Abstract
Objective:
The value of robotic pancreaticoduodenectomy (RPD) compared with laparoscopic pancreaticoduodenectomy (LPD) for pancreatic and periampullary tumors is controversial. This study aims to assess the available literature and compare the short outcomes of RPD and LPD.
Methods:
The PubMed, Cochrane Library, Embase, and Web of Science databases were searched to identify available research published up to 24 July, 2024. Risk ratios (RRs) and mean differences (MDs) with 95% confidence intervals (CIs) were calculated.
Results:
Seventeen studies with a total of 9417 patients (RPD group: 3334 patients; LPD group: 6083 patients) were included in this meta-analysis. The RPD group had lower overall morbidity (RR, 0.79), conversion (RR, 0.29) and blood transfusion rates (RR, 0.61), shorter length of stay (MD, -0.72 days), and higher number of harvested lymph nodes (MD, 0.62) than the LPD group. There were no significant differences in 90-day mortality (RR, 0.89), major complications (RR, 0.87), operative time (MD, -3.74 mins), blood loss (MD, -24.14 mL), reoperation (RR, 0.94), bile leak (RR, 0.62), postoperative pancreatic hemorrhage (RR, 0.96), postoperative pancreatic fistula (RR, 0.74), delayed gastric emptying (RR, 1.24), and R0 resection (RR, 1.00) between the groups.
Conclusions:
Compared with LPD, RPD for pancreatic and periampullary tumors could be safe and effective, and it has superior surgical outcomes. Further randomized controlled trials to verify the potential advantages of RPD over LPD are necessary.
Systematic review registration:
https://www.crd.york.ac.uk/PROSPERO/display_record.php?RecordID=581133, identifier CRD42024581133.
1 Introduction
Pancreaticoduodenectomy (PD) is a standard procedure for pancreatic and periampullary tumors (1, 2). PD is considered one of the most challenging procedures in hepatobiliary and pancreatic surgery due to the complex internal organ anatomy and digestive tract reconstruction required (1, 3). In recent years, despite improvements in surgical techniques and perioperative management, the postoperative complication rate of open PD remains as high as 46% in high-volume centers (4). Postoperative complications not only increase the economic burden of patients, but also damage the long-term survival of patients (5, 6).
Minimally invasive surgery (such as laparoscopic and robotic surgery) is a potential strategy to reduce perioperative morbidity due to less trauma, lower intraoperative blood loss and faster postoperative recovery (7). It has been widely used and offers proven advantages over open techniques in terms of short-term outcomes for various abdominal surgeries such as gastrectomy, colorectal surgery and prostate cancer surgery (8–11). Similarly, minimally invasive surgery has been increasingly used in pancreatic surgery in recent years. Compared to laparoscopic surgery, the robotic platform can provide more flexible operating instruments and a clearer and wider field of view (1). In theory, these advantages of robotic surgery could lead to better surgical outcomes. However, some recent clinical studies comparing robotic pancreaticoduodenectomy (RPD) and laparoscopic pancreaticoduodenectomy (LPD) have had conflicting results. The study by Farah et al. (12) showed that RPD has fewer complications and lower perioperative mortality compared to LPD. Zhang et al. ‘s study (13) included 2255 patients receiving PD, and the results showed no significant difference in postoperative morbidity and mortality between the RPD group and the LPD group. Unfortunately, systematic reviews and meta-analyses comparing the short-term outcomes of RPD and LPD in pancreatic and periampullary tumors are still lacking.
Therefore, we conducted a comprehensive collection of the currently published evidence and performed a meta-analysis to compare the efficacy and safety of RPD and LPD in the treatment of pancreatic and periampullary tumors. These results may help provide a valuable reference for surgeons in selecting surgical approaches.
2 Methods
2.1 Search strategy
This meta-analysis was follows the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (14). The study was registered in the PROSPERO database.
Two investigators independently conducted a comprehensive literature search using the Web of Science, PubMed, EMBASE, and Cochrane Library databases to identify studies published before 24 July, 2024. The details of the searching record were presented in Table 1. In addition, we checked the reference lists of the identified articles and related reviews to further screen for eligible studies. No language restrictions were applied during the search process.
Table 1
| Database | Search term | Number |
|---|---|---|
| PubMed (Title/Abstract) | #1: da Vinci OR robot* OR robot-assisted OR robotic-assisted | #1: 82243 |
| #2: laparoscopy OR Laparoscop* | #2: 16122 | |
| #3: pancreatoduodenectomy OR Pancreaticoduodenectom* OR Duodenopancreatectom* OR Whipple OR Whipple’s procedure OR Kausch-Whipple OR Kausch-Whipple procedure | #3: 18114 | |
| #4: #1 AND #2 AND #3 | #4: 314 | |
| Embase (Title Abstract Keyword) |
#1: pancreatoduodenectomy OR Pancreaticoduodenectom* OR Duodenopancreatectom* OR Whipple’s procedure OR Kausch-Whipple OR Kausch-Whipple procedure | #1: 23167 |
| #2: Da Vinci OR Robot* OR Robot-assisted OR Robotic-assisted | #2: 118850 | |
| #3: laparoscopy or Laparoscop* | #3: 264201 | |
| #4: #1 AND #2 AND #3 | #4: 561 | |
| Cochrane Library (Title Abstract Keyword) | #1: (Pancreatoduodenectomy) OR (Pancreaticoduodenectom*) OR (Duodenopancreatectom*) OR (Whipple’s procedure) OR (Kausch-Whipple) OR (Kausch-Whipple procedure) | #1: 1437 |
| #2: (Da Vinci) OR Robot* OR Robot-assisted OR Robotic-assisted | #2: 7976 | |
| #3: laparoscopy OR Laparoscop* | #3: 28629 | |
| #4: #1 AND #2 AND #3 | #4: 29 | |
| Web of Science (Topic) |
#1:(Da Vinci) OR (Robot*) OR (Robot-assisted) OR (Robotic-assisted) | #1: 558243 |
| #2: (laparoscopy) OR (Laparoscop*) | #2: 247721 | |
| #3:(Pancreatoduodenectomy) OR (Pancreaticoduodenectom*) OR (Duodenopancreatectom*) OR (Whipple’s procedure) OR (Kausch-Whipple) OR (Kausch-Whipple procedure) | #3: 23551 | |
| #4: #1 AND #2 AND #3 | #4: 601 |
Electronic search strategy.
2.2 Study selection
Inclusion criteria were as follows (1): Patient: Patients diagnosed with pancreatic or periampullary (distal bile duct, ampulla, and duodenum) tumors (2); Intervention: RPD (3); Comparison: LPD (4); Outcomes: Primary outcomes encompassed 90-day mortality, overall morbidity, major complications, and length of stay. Secondary outcomes included blood loss, operative time, conversion, reoperation, bile leak, postoperative pancreatic fistula (POPF), postoperative pancreatic hemorrhage, delayed gastric emptying, blood transfusion, number of lymph nodes harvested, and R0 resection (5); Study type: randomized controlled trials (RCTs), cohort studies, and case-control studies.
The exclusion criteria were as follows: single-arm studies, animal studies, repeated publications, reviews, case reports, conference abstracts, and letters were excluded.
2.3 Data extraction
Data from all eligible studies were independently extracted by two investigators, and any disagreements were resolved by discussion with a third-party independent reviewer. The following data were extracted: author name, year of publication, country, study design, study population (sample size, age, and sex), and outcomes (90-day mortality, morbidity, length of stay, blood loss, operative time, conversion, reoperation, bile leak, POPF, postoperative pancreatic hemorrhage, delayed gastric emptying, blood transfusion, number of lymph nodes harvested, and R0 resection).
2.4 Quality assessment
The risk of bias in RCTs was assessed independently by two authors using the Cochrane risk-of-bias tool 2 (15) (1): randomization process (2), deviations from intended interventions (3), missing outcome data (4), measurement of the outcome (5), selection of reported results, and (6) overall risk of bias. For non-RCTs, the quality assessment was conducted independently by two authors using the Newcastle-Ottawa Scale (NOS), which assigns a score on a 9-point scale. A score of ≥7 indicates high quality, and scores of 5–6 indicate moderate quality. Any discrepancies were resolved through discussion, with intervention by a third author whenever necessary.
2.5 Statistical analysis
The meta-analysis was performed using the Review manager 5.3. Mean difference (MD) with corresponding 95% confidence intervals (CI) were calculated for quantitative data and risk ratios (RR) for qualitative variables. The I² statistic was used to assess the degree of statistical heterogeneity between included studies. A random-effects model was used if I² > 50%; otherwise, a fixed-effects model was employed (16). To explore the robustness of the results, we adopted the one-study exclusion method to evaluate the impact of each study on the total effect size. The potential publication bias was assessed using funnel plot and Egger’s tests if 10 or more studies were identified. Statistical significance was set at P value < 0.05.
3 Results
3.1 Literature retrieval
A total of 1508 articles were retrieved from four databases, and 488 duplicates were excluded. After reviewing titles and abstracts, 972 studies were excluded, and the full texts of the remaining 48 studies were evaluated. Finally, 17 studies (12, 13, 17–31) were included in the final analysis (Figure 1).
Figure 1
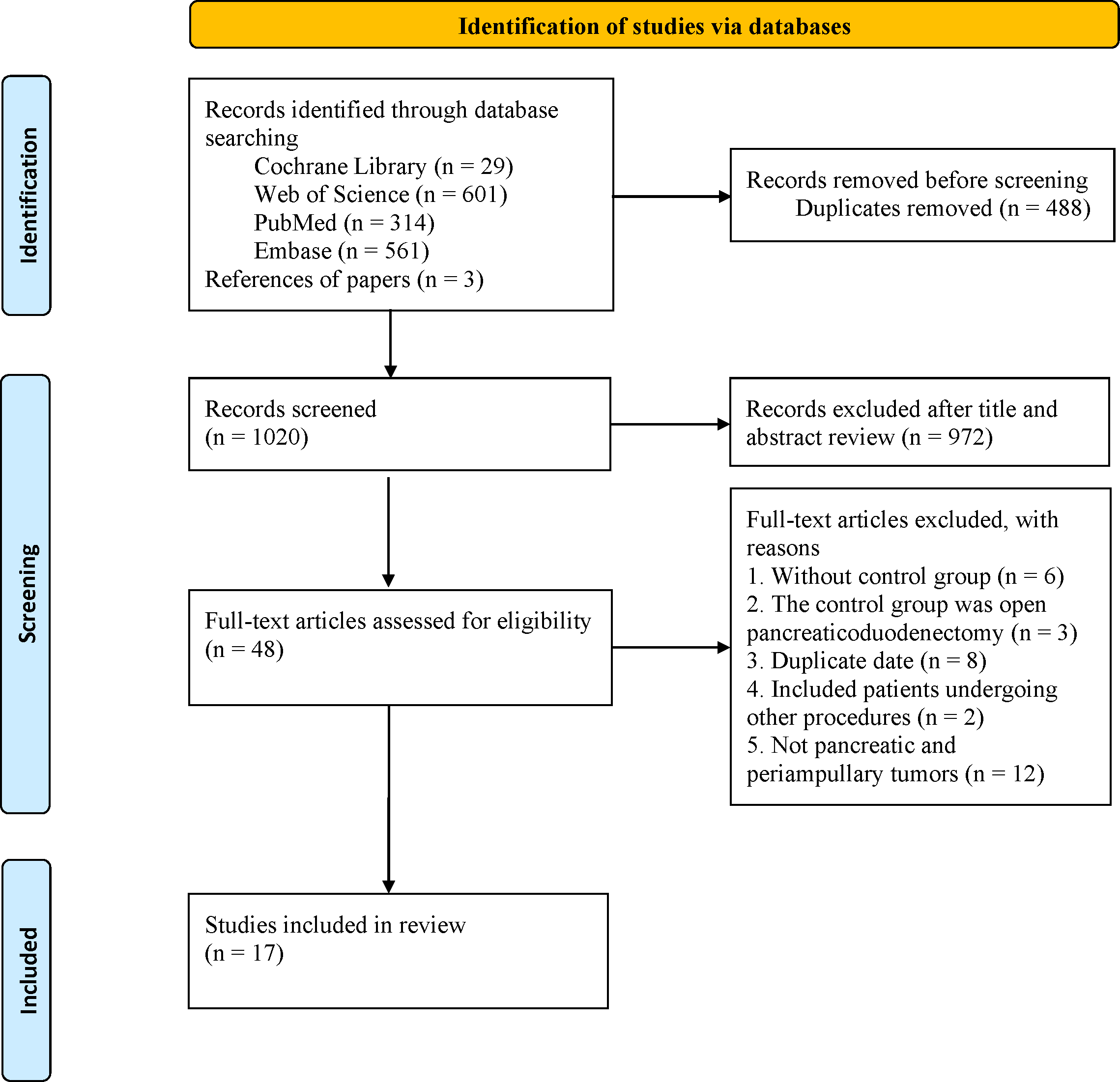
The PRISMA flowchart.
3.2 Study characteristics and quality assessment
The main characteristics of the included studies (12, 13, 17–30) are summarized in Table 2. The studies were published between 2016 and 2024 and included 15812 patients (RPD group: 3334 patients; LPD group: 6083 patients). Four of the studies adopted the PSM design (13, 23, 30, 31). The included patients were mainly from China (13, 17, 19, 22, 25, 29), the United States (23, 26, 30), Korea (21, 27, 31), UK (12, 20), Russia (24), and Singapore (18). All studies (12, 13, 17–31) were considered of moderate to high quality, achieving a score of ≥6 based on the NOS.
Table 2
| Author, year | Country | Period of study | Male | Study design | Age | Sample size | Robotic platforms | Included diseases | NOS |
|---|---|---|---|---|---|---|---|---|---|
| Liu 2017 (17) | China | 2015-2016 | RPD:14 LPD: 12 |
RCS | RPD: 57.16(68.56) LPD: 60.54(18.25) |
RPD: 27 LPD: 25 |
The da Vinci®S Surgical System (Intuitive Surgical Inc., Sunnyvale, CA) | Periampullary neoplasms (9 benign lesions and 43 malignant lesions) | 6/9 |
| Goh 2019 (18) | Singapore | 2014-2017 | RPD:5 LPD: 16 |
RCS | RPD: 70(24-79) LPD: 62.5(24-79) |
RPD: 10 LPD: 20 |
The da Vinci Si robotic platform | Periampullary tumours (7 benign lesions and 23 malignant lesions) | 7/9 |
| Zhang 2018 (19) | China | 2013-2017 | RPD:12 LPD: 11 |
RCS | RPD: 68(50-78) LPD: 64(42-76) |
RPD: 20 LPD: 20 |
NA | Periampullary tumors (6 benign lesions and 34 malignant lesions) | 7/9 |
| Gall 2020 (20) | UK | 2017-2019 | RPD: 16 LPD: 19 |
RCS | RPD: 60.93(12.52) LPD: 65.18(11.36) |
RPD: 25 LPD: 41 |
The Da Vinci Si and Xi models |
Malignancies of the pancreatic head | 7/9 |
| Park 2021 (21) | Korea | 2016-2020 | RPD: 26 LPD: 30 |
RCS | RPD: 66.65(10.97) LPD: 65.70(12.97) |
RPD: 49 LPD: 43 |
The da Vinci Xi Surgical System (Intuitive Surgical Inc., Sunnyvale, CA, USA) | Tumors confined to the pancreatic head or periampullary region (16 benign lesions and 76 malignant lesions) | 8/9 |
| Choi 2022 (31) | Korea | 2012-2020 | RPD: 26 LPD: 29 |
RCS, PSM | RPD: 60.02(11.97) LPD: 60.42(11.14) |
RPD: 50 LPD: 50 |
NA | Periampullary tumors (44 benign lesions and 56 malignant lesions) | 8/9 |
| Guo 2022 (22) | China | 2016-2020 | RPD: 21 LPD: 12 |
RCS | RPD: 53.7(14.4) LPD: 52.1(13.5) |
RPD: 32 LPD: 21 |
NA | Periampullary tumors (14 benign lesions and 39 malignant lesions) | 6/9 |
| Naffouje 2022 (23) | USA | 2004-2017 | RPD: 181 LPD: 553 |
RCS, PSM | RPD: 67.79(10.69) LPD: 67.86(10.31) |
RPD: 358 LPD: 1074 |
NA | Stage I–III (T1–3 Nany M0) pancreatic adenocarcinoma | 9/9 |
| Tyutyunnik 2022 (24) | Russia | 2007-2015 | RPD: 43 LPD: 42 |
RCS | RPD: 62.5 (25–84) LPD: 62(34-82) |
RPD: 100 LPD: 100 |
NA | Malignant and benign tumors of the head of the pancreas and periampullary area (57 benign lesions and 143 malignant lesions) | 7/9 |
| Zong 2022 (25) | China | 2018-2022 | RPD: 36 LPD: 77 |
RCS | RPD: 58.2(1.7) LPD: 58.1(1.4) |
RPD: 76 LPD: 114 |
The Da Vinci Si Surgical System (Intuitive Surgical, Sunnyvale, CA, USA). |
Periampullary benign or malignant tumors (42 benign lesions and 148 malignant lesions) | 7/9 |
| Kalabin 2023 (26) | USA | 2010-2018 | RPD: 347 LPD: 1390 |
RCS | RPD: 65.36(64.47-66.25) LPD: 64.97(64.55-65.39) |
RPD: 676 LPD: 2677 |
NA | Pancreatic adenocarcinoma | 7/9 |
| Lee 2023 (27) | Korea | 2015-2019 | RPD: 10 LPD: 28 |
RCS | RPD: 57.7(11.6) LPD: 68.2(8.5) |
RPD: 21 LPD: 60 |
NA | Distal bile duct cancer | 7/9 |
| Uijterwijk 2023 (28) | 8 centers (6 in Europe, 1 in Australia, and 1 in Asia) | 2010-2021 | RPD: NA LPD: NA |
RCS | RPD: NA LPD: NA |
RPD: 37 LPD: 53 |
NA | Distal cholangiocarcinoma | 6/9 |
| Zhang 2023 (13) | China | 2015-2022 | RPD: 612 LPD: 622 |
RCS, PSM | RPD: 60.5(52.0-67.0) LPD: 61.0(52.0-67.0) |
RPD: 1006 LPD: 1006 |
NA | Benign, premalignant, or resectable malignant or borderline resectable tumors of the pancreatic and periampullary region (383 benign lesions and 1629 malignant lesions) | 9/9 |
| Dai 2024 (29) | China | 2016-2023 | RPD: 27 LPD: 32 |
RCS | RPD: 59.8(10.6) LPD: 60.5(12.2) |
RPD: 47 LPD: 54 |
The da Vinci Xi (or Si) Surgical System | Pancreatic cancer | 8/9 |
| Farah 2024 (12) | UK | 2014-2021 | RPD: NA LPD: NA |
RCS | RPD: NA LPD: NA |
RPD: 175 LPD: 100 |
NA | Pancreatic cancer | 7/9 |
| Wehrle 2024 (30) | USA | 2010-2020 | RPD: 323 LPD: 332 |
RCS, PSM | RPD: 66.5(10.4) LPD: 65.6(10.1) |
RPD: 625 LPD: 625 |
NA | Pancreatic cancer | 9/9 |
Study Characteristics of the 17 included studies.
LPD, laparoscopic pancreaticoduodenectomy; NA, not available; PSM, propensity score matching; RCS, retrospective cohort study; RPD, robotic pancreaticoduodenectomy.
3.3 Meta-analysis
3.3.1 90-day mortality
Seven studies (18, 20–22, 24, 26, 29) reported data on 90-day mortality. The combined results of the 7 studies showed that there was no significant difference between the RPD group and the LPD group regarding this outcome with low heterogeneity (RR 0.89, 95% CI 0.59, 1.36; Heterogeneity: I2 = 0%, P = 0.85) (Figure 2A) (Table 3).
Figure 2
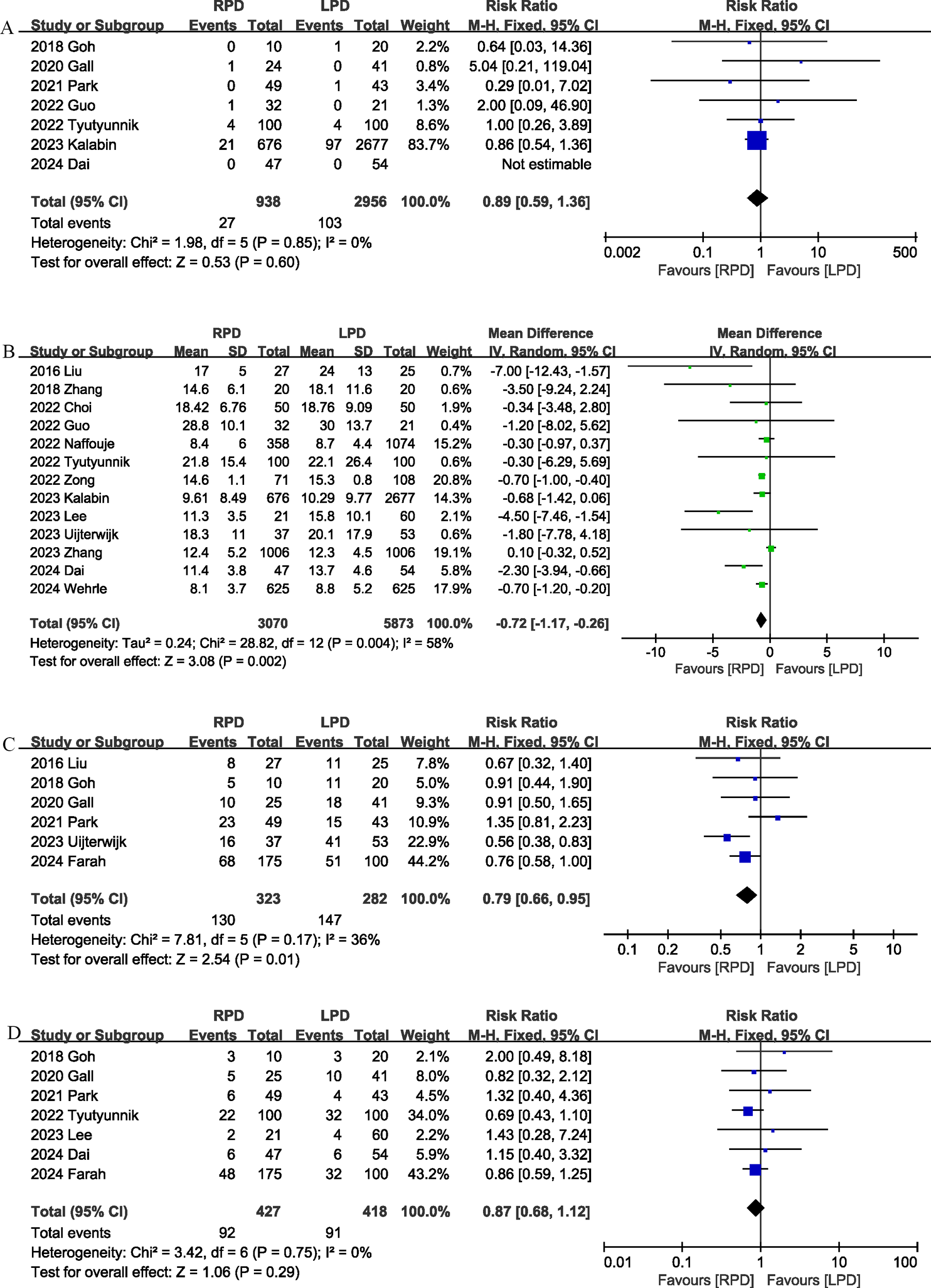
Comparison of primary outcomes between the two groups. (A) 90-day mortality, (B) length of stay, (C) overall morbidity, and (D) major complications.
Table 3
| Outcomes | No. of studies | Events for RPD | Events for LPD | Effect size | 95%CI | P | I2 (%) |
|---|---|---|---|---|---|---|---|
| Overall complications | 6 | 130/323 | 147/282 | 0.79 | 0.66, 0.95 | 0.01 | 36 |
| 90-day Mortality | 7 | 27/938 | 103/2956 | 0.89 | 0.59, 1.36 | 0.60 | 0 |
| Major complications | 7 | 92/427 | 91/418 | 0.87 | 0.68, 1.12 | 0.29 | 0 |
| Postoperative pancreatic fistula | 11 | 66/585 | 97/616 | 0.74 | 0.55, 1.00 | 0.05 | 0 |
| Bile leak | 8 | 22/383 | 39/424 | 0.62 | 0.37, 1.04 | 0.07 | 0 |
| Delayed gastric emptying | 8 | 95/526 | 63/503 | 1.24 | 0.67, 2.29 | 0.49 | 68 |
| Postoperative pancreatic hemorrhage | 5 | 25/216 | 28/219 | 0.96 | 0.57, 1.61 | 0.87 | 1 |
| Blood transfusion | 7 | 55/439 | 91/482 | 0.61 | 0.45, 0.83 | 0.002 | 27 |
| R0 resection | 6 | 717/896 | 2380/2957 | 1.00 | 0.93, 1.08 | 0.94 | 65 |
| Reoperation | 8 | 9/279 | 11/330 | 0.94 | 0.44, 2.02 | 0.87 | 0 |
| Conversion | 8 | 33/492 | 94/475 | 0.29 | 0.20, 0.42 | <0.00001 | 31 |
| Blood loss | 13 | – | – | -24.14 | -55.98, 7.71 | 0.14 | 83 |
| Operation time | 11 | – | – | -3.74 | -22.74, 15.26 | 0.70 | 94 |
| Number of lymph nodes harvested | 8 | – | – | 0.62 | 0.28, 0.95 | 0.0003 | 36 |
| Hospital stay | 13 | – | – | -0.72 | -1.17, -0.26 | 0.002 | 58 |
Summary of results from all outcomes.
3.3.2 Length of stay
The length of the hospital stay was reported in 13 studies (13, 17, 19, 22–31). According to the results of this meta-analysis, RPD significantly reduced the length of hospital stay (MD, -0.72 days; 95% CI, -1.17, -0.26, P = 0.002) (Figure 2B).
3.3.3 Morbidity
Six studies (12, 17, 18, 20, 21, 28) assessed overall complication. The pooled results suggested that RPD significantly reduced the overall complication rate (RR 0.79, 95% CI 0.66, 0.95, P = 0.01), with low heterogeneity (I2 = 36%, P = 0.17) (Figure 2C). Combined data from 7 studies (12, 18, 20, 21, 24, 27, 29) showed that the rates of major complications (Clavien–Dindo ≥ 3) were comparable between the RPD and LPD groups (RR 0.87, 95% CI 0.68, 1.12; Heterogeneity: I2 = 0%, P = 0.75) (Figure 2D).
3.3.4 Blood loss
Thirteen studies (13, 17–22, 24, 25, 27–29, 31) provided information on intraoperative blood loss. The combined results showed that the intraoperative blood loss was similar between the RPD group and the LPD group (MD, -24.14 mL; 95% CI, -55.98, 7.71, P = 0.14; I2 = 83%) (Figure 3A).
Figure 3
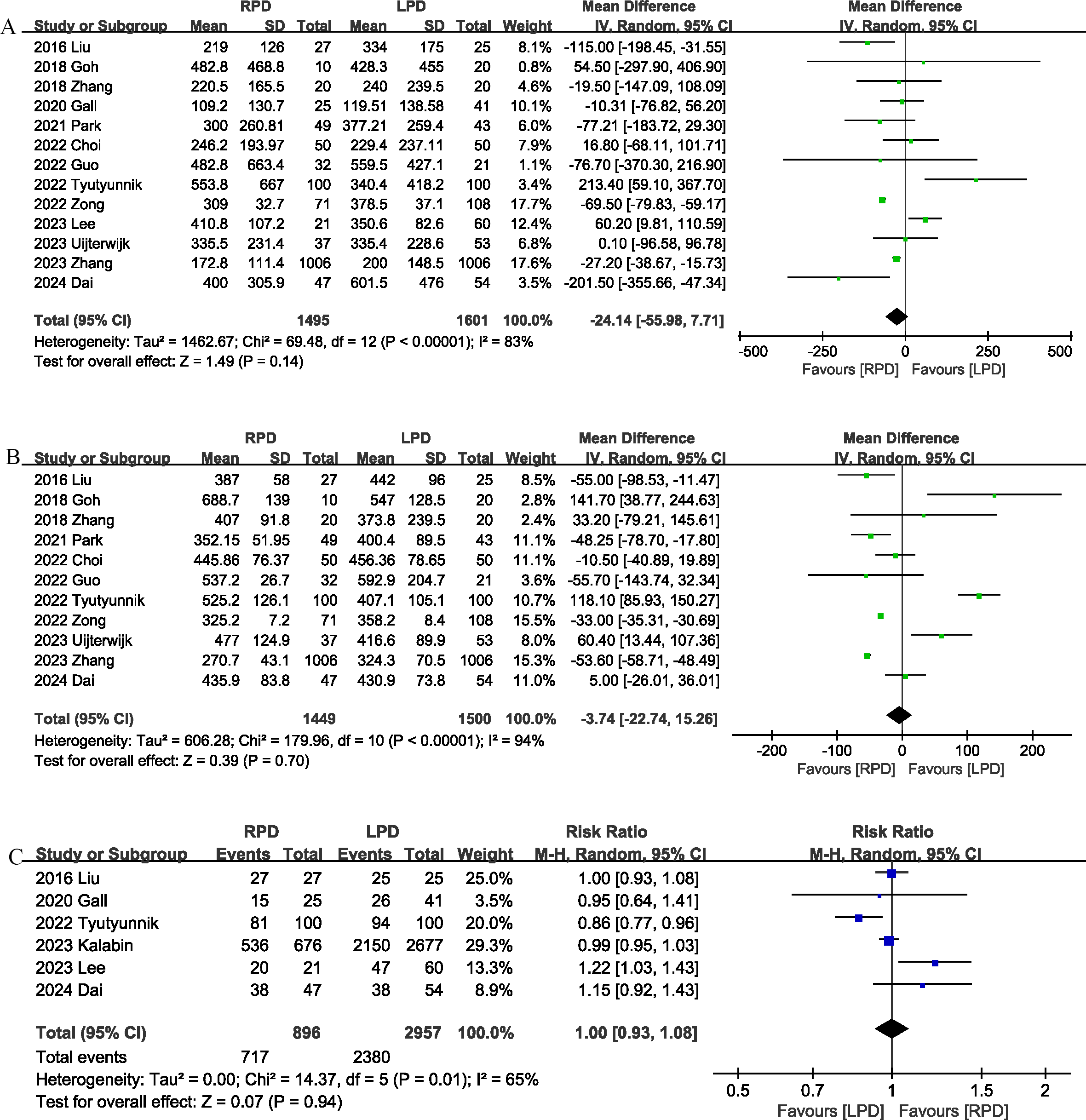
Comparison of secondary outcomes between the two groups. (A) intraoperative blood loss, (B) operative time, and (C) R0 resection.
3.3.5 Operation time
The operation time was reported in 11 studies (13, 17–19, 21, 22, 24, 25, 28, 29, 31). The combined results showed that the RPD group has similar operation time as compared with the LPD group (MD, -3.74 mins; 95% CI, -22.74, 15.26, P = 0.70) (Figure 3B).
3.3.6 R0 resection
R0 resection was reported in 6 studies (17, 20, 24, 26, 27, 29), and the combined effect size suggested that the R0 resection rates were comparable between the two groups (RR 1.00, 95% CI 0.93, 1.08, P = 0.94; I2 = 65%) (Figure 3C).
3.3.7 Number of lymph nodes harvested
Eight trials (13, 17, 22, 23, 26, 28–30) reported the number of lymph nodes harvested. Compared with LPD, RPD significantly increased the number of lymph nodes harvested (MD, 0.62; 95% CI, 0.28, 0.95, P = 0.0003; I2 = 36%) (Figure 4A).
Figure 4
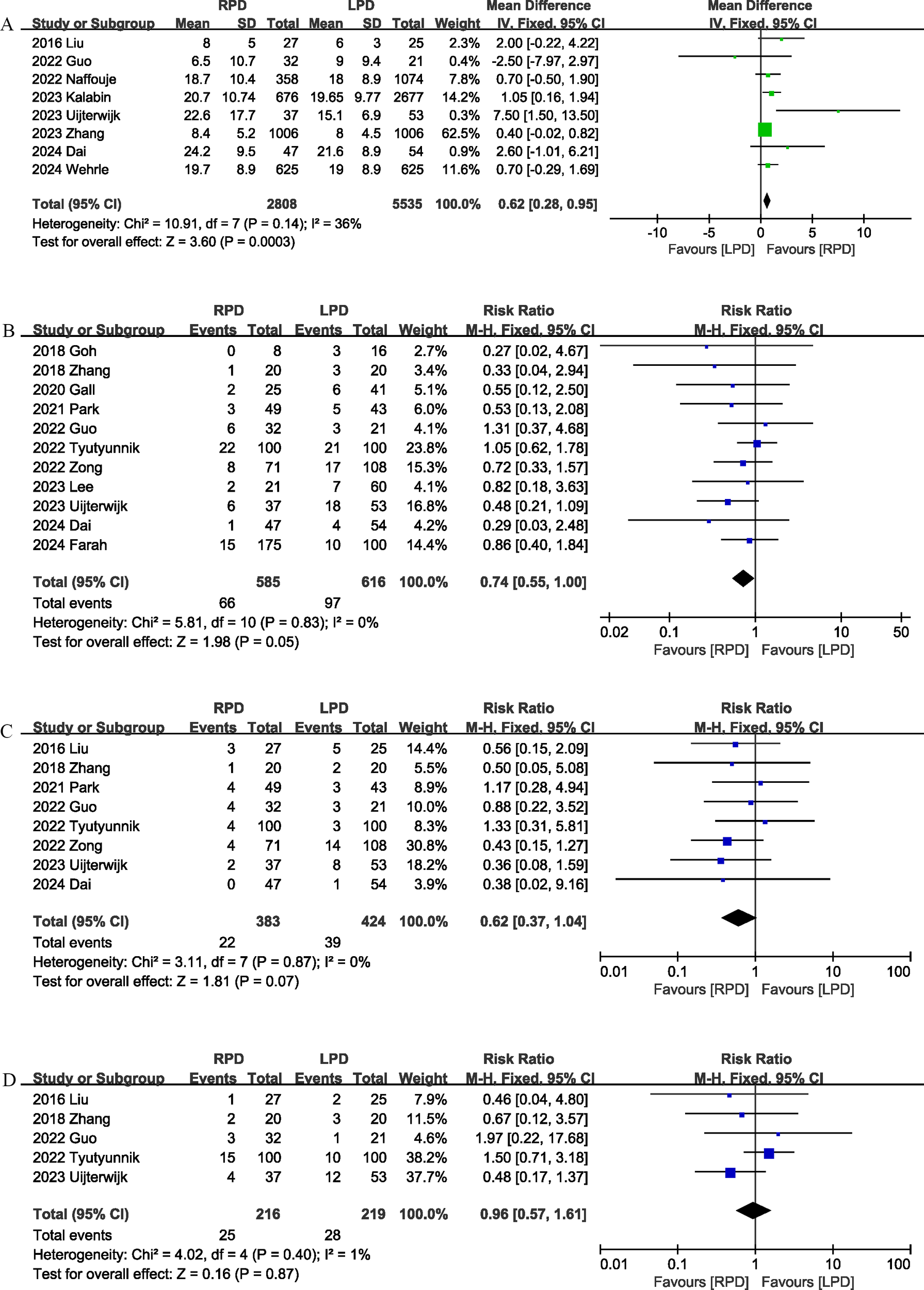
Comparison of secondary outcomes between the two groups. (A) number of lymph nodes harvested, (B) postoperative pancreatic fistula, (C) bile leak, and (D) postoperative pancreatic hemorrhage.
3.3.8 Postoperative pancreatic fistula
Eleven studies (12, 18–22, 24, 25, 27–29) evaluated the POPF. There was no significant difference in the incidence of POPF (RR 0.74, 95% CI 0.55, 1.00, P = 0.05) (Figure 4B) between the RPD and LPD groups.
3.3.9 Bile leak
Eight studies (17, 19, 21, 22, 24, 25, 28, 29) reported bile leaks. No significant differences were observed between the two groups (RR 0.62, 95% CI 0.37, 1.04, P = 0.07), and heterogeneity was low (I2 = 0%, P = 0.87) (Figure 4C).
3.3.10 Postoperative pancreatic hemorrhage
Postoperative pancreatic hemorrhage was reported in 5 studies (17, 19, 22, 24, 28), and the combined effect size suggested that the postoperative pancreatic hemorrhage rates were comparable between the two groups (RR 0.96, 95% CI 0.57, 1.61, P = 0.87; I2 = 1%) (Figure 4D).
3.3.11 Conversion rate
Conversion rate was evaluated in 8 studies (12, 17, 18, 20, 22, 24, 25, 29), and the pooled results showed that RPD had lower conversion rate than LPD (RR 0.29, 95% CI 0.20, 0.42; heterogeneity: I2 = 31%, P = 0.18) (Figure 5A).
Figure 5
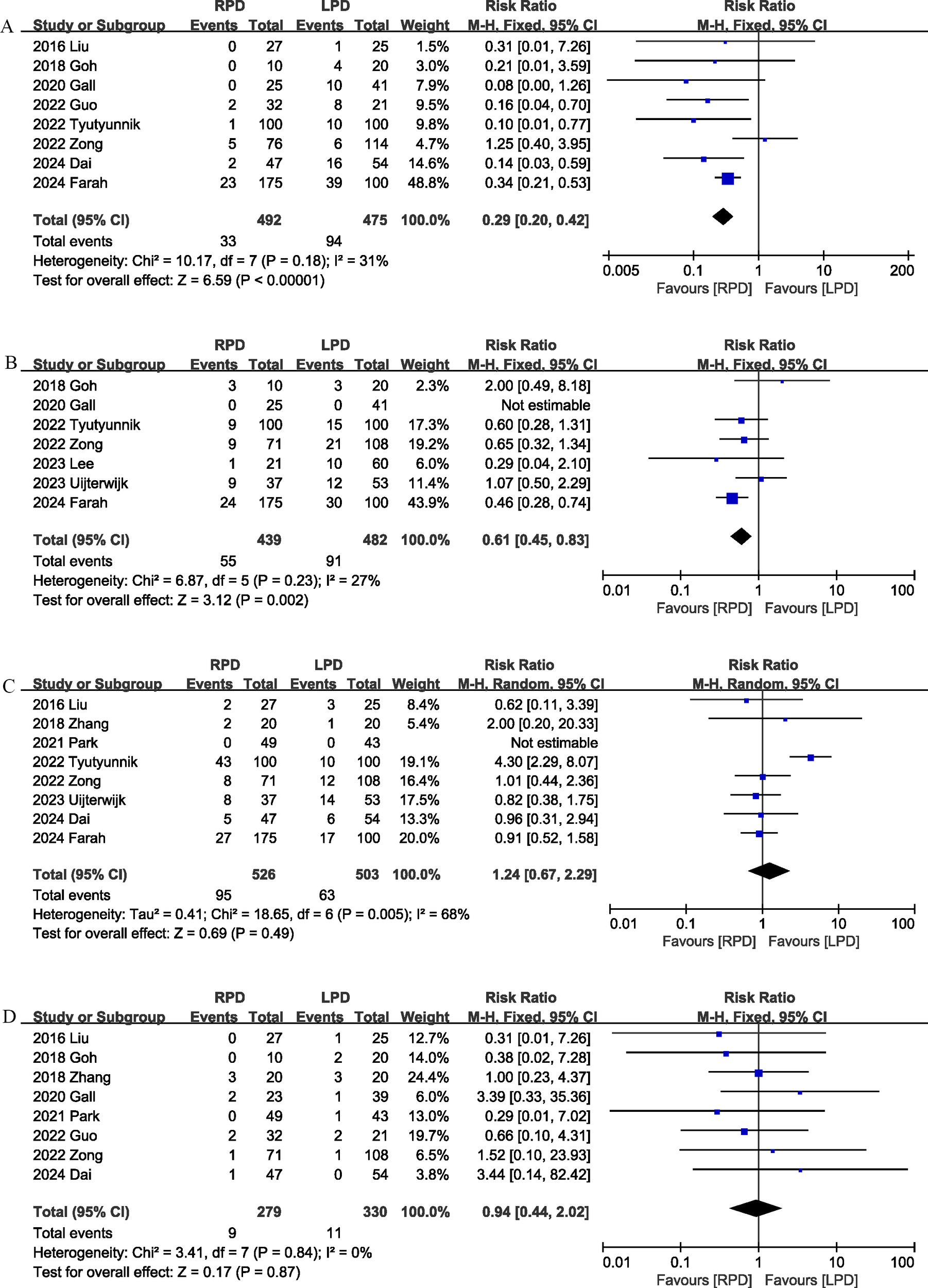
Comparison of secondary outcomes between the two groups. (A) Conversion rate, (B) blood transfusion, (C) delayed gastric emptying, and (D) reoperation.
3.3.12 Blood transfusion
Seven studies (12, 18, 20, 24, 25, 27, 28) compared blood transfusion rates between the RPD and LPD groups. The combined results showed that RPD was effective in reducing the blood transfusion rate (RR 0.61, 95% CI 0.45, 0.83, P = 0.002) (Figure 5B).
3.3.13 Delayed gastric emptying
Delayed gastric emptying was reported in 8 studies (12, 17, 19, 21, 24, 25, 28, 29), and there was no significant difference in the incidence of delayed gastric emptying (RR 1.24, 95% CI 0.67, 2.29, P = 0.49) (Figure 5C) between the two groups.
3.3.14 Reoperation
Eight trials (17–22, 25, 29) reported the reoperation rates. There were no significant differences between the two groups, and heterogeneity was low (RR 0.94, 95% CI 0.44, 2.02; Heterogeneity: I2 = 0%, P = 0.84; Figure 5D).
3.4 Publication bias and sensitivity analysis
According to the funnel plots and Egger tests (Figure 6), and no significant publication bias was observed for operation time, blood loss, POPF, and length of stay. Sensitivity analysis showed that no single study affected the overall effect size of the 90-day mortality, major complications, length of stay, conversion, reoperation, postoperative pancreatic hemorrhage, delayed gastric emptying, number of harvested lymph nodes, and R0 resection. The sensitivity analysis suggested that the total effect size of overall morbidity changed significantly when the study by Farah et al. (12) (RR 0.82, 95% CI 0.64, 1.04; I2 = 48%, P = 0.10) or the study by Uijterwijk et al. (28) (RR 0.86, 95% CI 0.71, 1.06; I2 = 7%, P = 0.37) was excluded. The total effect size of POPF changed significantly when the study by Guo et al. (22) (RR 0.72, 95% CI 0.53, 0.97; I2 = 0%, P = 0.83) or the study by Tyutyunnik et al. (24) (RR 0.64, 95% CI 0.45, 0.93; I2 = 0%, P = 0.92) was excluded. The total effect size of bile leak changed significantly when the study by Park et al. (21) (RR 0.57, 95% CI 0.33, 0.99; I2 = 0%, P = 0.89) or the study by Tyutyunnik et al. (24) (RR 0.56, 95% CI 0.32, 0.97; I2 = 0%, P = 0.92) was excluded. The total effect size of blood transfusion rates changed significantly when the study by Farah et al. (12) (RR 0.74, 95% CI 0.49, 1.10; I2 = 3%, P = 0.39) was excluded. The total effect size of operation time changed significantly when the study by Tyutyunnik et al. (24) (MD, -22.61 mins; 95% CI, -38.40, -6.82, P = 0.005) was excluded. The total effect size of blood loss changed significantly when the study by Lee et al. (27) (MD, -37.11 mL; 95% CI, -68.24, -5.99, P = 0.02; I2 = 79%) or the study by Tyutyunnik et al. (24) (MD, -32.39 mL; 95% CI, -62.94, -1.84, P = 0.04; I2 = 81%) was excluded.
Figure 6
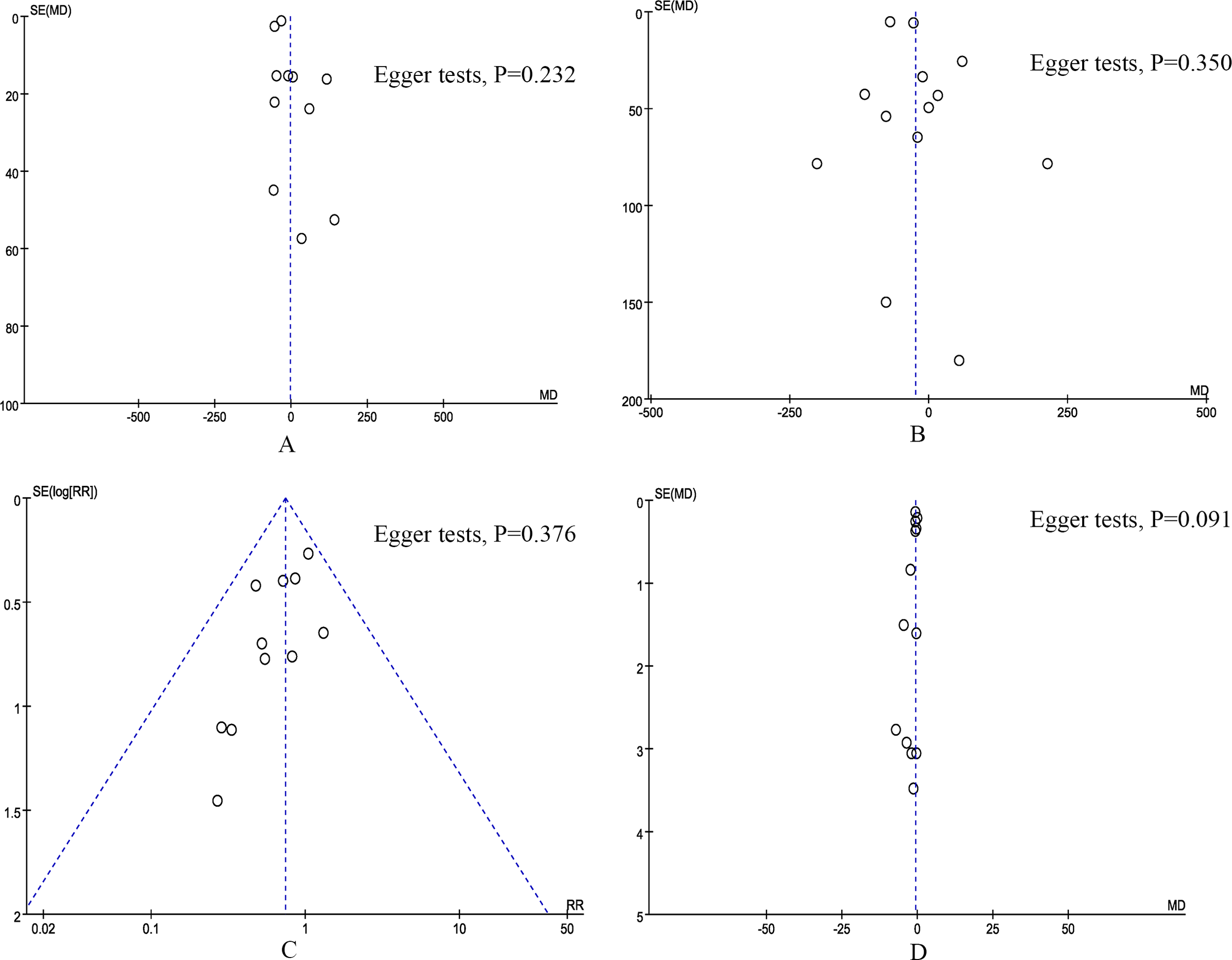
Funnel plot of primary outcomes. (A) operation time, (B) blood loss, (C) postoperative pancreatic fistula, and (D) length of stay.
4 Discussion
To our knowledge, this is the first meta-analysis to compare RPD and LPD for pancreatic and periampullary tumors. Based on evidence from 17 medium to high-quality studies, our meta-analysis showed that RPD significantly reduced overall postoperative complication rates, blood transfusion rates, and conversion rates, improved the number of lymph nodes harvested, and reduced length of hospital stay. In addition, there were no significant differences between RPD and LPD in postoperative mortality, major complication rate, reoperation rate, R0 resection rate, operative time, and blood loss. Our results have important clinical value because we provide evidence that short-term outcomes for RPD are not inferior to LPD, and this information may help surgeons select the appropriate surgical approach for patients with pancreatic and periampullary tumors.
The high morbidity and mortality after PD is an urgent problem for surgeons to solve. Minimally invasive surgical techniques offer a potential strategy for reducing postoperative morbidity in hepatobiliary and pancreatic surgery (32–34). LPD and RPD are two important minimally invasive procedures. Due to the shortcomings of laparoscopic surgery such as limited movement, unstable camera platform and two-dimensional imaging, LPD is more dependent on the surgical technique of the surgeon. Compared with laparoscopic surgery, robotic surgery retains the advantages of minimally invasive surgery, but also has a 3D visual surgical field of view and flexible operating instruments (6, 35). These advantages of robotic surgery may offer potential benefits in reducing complications after PD. This was also confirmed by our study, and our pooled results showed that RPD significantly reduced the total postoperative complication rate compared with LPD. In addition, in other surgical procedures, such as radical resection of rectal cancer and gastric cancer, similar results were seen in comparison of robotic surgery with laparoscopic surgery (36, 37). Pancreatic specific complications such as postoperative pancreatic hemorrhage, POPF and biliary fistula are common complications after PD, and are the main causes of perioperative death (2). Zhang et al.’s study (13) showed that POPF, PPH and reoperation were independent risk factors for postoperative mortality in PD. Consistent with the findings of several existing clinical studies (38–40), our meta-analysis suggests that the rates of these complications (postoperative pancreatic hemorrhage, POPF, and biliary fistula) for RPD and LPD are comparable. Similarly, several previous meta-analyses (41, 42) have shown no significant difference in pancreas-specific complications between the robotic approach and the laparoscopic approach in pancreatic surgery.
R0 resection and lymph node dissection are two important measures of oncology efficacy. R0 resection was associated with long-term survival (43). A meta-analysis of 61 studies including 62,529 patients by Kamarajah et al. (43) showed no significant difference in R0 resection rates between surgical approaches (RPD, LPD, and open PD). Similar results were observed in our study. Adequate lymph node dissection is essential for accurate staging (44). Malleo et al. (45) analyzed data from 1218 patients and showed that patients with pancreatic ductal adenocarcinoma need to obtain at least 28 lymph nodes. The RPD has an enlarged 3D field of view and a tremor filter that may aid in precise lymph node dissection (41). Our meta-analysis showed that RPD significantly increased the number of lymph nodes obtained in malignant. This is similar to a meta-analysis by Ouyang et al. (41), which included patients receiving PD for benign or malignant disease and showed that significantly more lymph nodes were obtained in the RPD group than in the LPD group.
A potential challenge to the popularity of RPD is the lengthening of surgical time. Some early studies (46–48) have shown that surgery time for RPD is significantly longer than for LPD. However, the results of this meta-analysis suggest that RPD does not extend the duration of surgery compared to LPD. This may be related to surgeons’ lack of experience with robotic surgery in earlier studies. Zhang et al. ‘s (13) study showed that when PD was performed by a surgeon who completed the RPD learning curve, the operation time in the RPD group was even shorter than that in the LPD group. The robotic platform provides enlarged images, reduces hand tremors, enables precise sutures, and may have potential advantages in reducing intraoperative blood loss. This study showed that although intraoperative blood loss was comparable between the RPD and LPD groups, the perioperative blood transfusion rate for RPD (12.5%) was significantly lower than for LPD (18.8%).
Conversion to open surgery is associated with poorer surgical outcomes. A recently published meta-analysis suggests that conversion to open in minimally invasive PD is associated with an increase in major postoperative complications and delayed postoperative recovery (49). Our meta-analysis showed that RPD had significantly lower transfer rates than LPD. Similarly, some previous meta-analyses (41, 50, 51) have shown that the conversion rate for hepatobiliary and pancreatic surgery is significantly lower in the robotic group than in the laparoscopic group. In addition, our study showed shorter hospital stays in the RPD group, which may be related to lower postoperative morbidity and conversion rates in the RPD group.
There are several limitations to our meta-analysis. First, the studies we included were all non-RCTs and were limited by the inherent limitations of retrospective studies. Second, there was a lack of evaluation of the long-term efficacy of RPD in the included studies. Considering the advantages of RPD in reducing postoperative morbidity and conversion rate and increasing the number of lymph nodes harvested, further evaluation of the difference in long-term prognosis between RPD and LPD is warranted. In addition, hospital volume may have an impact on surgical outcomes of RPD versus LPD. In our meta-analysis, some of the included studies included data from both high-volume and low-volume centers, so a subgroup analysis could not be performed to further evaluate the effect of hospital volume on the results. Future, high-quality studies with large samples to assess the effect of surgical center volume on the outcome of RPD versus LPD are warranted. Finally, there was high heterogeneity in some outcomes (operation time and blood loss), so these results should be treated with caution.
In conclusion, this meta-analysis showed that RPD can provide short-term perioperative outcomes that are not inferior to those of LPD in pancreatic and periampullary tumors. In addition, compared with LPD, RPD has potential advantages in reducing postoperative complications, blood transfusion and conversion to laparotomy, shortening hospital stay, and increasing the number of lymph nodes harvested. Further high-quality RCTs are necessary to demonstrate the benefits and clinical value of RPD.
Statements
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Author contributions
GT: Conceptualization, Data curation, Formal analysis, Methodology, Project administration, Software, Writing – original draft, Writing – review & editing. FC: Conceptualization, Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. RC: Conceptualization, Methodology, Writing – original draft, Writing – review & editing. RZ: Conceptualization, Funding acquisition, Project administration, Writing – original draft, Writing – review & editing. JZ: Conceptualization, Formal analysis, Methodology, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was financially supported by National Natural Science Foundation of China (22004088) and Science & Technology SupportProject of Sichuan Province (2023YFS0183).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
Podda M Gerardi C Di Saverio S Marino MV Davies RJ Pellino G et al . Robotic-assisted versus open pancreaticoduodenectomy for patients with benign and Malignant periampullary disease: a systematic review and meta-analysis of short-term outcomes. Surg Endosc. (2020) 34:2390–409. doi: 10.1007/s00464-020-07460-4
2
Yan Y Hua Y Chang C Zhu X Sha Y Wang B . Laparoscopic versus open pancreaticoduodenectomy for pancreatic and periampullary tumor: A meta-analysis of randomized controlled trials and non-randomized comparative studies. Front Oncol. (2022) 12:1093395. doi: 10.3389/fonc.2022.1093395
3
Sattari SA Sattari AR Makary MA Hu C He J . Laparoscopic versus open pancreatoduodenectomy in patients with periampullary tumors: A systematic review and meta-analysis. Ann Surg. (2023) 277:742–55. doi: 10.1097/sla.0000000000005785
4
Reis PCA Bittar V Almirón G Schramm AJ Oliveira JP Cagnacci R et al . Laparoscopic versus open pancreatoduodenectomy for periampullary tumors: A systematic review and meta-analysis of randomized controlled trials. J Gastrointest Cancer. (2024) 55:1058–68. doi: 10.1007/s12029-024-01091-x
5
Jajja MR Mustansir F Nadeem SO Lovasik BP Blair CM Sarmiento JM . Counting the cost: financial implications of complications following pancreaticoduodenectomy. HPB (Oxford). (2022) 24:1177–85. doi: 10.1016/j.hpb.2021.12.012
6
Tang G Zhang L Xia L Zhang J Chen R Zhou R . Comparison of short-term outcomes of robotic versus open Pancreaticoduodenectomy: A Meta-Analysis of randomized controlled trials and Propensity-Score-Matched studies. Int J Surg. (2024). doi: 10.1097/js9.0000000000001871
7
Palacio J Sanchez D Samuels S Ainuz BY Vigue RM Hernandez WE et al . Impact of conversion at time of minimally invasive pancreaticoduodenectomy on perioperative and long-term outcomes: Review of the National Cancer Database. Ann Hepatobiliary Pancreat Surg. (2023) 27:292–300. doi: 10.14701/ahbps.22-101
8
Davey MG Temperley HC O’Sullivan NJ Marcelino V Ryan OK Ryan ÉJ et al . Minimally invasive and open gastrectomy for gastric cancer: A systematic review and network meta-analysis of randomized clinical trials. Ann Surg Oncol. (2023) 30:5544–57. doi: 10.1245/s10434-023-13654-6
9
Ammirati CA Passera R Beltrami E Peluso C Francis N Arezzo A . Laparoscopic and robotic surgery for colorectal cancer in older patients: a systematic review and meta-analysis. Minim Invasive Ther Allied Technol. (2024), 33:253–69. doi: 10.1080/13645706.2024.2360094
10
Gang DY Dong L DeChun Z Yichi Z Ya L . A systematic review and meta-analysis of minimally invasive total mesorectal excision versus transanal total mesorectal excision for mid and low rectal cancer. Front Oncol. (2023) 13:1167200. doi: 10.3389/fonc.2023.1167200
11
Wang CJ Chen CX Liu Y Wen Z Li HY Huang HT et al . Comparative analysis of perioperative outcomes in obese patients undergoing robot-assisted radical prostatectomy (RARP) versus open radical prostatectomy (ORP): a systematic review and meta-analysis. J Robot Surg. (2024) 18:248. doi: 10.1007/s11701-024-02010-9
12
Farah E Al Abbas A Abreu AA Cheng M Yopp A Wang S et al . Minimally invasive pancreaticoduodenectomy: A favorable approach for frail patients with pancreatic cancer. Surgery. (2024) 175:1168–75. doi: 10.1016/j.surg.2023.12.022
13
Zhang XP Xu S Zhao ZM Yu GS Han B Chen X et al . Outcomes of robotic versus laparoscopic pancreatoduodenectomy following learning curves of surgeons: A multicenter study on 2255 patients. Ann Surg. (2023). doi: 10.1097/sla.0000000000006167
14
Page MJ McKenzie JE Bossuyt PM Boutron I Hoffmann TC Mulrow CD et al . The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Int J Surg. (2021) 88:105906. doi: 10.1016/j.ijsu.2021.105906
15
Sterne JAC Savović J Page MJ Elbers RG Blencowe NS Boutron I et al . RoB 2: a revised tool for assessing risk of bias in randomised trials. Bmj. (2019) 366:l4898. doi: 10.1136/bmj.l4898
16
Higgins JP Thompson SG . Quantifying heterogeneity in a meta-analysis. Stat Med. (2002) 21:1539–58. doi: 10.1002/sim.1186
17
Liu R Zhang T Zhao ZM Tan XL Zhao GD Zhang X et al . The surgical outcomes of robot-assisted laparoscopic pancreaticoduodenectomy versus laparoscopic pancreaticoduodenectomy for periampullary neoplasms: a comparative study of a single center. Surg Endosc. (2017) 31:2380–6. doi: 10.1007/s00464-016-5238-6
18
Goh BKP Low TY Kam JH Lee SY Chan CY . Initial experience with laparoscopic and robotic surgery for the treatment of periampullary tumours: single institution experience with the first 30 consecutive cases. ANZ J Surg. (2019) 89:E137–e41. doi: 10.1111/ans.15033
19
Zhang Y Hong D Zhang C Hu Z . Total laparoscopic versus robot-assisted laparoscopic pancreaticoduodenectomy. Biosci Trends. (2018) 12:484–90. doi: 10.5582/bst.2018.01236
20
Gall TM Pencavel TD Cunningham D Nicol D Jiao LR . Transition from open and laparoscopic to robotic pancreaticoduodenectomy in a UK tertiary referral hepatobiliary and pancreatic centre - Early experience of robotic pancreaticoduodenectomy. HPB (Oxford). (2020) 22:1637–44. doi: 10.1016/j.hpb.2020.03.008
21
Park SE Choi HJ You YK Hong TH . Effectiveness and stability of robot-assisted anastomosis in minimally invasive pancreaticoduodenectomy. Ann Surg Treat Res. (2021) 100:329–37. doi: 10.4174/astr.2021.100.6.329
22
Guo W Ye X Li J Lu S Wang M Wang Z et al . Comparison of surgical outcomes among open, laparoscopic, and robotic pancreatoduodenectomy: a single-center retrospective study. BMC Surg. (2022) 22:348. doi: 10.1186/s12893-022-01797-4
23
Naffouje SA Kamarajah SK Denbo JW Salti GI Dahdaleh FS . Surgical Approach does not Affect Return to Intended Oncologic Therapy Following Pancreaticoduodenectomy for Pancreatic Adenocarcinoma: A Propensity-Matched Study. Ann Surg Oncol. (2022) 29:7793–803. doi: 10.1245/s10434-022-12347-w
24
Tyutyunnik P Klompmaker S Lombardo C Lapshyn H Menonna F Napoli N et al . Learning curve of three European centers in laparoscopic, hybrid laparoscopic, and robotic pancreatoduodenectomy. Surg Endosc. (2022) 36:1515–26. doi: 10.1007/s00464-021-08439-5
25
Zong K Luo K Chen K Ye J Liu W Zhai W . A comparative study of robotics and laparoscopic in minimally invasive pancreatoduodenectomy: A single-center experience. Front Oncol. (2022) 12:960241. doi: 10.3389/fonc.2022.960241
26
Kalabin A Mani VR Kruse RL Schlesselman C Li KY Staveley-O’Carroll KF et al . New perspectives on robotic pancreaticoduodenectomy: An analysis of the National Cancer Database. World J Gastrointest Surg. (2023) 15:60–71. doi: 10.4240/wjgs.v15.i1.60
27
Lee W Song KB Hong S Park Y Kwak BJ Jun E et al . Minimally invasive versus open pancreaticoduodenectomy for distal bile duct cancer: an inverse probability of treatment weighting analysis of outcomes. Surg Endosc. (2023) 37:881–90. doi: 10.1007/s00464-022-09533-y
28
Uijterwijk BA Lemmers DHL Bolm L Luyer M Koh YX Mazzola M et al . Long-term outcomes after laparoscopic, robotic, and open pancreatoduodenectomy for distal cholangiocarcinoma: an international propensity score-matched cohort study. Ann Surg. (2023) 278:e570–e9. doi: 10.1097/sla.0000000000005743
29
Dai M Chen L Xu Q Cui M Li P Liu W et al . Robotic versus laparoscopic pancreaticoduodenectomy for pancreatic cancer: evaluation and analysis of surgical efficacy. Ann Surg Oncol. (2024) 31:7043–51. doi: 10.1245/s10434-024-15764-1
30
Wehrle CJ Chang JH Gross AR Woo K Naples R Stackhouse KA et al . Comparing oncologic and surgical outcomes of robotic and laparoscopic pancreatoduodenectomy in patients with pancreatic cancer: a propensity-matched analysis. Surg Endosc. (2024) 38:2602–10. doi: 10.1007/s00464-024-10783-1
31
Choi M Rho SY Kim SH Hwang HK Lee WJ Kang CM . Total laparoscopic versus robotic-assisted laparoscopic pancreaticoduodenectomy: which one is better? Surg Endoscopy. (2022) 36:8959–66. doi: 10.1007/s00464-022-09347-y
32
DeLaura I Sharib J Creasy JM Berchuck SI Blazer DG 3rd Lidsky ME et al . Defining the learning curve for robotic pancreaticoduodenectomy for a single surgeon following experience with laparoscopic pancreaticoduodenectomy. J Robot Surg. (2024) 18:126. doi: 10.1007/s11701-023-01746-0
33
Farrarons SS van Bodegraven EA Sauvanet A Hilal MA Besselink MG Dokmak S . Minimally invasive versus open central pancreatectomy: Systematic review and meta-analysis. Surgery. (2022) 172:1490–501. doi: 10.1016/j.surg.2022.06.024
34
Zhang J Shi M Ding W Duan M Dai Z Chen Y . Effect of minimally invasive versus open surgery in hepatectomy on postoperative wound complications in patients with hepatocellular carcinoma: A meta-analysis. Int Wound J. (2023) 20:4159–65. doi: 10.1111/iwj.14313
35
Yan Q Xu LB Ren ZF Liu C . Robotic versus open pancreaticoduodenectomy: a meta-analysis of short-term outcomes. Surg Endosc. (2020) 34:501–9. doi: 10.1007/s00464-019-07084-3
36
Li Z Zhou W Yang W Miao Y Zhang Y Duan L et al . Efficacy and safety of robotic vs. laparoscopic gastrectomy for patients with gastric cancer: systematic review and meta-analysis. Int J Surg. (2024). doi: 10.1097/js9.0000000000001826
37
Shi H Yi X Yan X Wu W Ouyang H Ou C et al . Meta-analysis of the efficacy and safety of robot-assisted comparative laparoscopic surgery in lateral lymph node dissection for rectal cancer. Surg Endosc. (2024) 38:5584–95. doi: 10.1007/s00464-024-11111-3
38
Khachfe HH Nassour I Hammad AY Hodges JC AlMasri S Liu H et al . Robotic pancreaticoduodenectomy: increased adoption and improved outcomes: is laparoscopy still justified? Ann Surg. (2023) 278:e563–e9. doi: 10.1097/sla.0000000000005687
39
Emmen A Görgec B Zwart MJW Daams F Erdmann J Festen S et al . Impact of shifting from laparoscopic to robotic surgery during 600 minimally invasive pancreatic and liver resections. Surg Endosc. (2023) 37:2659–72. doi: 10.1007/s00464-022-09735-4
40
Chao YJ Lu WH Liao TK Su PJ Wang CJ Lai CH et al . Feasibility of simultaneous development of laparoscopic and robotic pancreaticoduodenectomy. Sci Rep. (2023) 13:6190. doi: 10.1038/s41598-023-33269-x
41
Ouyang L Zhang J Feng Q Zhang Z Ma H Zhang G . Robotic versus laparoscopic pancreaticoduodenectomy: an up-to-date system review and meta-analysis. Front Oncol. (2022) 12:834382. doi: 10.3389/fonc.2022.834382
42
Kamarajah SK Sutandi N Robinson SR French JJ White SA . Robotic versus conventional laparoscopic distal pancreatic resection: a systematic review and meta-analysis. HPB (Oxford). (2019) 21:1107–18. doi: 10.1016/j.hpb.2019.02.020
43
Kamarajah SK Bundred JR Marc OS Jiao LR Hilal MA Manas DM et al . A systematic review and network meta-analysis of different surgical approaches for pancreaticoduodenectomy. HPB (Oxford). (2020) 22:329–39. doi: 10.1016/j.hpb.2019.09.016
44
Resende V Endo Y Munir MM Khalil M Rashid Z Lima HA et al . Prognostic value of nodal staging classification and number of examined lymph nodes among patients with ampullary cancer. J Gastrointest Surg. (2024) 28:33–9. doi: 10.1016/j.gassur.2023.11.008
45
Malleo G Maggino L Qadan M Marchegiani G Ferrone CR Paiella S et al . Reassessment of the optimal number of examined lymph nodes in pancreatoduodenectomy for pancreatic ductal adenocarcinoma. Ann Surg. (2022) 276:e518–e26. doi: 10.1097/sla.0000000000004552
46
Kim H Choi SH Jang JY Choi M Lee JH Kang CM . Multicenter comparison of totally laparoscopic and totally robotic pancreaticoduodenectomy: Propensity score and learning curve-matching analyses. J Hepatobiliary Pancreat Sci. (2022) 29:311–21. doi: 10.1002/jhbp.1078
47
Klompmaker S van Hilst J Wellner UF Busch OR Coratti A D’Hondt M et al . Outcomes after minimally-invasive versus open pancreatoduodenectomy: A pan-european propensity score matched study. Ann Surg. (2020) 271:356–63. doi: 10.1097/sla.0000000000002850
48
van Oosten AF Ding D Habib JR Irfan A Schmocker RK Sereni E et al . Perioperative outcomes of robotic pancreaticoduodenectomy: a propensity-matched analysis to open and laparoscopic pancreaticoduodenectomy. J Gastrointest Surg. (2021) 25:1795–804. doi: 10.1007/s11605-020-04869-z
49
Li ZL Li M Xiong JJ Lu HM . The impact of conversion during minimally invasive pancreatoduodenectomy: A meta-analysis. Asian J Surg. (2023) 46:1539–40. doi: 10.1016/j.asjsur.2022.09.074
50
Liu J Yao J Zhang J Wang Y Shu G Lou C et al . A comparison of robotic versus laparoscopic distal pancreatectomy for benign or Malignant lesions: A meta-analysis. J Laparoendosc Adv Surg Tech A. (2023) 33:1146–53. doi: 10.1089/lap.2023.0231
51
Liang B Peng Y Yang W Yang Y Li B Wei Y et al . Robotic versus laparoscopic liver resection for posterosuperior segments: a systematic review and meta-analysis. HPB (Oxford). (2024) 26:1089–02. doi: 10.1016/j.hpb.2024.06.003
Summary
Keywords
robotic pancreaticoduodenectomy, laparoscopic pancreaticoduodenectomy, periampullary tumor, morbidity, meta-analysis
Citation
Tang G, Chen F, Chen R, Zhou R and Zhang J (2024) Robotic versus laparoscopic pancreaticoduodenectomy for pancreatic and periampullary tumors: a meta-analysis. Front. Oncol. 14:1486504. doi: 10.3389/fonc.2024.1486504
Received
26 August 2024
Accepted
01 November 2024
Published
19 November 2024
Volume
14 - 2024
Edited by
Tudor Mocan, University of Medicine and Pharmacy Iuliu Hatieganu, Romania
Reviewed by
Tevfiktolga Sahin, İnönü University, Türkiye
Tiago Correia De Sá, Centro Hospitalar do Tâmega e Sousa, Portugal
Updates
Copyright
© 2024 Tang, Chen, Chen, Zhou and Zhang.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rongxing Zhou, rongxingzhou@126.com; Jingyi Zhang, jingyi223@163.com
†These authors have contributed equally to this work and share first authorship
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.