- Department of Women Health Care, Hunan Provincial Maternal and Child Health Care Hospital, Hunan, China
Objective: To describe the incidence and distribution of cervical intraepithelial neoplasia (CIN) and cervical cancer (CC) for rural women aged 35-64 in Hunan Province, China, 2020-2023.
Methods: Data were from the Hunan Provincial Cervical Cancer Screening Program in Rural Areas. Most rural women aged 35-64 in Hunan Province attend the program. All women diagnosed with CINs and CCs will be asked to register detailed information, including pelvic examination, diagnosis, age, education level, etc. CINs included low-grade squamous intraepithelial lesions (LSIL) (CIN1), high-grade squamous intraepithelial lesions (HSIL) (CIN2 and 3); CCs included adenocarcinoma in situ (AIS), early invasive cervical cancer (EICC) (stage Ia1 and Ia2) and invasive cervical cancer (ICC) (stage Ib and above). The incidence of CIN and CC is the number of cases per 1000 women. Chi-square tests (χ2) were used to examine if there were significant differences in proportions among different groups.
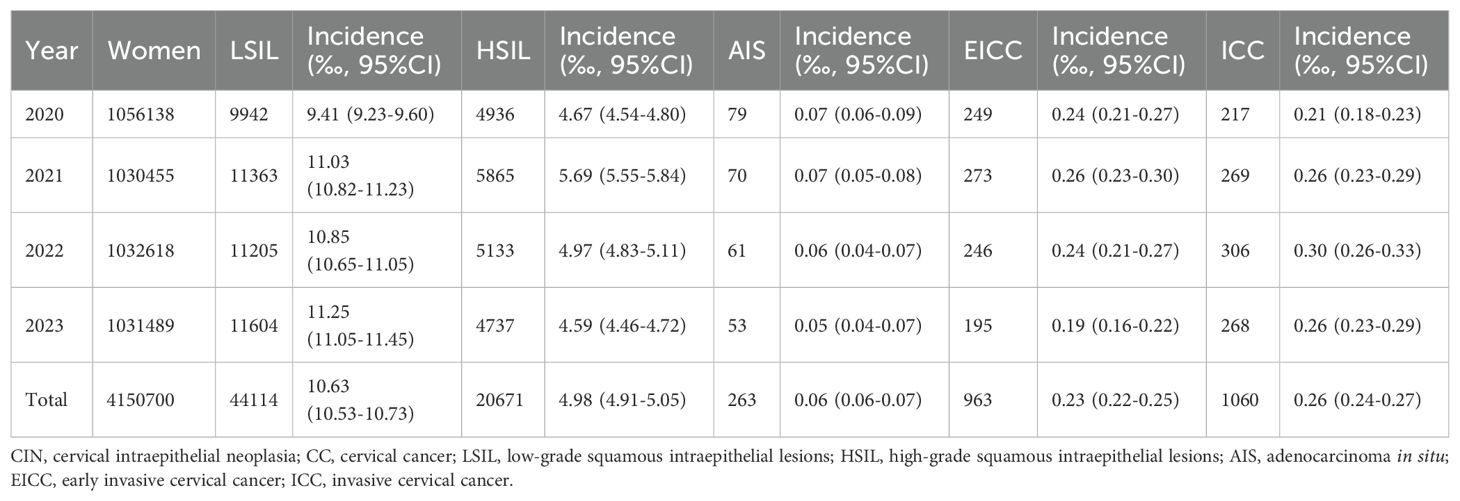
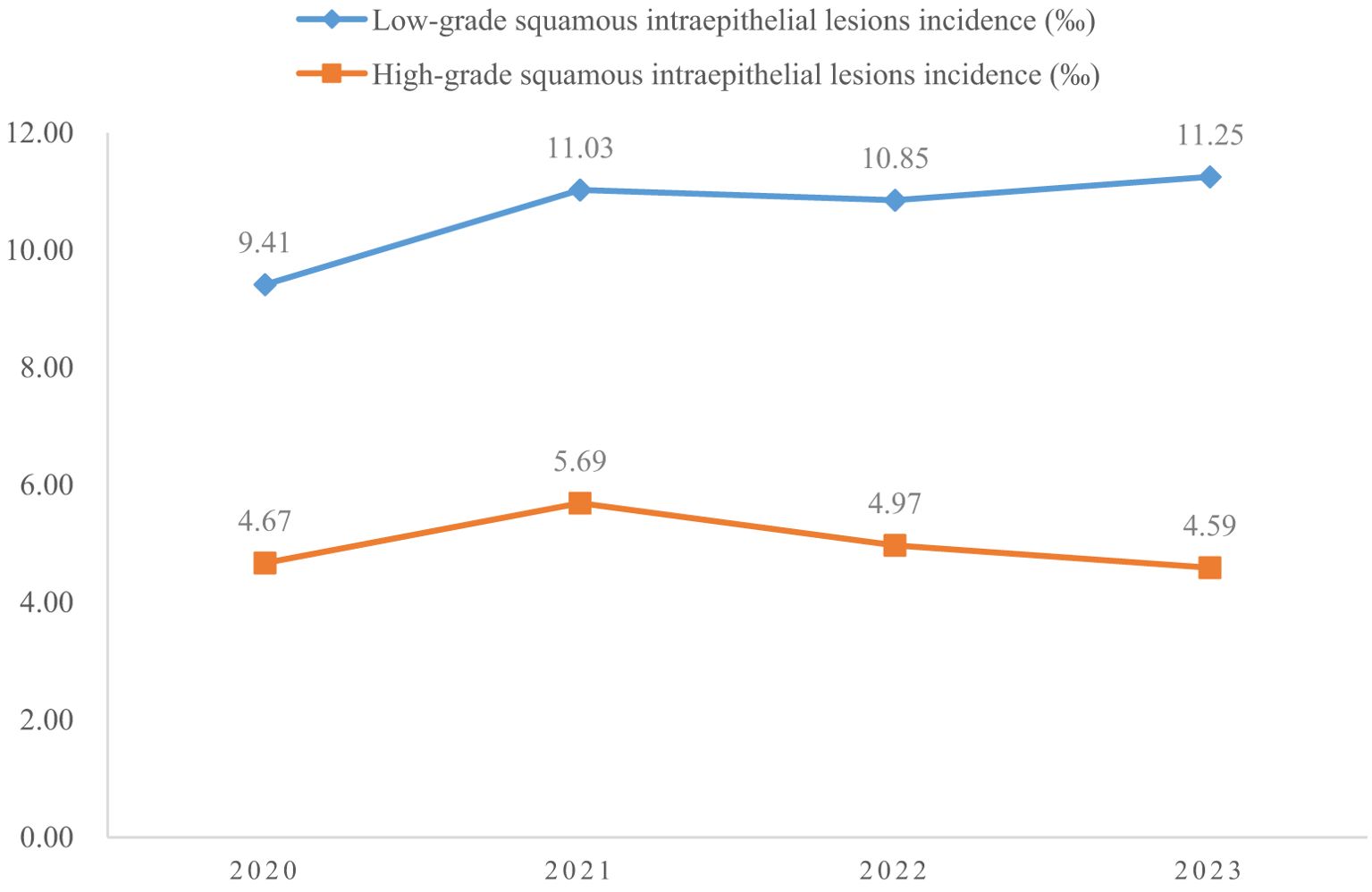
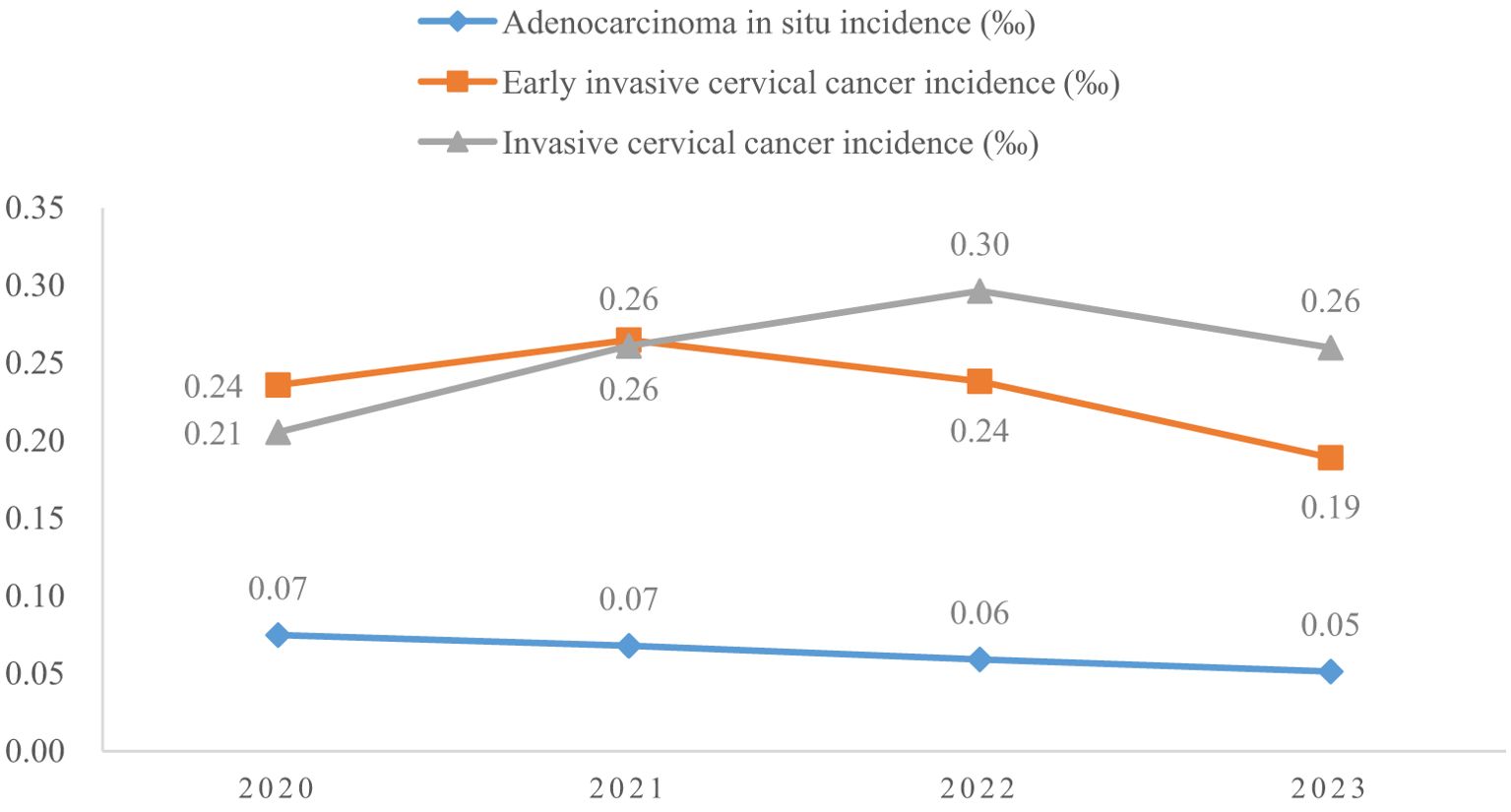
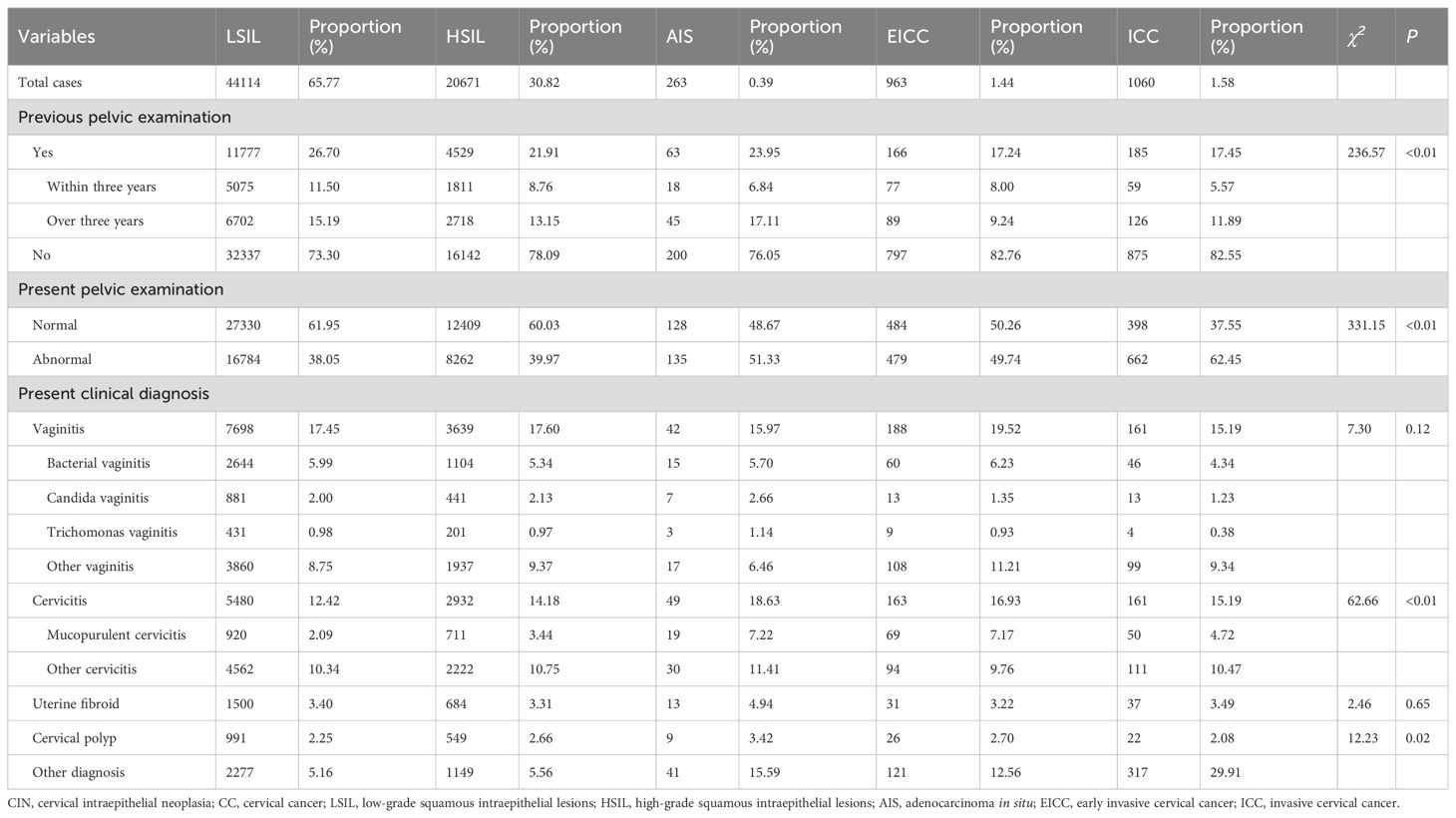
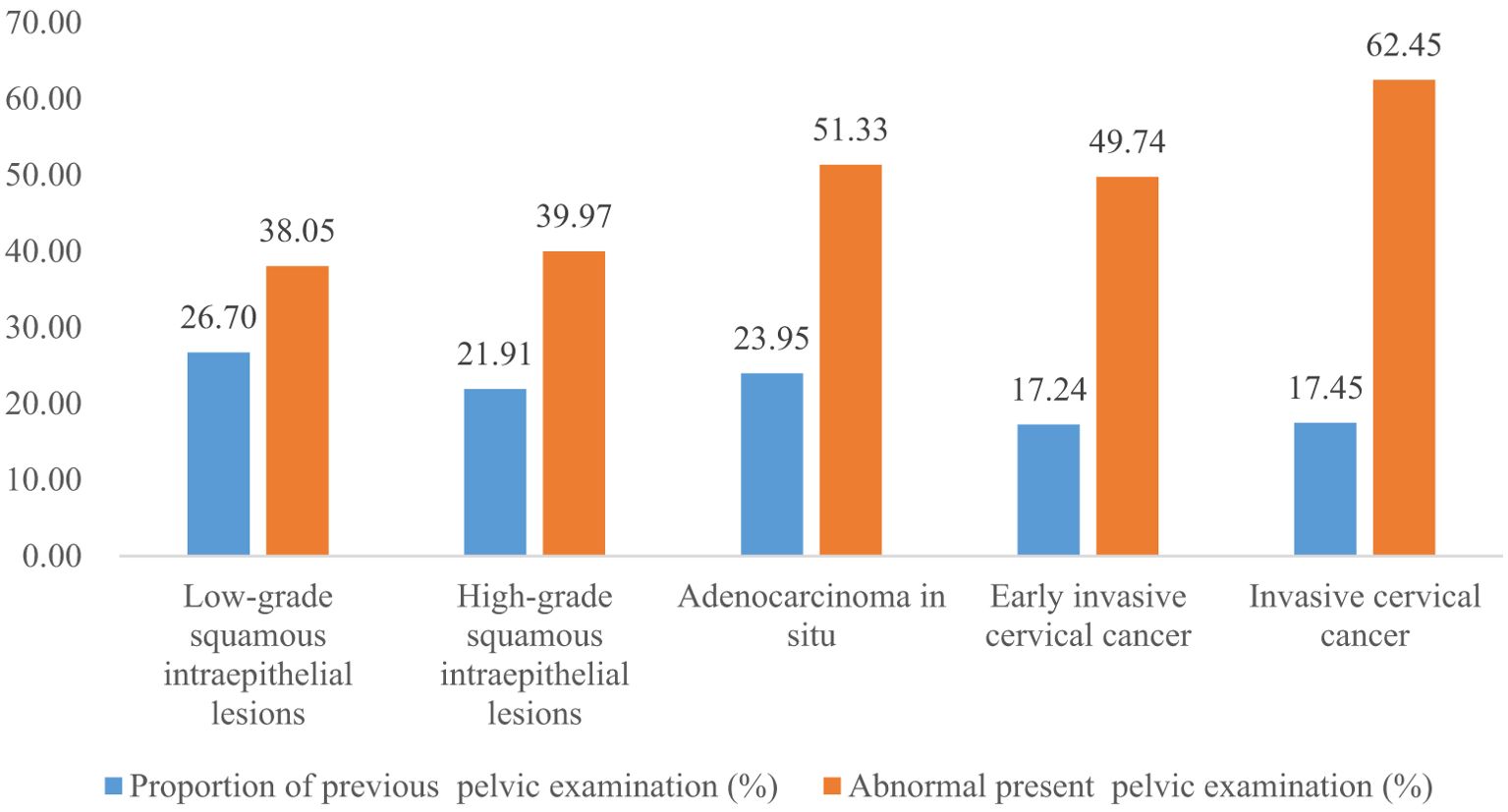
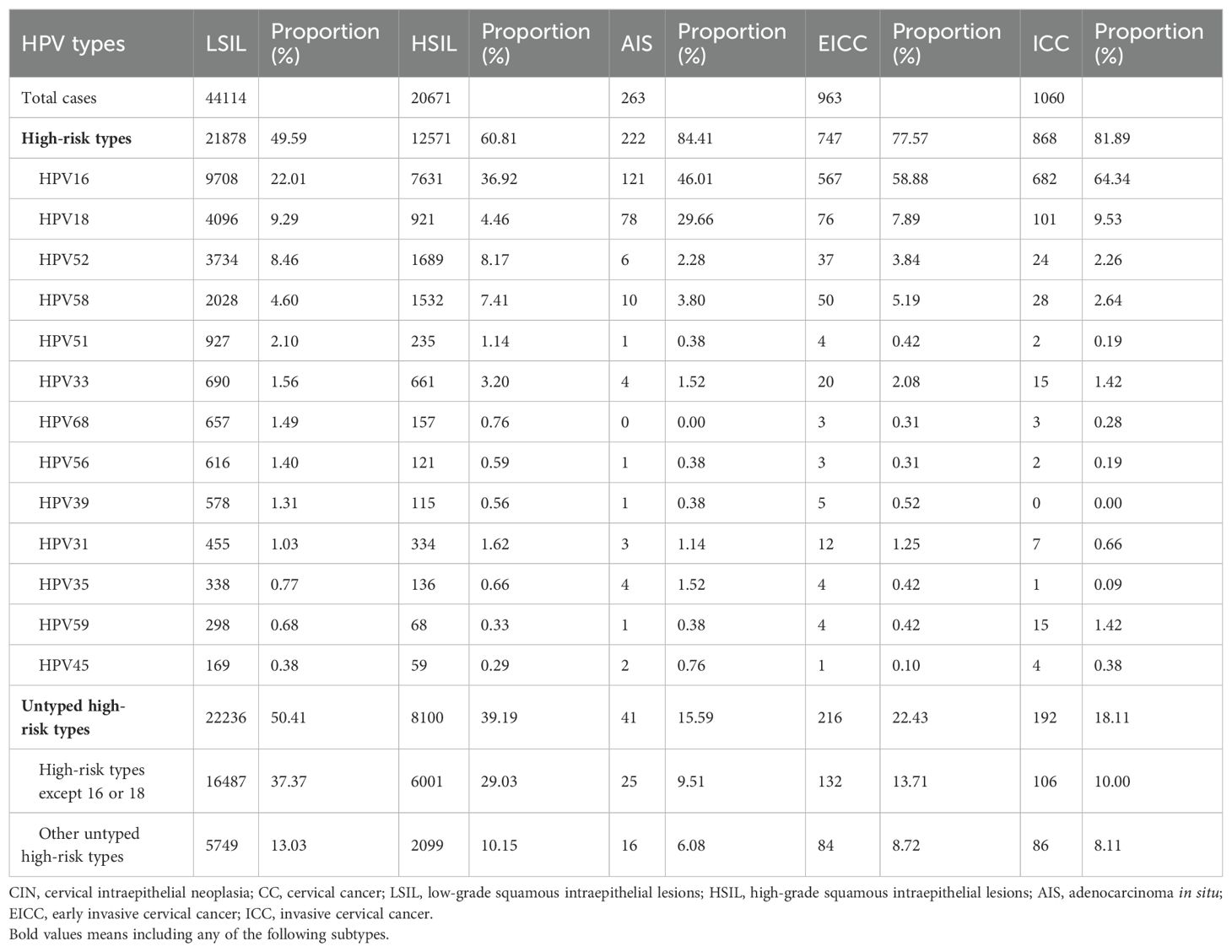
Results: A total of 4150700 women were included, and 67071 CINs and CCs were identified. The incidence of LSIL, HSIL, AIS, EICC and ICC were 10.63‰(95%CI: 10.53-10.73), 4.98‰(95%CI: 4.91-5.05), 0.06‰(95%CI: 0.06-0.07), 0.23‰(95%CI: 0.22-0.25), and 0.26‰(95%CI: 0.24-0.27), respectively. The proportion of previous pelvic examinations was relatively low in EICC (17.24%) and ICC (17.45%) (χ2 = 236.57, P <0.01), present abnormal examination was relatively high in AIS (51.33%), EICC (49.74%) and ICC (62.45%) (χ2 = 331.15, P <0.01). HPV16 was the most common high-risk type for LSIL (22.01%), HSIL (36.92%), AIS (46.01%), EICC (58.88%), and ICC (64.34%). The proportion of HSIL was relatively high in women aged 35-44 (27.03%), AIS was relatively high in women aged 45-54 (46.39%), EICC (44.24%), and ICC (48.58%) was relatively high in women aged 55-64. The proportion of ICC was relatively high in women with elementary school (38.68%), HSIL (15.10%) and AIS (17.49%) was relatively high in women with senior high school, AIS (1.52%), EICC (0.62%) and ICC (0.75%) was relatively low in women with university and above. (P <0.01).
Conclusion: We have described the incidence and distribution of CIN and CC among rural women aged 35-64. These findings were clinically relevant and were useful for clinical counseling and early diagnosis of CC.
1 Introduction
Cervical cancer (CC) is the fourth most common cancer among women globally (1). Globally in 2020, there were an estimated 604 127 CC cases and 341 831 deaths, with a corresponding age-standardized incidence of 13.3 cases per 100 000 women-years (95% CI 13.3–13.3) and mortality rate of 7.2 deaths per 100 000 women-years (95% CI 7.2–7.3) (2). CC is a global public health problem, with a particularly high burden in many low-income and middle-income countries (2), and is the most frequent gynecological cancer in developing countries (3). CC is also the leading cause of cancer-related deaths among women in low- and middle-income countries. Each year, China accounts for about 1/5 of the world’s CC incidence and deaths (4, 5). Moreover, the incidence and mortality of CC have shown an upward trend in recent years in China (6, 7). It has been one of the major threats to women’s health (6). Persistent high-risk human papillomavirus (HPV) infection is strongly and consistently associated with high-grade cervical intraepithelial neoplasia (CIN) acquisition and is considered essential for the progression of CC (8). Therefore, the study on CIN and CC is significant and deserves more attention.
In the early stages, CC is often asymptomatic; as CC progresses, nonspecific symptoms such as vaginitis and cervicitis may develop (9). Previous studies showed that persistent genital high-risk human papillomavirus (HPV) infection was the leading cause of CC (9). Okunade reported that about 99.7% of CC cases were caused by persistent genital high-risk human papillomavirus (HPV) infection (10). To reduce the incidence and mortality of CC, HPV screening and HPV vaccines for CC are becoming more and more widely used (11–13).
In 2009, the National Health Commission of the People’s Republic of China began to implement China’s National Cervical Cancer Screening Program in Rural Areas in some selected counties, which provides free screening for rural women aged 15-64 years and to encourage early diagnosis, detection, prevention, and treatment of CC (14, 15). In 2012, Hunan Province began implementing the Hunan Provincial Cervical Cancer Screening Program in Rural Areas (HPCCSPRA) in all counties, providing free CC HPV screening, diagnosis, and treatment for rural women aged 35-64.
Although there have been some studies on CC screening, diagnosis, and treatment (3, 16, 17), to the best of our knowledge, there are fewer studies on the incidence and distribution of CIN and CC based on long-term, large-sample population data, especially in Hunan Province, China.
Hunan Province is located in south-central China and covers a population of about 65 million. In this study, we conducted a systematic study using data from the HPCCSPRA (2020-2023) to describe the incidence and distribution of CIN and CC for rural women aged 35-64. This information may be useful for clinical counseling and early diagnosis of CC.
2 Methods
2.1 Data sources
Data sources were obtained from the HPCCSPRA (2020-2023). Rural women aged 35-64 years volunteered to attend the HPCCSPRA. From 2020 to 2023, most rural women in Hunan Province attend the HPCCSPRA.
The service for HPCCSPRA is as follows: (1) All women who attend the HPCCSPRA will receive pelvic examinations, microscopic detection of vaginal secretions (wet film) or Gram stain test, and high-risk HPV testing. The main items of pelvic examinations include vaginitis (including bacterial vaginitis, candida vaginitis, trichomonas vaginitis, et al.), cervicitis, uterine fibroid, cervical polyp, condyloma acuminatum, et al. (2) Women with high-risk HPV will receive further cervical cytology. If the result of the cervical cytology is abnormal or suspected, they will receive further colposcopy. Women with HPV 16 or 18 will receive colposcopy directly. According to the International Agency for Research on Cancer, of the 30-40 HPV types that infect the anogenital tract, there are 13 high-risk (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and 68) HPV types (18). (3) Women with abnormal or suspicious colposcopy will receive histopathological examination. (4) All women diagnosed with CINs and CCs will be asked to register detailed information, including previous pelvic examination, present pelvic examination, diagnosis, age, education level, etc. Detailed information about the data collection has been reported elsewhere (19, 20).
2.2 Ethics approval and consent to participate
The Hunan Provincial Health Commission routinely collected that data, and the government developed the “Working Manual for HPCCSPRA” to collect that data. Therefore, there is no additional written informed consent. The Medical Ethics Committee of Hunan Provincial Maternal and Child Health Care Hospital approved the study. (NO: 2024-S041). It is a retrospective study of medical records; all data were fully anonymized before we accessed them. Moreover, we de-identified the patient records before analysis. We confirmed that all operations were following relevant guidelines and regulations.
2.3 Data quality control
The Hunan Provincial Health Commission developed a quality control manual for the province. Data were collected and reported by experienced and trained doctors and nurses. To ensure data consistency and accuracy, all collectors must be trained and qualified before starting work. The Hunan Provincial Health Commission asks the technical guidance departments to conduct comprehensive quality control yearly to reduce surveillance data integrity and information error rates.
2.4 Definitions
According to the International Federation of Gynecology and Obstetrics and Bethesda System for cervical cytology, CINs were classified as low-grade squamous intraepithelial lesions (LSIL) (including CIN1), high-grade squamous intraepithelial lesions (HSIL) (including CIN2 and 3); CCs were classified as adenocarcinoma in situ (AIS), early invasive cervical cancer (EICC) (encompassing: stage Ia1 and Ia2) and invasive cervical cancer (ICC) (encompassing: stage Ib and above) according to the types and stages (21, 22). The incidence of CIN and CC is defined as the number of cases per 1000 women. Since almost all CINs and CCs are associated with HPV, the incidence in this study is similar to the actual incidence.
2.5 Statistical analysis
The incidence of CIN and CC with 95% confidence intervals (CI) was calculated by the log-binomial method (23). Chi-square tests (χ2) were used to examine if there were significant differences in proportion among different groups.
Statistical analyses were performed using SPSS 22.0 (IBM Corp., NY, USA).
3 Results
3.1 Incidence of CIN and CC in Hunan Province, China, 2020-2023
A total of 4150700 women were included in this study, and 67071 CINs and CCs were identified. The incidence of LSIL, HSIL, AIS, EICC and ICC were 10.63‰(95%CI: 10.53-10.73), 4.98‰(95%CI: 4.91-5.05), 0.06‰(95%CI: 0.06-0.07), 0.23‰(95%CI: 0.22-0.25), and 0.26‰(95%CI: 0.24-0.27), respectively. The incidence of CIN (including LSIL and HSIL) was 15.61‰(95%CI: 15.49-15.73), and the incidence of CC (including AIS, EICC, and ICC) was 0.55‰ (95%CI: 0.53-0.57). Table 1 shows the details of the incidence of CIN and CC in Hunan Province, China, 2020-2023. (Table 1; Figures 1, 2).
3.2 Pelvic examinations of CIN and CC
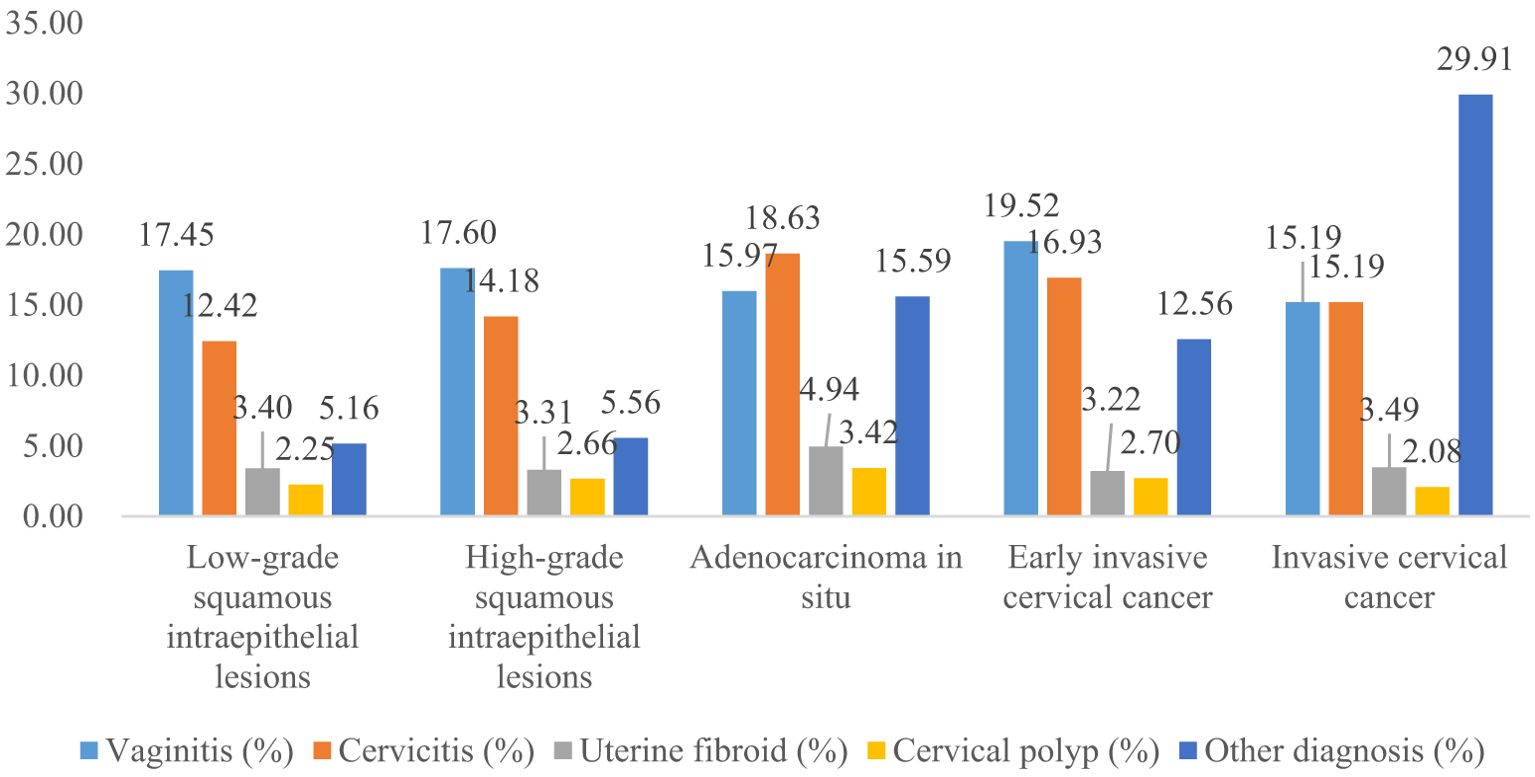
The proportion of previous pelvic examinations in LSIL, HSIL, AIS, EICC, and ICC was 26.70%, 21.91%, 23.95%, 17.24%, and 17.45%, respectively, and was relatively low in EICC and ICC (χ2 = 236.57, P <0.01). In the present pelvic examination, the proportion of abnormal examination results in LSIL, HSIL, AIS, EICC, and ICC was 38.05%, 39.97%, 51.33%, 49.74%, and 62.45%, respectively, and was relatively high in AIS, EICC and ICC (χ2 = 331.15, P <0.01). In the present clinical diagnosis, the proportion of vaginitis in LSIL, HSIL, AIS, EICC, and ICC was 17.45%, 17.60%, 15.97%, 19.52%, and 15.19%, respectively, without significant differences (χ2 = 7.30, P =0.12); The proportion of cervicitis in LSIL, HSIL, AIS, EICC, and ICC was 12.42%, 14.18%, 18.63%, 16.93%, and 15.19%, respectively, and was relatively high in AIS, EICC, and ICC (χ2 = 62.66, P <0.01); The proportion of uterine fibroid in LSIL, HSIL, AIS, EICC, and ICC was 3.40%, 3.31%, 4.94%, 3.22%, and 3.49%, respectively, without significant differences (χ2 = 2.46, P =0.65); The proportion of cervical polyps in LSIL, HSIL, AIS, EICC, and ICC was 2.25%, 2.66%, 3.42%, 2.70%, and 2.08%, respectively, and was relatively high in AIS (χ2 = 12.23, P =0.02). Table 2 shows the details of the pelvic examination of CIN and CC. (Table 2; Figures 3, 4).
3.3 High-risk HPV types in CIN and CC
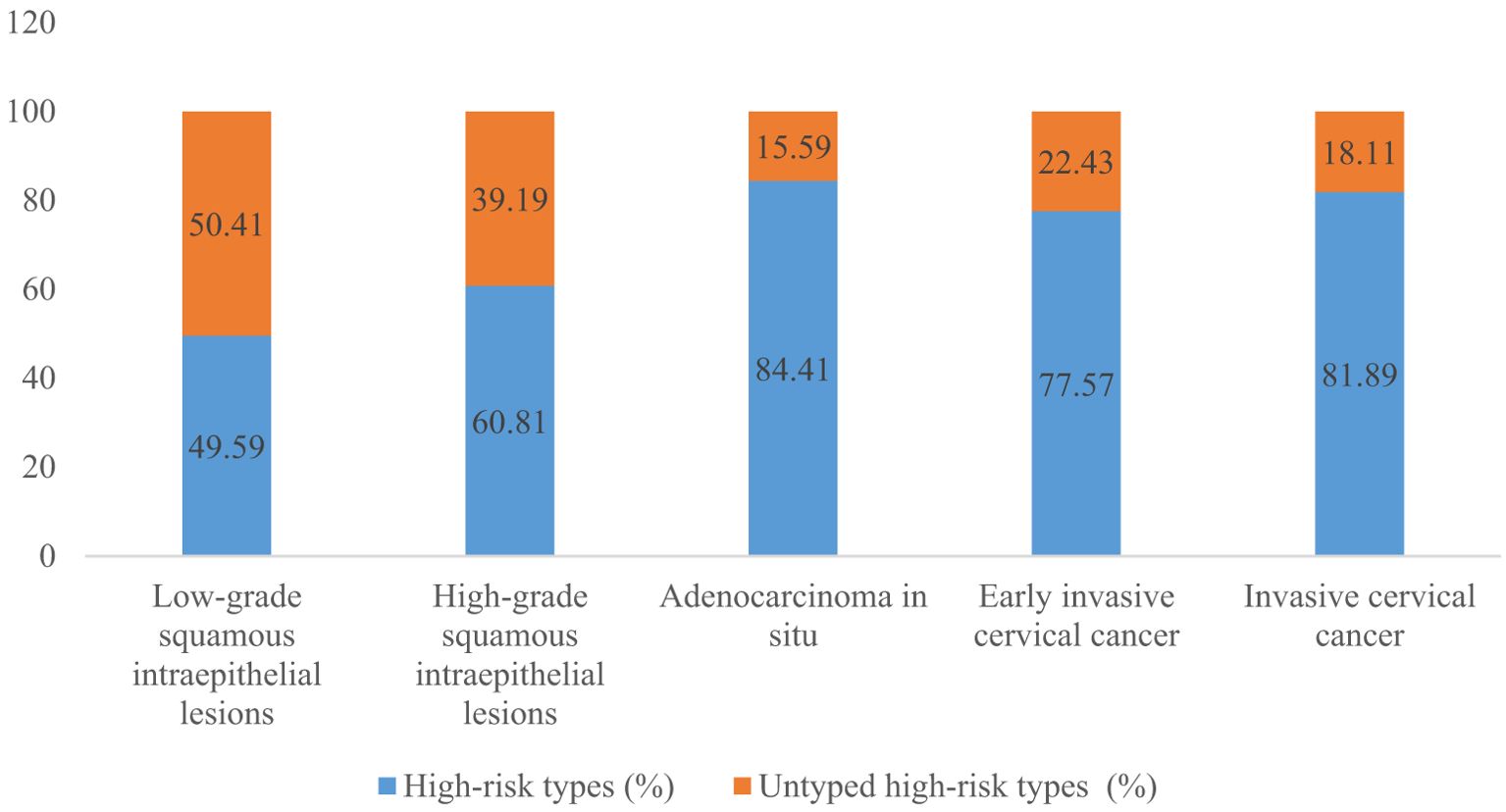
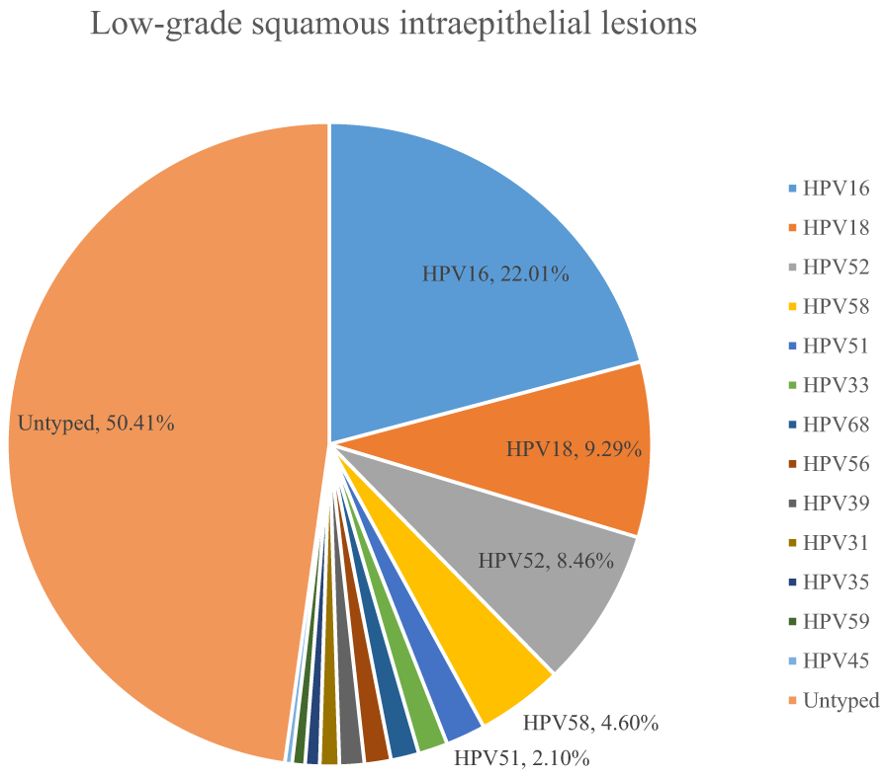
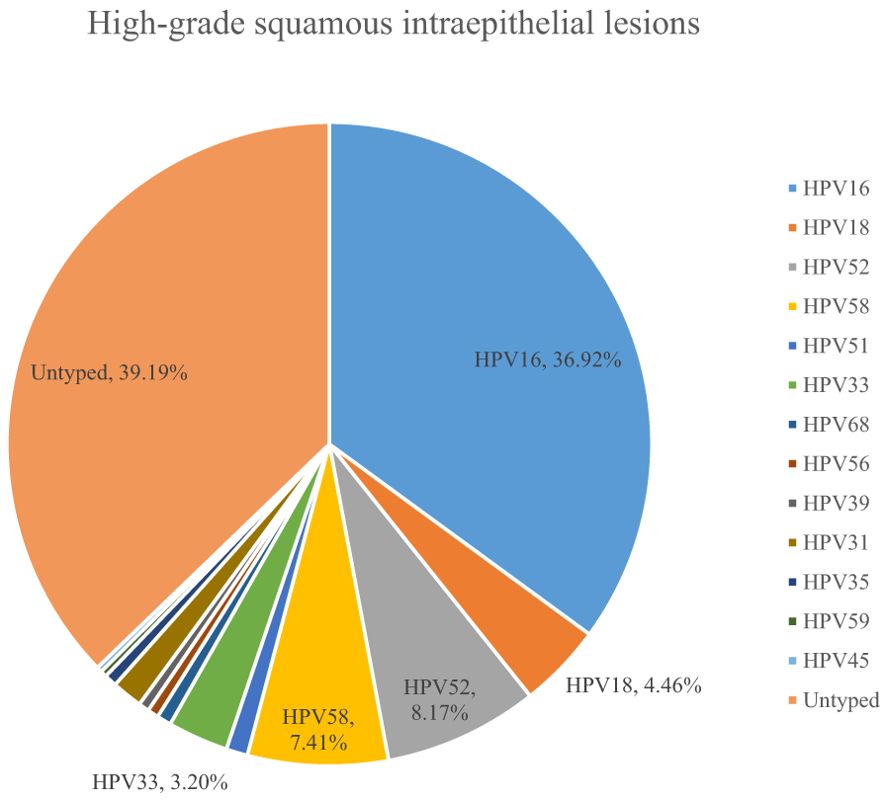
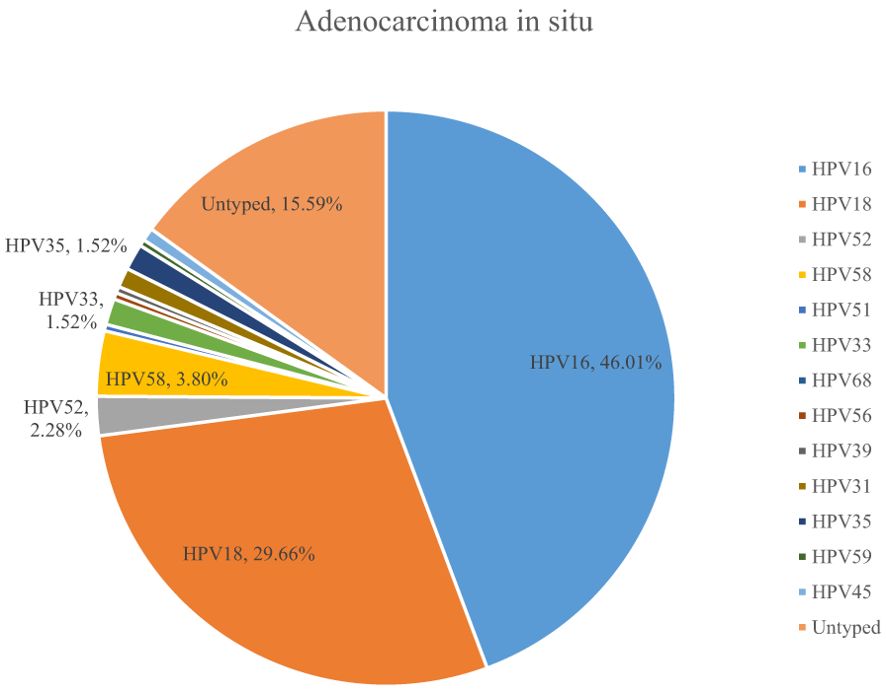
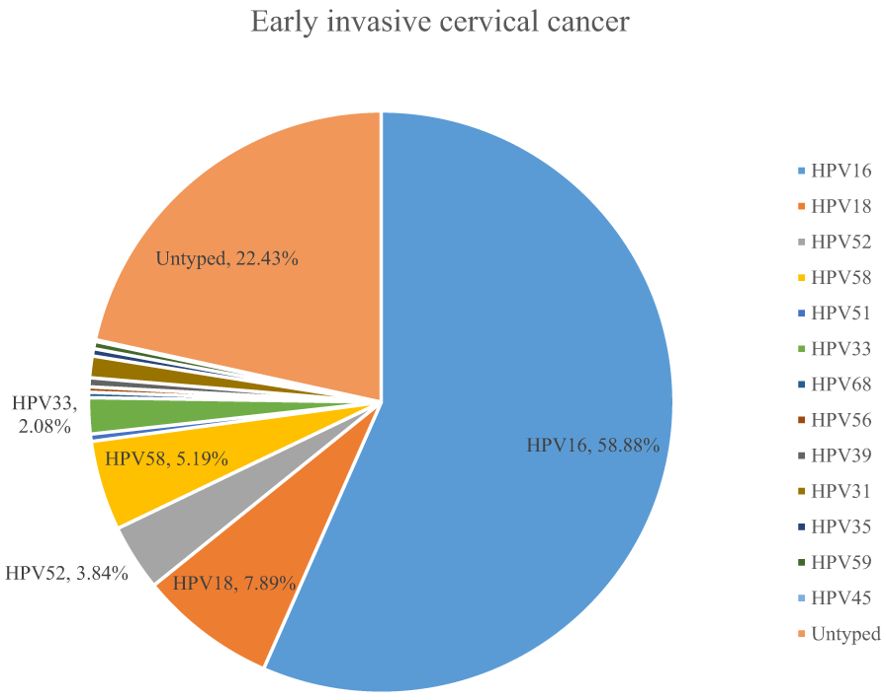
A total of 54.10% (36286/67071) of CINs and CCs identified a specific high-risk HPV type. The proportion of untyped high-risk types in LSIL, HSIL, AIS, EICC, and ICC was 50.41%, 39.19%, 15.59%, 22.43%, and 18.11%, respectively, and was relatively high in LSIL and HSIL (χ2 = 1376.50, P <0.01). HPV16 was the most common high-risk type for LSIL (22.01%), HSIL (36.92%), AIS (46.01%), EICC (58.88%), and ICC (64.34%). There were significant differences in the proportions (χ2 = 2799.41, P <0.01). The proportion of HPV16 was relatively high for AIS, EICC, and ICC. HPV18 was the second most common high-risk type for LSIL (9.29%), AIS (29.66%), EICC (7.89%), and ICC (9.53%), and was the fourth most common high-risk type for HSIL (4.46%). There were also significant differences in the proportions (χ2 = 631.12, P <0.01). The proportion of HPV18 was relatively high for AIS. HPV52 (8.17%) and HPV58 (7.41%) were the second and third most common high-risk types for HSIL, respectively, and the proportions were significantly higher than HPV18 (4.46%) (χ2 = 255.55, P <0.01). Table 3 shows the details of high-risk HPV types in CINs and CCs. (Table 3; Figures 5–10).
3.4 Distribution of CIN and CC by epidemiological characteristics
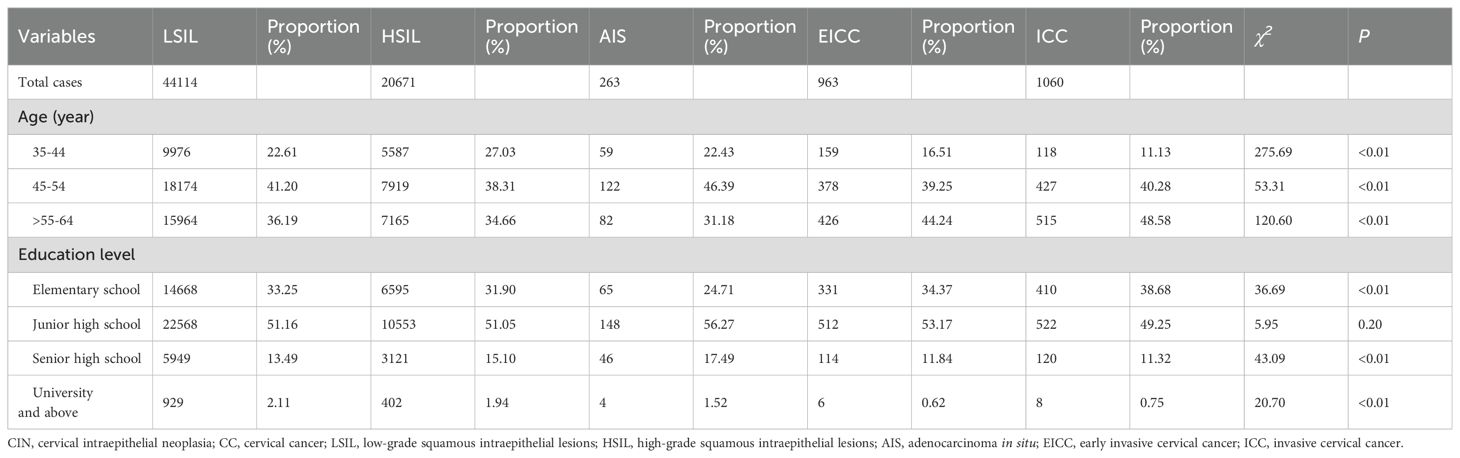
Women aged 35-44, 45-54, and 55-64 years accounted for 22.61%, 41.20%, and 36.19% of LSIL, respectively. Compared to LSIL, the proportion of HSIL was relatively high in women aged 35-44 years (27.03%), the proportion of AIS was relatively high in women aged 45-54 years (46.39%), the proportion of EICC (44.24%) and ICC (48.58%) was relatively high in women aged 55-64 years. (P <0.01).
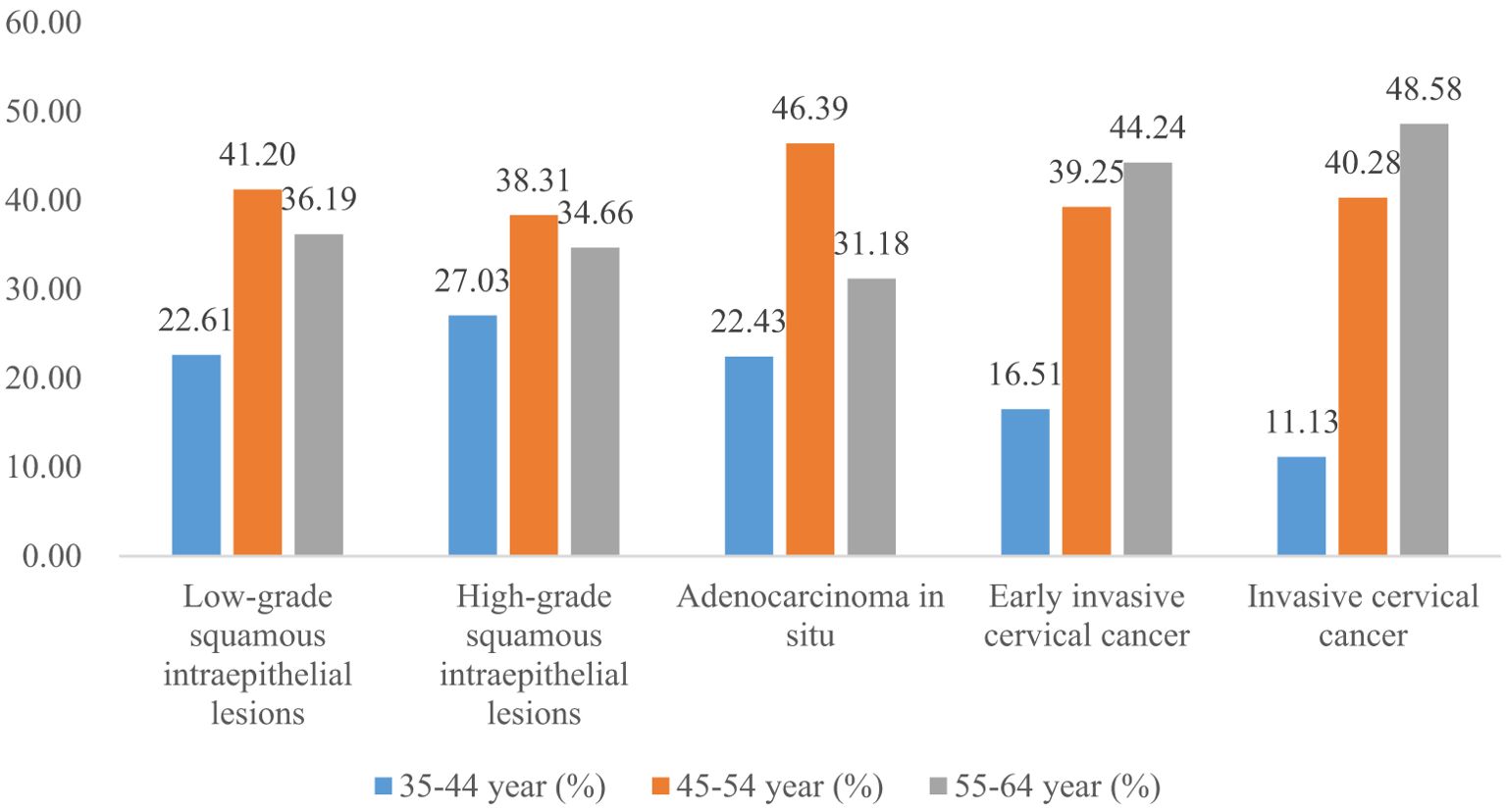
Women with elementary school, junior high school, senior high school, and university and above accounted for 33.25%, 51.16%, 13.49%, and 2.11% of all LSIL, respectively. Compared to LSIL, the proportion of ICC was relatively high in women with elementary school (38.68%), the proportion of HSIL (15.10%) and AIS (17.49%) was relatively high in women with senior high school, the proportion of AIS (1.52%), EICC (0.62%) and ICC (0.75%) was relatively low in women with university and above. (P <0.01). (Table 4; Figures 11, 12).
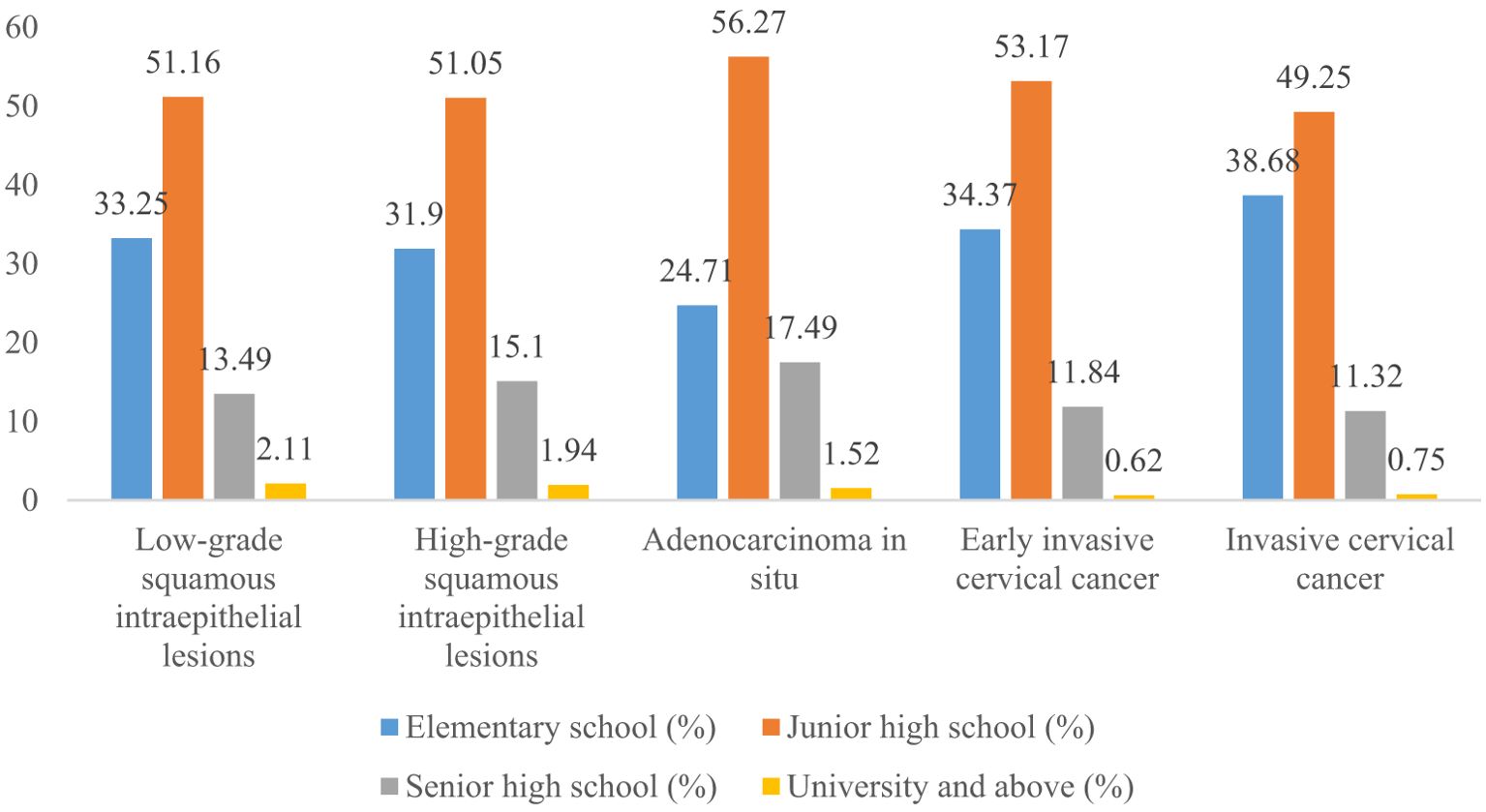
Figure 12. Distribution of cervical intraepithelial neoplasia and cervical cancer by education level.
4 Discussion
Overall, we have described the incidence and distribution of CIN and CC among rural women aged 35-64. To the best of our knowledge, previous studies rarely address this issue. The study was clinically relevant, and our findings may be useful for clinical counseling and early diagnosis of CC.
First, we have reported the incidence of CIN and CC for rural women aged 35-64. To the best of our knowledge, few studies have reported the incidence of CIN and CC for rural women aged 35-64 years based on a large population, including in China. Some studies have reported the overall incidence of CC, or the incidence of CIN, for women aged 35-64. For example, Gu et al. reported that the CC incidence was 0.28‰ for the total population aged 35-64 years in China (2000-2015) (24), and the CC incidence for women aged 35-64 years was about 0.56‰ (half of the total). It is consistent with the CC incidence reported in this study. Zhao et al. reported that the CC incidence was 26.54/100000, and the HSIL incidence was 223.89/100000 for women aged 35-64 years in rural areas in China (2018) (19); Shen et al. reported that the incidence of HSIL and CC was 395.6/100000 for women aged 35-64 years in Beijing City, China (2014-2015) (25); Lu et al. reported that the incidence of LSIL, HSIL, and CC was 1.89%, 0.60% and 0.03%, respectively, for women in minority area, Guangxi Province, China (2012-2019) (26); Shahmoradi et al. reported that the CC incidence among US women aged 35-64 years was from 14.38/100000 to 25.51/100000 (2001-2019) (27); Cleveland et al. reported that AIS incidence for women aged 40-64 years was about 2.4/100000 to 3.4/100000 (US, 2008-2015) (28). There are significant differences between these studies and the present study. On the one hand, it may be associated with differences in economic and medical conditions in different areas, which may affect CC screening and diagnosis rates (29). On the other hand, some previous studies were limited in study data, such as the study populations were from several hospitals, from small areas, or over a shorter period. To the best of our knowledge, no study has reported the incidence of CIN and CC in Hunan Province based on province-wide data. In addition, we have reported the incidence of CIN and CC by stages. It is of great significance for public health.
Second, among LSIL, HSIL, AIS, EICC, and ICC, there were significant differences in the proportion of previous pelvic examination and abnormal present pelvic examination, with a relatively low proportion of previous pelvic examination in EICC and ICC and a relatively high proportion of abnormal present pelvic examination in AIS, EICC, and ICC. It suggests that the more severe the CC, the more common the associated abnormal pelvic examinations are, but the lower the proportion of pelvic examinations. Moreover, it also suggests that women suffering from CC may be associated with a lack of pelvic examination. Previous studies support these findings (30). In this study, among LSIL, HSIL, AIS, EICC, and ICC, there was no significant difference in the proportion of vaginitis and uterine fibroid. At the same time, there were significant differences in the proportion of cervicitis and cervical polyp. The proportion of cervicitis was relatively high in AIS, EICC, and ICC, and the proportion of cervical polyps was relatively high in AIS. Overall, fewer studies address the above issues.
Although details of the negative population were not available in this study, if we regard LSIL as the reference, some previous studies were consistent with this study. For example, Long et al. reported that severe cervical inflammation was positively related to HSIL (31). Zhang et al. reported that cervical inflammation was a risk factor for cervical lesions (32). Zhao et al. reported that concurrent reproductive tract infections (such as cervicitis and cervical erosion) were risk factors for CC (33). Levy et al. reported an increased incidence of atypical squamous cells of undetermined significance, and atypical glandular cells not otherwise specified pap diagnoses were found in women with benign polyps on biopsy (34). Kucukyıldız et al. reported that endocervical polyps may be more common in patients with high-risk HPV infections (35). However, some previous studies were inconsistent with this study. For example, Brusselaers et al. found a causal link between vaginal dysbiosis and CC along the oncogenic human papillomavirus acquisition, persistence, and cervicovaginal dysplasia development pathway (36). Tao et al. reported that risk factors for HSIL included both Trichomonas vaginalis infection and cervical inflammation (37). The inconsistent results described above may be related to various factors (30, 38), such as different economic and medical conditions. Our findings suggest that there may be a correlation, perhaps a causal relationship, between CIN and CC and a broad range of abnormal pelvic examinations. It deserves in-depth research. Our findings may be useful for future in-depth studies.
Third, previous studies showed that almost all CCs are associated with HPV infection (10). In this study, we have presented the 13 high-risk HPV types for CIN and CC in detail, which have rarely been addressed in previous studies. HPV16 and HPV 18 were the most common high-risk types for all stages except for HSIL, and the more severe the CC, the higher the proportion of HPV16. It is consistent with previous studies (39, 40). However, there were significant differences in HPV types and proportions between this study and previous studies. For example, Muñoz et al. reported that HPV45 (4.4%) and HPV31 (3.4%) were common high-risk types (11 studies in nine countries, from 1985 to 1997) (40). Yang et al. reported that the most common high-risk HPV types were HPV52 (22.02%), HPV58 (12.79%) and HPV16 (12.60%) in LSIL; HPV16 (28.31%), HPV52 (20.63%), and HPV58 (17.73%) in HSIL; HPV16 (37.17%), HPV52 (13.77%), and HPV18 (8.39%) in EICC and ICC (Guangzhou City, China, 2015-2021) (41). Zhang et al. reported that the most common high-risk HPV types were HPV52 (20.31%), HPV16 (16.81%), HPV58 (14.44%), HPV18 (6.44%), and HPV53 (5.76%) in CIN1 (LSIL); HPV16 (45.69%), HPV58 (15.50%), HPV52 (11.74%), HPV33 (9.35%), and HPV31 (4.34%) in CIN2/3 (HSIL) (China, 2000-2019) (42). Cleveland et al. reported that HPV16 was the most frequently detected high-risk type in both AIS (57%) and CIN3 (58%), whereas HPV18 was the second most common high-risk type in AIS (38%) and less common in CIN3 (5%) (US, 2008-2015) (28). It is inconsistent with this study. These differences may be related partly to the fact that detailed HPV types were not reported in many cases, especially in LSIL and HSIL. It may lead to difficulties in comparing this study with other studies. It is a shortcoming in this study. However, due to the large sample size and comprehensive presentation of various HPV types in this study, this remains an important reference, for example, for vaccine research (43).
Fourth, compared to LSIL, the proportion of EICC and ICC was relatively high in women aged 55-64; the proportion of AIS, EICC, and ICC was relatively low in women with university and above. Details of the negative population were unavailable in this study. If we regard LSIL as the reference, we would get the following findings: the proportion of severe CC was relatively high in the older age groups and relatively low in women with high education levels. It is consistent with previous studies (26, 32, 37, 44, 45). The proportion of severe CC was relatively high in the older age groups, possibly associated with several factors. On the one hand, in the early stages, CC is often asymptomatic, resulting in most women not being screened or examined (9, 46). When severe CC appears, it often develops over a long time. On the other hand, women’s economic conditions, medical conditions, and health knowledge increase with age, increasing CC screening rates (4, 29, 47, 48). Compared to older women, younger women may lack systematic screening and pelvic examination. The proportion of severe CC was relatively low in highly educated people, which may be mainly associated with better health knowledge and economic conditions. Health knowledge is one of the main factors in CC screening; a higher level of education and confidence that any potential symptom would be identified were associated with increased awareness (47–50). Education level is associated with economic conditions, which may have an important impact on CC screening, diagnosis, and treatment (29, 51). Women with high education levels may access earlier screening and diagnosis of CC, reducing the proportion of severe CC.
Some things could be improved in this study. First, due to data limitations, some demographic characteristics, such as economic conditions and ethnic groups, were not included in this study. In addition, there is no detailed information on the medical cost of CIN and CC. Second, detailed HPV types were not reported in many patients, especially in LSIL and HSIL, which may lead to difficulties in comparing this study with other studies. Third, this study focused on the pelvic examination and epidemiology of patients. Due to the lack of detailed information on the negative population, we could not conduct some analyses, such as calculating the incidence of CIN and CC by age and education level and analyzing risk factors for CIN and CC. Fourth, all the patients in this study were women with high-risk HPV. However, although almost all CINs and CCs are associated with high-risk HPV, there are still some that may not be associated with high-risk HPV. Therefore, the incidence of CIN and CC may be slightly underestimated.
Despite some limitations, this study is still of great significance. On the one hand, this study involved many people and covered a wide area, which was well representative. On the other hand, the research limitations pointed out above also provided directions for further in-depth research, and our findings may be useful for future in-depth studies.
5 Conclusion
We have described the incidence and distribution of CIN and CC among rural women aged 35-64. These findings were clinically relevant and were useful for clinical counseling and early diagnosis of CC.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author/s.
Ethics statement
The studies involving humans were approved by The Medical Ethics Committee of Hunan Provincial Maternal and Child Health Care Hospital approved the study. (NO: 2024-S041). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
XZ: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. TH: Data curation, Investigation, Resources, Writing – original draft. TG: Data curation, Investigation, Resources, Writing – original draft. YL: Data curation, Investigation, Resources, Writing – original draft. HL: Data curation, Investigation, Resources, Writing – original draft. WY: Data curation, Funding acquisition, Investigation, Project administration, Resources, Validation, Writing – original draft. YW: Conceptualization, Data curation, Investigation, Project administration, Resources, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. Hunan Provincial Science and Technology Department clinical medical technology innovation guidance project (NO: 2021SK50611). (from WY) National Key Clinical Specialty Scientific Research Project (grant number: Z2023106). (from YW)
Acknowledgments
The authors thank all the women who participated in the project.
Conflict of interest
The authors declare that the research was conducted without any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Vu M, Yu J, Awolude OA, Chuang L. Cervical cancer worldwide. Curr problems cancer. (2018) 42:457–65. doi: 10.1016/j.currproblcancer.2018.06.003
2. Singh D, Vignat J, Lorenzoni V, Eslahi M, Ginsburg O, Lauby-Secretan B, et al. Global estimates of incidence and mortality of cervical cancer in 2020: a baseline analysis of the WHO Global Cervical Cancer Elimination Initiative. Lancet Global Health. (2023) 11:e197–206. doi: 10.1016/s2214-109x(22)00501-0
3. Tsikouras P, Zervoudis S, Manav B, Tomara E, Iatrakis G, Romanidis C, et al. Cervical cancer: screening, diagnosis and staging. J BUON: Off J Balkan Union Oncol. (2016) 21:320–5.
4. Arbyn M, Weiderpass E, Bruni L, de Sanjosé S, Saraiya M, Ferlay J, et al. Estimates of incidence and mortality of cervical cancer in 2018: a worldwide analysis. Lancet Global Health. (2020) 8:e191–203. doi: 10.1016/s2214-109x(19)30482-6
5. Shrestha AD, Neupane D, Vedsted P, Kallestrup P. Cervical cancer prevalence, incidence and mortality in low and middle income countries: A systematic review. Asian Pacific J Cancer prevention: APJCP. (2018) 19:319–24. doi: 10.22034/apjcp.2018.19.2.319
6. Guo M, Xu J, Du J. Trends in cervical cancer mortality in China from 1989 to 2018: an age-period-cohort study and Joinpoint analysis. BMC Public Health. (2021) 21:1329. doi: 10.1186/s12889-021-11401-8
7. Yuan M, Zhao X, Wang H, Hu S, Zhao F. Trend in cervical cancer incidence and mortality rates in China, 2006-2030: A bayesian age-period-cohort modeling study. Cancer epidemiology Biomarkers prevention: Publ Am Assoc Cancer Research cosponsored by Am Soc Prev Oncol. (2023) 32:825–33. doi: 10.1158/1055-9965.Epi-22-0674
8. Hoffman SR, Le T, Lockhart A, Sanusi A, Dal Santo L, Davis M, et al. Patterns of persistent HPV infection after treatment for cervical intraepithelial neoplasia (CIN): A systematic review. Int J cancer. (2017) 141:8–23. doi: 10.1002/ijc.30623
9. Cohen PA, Jhingran A, Oaknin A, Denny L. Cervical cancer. Lancet (London England). (2019) 393:169–82. doi: 10.1016/s0140-6736(18)32470-x
10. Okunade KS. Human papillomavirus and cervical cancer. J obstetrics gynaecology: J Institute Obstetrics Gynaecology. (2020) 40:602–8. doi: 10.1080/01443615.2019.1634030
11. Bedell SL, Goldstein LS, Goldstein AR, Goldstein AT. Cervical cancer screening: past, present, and future. Sexual Med Rev. (2020) 8:28–37. doi: 10.1016/j.sxmr.2019.09.005
12. Bhatla N, Singhal S. Primary HPV screening for cervical cancer. Best Pract Res Clin obstetrics gynaecology. (2020) 65:98–108. doi: 10.1016/j.bpobgyn.2020.02.008
13. Wang R, Pan W, Jin L, Huang W, Li Y, Wu D, et al. Human papillomavirus vaccine against cervical cancer: Opportunity and challenge. Cancer letters. (2020) 471:88–102. doi: 10.1016/j.canlet.2019.11.039
14. Zhang M, Bao HL, Wang LM, Zhao ZP, Huang ZJ, Zhang X, et al. Analysis of cervical cancer screening and related factors in China. Zhonghua yi xue za zhi. (2021) 101:1869–74. doi: 10.3760/cma.j.cn112137-20210108-00054
15. National-Health-Commission-of-the-People's-Republic-of-China. Cervical Cancer Screening Program for Rural Women (2009). Available online at: http://www.nhc.gov.cn/wjw/gfxwj/201304/f02672de69334e32be55acb7e3520ae8.shtml (Accessed June 1, 2024).
16. Sharma S, Deep A, Sharma AK. Current treatment for cervical cancer: an update. Anti-cancer Agents medicinal Chem. (2020) 20:1768–79. doi: 10.2174/1871520620666200224093301
17. Hill EK. Updates in cervical cancer treatment. Clin obstetrics gynecology. (2020) 63:3–11. doi: 10.1097/grf.0000000000000507
18. IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Human papillomaviruses 2024. Available at: https://publications.iarc.fr/Book-And-Report-Series/Iarc-Monographs-On-The-Identification-Of-Carcinogenic-Hazards-To-Humans/Human-Papillomaviruses-2007 (Accessed June 1, 2024).
19. Zhao YX, Ma L, Ren WH, Song B, Wang LH, Di JL, et al. Analysis of the reported data of National Cervical Cancer Screening Program in Rural Areas in China from 2009 to 2018. Zhonghua yi xue za zhi. (2021) 101:1863–8. doi: 10.3760/cma.j.cn112137-20210111-00075
20. Zhao Y, Bao H, Ma L, Song B, Di J, Wang L, et al. Real-world effectiveness of primary screening with high-risk human papillomavirus testing in the cervical cancer screening programme in China: a nationwide, population-based study. BMC Med. (2021) 19:164. doi: 10.1186/s12916-021-02026-0
21. Saleh M, Virarkar M, Javadi S, Elsherif SB, de Castro, Bhosale P. Cervical cancer: 2018 revised international federation of gynecology and obstetrics staging system and the role of imaging. AJR Am J roentgenology. (2020) 214:1182–95. doi: 10.2214/ajr.19.21819
22. Solomon D, Davey D, Kurman R, Moriarty A, O'Connor D, Prey M, et al. The 2001 Bethesda System: terminology for reporting results of cervical cytology. Jama. (2002) 287:2114–9. doi: 10.1001/jama.287.16.2114
23. Petersen MR, Deddens JA. A comparison of two methods for estimating prevalence ratios. BMC Med Res methodology. (2008) 8:9. doi: 10.1186/1471-2288-8-9
24. Gu X, Sun G, Zheng R, Zhang S, Zeng H, Sun K, et al. Incidence and mortality of cervical cancer in China in 2015. J Natl Cancer Center. (2022) 2:70–7. doi: 10.1016/j.jncc.2022.01.002
25. Shen J, Gao LL, Zhang Y, Han LL, Wang JD. Prevalence of high-risk HPV and its distribution in cervical precancerous lesions among 35-64 years old women who received cervical cancer screening in Beijing. Zhonghua yu fang yi xue za zhi [Chinese J Prev medicine]. (2018) 52:493–7. doi: 10.3760/cma.j.issn.0253-9624.2018.05.008
26. Lu H, He H, Qin J, Chen M, Liu Q, Li M, et al. Populations at high risk of cervical cancer in Guangxi Province: Findings from two screening projects in a minority area of South China. J Med screening. (2022) 29:44–52. doi: 10.1177/09691413211039254
27. Shahmoradi Z, Damgacioglu H, Clarke MA, Wentzensen N, Montealegre J, Sonawane K, et al. Cervical cancer incidence among US women, 2001-2019. Jama. (2022) 328:2267–9. doi: 10.1001/jama.2022.17806
28. Cleveland AA, Gargano JW, Park IU, Griffin MR, Niccolai LM, Powell M, et al. Cervical adenocarcinoma in situ: Human papillomavirus types and incidence trends in five states, 2008-2015. Int J cancer. (2020) 146:810–8. doi: 10.1002/ijc.32340
29. Bruni L, Serrano B, Roura E, Alemany L, Cowan M, Herrero R, et al. Cervical cancer screening programmes and age-specific coverage estimates for 202 countries and territories worldwide: a review and synthetic analysis. Lancet Global Health. (2022) 10:e1115–e27. doi: 10.1016/s2214-109x(22)00241-8
30. Aballéa S, Beck E, Cheng X, Demarteau N, Li X, Ma F, et al. Risk factors for cervical cancer in women in China: A meta-model. Women's Health (London England). (2020) 16:1745506520940875. doi: 10.1177/1745506520940875
31. Long T, Long L, Chen Y, Li Y, Tuo Y, Hu Y, et al. Severe cervical inflammation and high-grade squamous intraepithelial lesions: a cross-sectional study. Arch gynecology obstetrics. (2021) 303:547–56. doi: 10.1007/s00404-020-05804-y
32. Zhang Q, Xie W, Wang F, Li RH, Cui L, Wang H, et al. Epidemiological investigation and risk factors for cervical lesions: cervical cancer screening among women in rural areas of Henan Province China. Med Sci monitor: Int Med J Exp Clin Res. (2016) 22:1858–65. doi: 10.12659/msm.894663
33. Zhao M, Gu RY, Ding SR, Luo L, Jia Y, Gao CX, et al. Risk factors of cervical cancer among ethnic minorities in Yunnan Province, China: a case-control study. Eur J Cancer prevention: Off J Eur Cancer Prev Organisation (ECP). (2022) 31:287–92. doi: 10.1097/cej.0000000000000704
34. Levy RA, Kumarapeli AR, Spencer HJ, Quick CM. Cervical polyps: Is histologic evaluation necessary? Pathology Res Pract. (2016) 212:800–3. doi: 10.1016/j.prp.2016.06.010
35. Kucukyıldız I, Karaca M, Akgor U, Turkyılmaz M, Keskinkılıc B, Kara F, et al. Endocervical polyps in high risk human papillomavirus infections. Ginekologia polska. (2022) 93:7–10. doi: 10.5603/GP.a2021.0207
36. Brusselaers N, Shrestha S, van de Wijgert J, Verstraelen H. Vaginal dysbiosis and the risk of human papillomavirus and cervical cancer: systematic review and meta-analysis. Am J obstetrics gynecology. (2019) 221:9–18.e8. doi: 10.1016/j.ajog.2018.12.011
37. Tao L, Han L, Li X, Gao Q, Pan L, Wu L, et al. Prevalence and risk factors for cervical neoplasia: a cervical cancer screening program in Beijing. BMC Public Health. (2014) 14:1185. doi: 10.1186/1471-2458-14-1185
38. Johnson CA, James D, Marzan A, Armaos M. Cervical cancer: an overview of pathophysiology and management. Semin Oncol nursing. (2019) 35:166–74. doi: 10.1016/j.soncn.2019.02.003
39. de Martel C, Plummer M, Vignat J, Franceschi S. Worldwide burden of cancer attributable to HPV by site. country HPV type. (2017) 141:664–70. doi: 10.1002/ijc.30716
40. Muñoz N, Bosch FX, de Sanjosé S, Herrero R, Castellsagué X, Shah KV, et al. Epidemiologic classification of human papillomavirus types associated with cervical cancer. New Engl J Med. (2003) 348:518–27. doi: 10.1056/NEJMoa021641
41. Yang X, Li Y, Tang Y, Li Z, Wang S, Luo X, et al. Cervical HPV infection in Guangzhou, China: an epidemiological study of 198,111 women from 2015 to 2021. Emerging Microbes infections. (2023) 12:e2176009. doi: 10.1080/22221751.2023.2176009
42. Zhang J, Cheng K, Wang Z. Prevalence and distribution of human papillomavirus genotypes in cervical intraepithelial neoplasia in China: a meta-analysis. Arch gynecology obstetrics. (2020) 302:1329–37. doi: 10.1007/s00404-020-05787-w
43. Arbyn M, Xu L. Efficacy and safety of prophylactic HPV vaccines. A Cochrane review of randomized trials. Expert Rev Vaccines. (2018) 17:1085–91. doi: 10.1080/14760584.2018.1548282
44. Huiyun J, Jie D, Huan W, Yuebo Y, Xiaomao L. Prevalence and characteristics of cervical human papillomavirus genotypes and cervical lesions among 58630 women from Guangzhou, China. J infection Public Health. (2023) 16:1531–6. doi: 10.1016/j.jiph.2023.07.013
45. Mijiti Y, Yusupu H, Liu H, Zhang X, Maimaiti G, Kawuli R, et al. Survey on cervical cancer knowledge and its influencing factors among 2,578 women in Shache county, Kashi, China. BMC women's Health. (2023) 23:246. doi: 10.1186/s12905-023-02390-4
46. Wang Z, Wang T, Yang J, Wang W, Zhang L, Su X, et al. Diagnostic yield and performance of a large population-based cervical cancer screening program in high-risk rural China. J Cancer. (2020) 11:4000–6. doi: 10.7150/jca.41472
47. Dalla V, Panagiotopoulou EK, Deltsidou A, Kalogeropoulou M, Kostagiolas P, Niakas D, et al. Level of awareness regarding cervical cancer among female Syrian refugees in Greece. J Cancer education: Off J Am Assoc Cancer Education. (2022) 37:717–27. doi: 10.1007/s13187-020-01873-4
48. Yimer NB, Mohammed MA, Solomon K, Tadese M, Grutzmacher S, Meikena HK, et al. Cervical cancer screening uptake in Sub-Saharan Africa: a systematic review and meta-analysis. Public Health. (2021) 195:105–11. doi: 10.1016/j.puhe.2021.04.014
49. Damiani G, Basso D, Acampora A, Bianchi CB, Silvestrini G, Frisicale EM, et al. The impact of level of education on adherence to breast and cervical cancer screening: Evidence from a systematic review and meta-analysis. Prev Med. (2015) 81:281–9. doi: 10.1016/j.ypmed.2015.09.011
50. Lim JN, Ojo AA. Barriers to utilisation of cervical cancer screening in Sub Sahara Africa: a systematic review. Eur J Cancer Care. (2017) 26:1–9. doi: 10.1111/ecc.12444
Keywords: cervical cancer, cervical intraepithelial neoplasia, incidence, pelvic examination, human papillomavirus
Citation: Zhou X, Han T, Guo T, Liu Y, Li H, Yingxia W and Wu Y (2024) Cervical intraepithelial neoplasia and cervical cancer in Hunan Province, China, 2020-2023. Front. Oncol. 14:1480983. doi: 10.3389/fonc.2024.1480983
Received: 15 August 2024; Accepted: 06 November 2024;
Published: 04 December 2024.
Edited by:
Louis Dubeau, University of Southern California, United StatesReviewed by:
Suvankar Ghorai, Florida International University, United StatesXiaoyan Zhang, Fudan University, China
Ana Afonso, NOVA University of Lisbon, Portugal
Copyright © 2024 Zhou, Han, Guo, Liu, Li, Yingxia and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wang Yingxia, MTkwNDA4MjI2QHFxLmNvbQ==; Yinglan Wu, Mjc1NTEzNDM1QHFxLmNvbQ==
 Xu Zhou
Xu Zhou Ting Han
Ting Han