
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Oncol. , 13 January 2025
Sec. Gynecological Oncology
Volume 14 - 2024 | https://doi.org/10.3389/fonc.2024.1488040
Introduction: Cervical cancer (CC) is a highly prevalent malignancy of the reproductive system. This study aimed to methodically assess the function of circular RNAs (circRNAs) as possible indicators of CC, with a specific emphasis on their usefulness in the identification, prediction, and correlation with clinicopathological elements.
Methods: A comprehensive literature search was conducted using databases such as PubMed, Cochrane Library, Web of Science, Embase, and the China National Knowledge Infrastructure (CNKI). The latest data were extracted on May 3rd, 2024. The diagnostic potential of circRNA expression was evaluated using a range of metrics including sensitivity, specificity, and area under the receiver operating characteristic curve (AUC). The importance of circRNAs was further evaluated in terms of clinical relevance, pathological features, and prognostic value using pooled odds ratios (ORs) and hazard ratios (HRs).
Results: The meta-analysis included 27 studies, which were categorised based on diagnostic applications (n=3), clinicopathological correlations (n=15), and prognostic evaluations (n=23). Elevated expression levels of oncogenic circRNAs were significantly associated with poor clinical indicators, including tumour size (odds ratio [OR] = 0.425, 95% confidence interval [CI]: 0.267–0.676), International Federation of Gynaecology and Obstetrics (FIGO) stage (OR = 0.315, 95% CI: 0.224–0.443), and lymph node metastasis (OR = 2.975, 95% CI: 1.816–4.872). This upregulation of oncogenic circRNA was also identified as a predictor of worse survival outcomes, with a hazard ratio (HR) of 2.13 (95% CI: 1.73–2.62, P < 0.001). The downregulation of circRNAs with tumour-suppressor properties was similarly associated with poor clinical parameters, such as tumour size (OR = 0.310, 95% CI: 0.102–0.941), FIGO stage (OR = 0.231, 95% CI: 0.101–0.527), and lymph node metastasis (OR = 2.430, 95% CI: 1.156–5.110), and was indicative of a worsened prognosis (HR = 2.20, 95% CI: 1.03–4.70, P = 0.042). In terms of diagnostic value, the pooled sensitivity, specificity, and area under the curve (AUC) were calculated to be 0.85, 0.83, and 0.91, respectively.
Conclusion: The results of our meta-analysis indicate that circRNAs have the potential to serve as promising biomarkers for CC diagnosis, prognosis, and clinicopathology.
Systematic review registration: https://www.crd.york.ac.uk/PROSPERO/, identifier CRD42024544997.
Cervical cancer (CC), predominantly caused by high-risk human papillomavirus (HPV) infection, is the fourth most common malignancy in women worldwide (1). It was associated with approximately 660,000 new cases and 350,000 deaths in 2022 alone, disproportionately affecting women in low- and middle-income areas and those who were human immunodeficiency virus (HIV)-positive (2). In recent years, China has experienced a troubling increase in the incidence of CC, characterised by increasing prevalence and a shift toward younger age groups. This trend contrasts with global patterns, which are influenced by disparate levels of healthcare access and screening programs (3, 4). In the international medical community, the standard approach for the diagnosis and treatment of CC is well-established and includes a combination of screening, surgical intervention, radiotherapy, and chemotherapy. Screening typically involves Pap smear and HPV DNA testing, which significantly reduces the incidence of CC in areas with robust screening programs (5). When CC is detected, the FIGO (International Federation of Gynecology and Obstetrics) staging system is commonly used to guide treatment decisions (6). Early stage CC is often treated surgically, including radical hysterectomy and pelvic lymph node dissection (7). Advanced cancer stages may require concurrent chemoradiation, in which radiation therapy is combined with chemotherapy to enhance the radiosensitivity of cancer cells (8). The 5-year survival rate for CC varies according to the stage at diagnosis, with higher rates for early stage disease (approximately 92% for stage I) and lower rates for later stages (approximately 56% for stage IV) (9). These findings highlight the importance of early detection and treatment. Therefore, it is imperative to develop biomarkers that can facilitate early diagnosis and enable the accurate assessment of prognosis in individuals with CC.
Circular RNAs (circRNAs) are non-coding RNA that create covalently closed continuous loops. These loops control gene expression by silencing miRNAs or attaching proteins that influence cellular processes (10). Recently, circRNAs have garnered considerable attention owing to their role in tumour biology as crucial regulators of gene expression and cellular function. For example, specific circRNAs have been shown to modulate the miR-187-3p/RTKN2 pathway in hepatocellular carcinoma, thereby promoting tumour growth and metastasis (11). Similarly, in glioblastomas, circRNAs can act as miRNAs, affecting the expression of genes pivotal to cell proliferation and survival (12). These examples underscore the multifaceted influence of circRNAs in shaping the tumour microenvironment and highlight their potential as biomarkers and therapeutic targets.
CircRNAs have been acknowledged as important regulators of CC. For example, Zhang et al. (13) showed that circCDKN2B-AS1 enhances aerobic glycolysis by absorbing IMP3 protein to stabilise HK2 mRNA, enhancing the malignant characteristics of CC, which may offer a prospective strategy for CC treatment. Abnormal expression of circRNAs such as circSLC26A4 and circ-ATP8A2 has been linked to the promotion of CC progression through the miR-1287-5p/HOXA7 axis and other molecular mechanisms (14). These findings highlight the potential of circRNAs as biomarkers for early diagnosis and as therapeutic targets for intervention in CC. However, these studies had several limitations. First, many of the available studies had small sample sizes, limiting the generalisability and reliability of the findings. Second, most studies focused on specific types of circRNAs, whereas the roles of other types of circRNAs in CC have not yet been fully explored. This limits our understanding of the role of circRNAs in CC.
In this meta-analysis, we conducted a comprehensive review of the literature on the role of circRNAs in CC, with particular focus on their potential as diagnostic, clinicopathological, and prognostic biomarkers. The primary objective of this study was to quantitatively synthesise available data to assess the effectiveness of circRNAs in the above contexts. We sought to reconcile the heterogeneity among studies, evaluate the methodological rigor of the included studies, and scrutinise potential publication biases that could affect the robustness of our conclusions. Here, we provide a comprehensive and systematic assessment of the role of circRNAs in CC, which will contribute to the refinement of personalised treatment strategies and enhance the clinical management of affected patients.
The methodology of this study was meticulously pre-registered on the PROSPERO platform under registration number CRD42024544997. This ensured transparency and reproducibility of our research.
The literature review was conducted in compliance with the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) statement.
The databases PubMed, Cochrane Library, Web of Science, Embase, and China National Knowledge Infrastructure (CNKI) were searched for scholarly articles on circRNAs and CC, focusing on publications up to the date of May 3rd, 2024.
To ensure comprehensiveness, literature retrieval was performed by two researchers working independently using both Medical Subject Headings (MeSH) and free-text keywords. In case of divergence, a third researcher’s input was sought. The search strategy encompassed the following MeSH terms and keywords (1): “Uterine Cervical Neoplasms”, “Cervical Cancer”, and “Cancer of the Cervix”; (2) “Circular RNA”, “CiRNA”, and “circRNAs”. The authors of relevant articles were contacted for further information when necessary.
Two researchers, working independently, evaluated the suitability of the studies and gathered relevant data. Any conflicts were addressed through consultation with an additional researcher. Studies that explored the significance of circRNA expression levels in relation to CC prognosis, diagnosis, and clinicopathological features were deemed eligible for meta-analysis.
The inclusion criteria were as follows: (1) studies in which included participants were diagnosed as having CC using pathology, (2) studies presenting circRNA levels categorised as either elevated or diminished, (3) research encompassing data to assess diagnostic, prognostic, and clinicopathological aspects, and (4) investigations of a cohort or case-control design.
The exclusion criteria were as follows: (1) redundant studies that constituted duplicates; (2) publications falling under the categories of reviews, meta-analyses, correspondence pieces, abstracts from conferences, or individual case reports; and (3) research endeavours with ambiguous or insufficient data, which precludes the conduct of an appropriate statistical analysis.
Two researchers independently gathered data from the selected studies, adhering to consistent criteria. The following data were collected: (1) prognostic data, including the expression level, threshold value, sample size, assay method, duration of follow-up, survival results, and survival results, including the hazard ratios (HRs) of overall survival (OS) or disease-free survival (DFS) and 95% confidence intervals (CIs). (2) Diagnostic data, including odds ratios (ORs) and 95% CIs, were used to integrate information and assess diagnostic accuracy. This was achieved using the following parameters: true positives (TP), false positives (FP), false negatives (FN), true negatives (TN), and the area under the receiver operating characteristic curve (ROC) (AUC) of each study. This enabled the evaluation of sensitivity (SEN) and specificity (SPE). (3) The clinicopathological characteristics included age, tumour size, FIGO stage, lymph node metastasis, and tumour differentiation. In instances where the HRs and their 95% CIs were not directly reported, these data were extracted from Kaplan–Meier (KM) curves using the Engauge Digitiser software, version 4.1. HRs and the accompanying 95% CIs were subsequently derived using the Excel program provided by Tierney et al. (15). In instances where TP, TN, FP, and FN were not explicitly stated, these metrics were inferred from the SEN and SPE values of the ROC curve facilitated by the GetData Graph Digitizer software (16).
Quality assessment was independently conducted by two researchers, with the input of a third researcher in cases of discrepancy. The methodological quality of the included diagnostic studies was evaluated in accordance with the four domains of the Diagnostic Accuracy Studies-2(QUADAS-2)tool: patient selection, index tests employed, reference standards applied, and flow and timing of the study design. The risk of bias in these domains was classified into three categories: high (H), low (L), or unclear (U). The quality of the prognostic studies was evaluated using the Newcastle-Ottawa Scale (NOS). A score of 7 or higher was considered indicative of a high level of methodological quality.
Statistical analysis was conducted using Review Manager 5.3 and Stata 12.0 software. The degree of heterogeneity among the included studies was evaluated using the I² statistic. A threshold of I² greater than 50%, in conjunction with a P-value of < 0.05, was established to indicate significant heterogeneity. In such instances, a random-effects model was used to aggregate the study results. Conversely, in instances where heterogeneity was deemed insignificant, a fixed-effects model was employed for the analysis. The threshold for statistical significance was set at a P-value of less < 0.05.
In the diagnostic meta-analysis, the TP, FP, FN, and TN were combined to calculate the pooled SEN and SPE of circRNA expression. The diagnostic accuracy was evaluated by calculating the area under the summary ROC (SROC). Deeks’ funnel plot asymmetry test was used to examine the potential for publication bias. A P value exceeding 0.1 indicates the absence of publication bias. In the prognostic meta-analysis, HRs and their corresponding 95% confidence intervals (CIs) were used to quantify the prognostic significance of the circRNAs. The presence of publication bias was evaluated using Egger’s test, with a P-value greater than 0.1 indicating the absence of such bias. Sensitivity analysis was conducted to ensure the robustness of the pooled HRs. Finally, pooled ORs and 95% CIs were calculated to investigate the correlation between circRNA expression levels and various clinicopathological features.
The literature search and screening processes used in this study are illustrated in Figure 1. The preliminary search yielded 1,119 potentially relevant studies from various databases, including PubMed (300 articles), Cochrane Library (one article), Web of Science (372 articles), Embase (357 articles), and CNKI (89 articles). In total, 214 unique articles were identified after removing duplicate records. Of the aforementioned studies, the full texts of 69 were not accessible. After comprehensively reviewing the remaining full-text articles, 113 were excluded. Fourteen studies were not case-control or cohort studies, 24 lacked comprehensive clinical data, and 81 did not provide the data required for analysis. A total of 27 studies published between 2018 and 2023 were selected for inclusion in the meta-analysis, which was conducted using the following categories: prognostic analyses (17–39), diagnostic assessments (21, 40, 41), and clinicopathological features (17, 18, 20, 22, 24, 28–36, 42).
Tables 1–4 present the main characteristics of the mentioned studies. Between 2018 and 2023, 27 circRNAs were identified and published. The studies that had been included were all from China. A total of 23 circRNAs were used in 23 investigations (Table 1), offering fundamental data for prognostic analysis. The duration of the patients’ follow-up varied from 36 to 140 months, and 20 to 192 samples were taken. On each of the nine dimensions, the NOS scores demonstrated the strength of the included studies for prognostic and clinical parameter analysis (Table 2).
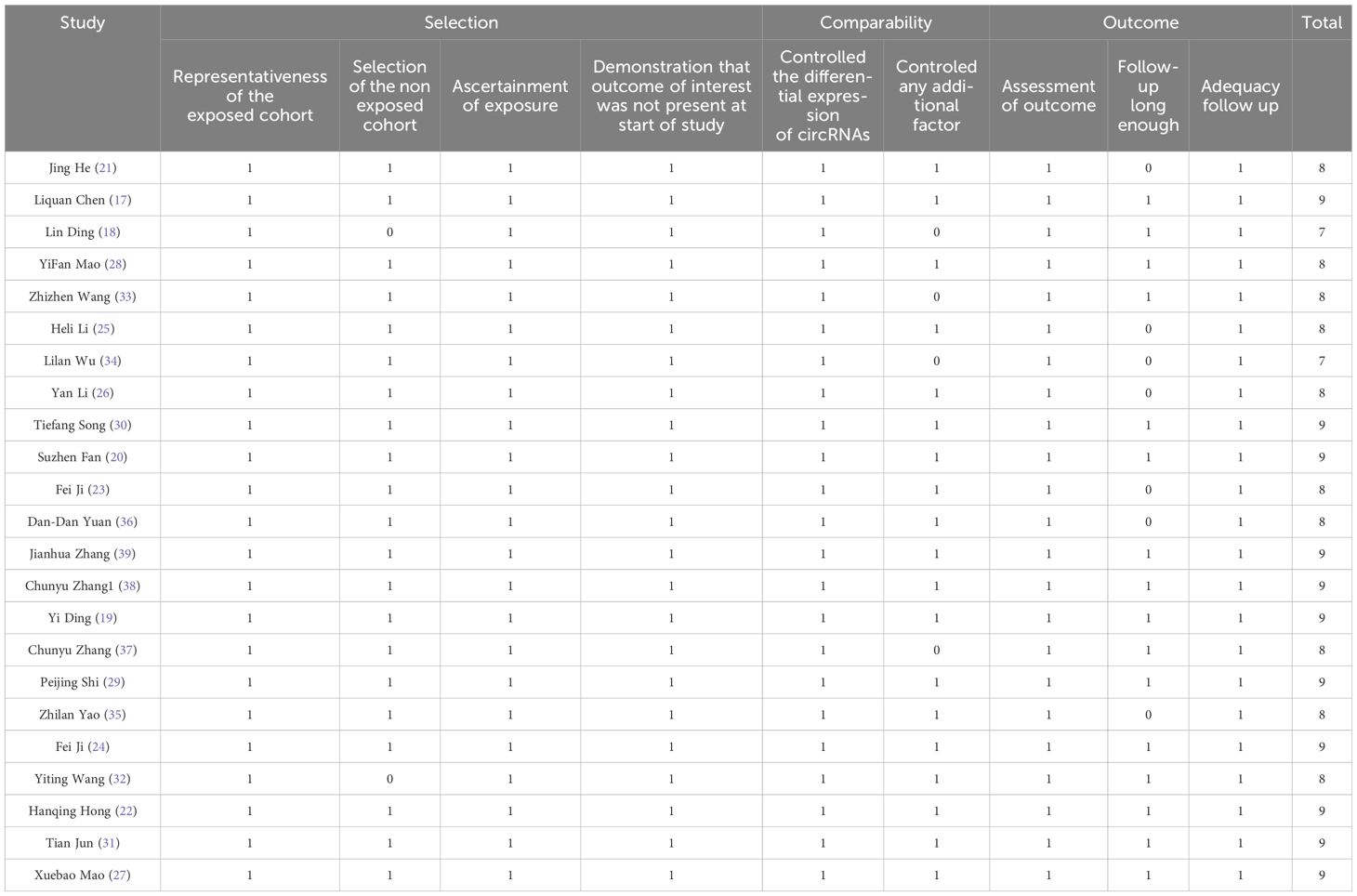
Table 2. Assessment of qualifying clinical parameter and prognostic research using the Newcastle-Ottawa Scale.
Table 3 contains information on several components used in the diagnostic analysis, including the SEN, SPE, and AUC. The sample sizes ranged from 30 to 192. Applicability issues and risk of bias were considered when evaluating the quality of the included studies, and the findings demonstrated that the included studies were of high quality (Figure 2). Table 4 shows the correlations between circRNAs and clinicopathological features.
Overall, 1,689 people from 23 studies were included in the prognosis meta-analysis. Fixed-effects models (I2 = 0.0%, P = 1.000) were used to determine the function of elevated circRNAs in CC prognosis. The findings showed that overexpression of carcinogenic circRNAs was associated with a poor prognosis (HR = 2.13, 95% CI: 1.73–2.62, P < 0.001) (Figure 3A). Concurrently, a significantly poorer prognosis for cancer patients with downregulated tumour-suppressor circRNAs was linked to them (HR = 2.20, 95% CI:1.03–4.70, P = 0.042). A fixed-effects model was used because of the lack of heterogeneity among the trials (I2 = 0%, P = 0.852) (Figure 3B). Furthermore, we summarised and analysed the correlation between circRNAs and DFS in patients with CC. We found that overexpression of oncogenic circRNAs affects the DFS of cervical cancer (HR=2.11, 95% CI=1.47–3.02, P<0.001), while downregulation of oncogenic circRNAs is not statistically significant for the DFS of cervical cancer (HR=2.23, 95% CI=0.94–5.31, P=0.070). We used fixed-effects models for our analysis (I2 = 0.0%, P = 0.887; I2 = 0.0%, P = 0.518). The precise numerical values are shown in Figure 4.
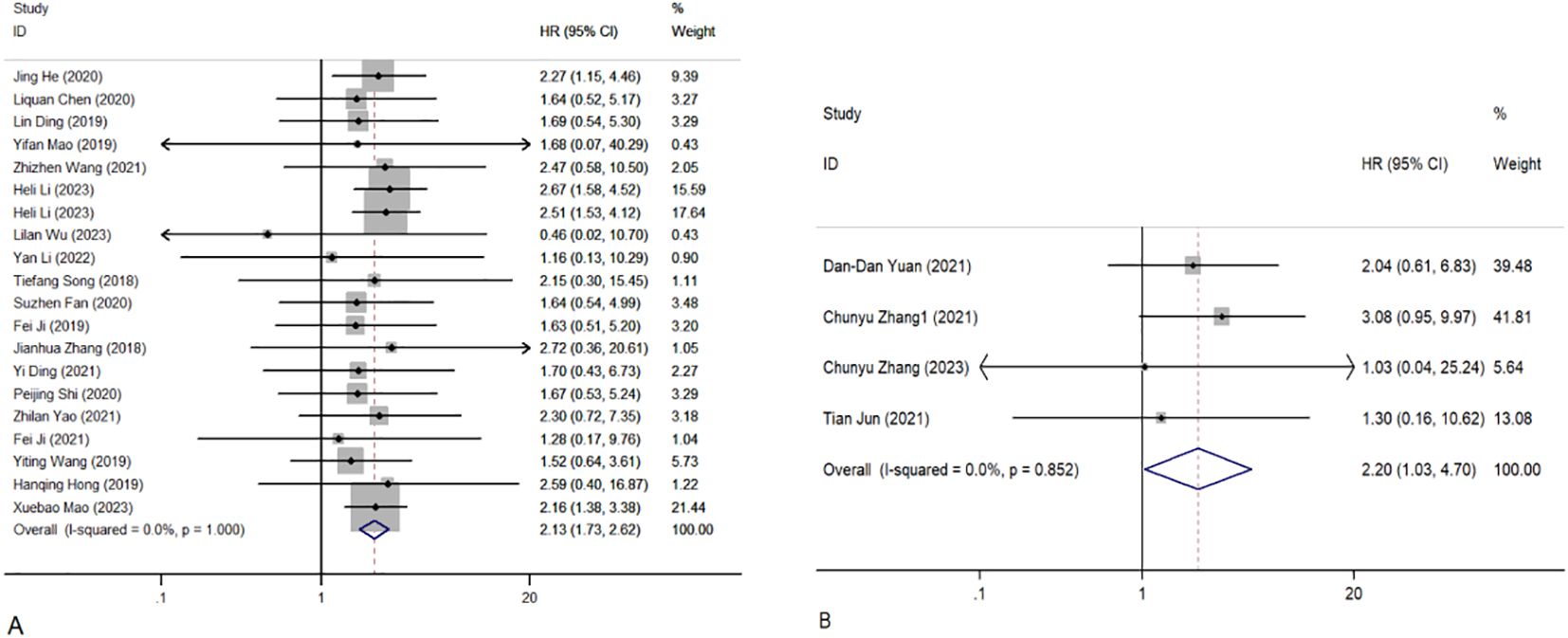
Figure 3. Forest plots of the OS of CC patients for circRNAs. (A) Upregulated circRNAs. (B) Downregulated circRNAs. HR, hazard ratios.

Figure 4. Forest plots of the DFS of CC patients for circRNAs. (A) Upregulated circRNAs. (B) Downregulated circRNAs. HR, hazard ratios.
In the subsequent subgroup analysis, we further found that follow-up time ≤60 months (HR=2.16, 95% CI=1.76-2.67) and upregulation of circRNAs (HR=2.16, 95% CI=1.76-2.66) were risk factors affecting OS (Figure 5). However, the two subgroups of cutoff values and sample sizes did not seem to significantly change the relationship between circRNAs and CC prognosis. In the subgroup analysis related to DFS, we found that only the upregulation of circRNAs (HR=2.11, 95% CI=1.47-3.02) was a risk factor for prognosis, while there was no strong association between the follow-up time and the cut-off value with CircRNAs (Figure 6). Nevertheless, it should be noted that this conclusion is based on the statistical analysis of existing data, and the actual biological significance may require further experiments and research to verify.
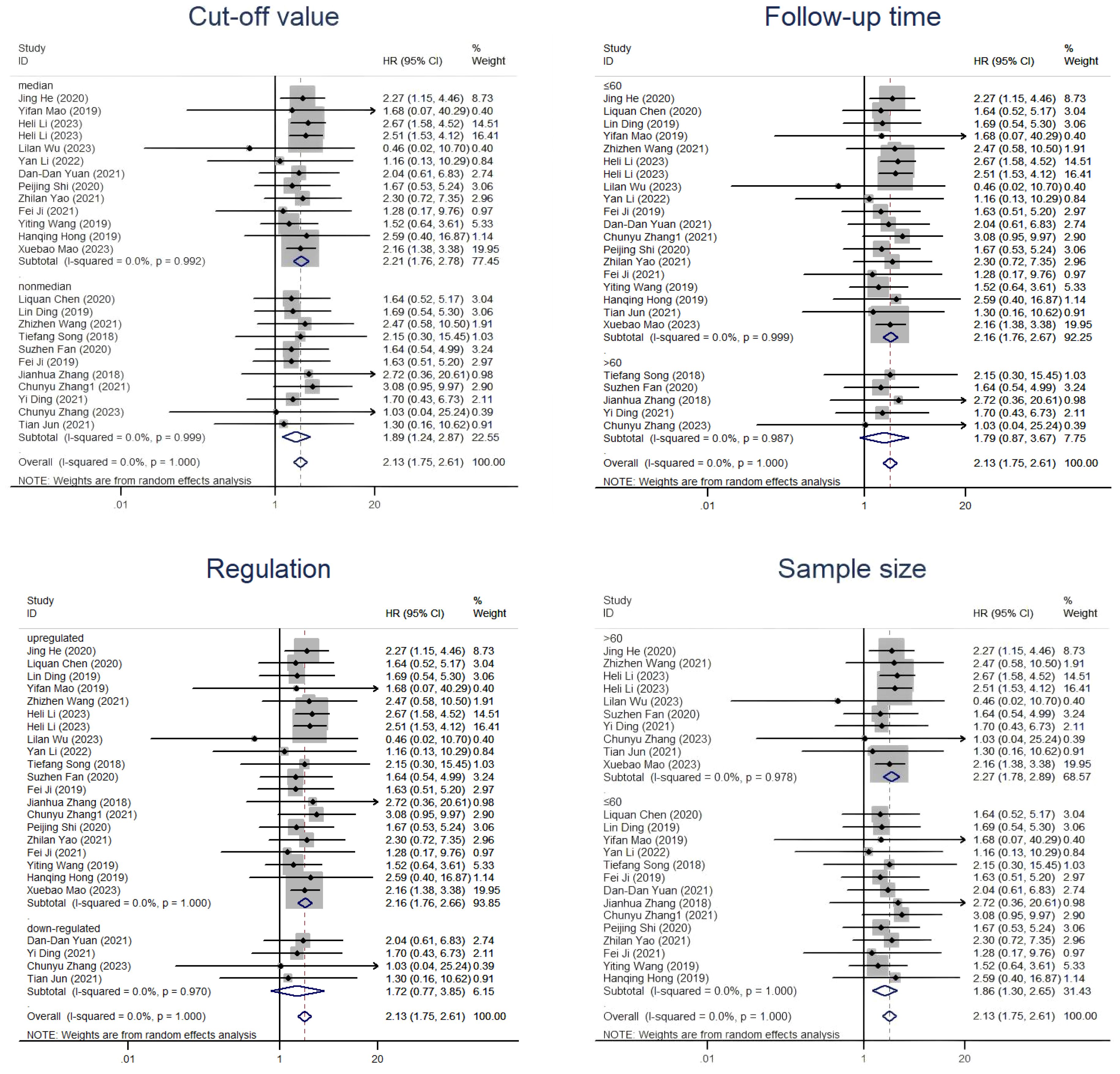
Figure 5. Subgroup analyses of OS for circRNAs, stratified by cut-off value, follow-up time, regulation and sample size.
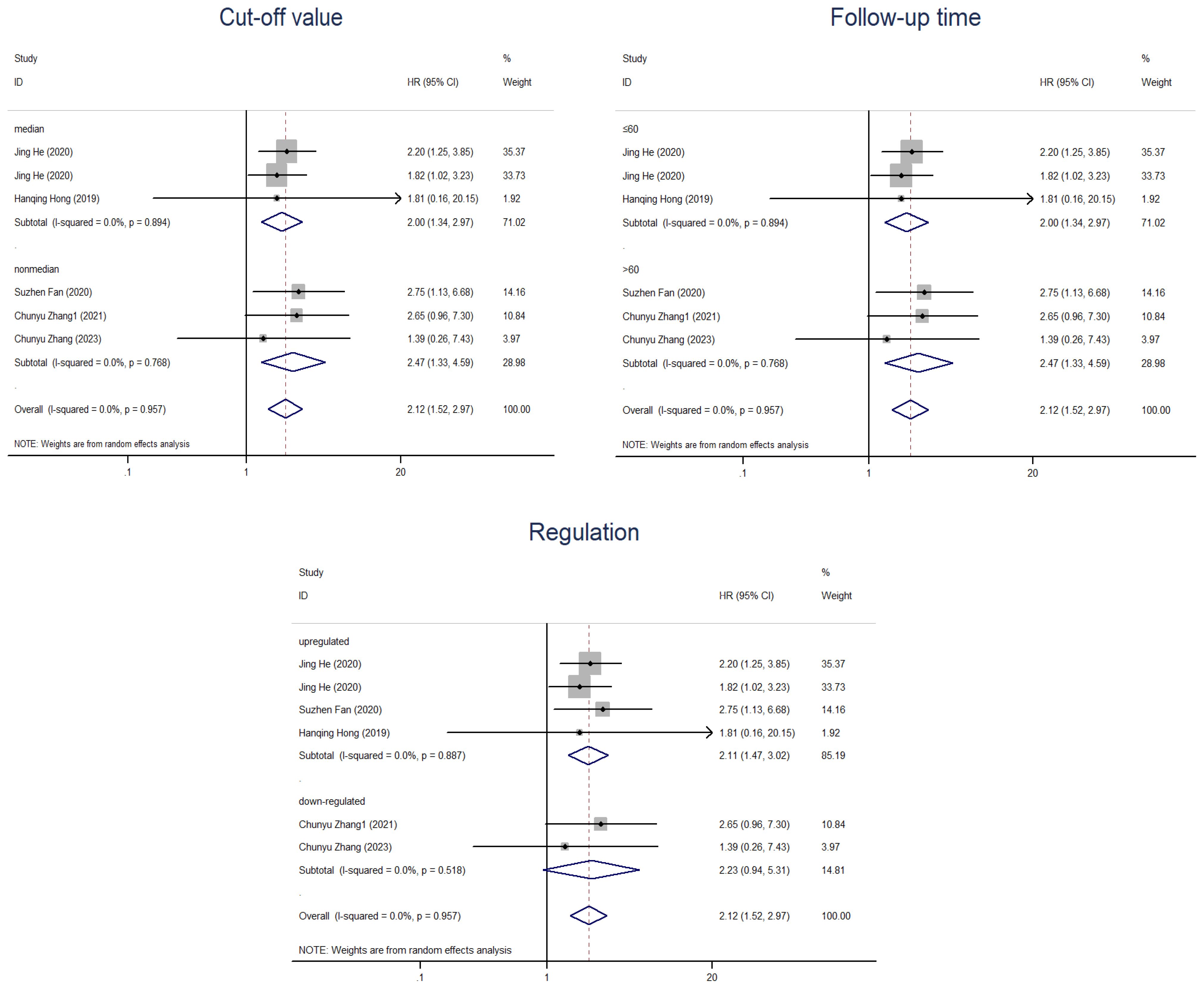
Figure 6. Subgroup analyses of DFS for circRNAs, stratified by cut-off value, follow-up time and regulation.
The diagnostic meta-analysis included 274 eligible patients selected from three qualifying trials. The combined SEN and SPC were computed to assess the diagnostic effectiveness of the circRNAs, and the results are presented in Figure 7. Analysis of the combined data revealed a sensitivity of 0.86 (95% CI:0.82–0.89) and a specificity of 0.83 (95% CI:0.78–0.88). Furthermore, the study of the SROC curve revealed an AUC of 0.91 (95% CI:0.88–0.93, Figure 8). These findings indicated that the tested circRNAs have diagnostic importance in CC, demonstrating high sensitivity and specificity.
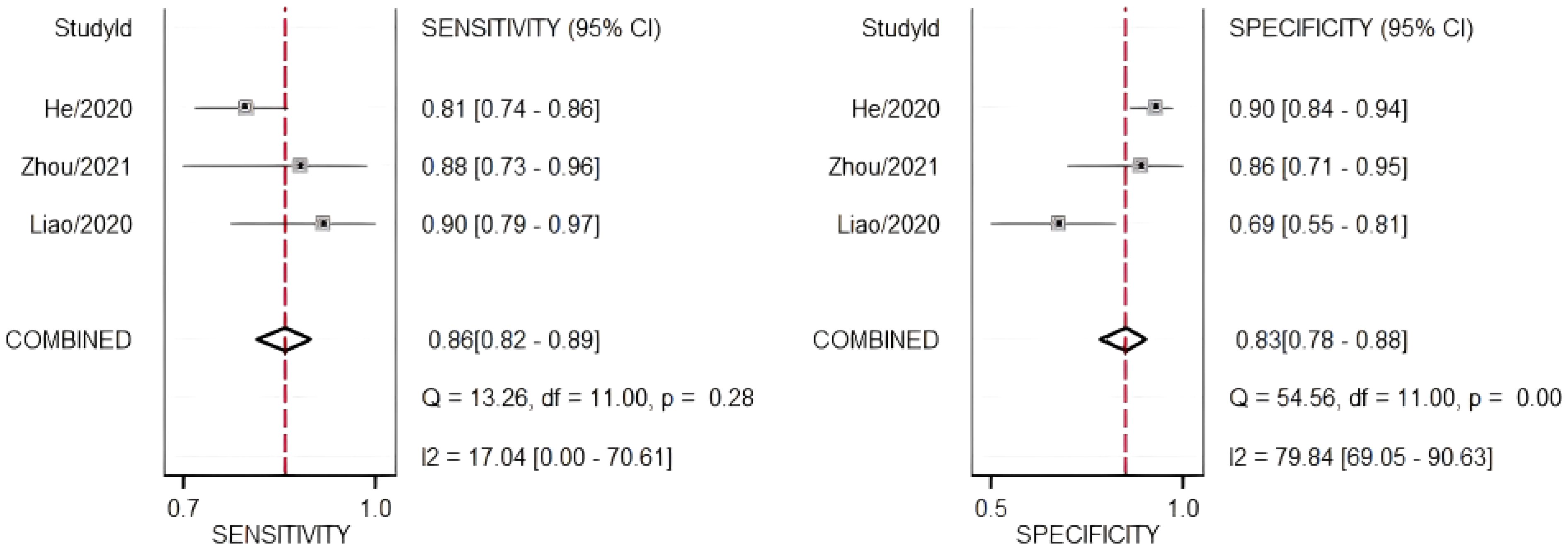
Figure 7. Forest plots of summary sensitivity and specificity to illustrate the diagnostic value of circRNAs for CC.
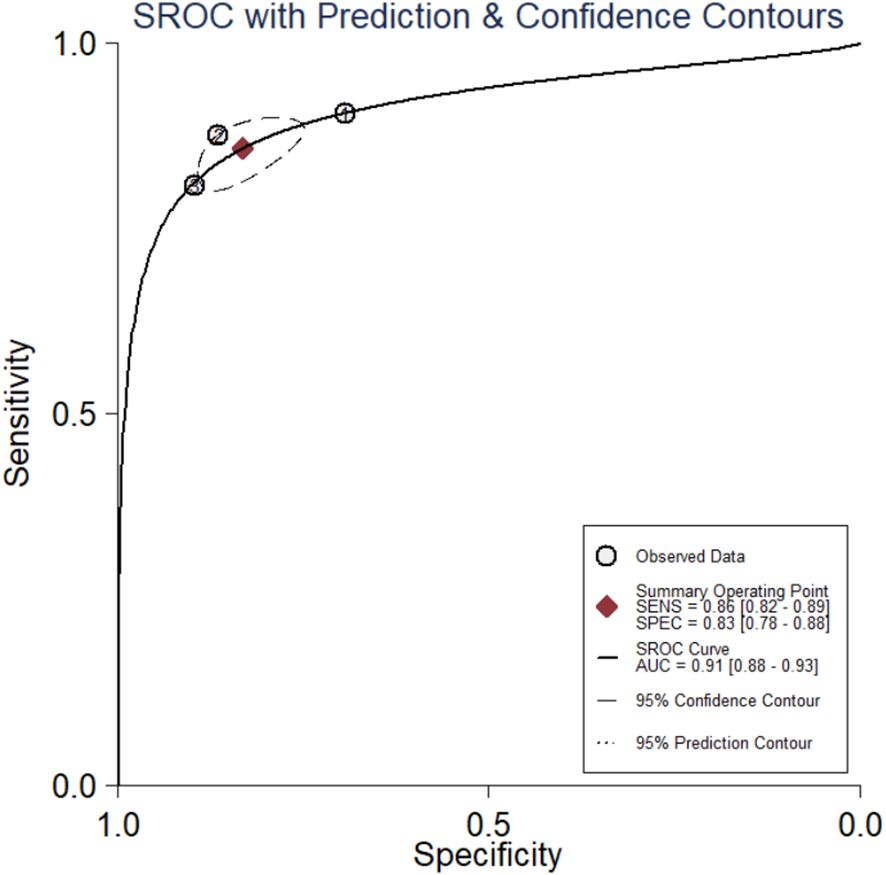
Figure 8. The summary receiver operating characteristic (SROC) curve based on circRNAs for diagnosis analysis. ROC, receiver operator characteristic.
In addition to their function in assessing the prognosis of patients with CC and aiding in the diagnosis of cancer, circRNAs are closely associated with other clinicopathological markers in patients with cancer. We compiled data from 15 studies, totalling 765 participants, for our meta-analysis to quantify the association between circRNAs and clinicopathological features of CC (Table 4). We then summarised the information contained in each article among the 15 studies that included the same influencing factors.
Elevated expression of cancer-causing circRNAs was associated with negative clinical features (tumour size: OR=0.425,95% CI: 0.267–0.676; FIGO stage: OR = 0.315, 95% CI: 0.224–0.443; lymph node metastasis: OR =2.975, 95% CI: 1.816–4.872). Reduced expression of tumour suppressor circRNAs was similarly associated with poorer clinical outcomes (tumour size: OR = 0.310, 95% CI: 0.102–0.941; FIGO stage: OR = 0.231, 95% CI: 0.101–0.527; lymph node metastasis: OR =2.430, 95% CI: 1.156–5.110). In addition, patient age and the degree of tumour differentiation were not statistically significant in relation to circRNA expression.
Deeks’ funnel plot asymmetry test was used to assess possible publication bias in the diagnostic meta-analysis. The results indicated the absence of publication bias (P = 0.80) (Supplementary Figure S1). Egger’s tests were used to examine the prognostic markers of CC in relation to circRNA expression and to assess the presence of potential publication bias in the operating system. Figure 9A demonstrates that the results of Egger’s tests for OS showed a P value of 0.003, suggesting the presence of publication bias in the included studies. For DFS, the result showed P = 0.942 for Egger’s test, indicating no significant publication bias (Figure 9B). After implementing the trim-and-fill method, the adjusted effect size was recalculated and the funnel plot was re-evaluated for symmetry. Despite adjusting for potential publication bias, the overall effect size remained significant (P < 0.05), suggesting that the initial findings were not significantly influenced by the presence of publication bias (Supplementary Figure S2). Concurrently, our study showed that the combined findings of the prognostic meta-analysis remained consistent when subjected to sensitivity analysis (Figures 9C, D).
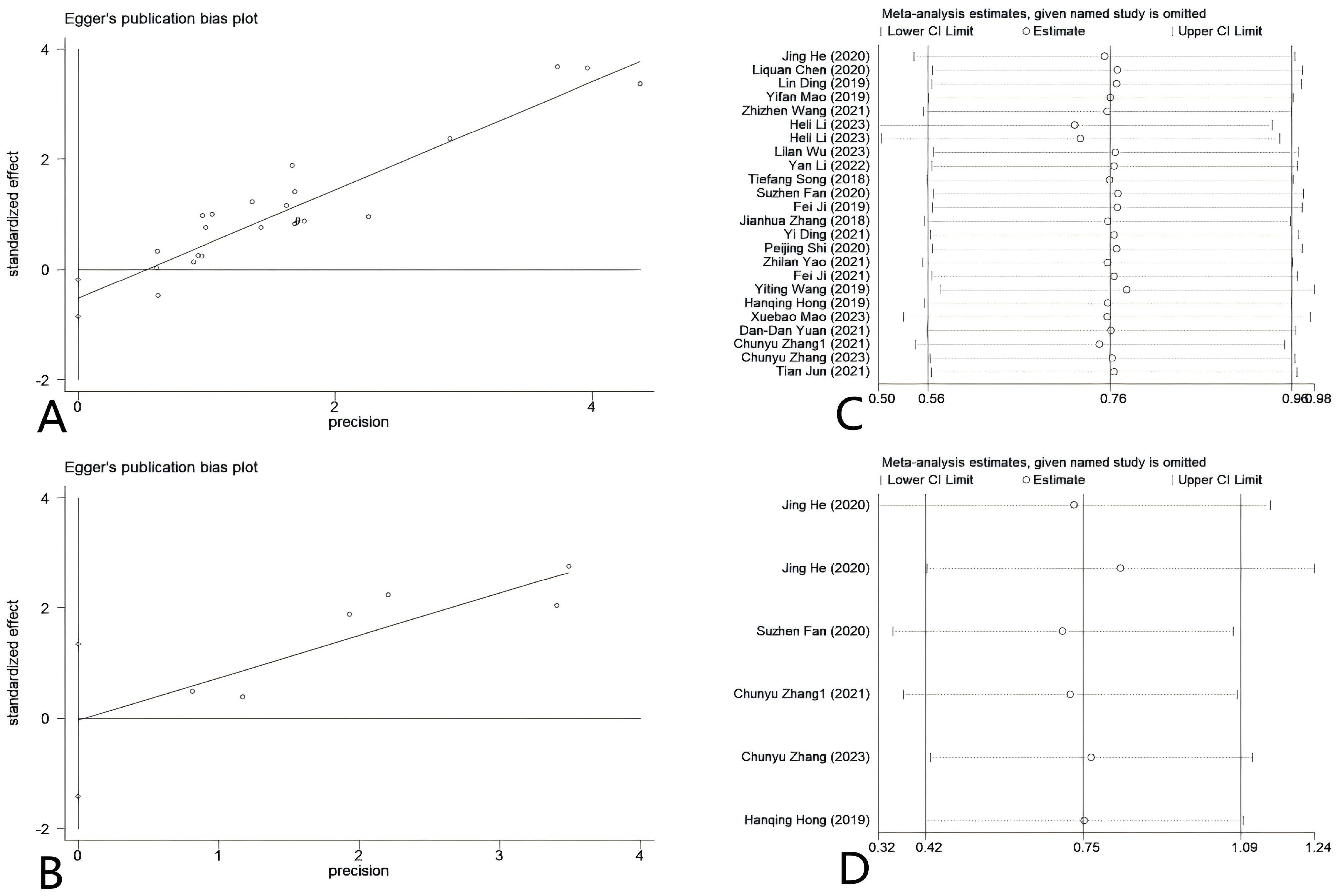
Figure 9. (A) Egger’s tests of OS (P = 0.003). (B) Egger’s tests of DFS(P = 0.942). (C) Sensitivity analysis of OS. (D) Sensitivity analysis of DFS.
Cervical cancer (CC) is a significant contributor to the global mortality from gynaecological cancers. The primary aetiology of CC is a persistent infection with high-risk HPV types. Cancer development is influenced by a complex interplay of genetic and epigenetic factors that facilitate disease progression. The most common histological subtype of CC is squamous cell carcinoma (SCC), which constitutes approximately 80% of all cases, followed by adenocarcinoma (AC), which accounts for approximately 20%. The implementation of robust CC screening programs and widespread HPV vaccination in developed countries have resulted in a considerable reduction in the incidence and mortality of SCC (43). However, a contrasting pattern has been observed for AC and SCC, the incidence of which has increased over the past three decades. This increase may be due to the limitations of traditional cytological screening methods, which are unable to effectively detect these subtypes, given their location deep within the cervical canal (44, 45).
The current diagnostic and treatment landscape for CC is multifaceted, and encompasses surgical interventions, radiotherapy, chemotherapy, emerging immunotherapies, and targeted therapies. Despite these advances, challenges such as the side effects of chemoradiation, limitations of immunotherapy, and the need for more effective treatments, particularly for metastatic disease, persist. The standard of care often involves concurrent chemoradiation of locally advanced tumours and a combination of chemotherapy with bevacizumab for metastatic disease. However, these treatments can be limited by toxicity and immunosuppression (46).
In the quest for novel therapeutic approaches, circRNAs have emerged as promising candidates for CC research. CircRNAs are a class of non-coding RNAs that exhibit distinctive expression patterns in eukaryotic transcriptomes owing to their covalently closed continuous-loop topology. They have been found to play a role in various cellular processes including cell growth and development, and their abnormal expression has been associated with CC development (14). The potential use of circRNAs in CC lies in their ability to serve as diagnostic biomarkers and therapeutic targets. They regulate cell proliferation, migration, invasion, and apoptosis. Their unique profiles in CC offer opportunities for early detection and personalised treatment strategies (47). As our understanding of the role of circRNAs in CC increases, these molecules may provide new avenues for improving patient outcomes and developing effective treatments.
Recently, several studies have focused on the function of circRNAs. However, there is a notable absence of relevant meta-analyses examining circRNA expression in CC. In previous meta-analyses, only one study has detected an association between circRNAs and CC. Liu et al. (48) identified circRNAs as potential biomarkers for both cervical and ovarian cancers, demonstrating their diagnostic and prognostic value. However, the scope of their study, although comprehensive, resulted in a less focused analysis of CC and did not incorporate clinicopathological data. Recent reviews have explored the role of circRNAs in CC development and progression. Chaichian et al. (49) highlighted the substantial influence of circRNAs on CC progression, and Najafi et al. (50) expanded the discourse to include the prognostic, diagnostic, and therapeutic implications of circRNAs in clinical settings. However, the absence of quantitative data indicates that these findings require empirical validation.
A total of 1983 patients with cancer from 27 suitable studies were included in this analysis in accordance with the specified inclusion and exclusion criteria. These studies included three studies that focused on diagnosis, 23 focused on prognosis, and 15 focused on clinicopathological aspects. Regarding prognostic risk factors, 19 circRNAs were linked to OS in patients with CC, whereas three circRNAs were linked to DFS. Overall, increased expression of oncogenic circRNAs is linked to a much higher risk of death in patients, being 2.13 times greater than those without such increase (95% CI: 1.73–2.62). Similarly, the downregulation of tumour-suppressor circRNAs confers a 2.20-fold increased risk of death, albeit with a wider confidence interval reflecting less certainty (95% CI: 1.03–4.70). In our prognostic meta-analysis that focused on OS, Egger’s test indicated the potential for publication bias, which merits further examination. This bias indicates that studies with insignificant or less impressive results may be less likely to be published, which could potentially bias the pooled results. Nevertheless, the application of the trim-and-fill method to account for this asymmetry did not yield a statistically significant difference in the results, indicating that despite the potential influence of publication bias, it had a minimal effect on the overall findings of our study.
In the context of CC diagnosis, a limited number of three studies were identified, which reported an AUC of 0.91 for circRNAs as potential diagnostic biomarkers. These studies indicated a sensitivity of 0.85 and a specificity of 0.83, suggesting that circRNAs can be used to effectively distinguish between healthy individuals and those with CC. In addition, some studies have highlighted the significant role of circRNAs in screening for cervical intraepithelial neoplasia (CIN). Luo et al. (51)analysed the temporal transcriptomic landscapes of mRNAs and circRNAs, identified functional circRNAs in cervical squamous cell carcinoma (CSCC), and improved our understanding of the pathogenesis and molecular biomarkers of CSCC and high-grade squamous intraepithelial lesions (HSIL). Zhou et al. (52) found that ciRS-7 could be used to discriminate between CC patients and healthy controls, as well as between CC and CIN patients, demonstrating great potential in clinical diagnosis. In conclusion, circRNAs may serve as potential biomarkers of CC and CIN. Therefore, circRNAs can be considered candidate biomarkers for screening CIN, including atypical hyperplasia. However, further studies are needed to verify the accuracy, sensitivity, and specificity of these circRNAs as clinical diagnostic tools.
In conclusion, we conducted a meta-analysis of the clinicopathological data from 765 patients with CC who participated in 15 different investigations. Notably, tumour size, FIGO stage, and lymph node metastasis were significantly associated with the expression of both upregulated and downregulated circRNAs, suggesting their integral involvement in tumour progression and metastatic potential.
Changes in circRNA expression are noted in response to chemotherapy, radiation therapy, and immunotherapy. For example, by reducing the expression of HSPB1, crVDAC3 induces ferroptosis in breast cancer cells, thereby mediating the resistance of HER2-low breast cancer to trastuzumab-deruxtecan (53). A growing body of evidence suggests that circRNAs may serve as prognostic biomarkers for predicting clinical response to cancer chemotherapy. However, more detailed experimental research is required to support their translational applications in therapy and prognosis (54). In addition, studies have shown that changes in the tumour microenvironment such as hypoxia, immune cell infiltration, and the presence of secreted factors can potentially affect the expression and function of circRNAs, thereby affecting the accuracy of circRNA detection (55). Therefore, it is necessary to consider the complexity and dynamic changes in the tumour microenvironment when performing circRNA detection to ensure the accuracy and reliability of the results.
These characteristics of circRNAs will not only be able to guide clinical decisions but also act as therapeutic targets or agents, paving the way for new treatment interventions. Due to the stable and non-degradable nature of circRNAs, several circRNA vaccines have been synthesised and tested both in vitro and in vivo (56). As research progresses, circRNAs are expected to open new chapters in precision medicine and become important platforms for future clinical diagnoses and treatments.
The results of this meta-analysis should be interpreted with caution owing to several constraints. First, all the analysed research originated in China. Hence, the generalisability of our findings to other populations may have been affected. Second, the diagnostic analysis was affected by the limited dataset of only three studies, and more data are needed to validate the results. Furthermore, the lack of explicit hazard ratio (HR) data in some studies required data extraction from the Kaplan-Meier (KM) curves, which could have introduced bias. Finally, the study did not incorporate bioinformatics prediction or validation of potential pathways, which represents an area for future enhancement. In the subsequent studies, we aimed to bridge this gap by integrating computational analyses to delineate the underlying mechanisms of circRNAs in CC. This approach refined our understanding of their roles and potentially uncovered novel targets for intervention, thereby enriching the existing body of research.
The results of our study indicate a significant correlation between circRNA dysregulation in patients with CC and key clinical parameters, including diagnosis, prognosis, and tumour characteristics. These findings have profound implications for biological understanding and clinical management of CC. Future research should elucidate the mechanistic roles of circRNAs as this may aid in the identification of novel therapeutic targets and foster innovative diagnostic and treatment strategies for CC.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
YX: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. CL: Funding acquisition, Investigation, Writing – review & editing. LC: Methodology, Visualization, Writing – review & editing. SW: Software, Supervision, Writing – review & editing. YW: Conceptualization, Data curation, Writing – review & editing. SL: Project administration, Visualization, Writing – review & editing. ML: Validation, Visualization, Writing – review & editing. XT: Data curation, Formal analysis, Funding acquisition, Investigation, Supervision, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the Nanchang Science and Technology Support Project (Hong Kezi (2021) 129 Item 24) and the Jiangxi Provincial Health Commission Science and Technology Plan Project (202322332).
We thank Anita Stephanie Savell, MD, from UC Davis for language support.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2024.1488040/full#supplementary-material
1. Burd EM. Human papillomavirus and cervical cancer. Clin Microbiol Rev. (2003) 16:1–17. doi: 10.1128/cmr.16.1.1-17.2003
2. Singh D, Vignat J, Lorenzoni V, Eslahi M, Ginsburg O, Lauby-Secretan B, et al. Global estimates of incidence and mortality of cervical cancer in 2020: A baseline analysis of the who global cervical cancer elimination initiative. Lancet Glob Health. (2023) 11:e197–206. doi: 10.1016/s2214-109x(22)00501-0
3. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
4. Guo M, Xu J, Du J. Trends in cervical cancer mortality in China from 1989 to 2018: an age-period-cohort study and joinpoint analysis. BMC Public Health. (2021) 21:1329. doi: 10.1186/s12889-021-11401-8
5. Koh WJ, Abu-Rustum NR, Bean S, Bradley K, Campos SM, Cho KR, et al. Cervical cancer, version 3.2019, nccn clinical practice guidelines in oncology. J Natl Compr Canc Netw. (2019) 17:64–84. doi: 10.6004/jnccn.2019.0001
6. Pecorelli S. Revised figo staging for carcinoma of the vulva, cervix, and endometrium. Int J Gynaecol Obstet. (2009) 105:103–4. doi: 10.1016/j.ijgo.2009.02.012
7. Poddar P, Maheshwari A. Surgery for cervical cancer: consensus & Controversies. Indian J Med Res. (2021) 154:284–92. doi: 10.4103/ijmr.IJMR_4240_20
8. Mayadev JS, Ke G, Mahantshetty U, Pereira MD, Tarnawski R, Toita T. Global challenges of radiotherapy for the treatment of locally advanced cervical cancer. Int J Gynecol Cancer. (2022) 32:436–45. doi: 10.1136/ijgc-2021-003001
9. Jiang K, Ai Y, Li Y, Jia L. Nomogram models for the prognosis of cervical cancer: A seer-based study. Front Oncol. (2022) 12:961678. doi: 10.3389/fonc.2022.961678
10. Chen LL. The expanding regulatory mechanisms and cellular functions of circular rnas. Nat Rev Mol Cell Biol. (2020) 21:475–90. doi: 10.1038/s41580-020-0243-y
11. Huang G, Liang M, Liu H, Huang J, Li P, Wang C, et al. Circrna hsa_Circrna_104348 promotes hepatocellular carcinoma progression through modulating mir-187-3p/rtkn2 axis and activating wnt/B-catenin pathway. Cell Death Dis. (2020) 11:1065. doi: 10.1038/s41419-020-03276-1
12. Guo X, Piao H. Research progress of circrnas in glioblastoma. Front Cell Dev Biol. (2021) 9:791892. doi: 10.3389/fcell.2021.791892
13. Zhang Y, Zhao L, Yang S, Cen Y, Zhu T, Wang L, et al. Circcdkn2b-as1 interacts with imp3 to stabilize hexokinase 2 mrna and facilitate cervical squamous cell carcinoma aerobic glycolysis progression. J Exp Clin Cancer Res. (2020) 39:281. doi: 10.1186/s13046-020-01793-7
14. Begliarzade S, Sufianov A, Ilyasova T, Shumadalova A, Sufianov R, Beylerli O, et al. Circular rna in cervical cancer: fundamental mechanism and clinical potential. Noncoding RNA Res. (2024) 9:116–24. doi: 10.1016/j.ncrna.2023.11.009
15. Jayne FT, Lesley AS, Davina G, Sarah B, Matthew RS. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. (2007) 8:16. doi: 10.1186/1745-6215-8-16
16. Ruo W, Qigu Y, Wenyi C, Feiqiong G, Pan L, Jian W, et al. Stem cell therapy for crohn's disease: systematic review and meta-analysis of preclinical and clinical studies. Stem Cell Res Ther. (2021) 12:463. doi: 10.1186/s13287-021-02533-0
17. Chen LQ, Zhang XW, Wang S, Lin XT, Xu LZ. Circ_0084927 facilitates cervical cancer development via sponging mir-142-3p and upregulating arl2. Cancer Manage Res. (2020) 12:9271–83. doi: 10.2147/cmar.S263596
18. Ding L, Zhang HT. Circ-atp8a2 promotes cell proliferation and invasion as a cerna to target egfr by sponging mir-433 in cervical cancer. Gene. (2019) 705:103–8. doi: 10.1016/j.gene.2019.04.068
19. Ding Y, Yuan X, Gu WW. Circular rna rbm33 contributes to cervical cancer progression via modulation of the mir-758-3p/pum2 axis. J Mol Histol. (2021) 52:173–85. doi: 10.1007/s10735-020-09933-1
20. Fan S, Zhao S, Gao X, Qin Q, Guo Y, Yuan Z, et al. Circular rna circgse1 promotes cervical cancer progression through mir-138-5p/vimentin. Onco Targets Ther. (2020) 13:13371–86. doi: 10.2147/ott.S282425
21. He J, Lv X, Zeng Z. A potential disease monitoring and prognostic biomarker in cervical cancer patients: the clinical application of circular rna_0018289. J Clin Lab Anal. (2020) 34:e23340. doi: 10.1002/jcla.23340
22. Hong HQ, Zhu H, Zhao SJ, Wang KL, Zhang N, Tian Y, et al. The novel circclk3/mir-320a/foxm1 axis promotes cervical cancer progression. Cell Death Dis. (2019) 10:950. doi: 10.1038/s41419-019-2183-z
23. Ji F, Du R, Chen TF, Zhang M, Zhu YF, Luo X, et al. Circular rna circslc26a4 accelerates cervical cancer progression via mir-1287-5p/hoxa7 axis. Mol Therapy-Nucleic Acids. (2020) 19:413–20. doi: 10.1016/j.omtn.2019.11.032
24. Ji F, Lu Y, Chen S, Yu Y, Lin X, Zhu Y, et al. Igf2bp2-modified circular rna circarhgap12 promotes cervical cancer progression by interacting M(6)a/foxm1 manner. Cell Death Discovery. (2021) 7:215. doi: 10.1038/s41420-021-00595-w
25. Li HL, Liang DX, Wang QY, Jiang P, Zhu JY, Guo Z. Expression of circprmt5 and ezh2 in cervical cancer tissues and its relationship with prognosis. Chin J Gerontology. (2023) 43:5411–5. doi: 10.3969/j.issn.1005-9202.2023.22.011
26. Li Y, Meng FD, Sui CG, Wang Y, Cheng DL. Circrna hsa_Circ_0001627 aggravates cervical cancer progression through upregulation of fndc3b and activating pi3k/mtor signaling pathway. J Cell Communication Signaling. (2023) 17:627–38. doi: 10.1007/s12079-022-00696-w
27. Mao XB, Wang XH, Qiu B, Li ML, Mao D, Jiang JP. Expression and clinical significance of cyclic rna circ:0010423 in cervical cancer. Oncol Prog J. (2023) 21:2150–4. doi: 10.11877/j.issn.1672-1535.2023.21.19.16
28. Mao YF, Zhang LY, Li Y. Circeif4g2 modulates the Malignant features of cervical cancer via the mir-218/hoxa1 pathway. Mol Med Rep. (2019) 19:3714–22. doi: 10.3892/mmr.2019.10032
29. Shi PJ, Zhang XY, Lou CX, Xue YX, Guo RB, Chen SZ. Hsa_Circ_0084927 regulates cervical cancer advancement via regulation of the mir-634/tpd52 axis. Cancer Manage Res. (2020) 12:9435–48. doi: 10.2147/cmar.S272478
30. Song TF, Xu AL, Zhang ZF, Gao F, Zhao L, Chen XH, et al. Circrna hsa_Circrna_101996 increases cervical cancer proliferation and invasion through activating tpx2 expression by restraining mir-8075. J Cell Physiol. (2019) 234:14296–305. doi: 10.1002/jcp.28128
31. Tian J, Wang C, Cheng HL, Wang N, Cao QX. The novel circular rna circ-pgap3 retards cervical cancer growth by regulating the mir-769-5p/P53 axis. Hum Cell. (2021) 34:878–88. doi: 10.1007/s13577-021-00493-4
32. Wang YT, Wang L, Wang WW, Guo X. Overexpression of circular rna hsa_Circ_0001038 promotes cervical cancer cell progression by acting as a cerna for mir-337-3p to regulate cyclin-M3 and metastasis-associated in colon cancer 1 expression. Gene. (2020) 733:144273. doi: 10.1016/j.gene.2019.144273
33. Wang Z, Chen Y, Wang W, Wang H, Liu R. Circmyc promotes cell proliferation, metastasis, and glycolysis in cervical cancer by up-regulating met and sponging mir-577. Am J Trans Res. (2021) 13:6043–54. doi: 10.34306343
34. Wu LL, Xiao HQ, Hong YQ, Xie MH, Yu YX, Jiang LJ. Circrna circ_0000118 regulates Malignancy of cervical cancer cells by regulating mir-211-5p/mir-377-3p/akt2 axis. Biochem Genet. (2023) 61:1625–44. doi: 10.1007/s10528-023-10332-w
35. Yao ZL, Shu LP, Yi Y, Qiao LF. Hsa_Circrna_000543 predicts poor prognosis and promotes cervical cancer cell progression through regulating mir-567/znf268 axis. Cancer Manage Res. (2021) 13:5211–22. doi: 10.2147/cmar.S302201
36. Yuan DD, Jia CD, Yan MY, Wang J. Circular rna hsa_Circ_0000730 restrains cell proliferation, migration, and invasion in cervical cancer through mir-942-5p/pten axis. Kaohsiung J Med Sci. (2021) 37:964–72. doi: 10.1002/kjm2.12443
37. Zhang CY, Jiang HY, Yuan L, Liao YD, Liu P, Du QQ, et al. Circvprbp inhibits nodal metastasis of cervical cancer by impeding rack1 O-glcnacylation and stability. Oncogene. (2023) 42:793–807. doi: 10.1038/s41388-023-02595-9
38. Zhang CY, Liu P, Huang JM, Liao YD, Pan CY, Liu JX, et al. Circular rna hsa_Circ_0043280 inhibits cervical cancer tumor growth and metastasis via mir-203a-3p/paqr3 axis. Cell Death Dis. (2021) 12:888. doi: 10.1038/s41419-021-04193-7
39. Zhang JH, Zhao XY, Zhang J, Zheng XR, Li FX. Circular rna hsa_Circ_0023404 exerts an oncogenic role in cervical cancer through regulating mir-136/tfcp2/yap pathway. Biochem Biophys Res Commun. (2018) 501:428–33. doi: 10.1016/j.bbrc.2018.05.006
40. Liao W, He J, Disoma C, Hu Y, Li J, Chen G, et al. Hsa_Circ_0107593 suppresses the progression of cervical cancer via sponging hsa-mir-20a-5p/93-5p/106b-5p. Front Oncol. (2020) 10:590627. doi: 10.3389/fonc.2020.590627
41. Zhou MY, Yang Z, Wang DB, Chen P, Zhang Y. The circular rna circzfr phosphorylates rb promoting cervical cancer progression by regulating the ssbp1/cdk2/cyclin E1 complex. J Exp Clin Cancer Res. (2021) 40:48. doi: 10.1186/s13046-021-01849-2
42. Liu JM, Wang DB, Long ZQ, Liu J, Li WS. Circrna8924 promotes cervical cancer cell proliferation, migration and invasion by competitively binding to mir-518d-5p /519-5p family and modulating the expression of cbx8. Cell Physiol Biochem. (2018) 48:173–84. doi: 10.1159/000491716
43. Olusola P, Banerjee HN, Philley JV, Dasgupta S. Human papilloma virus-associated cervical cancer and health disparities. Cells. (2019) 8:622. doi: 10.3390/cells8060622
45. Stolnicu S, Barsan I, Hoang L, Patel P, Terinte C, Pesci A, et al. International endocervical adenocarcinoma criteria and classification (Iecc): A new pathogenetic classification for invasive adenocarcinomas of the endocervix. Am J Surg Pathol. (2018) 42:214–26. doi: 10.1097/pas.0000000000000986
46. Ferrall L, Lin KY, Roden RBS, Hung CF, Wu TC. Cervical cancer immunotherapy: facts and hopes. Clin Cancer Res. (2021) 27:4953–73. doi: 10.1158/1078-0432.Ccr-20-2833
47. Chen S, Yang X, Yu C, Zhou W, Xia Q, Liu Y, et al. The potential of circrna as a novel diagnostic biomarker in cervical cancer. J Oncol. (2021) 2021:5529486. doi: 10.1155/2021/5529486
48. Liu F, Wu X, Zhu H, Wang F. Dysregulated expression of circular rnas serve as diagnostic and prognostic markers in ovarian and cervical cancer: A prisma-compliant systematic review and meta-analysis. Med (Baltimore). (2021) 100:e27352. doi: 10.1097/md.0000000000027352
49. Chaichian S, Shafabakhsh R, Mirhashemi SM, Moazzami B, Asemi Z. Circular rnas: A novel biomarker for cervical cancer. J Cell Physiol. (2020) 235:718–24. doi: 10.1002/jcp.29009
50. Najafi S. Circular rnas as emerging players in cervical cancer tumorigenesis; a review to roles and biomarker potentials. Int J Biol Macromol. (2022) 206:939–53. doi: 10.1016/j.ijbiomac.2022.03.103
51. Luo H, Li Y, Zhao Y, Chang J, Zhang X, Zou B, et al. Comprehensive analysis of circrna expression profiles during cervical carcinogenesis. Front Oncol. (2021) 11:676609. doi: 10.3389/fonc.2021.676609
52. Zhou Y, Shen L, Wang YZ, Zhou CC. The potential of cirs-7 for predicting onset and prognosis of cervical cancer. Neoplasma. (2020) 67:312–22. doi: 10.4149/neo_2019_190415N334
53. Zou Y, Yang A, Chen B, Deng X, Xie J, Dai D, et al. Crvdac3 alleviates ferroptosis by impeding hspb1 ubiquitination and confers trastuzumab deruxtecan resistance in her2-low breast cancer. Drug Resist Update. (2024) 77:101126. doi: 10.1016/j.drup.2024.101126
54. To KKW, Zhang H, Cho WC. Competing endogenous rnas (Cernas) and drug resistance to cancer therapy. Cancer Drug Resist. (2024) 7:37. doi: 10.20517/cdr.2024.66
55. Marangon D, Lecca D. Exosomal non-coding rnas in glioma progression: insights into tumor microenvironment dynamics and therapeutic implications. Front Cell Dev Biol. (2023) 11:1275755. doi: 10.3389/fcell.2023.1275755
Keywords: cervical cancer, circular RNAs, meta-analysis, diagnosis, prognosis, clinicopathological
Citation: Xu Y, Li C, Cheng L, Wang S, Wu Y, Li S, Liu M and Tao X (2025) Prognostic and diagnostic value of circRNA expression in cervical cancer: a meta analysis. Front. Oncol. 14:1488040. doi: 10.3389/fonc.2024.1488040
Received: 29 August 2024; Accepted: 13 December 2024;
Published: 13 January 2025.
Edited by:
Sujit Nair, Phytoveda Pvt. Ltd, IndiaReviewed by:
Xiaoqian Hu, The University of Hong Kong, Hong Kong SAR, ChinaCopyright © 2025 Xu, Li, Cheng, Wang, Wu, Li, Liu and Tao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaohua Tao, dGFveGlhb2h1YUAxMjYuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.