
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Oncol., 28 November 2024
Sec. Gastrointestinal Cancers: Gastric and Esophageal Cancers
Volume 14 - 2024 | https://doi.org/10.3389/fonc.2024.1484802
This article is part of the Research TopicExploring DNA Repair Defects and Metabolic Vulnerability to Enhance Immunotherapy responseView all 3 articles
Background: Microsatellite instability-high (MSI-H) or deficient mismatch repair (dMMR) represents a distinct molecular phenotype observed in malignant tumors. These tumors typically exhibit high levels of programmed cell death 1 ligand 1 (PD-L1) expression and high tumor mutational burden (TMB), resulting in an enhanced response to immune checkpoint inhibitors (ICI) therapy. The emergence of ICI has transformed the therapeutic strategy of gastric cancer (GC). Immune checkpoint blockade significantly improves the survival of gastric cancer patients, especially those with MSI-H or dMMR. However, it’s worth noting that not all patients with MSI-H respond favorably to this treatment. It has been reported that factors such as tumor heterogeneity, alterations in the tumor microenvironment, and aberrant activation of tumor-related signaling pathways have been linked with resistance to ICI therapy.
Case presentation: Here, we describe a case of dMMR and MSI-H GC with adenomatous polyposis coli (APC) and phosphatase and tensin homolog deleted on chromosome ten (PTEN) mutations that failed to respond to anti-PD-1 combined with anti-HER2 (human epidermal growth factor receptor-2) therapy and chemotherapy. We attempted to elucidate the underlying causes and mechanisms behind this lack of response, and to provide new insights into treatment options for these patients.
Conclusions: Mutations of key genes within tumor-related signaling pathways and the infiltration of CD8+T cells in the tumor microenvironment may influence the efficacy of immunotherapy for MSI-H solid tumors.
Gastric cancer (GC) remains one of the leading and fatal malignant tumors with high heterogeneity and aggressiveness, ranking fourth for mortality and fifth for incidence globally (1). Unfortunately, more than 50% of patients are diagnosed with locally advanced and metastatic stages, eliminating the option for surgical resection and leading to a poor prognosis. Recently, ICI therapy has emerged as a new standard of treatment for advanced and metastatic GC, and has shown favorable clinical benefits in some populations (2–4). In addition, the combination of pembrolizumab, trastuzumab and chemotherapy revealed favorable clinical benefits as first-line therapy for HER2-positive advanced GC patients (5). However, the GC populations who benefit from immunotherapy are very limited in the real world.
GC with dMMR accounts for approximately 5% to 20% (6). Tumors with dMMR are particularly subject to mutations in repetitive DNA sequences, leading to high levels of microsatellite instability (MSI-H) (7, 8). KEYNOTE-177 phase III clinical study demonstrated that pembrolizumab significantly extended progression-free survival(PFS)compared to chemotherapy for MSI-H or dMMR metastatic colorectal cancer patients in the first-line setting (9). In addition to colorectal cancer, ICI therapy has also demonstrated significant efficacy in GC with MSI-H. Regardless of the line of therapy, MSI-H status predicts preferable survival outcomes in advanced gastric or gastroesophageal junction cancer patients treated with pembrolizumab (10). Nevertheless, there are a subset of MSI-H/dMMR GC patients who do not respond to it. Findings from a small sample demonstrated that low TMB and PTEN mutations, particularly mutations in the domain of phosphatases may be negatively associated with the response to anti-PD-1 therapy in patients with MSI-H/dMMR gastrointestinal tumors (11). Alterations in tumor cell intrinsic and extrinsic factors, as well as aberrant activation of tumor-related signaling pathways contribute to the underlying resistance mechanisms of ICI therapy (12). With the development of next-generation sequencing (NGS), genetic analysis of the MSI-H/dMMR phenotype of GC has hinted that certain gene mutations may lead to primary or acquired resistance to ICI therapy. In this case study, we present a GC case with dMMR/MSI-H status, HER-2-positive, as well as APC and PTEN mutations who suffered a negative response to anti-PD-1 therapy. Additionally, the potential causes and mechanisms of resistance were also tentatively explored.
A 65-year-old female patient was admitted to the hospital with upper abdominal pain and tarry stool for one week in October 2021. A gastroscopy examination revealed an ulcerative lesion in the gastric body. The gastroscopic biopsy confirmed adenocarcinoma of the gastric body, and computed tomography (CT) examination indicated no distant metastasis. This patient then underwent a radical total gastrectomy in our hospital. Hematoxylin-eosin (HE) staining of postoperative pathological tissue was shown in Figure 1A. Pathological examination of the surgical specimen revealed ulcerated poorly differentiated adenocarcinoma, classified as pT3N0M0, HER2-positive [immunohistochemistry (IHC) 3+, fluorescence in-situ hybridization (FISH) positive] and PD-L1-negative (IHC -), with a deficiency of PMS2 protein indicating dMMR (Figures 1E-H). Helicobacter pylori (H. pylori) was negative. There was no recurrence or metastasis in this patient’s postoperative baseline assessment. Postoperatively, the patient received six cycles of XELOX chemotherapy.
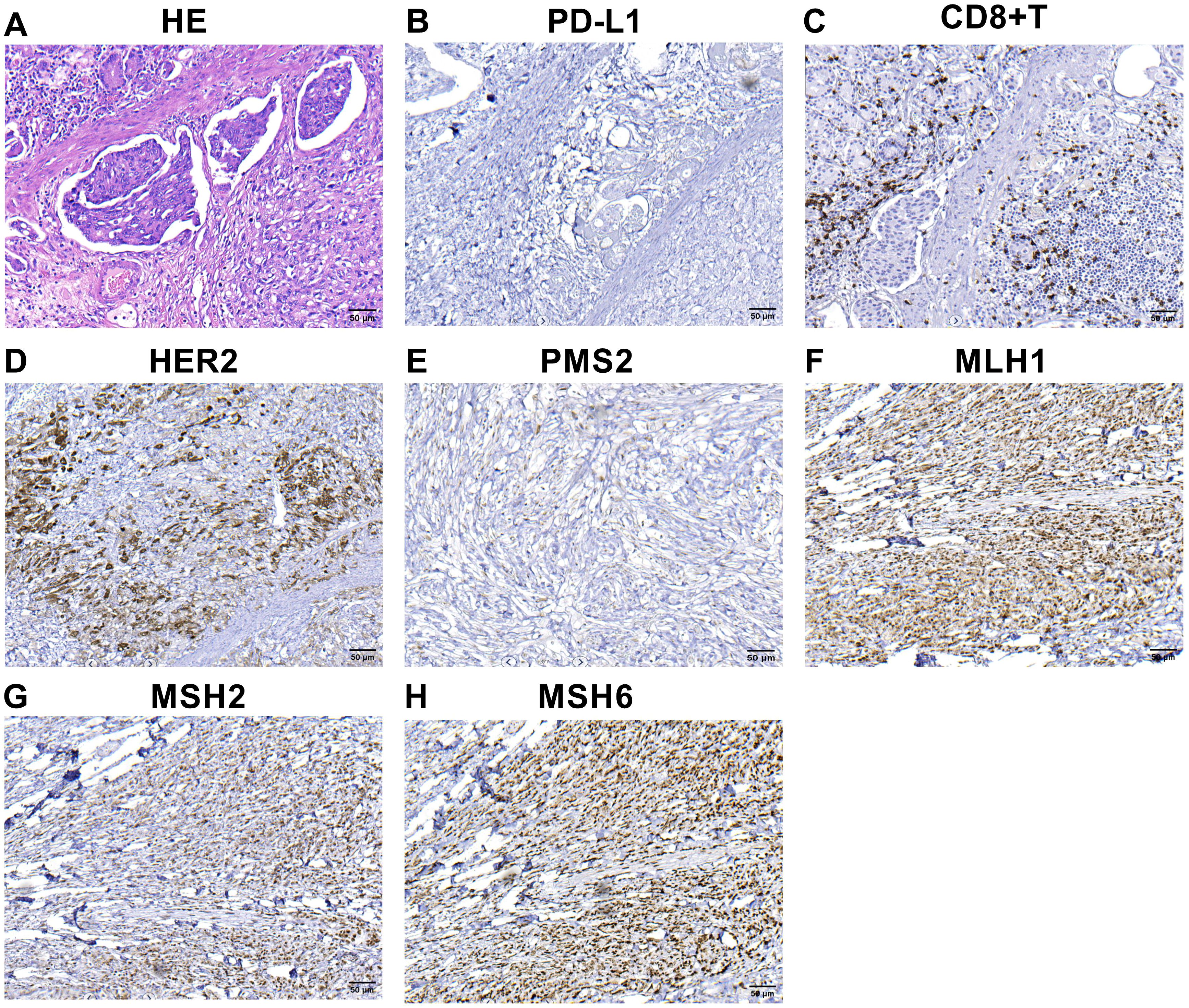
Figure 1. IHC and hematoxylin-eosin (HE) staining findings of primary tumor (A–H) HE staining of primary tumor (A).The combined positive score of PD-L1 expression in primary tumor before treatment (B). Infiltration of CD8+ T cells in the microenvironment of primary tumor before treatment (C). HER2 expression in the primary tumor (D). The expression of the protein PMS2 (E), MLH1 (F), MSH2 (G) and MSH6 (H). MLH1, MSH2 and MSH6 were positively expressed, whereas PMS2 was lacking. The microscopic magnification of all images is 200.
However, the patient experienced a recurrence in retroperitoneal lymph nodes 17 months after surgery (Figure 2A). The physical examination did not reveal any palpable superficial enlarged lymph nodes throughout the body. We reviewed the results of tumor tissue IHC, and found a deficiency of PMS2 protein (Figure 1E) and HER2-positive (Figure 1D). MSI-H status was confirmed further by PCR and NGS in this patient. The PCR test indicated that three single nucleotide sites (BAT25, BAT26, and D2S123) were changed. NGS also revealed a high TMB in both peripheral blood and tumor tissue, suggesting that this patient may benefit from ICI therapy. Based on the findings of KEYNOTE-811 clinical trial, the patient was subsequently treated with anti-PD-1(sintilimab, 200mg) in combination with anti-HER2 (trastuzumab, the first loading dose was 8 mg/kg, and the maintenance dose was 6 mg/kg) therapy and chemotherapy (S-1, 50mg, twice a day).
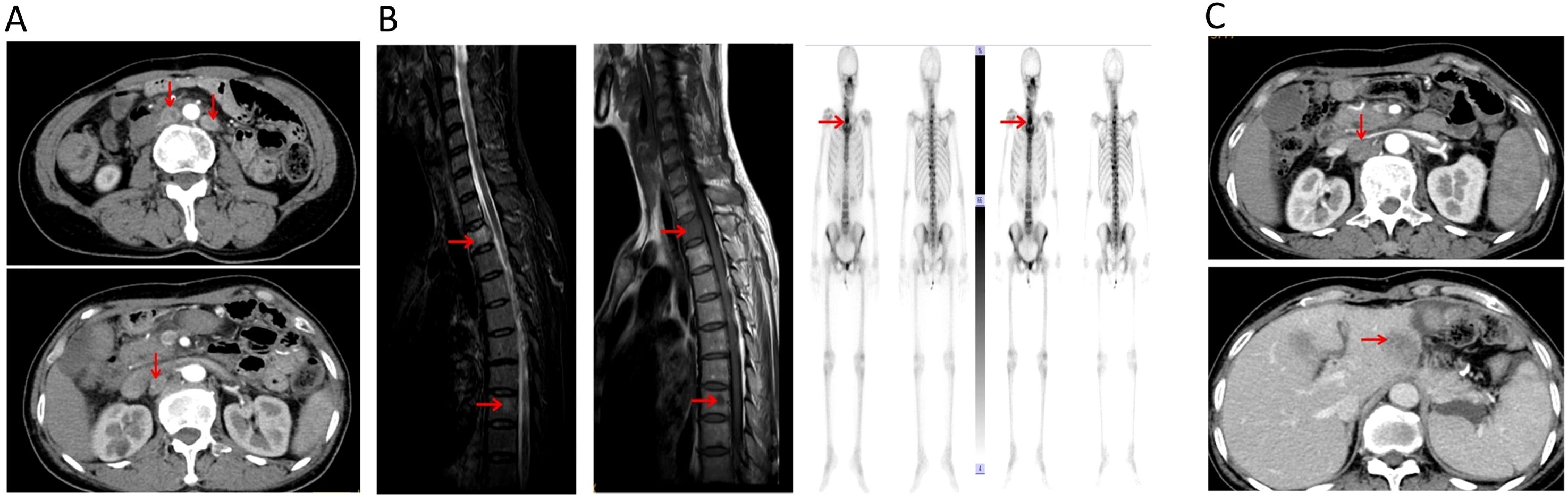
Figure 2. (A) Radiologic images of retroperitoneal lymph node metastases 17 months after operation. (B) Radiologic images of bone metastases after the first-line treatment. (C) Radiologic images of another retroperitoneal lymph node and liver metastases after the second-line treatment.
Unfortunately, after 3 months of the first-line treatment, this patient presented with back pain and weakness in both upper limbs. During the physical examination, no tenderness was elicited upon palpation of the back region. Emission Computed Tomography(ECT)and magnetic resonance imaging (MRI) confirmed metastatic bone destruction in the second and eighth thoracic vertebrae (Figure 2B). The patient’s second thoracic vertebra metastatic lesion was performed with local radiotherapy, synchronized with apatinib treatment. The patient’s back pain was significantly relieved at the end of radiation therapy. Subsequently, the patient initiated a second-line treatment regimen comprising anti-PD-1(sintilimab, 200mg) in combination with chemotherapy (albumin-bound paclitaxel, 200mg) and apatinib every three weeks.
However, after two months on the second-line treatment, this patient developed another retroperitoneal lymph node and liver metastases (Figure 2C). The physical examination did not reveal any palpable superficial enlarged lymph nodes throughout the body. Owing to the patient’s compromised physical condition, the decision was made to discontinue anti-tumor treatment in December 2023, and the patient transitioned to best supportive care. The entire treatment process of this case was shown in Figure 3.
IHC assay was used to detect the expression level of PMS2, MLH1, MSH6, MSH2, as well as PD-L1-positive and CD8+T cells in the formalin-fixed, paraffin-embedded (FFPE) tumor tissues. For quantitative analysis, image Pro-Plus software was used to analyze the density of CD8+T cells and PD-L1-positive tumor cells. The number of CD8 + T cells within tumor tissues and stroma was 44 cells/mm2 and 268 cells/mm2, respectively. Notably, no PD-L1 positive tumor cells were detected. These data revealed that very few CD8+T cells were infiltrated in the tumor, and large numbers of CD8+T cells were infiltrated in the stroma (Figure 1C). Furthermore, negative expression of PD-L1 protein and a deficiency of PMS2 protein were observed in this case (Figures 1B, E).
The tissue DNAs and circulating tumor DNAs (ctDNA) were extracted using the GeneRead DNA FFPE Kit (Qiagen) and Qiagen DNA blood mini kit (Qiagen), respectively. The extracted DNAs were amplified, purified and analyzed using a panel (YuceOneTM Plus X, Yucebio, China). TMB was calculated based on non-silencing somatic mutations, including coding base substitutions and insertions or deletions. TMB-High (TMB-H) was defined as TMB > 20 muts/Mb. The FFPE –derived results disclosed three gene mutations with notable frequencies, including APC R1114*, APC R1450* and PTEN R233Q *. Conversely, the peripheral blood test results showed five gene mutations with high frequency, including PTEN K260T, EGFR R677C, APC R1114*, APC R1450*and KIT R135C. Besides, the results of TMB from tumor tissue and peripheral blood were 66.5 muts/Mb and 45 muts/Mb, respectively.
This case pertains to a patient with MSI-H GC presenting APC and PTEN mutations and exhibiting unresponsiveness to ICI therapy. This case presents notable characteristics encompassing PD-L1 expression, TMB, HER2 status, and specific genetic alterations in a MSI-H GC patient. We attempted to expound the causes for resistance to ICI therapy in this case. Two methods are usually used to screen MSI and MMR status. In this instance, IHC showed a deficiency of PMS2 protein, and the PCR test also revealed alterations at three single nucleotide sites (BAT25, BAT26 and D2S123), thereby affirming the presence of dMMR) or MSI-H traits in this patient.
The PTEN gene mutations observed in the tumor tissues differ from those in peripheral blood in our case. The potential reasons for this result are as follows. The tumor cells exhibit high heterogeneity. This heterogeneity may stem from the presence of multiple concurrent tumor cell clones, each characterized by a distinct genotype. The genetic composition of tumor cells found in tumor tissue and peripheral blood may be derived from different clonal populations. Similarly, such genotypic differences may exist between metastases and primary tumors. Therefore, there may be variations in the results of genetic testing.
Increasing evidence has confirmed that ICI therapy revealed robust and sustained antitumor activity, and a favorable response in solid tumors (9, 13)with MSI-H/dMMR, including GC (10). However, approximately 50% of MSI-H cancer patients failed to respond to ICI therapy, suggesting that certain factors in the tumor microenvironment may affect the success of immune checkpoint blockade (14). In a small sample study of MSI-H gastric cancer, the level of PD-L1 expression on tumor cells or on immune cells was observed to be closely associated with survival outcomes (15). In this case, the IHC test showed PD-L1-negative, which may have partly contributed to the resistance to immunotherapy.
TMB is considered another biomarker associated with immunotherapy efficacy.
Pembrolizumab has obtained FDA approval for the treatment of solid tumors with tissue TMB-H based on the results of the KEYNOTE-158 study (16). In addition, TMB has been reported to be an important independent predictor within MSI-H mCRC treated with ICI therapy (17). However, there is currently no consistent cutoff value for TMB. An interesting study revealed substantial overlap between MSI-H and TMB-H, and the relationship between some significant mutant genes and phenotypes in 330 GC patients, providing new insights into the treatment of GC (18). In this patient, NGS results of both tumor tissue and peripheral blood showed high TMB, suggesting that other factors may be leading to immune tolerance in the tumor microenvironment.
Previous evidence has demonstrated that tumor infiltrating lymphocytes (TILs) are highly relevant to the host immune response to tumors and may predict clinical response to immunotherapy in several tumors (19–21). Cytotoxic CD8 + T cells are the main anti-tumor immune cells that clear tumor cells. CD8 + T cells have been observed to be associated with significant efficacy of immunotherapy in dMMR colorectal cancer (22). In addition, recent evidence (23) has demonstrated that a subset of CD8+TILs within the tumor microenvironment can recognize tumor neoantigens, suggesting an indirect anti-tumor effect. In this case, the IHC revealed that very few CD8+T cells were infiltrated within the tumor, and a significant number of CD8+T cells were filled in the stroma, which may lead to resistance to immunotherapy to some extent.
Abnormal expression or mutation of certain key genes and aberrant activation of tumor-related signaling pathways can prevent immune cell infiltration or function in the tumor microenvironment, contributing to resistance to immunotherapy (12). The mitogen-activated protein kinase (MAPK) pathway, the PTEN-PI3K-AKT signaling pathway, the WNT/b-catenin signaling pathway, interferon-gamma (IFNγ) signaling pathways, and lack of tumor antigen expression have been identified to be associated with tumor immune escape (24–29). Notably, mutations in the PTEN phosphatase domain were significantly associated with shorter survival and the presence of an immunosuppressive tumor microenvironment in MSI-H/dMMR gastrointestinal tumors patients treated with anti-PD-1 therapy (11). In addition, PTEN mutations in the phosphatase domain lead to reduced PTEN mRNA expression and loss of PTEN protein. as well as enrichment of the PI3K/AKT/mTOR and MTORC1 signaling pathways, indicative of PTEN dysfunction and possible association with anti-PD1 therapy resistance. PTEN mutation in this case may be associated with resistance to anti-PD1 therapy. It is well known that APC gene mutations play a pivotal role in tumorigenesis and progression. APC gene mutations play a crucial role in the carcinogenesis of intestinal type gastric cancer, independently of MSI status (30). Feng et al. reported that APC gene mutations may be correlated with worse response and efficacy for immunotherapy in CRC patients irrespective of MSI phenotype (31). In our case, NGS findings revealed two APC gene mutations in both the tumor tissue and peripheral blood, which may result in resistance to anti-PD1 therapy. Combined with the factors mentioned above, the tumor immune microenvironment is extremely complex, presenting great challenges in predicting the efficacy of immunotherapy. Therefore, it is necessary to comprehensively evaluate the tumor microenvironment characteristics and detailed genomic status of patients prior to ICI therapy, even for MSI-H tumors.
H. pylori colonizes the gastric mucosa and plays a significant role in the development of gastric cancer. H. pylori infection can affect gastric mucosal tumor microenvironment (TME), T cell function, and PD-L1 expression, suggesting the potential impact of H. pylori infection on immunotherapy (32). Two studies (33, 34) have shown that H. pylori infection is linked to adverse outcomes for immunotherapy in patients with advanced gastric cancer. Conversely, a large retrospective study (35) involving 10,122 patients with various cancer types indicated that H. pylori infection could be a favorable factor for immunotherapy in gastric cancer. The influence of H. pylori infection on the efficacy of immunotherapy for gastric cancer remains controversial, and the mechanisms by which H. pylori affects immunotherapy effectiveness are not yet fully understood. In our case, Helicobacter pylori negative status may have some effect on the efficacy of immunotherapy.
However, limitations of our study have to be acknowledged. Firstly, selection bias is inevitable in the present study due to a case report. Secondly, we did a small panel not gene signatures.
This case has important clinical implications and provides novel insights into the treatment of solid tumors with MSI-H. The efficacy of immunotherapy for MSI-H solid tumors may be affected by mutations in key genes in tumor-related signaling pathways and the degree of CD8+ T cell infiltration in the tumor microenvironment.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
The studies involving humans were approved by Ethics Committee of The Affiliated Xinghua People’s Hospital, Medical School of Yangzhou University. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin. Written informed consent was obtained from the individual(s), and minor(s)’ legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
JL: Conceptualization, Data curation, Formal Analysis, Supervision, Writing – original draft, Writing – review & editing. XZ: Conceptualization, Data curation, Formal Analysis, Investigation, Writing – original draft. QR: Conceptualization, Data curation, Supervision, Writing – original draft. CS: Conceptualization, Data curation, Formal Analysis, Writing – original draft. JY: Conceptualization, Data curation, Writing – review & editing. YC: Conceptualization, Data curation, Formal Analysis, Writing – review & editing. DC: Data curation, Methodology, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
2. Kang YK, Boku N, Satoh T, Ryu MH, Chao Y, Kato K, et al. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet (London England). (2017) 390:2461–71. doi: 10.1016/S0140-6736(17)31827-5
3. Janjigian YY, Shitara K, Moehler M, Garrido M, Salman P, Shen L, et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet (London England). (2021) 398:27–40. doi: 10.1016/S0140-6736(21)00797-2
4. Fuchs CS, Doi T, Jang RW, Muro K, Satoh T, Machado M, et al. Safety and efficacy of Pembrolizumab monotherapy in patients with previously treated advanced gastric and gastroesophageal junction cancer: phase 2 clinical KEYNOTE-059 trial. JAMA Oncol. (2018) 4:e180013. doi: 10.1001/jamaoncol.2018.0013
5. Janjigian YY, Kawazoe A, Yañez P, Li N, Lonardi S, Kolesnik O, et al. The KEYNOTE-811 trial of dual PD-1 and HER2 blockade in HER2-positive gastric cancer. Nature. (2021) 600:727–30. doi: 10.1038/s41586-021-04161-3
6. Hause RJ, Pritchard CC, Shendure J, Salipante SJ. Classification and characterization of microsatellite instability across 18 cancer types. Nat Med. (2016) 22:1342–50. doi: 10.1038/nm.4191
7. Dudley JC, Lin MT, Le DT, Eshleman JR. Microsatellite instability as a biomarker for PD-1 blockade. Clin Cancer Res. (2016) 22:813–20. doi: 10.1158/1078-0432.CCR-15-1678
8. Muzny DM, Bainbridge MN, Chang K, Dinh HH, Drummond JA, Fowler G, et al. Comprehensive molecular characterization of human colon and rectal cancer. Nature. (2012) 487:330–7. doi: 10.1038/nature11252
9. André T, Shiu KK, Kim TW, Jensen BV, Jensen LH, Punt C, et al. Pembrolizumab in microsatellite-instability-high advanced colorectal cancer. New Engl J Med. (2020) 383:2207–18. doi: 10.1056/NEJMoa2017699
10. Chao J, Fuchs CS, Shitara K, Tabernero J, Muro K, Van Cutsem E, et al. Assessment of pembrolizumab therapy for the treatment of microsatellite instability-high gastric or gastroesophageal junction cancer among patients in the KEYNOTE-059, KEYNOTE-061, and KEYNOTE-062 clinical trials. JAMA Oncol. (2021) 7:895–902. doi: 10.1001/jamaoncol.2021.0275
11. Chida K, Kawazoe A, Kawazu M, Suzuki T, Nakamura Y, Nakatsura T, et al. A low tumor mutational burden and PTEN mutations are predictors of a negative response to PD-1 blockade in MSI-H/dMMR gastrointestinal tumors. Clin Cancer Res. (2021) 27:3714–24. doi: 10.1158/1078-0432.CCR-21-0401
12. Sharma P, Hu-Lieskovan S, Wargo JA, Ribas A. Primary, adaptive, and acquired resistance to cancer immunotherapy. Cell. (2017) 168:707–23. doi: 10.1016/j.cell.2017.01.017
13. O'Malley DM, Bariani GM, Cassier PA, Marabelle A, Hansen AR, De Jesus Acosta A, et al. Pembrolizumab in patients with microsatellite instability-high advanced endometrial cancer: results from the KEYNOTE-158 study. J Clin Oncol. (2022) 40:752–61. doi: 10.1200/JCO.21.01874
14. Mestrallet G, Brown M, Bozkus CC, Bhardwaj N. Immune escape and resistance to immunotherapy in mismatch repair deficient tumors. Front Immunol. (2023) 14:1210164. doi: 10.3389/fimmu.2023.1210164
15. Cho J, Lee J, Bang H, Kim ST, Park SH, An JY, et al. Programmed cell death-ligand 1 expression predicts survival in patients with gastric carcinoma with microsatellite instability. Oncotarget. (2017) 8:13320–8. doi: 10.18632/oncotarget.14519
16. Marabelle A, Fakih M, Lopez J, Shah M, Shapira-Frommer R, Nakagawa K, et al. Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: prospective biomarker analysis of the multicohort, open-label, phase 2 KEYNOTE-158 study. Lancet Oncol. (2020) 21:1353–65. doi: 10.1016/S1470-2045(20)30445-9
17. Schrock AB, Ouyang C, Sandhu J, Sokol E, Jin D, Ross JS, et al. Tumor mutational burden is predictive of response to immune checkpoint inhibitors in MSI-high metastatic colorectal cancer. Ann Oncol. (2019) 30:1096–103. doi: 10.1093/annonc/mdz134
18. Cho J, Ahn S, Son DS, Kim NK, Lee KW, Kim S, et al. Bridging genomics and phenomics of gastric carcinoma. Int J Cancer. (2019) 145:2407–17. doi: 10.1002/ijc.v145.9
19. Denkert C, von Minckwitz G, Darb-Esfahani S, Lederer B, Heppner BI, Weber KE, et al. Tumour-infiltrating lymphocytes and prognosis in different subtypes of breast cancer: a pooled analysis of 3771 patients treated with neoadjuvant therapy. Lancet Oncol. (2018) 19:40–50. doi: 10.1016/S1470-2045(17)30904-X
20. Xing X, Guo J, Ding G, Li B, Dong B, Feng Q, et al. Analysis of PD1, PDL1, PDL2 expression and T cells infiltration in 1014 gastric cancer patients. Oncoimmunology. (2018) 7:e1356144. doi: 10.1080/2162402X.2017.1356144
21. Rozek LS, Schmit SL, Greenson JK, Tomsho LP, Rennert HS, Rennert G, et al. Tumor-infiltrating lymphocytes, Crohn's-like lymphoid reaction, and survival from colorectal cancer. J Natl Cancer Institute. (2016) 108. doi: 10.1093/jnci/djw027
22. Li J, Hu H, Qin G, Bai F, Wu X, Ke H, et al. Biomarkers of pathologic complete response to neoadjuvant immunotherapy in mismatch repair-deficient colorectal cancer. Clin Cancer Res. (2024) 30:368–78. doi: 10.1158/1078-0432.CCR-23-2213
23. Simoni Y, Becht E, Fehlings M, Loh CY, Koo SL, Teng KWW, et al. Bystander CD8(+) T cells are abundant and phenotypically distinct in human tumour infiltrates. Nature. (2018) 557:575–9. doi: 10.1038/s41586-018-0130-2
24. Liu C, Peng W, Xu C, Lou Y, Zhang M, Wargo JA, et al. BRAF inhibition increases tumor infiltration by T cells and enhances the antitumor activity of adoptive immunotherapy in mice. Clin Cancer Res. (2013) 19:393–403. doi: 10.1158/1078-0432.CCR-12-1626
25. Peng W, Chen JQ, Liu C, Malu S, Creasy C, Tetzlaff MT, et al. Loss of PTEN promotes resistance to T cell-mediated immunotherapy. Cancer Discovery. (2016) 6:202–16. doi: 10.1158/2159-8290.CD-15-0283
26. De Henau O, Rausch M, Winkler D, Campesato LF, Liu C, Cymerman DH, et al. Overcoming resistance to checkpoint blockade therapy by targeting PI3Kγ in myeloid cells. Nature. (2016) 539:443–7. doi: 10.1038/nature20554
27. Luke JJ, Bao R, Sweis RF, Spranger S, Gajewski TF. WNT/β-catenin pathway activation correlates with immune exclusion across human cancers. Clin Cancer Res. (2019) 25:3074–83. doi: 10.1158/1078-0432.CCR-18-1942
28. Gao J, Shi LZ, Zhao H, Chen J, Xiong L, He Q, et al. Loss of IFN-γ Pathway genes in tumor cells as a mechanism of resistance to anti-CTLA-4 therapy. Cell. (2016) 167:397–404.e399. doi: 10.1016/j.cell.2016.08.069
29. Gubin MM, Zhang X, Schuster H, Caron E, Ward JP, Noguchi T, et al. Checkpoint blockade cancer immunotherapy targets tumour-specific mutant antigens. Nature. (2014) 515:577–81. doi: 10.1038/nature13988
30. Fang DC, Luo YH, Yang SM, Li XA, Ling XL, Fang L. Mutation analysis of APC gene in gastric cancer with microsatellite instability. World J Gastroenterol. (2002) 8:787–91. doi: 10.3748/wjg.v8.i5.787
31. Feng F, Sun H, Zhao Z, Sun C, Zhao Y, Lin H, et al. Identification of APC mutation as a potential predictor for immunotherapy in colorectal cancer. J Oncol. (2022) 2022:6567998. doi: 10.1155/2022/6567998
32. Zhong X, Zheng H, Zhao S, Wang Z, Su Y, Zhong K, et al. Effects and mechanisms of Helicobacter pylori on cancers development and immunotherapy. Front Immunol. (2024) 15:1469096. doi: 10.3389/fimmu.2024.1469096
33. Magahis PT, Maron SB, Cowzer D, King S, Schattner M, Janjigian Y, et al. Impact of Helicobacter pylori infection status on outcomes among patients with advanced gastric cancer treated with immune checkpoint inhibitors. J immunotherapy Cancer. (2023) 11. doi: 10.1136/jitc-2023-007699
34. Che H, Xiong Q, Ma J, Chen S, Wu H, Xu H, et al. Association of Helicobacter pylori infection with survival outcomes in advanced gastric cancer patients treated with immune checkpoint inhibitors. BMC Cancer. (2022) 22:904. doi: 10.1186/s12885-022-10004-9
Keywords: microsatellite instability high, mismatch repair-deficient, gastric cancer, immunotherapy, case report
Citation: Liu J, Zhang X, Ren Q, Song C, Yu J, Cai Y and Chen D (2024) Negative response to immunotherapy in dMMR or MSI-H gastric cancer with APC and PTEN mutations: a case report. Front. Oncol. 14:1484802. doi: 10.3389/fonc.2024.1484802
Received: 22 August 2024; Accepted: 11 November 2024;
Published: 28 November 2024.
Edited by:
Dawit Kidane-Mulat, Howard University, United StatesReviewed by:
Keren Jia, Peking University, ChinaCopyright © 2024 Liu, Zhang, Ren, Song, Yu, Cai and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiang Liu, bGl1amlhbmc4OTAxQDE2My5jb20=; Dadong Chen, Y2RkdnNjYTE5ODlAMTI2LmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.