- 1Department of Cervical Disease, Xuzhou Maternity and Child Health Hospital, Jiangsu, China
- 2Department of Gynecology, Yueyang Central Hospital, Hunan, China
- 3Department of Medical Laboratory, Beijing Origin-Poly Bio-Tec Co., Ltd., Beijing, China
- 4Department of Clinical Laboratory, Xuzhou Maternity and Child Health Hospital, Jiangsu, China
- 5Department of Gynecology, Changsha Women and Child Health Care Hospital affiliated to Hunan Normal University, Hunan, China
Objective: This study aimed to evaluate the clinical utility of PAX1/JAM3 methylation (CISCER) test in triaging high-risk human papillomavirus (hrHPV)-positive women.
Methods: We enrolled women who underwent opportunistic screening at Cervical Disease outpatient clinics of Xuzhou Maternity and Child Health Hospital, and Yueyang Central Hospital from December 2022 to May 2024. The effectiveness of CISCER and cytology tests in triaging hrHPV+ patients was analyzed.
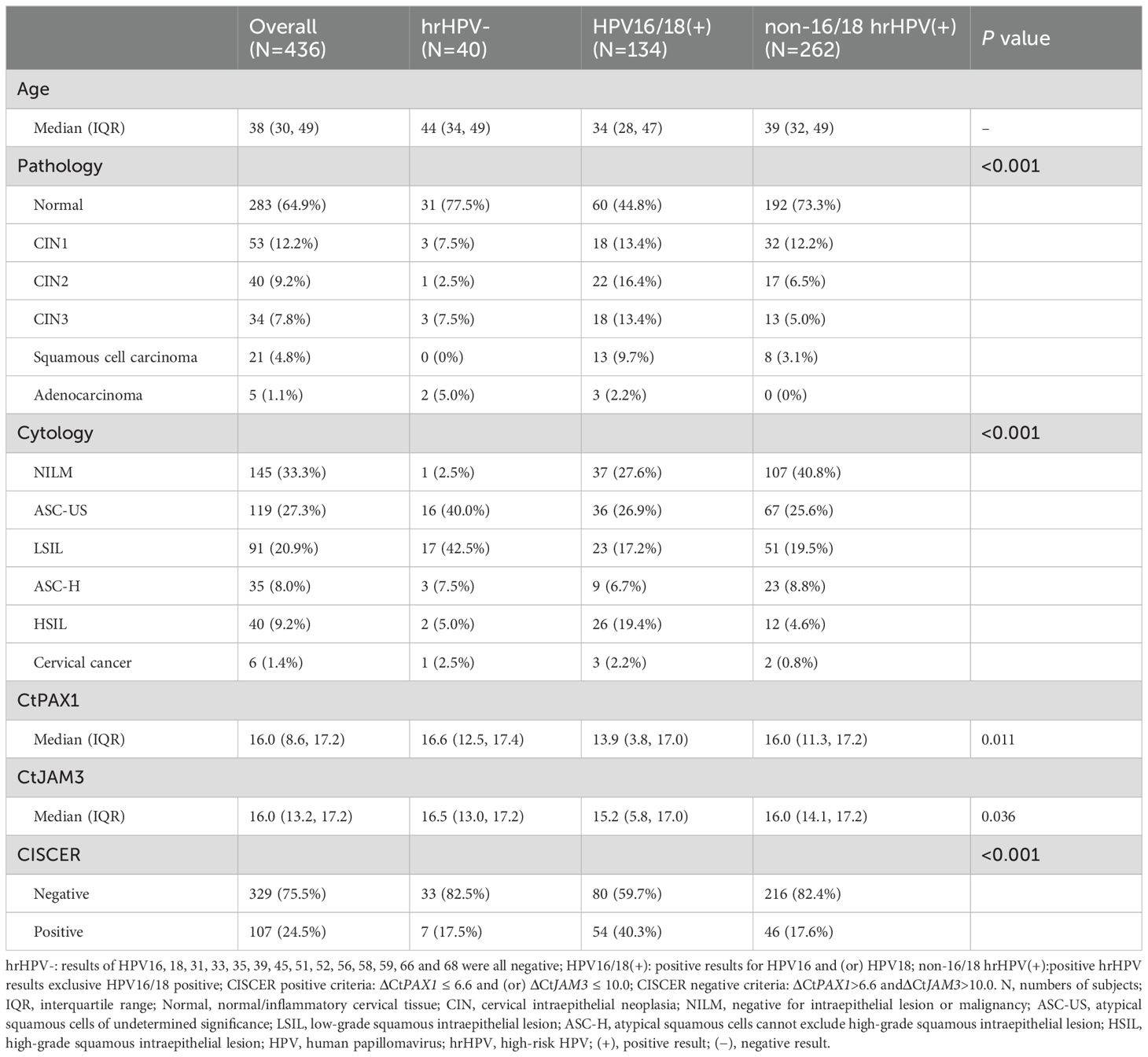
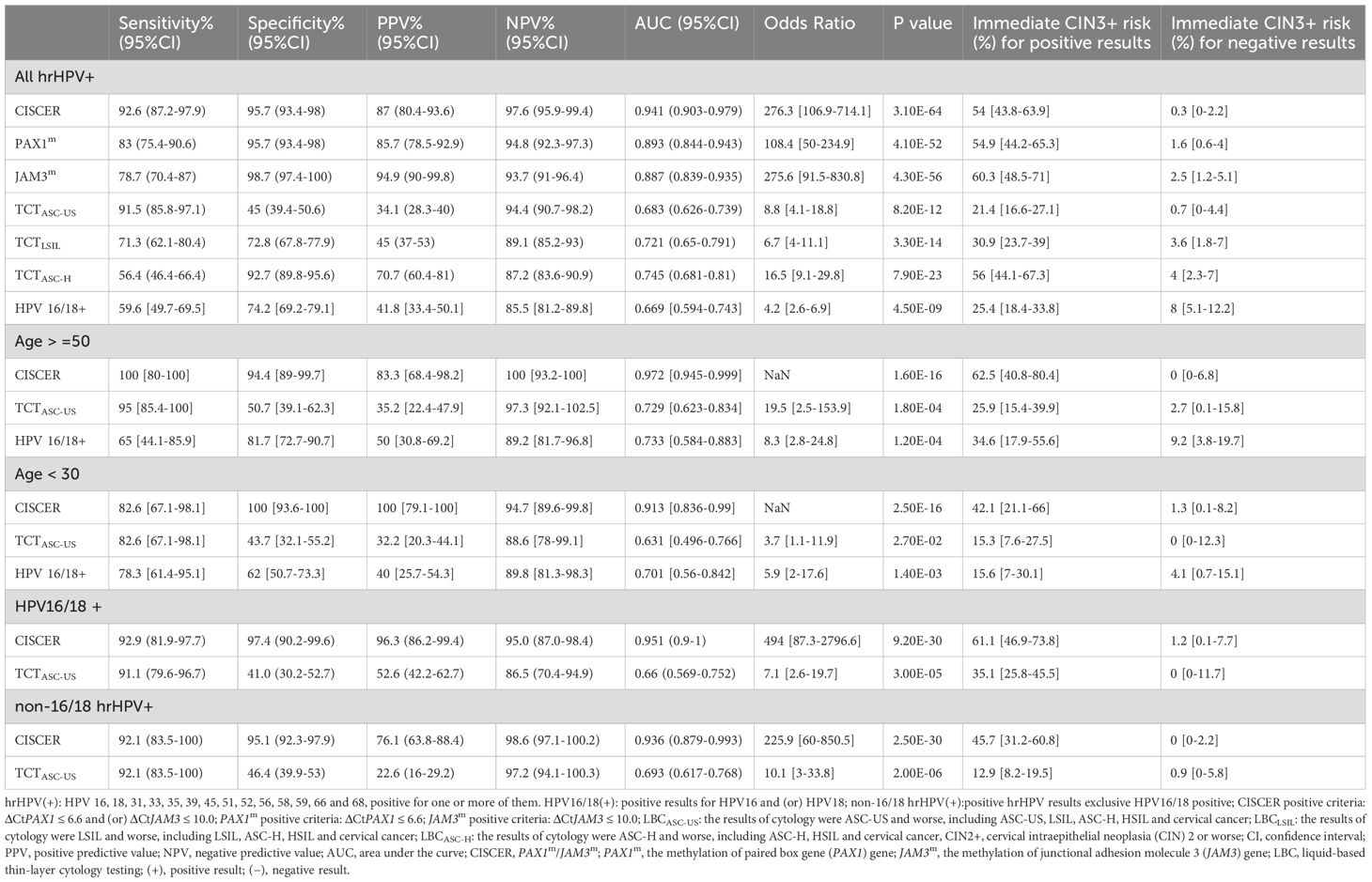
Results: Among the 436 study participants, 283 (64.9%) had no cervical intraepithelial neoplasia (CIN), while 53 (12.2%) had CIN1, 40 (9.2%) had CIN2, 34 (7.8%) had CIN3, and 26 (5.9%) had cervical cancers. The CISCER tests identified all cases of cervical cancer, particularly 2 hrHPV-negative adenocarcinoma cases. In 396 hrHPV+ individuals, the sensitivity of CISCER tests for detecting CIN2+ lesions was 92.6% (95% CI: 87.2-97.9%), with a specificity of 95.7% (95% CI: 93.4-98%), and an area under the receiver operating characteristic curve (AUC) of 0.941 (95% CI: 0.903-0.979), outperforming cytology tests in both HPV16/18+ and non-16/18 hrHPV+ women. Notably, CISCER demonstrated 100% (95% CI: 90-100%) sensitivity in women aged≥50 and 100% (95%CI: 93.6-100%) specificity in women aged<30. Among CIN2+ women, 37.2% (including 3 cancer) showed low-grade cytological changes that could be detected by CISCER. Meanwhile, 52% of CIN2- women exhibited cytological abnormalities but had negative CISCER results. The immediate CIN3+ risk based on positive CISCER results was 54% (95% CI: 43.8-63.9%).
Conclusion: The PAX1/JAM3 methylation detection using cervical exfoliated cells showed superior triage performance for hrHPV-positive patients compared to traditional strategies.
Introduction
Globally, cancers of the female reproductive organs (vulva, vagina, cervix, uterus, ovaries) represent approximately 15% of all female cancer cases and deaths (1). In February 2024, the International Agency for Research on Cancer (IARC) reported that 2022 witnessed roughly 1,473,427 new cases and 680,372 deaths in female reproductive organs cancers worldwide (2). Data from China’s National Central Cancer Registry and the World Cancer Research Fund International (WCRF International) for 2022 revealed a persistent increase in new cervical cancer cases in China, with an estimated 150,700 new cases, positioning China as the country with the highest incidence of new cervical cancer cases globally. During the same period, there were approximately 55,700 cervical cancer-related deaths in China (3, 4).
Over 90% of cervical cancers are caused by human papillomavirus (HPV) infection (5). Currently, high-risk HPV (hrHPV) DNA testing is recommended as the primary screening method for cervical cancer in China, with liquid-based cytology testing (LBC) used in resource-limited settings (6). Following infection with hrHPV, patients undergo epigenetic changes that can lead to the development of cervical intraepithelial neoplasia (CIN) 1-3 and, ultimately, cervical cancer (7). HrHPV infection induces mild to moderate cellular abnormalities, which are histologically classified as CIN 1 or CIN 2, whereas most HPV-positive cases are transient and resolve spontaneously, with only a minority progressing to CIN2 or worse (CIN2+). High-grade lesions CIN 2 and CIN 3, both of which are classified by the American Society for Colposcopy and Cervical Pathology (ASCCP) as primary screening targets in precancers, may take decades to progress to cervical cancer (7–9). Thus, HPV positivity for some individuals, especially for women in their 20s, may only signify an infection stage rather than a precancerous state, while misinterpretation of HPV positivity potentially causes undue anxiety, unnecessary treatment, and even increased risk of obstetric complications among screened women (6, 10). Despite high specificity and immediate risk assessment capabilities, cytology’s lower sensitivity and negative predictive value (NPV) limit its effectiveness in long-term risk prediction compared to hrHPV DNA testing (11). While introduction of HPV vaccination has notably reduced cervical cancer caused by vaccine-covered hrHPV genotypes (12–14), the rising incidence of non-vaccine hrHPV genotypes and HPV-unrelated adenocarcinomas is still noteworthy (15, 16). Therefore, within the framework of current guidelines recommending screening methods and comprehensively promoting HPV vaccination, there remains a continuing need to identify appropriate biomarkers as auxiliary screening indicators.
Epigenetic modification mainly involves alterations in gene expression levels driven by non-sequence-based changes. Dysregulated DNA methylation, one of the primary epigenetic mechanisms, contributes considerably to cancer development, invasion, and metastasis (8, 17). Accumulation of DNA methylation in specific genes, acting as an early indicator of malignant transformation, offers new avenues for early prevention, recurrence monitoring, and prognosis assessment (18, 19). Paired box gene 1 (PAX1) methylation emerged as a triage tool for detecting CIN3+ among hrHPV-positive women, demonstrating comparable clinical performance with LBC and superior testing efficacy compared to HPV16/18 (20, 21). Additionally, junctional adhesion molecule 3 (JAM3) methylation showed good diagnostic accuracy for CIN2+, serving as an effective triage marker for hrHPV-positive patients and stratification method for patients with atypical squamous cells of undetermined significance (ASC-US) (22–24). The combination of these dual genes has also been validated as a tool for cervical cancer screening and stratification. In a multicenter prospective study, PAX1/JAM3 methylation demonstrated superior clinical efficacy, especially the area under the curve (AUC), for testing CIN3+ compared to cytology, leading to a reduction in unnecessary colposcopy referrals (25). Furthermore, elevated PAX1/JAM3 methylation levels correlated significantly with persistent HPV infections lasting over three years, suggesting their potential utility in identifying continuous HPV infection (26). In this report, we conducted a real-world study to evaluate the triage efficacy of PAX1/JAM3 methylation (CISCER) test among hrHPV-positive patients.
Methods
Study population
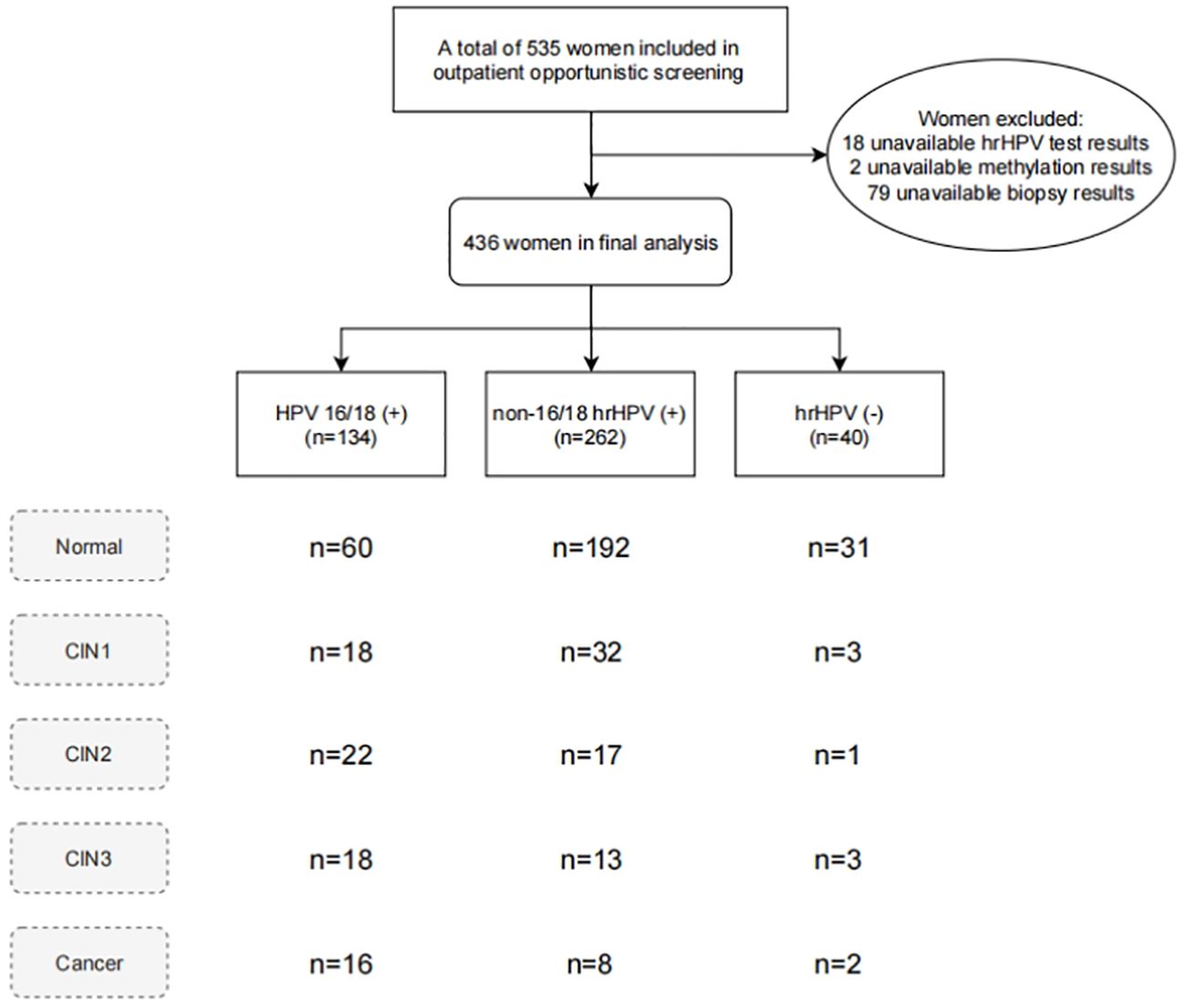
This study recruited women undergoing opportunistic cervical cancer screening at Cervical Disease outpatient clinics of Xuzhou Maternity and Child Health Hospital, and Yueyang Central Hospital from December 2022 to May 2024. Participants were enrolled based on the following inclusion criteria: 1) age over 18 years; 2) complete medical records and voluntary signing of informed consent. The exclusion criteria were as follows: 1) history of HIV infection or immunodeficiency disorders, and related treatments; 2) sexual activity or vaginal douching in the past 7 days; 3) pregnant or lactating; 4) significant cardiovascular, respiratory, digestive, urinary, hematologic, or psychiatric diseases, and other types of tumors. The study protocol was approved by the ethics committee of Xuzhou Maternity and Child Health Hospital (2023–06) and Yueyang Central Hospital (2024–002). A total of 535 women undergoing cervical screening were included, and the final analysis excluded those without available detection results (Figure 1).
Cytology testing
Cervical exfoliated cell samples were collected using cervical brushes and preserved in cell preservation solution (Landing Intelligent Medicine Co., Ltd, Wuhan, China). These samples were processed into slides using a liquid-based cytology method. Cytology results were classified according to The Bethesda System (TBS) 2014 (27), including categories: negative for intraepithelial lesion or malignancy (NILM), ASC-US, low-grade squamous intraepithelial lesion (LSIL), atypical squamous cells cannot exclude high-grade squamous intraepithelial lesion (ASC-H), high-grade squamous intraepithelial lesion (HSIL), and cervical cancer.
hrHPV DNA testing
HPV Genotyping Kit for 15 subtypes (Yaneng BIO, Shenzhen, China) was used for genotyping, including 14 hrHPV types: HPV16, HPV18, and 12 other hrHPV types (31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66 and 68). The collected cervical exfoliated cell samples were stored at 4°C in standard cell media provided by the Kit, and then sent to the laboratory for HPV testing within 48 hours. HPV tests were conducted via polymerase chain reaction (PCR)-reverse dot blot hybridization technology and the testing followed the manufacturer’s instructions as described previously (28).
DNA isolation and PAX1/JAM3 methylation detection
DNA was isolated from cervical samples using the JH-DNA Isolation and Purifying kit (OriginPoly Bio-Tec Co., Ltd., Beijing, China) according to the manufacturer’s protocol. The DNA concentration was measured with the NanoDrop 2000c spectrophotometer (Thermo Fisher Scientific, DE, USA). Subsequently, 200–1000 ng of DNA underwent bisulfite conversion using the JH-DNA Methylation-Lightning MagPrep kit (OriginPoly Bio-Tec Co., Ltd., Beijing, China).
PAX1 and JAM3 gene methylation were assessed using the “Human PAX1 and JAM3 Gene Methylation Detection Kit (PCR-fluorescence probe method)” (Origin Biotechnology Co., Ltd., Beijing, China), approved by the National Medical Products Administration as Class III medical device (Registration No. 20233400253). Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as an internal reference (OriGene Technologies, Beijing, China). The analysis was performed using the SLAN-96S fully automated medical PCR analysis system (Hongshi Medical Technology Co., Ltd., Shanghai, China). The PCR reactions started with a single cycle of initial incubation at 96°C for 10 minutes, followed by 45 cycles consisting of denaturation at 94°C for 15 seconds, annealing at 64°C for 5 seconds, and extension at 60°C for 30 seconds. The reaction concluded with a cooling step to 25°C for 1 minute. Clinical personnel were blinded to patient clinical information, LBC results, and cervical tissue pathology results. The hypermethylation status of the PAX1 and JAM3 genes was determined by calculating the difference between their respective cycle threshold (Ct) values and that of GAPDH (ΔCtPAX1 = CtPAX1 - CtGAPDH; ΔCtJAM3 = CtJAM3 - CtGAPDH). The criteria for defining positive CISCER (PAX1/JAM3 methylation testing) results were ΔCtPAX1≤ 6.6 or ΔCtJAM3 ≤10.0, with specific experimental steps and criteria referenced from previous protocols (26). Samples with insufficient DNA concentration for methylation testing were excluded from final analysis.
Colposcopy and tissue pathology
Patients with abnormal hrHPV DNA or cytology results were referred for colposcopy, and colposcopy-directed biopsies were performed on visible lesions or 1-2 random biopsies were taken from the normal-appearing cervix, unless the patient refused to undergo a biopsy. For women with cervical transformation zone 3 (TZ3), endocervical curettage (ECC) was performed. The positive methylation results were not used as a colposcopy referral indicator.
Histological assessment was performed by two experienced pathologists according to the “Guidelines for cervical cancer screening in China” (6), “Chinese Expert Consensus on Management of Cervical Low-grade Squamous Intraepithelial Lesions” (29), and “Chinese Expert Consensus on Management of Cervical High-grade Squamous Intraepithelial Lesions” (30). Lesion staging included: normal/inflammatory cervical tissue (Normal), CIN1, CIN2, CIN3, squamous cell carcinoma, and adenocarcinoma.
Statistical analysis
All statistical analyses were conducted using R version 4.4.0 (2024–04–24). The pROC package (version 1.18.4) was used to generate Receiver Operating Characteristic (ROC) curves, AUC, and corresponding 95% Confidence Intervals (95% CI). Sensitivity, specificity, Positive Predictive Value (PPV), and Negative Predictive Value (NPV) with their 95% CI were calculated using functions from the epiR (version 2.0.75) package. Categorical variables were presented as percentages, while continuous variables were presented as medians with interquartile ranges (Q1-Q3). Kruskal-Wallis rank sum test was used for comparing continuous variables between groups, and chi-square tests or Fisher’s exact tests were used for categorical variables. A P-value of 0.05 was considered statistically significant.
Results
Basic characteristics of patients
A total of 436 women, with a median age of 38 years (range 30 to 49 years), participated in the study (Table 1). Histopathological results indicated that among the participants: 298 (66.1%) had normal/inflammatory cervical tissue, followed by 53 (11.8%) with CIN1, 40 (8.9%) with CIN2, 34 (7.5%) with CIN3, 21 (4.7%) with squamous cell carcinoma (SCC), and 5 (1.1%) with adenocarcinoma, totaling 26 cases (5.8%) of cervical cancer. The results of hrHPV DNA testing revealed 40 hrHPV-negative cases, 134 HPV16/18-positive cases, and 262 cases positive for non-16/18 hrHPV. In women who were hrHPV-negative, HPV16/18-positive and non-16/18 hrHPV-positive, the numbers with CIN2+ lesions were 6 (15%), 56 (41.8%), and 38 (14.5%) respectively, with 2 (3.6%), 16 (11.9%), and 8 (3.1%) diagnosed with cervical cancer. This indicates that the proportion of women infected with HPV16/18 developing high grade lesions is significantly higher than those infected with non-16/18 hrHPV (P < 0.001), while the proportion of women with abnormal LBC results were lower in women with non-16/18 hrHPV than HPV 16/18. It is noteworthy that two cases of cervical adenocarcinoma had negative hrHPV DNA test results. One of these cases even one showed NILM in LBC results. However, they exhibited high methylation levels of PAX1 and JAM3 genes.
The methylation levels of PAX1 and JAM3 genes in hrHPV-positive patients
Figure 2A illustrated the ΔCt values of PAX1 and JAM3 genes in hrHPV-positive patients with different cervical lesions. The methylation levels of these two genes showed no differences between patients with normal/inflammatory cervical tissue and CIN1, but significantly elevated in CIN2 lesions and continued to increase with disease progression. The mean values of ΔCtPAX1 and ΔCtJAM3 in hrHPV-positive patients with CIN2+ were below 6.6 and 10.0, respectively, while they were above these thresholds in those with normal/inflammatory cervical tissue and CIN1. All cervical cancer cases exhibited extremely high methylation levels, and none were missed by CISCER test. Furthermore, we found no statistically significant difference in ΔCtPAX1 and ΔCtJAM3 between CIN2+ patients infected with HPV 16/18 and non-16/18 hrHPV (Figure 2B). This suggests that once high-grade lesions have developed, the severity of the disease does not differ according to the HPV type.
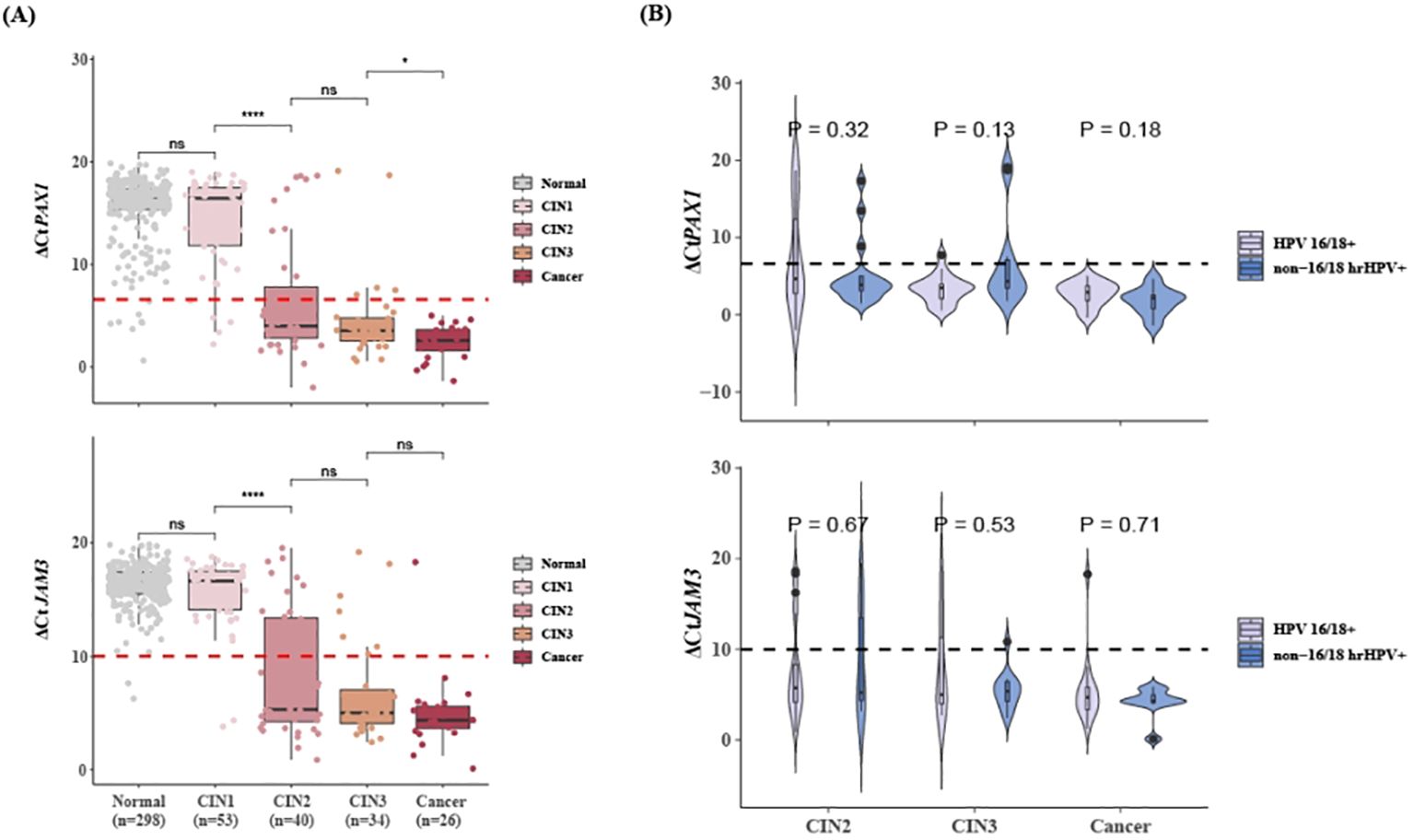
Figure 2. Methylation levels of PAX1 and JAM3 gene in hrHPV-positive women with different lesions. (A) Distribution plots of ΔCtPAX1 and ΔCtJAM3 in hrHPV+ patients with different lesions. (B) Distribution plots of ΔCtPAX1 and ΔCtJAM3 in CIN2+ patients who infected with HPV 16/18 or non-16/18 hrHPV. Dashed line means ΔCtPAX1 = 6.6, ΔCtJAM3 = 10.0. `x± s, nsP>0.05, *P<0.05, ****P<0.0001. PAX1, paired box gene 1; JAM3, junctional adhesion molecule 3; CIN, cervical intraepithelial neoplasia; hrHPV+, Positive for HPV 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66 or 68; non-16/18 hrHPV+, positive hrHPV results excluding HPV16/18.
Clinical performance of PAX1/JAM3 methylation in hrHPV-positive women
The clinical efficacy of PAX1 and JAM3 gene methylation detection in triaging hrHPV-positive women was shown in Table 2. Among hrHPV-positive women with CIN2+, CISCER detection demonstrated the highest sensitivity at 92.6% (95% CI: 87.2-97.9%), maintaining a high specificity of 95.7% (95% CI: 93.4-98%). In contrast, LBCASC-US+ had comparable sensitivity (91.5%: 85.8-97.1%) to CISCER tests but markedly lower specificity (45%: 39.4-50.6%), while LBCASC-H+ demonstrated similar specificity (92.7%: 89.8-95.6%) to CISCER tests yet much lower sensitivity (56.4%: 46.4-66.4%). Thus, the CISCER tests achieved a high AUC of 0.941 (95% CI: 0.903-0.979), significantly superior to LBC detection [LBCASC-US+: 0.683(0.626-0.739), LBCLSIL: 0.721(0.65-0.791), LBCASC-H+:0.745(0.681-0.81)]. Furthermore, this superiority was observed in women infected with HPV 16/18 and other hrHPV types (Table 2, Supplementary Table S1).
The immediate CIN3+ risk based on positive CISCER results would be 54% (95% CI: 43.8-63.9%), significantly higher than that of LBCASC-US (21.4%: 16.6-27.1%) and LBCLSIL (30.9%: 23.7-39%). When the cytology test result showed ASC-H, HSIL or cancer, the patient’s risk of CIN3+ was 56% (95%CI: 44.1-67.3%), slightly higher than CISCER (54%: 43.8-63.9). Conversely, with LBC results of NILM, ASC-US or LSIL, the risk of CIN3+ was 4% (95%CI: 44.1-67.3%), markedly higher than that of CISCER-negative (0.3%: 0-2.2%). This suggests that low-grade cytology results could experience 4% risk of missing the diagnosis of CIN3+, a risk that CISCER can effectively mitigate.
Notably, the sensitivity of CISCER tests achieved 100% (95% CI: 80-100%) in women aged over 50, with a high specificity of 94.4% (95% CI: 94.4-99.7%). In this demographic, a positive CISCER result indicated a 62.5% risk of CIN3+ (95% CI: 40.8-80.4%), while a negative result reduces the risk of CIN3+ to 0%. Additionally, CISCER achieved 100% specificity in women under 30, minimizing unnecessary interventions and protecting fertility in this younger population (More information in Supplementary Table S1).
Comparison of PAX1/JAM3 methylation and cytology detection
We further analyzed the proportions of women based on their CISCER test and LBC results, stratified for histology (Figure 3). In hrHPV-positive patients with CIN2+ lesions (including one cervical squamous carcinoma and two adenocarcinoma cases), approximately 45% (43.6%) showed cytology results of NILM, ASC-US or LSIL, whereas the CISCER test yielded positive results in 37.2% of these women, underscoring the potential of methylation tests to capture a substantial proportion of missed diagnoses caused by inaccurate cytological results (Figure 3A). In addition, this proportion was 37.5% for women positive for HPV 16/18 and 36.8% for those positive for non-16/18 hrHPV (Figure 3A).
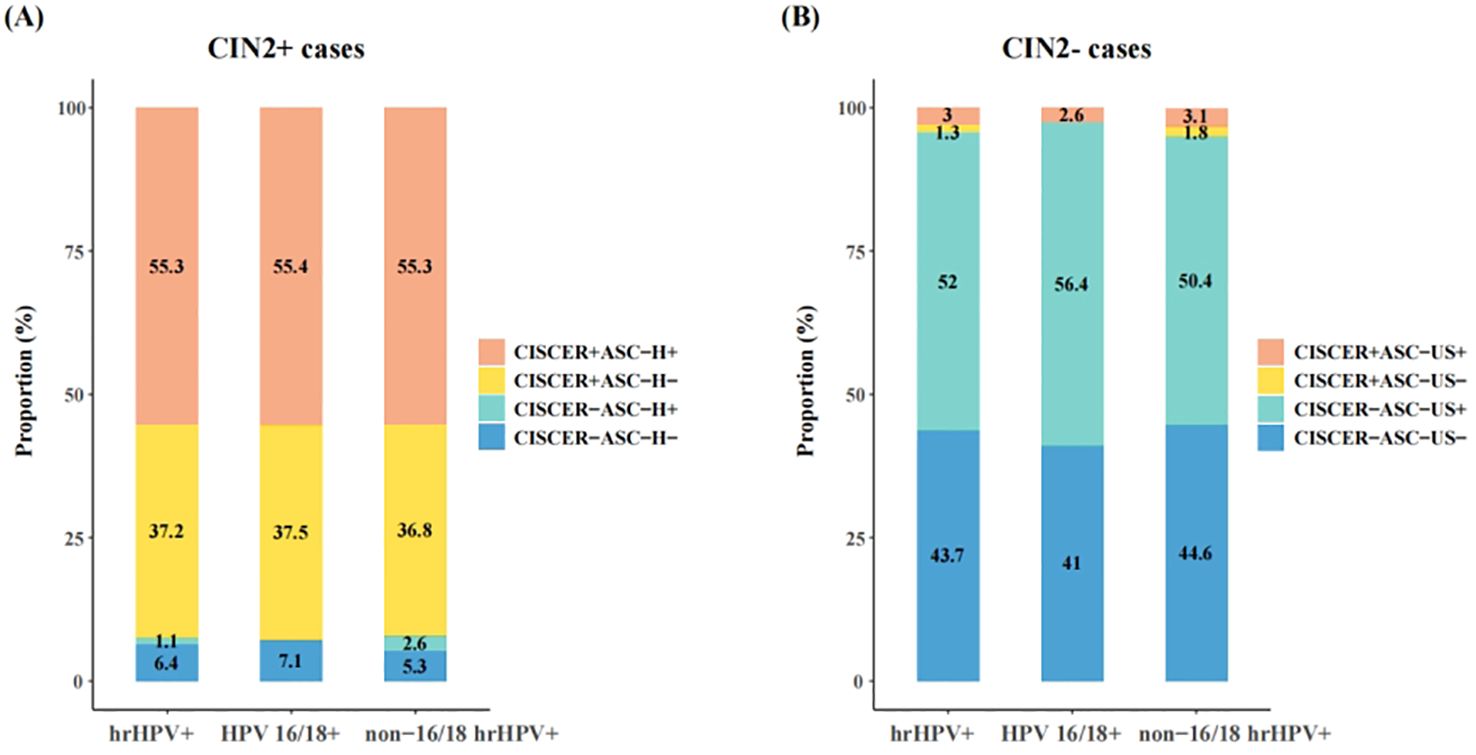
Figure 3. Comparison between CISCER and cytology results in hrHPV+ patients with CIN2+ or CIN2- lesions. (A) Results of CISCER and cytology tests in hrHPV+ patients with CIN2+ lesions. (B) Results of CISCER and cytology tests in hrHPV+patients with CIN2- lesions. CIN, cervical intraepithelial neoplasia; CIN2+ cases, patients with CIN 2 or severe lesions; CIN2- cases, patients with normal/inflammatory cervical tissue or CIN1; ASC-H-, the results of cytology were NILM, ASC-US or LSIL; ASC-H+, the results of cytology were ASC-H, HSIL or cervical cancer; CISCER+, ΔCtPAX1 ≤ 6.6 and (or) ΔCtJAM3 ≤ 10.0; CISCER-, ΔCtPAX1>6.6 and ΔCtJAM3>10.0; ASC-US-, the results of cytology were NILM; ASC-US+, the results of cytology were ASC-US, LSIL, ASC-H, HSIL or cervical cancer.
On the other hand, abnormal LBC results (ASC-US+) were observed in 55% of women with normal/inflammatory cervical tissue or CIN1, which may lead to unnecessary medical resource utilization and reduced patient compliance if all these women were referred to colposcopy or treatment (Figure 3B). The high specificity of CISCER tests effectively mitigated this issue, achieving negative results in 52% of these cases (Figure 3B). These findings once again underscore the CISCER test can serve as a powerful tool for stratifying hrHPV-positive women without relying on assessment of cellular morphology.
Discussion
This study evaluated the clinical efficacy of PAX1/JAM3 methylation detection in triaging HPV16/18-positive and non-16/18 hrHPV-positive patients within a cohort of 436 enrolled women, including 396 who were hrHPV-positive. Our findings demonstrated robust performance of CISCER test with sensitivity and specificity exceeding 92%. In addition, PAX1 and JAM3 methylation detection, whether alone or in combination, achieved a more balanced sensitivity and specificity profile and higher AUC values compared to these of cytology. Importantly, our study highlighted that the CISCER tests successfully identified all cervical cancer cases among the enrolled patients, including two adenocarcinoma cases that were hrHPV- negative.
Cervical cancer stands as the most predominant malignancy affecting the female reproductive system and a leading cause of female cancer-related deaths worldwide, accounting for approximately 6.9% of all cancer cases and 8.1% of fatalities as of February 2024 (2). Recent data from the National Cancer Center of China reveal a rising trend in cervical cancer incidence and mortality rate among Chinese women from 2010 to 2018 (4). Adding to the problem, cervical cancer screening coverage remains inadequate, with only 36.8% of women aged 35-64 undergoing screening between 2018 and 2019 in China, particularly in rural areas, as well as in the central and western regions (31). While cytology offers high specificity, its limited sensitivity (50-70%) poses challenges in detecting high-grade lesions and contributes to potential underdiagnosis issues (32, 33). Since 97% of precancerous lesions are HPV-positive, there is limited additional information that can be provided by performing both cytology and HPV DNA testing compared to HPV DNA testing alone (34, 35).
DNA methylation has garnered international recognition as a pivotal tool in cervical cancer screening, triage, and management, due to its reliable performance in efficiently detecting precancerous lesions (7, 36, 37). The latest expert consensus released by the Tumor marker committee of Chinese anti-cancer association in 2024 proposes that DNA methylation testing could play a role in cervical cancer screening management and post-treatment monitoring (38). Previous research, as evidenced by study including conditional inference tree model, had shown that methylation assay was more pertinent to the pathological diagnosis than cytology in diagnosing both high- and low-grade cervical lesions, and the assay can effectively differentiate high-grade cervical lesions regardless of cytology test (39). DNA methylation testing could mitigate overtreatment in patients diagnosed as CIN2+, where a positive result for specific methylation markers indicates a heightened risk of short-term progression to cervical cancer (7). In this study, we observed that over 35% of CIN2+ patients had negative cytology or low-grade lesions (ASC-US and LSIL) but positive CISCER results, including 3 cancer cases. Additionally, more than 50% of CIN2- patients was abnormal cytology (ASC-US+) but negative for CISCER tests. Moreover, CISCER’s immediate CIN3+ risk for negative results in hrHPV-positive women was much lower than LBC. These finding indicates that CISCER tests held a lower missed diagnosis rate in high-grade lesions and also resulted in fewer referrals for colposcopy in women with low-grade lesions. This highlights the potential of PAX1/JAM3 methylation to complement hrHPV tests in triaging HPV-positive women rather than relying on cytology detection.
Cervical cancer is frequently diagnosed in populations lacking adequate screening (40). Despite hrHPV DNA test being recommended as the primary screening tool for cervical cancer (6, 41), not all cases are associated with HPV infection, such as gastric-type endocervical adenocarcinoma (G-EAC). In our study cohort, two adenocarcinoma patients tested negative for hrHPV DNA test, but were successfully identified by CISCER tests. Specifically, these two cases exhibited notably high levels of PAX1 and JAM3 methylation (Patient 1: ΔCtPAX1 = 2.52, ΔCtJAM3 = 5.28, LBC: NILM; Patient 2: ΔCtPAX1 = 1.82, ΔCtJAM3 = 3.72, LBC: adenocarcinoma). Thus, DNA methylation tests in our study provided more comprehensive identification of cervical cancer patients compared to hrHPV DNA tests, consistent with previous findings (42). Therefore, PAX1/JAM3 methylation detection could not only reduce the number of patients who were missed or misdiagnosed by cytology, but also offers a highly sensitive marker panel for cancer, especially those unrelated to HPV infection.
We observed that CISCER had higher sensitivity in women aged≥50 and higher specificity in women aged<30. An analysis of cervical cancer trends among Chinese women from 1990 to 2019 reveals that the highest incidence and mortality rates were observed in the 50-54 age group, followed by women aged 55 and older (43). This pattern may be influenced by hormonal changes in postmenopausal women, which lead to genital tract atrophy and adhesions and then complicate the collection of adequate samples during gynecological examinations (44–46), which may cause anxiety in healthy women. Even worse, the squamous-columnar junction of the cervix shifts upward for elderly women, causing common sites of cervical cancer and precancerous lesions to retract into the cervical canal. This anatomical change often results in missed or inaccurate diagnoses during cervical cytology, colposcopy biopsy, or endocervical curettage (ECC), leading to delays in the initiation of appropriate treatment (47). These changes have introduced significant challenges for cervical cancer screening in postmenopausal women. The high sensitivity of CISCER tests in our study suggests its effectiveness in accurately identifying high-risk patients, which is essential for optimizing cervical cancer screening strategies for postmenopausal women.
Efforts to eliminate cervical cancer in China include extensive HPV vaccination campaigns nationwide, aiming at increasing vaccination rates among women (48, 49). A national survey identified HPV 52, 58, 53, 16, and 51 as the predominant genotypes, varying significantly by age groups and geographic locations in China (50). HPV vaccination efforts expand, necessitating effective cervical cancer screening methods for vaccinated and non-vaccinated individuals and also those infected with non-vaccinated hrHPV genotypes. A long-term follow-up of Dutch women vaccinated with the bivalent vaccine in adolescence revealed high-grade cervical lesions were still detectable in women who were vaccinated early, and the main types of HPV infection were HPV52, 59, 51, 58, and 33, which were non-vaccinated HPV genotypes (12). We observed consistent performance of PAX1 and JAM3 methylation in screening hrHPV-positive patients with CIN2+ regardless of HPV16/18 or non-16/18 hrHPV infections, and PAX1/JAM3 methylation levels were comparable between high-grade cervical lesions caused by non-16/18 hrHPV and HPV16/18. Given the direct correlation where higher levels of methylation correspond to more advanced cervical disease (51), it suggested that the severity of cervical disease did not vary based on hrHPV genotypes once high grade lesions had emerged. Hence, cervical diseases caused by non-16/18 hrHPV genotypes are also noteworthy, as women may not experience reduced severity of cervical lesions due to infection with these genotypes. Methylation tests hold the ability to detect high-grade cervical lesions caused by non-vaccinated hrHPV genotypes, which underscores the potential for them to perform well in screening programs as more vaccinated individuals enter the screening cohort in the future.
Limitations of our study included its simple size, which might limit the generalizability of the findings due to the lack of extensive research data supporting the results. In addition, the histopathological results relied on biopsies from patients referred to colposcopy outpatients rather than surgical pathological results, potentially impacting the accuracy of the histopathological assessments. Future multicenter prospective studies with larger sample sizes and rigorous clinical evaluations are needed to further validate the clinical utility of DNA methylation testing in cervical cancer screening.
Conclusions
Our findings indicate that PAX1/JAM3 methylation test significantly outperforms cytology in triaging hrHPV-positive patients. This could help mitigate the issue of missed diagnoses in postmenopausal women and address fertility preservation concerns in younger women. Furthermore, PAX1/JAM3 methylation test could be valuable for detecting cervical lesions associated with non-vaccine HPV types.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The study protocol was approved by the ethics committee of Xuzhou Maternity and Child Health Hospital (2023–06) and Yueyang Central Hospital (2024–002). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
HL: Conceptualization, Investigation, Writing – original draft, Writing – review & editing. YL: Conceptualization, Investigation, Writing – original draft, Writing – review & editing. SY: Formal analysis, Visualization, Writing – original draft, Writing – review & editing. MJ: Data curation, Writing – review & editing. QD: Data curation, Writing – review & editing. HW: Data curation, Writing – review & editing. JL: Methodology, Writing – review & editing. YC: Validation, Visualization, Writing – review & editing. PL: Conceptualization, Project administration, Writing – review & editing. JW: Formal analysis, Writing – review & editing. YW: Methodology, Writing – review & editing. ZW: Conceptualization, Investigation, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by 2022 Xuzhou Special Fund for Promoting Scientific and Technological Innovation Key R&D Program (Social Development, Medicine and Health-General) Project Number: KC22195.
Conflict of interest
Authors SY, PL, JW, and YW were employed by the company Beijing Origin-Poly Bio-Tec Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2024.1481626/full#supplementary-material
References
1. Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clin. (2024) 74:229–63. doi: 10.3322/caac.21834
2. International Agency for Research on Cancer. Cancer Today: Data visualization tools for exploring the global cancer burden in 2022. Globocan 2022 (version 11) - 08022024 . Available online at: https://gco.iarc.who.int/today/en (Accessed July 22, 2024).
3. WCRF International. Cervical cancer statistics (2024). Available online at: https://www.wcrf.org/cancer-trends/cervical-cancer-statistics/ (Accessed July 20, 2024).
4. Sun K, Han B, Zeng H, Wang S, Li L, Chen R, et al. Incidence and mortality of cancers in female genital organs - China, 2022. China CDC weekly. (2024) 6:195–202. doi: 10.46234/ccdcw2024.040
5. Tabibi T, Barnes JM, Shah A, Osazuwa-Peters N, Johnson KJ, Brown DS. Human papillomavirus vaccination and trends in cervical cancer incidence and mortality in the US. JAMA Pediatr. (2022) 176:313–6. doi: 10.1001/jamapediatrics.2021.4807
6. Li M, Wei L, Sui L, Ma D, Kong B, Wu X, et al. Guidelines for cervical cancer screening in China. Chin J Clin Obstet Gynecol. (2023) 24:437–42. doi: 10.13390/j.issn.1672-1861.2023.04.029
7. Steenbergen RD, Snijders PJ, Heideman DA, Meijer CJ. Clinical implications of (epi) genetic changes in HPV-induced cervical precancerous lesions. Nat Rev Cancer. (2014) 14:395–405. doi: 10.1038/nrc3728
8. Doorbar J, Egawa N, Griffin H, Kranjec C, Murakami I. Human papillomavirus molecular biology and disease association. Rev Med Virol. (2015) 25:2–23. doi: 10.1002/rmv.1822
9. Fontham ETH, Wolf AMD, Church TR, Etzioni R, Flowers CR, Herzig A, et al. Cervical cancer screening for individuals at average risk: 2020 guideline update from the American cancer society. CA: Cancer J Clin. (2020) 70:321–46. doi: 10.3322/caac.21628
10. Bruinsma F, Quinn M. The risk of preterm birth following treatment for precancerous changes in the cervix: a systematic review and meta-analysis. BJOG: Int J Obstetrics Gynaecology. (2011) 118:1031–41. doi: 10.1111/j.1471-0528.2011.02944.x
11. Perkins RB, Guido RS, Castle PE, Chelmow D, Einstein MH, Garcia F, et al. 2019 ASCCP risk-based management consensus guidelines for abnormal cervical cancer screening tests and cancer precursors. J lower genital tract Dis. (2020) 24:102–31. doi: 10.1097/LGT.0000000000000525
12. Louvanto K, Verhoef L, Pimenoff V, Eriksson T, Leppälä S, Lagheden C, et al. Low methylation marker levels among human papillomavirus-vaccinated women with cervical high-grade squamous intraepithelial lesions. Int J Cancer. (2024) 155(9):1549–57. doi: 10.1002/ijc.35044
13. Patel C, Brotherton JM, Pillsbury A, Jayasinghe S, Donovan B, Macartney K, et al. The impact of 10 years of human papillomavirus (HPV) vaccination in Australia: what additional disease burden will a nonavalent vaccine prevent? Euro surveillance : Bull Europeen sur les maladies transmissibles = Eur communicable Dis Bull. (2018) 23:1700737. doi: 10.2807/1560-7917.ES.2018.23.41.1700737
14. Falcaro M, Castañon A, Ndlela B, Checchi M, Soldan K, Lopez-Bernal J, et al. The effects of the national HPV vaccination programme in England, UK, on cervical cancer and grade 3 cervical intraepithelial neoplasia incidence: a register-based observational study. Lancet. (2021) 398:2084–92. doi: 10.1016/S0140-6736(21)02178-4
15. Seyoum A, Seyoum B, Gure T, Alemu A, Alemayehu DH, Alemu A, et al. High rate of non-vaccine targeted high-risk HPV genotypes circulate among women in eastern Ethiopia. Sci Rep. (2024) 14:958. doi: 10.1038/s41598-024-51594-7
16. Pimenoff VN, Gray P, Louvanto K, Eriksson T, Lagheden C, Soderlund-Strand A, et al. Ecological diversity profiles of non-vaccine-targeted HPVs after gender-based community vaccination efforts. Cell Host Microbe. (2023) 31:1921+. doi: 10.1016/j.chom.2023.10.001
17. Li C, Ke J, Liu J, Su J. DNA methylation data–based molecular subtype classification related to the prognosis of patients with cervical cancer. J Cell Biochem. (2020) 121:2713–24. doi: 10.1002/jcb.29491
18. Zhu H, Zhu H, Tian M, Wang D, He J, Xu T. DNA methylation and hydroxymethylation in cervical cancer: diagnosis, prognosis and treatment. Front Genet. (2020) 11:347. doi: 10.3389/fgene.2020.00347
19. Chen Y, Zou Q, Chen Q, Wang S, Du Q, Mai Q, et al. Methylation-related differentially expressed genes as potential prognostic biomarkers for cervical cancer. Heliyon. (2024) 10:e36240. doi: 10.1016/j.heliyon.2024.e36240
20. Chang C-L, Ho S-C, Su Y-F, Juan Y-C, Huang C-Y, Chao A-S, et al. DNA methylation marker for the triage of hrHPV positive women in cervical cancer screening: Real-world evidence in Taiwan. Gynecologic Oncol. (2021) 161:429–35. doi: 10.1016/j.ygyno.2021.02.011
21. Li M, Zhao C, Zhao Y, Li J, Zhang X, Zhang W, et al. Association and effectiveness of PAX1 methylation and HPV viral load for the detection of cervical high-grade squamous intraepithelial lesion. Pathogens. (2022) 12:63. doi: 10.3390/pathogens12010063
22. Kong L, Wang L, Wang Z, Xiao X, You Y, Wu H, et al. Cytological DNA methylation for cervical cancer screening: a validation set. Front Oncol. (2023) 13:1181982. doi: 10.3389/fonc.2023.1181982
23. Yin A, Zhang Q, Kong X, Jia L, Yang Z, Meng L, et al. JAM3 methylation status as a biomarker for diagnosis of preneoplastic and neoplastic lesions of the cervix. Oncotarget. (2015) 6:44373. doi: 10.18632/oncotarget.6250
24. Kong L, Wang L, Wang Z, Xiao X, You Y, Wu H, et al. DNA methylation for cervical cancer screening: a training set in China. Clin Epigenet. (2020) 12:1–10. doi: 10.1186/s13148-020-00885-7
25. Chen X, Jin X, Kong L, Liou Y, Liu P, Dong Z, et al. Triage performance of PAX1\textsuperscriptm/JAM3\textsuperscriptm in opportunistic cervical cancer screening of non-16/18 human papillomavirus-positive women: A multicenter prospective study in China. Clin Epigenet. (2024) 16:108. doi: 10.1186/s13148-024-01731-w
26. Li M, Zhao C, Zhang X, Li J, Zhao Y, Zhang W, et al. PAX1/JAM3 methylation and HPV viral load in women with persistent HPV infection. Cancers. (2024) 16:1430. doi: 10.3390/cancers16071430
27. Nayar R, Wilbur DC. The pap test and bethesda 2014. Cancer cytopathology. (2015) 123:271–81. doi: 10.1002/cncy.21521
28. Xie F, Zhang L, Zhao D, Wu X, Wei M, Zhang X, et al. Prior cervical cytology and high-risk HPV testing results for 311 patients with invasive cervical adenocarcinoma: a multicenter retrospective study from China’s largest independent operator of pathology laboratories. BMC Infect Dis. (2019) 19:962. doi: 10.1186/s12879-019-4614-y
29. Bi H, Li M, Zhao C, Zhao Y, You Z, Gen L LIJ, et al. Chinese expert consensus on the management of low-grade squamous intraepithelial lesions of the cervix. Chin J Clin Obstetrics Gynecology. (2022) 23:443–5. doi: 10.13390/j.issn.1672-1861.2022.04.036
30. Zhao C, Bi H, Zhao Y, Geng L, Liu J, Sheng D, et al. Chinese expert consensus on the management of high-grade squamous intraepithelial lesions of the cervix. Chin J Clin Obstetrics Gynecology. (2022) 23:220. doi: 10.13390/j.issn.1672-1861.2022.02.038
31. Zhang M, Zhong Y, Wang L, Bao H, Huang Z, Zhao Z, et al. Cervical cancer screening coverage—China, 2018–2019. China CDC weekly. (2022) 4:1077. doi: 10.46234/ccdcw2022.217
32. Nishimura H, Yeh PT, Oguntade H, Kennedy CE, Narasimhan M. HPV self-sampling for cervical cancer screening: a systematic review of values and preferences. BMJ Global Health. (2021) 6:e003743. doi: 10.1136/bmjgh-2020-003743
33. Zhao Y, Zhao C, Li M, Li J, Wei L. Screening for cervical cancer and diversion of abnormal screening results. J Chin Physician. (2023) 25(5): 649–51. doi: 10.3760/cma.j.cn431274-20230413-00480
34. Schiffman M, Kinney WK, Cheung LC, Gage JC, Fetterman B, Poitras NE, et al. Relative performance of HPV and cytology components of cotesting in cervical screening. J Natl Cancer Institute. (2018) 110:501–8. doi: 10.1093/jnci/djx225
35. Egemen D, Cheung LC, Chen X, Demarco M, Perkins RB, Kinney W, et al. Risk estimates supporting the 2019 ASCCP risk-based management consensus guidelines. J lower genital tract Dis. (2020) 24:132–43. doi: 10.1097/LGT.0000000000000529
36. Kremer W, Steenbergen R, Heideman D, Kenter G, Meijer C. The use of host cell DNA methylation analysis in the detection and management of women with advanced cervical intraepithelial neoplasia: a review. BJOG: Int J Obstetrics Gynaecology. (2021) 128:504–14. doi: 10.1111/1471-0528.16395
37. Zhu P, Xiong J, Yuan D, Li X, Luo L, Huang J, et al. ZNF671 methylation test in cervical scrapings for cervical intraepithelial neoplasia grade 3 and cervical cancer detection. Cell Rep Med. (2023) 4:101143. doi: 10.1016/j.xcrm.2023.101143
38. Tumor marker committee of Chinese anti-cancer association. Expert Consensus on detection and clinical application of tumor DNA methylation markers (Version 2024). Chin J Oncol Prev Treat. (2024) 16:129–42.
39. Li X, He S, Zhao X, Sun D, Wu S, Xu D, et al. High-grade cervical lesions diagnosed by JAM3/PAX1 methylation in high-risk human papillomavirus-infected patients. Zhong nan da xue bao Yi xue ban= J Cent South Univ Med Sci. (2023) 48:1820–9. doi: 10.11817/j.issn.1672-7347.2023.230175
40. Cohen CM, Wentzensen N, Castle PE, Schiffman M, Zuna R, Arend RC, et al. Racial and ethnic disparities in cervical cancer incidence, survival, and mortality by histologic subtype. J Clin oncology : Off J Am Soc Clin Oncol. (2023) 41:1059–68. doi: 10.1200/JCO.22.01424
41. Force UPST, Curry SJ, Krist AH, Owens DK, Barry MJ, Caughey AB, et al. Screening for cervical cancer: US preventive services task force recommendation statement. JAMA. (2018) 320:674–86. doi: 10.1001/jama.2018.10897
42. Shang X, Kong L, Xiao X, Wan R, Wang J, Wu H, et al. A multicenter study on the accuracy of PAX1/JAM3 dual genes methylation testing for screening cervical cancer. Zhonghua yi xue za zhi. (2024) 104:1852–9. doi: 10.3760/cma.j.cn12137-20231004-00630
43. Li S, Huang M, Zhu Y, Zeng H, Zhang F. Temporal trends in incidence and mortality of cervical cancer in China from 1990 to 2019 and predictions for 2034. Eur J Cancer Prev. (2023) 33(3): 10–1097. doi: 10.1097/CEJ.0000000000000849
44. Durkin N. An introduction to medical science: A comprehensive guide to anatomy, biochemistry and physiology. Springer Sci Business Media. (2012).
45. Pandit L, Ouslander JG. Postmenopausal vaginal atrophy and atrophic vaginitis. Am J Med Sci. (1997) 314:228–31. doi: 10.1097/00000441-199710000-00004
46. Asciutto KC, Forslund O, Borgfeldt C. Prevalence of high-risk HPV in postmenopausal women with benign cervical cytology - A population-based cohort study. Anticancer Res. (2018) 38:4221–8. doi: 10.21873/anticanres.12718
47. He Y, Wu Y, Wang J, Zhang S, Zhao H, Ai H, et al. Cervical lesions in older women(2023 edition). Chin J Pract Gynecology Obstetrics. (2024) 39:531–6. doi: 10.19538/j.fk2023050112
48. Health Commission, Ministry of Education, Ministry of Civil Affairs, Ministry of Finance, Medical Insurance Bureau, Bureau of Traditional Chinese Medicine, et al. Action plan to accelerate the elimination of cervical cancer (2022-2030). In: Notice on the issuance of the Action Plan for Accelerating the Elimination of Cervical Cancer (2023-2030) (2023) National Health Commission of the People's Republic of China:Department of Maternal and Health. Available at: https://www.gov.cn/zhengce/zhengceku/2023-01/21/content_5738364.htm.
49. Zhao C, Li M, Deng H, Wei L. Cervical cancer prevention and current issues in 2023. J Multidiscip Cancer Management(Electronic Version). (2024) 10:63–8. doi: 10.12151/JMCM.2024.01-10
50. Bao H-L, Jin C, Wang S, Song Y, Xu Z-Y, Yan X-J, et al. Prevalence of cervicovaginal human papillomavirus infection and genotypes in the pre-vaccine era in China: A nationwide population-based study. J infection. (2021) 82:75–83. doi: 10.1016/j.jinf.2021.02.017
Keywords: junctional adhesion molecule 3, paired box gene 1, high-risk HPV positive, cervical cancer, PAX1/JAM3 methylation
Citation: Liang H, Liu Y, Yin S, Jiang M, Dou Q, Wang H, Liu J, Chen Y, Liu P, Wang J, Wang Y and Wu Z (2024) Assessment of PAX1 and JAM3 methylation triage efficacy across HPV genotypes and age groups in high-risk HPV-positive women in China. Front. Oncol. 14:1481626. doi: 10.3389/fonc.2024.1481626
Received: 16 August 2024; Accepted: 12 November 2024;
Published: 26 November 2024.
Edited by:
Elzbieta Pluciennik, Medical University of Lodz, PolandReviewed by:
Gh Rasool Bhat, Sher - i - Kashmir Institute of Medical Sciences, IndiaGuy-Armel Bounda, China Pharmaceutical University, China
Copyright © 2024 Liang, Liu, Yin, Jiang, Dou, Wang, Liu, Chen, Liu, Wang, Wang and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hui Liang, Y2FuZHkyNjY2QDE2My5jb20=; Zhe Wu, MjM1NDU2MjAyMkBxcS5jb20=
†These authors have contributed equally to this work
 Hui Liang1*†
Hui Liang1*† Suyue Yin
Suyue Yin Jing Wang
Jing Wang