
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Oncol. , 09 January 2025
Sec. Head and Neck Cancer
Volume 14 - 2024 | https://doi.org/10.3389/fonc.2024.1489451
This article is part of the Research Topic Advances in Neoadjuvant Therapy for Head and Neck Squamous Cell Carcinoma View all 6 articles
Background: Head and neck squamous cell carcinoma (HNSCC) is one of the most common types of cancer worldwide and immune checkpoint inhibitors have shown favorable therapeutic effects in recurrent or metastatic or locally advanced head and neck squamous cell carcinoma (R/M/LA HNSCC). However, the effects of immunotherapy in HNSCC are still inconsistent because of complicating factors. This meta-analysis tries to provide a more precise assessment of the efficacy and safety of this integrated approach in HNSCC.
Methods: We conducted a systematic review and meta-analysis of randomized clinical trials according to Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guidelines. The outcomes were overall survival (OS), progression-free survival (PFS), and treatment-related adverse events (TRAEs). A total of 8 out of 2445 articles were analyzed, including 5067 HNSCC patients, including 823 and 4244 patients with LA HNSCC and R/M HNSCC.
Results: The combined data revealed that immunotherapy has an apparent difference in OS (HR 0.86 95% CI 0.77-0.98) compared with standard of care (Soc, like fluoropyrimidine, methotrexate, docetaxel, or cetuximab) but was equal with the other treatment in PFS (HR 1.08, 95% CI 0.85-1.37). Furthermore, the occurrence of grade 3 or higher adverse events related to the drugs was lower than systematic therapy (OR 0.35, 95% CI 0.17-0.73).
Conclusions: The study has provided compelling evidence that immunotherapy is a significant benefit in OS for HNSCC patients, either R/M HNSCC or LA HNSCC, immunochemotherapy may benefit more for these patients, but double-agent immunotherapy showed no more benefit for R/M HNSCC patients.
Systematic Review Registration: https://www.crd.york.ac.uk/, identifier CRD42023471570.
Head and neck squamous cell carcinomas (HNSCC) are derived from the mucosal epithelium in the oral cavity, pharynx, and larynx and are the most common malignancies that arise in the head and neck (1). HNSCC is the sixth most common cancer worldwide, with 890,000 new cases and 450,000 deaths in 2018. The incidence of HNSCC continues to rise and is anticipated to increase by 30% (that is 1.08 million new cases annually) by 2030 (2). Due to the complex anatomy of the head and neck, more than 50% of the HNSCC patients were diagnosed in clinical stage III or IV, and the survival rate is only 40~50%. Besides, local recurrence or metastasis also leads to the poor prognosis of HNSCC (3). Multimodal treatments include surgery, radiotherapy, chemotherapy, and molecular-targeted therapy. Despite the continuous innovation of treatment methods, there are still problems such as insufficient efficacy and excessive toxicity (4).
Recent understanding of the role of immune dysfunction in HNSCC has quickly established immunotherapy (IMT) as a promising treatment avenue (5). The monoclonal antibodies (mAbs) anti-programmed death protein-1 (anti-PD-1) nivolumab and pembrolizumab are the first immune checkpoint inhibitors (ICIs) approved for the treatment of patients with recurrent or metastatic HNSCC (R/M HNSCC) (6, 7). Anti-programmed death ligand-1 (anti-PD-L1) checkpoint inhibitors such as durvalumab and avelumab have been approved for the treatment of patients with locally advanced or metastatic HNSCC under phase III clinical trials since 2017 (8). In particular, the PD-1 therapy effect is mediated by the binding with T lymphocytes resulting in a systemic effect, whereas the activity of anti-PD-L1 therapy is directed against the receptor expressed on tumor cells (9, 10). Immune checkpoint molecules cytotoxic T lymphocyte antigen 4 (CTLA-4) like tremelimumab and ipilimumab also have been used in HNSCC (11).
Unfortunately, the most optimum regime for HNSCC is still unclear. Many recommendations are based on single RCT results or anti-PD-1/PD-L1 pathway IMT meta-analysis. In this review, we appraised RCTs evaluating the efficacy and safety of immunotherapy in patients with HNSCC through a systematic review and meta-analysis. The activity of systemic treatment will be assessed through overall survival (OS), progression-free survival (PFS), overall response rate (ORR), duration of response (DoR) (12), and treatment-related adverse events (TRAEs).
We performed a systematic literature review and meta-analysis of peer-reviewed journals published between 2004 to December 2023 from PubMed, Medline, Embase, and Web of Science. The search strategies used a combination of subject headings (e.g., MeSH in PubMed) and keywords such as head and neck squamous cell carcinoma, immunotherapy, and randomized clinical trials (RCTs). The English language only was applied to the search. Clinical trials were restricted to phase II and phase III RCTs (Figure 1).
Bibliographies of review articles and editorials were manually searched. The literature review process followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement checklist and flowchart (13). Two authors (C.L Jiang and S.Q Wang) independently conducted the literature search, evaluated data from eligible studies, and data extraction, which were then checked by a third author (L.J Zhu). If a trial was reported by several publications, we included the most recent results.
Inclusion criteria were: ① patients with histologically confirmed HNSCC; ② patients received immunotherapy; ③ the study compared systemic therapy; ④ the study provided the hazard ratios (HRs) for OS or reported information to calculate these; ⑤ phase II~III, RCTs. Exclusion criteria were: ① nasopharyngeal cancer or esophageal carcinoma; ② trial in abstract forms or protocol report; ③ studies lacking relevant statistics.
The selected studies were assessed with the Cochrane Collaboration’s risk of bias tool, which is used to assign a rating of low, unclear risk of bias, or high for the domains of selection, performance, detection, attrition, reporting, and others (Supplementary Figure 1A).
The following data were extracted from each article: trial identifier, author, publication year, journal, study design, intention-to-treat (ITT) population, age, gender, race, cancer stage, drug and dose, follow-up, OS, PFS, ORR, DoR, and TRAEs.
The primary endpoint was OS, secondary endpoints were PFS, ORR, DoR, and TRAEs. The OS, PFS, and DoR were expressed as HR for the meta-analysis, and ORR and TRAEs were reported as odds ratio (OR). If there were no (or all) events reported in both treatment groups, the study was excluded from the meta-analysis. A forest plot was constructed, including the overall effect, Cochran’s Q chi-square test, and I² statistics. The Cochran’s Q chi-square test and I² statistics were used to determine heterogeneity across the included trials, and I² values of 25%, 50%, and 75% were considered to indicate low, moderate, and high inconsistencies, respectively. A fixed effects model was used if the studies had low heterogeneity (P>0.1, I²<50%). Otherwise, a random-effect model was applied. All statistical analyses were conducted with Review Manager version 5.3. A significant result was indicated by a p-value of <0.05 for any measured outcomes.
Study selection was conducted according to the PRISMA flowchart (Figure 1). The initial database query retrieved 1120 studies, where 28 full-text articles were screened for eligibility. Eight RCTs met the inclusion criteria and were included in the meta-analysis of randomized clinical trials (supplement data extraction). Characteristics of the included studies are described in Table 1. Seven studies were phase III (7, 14–19)and one study was phase II (20). A total of 4207 patients were included, most patients were male (81.54%), and the median age was 58.8 years. The median follow-up was 21.3 months. No trial had a low bias risk (Supplementary Figure 1).
The patients in all trials were combined for the meta-analysis of OS demonstrated significant differences between experiment groups and control groups (HR 0.86, 95% CI 0.77-0.98, P=0.02, Figures 2A, B. Based on PFS, the result indicated that no significant efficacy difference between immunotherapy and non-immunotherapy (HR 1.08, 95% CI 0.85-1.37, P=0.52, Figure 3). For ORR, five articles with 2558 patients were analyzed, the immunotherapy group showed better response than Soc (OR 0.69, 95% CI 0.34-1.41, P=0.31, Figure 4). Besides, three trials (14, 16, 18) with a total of 2037 patients were included in the DoR analysis. A trend towards a significantly increased DoR in experimental groups than in control groups (HR 0.34, 95% CI 0.31-0.37, P<0.01, Figure 5
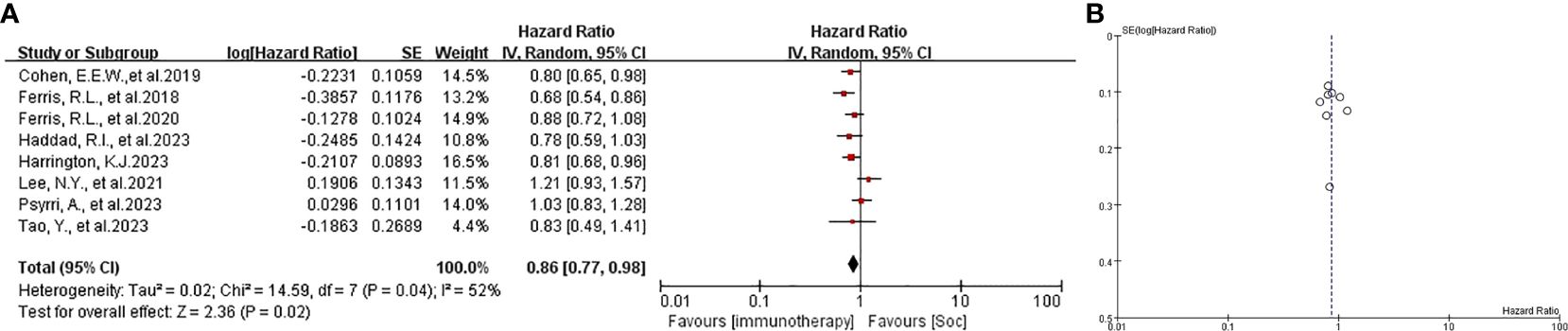
Figure 2. (A) forest plot OS analysis of immunotherapy in HNSCC. (B) funnel plot of survival benefits associated with immunotherapy versus SoC. OS, overall survival. SoC, standard of care. HNSCC, head and neck of squamous cell carcinoma.
In this meta-analysis, grade 3-4 treatment-related adverse events were administered with an acceptable safety profile in immunotherapy groups, supported by eight included studies (OR 0.35, 95% CI 0.17-0.73, P=0.01, Figure 6). The incidence of grade 3-4 TRAEs was 30.42% vs 54.65% compared with experiment groups and control groups. The most common > 3-grade treatment-related events of immunotherapy were fatigue, anemia, diarrhea, and skin reactions like rash (7, 16, 18). The most severe immune-related adverse events (irAE) were hepatitis, myocarditis, and Sjogren syndrome. All analyzed data are shown in Table 2. Prior similar meta-analysis outcomes are shown in Supplementary Table 1.
The therapeutic arsenal of HNSCC is rapidly evolving because of the introduction of new immunotherapeutic agents, which have been shown to improve treatment outcomes and OS in recurrent and metastatic disease and local advanced HNSCC (R/M/LA-HNSCC) (21). Our meta-analysis confirms immunotherapy was effective in OS with the same benefits observed in PFS, which is consistent with the Paderno, A.et al study (22) but inconsistent with Chen, L. et al. report (23). It may need to subdivision PD-L1 expression and HPV status and other so-on subgroups to analyze the reason.
CheckMate 651 (ClinicalTrial.gov identifier: NCT02741570) showed no statistically significant differences in OS with nivolumab plus ipilimumab versus EXTREME (platinum, 5-fluorouracil, and cetuximab in all randomly assigned R/M HNSCC (HR 0.95, 97.9% CI 0.80-1.13, P=0.4951), this study manifested no impact of median OS from the different primary site including oral cavity, oropharynx, hypopharynx, and larynx (HR 0.99, 95% CI 0.87-1.12, P=0.85) (16). JAVELIN Head and Neck 100 (ClinicalTrials.gov identifier: NCT02952586) informed avelumab was not prolonging PFS in patients with locally advanced squamous cell carcinoma of the head and neck (HR 1.21, 95% CI 0.93-1.57, P=0.92). Moreover, the subgroup of the primary site was generally consistent with the primary outcome in PFS (HR 1.14, 95% CI 0.87-1.48, P=0.34) (17).
KESTREL (ClinicalTrial.gov identifier: NCT02551159) and EAGLE (ClinicalTrial.gov identifier: NCT02369874) confirmed durvalumab plus tremelimumab was not superior to EXTREME or SoC (cetuximab, a taxane, methotrexate or a fluoropyrimidine) (14, 18). This means an addition of CTLA-4 inhibitors doesn’t improve either OS or PFS compared with PD-L1 inhibitor monotherapy in patients with R/M HNSCC. A meta-analysis supported this conclusion that a combination of durvalumab plus tremelimumab may achieve comparable outcomes in terms of ORR, OS, PFS, and DoR (24). KEYNOTE-048 (ClinicalTrial.gov identifier: NCT02358031) verified pembrolizumab-chemotherapy improved OS (HR 0.71, 95% CI 0.59-0.85) and PFS (HR 0.73, 95% CI 0.61-0.88) (15). A meta-analysis figured out that combining chemotherapy and PD-1 inhibitors in neoadjuvant treatment could improve ORR compared with immunotherapy only (61% vs 22%) (25). Patil, V. M. et al. reported that low-dose nivolumab plus triple metronomic chemotherapy (TMC-I) improved OS more than TMC in recurrent or newly diagnosed advanced HNSCC (HR 0.545, 95% CI 0.36-0.82) (26).
This study had several limitations. First, the objective response rate was not investigated due to the high heterogeneity of data. Second, there was substantial diversity in the included studies in terms of the treatment regimens and agents. This clinical heterogeneity could be considered a potential problem in interpreting the results of the present meta-analysis. Third, the included RCTs are most of the open-label designs and were supported by pharmaceutical industry funding. Fourth, the efficacy biomarker of immunotherapy in HNSCC is still not unique, such as combined positive score (CPS) expression and human papillomavirus (HPV) status not enough to indicate the prognosis. Finally, long-term clinical outcomes like 5-year OS have not been evaluated.
To the best of our knowledge, this is the largest inclusion of HNSCC patients study of meta-analysis and the first focus on the efficacy of CTLA-4 immunotherapy in HNSCC review. This meta-analysis indicated that HNSCC patients could get clinical benefits from immunotherapy, either R/M HNSCC or LA HNSCC, especially PD-1 inhibitors. However, its routine use is hindered by its expense and the challenge of selecting patients who will truly benefit. More convincing results need more immunotherapy-applied studies involved. The excellent treatment protocol of HNSCC needs more long-term clinical data. In the future, we may try to explore the efficacy of neoadjuvant low-dose immuno-chemotherapy in LA-HNSCC and attempt to define the boundaries of tumor reduction surgery results in protecting more organ functions.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
CJ: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Software, Writing – original draft, Writing – review & editing. SW: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Project administration, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. LZ: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. Guangzhou Municipal Science and Technology Bureau funding-Basic and Applied Basic Research Topic of 2024 (Program Number: SL2023A04J00854)
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2024.1489451/full#supplementary-material
1. Johnson DE, Burtness B, Leemans CR, Lui VWY, Bauman JE, Grandis JR. Head and neck squamous cell carcinoma. Nat Rev Dis Primers. (2020) 6:92. doi: 10.1038/s41572-020-00224-3
2. Singh D, Vignat J, Lorenzoni V, Eslahi M, Ginsburg O, Lauby-Secretan B, et al. Global estimates of incidence and mortality of cervical cancer in 2020: a baseline analysis of the WHO Global Cervical Cancer Elimination Initiative. Lancet Glob Health. (2023) 11:e197–206. doi: 10.1016/S2214-109X(22)00501-0
3. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
4. Haddad R, O'Neill A, Rabinowits G, Tishler R, Khuri F, Adkins D, et al. Induction chemotherapy followed by concurrent chemoradiotherapy (sequential chemoradiotherapy) versus concurrent chemoradiotherapy alone in locally advanced head and neck cancer (PARADIGM): a randomised phase 3 trial. Lancet Oncol. (2013) 14:257–64. doi: 10.1016/S1470-2045(13)70011-1
5. Bhatia A, Burtness B. Treating head and neck cancer in the age of immunotherapy: A 2023 update. Drugs. (2023) 83:217–48. doi: 10.1007/s40265-023-01835-2
6. Ferris RL, Blumenschein G Jr., Fayette J, Guigay J, Colevas AD, Licitra L, et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med. (2016) 375:1856–67. doi: 10.1056/NEJMoa1602252
7. Cohen EEW, Soulieres D, Le Tourneau C, Dinis J, Licitra L, Ahn MJ, et al. Pembrolizumab versus methotrexate, docetaxel, or cetuximab for recurrent or metastatic head-and-neck squamous cell carcinoma (KEYNOTE-040): a randomised, open-label, phase 3 study. Lancet. (2019) 393:156–67. doi: 10.1016/S0140-6736(18)31999-8
8. Syed YY. Erratum to: durvalumab: first global approval. Drugs. (2017) 77:1817. doi: 10.1007/s40265-017-0826-x
9. Yamaguchi H, Hsu JM, Yang WH, Hung MC. Mechanisms regulating PD-L1 expression in cancers and associated opportunities for novel small-molecule therapeutics. Nat Rev Clin Oncol. (2022) 19:287–305. doi: 10.1038/s41571-022-00601-9
10. Yi M, Zheng X, Niu M, Zhu S, Ge H, Wu K. Combination strategies with PD-1/PD-L1 blockade: current advances and future directions. Mol Cancer. (2022) 21:28. doi: 10.1186/s12943-021-01489-2
11. Mei Z, Huang J, Qiao B, Lam AK. Immune checkpoint pathways in immunotherapy for head and neck squamous cell carcinoma. Int J Oral Sci. (2020) 12:16. doi: 10.1038/s41368-020-0084-8
12. Yu SL, Hsiao YJ, Cooper WA, Choi YL, Aviles-Salas A, Chou TY, et al. The Ring Study: an international comparison of PD-L1 diagnostic assays and their interpretation in non-small cell lung cancer, head and neck squamous cell cancer and urothelial cancer. Pathology. (2023) 55:19–30. doi: 10.1016/j.pathol.2022.07.016
13. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Int J Surg. (2021) 88:105906. doi: 10.1016/j.ijsu.2021.105906
14. Psyrri A, Fayette J, Harrington K, Gillison M, Ahn MJ, Takahashi S, et al. Durvalumab with or without tremelimumab versus the EXTREME regimen as first-line treatment for recurrent or metastatic squamous cell carcinoma of the head and neck: KESTREL, a randomized, open-label, phase III study. Ann Oncol. (2023) 34:262–74. doi: 10.1016/j.annonc.2022.12.008
15. Harrington KJ, Burtness B, Greil R, Soulières D, Tahara M, de Castro G Jr., et al. Pembrolizumab with or without chemotherapy in recurrent or metastatic head and neck squamous cell carcinoma: updated results of the phase III KEYNOTE-048 study. J Clin Oncol. (2023) 41:790–802. doi: 10.1200/JCO.21.02508
16. Haddad RI, Harrington K, Tahara M, Ferris RL, Gillison M, Fayette J, et al. Nivolumab plus ipilimumab versus EXTREME regimen as first-line treatment for recurrent/metastatic squamous cell carcinoma of the head and neck: the final results of checkMate 651. J Clin Oncol. (2023) 41:2166–80. doi: 10.1200/JCO.22.00332
17. Lee NY, Ferris RL, Psyrri A, Haddad RI, Tahara M, Bourhis J, et al. Avelumab plus standard-of-care chemoradiotherapy versus chemoradiotherapy alone in patients with locally advanced squamous cell carcinoma of the head and neck: a randomised, double-blind, placebo-controlled, multicentre, phase 3 trial. Lancet Oncol. (2021) 22:450–62. doi: 10.1016/S1470-2045(20)30737-3
18. Ferris RL, Haddad R, Even C, Tahara M, Dvorkin M, Ciuleanu TE, et al. Durvalumab with or without tremelimumab in patients with recurrent or metastatic head and neck squamous cell carcinoma: EAGLE, a randomized, open-label phase III study. Ann Oncol. (2020) 31:942–50. doi: 10.1016/j.annonc.2020.04.001
19. Ferris RL, Blumenschein G Jr., Fayette J, Guigay J, Colevas AD, Licitra L, et al. Nivolumab vs investigator's choice in recurrent or metastatic squamous cell carcinoma of the head and neck: 2-year long-term survival update of CheckMate 141 with analyses by tumor PD-L1 expression. Oral Oncol. (2018) 81:45–51. doi: 10.1016/j.oraloncology.2018.04.008
20. Tao Y, Biau J, Sun XS, Sire C, Martin L, Alfonsi M, et al. Pembrolizumab versus cetuximab concurrent with radiotherapy in patients with locally advanced squamous cell carcinoma of head and neck unfit for cisplatin (GORTEC 2015-01 PembroRad): a multicenter, randomized, phase II trial. Ann Oncol. (2023) 34:101–10. doi: 10.1016/j.annonc.2022.10.006
21. Le X, Ferrarotto R, Wise-Draper T, Gillison M. Evolving role of immunotherapy in recurrent metastatic head and neck cancer. J Natl Compr Canc Netw. (2020) 18:899–906. doi: 10.6004/jnccn.2020.7590
22. Paderno A, Petrelli F, Lorini L, Capriotti V, Gurizzan C, Bossi P. The predictive role of PD-L1 in head and neck cancer: A systematic review and meta-analysis. Oral Oncol. (2024) 153:106799. doi: 10.1016/j.oraloncology.2024.106799
23. Chen L, Mo DC, Hu M, He W, Yang QW, Tang J. Combination therapy with immune checkpoint inhibitors in recurrent or metastatic squamous cell carcinoma of the head and neck: A meta-analysis. Int Immunopharmacol. (2023) 119:110270. doi: 10.1016/j.intimp.2023.110270
24. Han X, Zhang H, Sun K, Li J, Wu W, Liu K, et al. Durvalumab with or without tremelimumab for patients with recurrent or metastatic squamous cell carcinoma of the head and neck: a systematic review and meta-analysis. Front Immunol. (2023) 14:1302840. doi: 10.3389/fimmu.2023.1302840
25. Chen S, Yang Y, Wang R, Fang J. Neoadjuvant PD-1/PD-L1 inhibitors combined with chemotherapy had a higher ORR than mono-immunotherapy in untreated HNSCC: Meta-analysis. Oral Oncol. (2023) 145:106479. doi: 10.1016/j.oraloncology.2023.106479
Keywords: immunotherapy, squamous cell carcinoma of head and neck (HNSCC), overall survival (OS), progression-free survival (PFS), treat-related adverse events (TRAEs)
Citation: Jiang C, Wang S and Zhu L (2025) Efficacy and safety of immunotherapy for head and neck squamous cell carcinoma: a meta-analysis of randomized clinical trials. Front. Oncol. 14:1489451. doi: 10.3389/fonc.2024.1489451
Received: 01 September 2024; Accepted: 09 December 2024;
Published: 09 January 2025.
Edited by:
Andreas Dietz, Leipzig University, GermanyReviewed by:
Mohamed Rahouma, NewYork-Presbyterian, United StatesCopyright © 2025 Jiang, Wang and Zhu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lijun Zhu, emh1bGlqdW5AZ2RwaC5vcmcuY24=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.