- 1Department of Oncology, Affliated Xiaoshan Hospital, Hangzhou Normal University, Hangzhou, China
- 2Department of GCP, Affliated Xiaoshan Hospital, Hangzhou Normal University, Hangzhou, China
Background: Non-clear cell renal cell carcinoma (nccRCC) represents a heterogeneous group of malignancies with substantial differences in morphology, genetic profiles, clinical behavior, and prognosis. Optimal treatment for nccRCC remains unclear, largely extrapolated from evidence available for clear cell renal cell carcinoma (ccRCC). This study aimed to compare the efficacy of current mainstream drug treatments for nccRCC to provide clinical treatment guidance for advanced cases.
Methods: We systematically searched PubMed, Embase, and Cochrane databases for trials published up to January 2, 2024, including controlled and single-arm trials. Primary outcomes included overall response rate (ORR), disease control rate (DCR), progression-free survival (PFS), and overall survival (OS).
Results: We selected six randomized controlled trials (RCTs) comparing mammalian target of rapamycin inhibitors (mTORi) with vascular endothelial growth factor receptor tyrosine kinase inhibitors (VEGFR-TKIs). These trials included four first-line and two second-line studies, with a total of 398 advanced nccRCC patients. Pooled results showed that VEGFR-TKIs significantly improved PFS compared to mTORi in first-line treatment (relative risk [RR] = 1.387; 95% confidence interval [CI]: 1.04-1.85; p = 0.026). In a single-arm meta-analysis, we included 22 VEGFR-TKI trials, three mTORi trials, 12 immune checkpoint inhibitor (ICI) therapies, five chemotherapy trials, and 10 combination therapy trials. The pooled ORR ranged from 6% (95% CI: 0–16%) to 36% (95% CI: 27–44%), and the pooled DCR ranged from 54% (95% CI: 50–58%) to 81% (95% CI: 70–91%). Subgroup analysis of ICI showed a higher ORR in the PD-L1 positive group compared to the PD-L1 negative group (RR = 3.044; 95% CI: 1.623-5.709; p = 0.001).
Conclusion: This systematic review and meta-analysis demonstrate that VEGFR-TKIs improve PFS in first-line treatment compared to mTORi. The single-arm meta-analysis suggest that combination therapies with different mechanisms result in better ORR and DCR. Furthermore, PD-L1 positive patients showed significantly better therapeutic responses with ICI treatment than PD-L1 negative patients.
1 Introduction
Renal cell carcinoma (RCC) is a common malignancy of the urinary and reproductive systems, making up 2-3% of systemic malignancies and 80-90% of renal malignancies (1). According to the 2016 World Health Organization (WHO) guidelines, RCC is classified histologically into renal clear cell carcinoma (ccRCC) and non-clear cell carcinoma (nccRCC) (2). NccRCC accounts for 15-30% of renal tumor, and includes various malignancies with significant differences in morphology, genetic profiles, immunohistochemical features, and clinical behaviors (3). Notable subtypes of nccRCC include papillary, chromophobe, collecting duct carcinoma (CDC), renal medullary, spindle cell, mucinous tubular, Xp11.2 translocation, carcinoma associated with neuroblastoma, and other rare entities (2). Papillary RCC (pRCC) and chromophobe RCC (chRCC) are the most prevalent, accounting for 10-15% and 4-5% of renal tumors, respectively (2). The sarcomatoid variant is not a distinct histologic entity but a high-grade transformation seen in various RCC subtypes, characterized by frequent metastasis to the lungs and bones and associated with poor prognosis (4).
Recent advancements in targeted therapy and immunotherapy have significantly improved ccRCC treatment outcomes (5–7). However, due to the heterogeneity and rarity of nccRCC, most RCC clinical trials primarily include ccRCC patients or a small subset of nccRCC patients. As a result, systemic therapy options for nccRCC are largely derived from ccRCC trials and retrospective studies (8, 9). Despite these efforts, nccRCC patients have significantly lower survival rates compared to those with ccRCC (1, 3). The National Comprehensive Cancer Network (NCCN) guidelines recommend clinical trials as the preferred treatment option for nccRCC patients (1), highlighting it as a critical and challenging research area.
Numerous endeavors have focused on developing mesenchymal-epithelial transition (MET) inhibitors, given that MET proto-oncogene has been found to have mutations and copy number changes of pRCC (10). A systematic review and meta-analysis evaluated the efficacy and safety of MET inhibitor (METi) in advanced pRCC, results indicated that the objective response rate (ORR) was 36.38% for MET+ pRCC, and an overall population ORR of 20.56% (11). Subgroup analysis of drugs showed that patients on cabozantinib had an ORR of 26.14%, on savolitinib had an ORR of 15.35% (11). METi represents a promising target for precision therapies, nevertheless, the available data remains limited, requiring further validation of this approach.
Due to the limited clinical trial data and guidance for nccRCC therapy selection, we conducted a systematic review and meta-analysis of existing data. This study aimed to evaluate the antitumor efficacy of vascular endothelial growth factor receptor tyrosine kinase inhibitors (VEGFR-TKIs), mTORi, chemotherapy, immune checkpoint inhibitors (ICIs), and combination therapies in treating nccRCC to provide clinical treatment guidance. Additionally, we analyzed single-arm studies due to the scarcity of controlled trials.
2 Methods
2.1 Search strategy
This systematic review and meta-analysis evaluated the efficacy of VEGFR-TKIs, mTORi, chemotherapy, ICIs, and combination therapies for advanced nccRCC. Comprehensive searches were performed in the PubMed, Embase, and Cochrane databases for reports published up to January 2, 2024. Only English-language articles and abstracts from all available years were included, while case reports, case series, and review articles were excluded. The primary search term was “renal cell carcinoma”.
2.2 Inclusion and exclusion criteria
All trial designs were included, comprising randomized controlled trials (RCTs), non-randomized controlled trials, and single-arm studies.
Inclusion criteria: (1) Adult patients diagnosed histologically or cytologically with advanced nccRCC; (2) Controlled trials or single-arm studies assessing interventions such as VEGFR-TKIs, mTORi, chemotherapy, ICIs, or combination therapies; (3) Studies published in English; (4) Reports of at least one of the following outcomes: ORR, disease control rate (DCR), progression-free survival (PFS), or overall survival (OS); (5) When multiple reports were available from the same investigator for the same patient population, the most recent or comprehensive report was selected.
Exclusion criteria: (1) In vitro or in vivo studies; (2) Case reports, case series, or studies with fewer than 10 participants; (3) Reviews, abstracts, or letters; (4) Re-challenge therapies; (5) Non-English publications.
2.3 Literature screening and data extraction
Two researchers (Y.P.Z. and X.Y.W) independently screened titles and abstracts based on predefined inclusion and exclusion criteria. Data extraction adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines (12). Discrepancies were resolved through discussion; if consensus was not reached, a third investigator (J.C.) was consulted to arbitrate, with final decisions determined by majority vote. If inclusion could not be established from the abstract or if essential data were missing, the full text was reviewed. A standardized data extraction form was created to collect the following information: study characteristics (first author, publication year, identifier, study design, study phase, treatment type, histological subgroups of nccRCC, treatment line), patient characteristics (number of evaluable patients, number of treatment arms, number of control arms, median age, sex), and outcome assessments (complete response [CR], partial response [PR], stable disease [SD], disease progression [PD], ORR, DCR, PFS, or OS).
2.4 Quality assessment
Two investigators (X.L.C. and J.F.H) independently performed the quality assessment. The risk of bias was evaluated in duplicate, with any discrepancies resolved by consensus or by consulting a third reviewer (H.W.). The methodological quality of the included RCTs was assessed using the Cochrane Risk of Bias Tool, as outlined in the Cochrane Handbook for Systematic Reviews of Interventions (13). A risk of bias summary was compiled using Review Manager Version 5.4 (Copenhagen: Nordic Cochrane Centre, Cochrane Collaboration, 2020).
2.5 Statistical analysis
Statistical analyses were performed using Stata 12.0 software (Stata Corporation, College Station, TX). Dichotomous data, including ORR and DCR, were compared using relative risk (RR). Hazard ratios (HR) for OS and PFS, along with their 95% confidence intervals (CIs), were extracted from each RCT. Summary HRs were calculated using either random or fixed-effects models, depending on the heterogeneity of the studies. Heterogeneity was assessed using the I² statistic and Chi-squared test, with I² ≥ 50% indicating significant heterogeneity. The random-effects model was employed when heterogeneity was substantial (I² > 50% or P < 0.05); otherwise, the fixed-effects model was applied (14). Additionally, the Begg test and funnel plots were used to assess potential publication bias among the studies. A two-tailed p-value of less than 0.05 was considered statistically significant.
3 Results
3.1 Literature selection
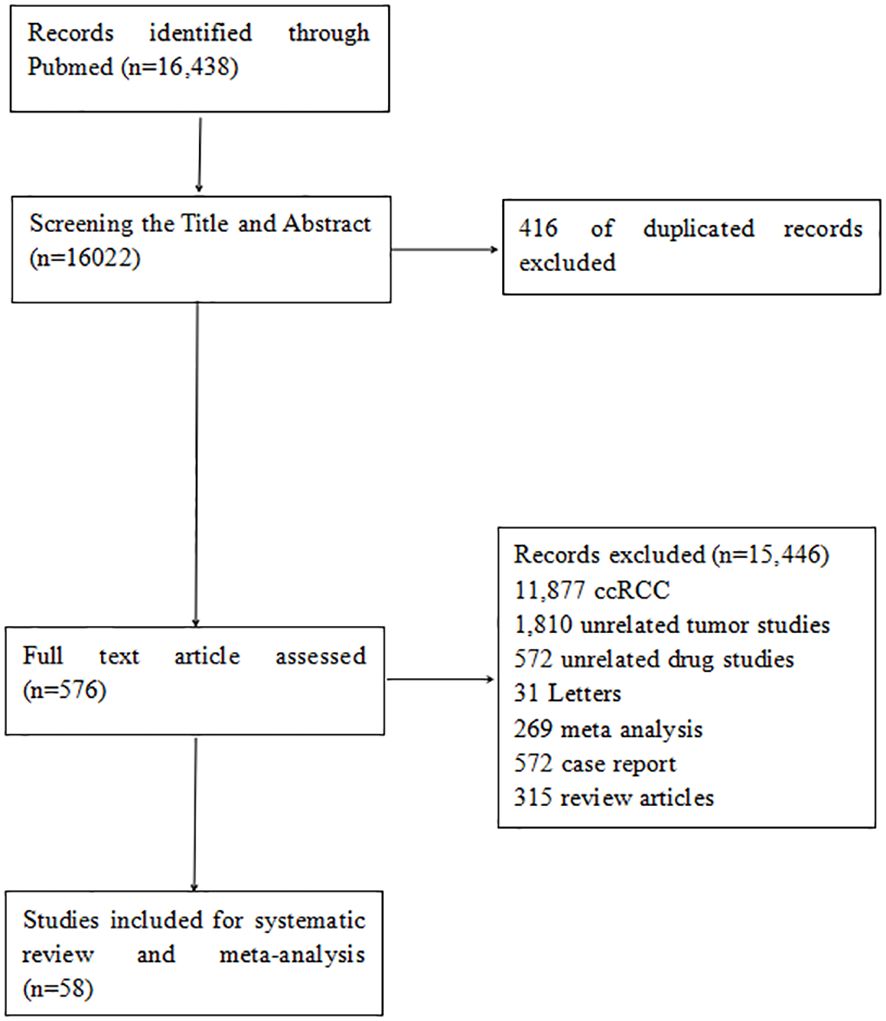
The electronic search identified 16,438 citations published between September 1994 and January 2024. After screening abstracts and titles, 576 full-text articles were reviewed, and 58 studies were included in the systematic review. These comprised six RCTs comparing the efficacy of mTORi and VEGFR-TKIs, 22 single-arm studies on VEGFR-TKIs, three on mTORi, 12 on immunotherapy, five on chemotherapy, and ten on combination therapy. The literature screening process is illustrated in the flowchart in Figure 1.
3.2 Characteristics of six RCTs comparing mTORi and VEGFR-TKIs
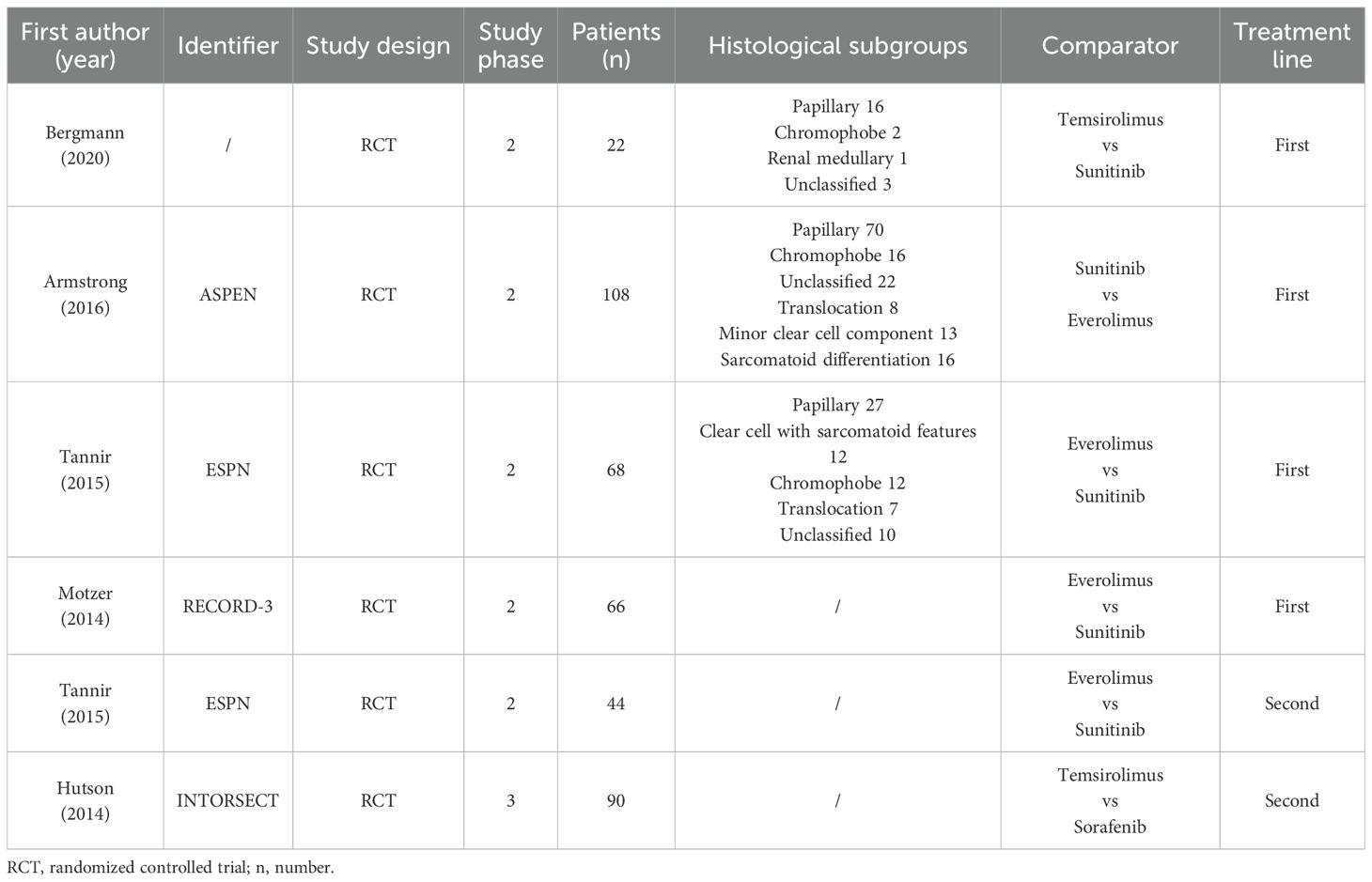
Four studies compared mTORi with VEGFR-TKIs as first-line treatments (15–18), while two studies focused on second-line treatments (15, 19). The ESPN trial provided data for both first-line and second-line treatments separately (15), and we analyzed them as distinct studies. The meta-analysis included 398 patients, with 203 receiving mTORi and 195 receiving VEGFR-TKIs. Among the VEGFR-TKI group, 45 patients were treated with sorafenib, and the rest received sunitinib. Five of the six studies were randomized phase II trials (15–18), and one was a randomized phase III trial (19). Four studies exclusively recruited nccRCC patients (15–17), while two included both ccRCC and nccRCC patients (18, 19), providing separate data for each subgroup. In the three RCTs that reported pathological subgroups (15–17), papillary histology was the most common nccRCC type (113 out of 198 patients). The characteristics and main outcomes of the six RCTs are summarized in Table 1 and Table 2, separately.
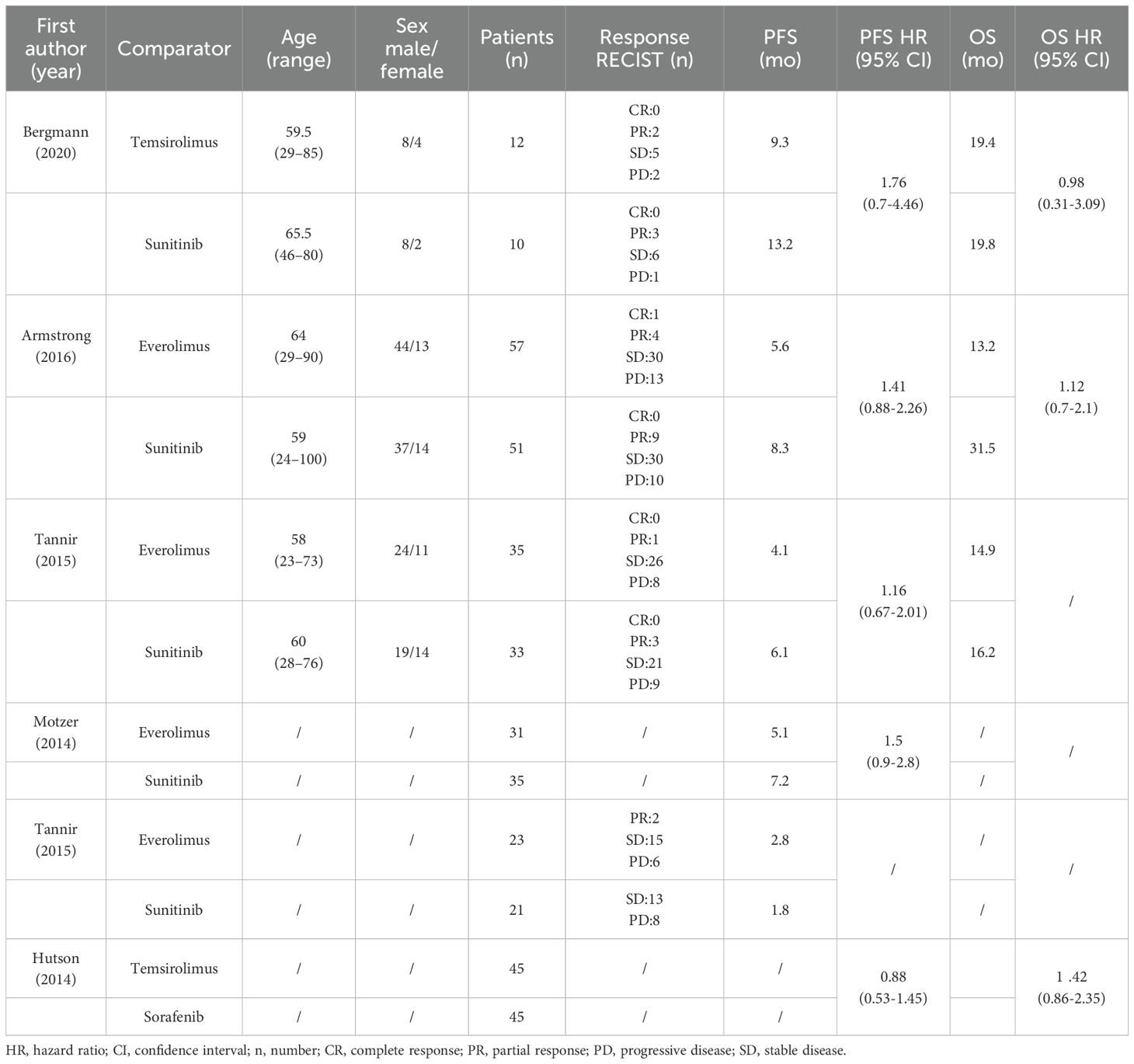
Table 2. Summary of main outcomes of the included RCTs: Response Evaluation Criteria in Solid Tumors (RECIST), progression - free survival (PFS) and overall survival (OS).
3.3 Assessment of study quality and risk of bias
A total of 398 patients were included in the six RCTs. Five studies reported PFS (15–19), four reported ORR and DCR (15–17), and three reported OS (16, 17, 19). The studies exhibited some risks of bias, primarily in blinding of participants and personnel (15–17, 19), random sequence generation (15, 17, 19) and allocation concealment (15, 17, 19). Figure 2 presents the risk of bias summary and graph.
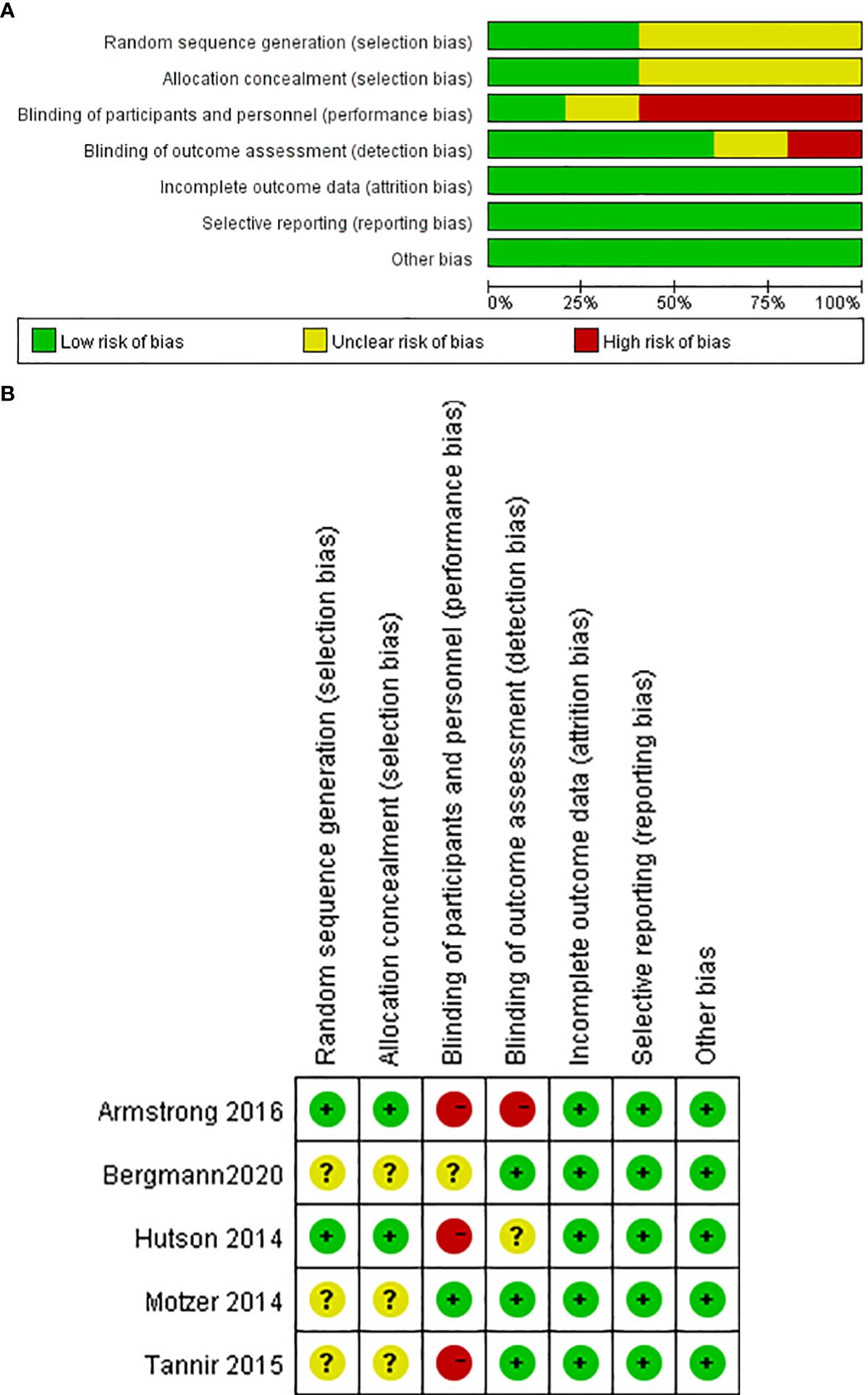
Figure 2. Risk of bias. (A) Graph: review authors’ judgments about each risk of bias item presented as percentages across all RCTs. (B) Summary: review authors’ judgments about each risk of bias item for each included RCT.
3.4 PFS and OS
A comparative meta-analysis of six RCTs examined the PFS of mTORi versus VEGFR-TKIs. Four of these trials were first-line treatments (15–18). The overall analysis showed no significant difference in PFS between the two treatment groups (RR = 1.240; 95% CI, 0.966-1.592; P = 0.091). However, a subgroup analysis revealed a significant PFS benefit for sunitinib over mTORi in first-line treatment (RR = 1.387; 95% CI, 1.040-1.850; P = 0.026) (Figure 3A). There was no significant heterogeneity among the studies.
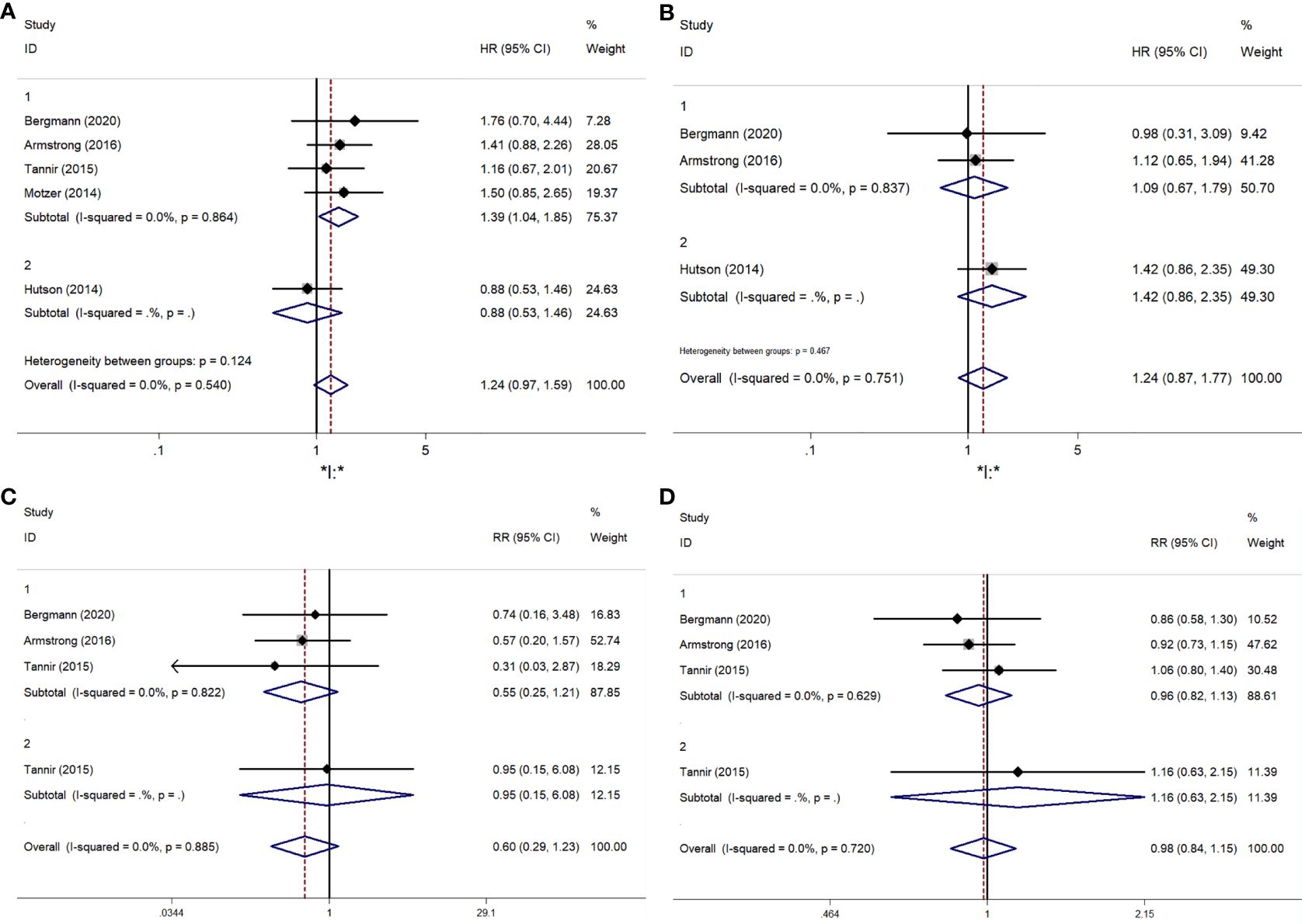
Figure 3. The forest plot comparing of PFS, OS, ORR, DCR between mTORi and VEGFR-TKIs. (A) PFS, (B) OS, (C) ORR, (D) DCR.
Three articles provided specific OS data: two compared mTORi to VEGFR-TKIs in first-line treatment (16, 17), and one in second-line treatment (19). The results showed no significant difference in OS between mTORi and VEGFR-TKIs (RR = 1.243; 95% CI, 0.874-1.769; P = 0.227) (Figure 3B). Similar results were observed in the subgroup analysis of first- and second-line treatments.
3.4.1 ORR and DCR
The meta-analysis results for ORR and DCR are shown in Figures 3C, D. Statistical tests indicated low heterogeneity for both ORR (I² = 0%, P = 0.885) and DCR (I² = 0%, P = 0.720). Sunitinib did not demonstrate a significant advantage over mTORi in ORR (RR = 0.597; 95% CI, 0.289-1.232; P = 0.163) or DCR (RR = 0.983; 95% CI, 0.837-1.153; P = 0.830). Subgroup analysis for first-line and second-line treatments also showed no significant differences in ORR (RR = 0.548; 95% CI, 0.248-1.209; P = 0.136) or DCR (RR = 0.960; 95% CI, 0.817-1.127; P = 0.616).
3.5 Single-arm trials with VEGFR-TKIs
3.5.1 Characteristics
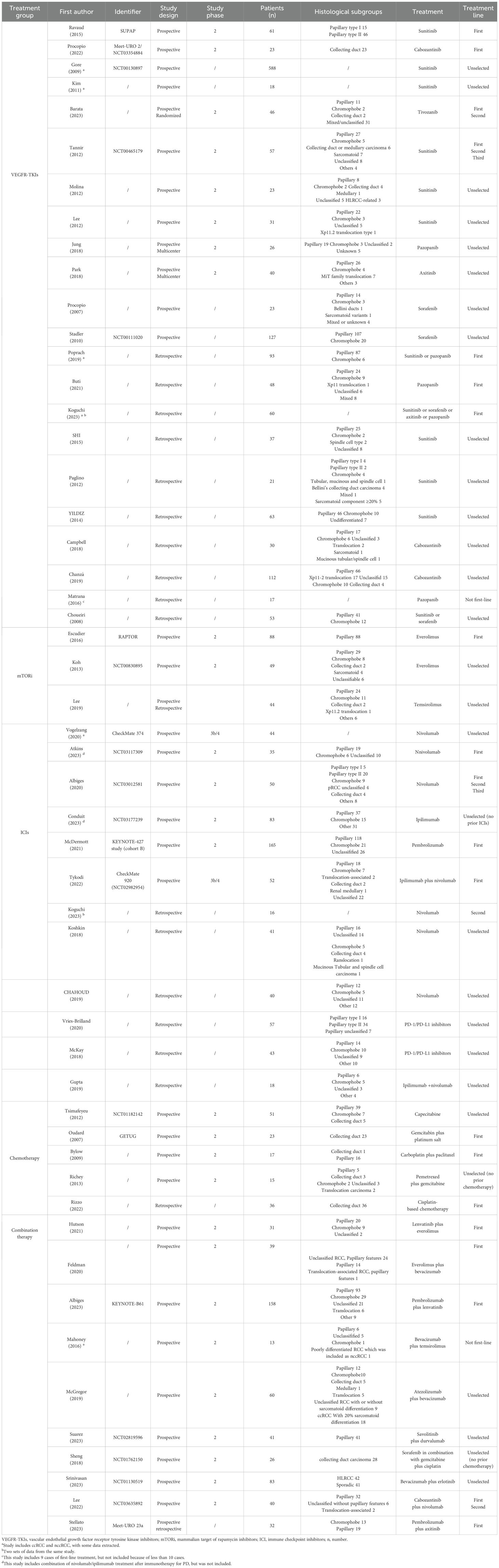
All included studies evaluated the efficacy of VEGFR-TKIs for advanced nccRCC. Among the 22 clinical trials, 12 were prospective (20–31) and 10 were retrospective (32–41), comprising a total of 1,597 patients. Of the 18 studies that specified pathological subgroups (21, 24–39, 41), papillary histology was the most common, accounting for 607 of 914 patients. The most frequently used VEGFR-TKI was sunitinib (1,025 patients), followed by sorafenib (192 patients), pazopanib (111 patients), axitinib (58 patients), and tivozanib (46 patients). Five studies involved only first-line treatments (20, 21, 32–34), while the remaining 17 either included patients with prior anti-tumor treatments or did not specify treatment history. Detailed information on these 22 single-arm experiments is provided in Table 3 and Table 4.
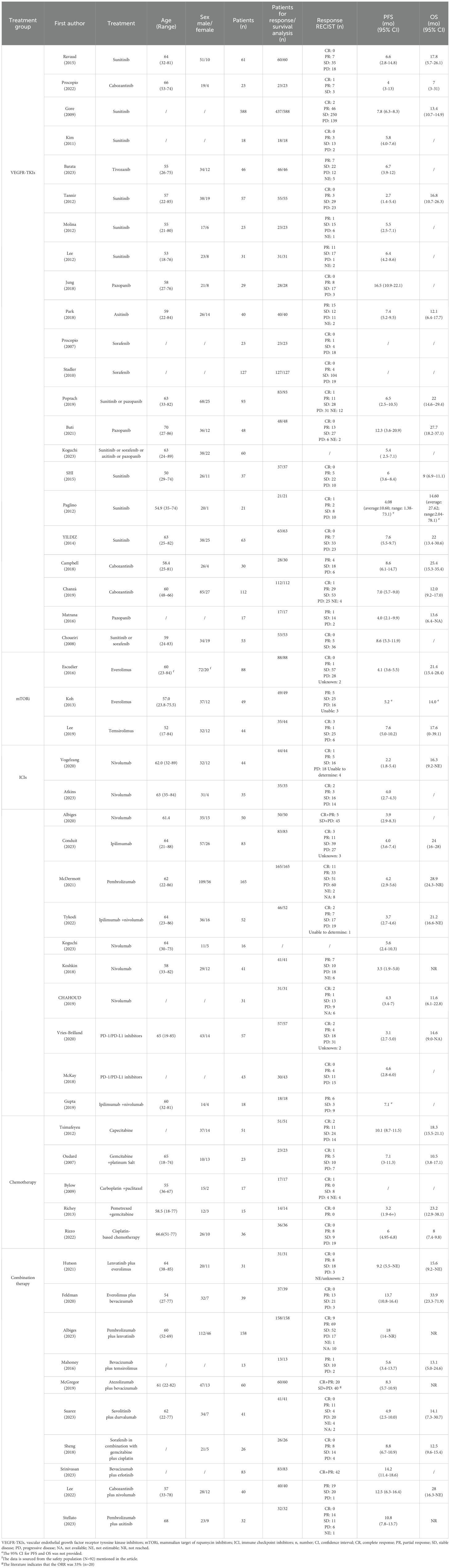
Table 4. Summary of main outcomes of the included studies: Response Evaluation Criteria in Solid Tumors (RECIST), progression-free survival (PFS) and overall survival (OS).
3.5.2 ORR and DCR
All studies, except one retrospective trial (32), provided analyzable data for ORR and DCR. Both ORR and DCR exhibited significant heterogeneity, with I² values over 50% and p-values below 0.01 in the Q-test, indicating notable variability among the studies. The ORR ranged from 3.15% to 35.48%, with a pooled ORR of 14% (95% CI: 11–18%) (Figure 4A). Subgroup analysis showed a pooled ORR of 14% (95% CI: 9–19%) in prospective studies and 15% (95% CI: 10–20%) in retrospective studies. The DCR varied from 21.74% to 90.21%, with a pooled DCR of 70% (95% CI: 64–76%) (Figure 5A). Subgroup analysis revealed a pooled DCR of 69% (95% CI: 61–78%) in prospective studies and 71% (95% CI: 63–80%) in retrospective studies.
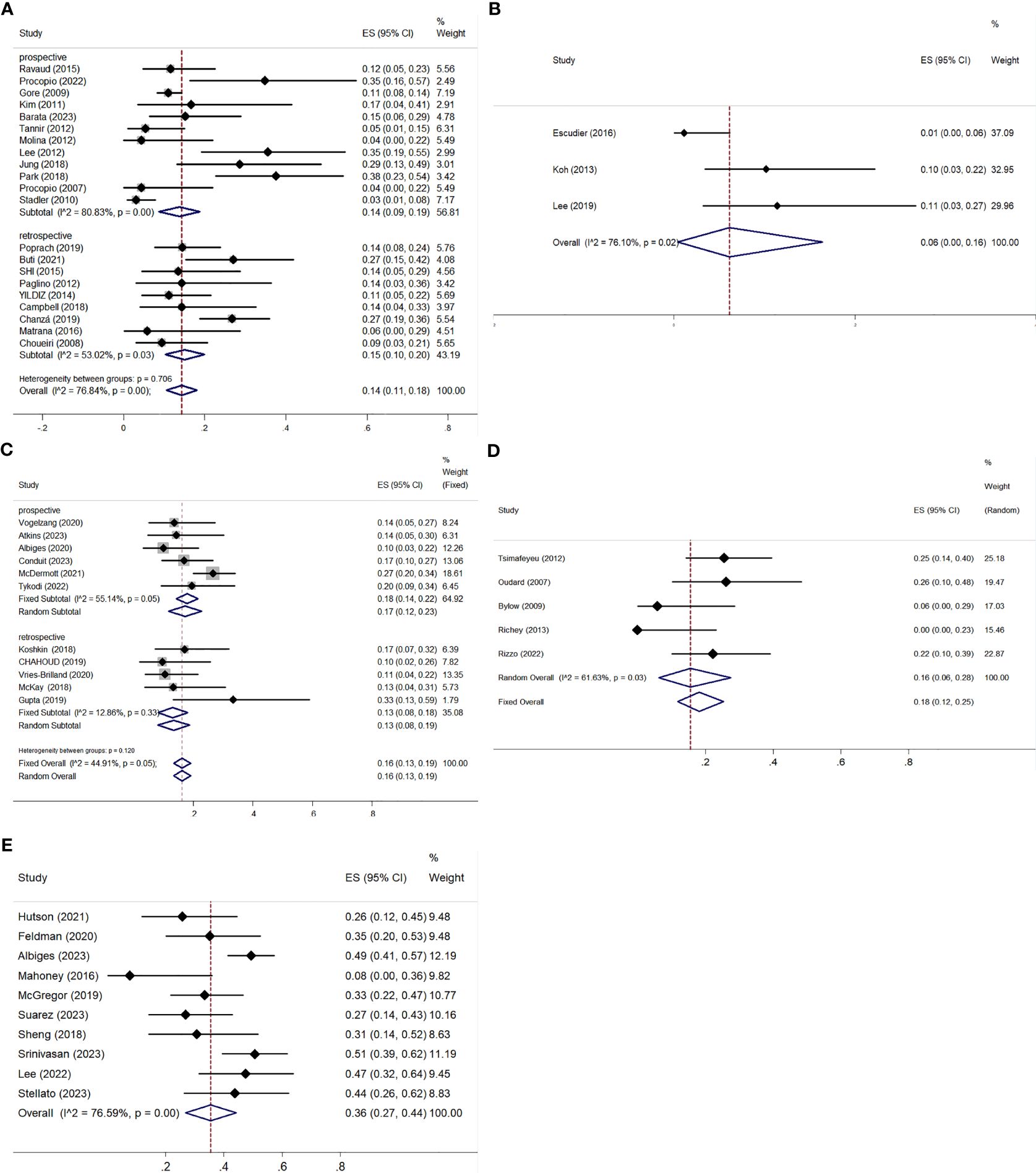
Figure 4. ORR for patients with nccRCC receiving VEGFR-TKIs, mTORi, ICIs, chemotherapy, and combination therapy. (A) VEGFR-TKIs, (B) mTORi, (C) ICIs, (D) chemotherapy, (E) combination therapy.
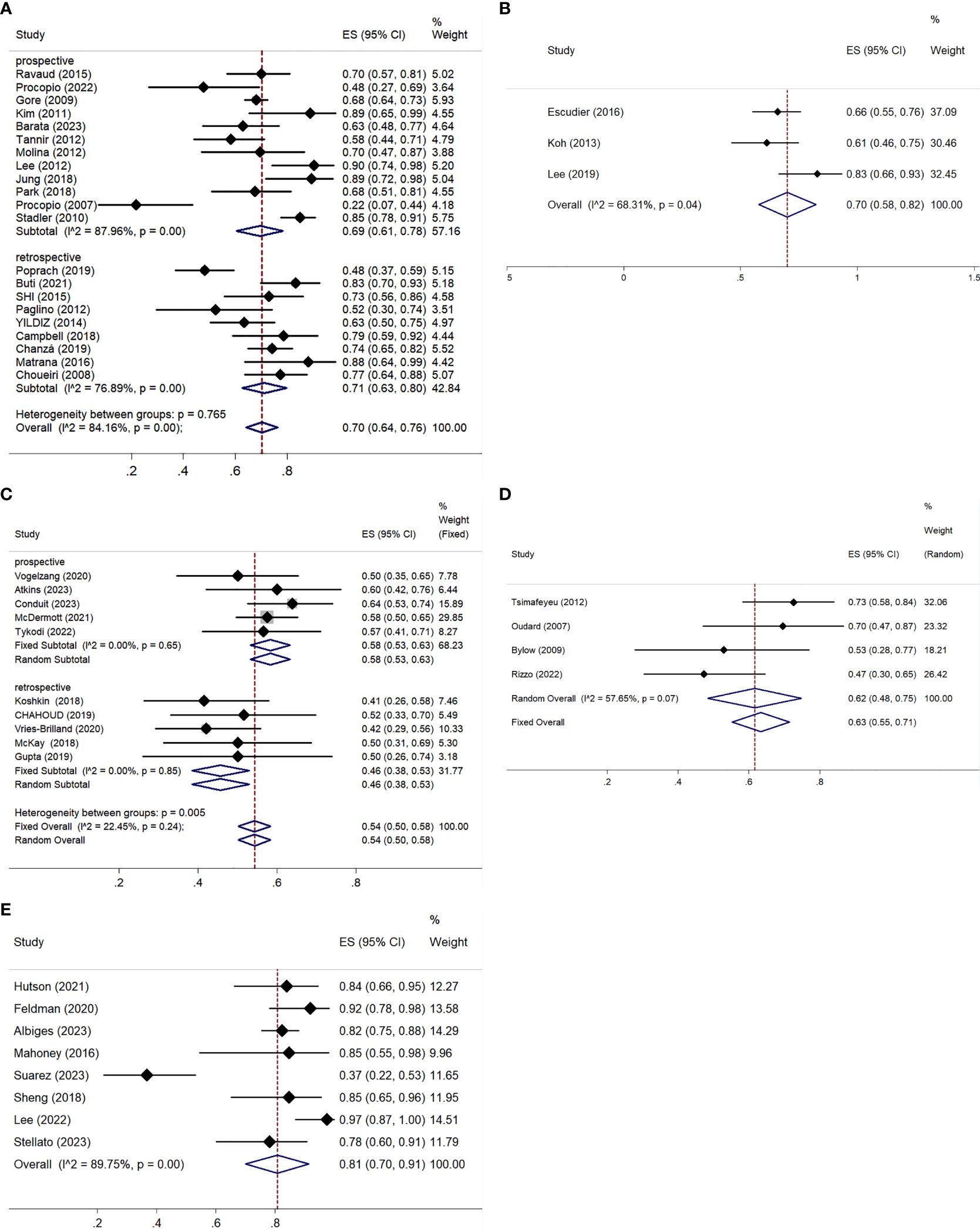
Figure 5. DCR for patients with nccRCC receiving VEGFR-TKIs, mTORi, ICIs, chemotherapy, and combination therapy. (A) VEGFR-TKIs, (B) mTORi, (C) ICIs, (D) chemotherapy, (E) combination therapy.
Sensitivity analysis for both ORR and DCR showed no significant interference from any single study, confirming the reliability of the meta-analysis results.
3.6 Single-arm trials with mTORi
3.6.1 Characteristics
Three relevant studies were included in this analysis after screening: RAPTOR (42), NCT00830895 (43), and Lee (44). RAPTOR (42) and NCT00830895 (43) were open-label, single-arm, non-randomized, multicenter studies, while Lee (16) integrated both prospective and retrospective data. A total of 215 patients were involved, with 181 in prospective analyses and 34 in retrospective studies. All 88 patients in RAPTOR (42) had pRCC. In the other two studies, pRCC was also the most common, followed by chRCC. Two studies used everolimus, while the third used temsirolimus. Only RAPTOR (42) included patients who had not received previous systemic therapy. Detailed information on these three single-arm studies is presented in Table 3 and Table 4.
3.6.2 ORR and DCR
The three articles provided data on ORR and DCR (42–44). The pooled ORR was 6% (95% CI: 0–16%), with significant heterogeneity (I² = 76.10%, P = 0.02) (Figure 4B). The pooled DCR was 70% (95% CI: 58–82%), also with high heterogeneity (I² = 68.31%, P = 0.04) (Figure 5B).
3.7 Single-arm trials with ICIs
3.7.1 Characteristics
A total of 622 patients participated in six prospective (45–50) and six retrospective studies (32, 51–55). Prior treatment with ICIs was excluded. Only three studies received first-line treatment (46–48). Among the prospective studies, two were Phase IIIb/IV trials (45, 48). Two retrospective studies analyzed various PD-1/PD-L1 inhibitors (53, 54). Nivolumab monotherapy was the most commonly used, appearing in six studies (32, 45, 46, 49, 51, 52). Combination therapy with ipilimumab and nivolumab was examined in two studies (48, 55). Both Vogelzang (45) and Koguchi (32) researched ccRCC and nccRCC but did not detail the nccRCC subtypes. Patients with pRCC were the most prevalent. Detailed information on these studies is presented in Table 3 and Table 4.
3.7.2 ORR and DCR
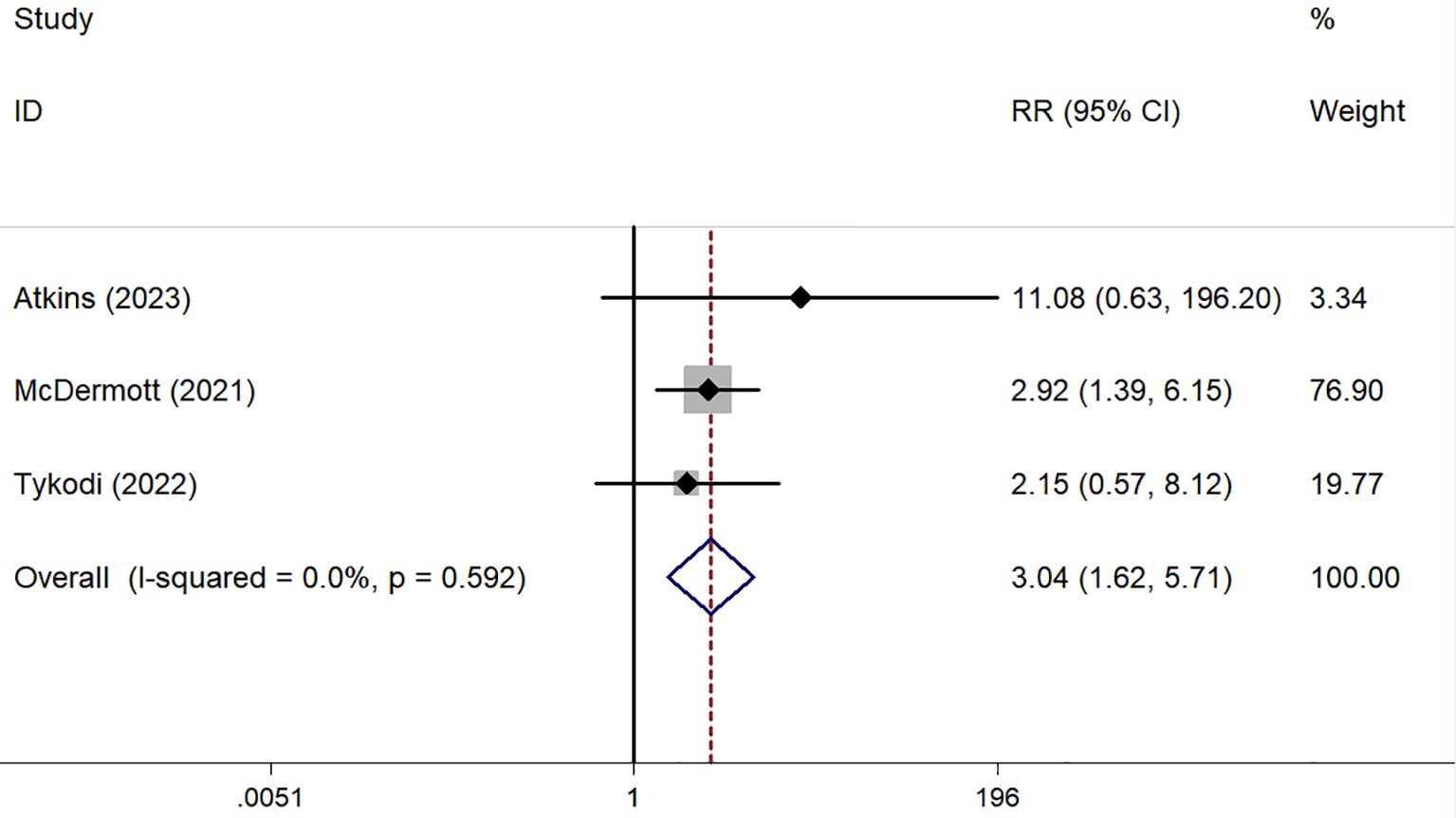
ORR was reported in six prospective (45–50) and five retrospective studies (51–55), while DCR was reported in five prospective (45–48, 50) and five retrospective studies (51–55). Both ORR and DCR showed no significant heterogeneity. The overall pooled ORR was 16% (95% CI: 13–19%) (Figure 4C). Subgroup analysis showed a pooled ORR of 18% (95% CI: 14–22%) in the prospective group and 13% (95% CI: 8–18%) in the retrospective group. The overall pooled DCR was 54% (95% CI: 50–58%), with a DCR of 58% (95% CI: 53–63%) in the prospective group and 46% (95% CI: 38–53%) in the retrospective group (Figure 5C). Additionally, ORR (RR = 3.044; 95% CI: 1.623-5.709%; P = 0.001) was higher in the PD-L1 positive group compared to the PD-L1 negative group (Figure 6).
3.8 Single-arm trials with chemotherapy
3.8.1 Characteristics
A total of 142 patients participated in four phase II trials (56–59) and one retrospective study (60), all excluding prior chemotherapy. Three patients received first-line treatment (56, 59, 60). Tsimafeyeu (58) focused on capecitabine monotherapy, while the other studies used combination chemotherapy. Oudard (56) and Rizzo (60) exclusively studied patients with CDC, whereas the remaining studies included various types of nccRCC. Detailed information is presented in Table 3 and Table 4.
3.8.2 ORR and DCR
Both ORR and DCR showed significant heterogeneity, with an I² > 50% in the Q-test. ORR ranged from 0% to 26.09% across studies, resulting in a pooled ORR of 16% (95% CI: 6–28%) (Figure 4D). DCR, which was not reported in Richey (57), ranged from 47.22% to 72.55%, with a pooled DCR of 62% (95% CI: 48–75%) (Figure 5D).
3.9 Single-arm trials with combination therapy
3.9.1 Characteristics
This analysis included ten studies with a total of 523 patients (61–70). Of these, one study incorporated both prospective and retrospective data (66), while the others were prospective phase II trials (61–65, 67–70). All studies investigated anti-angiogenesis therapies, using either small molecule targeted agents, such as VEGFR-TKIs, or large molecule monoclonal antibodies, such as bevacizumab, often in combination with mTORi, ICIs, or chemotherapy. Notably, Suarez (69) focused exclusively on pRCC, and Sheng (70) examined only CDC. The remaining studies included various subtypes of nccRCC, with pRCC being the most common subtype. Detailed information on these ten single-arm studies is presented in Table 3 and Table 4.
3.9.2 ORR and DCR
All studies provided data on ORR (61–70), while only eight included DCR (61–64, 67–70). Statistical analysis indicated high heterogeneity for both ORR (I² = 76.59%, P < 0.01) and DCR (I² = 89.75%, P < 0.01). ORR across studies ranged from 7.69% to 50.6%, with a pooled estimate of 36% (95% CI: 27–44%) (Figure 4E). DCR varied from 36.59% to 97.50%, resulting in a pooled DCR of 81% (95% CI: 70–91%) (Figure 5E). Subgroup analysis revealed five studies using bevacizumab (62–66), seven utilizing VEGFR-TKIs (61, 63, 66, 68, 70), and five based on ICIs (63, 65, 67–69). All subgroups demonstrated significant heterogeneity for ORR. The pooled ORRs were 31% (95% CI: 17–45%) for bevacizumab, 41% (95% CI: 31–50%) for VEGFR-TKIs, and 40% (95% CI: 31–50%) for ICIs.
4 Discussion
The optimal therapeutic strategy for nccRCC remains contentious. The heterogeneity of nccRCC complicates the establishment of robust evidence for specific therapies, as it hinders the conduct of prospective randomized trials. While systemic treatments effective for ccRCC show some activity in nccRCC, their response rates are significantly lower. Therefore, the panel recommends prioritizing clinical trial enrollment for nccRCC patients (1).
Our analysis of six RCTs compared the efficacy of mTORi and VEGFR-TKIs. Unfortunately, we did not achieve positive results in terms of effectiveness and survival. However, subgroup analysis of the four first-line studies showed that PFS was superior with sunitinib compared to mTORi (RR = 1.387; 95% CI: 1.04-1.85; p = 0.026). This advantage was not reflected in ORR and DCR, possibly due to limited data, as only three articles reported on ORR and DCR. Further validation through large-scale studies is needed.
NccRCC is a heterogeneous disease with significant variability among studies in histological subtypes, populations recruited, and risk factors, complicating comparisons across studies. Despite these challenges, current research suggests sunitinib as a potential treatment option for advanced nccRCC. The ESPN study found that sunitinib tended to prolong OS in first-line treatment of nccRCC without sarcomatoid features (15). Among 49 patients without sarcomatoid features, median OS was 31.6 months with sunitinib compared to 10.5 months with everolimus (p = 0.075) (15). The ASPEN trial highlighted the benefit of VEGFR-TKI therapy in patients with good or intermediate risk according to MSKCC criteria compared to everolimus (16). The RECORD-3 study, through prespecified subgroup analysis of MSKCC prognosis, found that median PFS was longer for patients with favorable and intermediate risk treated with first-line sunitinib compared to everolimus (18). However, this study included both ccRCC and nccRCC, and did not separately report results for the nccRCC group based on MSKCC criteria. These findings indicate that future nccRCC studies should focus on differences in efficacy based on MSKCC risk groups.
Sunitinib is a primary treatment option for nccRCC, but the search for a superior tyrosine kinase inhibitor (TKI) continues. Cabozantinib, an oral inhibitor of MET, VEGFR, and AXL, has shown promise. The randomized phase II SWOG 1500 trial (71) compared cabozantinib, crizotinib, and savolitinib with sunitinib in patients with advanced pRCC who had received up to one prior systemic therapy, excluding VEGFR- and MET-targeted TKIs. Only cabozantinib demonstrated significantly longer PFS and a higher ORR than sunitinib. As a dual VEGF-MET inhibitor, cabozantinib’s efficacy suggests that pRCC may involve both VEGF and MET signaling pathways. Although the SWOG 1500 trial focused on pRCC, the Meet-URO 2/NCT03354884 (20) trial treated 23 patients with CDC using cabozantinib, achieving an ORR of 34.78%, surpassing most previously reported targeted therapies. Consequently, NCCN guidelines now recommend cabozantinib as a preferred option alongside clinical trials (1).
Future research should focus on the genetic and molecular characteristics of nccRCC to better identify the target audience for these therapies. Given the limited number of RCTs, our analysis includes relevant single-arm trials to evaluate drug efficacy more comprehensively. Single-arm trials have shown limited efficacy of monotherapy for nccRCC, with mTORi demonstrating the lowest pooled ORR of only 6% (95% CI: 0-16%). In contrast, VEGFR-TKIs, chemotherapy, and ICIs had monotherapy effective rates of 14-16%. Koh (43) found that patients with chRCC treated with everolimus exhibited longer PFS and better ORR compared to other RCC subtypes. Conversely, Lee (44) reported no significant differences in PFS or OS among histological subtypes treated with temsirolimus, although patients with poor prognosis, as defined by ARCC criteria, had significantly shorter PFS and OS.
The pooled ORR for single-drug treatments with chemotherapy, VEGFR TKIs, and ICIs ranged from 14-16%. While the ORR and DCR of chemotherapy were similar to those of VEGFR-TKIs and ICIs, four of the five chemotherapy-related studies were published between 2007 and 2013, making them somewhat outdated. Additionally, the chemotherapy regimens varied: Tsimafeyeu (58) used oral capecitabine as a single agent for nccRCC, Richey (57) conducted a phase II trial with pemetrexed and gemcitabine, and the other three studies employed platinum-based combination chemotherapy (56, 59, 60).
Retrospective and recent prospective clinical trials have evaluated the antitumor activity of ICIs for nccRCC, either as monotherapy or combined with PD-1/PD-L1 inhibitors. Subgroup analysis revealed that PD-L1 positive patients had significantly better ORRs than PD-L1 negative patients, suggesting that PD-L1 expression levels could guide treatment choices for nccRCC patients. However, only three trials provided relevant data, and there was no standardized method for PD-L1 detection (46–48). Larger and more rigorous studies are needed to identify nccRCC patients who would benefit most from ICIs.
Treatment options for nccRCC are expanding, with drug combinations of different mechanisms entering clinical practice. Examples include lenvatinib plus everolimus, everolimus plus bevacizumab, pembrolizumab plus lenvatinib, and cabozantinib plus nivolumab. Our research indicates that combination therapies with different mechanisms show better ORR and DCR compared to single-agent therapies. Subgroup analysis found that combination therapies based on VEGFR-TKIs and ICIs achieved similar ORRs, both outperforming combination therapies based on bevacizumab.
5 Conclusions
In conclusion, the systematic review shows that sunitinib provides superior PFS compared to mTORi as a first-line treatment for advanced nccRCC. Due to limited data, single-arm trials were included to improve clinical guidance. The results indicated that PD-L1 positive patients had better ORR than PD-L1 negative patients, suggesting a need for further investigation. Additionally, combination therapies involving different mechanisms, especially those based on VEGFR-TKIs or ICIs, were more effective than single-agent treatments.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Author contributions
YZ: Conceptualization, Data curation, Writing – original draft. HF: Supervision, Visualization, Writing – review & editing. JC: Data curation, Writing – review & editing. XW: Data curation, Writing – review & editing. HW: Data curation, Writing – review & editing. XC: Data curation, Writing – review & editing. JH: Formal analysis, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
We thank all the researchers who contributed to this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Motzer RJ, Jonasch E, Agarwal N, Alva A, Bagshaw H, Baine M, et al. “National Comprehensive Cancer Network. Clinical practice guidelines in oncology”. In: Kidney Cancer, Version 3 (2024). Available at: https://www.nccn.org/professionals/physician_gls/pdf/kidney.pdf (Accessed March 11, 2024).
2. Moch H, Cubilla AL, Humphrey PA, Reuter VE, Ulbright TM. The 2016 WHO classification of tumours of the urinary system and male genital organs-part A: renal, penile, and testicular tumours. Eur Urol. (2016) 70:93–105. doi: 10.1016/j.eururo.2016.02.029
3. Barthélémy P, Rioux-Leclercq N, Thibault C, Saldana C, Borchiellini D, Chevreau C, et al. Non-clear cell renal carcinomas: Review of new molecular insights and recent clinical data. Cancer Treat Rev. (2021) 97:102191. doi: 10.1016/j.ctrv.2021.102191
4. Vera-Badillo FE, Templeton AJ, Duran I, Ocana A, de Gouveia P, Aneja P, et al. Systemic therapy for non-clear cell renal cell carcinomas: a systematic review and meta-analysis. Eur Urol. (2015) 67:740–9. doi: 10.1016/j.eururo.2014.05.010
5. Rini BI, Plimack ER, Stus V, Gafanov R, Hawkins R, Nosov D, et al. Pembrolizumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N Engl J Med. (2019) 380:1116–27. doi: 10.1056/NEJMoa1816714
6. Rini BI, Pal SK, Escudier BJ, Atkins MB, Hutson TE, Porta C, et al. Tivozanib versus sorafenib in patients with advanced renal cell carcinoma (TIVO-3): a phase 3, multicentre, randomised, controlled, open-label study. Lancet Oncol. (2020) 21:95–104. doi: 10.1016/S1470-2045(19)30735-1
7. Motzer RJ, Tannir NM, McDermott DF, Arén Frontera O, Melichar B, Choueiri TK, et al. Nivolumab plus Ipilimumab versus Sunitinib in Advanced Renal-Cell Carcinoma. N Engl J Med. (2018) 378:1277–90. doi: 10.1056/NEJMoa1712126
8. Ahrens M, Scheich S, Hartmann A, Bergmann L. Non-clear cell renal cell carcinoma - pathology and treatment options. Oncol Res Treat. (2019) 42:128–35. doi: 10.1159/000495366
9. Gulati S, Philip E, Salgia S, Pal SK. Evolving treatment paradigm in metastatic non clear cell renal cell carcinoma. Cancer Treat Res Commun. (2020) 23:100172. doi: 10.1016/j.ctarc.2020.100172
10. Albiges L, Guegan J, Le Formal A, Verkarre V, Rioux-Leclercq N, Sibony M, et al. MET is a potential target across all papillary renal cell carcinomas: result from a large molecular study of pRCC with CGH array and matching gene expression array. Clin Cancer Res. (2014) 20:3411–21. doi: 10.1158/1078-0432.CCR-13-2173
11. Moraes FCA, Vilbert M, Alves VFC, de Oliveira Almeida G, Priantti JN, Madeira T, et al. Mesenchymal-epithelial transition kinase inhibitor therapy in patients with advanced papillary renal-cell carcinoma: A systematic review and meta-analysis. Int J Mol Sci. (2023) 24:17582. doi: 10.3390/ijms242417582
12. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 29:372. doi: 10.1136/bmj.n71
13. Higgins JPT, Green S. Cochrane handbook for systematic reviews of interventions Vol. 4. New Jersey: John Wiley & Sons (2011).
14. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. (1986) 7:177–88. doi: 10.1016/0197-2456(86)90046-2
15. Tannir NM, Jonasch E, Albiges L, Altinmakas E, Ng CS, Matin SF, et al. Everolimus versus sunitinib prospective evaluation in metastatic non-clear cell renal cell carcinoma (ESPN): A randomized multicenter phase 2 trial. Eur Urol. (2016) 69:866–74. doi: 10.1016/j.eururo.2015.10.049
16. Armstrong AJ, Halabi S, Eisen T, Broderick S, Stadler WM, Jones RJ, et al. Everolimus versus sunitinib for patients with metastatic non-clear cell renal cell carcinoma (ASPEN): a multicentre, open-label, randomised phase 2 trial. Lancet Oncol. (2016) 17:378–88. doi: 10.1016/S1470-2045(15)00515-X
17. Bergmann L, Grünwald V, Maute L, Grimm MO, Weikert S, Schleicher J, et al. A randomized phase IIa trial with temsirolimus versus sunitinib in advanced non-clear cell renal cell carcinoma: an intergroup study of the CESAR central european society for anticancer drug research-EWIV and the interdisciplinary working group on renal cell cancer (IAGN) of the german cancer society. Oncol Res Treat. (2020) 43:333–9. doi: 10.1159/000508450
18. Motzer RJ, Barrios CH, Kim TM, Falcon S, Cosgriff T, Harker WG, et al. Phase II randomized trial comparing sequential first-line everolimus and second-line sunitinib versus first-line sunitinib and second-line everolimus in patients with metastatic renal cell carcinoma. J Clin Oncol. (2014) 32:2765–72. doi: 10.1200/JCO.2013.54.6911
19. Hutson TE, Escudier B, Esteban E, Bjarnason GA, Lim HY, Pittman KB, et al. Randomized phase III trial of temsirolimus versus sorafenib as second-line therapy after sunitinib in patients with metastatic renal cell carcinoma. J Clin Oncol. (2014) 32:760–7. doi: 10.1200/JCO.2013.50.3961
20. Procopio G, Sepe P, Claps M, Buti S, Colecchia M, Giannatempo P, et al. Cabozantinib as first-line treatment in patients with metastatic collecting duct renal cell carcinoma: results of the BONSAI trial for the italian network for research in urologic-oncology (Meet-URO 2 study). JAMA Oncol. (2022) 8:910–3. doi: 10.1001/jamaoncol.2022.0238
21. Ravaud A, Oudard S, De Fromont M, Chevreau C, Gravis G, Zanetta S, et al. First-line treatment with sunitinib for type 1 and type 2 locally advanced or metastatic papillary renal cell carcinoma: a phase II study (SUPAP) by the French Genitourinary Group (GETUG)†. Ann Oncol. (2015) 26:1123–8. doi: 10.1093/annonc/mdv149
22. Gore ME, Szczylik C, Porta C, Bracarda S, Bjarnason GA, Oudard S, et al. Safety and efficacy of sunitinib for metastatic renal-cell carcinoma: an expanded-access trial. Lancet Oncol. (2009) 10:757–63. doi: 10.1016/S1470-2045(09)70162-7
23. Kim HS, Hong MH, Kim K, Shin SJ, Ahn JB, Jeung HC, et al. Sunitinib for Asian patients with advanced renal cell carcinoma: a comparable efficacy with different toxicity profiles. Oncology. (2011) 80:395–405. doi: 10.1159/000330361
24. Barata PC, Chehrazi-Raffle A, Allman KD, Asnis-Alibozek A, Kasturi V, Pal SK. Activity of tivozanib in non-clear cell renal cell carcinoma: subgroup analysis from a phase II randomized discontinuation trial. Oncologist. (2023) 28:894–900. doi: 10.1093/oncolo/oyad132
25. Tannir NM, Plimack E, Ng C, Tamboli P, Bekele NB, Xiao L, et al. A phase 2 trial of sunitinib in patients with advanced non-clear cell renal cell carcinoma. Eur Urol. (2012) 62:1013–9. doi: 10.1016/j.eururo.2012.06.043
26. Molina AM, Feldman DR, Ginsberg MS, Kroog G, Tickoo SK, Jia X, et al. Phase II trial of sunitinib in patients with metastatic non-clear cell renal cell carcinoma. Invest New Drugs. (2012) 30:335–40. doi: 10.1007/s10637-010-9491-6
27. Lee JL, Ahn JH, Lim HY, Park SH, Lee SH, Kim TM, et al. Multicenter phase II study of sunitinib in patients with non-clear cell renal cell carcinoma. Ann Oncol. (2012) 23:2108–14. doi: 10.1093/annonc/mdr586
28. Jung KS, Lee SJ, Park SH, Lee JL, Lee SH, Lim JY, et al. Pazopanib for the treatment of non-clear cell renal cell carcinoma: A single-arm, open-label, multicenter, phase II study. Cancer Res Treat. (2018) 50:488–94. doi: 10.4143/crt.2016.584
29. Park I, Lee SH, Lee JL. A multicenter phase II trial of axitinib in patients with recurrent or metastatic non-clear-cell renal cell carcinoma who had failed prior treatment with temsirolimus. Clin Genitourin Cancer. (2018) 16:e997–e1002. doi: 10.1016/j.clgc.2018.05.011
30. Procopio G, Verzoni E, Gevorgyan A, Mancin M, Pusceddu S, Catena L, et al. Safety and activity of sorafenib in different histotypes of advanced renal cell carcinoma. Oncology. (2007) 73:204–9. doi: 10.1159/000127387
31. Stadler WM, Figlin RA, McDermott DF, Dutcher JP, Knox JJ, Miller WH Jr. Safety and efficacy results of the advanced renal cell carcinoma sorafenib expanded access program in North America. Cancer. (2010) 116:1272–80. doi: 10.1002/cncr.v116:5
32. Koguchi T, Naito S, Hatakeyama S, Numakura K, Muto Y, Kato R, et al. The efficacy of molecular targeted therapy and nivolumab therapy for metastatic non-clear cell renal cell carcinoma: A retrospective analysis using the Michinoku Japan urological cancer study group database. Cancer Med. (2023) 12:20677–89. doi: 10.1002/cam4.v12.22
33. Poprach A, Rumanova K, Lakomý R, Chloupková R, Stanik M, Pokrivcak T. Tyrosine kinase inhibitors in the first-line treatment for metastatic nonclear cell renal carcinoma: A retrospective analysis of a national database. Urol Oncol. (2019) 37:294.e1–8. doi: 10.1016/j.urolonc.2018.12.017
34. Buti S, Bersanelli M, Massari F, De Giorgi U, Caffo O, Aurilio G, et al. First-line pazopanib in patients with advanced non-clear cell renal carcinoma: An Italian case series. World J Clin Oncol. (2021) 12:1037–46. doi: 10.5306/wjco.v12.i11.1037
35. Shi HZ, Tian J, Li CL. Safety and efficacy of sunitinib for advanced non-clear cell renal cell carcinoma. Asia Pac J Clin Oncol. (2015) 11:328–33. doi: 10.1111/ajco.2015.11.issue-4
36. Paglino C, Imarisio I, Ganini C, Morbini P, Vercelli A, Bregant C, et al. Sunitinib in advanced metastatic non-clear cell renal cell carcinoma: a single institution retrospective study. Future Oncol. (2012) 8:1605–12. doi: 10.2217/fon.12.145
37. Yildiz I, Ekenel M, Akman T, Kocar M, Uysal M, Kanitez M, et al. Sunitinib for patients with metastatic non-clear cell renal cell carcinoma: a Multicenter Retrospective Turkish Oncology Group trial. Anticancer Res. (2014) 34:4329–34. doi: 10.1200/jco.2014.32.15_suppl.e15602
38. Campbell MT, Bilen MA, Shah AY, Lemke E, Jonasch E, Venkatesan AM, et al. Cabozantinib for the treatment of patients with metastatic non-clear cell renal cell carcinoma: A retrospective analysis. Eur J Cancer. (2018) 104:188–94. doi: 10.1016/j.ejca.2018.08.014
39. Martínez Chanzá N, Xie W, Asim Bilen M, Dzimitrowicz H, Burkart J, Geynisman DM, et al. Cabozantinib in advanced non-clear-cell renal cell carcinoma: a multicentre, retrospective, cohort study. Lancet Oncol. (2019) 20:581–90. doi: 10.1016/S1470-2045(18)30907-0
40. Matrana MR, Baiomy A, Campbell M, Alamri S, Shetty A, Teegavarapu P, et al. Outcomes of patients with metastatic non-clear-cell renal cell carcinoma treated with pazopanib. Clin Genitourin Cancer. (2017) 15:e205–8. doi: 10.1016/j.clgc.2016.07.016
41. Choueiri TK, Plantade A, Elson P, Negrier S, Ravaud A, Oudard S, et al. Efficacy of sunitinib and sorafenib in metastatic papillary and chromophobe renal cell carcinoma. J Clin Oncol. (2008) 26:127–31. doi: 10.1200/JCO.2007.13.3223
42. Escudier B, Molinie V, Bracarda S, Maroto P, Szczylik C, Nathan P, et al. Open-label phase 2 trial of first-line everolimus monotherapy in patients with papillary metastatic renal cell carcinoma: RAPTOR final analysis. Eur J Cancer. (2016) 69:226–35. doi: 10.1016/j.ejca.2016.08.004
43. Koh Y, Lim HY, Ahn JH, Lee JL, Rha SY, Kim YJ, et al. Phase II trial of everolimus for the treatment of non clear-cell renal cell carcinoma. Ann Oncol. (2013) 24:1026–31. doi: 10.1093/annonc/mds582
44. Lee JB, Park HS, Park S, Lee HJ, Kwon KA, Choi YJ, et al. Temsirolimus in asian metastatic/recurrent non-clear cell renal carcinoma. Cancer Res Treat. (2019) 51:1578–88. doi: 10.4143/crt.2018.671
45. Vogelzang NJ, Olsen MR, McFarlane JJ, Arrowsmith E, Bauer TM, Jain RK, et al. Safety and efficacy of nivolumab in patients with advanced non-clear cell renal cell carcinoma: results from the phase IIIb/IV checkMate 374 study. Clin Genitourin Cancer. (2020) 18:461–8. doi: 10.1016/j.clgc.2020.05.006
46. Atkins MB, Jegede OA, Haas NB, McDermott DF, Bilen MA, Stein M, et al. Phase II study of nivolumab and salvage nivolumab/ipilimumab in treatment-naïve patients with advanced non-clear cell renal cell carcinoma (HCRN GU16-260-Cohort B). J Immunother Cancer. (2023) 11:e004780. doi: 10.1136/jitc-2022-004780
47. McDermott DF, Lee JL, Ziobro M, Suarez C, Langiewicz P, Matveev VB, et al. Open-label, single-arm, phase II study of pembrolizumab monotherapy as first-line therapy in patients with advanced non-clear cell renal cell carcinoma. J Clin Oncol. (2021) 39:1029–39. doi: 10.1200/JCO.20.02365
48. Tykodi SS, Gordan LN, Alter RS, Arrowsmith E, Harrison MR, Percent I, et al. Safety and efficacy of nivolumab plus ipilimumab in patients with advanced non-clear cell renal cell carcinoma: results from the phase 3b/4 CheckMate 920 trial. J Immunother Cancer. (2022) 10:e003844. doi: 10.1136/jitc-2021-003844
49. Albiges L, Pouessel D, Beylot-Barry M, Guido B, Diane P, Céline G, et al. Nivolumab in metastatic nonclear cell renal cell carcinoma: First results of the AcSe prospective study. J Clin Oncol. (2020) 38:699–9. doi: 10.1200/JCO.2020.38.6_suppl.699
50. Conduit C, Davis ID, Goh JC, Kichenadasse G, Gurney H, Harris CA, et al. A phase II trial of nivolumab followed by ipilimumab and nivolumab in advanced non-clear-cell renal cell carcinoma. BJU Int. (2024) 133:57–67. doi: 10.1111/bju.v133.S3
51. Koshkin VS, Barata PC, Zhang T, George DJ, Atkins MB, Kelly WJ, et al. Clinical activity of nivolumab in patients with non-clear cell renal cell carcinoma. J Immunother Cancer. (2018) 6:9. doi: 10.1186/s40425-018-0319-9
52. Chahoud J, Msaouel P, Campbell MT, Bathala T, Xiao L, Gao J, et al. Nivolumab for the treatment of patients with metastatic non-clear cell renal cell carcinoma (nccRCC): A single-institutional experience and literature meta-analysis. Oncologist. (2020) 25):252–8. doi: 10.1634/theoncologist.2019-0372
53. de Vries-Brilland M, Gross-Goupil M, Seegers V, Boughalem E, Beuselinck B, Thibault C, et al. Are immune checkpoint inhibitors a valid option for papillary renal cell carcinoma? A multicentre retrospective study. Eur J Cancer. (2020) 136:76–83. doi: 10.1016/j.ejca.2020.02.019
54. McKay RR, Bossé D, Xie W, Wankowicz SAM, Flaifel A, Brandao R, et al. The clinical activity of PD-1/PD-L1 inhibitors in metastatic non-clear cell renal cell carcinoma. Cancer Immunol Res. (2018) 6:758–65. doi: 10.1158/2326-6066.CIR-17-0475
55. Gupta R, Ornstein MC, Li H, Allman KD, Wood LS, Gilligan T, et al. Clinical activity of ipilimumab plus nivolumab in patients with metastatic non-clear cell renal cell carcinoma. Clin Genitourin Cancer. (2020) 18:429–35. doi: 10.1016/j.clgc.2019.11.012
56. Oudard S, Banu E, Vieillefond A, Fournier L, Priou F, Medioni J, et al. Prospective multicenter phase II study of gemcitabine plus platinum salt for metastatic collecting duct carcinoma: results of a GETUG (Groupe d'Etudes des Tumeurs Uro-Génitales) study. J Urol. (2007) 177:1698–702. doi: 10.1016/j.juro.2007.01.063
57. Richey SL, Tamboli P, Ng CS, Lin E, Lim ZD, Araujo JC, et al. Phase II trial of pemetrexed plus gemcitabine in patients with locally advanced and metastatic nonclear cell renal cell carcinoma. Am J Clin Oncol. (2013) 36:450–4. doi: 10.1097/COC.0b013e3182546a91
58. Tsimafeyeu I, Demidov L, Kharkevich G, Petenko N, Galchenko V, Sinelnikov I, et al. multicenter, uncontrolled trial of single-agent capecitabine in patients with non-clear cell metastatic renal cell carcinoma. Am J Clin Oncol. (2012) 35:251–4. doi: 10.1097/COC.0b013e31820dbc17
59. Bylow KA, Atkins MB, Posadas EM, Stadler WM, McDermott DF. Phase II trial of carboplatin and paclitaxel in papillary renal cell carcinoma. Clin Genitourin Cancer. (2009) 7:39–42. doi: 10.3816/CGC.2009.n.007
60. Rizzo M, Chiellino S, Gernone A, Porta C. Cisplatin-based chemotherapy for the treatment of metastatic collecting duct carcinomas: A real-world, retrospective analysis. Front Oncol. (2022) 12:939953. doi: 10.3389/fonc.2022.939953
61. Hutson TE, Michaelson MD, Kuzel TM, Agarwal N, Molina AM, Hsieh JJ, et al. A single-arm, multicenter, phase 2 study of lenvatinib plus everolimus in patients with advanced non-clear cell renal cell carcinoma. Eur Urol. (2021) 80:162–70. doi: 10.1016/j.eururo.2021.03.015
62. Feldman DR, Ged Y, Lee CH, Knezevic A, Molina AM, Chen YB, et al. Everolimus plus bevacizumab is an effective first-line treatment for patients with advanced papillary variant renal cell carcinoma: Final results from a phase II trial. Cancer. (2020) 126:5247–55. doi: 10.1002/cncr.v126.24
63. Albiges L, Gurney H, Atduev V, Suarez C, Climent MA, Pook D, et al. Pembrolizumab plus lenvatinib as first-line therapy for advanced non-clear-cell renal cell carcinoma (KEYNOTE-B61): a single-arm, multicentre, phase 2 trial. Lancet Oncol. (2023) 24:881–91. doi: 10.1016/S1470-2045(23)00276-0
64. Mahoney KM, Jacobus S, Bhatt RS, Song J, Carvo I, Cheng SC, et al. Phase 2 study of bevacizumab and temsirolimus after VEGFR TKI in metastatic renal cell carcinoma. Clin Genitourin Cancer. (2016) 14:304–13. doi: 10.1016/j.clgc.2016.02.007
65. McGregor BA, McKay RR, Braun DA, Werner L, Gray K, Flaifel A, et al. Results of a multicenter phase II study of atezolizumab and bevacizumab for patients with metastatic renal cell carcinoma with variant histology and/or sarcomatoid features. J Clin Oncol. (2020) 38:63–70. doi: 10.1200/JCO.19.01882
66. Stellato M, Buti S, Maruzzo M, Bersanelli M, Pierantoni F, De Giorgi U, et al. Pembrolizumab plus axitinib for metastatic papillary and chromophobe renal cell carcinoma: NEMESIA (Non clear MEtaStatic renal cell carcinoma pembrolizumab axitinib) study, a subgroup analysis of I-RARE observational study (Meet-URO 23a). Int J Mol Sci. (2023) 24:1096. doi: 10.3390/ijms24021096
67. Srinivasan R, Gurram S, Harthy MA, Singer EA, Linehan WM. Results from a phase II study of bevacizumab and erlotinib in subjects with advanced hereditary leiomyomatosis and renal cell cancer (HLRCC) or sporadic papillary renal cell cancer. J Clin Oncol. (2020) 38:5004–4. doi: 10.1200/JCO.2020.38.15_suppl.5004
68. Lee CH, Voss MH, Carlo MI, Chen YB, Zucker M, Knezevic A, et al. Phase II trial of cabozantinib plus nivolumab in patients with non-clear-cell renal cell carcinoma and genomic correlates. J Clin Oncol. (2022) 40:2333–41. doi: 10.1200/JCO.21.01944
69. Suárez C, Larkin JMG, Patel P, Valderrama BP, Rodriguez-Vida A, Glen H, et al. Phase II study investigating the safety and efficacy of savolitinib and durvalumab in metastatic papillary renal cancer (CALYPSO). J Clin Oncol. (2023) 41:2493–502. doi: 10.1200/JCO.22.01414
70. Sheng X, Cao D, Yuan J, Zhou F, Wei Q, Xie X, et al. Sorafenib in combination with gemcitabine plus cisplatin chemotherapy in metastatic renal collecting duct carcinoma: A prospective, multicentre, single-arm, phase 2 study. Eur J Cancer. (2018) 100:1–7. doi: 10.1016/j.ejca.2018.04.007
71. Pal SK, Tangen C, Thompson IM Jr, Balzer-Haas N, George DJ, Heng DYC, et al. A comparison of sunitinib with cabozantinib, crizotinib, and savolitinib for treatment of advanced papillary renal cell carcinoma: a randomised, open-label, phase 2 trial. Lancet. (2021) 397:695–703. doi: 10.1016/S0140-6736(21)00152-5
Keywords: non-clear cell renal cell carcinoma, vascular endothelial growth factor receptor tyrosine kinase inhibitors, mammalian target of rapamycin inhibitors, chemotherapy, immune checkpoint inhibitor
Citation: Zhang Y, Chen J, Wang X, Wang H, Chen X, Hong J and Fang H (2024) Effectiveness of systemic treatments for advanced non-clear cell renal cell carcinoma: a systematic review and meta-analysis. Front. Oncol. 14:1478245. doi: 10.3389/fonc.2024.1478245
Received: 09 August 2024; Accepted: 03 December 2024;
Published: 18 December 2024.
Edited by:
Lothar Bergmann, University Hospital Frankfurt, GermanyReviewed by:
Shiying Tang, Peking University Third Hospital, ChinaFrancisco Cezar Aquino de Moraes, Federal University of Pará, Brazil
Copyright © 2024 Zhang, Chen, Wang, Wang, Chen, Hong and Fang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hongming Fang, ZmFuZ2hvbmdtaW5nMDQxMkAxNjMuY29t
 Yaping Zhang1
Yaping Zhang1 Hongming Fang
Hongming Fang