevation in cytokine levels post-therapy, as well as the restoration of B lymphocyte counts within 6 months. Overall, it is preferable to believe that CAR-T therapy is not primarily responsible for hepatic malignancy development. The occurrence of cirrhosis cancerization following CAR-T therapy in this case may be coincidental; however, caution should still be exercised regarding this issue.
Recent studies have focused on the evaluation of ASCT bridging CAR-T therapy vs. CAR-T therapy for R/R DLBCL. One single-arm prospective clinical study (26) explored the efficacy and safety of ASCT bridging CD19/CD22 CAR-T therapy in 42 R/R aggressive B-cell non-Hodgkin lymphoma (B-NHL) patients, at a median follow-up of 24.3 months, the OR rate was 90.5%, the 2-year PFS rate was 83.3%, and the median PFS and OS were not reached. All cases of CRS and ICANS were reversible. Another retrospective cohort study (27) compared ASCT-CART to ASCT in 67 R/R DLBCL patients. The ASCT-CART group showed a higher CR rate (71% vs. 33%; p = 0.003), a superior 3-year PFS (80% vs. 44%; p = 0.036), and a lower 3-year relapse/progression rate (15% vs. 56%; p = 0.015) than the ASCT group. Although P3 in our study failed this combined therapy, the prospect of future trials to explore the efficacy and safety of ASCT bridging CAR-T therapy in a larger patient cohort is still promising. P3 achieved CR under lenalidomide maintenance 6 months after ASCT bridging CAR-T. Lenalidomide, an oral immunomodulatory drug, exerts its effects primarily through direct anti-tumor activity, immune modulation, and regulation of the tumor microenvironment (28). We conducted a study (29) evaluating lenalidomide maintenance after CAR-T therapy in R/R DLBCL patients, and we found that OS was significantly prolonged in the lenalidomide maintenance group; in addition, the in vitro test showed that the delayed exhaustion of CAR-T cells may contribute to the OS benefit. The remission observed in P3 cannot be attributed to delayed exhaustion of CAR-T cells, as these cells were undetectable prior to maintenance therapy. Nonetheless, the early introduction of lenalidomide maintenance therapy following CAR-T treatment may enhance efficacy and promote sustained peripheral blood levels of CAR-T cells. Looking back retrospectively, gene next-generation sequencing (NGS) could not be performed and many novel targeting drugs were not available; otherwise, genetic guided targeting treatment might have been an alternative choice for these patients.
There are some limitations in our report. The number of cases included is relatively small. Since the incidence rate of R/R DLBCL combined with cirrhosis is low, and the majority of the patients are CAR-T therapy ineligible due to patients’ physical limitations and high cost, there is difficulty in finding more candidates. Additionally, follow-up is still required to assess long-term efficacy and complications. In general, CAR-T therapy demonstrates effectiveness with tolerable treatment-related toxicities in treating our three refractory DLBCL patients with HBV-related cirrhosis. However, concerns persist regarding HBV reactivation and other long-term complications, despite the use of antiviral prophylaxis. Close monitoring of HBV-DNA levels, liver imaging, and liver function after CAR-T therapy is imperative. Further studies and data collection are warranted to ascertain whether CAR-T therapy is a suitable strategy for this unique patient population.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
The studies involving humans were approved by the Ethics Committee of the First Affiliated Hospital of Soochow University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
DK: Data curation, Formal analysis, Investigation, Writing – original draft. NP: Conceptualization, Project administration, Writing – review & editing. QZ: Data curation, Formal analysis, Supervision, Writing – review & editing. XZ: Methodology, Supervision, Writing – review & editing. JL: Software, Writing – review & editing. RZ: Supervision, Validation, Writing – original draft. DW: Funding acquisition, Project administration, Resources, Writing – review & editing. ZJ: Investigation, Project administration, Resources, Supervision, Validation, Visualization, Writing – review & editing. CQ: Formal analysis, Funding acquisition, Investigation, Writing – original draft, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by research grants from the National Natural Science Foundation of China (81400155 and 81600114), the Jiangsu Natural Science Foundation of China (BK20140374), and the Top-Notch Young Health Talents, 5th Suzhou Health Professionals Program (GSWS2019035).
The authors gratefully acknowledge all the members of the Department of Hematology, the First Affiliated Hospital of Soochow University for their help in treating the patients.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2024.1491100/full#supplementary-material
1. Ren W, Ye X, Su H, Li W, Liu D, Pirmoradian M, et al. Genetic landscape of hepatitis B virus-associated diffuse large B-cell lymphoma. Blood. (2018) 131:2670–81. doi: 10.1182/blood-2017-11-817601
2. Yan X, Zhou M, Lou Z, Mu Q, Sheng L, Zhang P, et al. Diffuse large B-cell lymphoma with concurrent hepatitis B virus infection in the MabThera era: Unique clinical features and worse outcomes. J Cancer Res Ther. (2018) 14:S248–S53. doi: 10.4103/0973-1482.187285
3. Locke FL, Ghobadi A, Jacobson CA, Miklos DB, Lekakis LJ, Oluwole OO, et al. Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): a single-arm, multicentre, phase 1-2 trial. Lancet Oncol. (2019) 20:31–42. doi: 10.1016/S1470-2045(18)30864-7
4. Schuster SJ, Bishop MR, Tam CS, Waller EK, Borchmann P, McGuirk JP, et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med. (2019) 380:45–56. doi: 10.1056/NEJMoa1804980
5. Kong D, Ping N, Gao X, Zou R, Wang P, Wu D, et al. Efficacy and safety of chimeric antigen receptor T cell therapy in relapsed/refractory diffuse large B-cell lymphoma with different HBV status: a retrospective study from a single center. Front Immunol. (2023) 14:1200748. doi: 10.3389/fimmu.2023.1200748
6. Loomba R, Liang TJ. Hepatitis B reactivation associated with immune suppressive and biological modifier therapies: current concepts, management strategies, and future directions. Gastroenterology. (2017) 152:1297–309. doi: 10.1053/j.gastro.2017.02.009
7. Kusumoto S, Tobinai K. Screening for and management of hepatitis B virus reactivation in patients treated with anti-B-cell therapy. Hematol Am Soc Hematol Educ Program. (2014) 2014:576–83. doi: 10.1182/asheducation-2014.1.576
8. Cao W, Wei J, Wang N, Xu H, Xiao M, Huang L, et al. Entecavir prophylaxis for hepatitis B virus reactivation in patients with CAR T-cell therapy. Blood. (2020) 136:516–9. doi: 10.1182/blood.2020004907
9. Yang C, Xie M, Zhang K, Liu H, Liang A, Young KH, et al. Risk of HBV reactivation post CD19-CAR-T cell therapy in DLBCL patients with concomitant chronic HBV infection. Leukemia. (2020) 34:3055–9. doi: 10.1038/s41375-020-0913-y
10. Wang Y, Liu Y, Tan X, Pan B, Ge J, Qi K, et al. Safety and efficacy of chimeric antigen receptor (CAR)-T-cell therapy in persons with advanced B-cell cancers and hepatitis B virus-infection. Leukemia. (2020) 34:2704–7. doi: 10.1038/s41375-020-0936-4
11. Chen CY, Tien FM, Cheng A, Huang SY, Chou WC, Yao M, et al. Hepatitis B reactivation among 1962 patients with hematological Malignancy in Taiwan. BMC Gastroenterol. (2018) 18:6. doi: 10.1186/s12876-017-0735-1
12. Qu C, Ping N, Kang L, Liu H, Qin S, Wu Q, et al. Radiation priming chimeric antigen receptor T-cell therapy in relapsed/refractory diffuse large B-cell lymphoma with high tumor burden. J Immunother. (2020) 43:32–7. doi: 10.1097/CJI.0000000000000284
13. Zhang Y, Li J, Lou X, Chen X, Yu Z, Kang L, et al. A prospective investigation of bispecific CD19/22 CAR T cell therapy in patients with relapsed or refractory B cell non-hodgkin lymphoma. Front Oncol. (2021) 11:664421. doi: 10.3389/fonc.2021.664421
14. Lee DW, Santomasso BD, Locke FL, Ghobadi A, Turtle CJ, Brudno JN, et al. ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol Blood Marrow Transplant. (2019) 25:625–38. doi: 10.1016/j.bbmt.2018.12.758
15. Visentini M, Casato M. HBV messing with the B-cell genome leads to DLBCL. Blood. (2018) 131:2602–3. doi: 10.1182/blood-2018-05-847525
16. Zhao X, Guo X, Xing L, Yue W, Yin H, He M, et al. HBV infection potentiates resistance to S-phase arrest-inducing chemotherapeutics by inhibiting CHK2 pathway in diffuse large B-cell lymphoma. Cell Death Dis. (2018) 9:61. doi: 10.1038/s41419-017-0097-1
17. Li J, Chen Y, Guo X, Bai X, Xu X, Han T, et al. lncNBAT1/APOBEC3A is a mediator of HBX-induced chemoresistance in diffuse large B cell lymphoma cells. Mol Ther Nucleic Acids. (2022) 27:1064–77. doi: 10.1016/j.omtn.2022.01.015
18. Song Z, Ma Y, Jiang D, Zhao R, Dong F. Long-term safety of rituximab in DLBCL patients with hepatitis B-related cirrhosis: A retrospective case series. Front Med (Lausanne). (2022) 9:890339. doi: 10.3389/fmed.2022.890339
19. Huang H, Li X, Zhu J, Ye S, Zhang H, Wang W, et al. Entecavir vs lamivudine for prevention of hepatitis B virus reactivation among patients with untreated diffuse large B-cell lymphoma receiving R-CHOP chemotherapy: a randomized clinical trial. JAMA. (2014) 312:2521–30. doi: 10.1001/jama.2014.15704
20. Park JW, Kwak KM, Kim SE, Jang MK, Suk KT, Kim DJ, et al. Comparison of the long-term efficacy between entecavir and tenofovir in treatment- naive chronic hepatitis B patients. BMC Gastroenterol. (2017) 17:39. doi: 10.1186/s12876-017-0596-7
21. Estevez J, Chen VL, Podlaha O, Li B, Le A, Vutien P, et al. Differential serum cytokine profiles in patients with chronic hepatitis B, C, and hepatocellular carcinoma. Sci Rep. (2017) 7:11867. doi: 10.1038/s41598-017-11975-7
22. Wei J, Zhu X, Mao X, Huang L, Meng F, Zhou J. Severe early hepatitis B reactivation in a patient receiving anti-CD19 and anti-CD22 CAR T cells for the treatment of diffuse large B-cell lymphoma. J Immunother Cancer. (2019) 7:315. doi: 10.1186/s40425-019-0790-y
23. West J, Card TR, Aithal GP, Fleming KM. Risk of hepatocellular carcinoma among individuals with different aetiologies of cirrhosis: a population-based cohort study. Aliment Pharmacol Ther. (2017) 45:983–90. doi: 10.1111/apt.2017.45.issue-7
24. Levrero M, Zucman-Rossi J. Mechanisms of HBV-induced hepatocellular carcinoma. J Hepatol. (2016) 64:S84–S101. doi: 10.1016/j.jhep.2016.02.021
25. Chen Y, Tian Z. HBV-induced immune imbalance in the development of HCC. Front Immunol. (2019) 10:2048. doi: 10.3389/fimmu.2019.02048
26. Cao Y, Xiao Y, Wang N, Wang G, Huang L, Hong Z, et al. CD19/CD22 chimeric antigen receptor T cell cocktail therapy following autologous transplantation in patients with relapsed/refractory aggressive B cell lymphomas. Transplant Cell Ther. (2021) 27:910 e1– e11. doi: 10.1016/j.jtct.2021.08.012
27. Wang T, Xu L, Gao L, Tang G, Chen L, Chen J, et al. Chimeric antigen receptor T-cell therapy combined with autologous stem cell transplantation improved progression-free survival of relapsed or refractory diffuse large B-cell lymphoma patients: A single-center, retrospective, cohort study. Hematol Oncol. (2022) 40:637–44. doi: 10.1002/hon.v40.4
28. Kotla V, Goel S, Nischal S, Heuck C, Vivek K, Das B, et al. Mechanism of action of lenalidomide in hematological Malignancies. J Hematol Oncol. (2009) 2:36. doi: 10.1186/1756-8722-2-36
Keywords: diffuse large B-cell lymphoma, cirrhosis, chimeric antigen receptor T-cell therapy, hepatitis B virus, case report
Citation: Kong D, Ping N, Zhu Q, Zhang X, Li J, Zou R, Wu D, Jin Z and Qu C (2024) Case report: CAR-T therapy demonstrated safety and efficacy in relapsed/refractory diffuse large B-cell lymphoma patients complicated with hepatitis B-related cirrhosis. Front. Oncol. 14:1491100. doi: 10.3389/fonc.2024.1491100
Received: 04 September 2024; Accepted: 11 November 2024;
Published: 05 December 2024.
Edited by:
Jia Wei, Huazhong University of Science and Technology, ChinaReviewed by:
Walter Hanel, The Ohio State University, United StatesCopyright © 2024 Kong, Ping, Zhu, Zhang, Li, Zou, Wu, Jin and Qu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Depei Wu, d3VkZXBlaTE4QDE2My5jb20=; Zhengming Jin, cXVjaGFuZ2p1QDEyNi5jb20=; Changju Qu, cWNqMzEwQHN1ZGEuZWR1LmNu
†These authors have contributed equally to this work and share first authorship
‡These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.