
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Oncol. , 21 November 2024
Sec. Gastrointestinal Cancers: Hepato Pancreatic Biliary Cancers
Volume 14 - 2024 | https://doi.org/10.3389/fonc.2024.1490717
Primary hepatic leiomyosarcoma (PHLS) is an extremely rare malignant tumor, which is often elusive in early diagnosis due to its rarity and nonspecific clinical and imaging presentations. Herein, we present a case of PHLS in a 66-year-old male and a review of the English literature from January 2000 to December 2023, focusing on the clinical and imaging characteristics of 30 patients with PHLS. The present patient was admitted to our hospital with complaints of abdominal distension, with history of hepatitis B. Tumor markers, including alpha-fetoprotein, carcinoembryonic antigen, and CA 19-9, were within the normal range. A hepatic tumor was incidentally identified during an abdominal ultrasound examination, further evaluated by contrast-enhanced CT and MR scans, which was preliminarily misdiagnosed as hepatocellular carcinoma. The tumor was surgically excised and definitively diagnosed as PHLS, characterized by two distinct areas with varying imaging features on contrast-enhanced CT and MR images. PHLS typically manifests as a well-defined, heterogeneously hypo- or iso-dense mass on CT, with a slightly prolonged T2 signal on MRI, and exhibits gradual enhancement during dynamic contrast-enhanced imaging. We advocate that the possibility of PHLS should be considered when the aforementioned imaging features are observed.
Leiomyosarcoma is a malignant mesenchymal tumor originating from smooth muscle lineage (1), which most commonly arises in the uterus, retroperitoneum, soft tissues and the alimentary tract. In clinic, primary hepatic leiomyosarcoma (PHLS) is extremely rare, which is difficult to make accurate pre-surgical diagnosis, due to its rarity, nonspecific clinical and imaging manifestations, and lack of recognition. In this study, we present a case of PHLS that was pathologically confirmed following surgical resection, and provides a systematic review of PHLS in the English literature for comparison, focusing on its clinical characteristics and imaging findings.
A 66-year-old man presented to a local hospital with a complaint of abdominal distension. An abdominal ultrasound examination revealed a large mass in the hepatogastric space. Subsequently, contrast-enhanced magnetic resonance (MR) imaging of the abdomen was performed (Figure 1) to better characterize the lesion. The mass demonstrated predominantly slight hyperintensity on T2-weighted images (T2WI) and hypointensity on T1-weighted images (T1WI). The presence of internal hemorrhage within the mass was suggested by hyperintensity on T1WI and hypointensity on T2WI. Diffusion-weighted imaging (DWI) showed restricted diffusion within the lesion. Following the administration of Gd-DTPA, the tumor exhibited heterogeneous enhancement during the arterial phase, with gradual enhancement observed in the portal and delayed phases.
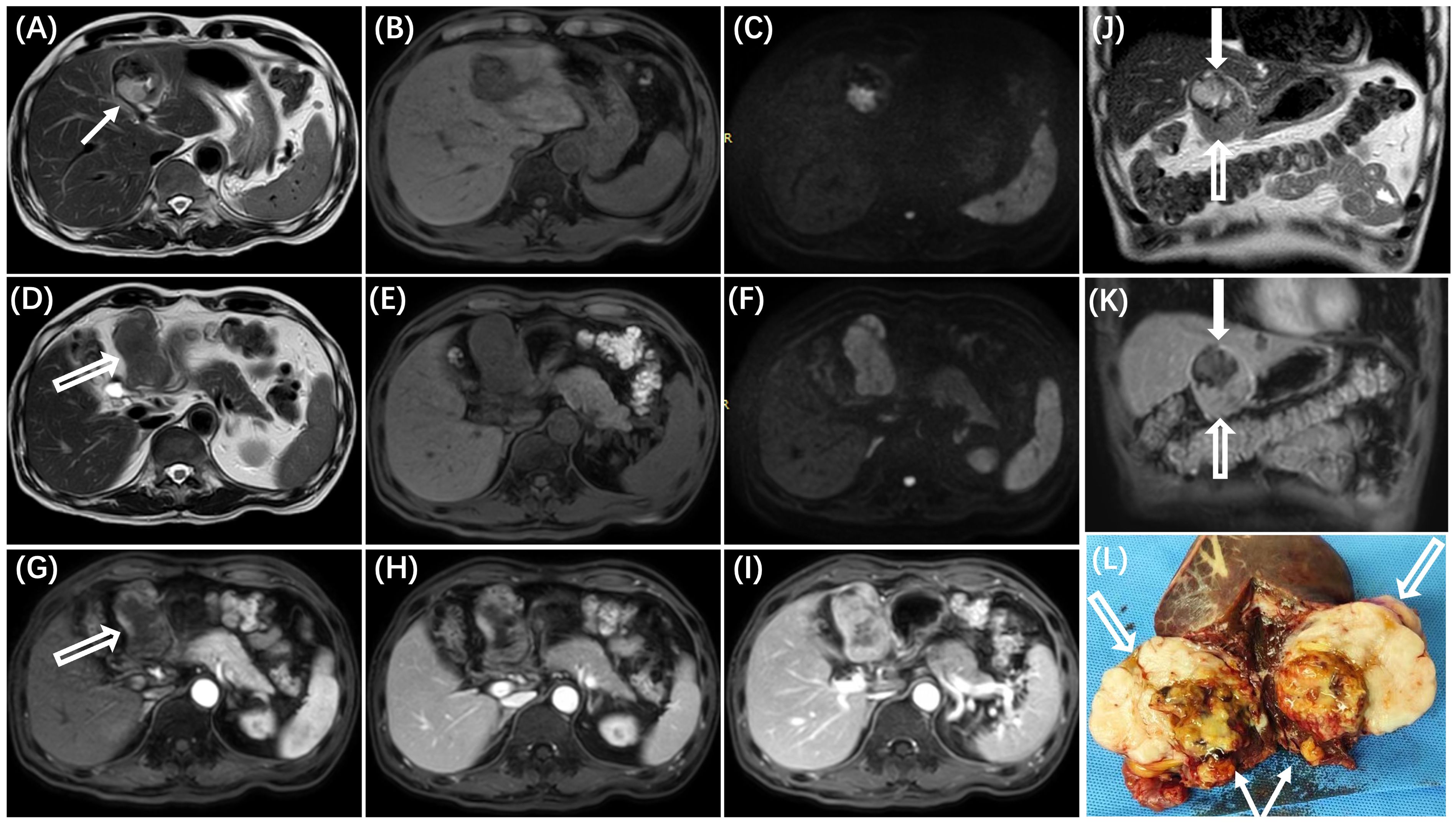
Figure 1. MR imaging reveals two distinct areas within the tumor, each with varying imaging characteristics. The area adjacent to the liver (A–C, white solid arrow in J) demonstrates mixed high and low signals on T2WI (A) and T1WI (B), corresponding to the undifferentiated region with necrosis and hemorrhage identified in pathological examination (white solid arrow in L). The area distant from the liver (D–F, white hollow arrow in J) exhibits mild hyperintensity on T2WI (D) and hypointensity on T1WI (E), correlating with the differentiated region observed in pathological examination (white hollow arrow in L). On dynamic contrast-enhanced images, the tumor displays heterogeneous enhancement during the arterial phase (G) and gradual enhancement in the portal venous and delayed phases (H, I), with different degrees of enhancement observed in the two areas (solid arrow and hollow arrow in the K). On diffusion-weighted imaging (DWI), the mass (white arrow) shows hyperintensity (C, F).
The patient was transferred to our hospital for further evaluation and treatment. Upon physical examination, the findings were predominantly unremarkable, except for mild tenderness in the right upper quadrant. The patient’s medical history is notable for a number of chronic conditions: chronic hepatitis B, which has been present for over 20 years, type 2 diabetes mellitus for the past 12 years, and a historical episode of tuberculosis that was effectively treated and resolved 30 years prior. No other significant personal or family medical histories were reported. Laboratory tests indicated a slight decrease in serum albumin level, while other liver function indices were within normal limits. Viral markers of hepatitis B were positive, including hepatitis B surface antigen (HBsAg), antibody to hepatitis B e (anti-HBe), and antibody to hepatitis B core (anti-HBc). The antibody to hepatitis B surface (anti-HBs) and hepatitis B e antigen (HBeAg) were negative. The serum levels of tumor markers, including alpha-fetoprotein (AFP), carcinoembryonic antigen (CEA), and CA 19-9, were within the normal range.
Further evaluation was conducted through pre- and post-contrast computed tomography (CT, Figure 2) of the chest, abdomen, and pelvis. A well-defined, heterogeneous mass with hypo- and iso-density was identified in the hepatogastric space, adjacent to the left branch of the portal vein and the ligamentum teres hepatis, measuring approximately 6.8 cm × 4.5 cm × 5.8 cm. Mild to moderate uneven enhancement was observed during the arterial phase, with gradual enhancement during the portal and equilibrium phases. Feeding arteries were noted to originate from the left hepatic artery, suggesting that the large exophytic mass originated from segment IV and III of the liver. No evidence of hepatic cirrhosis or other clinically significant lesions was identified. Given the imaging features and the patient’s history of hepatitis B, a preliminary diagnosis of hepatocellular carcinoma (HCC) with atypical imaging appearances was established. A laparoscopic left hemi-hepatectomy was subsequently performed, and the patient had a smooth postoperative course. He was discharged 7 days following the surgery, marking a total hospital stay of 14 days.
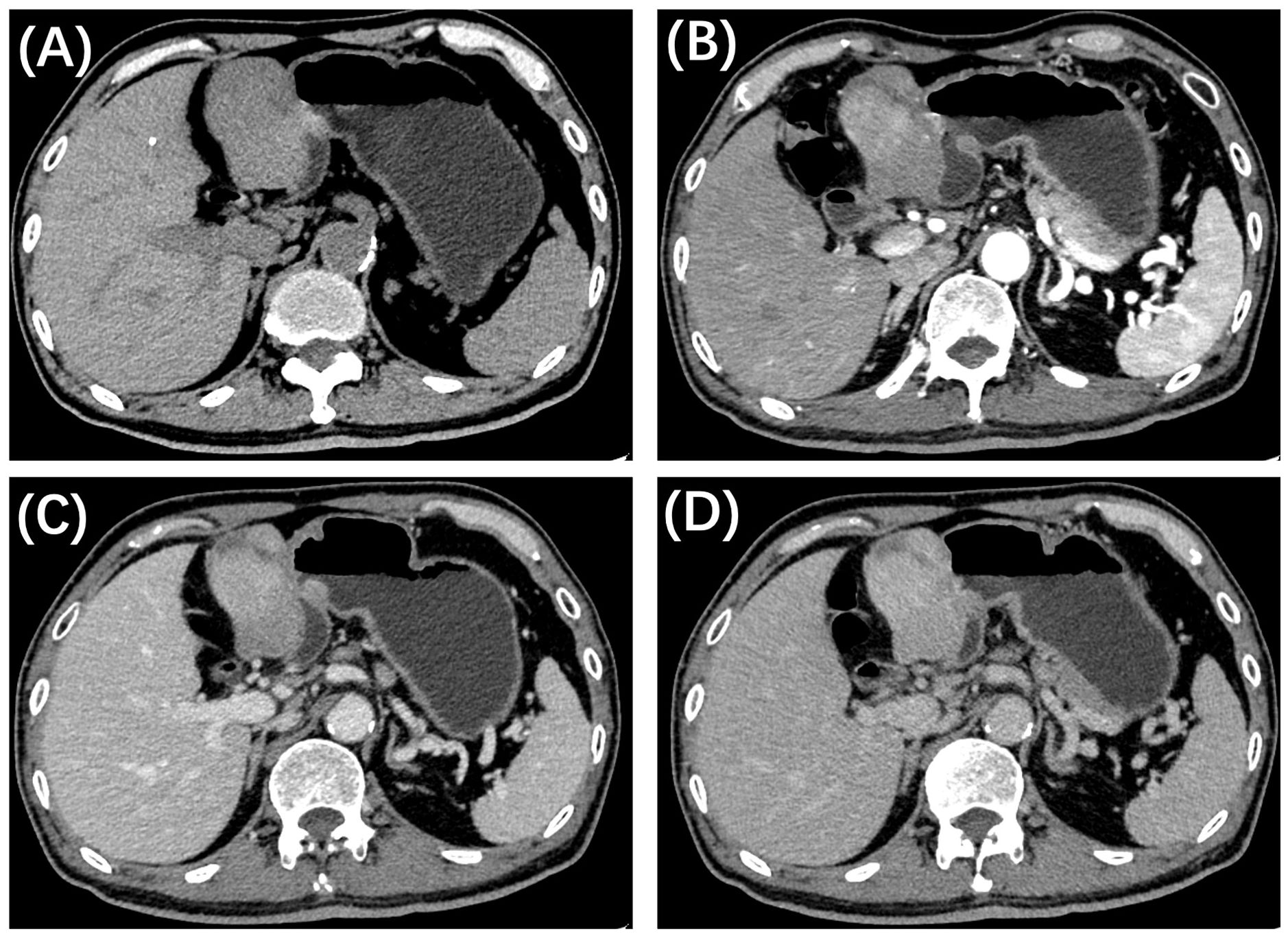
Figure 2. CT imaging of the present patient with primary hepatic leiomyosarcoma. (A) Axial precontrast CT reveals a well-defined, iso-dense mass in the left lobe of the liver, featuring a large exophytic component. Contrast-enhanced CT demonstrates uneven mild to moderate enhancement in the arterial phase (B), with gradual enhancement observed during the portal (C) and delayed (D) phases.
Gross pathological examination demonstrated a gray-white tumor mass, measuring 6×5×4 cm in size. The cut surface exhibited as a grayish-white hue with scattered areas of hemorrhage and necrosis. The margin was tumor-free, and there was no evidence of lymphovascular or perineural invasion.
Microscopic examination (Figure 3) revealed that the tumor was composed of spindle-shaped cells and had two discrete areas. One area was predominantly composed of ‘differentiated’ cells with moderate nuclear atypia, densely packed in a fascicular and interwoven pattern. These cells exhibited strong positivity for smooth muscle markers: desmin (3+), caldesmon (3+), and smooth muscle actin (3+). The other area was predominantly composed of ‘dedifferentiated’ cells with marked nuclear atypia and a high mitotic rate (approximately 40 per 5 mm²). These cells displayed irregular or epithelioid morphology alongside necrosis, and showed patchy positivity for desmin and caldesmon, and were negative for smooth muscle actin. The Ki-67 proliferation index was significantly elevated in both differentiated and dedifferentiated components. A final diagnosis of leiomyosarcoma was confirmed by a union discussion between the departments of Pathology of our hospital and another institution. The tumor was classified as grade 3 according to the Federation Nationale des Centres de Lutte Contre le Cancer (FNCLCC) grading system.
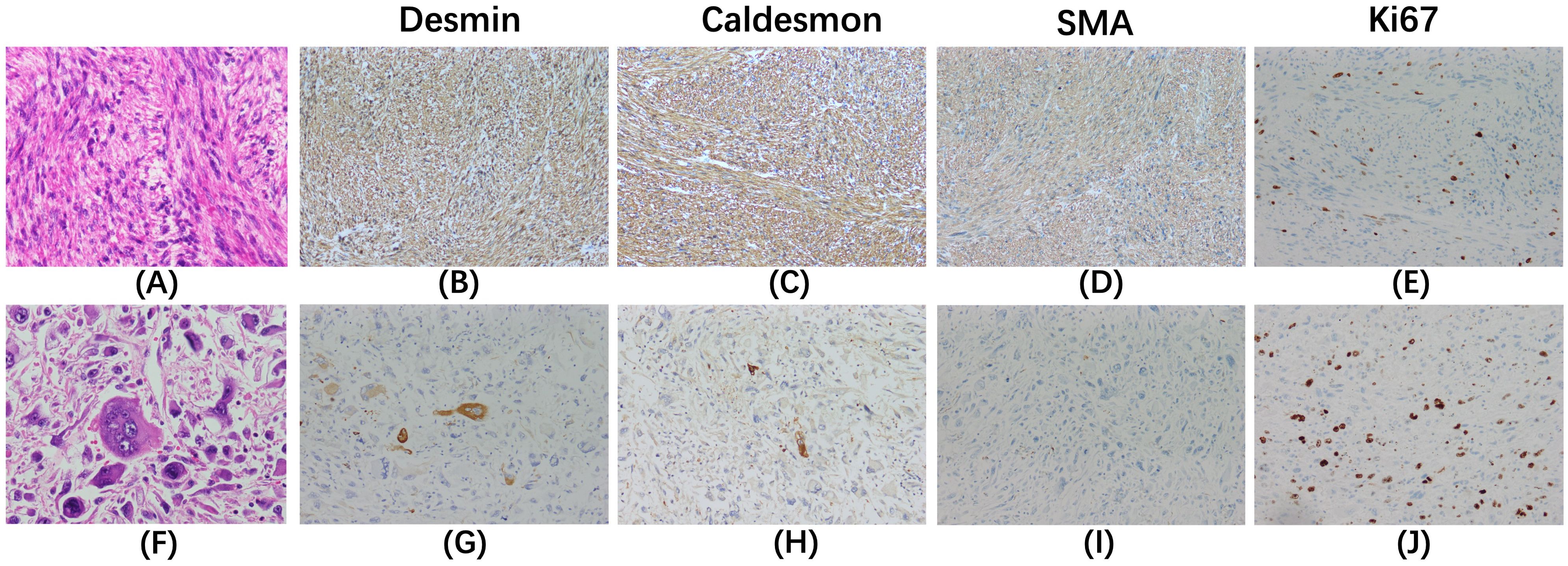
Figure 3. Hematoxylin-eosin staining (×400) and immunohistochemical staining (×200) are shown for the differentiated components (A–E) and the dedifferentiated components (F–J). In the differentiated components (A), cells with moderate nuclear atypia are densely packed in a fascicular and interwoven pattern. In contrast, the dedifferentiated components (F) display cells that are irregular or epithelioid, with marked nuclear atypia and a high mitotic rate. The differentiated area exhibits strong positivity for desmin (B), caldesmon (C), and smooth muscle actin (SMA, D). The dedifferentiated region shows patchy positivity for desmin (G) and caldesmon (H), and is negative for SMA (I). The Ki-67 index is significantly elevated in both the differentiated (E) and dedifferentiated components (J).
After the surgical procedure, the patient was informed about the potential benefits of adjuvant chemotherapy. Despite these considerations, the patient declined this treatment option and opted for regular follow-ups, which included periodic imaging and blood tests. After approximately 22 months of post-surgical monitoring, no local recurrence or metastasis was detected. Therefore, the diagnosis of primary hepatic leiomyosarcoma was comprehensive to made.
Primary hepatic sarcomas account for less than 1% of all malignant hepatic tumors, with PHLS being even rarer, making up only 14-29% of all primary hepatic sarcomas (2–4). PHLS is believed to originate from smooth muscle cells within intrahepatic vessels, bile ducts, or ligaments (3). Given the scarcity of valuable data on clinical and imaging features, coupled with the tumor’s rarity, PHLS is frequently misdiagnosed as HCC or other conditions, such as cholangiocarcinoma, hepatocellular adenoma, focal nodular hyperplasia, abscesses, and hydatid disease (5–9).
The preoperative diagnosis of hepatic leiomyosarcoma is essential due to its metastatic potential, which necessitates a thorough diagnostic workup to exclude other primary sites (10). Such precise diagnosis is vital for devising appropriate treatment strategies, including the consideration of neoadjuvant therapy and the planning of intricate surgeries with broader margins to accommodate the aggressive characteristics of PHLS (11). Correct identification of PHLS is also essential to prevent the administration of inappropriate treatments that are typically prescribed for more prevalent liver tumors, such as HCC.
Therefore, we present this case of PHLS and review the existing literature to raise awareness of this uncommon malignant tumor. Our aim is to contribute to the clinical and radiological understanding of PHLS, ultimately improving patient management and outcomes.
Case reports or case series of PHLS published in the English language from January 1, 2000, to December 31, 2023, were retrieved from PubMed and Google Scholar. After detailed screening of each article, 29 publications with 30 cases regarding the imaging features of PHLS were included in this study. The clinical manifestations and imaging findings of these 30 reported cases, along with the findings from the current case, are summarized in Tables 1 and 2, respectively.
The patients’ ages ranged from 5 months to 86 years, with a mean age of 52.4 years and three patients being under 18 years old. The prevalence was slightly higher among female patients, reflected in a female-to-male ratio of 1.4:1. The most common symptom was right upper quadrant abdominal pain. Other clinical presentations included abdominal distension, nausea, jaundice, weight loss, and a palpable abdominal mass, while some patients were incidentally discovered without apparent symptoms. Tumor marker levels, including AFP, CEA, and CA19-9, were typically within the normal range. Among the 30 reported cases, serum CA19-9 levels were slightly elevated in two patients, and serum AFP and CEA levels were each slightly increased in one patient. The current patient, an elderly male, presented with the clinical symptom of abdominal distension and had normal levels of tumor markers, a presentation similar to that of other abdominal malignancies and lacking specificity.
PHLS has been reported to be closely associated with immunocompromised states, including AIDS (12, 13), the post-renal transplant period (14, 15), and radiochemotherapy for Hodgkin’s lymphoma (9). This association is postulated to occur due to the uninhibited effects of the Epstein-Barr virus (EBV) on smooth muscle proliferation (12, 13). However, many patients diagnosed with PHLS were immunocompetent. Among the 31 patients, 3 (3/31, 9.6%) were immunodeficient, 4 (4/31, 12.9%) had a history of hepatitis B or C, with or without liver cirrhotic, and the remaining 24 (24/31, 77.4%) had no identifiable predisposing factors. Therefore, the etiology of PHLS remains unclear and requires further elucidation.
The non-specific imaging features of PHLS present a challenge for preoperative diagnosis. Conventional US was performed in 18 of the 31 patients, typically revealing a hypoechoic mass. CT scans were conducted on 29 of the 31 patients, with most showing well-defined hypo- or iso-dense masses that included heterogeneous areas indicative of necrosis or hemorrhage. MR imaging was performed in 20 of the 31 patients, showing hyperintensity on T2WI and hypointensity on T1WI. It may also exhibit internal heterogeneity, suggesting the presence of intratumoral hemorrhage or necrosis. The enhancement patterns of PHLS are comparable on both CT and MRI scans. Among the 31 cases, 23 patients received contrast-enhanced CT scans, and 15 received contrast-enhanced MR scans. A substantial proportion of these cases demonstrated peripheral, heterogeneous enhancement during the arterial phase, with persistent or gradual enhancement observed in the portal venous or delayed phases. Lv WF (16) and Kazawa N (17) have suggested that the gradual enhancement observed during the delayed phase on contrast-enhanced MR images may be a distinctive feature of PHLS. However, certain other hepatic tumors, including intrahepatic cholangiocarcinoma, atypical HCC, squamous cell carcinoma, and other sarcomas, frequently show delayed enhancement (18–20). Therefore, in our opinion, this feature could be a significant sign for the differential diagnosis of PHLS from typical HCC, but the diagnostic value for PHLS requires further study. In addition, some cases of PHLS reported in the literature exhibited the enhancement pattern described as “fast-in and fast-out” (6, 21), which is characteristic of the typical enhancement pattern seen in HCC. Diffusion-weighted imaging was performed in 7 of the 31 patients, revealing restricted diffusion in all cases.
In the present case, contrast-enhanced CT and MR imaging indicated a probable malignant hepatic lesion. Considering the patient’s history of hepatitis B, the initial misdiagnosis by radiologists and clinicians was HCC. However, with normal AFP levels and the scans showing gradual enhancement—features not aligning with HCC —the possibility of other malignant tumors necessitates consideration.
A definitive diagnosis of PHLS relies solely on histological and immunohistological examinations. Characteristic histological features include intersecting bundles of spindle-shaped cells with deeply eosinophilic cytoplasm and hyperchromatic nuclei. Immunostaining is positive for SMA and desmin, which are markers indicative of smooth muscle differentiation. A negative reaction for CD34, CD117, DOG1, cytokeratins, neuron-specific enolase (NSE), S-100 protein, and alpha-fetoprotein aids in ruling out other differential diagnosess (3, 16, 21).
MRI is considered the most useful modality for characterizing liver masses due to its superior soft-tissue contrast resolution. In the present case, MR images revealed two distinct areas within the tumor, each exhibiting different imaging characteristics that correspond to the underlying pathological changes of PHLS. The area adjacent to the liver showed mixed high and low signals on T2WI and T1WI, corresponding to the undifferentiated region with necrosis and hemorrhage identified in pathological examination. The solid component of this area demonstrated significant hyperintensity on T2WI and a gradual enhancement pattern on dynamic contrast-enhanced images. In contrast, the area more distant from the liver displayed homogeneously mild hyperintensity on T2WI and marked gradual enhancement on dynamic contrast-enhanced images, correlating with the differentiated region composed of densely packed spindle-shaped cells as observed in pathological examination.
In conclusion, we report a case of PHLS, which is rare and lacks characteristic clinical and imaging manifestations. Based on the current patient and previously published data, PHLS often presents as a well-defined, heterogeneously hypo- or iso-dense mass on CT, with slightly prolonged T2 signal on MRI, and shows gradual enhancement during dynamic contrast-enhanced imaging. Although a definitive diagnosis of PHLS requires pathological examination, differentiation should be made once the aforementioned imaging features are present.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
The studies involving humans were approved by Ethics Review Committee of Guangdong Provincial People’s Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent was obtained from the participant/patient(s) for the publication of this case report.
LY: Writing – original draft, Writing – review & editing, Data curation. RH: Data curation, Writing – original draft. SC: Writing – review & editing, Conceptualization, Funding acquisition. JC: Writing – review & editing, Data curation. JL: Writing – review & editing, Supervision.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by National Natural Scientific Foundation of China (No.82202095 and 82102147), Guangdong Basic and Applied Basic Research Foundation (No.2022A1515011650) and Guangdong Province’s Special Fund for Science and Technology Innovation Strategy (“Major project + Task List”) Project (No.221125141604534).
The authors declare the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Serrano C, George S. Leiomyosarcoma. Hematol Oncol Clin North Am. (2013) 27:957–74. doi: 10.1016/j.hoc.2013.07.002
2. Martins ACA, Costa Neto DCD, Silva J, Moraes YM, LeAo CS, Martins C. Adult primary liver sarcoma: systematic review. Rev Col Bras Cir. (2020) 47:e20202647. doi: 10.1590/0100-6991e-20202647
3. Ahmed H, Bari H, Nisar Sheikh U, Basheer MI. Primary hepatic leiomyosarcoma: A case report and literature review. World J Hepatol. (2022) 14:1830–9. doi: 10.4254/wjh.v14.i9.1830
4. Konstantinidis IT, Nota C, Jutric Z, Ituarte P, Chow W, Chu P, et al. Primary liver sarcomas in the modern era: Resection or transplantation? J Surg Oncol. (2018) 117:886–91. doi: 10.1002/jso.24979
5. Ghosh R, Halder A, Nim RK, Ray S, Chatterjee U. Primary hepatic leiomyosarcoma masquerading as focal nodular hyperplasia of liver: A wolf in sheep’s clothing. Indian J Pathol Microbiol. (2023). doi: 10.4103/ijpm.ijpm_735_22
6. Liu W, Liang W. Primary hepatic leiomyosarcoma presenting as a thick-walled cystic mass resembling a liver abscess: A case report. Med (Baltimore). (2018) 97:e13861. doi: 10.1097/MD.0000000000013861
7. Shamseddine A, Faraj W, Mukherji D, El Majzoub N, Khalife M, Soubra A, et al. Unusually young age distribution of primary hepatic leiomyosarcoma: case series and review of the adult literature. World J Surg Oncol. (2010) 8:56. doi: 10.1186/1477-7819-8-56
8. Morris CJ, Ghanta R. Masquerading primary liver leiomyosarcoma as a hemorrhagic hepatoma. Clin Gastroenterol Hepatol. (2010) 8:e26. doi: 10.1016/j.cgh.2009.09.021
9. Giuliante F, Sarno G, Ardito F, Pierconti F. Primary hepatic leiomyosarcoma in a young man after Hodgkin’s disease: diagnostic pitfalls and therapeutic challenge. Tumori. (2009) 95:374–7. doi: 10.1177/030089160909500318
10. Chi M, Dudek AZ, Wind KP. Primary hepatic leiomyosarcoma in adults: analysis of prognostic factors. Onkologie. (2012) 35:210–4. doi: 10.1159/000337416
11. Esposito F, Lim C, Baranes L, Salloum C, Feray C, Calderaro J, et al. Primary leiomyosarcoma of the liver: Two new cases and a systematic review. Ann Hepatobiliary Pancreat Surg. (2020) 24:63–7. doi: 10.14701/ahbps.2020.24.1.63
12. Metta H, Corti M, Trione N, Masini D, Monestes J, Rizzolo M, et al. Primary hepatic leiomyosarcoma–a rare neoplasm in an adult patient with AIDS: second case report and literature review. J Gastrointest Cancer. (2014) 45 Suppl:1: 36–9. doi: 10.1007/s12029-013-9525-3
13. Chelimilla H, Badipatla K, Ihimoyan A, Niazi M. A rare occurrence of primary hepatic leiomyosarcoma associated with epstein barr virus infection in an AIDS patient. Case Rep gastrointes. (2013) 2013:691862. doi: 10.1155/2013/691862
14. Jeribi A, Albano L, Berguignat M, Maaroufi A, Dewisme C, Favre G, et al. Epstein-Barr virus-associated hepatic leiomyosarcoma after renal transplantation: case report. Transplant Proc. (2010) 42:4356–8. doi: 10.1016/j.transproceed.2010.09.122
15. Fujita H, Kiriyama M, Kawamura T, Ii T, Takegawa S, Dohba S, et al. Primary hepatic leiomyosarcoma in a woman after renal transplantation: report of a case. Surg Today. (2002) 32:446–9. doi: 10.1007/s005950200073
16. Lv WF, Han JK, Cheng DL, Tang WJ, Lu D. Imaging features of primary hepatic leiomyosarcoma: A case report and review of literature. Oncol Lett. (2015) 9:2256–60. doi: 10.3892/ol.2015.3006
17. Kazawa N, Yamashita Y. Primary hepatic leiomyosarcoma (PHLS): A case report and literature review. Int J Radiol Diagn. Imaging. (2020) 3:80–6. doi: 10.33545/26644436.2020.v3.i4b.140
18. Zhao L, Zhou Y, Ding J, Qin Z, Zhou H, Jing X. Primary hepatic squamous cell carcinoma: case report and systematic review of the literature. Front Oncol. (2023) 13:1229936. doi: 10.3389/fonc.2023.1229936
19. Wu P, Kuo H, Sun C, Feng I, Li W, Ho C. Clinical characteristics, imaging features, and outcomes of primary hepatic angiosarcoma: A single-center study and literature review. Advances in Digestive Medicine (2024). doi: 10.1002/aid2.13387
20. Frittoli B, Castaldo A, Santarsiere M, Ascione R, Tanzi G, Ponsiglione A, et al. A unique case of lymphoepithelioma-like HCC with osteoclast-like giant cells: CT imaging features with pathologic correlations. Clin J Gastroenterol. (2024) 17:112–7. doi: 10.1007/s12328-023-01871-1
21. Mitra S, Rathi S, Debi U, Dhiman RK, Das A. Primary hepatic leiomyosarcoma: histopathologist’s perspective of a rare case. J Clin Exp Hepatol. (2018) 8:321–6. doi: 10.1016/j.jceh.2018.04.015
22. Vella S, Cortis K, Pisani D, Pocock J, Aldrighetti L. Case of primary hepatic leiomyosarcoma successfully treated with laparoscopic right hepatectomy. BMJ Case Rep. (2020) 13:e233567. doi: 10.1136/bcr-2019-233567
23. Zhu KL, Cai XJ. Primary hepatic leiomyosarcoma successfully treated by transcatheter arterial chemoembolization: A case report. World J Clin cases. (2019) 7:525–31. doi: 10.12998/wjcc.v7.i4.525
24. Feretis T, Kostakis ID, Damaskos C, Garmpis N, Mantas D, Nonni A, et al. Primary hepatic leiomyosarcoma: a case report and review of the literature. Acta Med (Hradec Kralove). (2018) 61:153–7. doi: 10.14712/18059694.2018.135
25. Xie P, Zhuang H. FDG PET/CT findings of primary hepatic leiomyosarcoma in an immunocompetent pediatric patient. Clin Nucl Med. (2017) 42:323–4. doi: 10.1097/RLU.0000000000001555
26. Shera I, More RB, Patel SK. Primary hepatic leiomyosarcoma A space occupying lesion in the liver: an enigma for diagnosis. J Gastroenterol Pancreatol Liver Disord. (2017) 4:1–3. doi: 10.15226/2374-815X/4/5/001102
27. Iida T, Maeda T, Amari Y, Yurugi T, Tsukamoto Y, Nakajima F. Primary hepatic leiomyosarcoma in a patient with autosomal dominant polycystic kidney disease. CEN Case Rep. (2017) 6:74–8. doi: 10.1007/s13730-017-0247-4
28. Giakoustidis D, Giakoustidis A, Goulopoulos T, Arabatzi N, Kainantidis A, Zaraboukas T. Primary gigantic leiomyosarcoma of the liver treated with portal vein embolization and liver resection. Ann Hepatobiliary Pancreat Surg. (2017) 21:228–31. doi: 10.14701/ahbps.2017.21.4.228
29. Gupta S. Primary hepatic leiomyosarcoma - a case report with review of literature. Ijcr. (2016) 2:53–7. doi: 10.32677/IJCR.2016.v02.i03.003
30. Majumder S, Dedania B, Rezaizadeh H, Joyal T, Einstein M. Tumor rupture as the initial manifestation of primary hepatic leiomyosarcoma. Gastrointest Cancer Res. (2014) 7:33–4.
31. Tsai PS, Yeh TC, Shih SL. Primary hepatic leiomyosarcoma in a 5-month-old female infant. Acta Radiol Short Rep. (2013) 2:2047981613498722. doi: 10.1177/2047981613498722
32. Takehara K, Aoki H, Takehara Y, Yamasaki R, Tanakaya K, Takeuchi H. Primary hepatic leiomyosarcoma with liver metastasis of rectal cancer. World J gastroentero. (2012) 18:5479–84. doi: 10.3748/wjg.v18.i38.5479
33. Shivathirthan N, Kita J, Iso Y, Hachiya H, Kyunghwa P, Sawada T, et al. Primary hepatic leiomyosarcoma: Case report and literature review. World J Gastrointest Oncol. (2011) 3:148–52. doi: 10.4251/wjgo.v3.i10.148
34. Liang X, Xiao-Min S, Jiang-Ping X, Jie-Yu Y, Xiao-Jun Z, Zhi-Ren F, et al. Liver transplantation for primary hepatic leiomyosarcoma: a case report and review of the literatures. Med Oncol. (2010) 27:1269–72. doi: 10.1007/s12032-009-9372-z
35. Surendrababu NR, Rao A, Samuel R. Primary hepatic leiomyosarcoma in an infant. Pediatr Radiol. (2006) 36:366. doi: 10.1007/s00247-005-0041-x
36. El Mesbahi O, Benhadji K, Paradis V, Belghiti J, Misset J, Raymond E. A bulky single mass of the liver that revealed a primary hepatic leiomyosarcoma. Oncologie. (2006) 8:927–30. doi: 10.1007/s10269-006-0516-x
37. Rokutanda N, Makita F, Ohwada S, Sakamoto I, Yoshimura S, Togo N, et al. Primary hepatic leiomyosarcoma with giant cyst formation: A case report. Kita Kanto Igaku. (2005) 55:165–8. doi: 10.2974/kmj.55.165
38. Kanazawa N, Izumi N, Tsuchiya K, Sakurai K, Hamano K, Itakura J, et al. A case of primary leiomyoma of the liver in a patient without evidence of immunosuppression. Hepatol Res. (2002) 24:80. doi: 10.1016/s1386-6346(02)00014-1
Keywords: liver, leiomyosarcoma, diagnostic imaging, tomography (X-ray computed), magnetic resonance imaging
Citation: Yan L, Huang R, Chen S, Chen J and Li J (2024) Imaging findings of primary hepatic leiomyosarcoma: a case report and literature review. Front. Oncol. 14:1490717. doi: 10.3389/fonc.2024.1490717
Received: 03 September 2024; Accepted: 30 October 2024;
Published: 21 November 2024.
Edited by:
Artur Rebelo, University Hospital in Halle, GermanyReviewed by:
Panagis M. Lykoudis, National and Kapodistrian University of Athens, GreeceCopyright © 2024 Yan, Huang, Chen, Chen and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jinglei Li, bGlqaW5nbGVpQGdkcGgub3JnLmNu
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.