- 1Clinical Nutrition, IRCCS San Raffaele Scientific Institute, Milano, Italy
- 2Department of Radiation Oncology, IRCCS San Raffaele Scientific Institute, Milano, Italy
- 3Translational Oncology ARCO Foundation, Cuneo, Italy
- 4Clinical Nutrition and Dietetics Unit, Fondazione IRCCS Policlinico San Matteo, Pavia, Italy
- 5General Medicine and Advanced Care Unit, IRCCS San Raffaele Scientific Institute, Milano, Italy
- 6Vita-Salute San Raffaele University, Milano, Italy
- 7Department of Otorhinolaryngology, IRCCS San Raffaele Scientific Institute, Milano, Italy
Introduction: Chemoradiotherapy in head and neck cancer patients has a curative intent but often deteriorates nutritional status leading to sarcopenia and cachexia.
Methods: In this observational and single-centered study, a prospective evaluation of several biochemical and anthropometrical parameters, weight loss, handgrip strength, visual analogue scale of appetite, questionnaires associated with malnutrition & quality of life and body composition (obtained by Bioelectrical Impedance Vector Analysis) was performed before and after high-dose cisplatin chemotherapy combined with radiotherapy in 60 patients affected by head and neck cancer. Oral nutritional supplements were used to reach the correct number of daily calories and proteins.
Results and discussion: All patients completed radiotherapy as planned and the 96,4% of them did not interrupt chemotherapy for toxicity, reaching a total dose of at least 200mg/m2. Despite a rapid deterioration of body composition during treatment, nutritional support helped patients to maintain (or in some cases improve) anthropometric parameters from the end of chemoradiotherapy to the following 3 months. Low prealbumin and albumin pre-treatment led to higher risk of toxicities with consequent reduction of cisplatin dose intensity, whereas weight at the end of the treatment seems to be an interesting predicting factor for disease free and overall survival (p=0.007; p=0.015).
Introduction
Head and neck cancers are a heterogeneous group of malignancies affecting the oral cavity, nasopharynx, oropharynx, hypopharynx, larynx, paranasal sinuses and salivary glands, which are challenging to treat due to their genetic complexity and aggressiveness. Globally, there are an estimated 890,000 new cases each year (about 4.5% of all cancer diagnoses) and 450,000 deaths (approximately 4.6% of global cancer deaths). Specifically, there are roughly 380,000 cases of lip and oral cavity cancers, 185,000 cases of laryngeal cancer, 133,000 cases of nasopharyngeal cancer, 98,000 cases of oropharyngeal cancer, 84,000 cases of hypopharyngeal cancer, and 54,000 cases of salivary gland cancer (1).
These cancers are primarily linked to tobacco use, alcohol abuse and viral infections, mainly Epstein–Barr virus and human papillomavirus (2). Other risk factors include radiation exposure, dietary patterns, alcohol-containing mouthwashes, poor oral hygiene and periodontal disease (3). The global distribution of risk factors affects the variability and incidence of these cancers, with worse prognosis for patients who receive a late diagnosis or have limited access to specialized care (4). For patients with locoregionally advanced head and neck squamous cell carcinoma (HNSCC) and unresectable tumors, or those with expected poor functional outcomes from surgery, primary concomitant chemoradiotherapy (CRT) with high-dose cisplatin (100 mg/m2) administered every three weeks for three cycles is the preferred treatment regimen (5–9). Moreover, the management is particularly complicated as some of these patients continue to use tobacco and alcohol during treatment, adversely affecting clinical outcomes (10).
Patients with locally advanced HNSCC often experience malnutrition, dysphagia and pain, leading to significant weight loss even before diagnosis (11, 12) and a loss of weight greater than 5% is an independent prognostic factor for poorer progression free survival (13). Malnutrition is associated with worse treatment outcomes, increased morbidity and mortality, treatment delays and unplanned hospitalizations (14). In several patients, malnutrition is present at diagnosis due to metabolic aberrations and difficulties with oral food intake, such as impaired swallowing or bolus passage, resulting in inadequate daily caloric intake. Therefore, nutritional support before, during and after treatment is crucial, even for overweight and obese patients whose high body mass index (BMI) might not initially suggest a risk for malnutrition (15). Additionally, nutritional counselling, which represents the first line of nutritional support, should be introduced before treatments begin to optimize body composition and address potential frailty (16, 17).
During treatments, patients frequently experience dysphagia, loss of appetite, fatigue, pain, dyspnea, xerostomia, sticky saliva and oral mucositis. These symptoms significantly impact nutritional status and the ability to maintain normal social relations, thereby affecting quality of life (18). Functional disturbances, such as issues with speech, swallowing, hearing and breathing, further complicate social interactions and contribute to physical and psychological difficulties, including changes in body image and loss of function (19).
The primary goal of nutritional support - provided before, during and after treatments - is to avoid weight loss and help patients complete the therapies with minimal toxicities (20). Cancer patients are often hypermetabolic compared to controls, making adequate nutrition critical to achieve this, oral nutritional supplements (ONS) help to ensure appropriate daily caloric and protein intake, as recommended by ESPEN guidelines (21).
This study aims to assess the impact of early and systematic nutritional counselling on the quality of life of patients with head and neck cancers, as well as on adherence to oncological treatments, tolerance, and overall response rates, with the goal of maintaining and potentially improving body composition.
Materials and methods
This is an observational, prospective, single-center study. From May 2021 to March 2023, 60 patients were consecutively enrolled at IRCCS San Raffaele Hospital in Milan, Italy. Specifically, all adults aged ≥ 18 years with head and neck cancer and a histological diagnosis of squamous cell carcinoma of the oral cavity, oropharynx, nasopharynx, larynx, hypopharynx, or an unknown squamous cell primary of head and neck origin were included. These cases were discussed in a Head and Neck team meeting (composed of surgeons, medical oncologists, radiation oncologists, nutritionists, head and neck radiologists, nuclear medicine physicians, pathologists, odontologists) and were deemed medically fit for a treatment plan involving either primary or adjuvant radiotherapy with concurrent chemotherapy (high-dose cisplatin). Conversely, women known to be pregnant or planning to become pregnant during the trial period, as well as patients requiring total parenteral nutrition, were excluded from the study. Comparisons shown in this study refer to 3 time points: T0 (at the beginning of the treatment), T1 (at the end of the treatment) and T2 (3 months after the treatment), at each time point, laboratory tests were collected (in particular: complete blood count, creatinine, albumin and prealbumin) as well as data regarding nutritional assessment and intervention.
The study received approval from the Institutional Ethical Committee (San Raffaele Hospital, Milan, approval date 08/06/2022) and all patients provided informed consent. Data were collected in compliance with the approved guidelines and according to good clinical practice.
Treatment
All patients were treated with helical TomoTherapy® (HT-Accuray, Maddison, WI, USA).
In the radical setting, a 18-Fluorodeoxyglucose computed tomography (CT) positron emission tomography (PET) scan was performed to identify biological target volume (BTV). For these patients, a hypofractionated schedule with a simultaneous integrated boost was used: 54 Gy in 30 fractions to bilateral neck nodes and 66 Gy to the tumor and high risk/PET positive nodes. These patients received primary concomitant Cisplatin at 100 mg/m2 on day 1, 22, and 43 during standard fractionated radiotherapy (or on day 1 and 22 during accelerated radiotherapy) (7–9).
In the postoperative setting, radiotherapy was prescribed according to histological examination findings: 54 Gy in 30 fractions to low-risk volumes and 61.5-64 Gy in 30 fractions to high-risk volumes. These patients received Cisplatin at 100 mg/m2 on day 1, 22, and 43 during radiotherapy or a flat dose of 50 mg weekly. In all cases, patients received a total dose of at least 200 mg/m2 of Cisplatin (22–25). Toxicities of the treatment were scored according to Common Terminology Criteria for Adverse Events (version 4.03).
Nutritional assessment and intervention
Nutritional risk screening
To evaluate the risk of malnutrition, Nutritional Risk Screening tool (NRS-2002) (26) and Mini Nutritional Assessment-Short Form tool (MNA-SF) (27) were used. For each patient, body weight and height were collected as well as the weight lost 3-6months before T0, BMI was calculated by dividing body weight (kg) by the height squared (m2), as described by the World Health Organization (28).
Body composition and functional assessment
A BIA 101 BIVA bioelectric impedance device (Akern®) was used to obtain the following parameters: standardized phase angle (SPA), body cellular mass index (BCMI), total body water (TBW, L), extracellular water (ECW, L), skeletal muscle mass (SM, kg), skeletal muscle index (SMI, kg/m2), appendicular skeletal muscle mass (ASMM, kg/m2) and fat mass index (FMI, kg/m2). BIVA, as a simple and non-invasive technique, estimates body composition by measuring the opposition (impedance) to an electrical current passing through the body. The impedance identifies cellular health and reflects membrane integrity, cell mass and hydration status (29). SPA was considered instead of Phase Angle to obtain measures independent from age, sex, ethnicity, and BMI (30).
Handgrip strength (HGS, kg) was assessed with a Camry Digital Hand Dynamometer (31) to determine muscle function and strength, this evaluation was considered together with data emerging from BIVA to identify patients at risk of sarcopenia and tailor nutritional counselling on that finding.
Appetite and quality of life
Patients reported their appetite levels using a visual analogue scale (VAS) to compare their sensations at different points in their therapeutic journey (32).
Quality of life was assessed using the Italian versions of FACT-H&N and EORTC QLQ-C35 questionnaires (33). The instruments consist of thirty-nine and thirty-eight questions, respectively, related to patient functioning and the severity of cancer-related symptoms. Final scores were calculated according to the scoring manual.
Nutritional requirements and intervention
Total daily energy requirements were calculated by multiplying the estimated resting energy expenditure (using the Harris‐Benedict equations) by a correcting factor of 1.5. The amount of ONS supplementation was set for each patient to reach calorie and protein needs as established by ESPEN guidelines (aiming for up to 25–30 kcal/kg/day and 1.5 g/kg/day of protein) (21), evaluating the specific food intake every three weeks from the beginning of chemoradiotherapy.
At each nutritional visit were considered symptoms such as dysgeusia, nausea, constipation and dysphagia to change consistency and possibly taste of the ONS, if needed to improve the compliance of the patients (maintaining the same nutritional values from a product to the respective alternative).
Nutritional counselling was conducted at the beginning of treatment and then every three weeks, selecting energy dense (up to 300-400 kcal/bottle) and/or high-protein ONS basing on each patient’s preferences and needs. The average protein content in the supplements was 20-40 g/day, adjusted to meet each patient’s estimated requirements and actual daily food intake. Due to rapid changes induced by the treatment, the supplementation plan was often adjusted every three weeks, taking into account alterations in body composition evaluated by BIVA.
Nutritional counselling included sample meal plans to create balanced breakfasts, lunches, dinners, and snacks with high concentrations of carbohydrates, proteins and fats to prevent the loss of weight, muscle mass and function. Meal plans were prepared in accordance with Italian Guidelines, in particular, were considered: “Linee Guida per una Sana Alimentazione” (Consiglio per la ricerca in agricoltura e l’analisi dell’economia agraria-Crea, 2018) and” IV Revisione dei Livelli di Assunzione di Riferimento di Nutrienti ed energia per la popolazione italiana-LARN” (Società Italiana di Nutrizione Umana-SINU, 2017). The plans were tailored to individual eating patterns and food preferences, with recipes suggestions to facilitate adherence.
When ONS were insufficient to meet nutritional goals or when oral intake of food or liquids by mouth was impossible due to treatment-related toxicities such as mucositis, artificial nutrition was initiated to prevent weight loss and subsequent malnutrition.
Statistical analysis
Due to the exploratory nature of the study, no a priori sample size calculation and statistical power analysis were performed.
Comparisons of each variable at different time points were conducted using the Friedman test for repeated measures. Differences in categorical variables were analyzed using the χ2-test or Fisher’s exact test. Correlations were assessed with Spearman’s rank correlation coefficient. A generalized linear mixed model (GLMM) was used to compare changes in weight, with the FACT variable as the target variable. Disease free survival (DFS) and overall survival (OS) were estimated using the Kaplan-Meier method and compared with the log-rank test. Cox regression analysis was performed to estimate hazard ratio (HR).
All analysis were conducted using SPSS V.24 (IBM SPSS Statistics for Windows, Version 24.0. Armonk, NY, USA). Correlations were plotted with JMP®, Version 17 Pro. SAS Institute Inc., Cary, NC, 1989–2024. In all tests, a P-value <0.05 was considered significant. The Benjamini–Hochberg (B-H) procedure was applied to control the false discovery rate at 10% (34).
Results
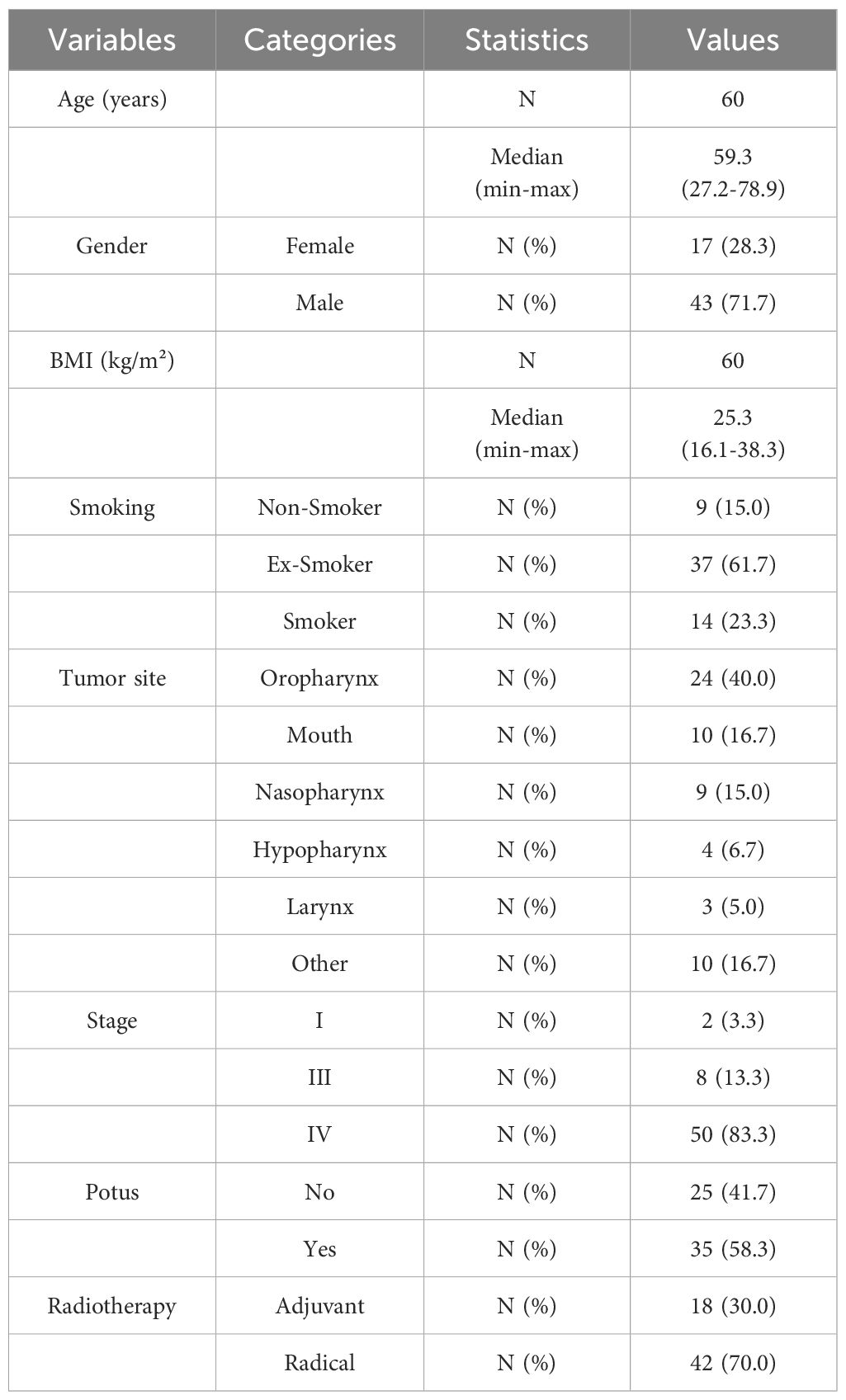
Sixty patients were consecutive enrolled and their characteristics are shown in Table 1.
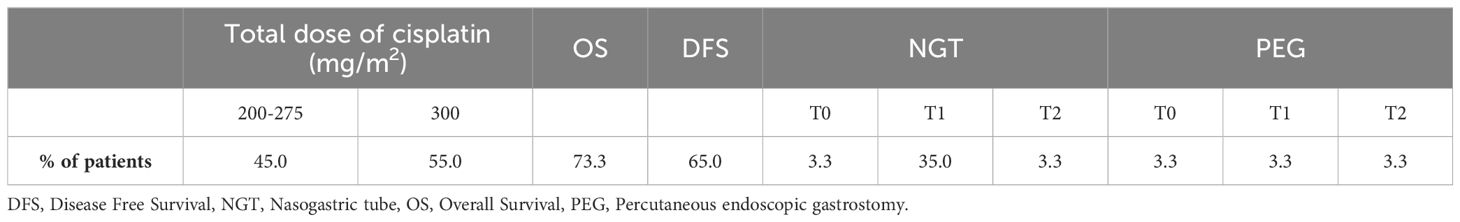
All patients completed radiotherapy as planned and 96.4% did not interrupt chemotherapy due to toxicity, achieving a total dose of at least 200 mg/m2 (with 55.0% completing 300 mg/m2). Regarding OS, 73.3% of the patients were still alive in March 2024 and 65.0% were surviving without disease (Table 2). All the patients received tailored nutritional counselling with ONS at T0, T1 and T2.
A small number of patients started treatment (T0) with prior artificial nutrition. By the end of the six weeks (T1), 35.0% of patients were being fed with nasogastric tube and 3.3% with a percutaneous endoscopic gastrostomy. None of the patients placed percutaneous endoscopic gastrostomy during treatment (the 3.3% indicated in Table 2 already had it at the beginning of the treatment), whereas some of them started at T0 with ONS and then placed nasogastric tube later when oral intake of food or liquids by mouth was impossible due to treatment-related toxicities.
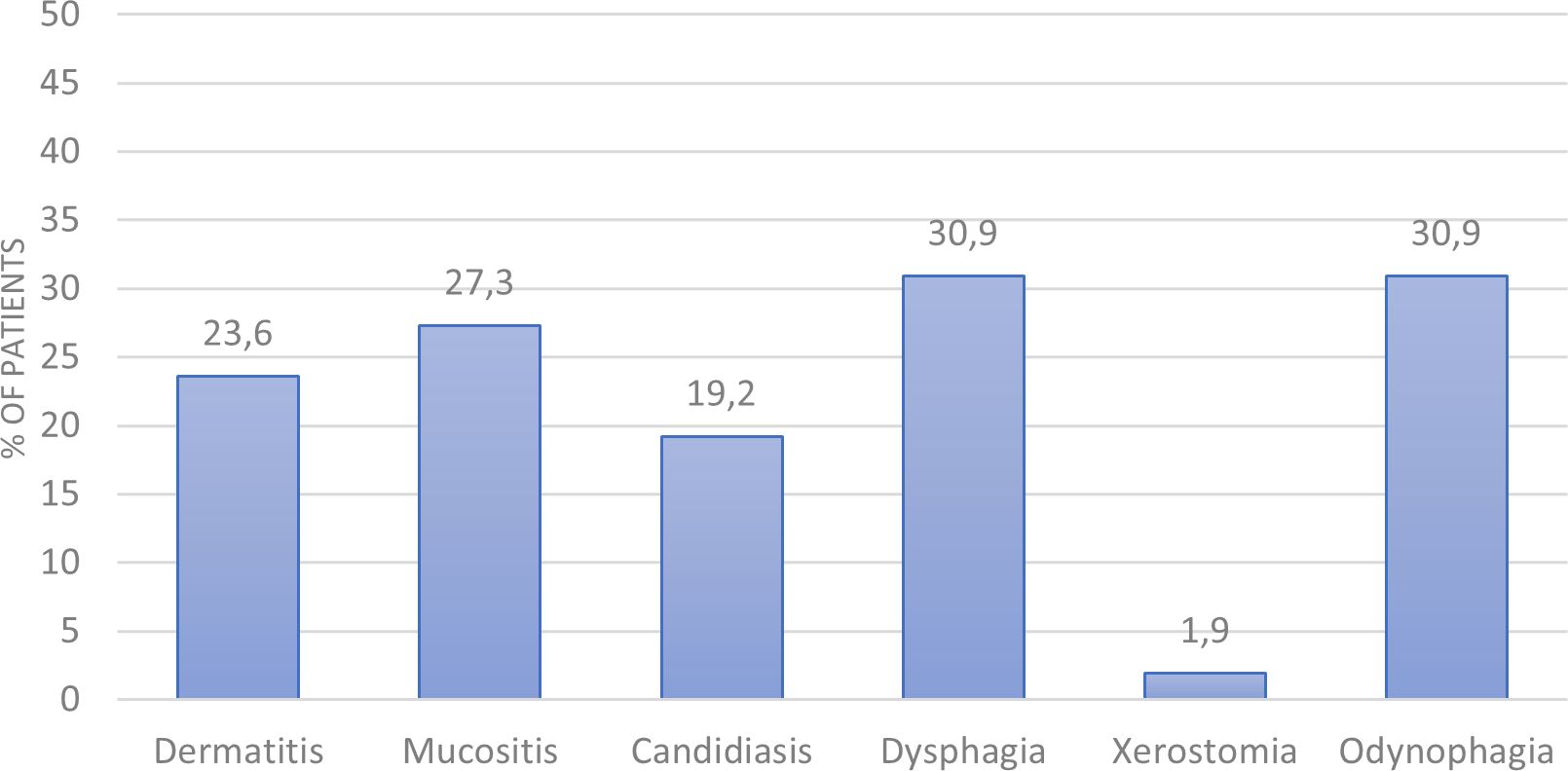
The most frequent grade 3 toxicities experienced by patients during treatment are shown in Figure 1. The most common toxicities were odynophagia, dysphagia and mucositis (30.9%, 30.9% and 27.3%, respectively). Others included dermatitis, candidiasis and xerostomia (23.6%, 19.2% and 1.9%, respectively).
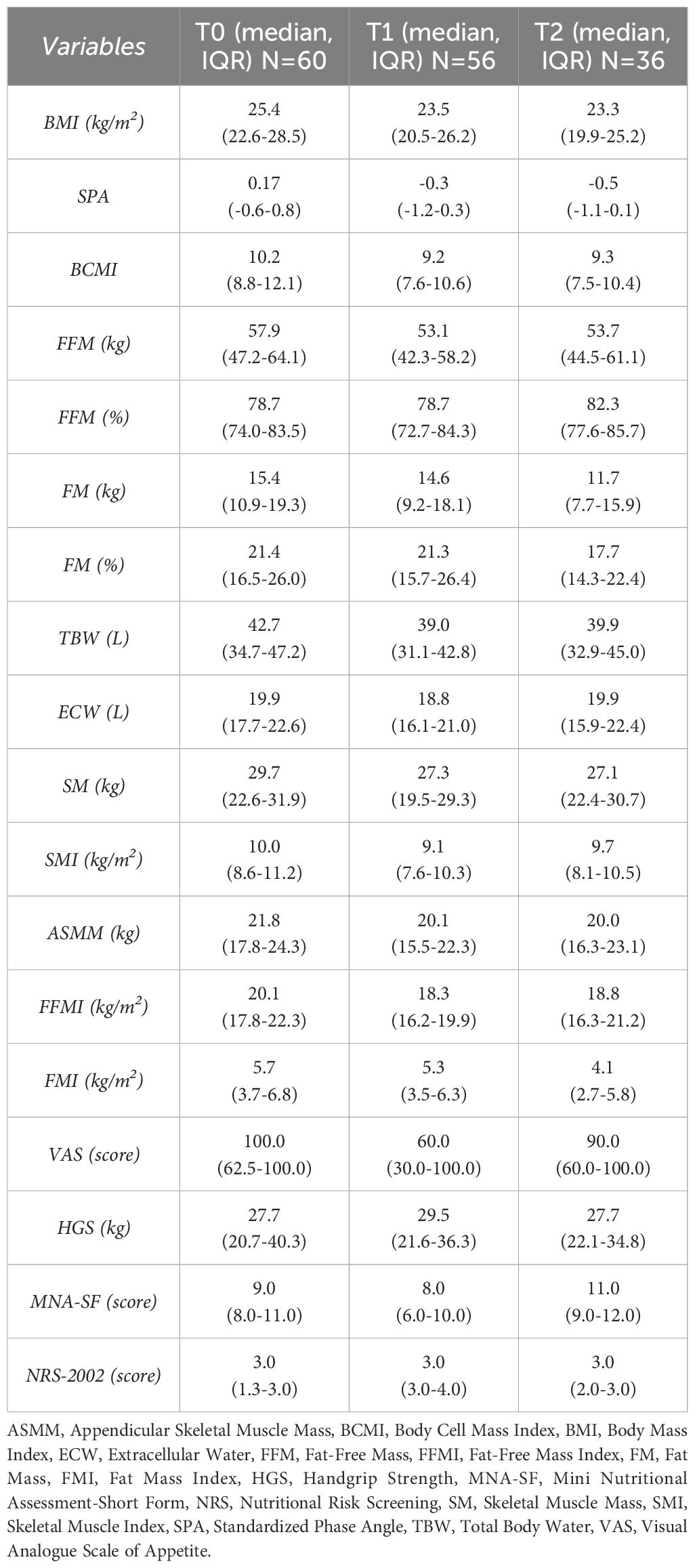
All collected variables at the start (T0) and end (T1) of CRT, as well as three months after (T2), are described in Table 3. The values are reported with median and interquartile range. Appetite, handgrip strength and nutritional risk did not deteriorate as rapidly as expected from T0 to T2. On the contrary, there was stability in VAS, HGS, MNA-SF and NRS-2002 scores from the beginning of the treatment to 3 months after.
Nutritional support helped patients maintain or, in some cases, improve their anthropometric parameters. For example, SMI increased from 9.1 kg/m2 at T1 to 9.7 kg/m2 at T2. While SM and SMI showed a significant decline from T0 to T1 (p=0.016 for both), this decline did not continue from T1 to T2 (p=1.000 for both). Data are presented in Table 4.
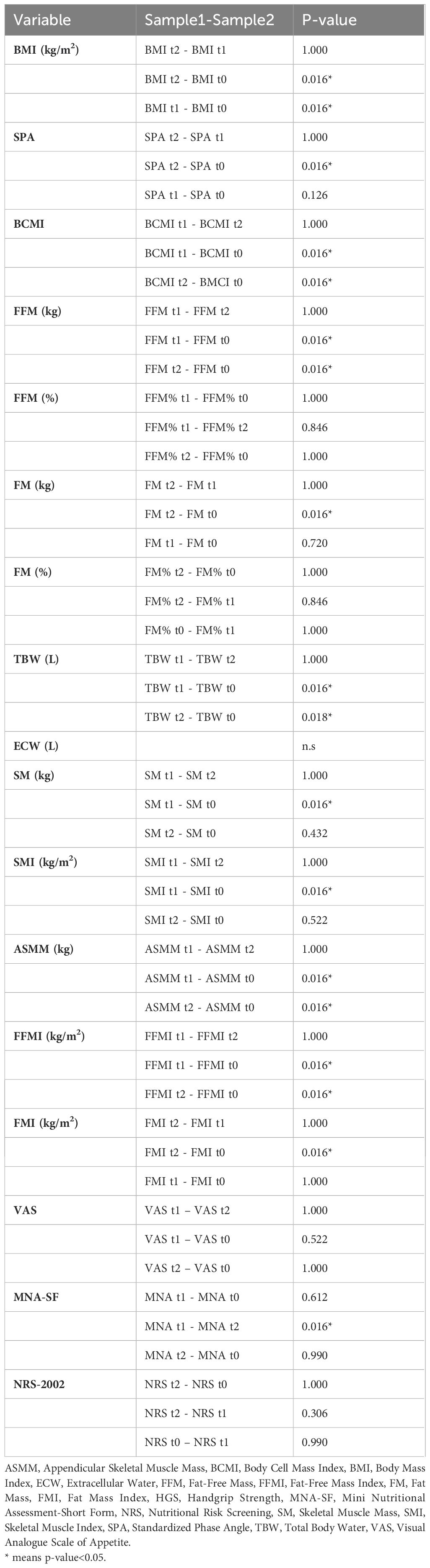
Table 4. Friedman test for repeated measures analysis of variance by ranks for anthropometric measure at T0, T1 and T2.
Low levels of prealbumin and albumin before treatment were associated with a higher risk of toxicities, which led to a reduction of cisplatin dose intensity. Pre-treatment prealbumin levels also showed a negative correlation with pre-treatment weight loss and several anthropometric parameters. As shown in Figure 2, pre-treatment prealbumin correlates with BCMI at T1 (p=0.016), FFM at T1 (p=0.020), TBW at T1 (p=0.037), ASMM at T1 (p=0.021) and FFMI at T1 (p=0.066). At T2, pre-treatment prealbumin correlates with BCMI (p=0.058) and FFM (p=0.097).
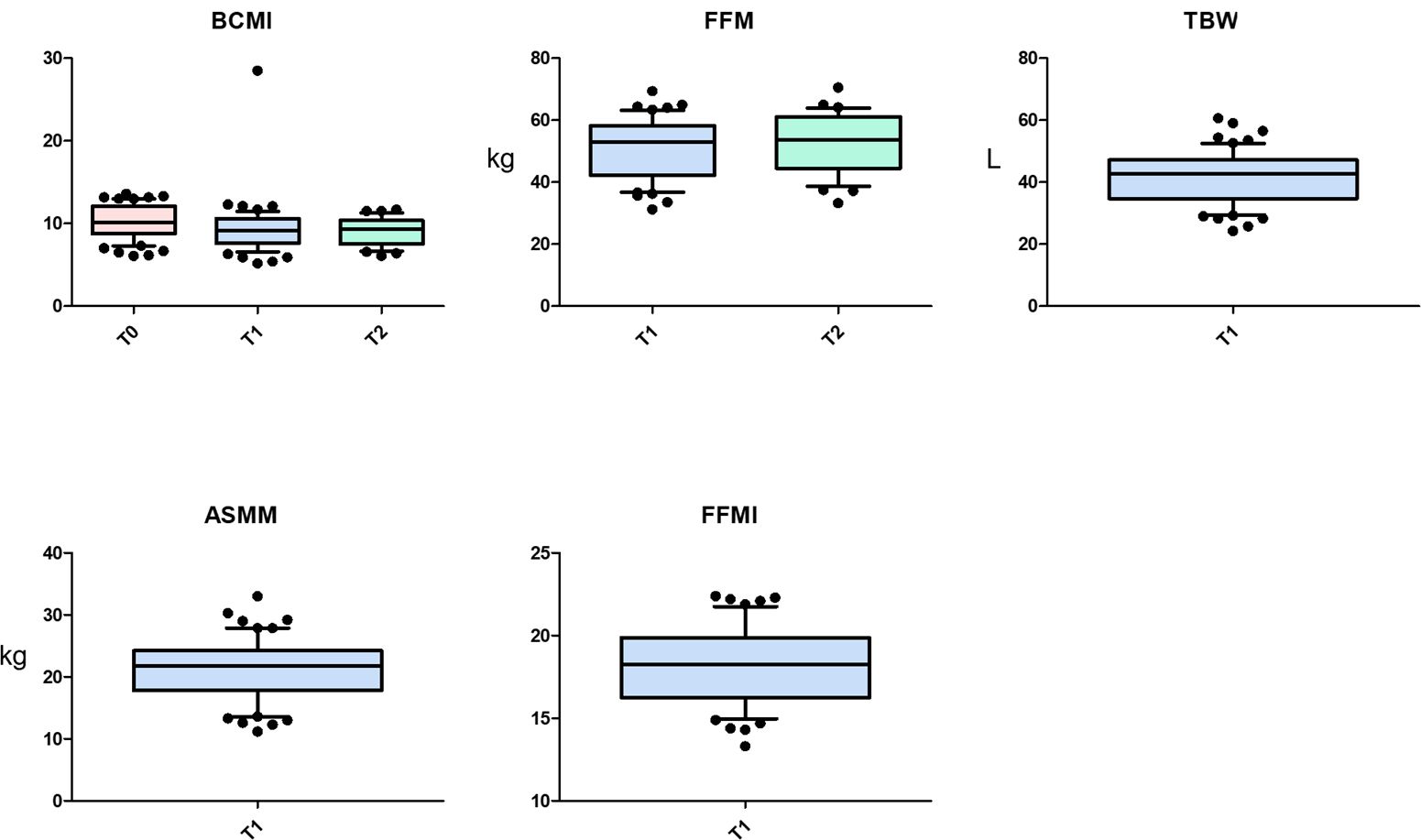
Figure 2. Correlations between pre-treatment prealbumin and anthropometric parameters. ASMM, Appendicular Skeletal Muscle Mass, BCMI, Body Cell Mass Index, FFM, Fat-Free Mass, FFMI, Fat-Free Mass Index, TBW, Total Body Water.
Quality of life questionnaires, FACTH&N and EORCT, showed a rapid deterioration in several variables from the beginning to the end of treatment, largely due to the severe side effects of CRT. However, there was an improvement in quality of life 3 months after treatment, in particular in areas such as sticky saliva, use of nutritional supplements, feeding tube use, weight loss/gain, pain, social eating, speech, coughing, open mouthing, dry mouth and swallowing.
The onset of toxicities was directly related to the dose intensity of cisplatin, as expected. However, there was no significant correlation between these toxicities and anthropometric measures or quality of life questionnaires at the end of CRT.
Table 5 present the relationship between various variables (creatinine pre-treatment, sex, age, weight after treatment, weight loss before treatment, neutrophil-to-lymphocyte ratio at the end of treatment), DFS and OS. Although the number of patients limits the certainty of these findings, weight at T1 seems to be a significant predictor for DFS (p=0.007) and OS (p= 0.015).
Discussion
Nutritional status is particularly important in HNSCC patients. The tumor itself can cause chewing difficulties, odynophagia, and dysphagia, leading to malnutrition. In addition, tooth extractions, usually performed before radiotherapy to minimize post-radiation complications or infections, further limit normal eating. Treatment side effects, especially from concomitant CRT, such as acute mucositis and long-term issues like dry mouth or sticky saliva, can also impair swallowing.
These challenges deeply affect patients’ impact body composition and quality of life, necessitating interventions to improve eating habits beyond treatment to prevent weight loss and reduced food intake. To mitigate malnutrition during CRT, prophylactic placement of a PEG tube might be considered. A randomized study showed that this approach resulted in fewer malnourished patients, longer enteral feeding and better quality of life 6 months after treatment without increased long-term dysphagia risk compared to standard clinical practice (35, 36). However, not all patients need enteral feeding, as seen in our study, where none required upfront PEG placement. Selecting high risk patients for malnutrition based on weight loss before treatment, age and radiotherapy dose to the constrictor muscles, can guide prophylactic PEG tube placement (37). Alternatively, nasogastric tube feeding effectively maintains body weight. In our Institution, this method is preferred due to its ease of placement and removal, as the optimal method for enteral feeding in HNSCC patients remain undetermined (38).
Protein-energy malnutrition can lead to sarcopenia, characterized by the loss of skeletal muscle mass and function (39). Sarcopenic patients with head and neck cancer have more than double the risk of severe treatment-related toxicity (40) and often require breaks in radiation treatment compared to non-sarcopenic patients (41). Dual-energy X-ray absorptiometry and magnetic resonance imaging are the gold standards for detecting low muscle mass but are limited in routine clinical practice due to high costs and radiation exposure concerns (42, 43). Another validated technique is CT-based body composition analysis at the third cervical vertebra (C3), which allow to identify the reduction of cervical muscles width (44).
For this study, we used BIVA and a dynamometer to detect sarcopenia, considering the strong relationship between isometric hand grip strength and lower extremity muscle power, knee extension torque and calf cross-sectional muscle area (45). These instruments are easy to use, inexpensive, reproducible and suitable for ambulatory patients.
Early intervention on sarcopenia with proactive exercise and nutritional counselling before treatment has been shown to reduce chemotherapy-related toxicity and improve OS, DFS and disease-specific survival (46). CRT strongly impacts food consumption, particularly affecting appetite and swallowing. ONS contribute to reach the nutritional requirements recommended by international guidelines (21). Patients benefit from high-quality protein sources, such as whey proteins, which are rapidly digested and increase plasma amino acid levels quickly (47). In cancer, protein breakdown is upregulated, leading to muscle weakness and dysfunction, but appropriate amounts of essential amino acids can preserve anabolic pathways and recovery (48). Whey protein supplementation improves body composition, muscle strength and treatment tolerance in malnourished advanced cancer patients, given their richness in cysteine, which supports glutathione synthesis and protects cells under chemotherapy-induced oxidative stress (49).
This study focused on the impact of nutritional counselling on treatment tolerance and outcomes. Our data showed that systematic nutritional counselling and regular body composition monitoring positively impacted on the onset of toxicities and significantly improved treatment adherence (100% completed radiotherapy, 96.4% chemotherapy). Although quality of life worsened during CRT, this trend was reversed within three months by continuing personalized nutritional counselling. Symptoms impacting the ability and desire to eat are common long-term issues for head and neck cancer survivors (50). Baseline-to-one-year changes in social eating problems are associated with swallowing-related quality of life, poor nutritional status, tumor site, age, and depressive symptoms (51). Early and systematic nutritional counselling is crucial for identifying and addressing patients’ critical issues from the start of oncologic treatment. As reported in several studies (52–54) and in our data, patients are frequently malnourished at diagnosis and treatment onset. Early nutritional intervention is facilitated by bioimpedance measurements at each nutritional visit, allowing immediate action if parameters worsen (e.g., BCMI and SPA). When ONS are insufficient to meet the energy requirements, nasogastric tubes are used for tailored enteral nutrition.
Serum albumin and prealbumin declines should be recognized as inflammatory markers rather than direct indicators of malnutrition. Indeed, visceral proteins decrease during the acute-phase response associated with acute and chronic illness and inflammation such as cancers and oncological treatments, regardless of underlying nutrition status, but normalization may indicate the resolution of inflammation, the reduction of nutritional risk, a transition to anabolism, and potentially lower calorie and protein requirements (55). In our study, prealbumin before CRT predicted body composition worsening at T1 and T2 and correlated with cisplatin dose intensity and treatment-related toxicities. Pre-treatment prealbumin was lower in patients with significant weight loss before CRT, likely due to its shorter half-life (2–3 days) compared to albumin (21 days).
The standard high-dose cisplatin regimen (100 mg/m2) for locally advanced squamous cell carcinoma of the head and neck often causes acute and late toxicities such as acute kidney injury (56), dysphagia, xerostomia, hypothyroidism, ototoxicity and osteoradionecrosis (57). In our study, dose reduction or treatment interruption was rare, and nutritional support was crucial to prevent and monitor complications, ensuring quality of life and body composition.
Finally, weight at the end of treatment emerged as a predictive factor for DFS and OS, suggesting that significant weight loss during treatment correlates with worsening health and slower recovery.
We deeply wanted to draw the attention on the changes in clinical outcome and quality of life of this set of patients when supported during the treatments by a multi-disciplinary approach that includes a nutritionist too, personalizing the pathway according to guidelines available for clinical nutrition (21). A similar customized structure is the key to ensure that the patients follow the instructions less laboriously and let them complete the set nutritional plan reducing the onset of toxicities related to chemoradiotherapy.
Our study’s limitations include a small sample size and the inability to evaluate average daily energy intake due to time constraints and the oncologic population’s linguistic/social issues. Future analysis aims to strengthen correlations between nutritional support and survival outcomes. Nutritional intervention was demonstrated, indeed, has been shown to improve three-year overall survival rates in head and neck cancer patients receiving nutritional counseling with/without ONS (58). Moreover, we considered a heterogeneous group of patients with different tumor types and stages, as well as confounding factors like artificial nutrition and surgery prior to treatment, which may influence chemoradiotherapy’s impact on body composition.
Conclusion
Nutritional counselling before, during and after chemoradiotherapy significantly impact the quality of life of head and neck cancer patients and helps them to complete their prescribed treatments with a lower rate of toxicities. Close monitoring of body composition using BIVA enable the early intervention for weight loss with ONS or enteral nutrition. This approach prevents muscle reduction in function and strength by counteracting the catabolism associated with high-dose cisplatin and radiotherapy, ultimately allowing patients to complete their treatments while maintaining a better quality of life.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Institutional Ethical Committee (San Raffaele Hospital, Milan, approval date 08/06/2022). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
SC: Conceptualization, Data curation, Investigation, Methodology, Writing – original draft, Writing – review & editing. CLD: Conceptualization, Data curation, Investigation, Methodology, Writing – original draft, Writing – review & editing. MP: Formal analysis, Methodology, Writing – review & editing. AC: Formal analysis, Methodology, Writing – review & editing. AP: Writing – review & editing. AM: Writing – review & editing. MT: Writing – review & editing. LG: Supervision, Writing – review & editing. AC: Data curation, Writing – review & editing. IDO: Data curation, Writing – review & editing. NGDM: Supervision, Writing – review & editing. RC: Conceptualization, Supervision, Writing – review & editing. AM: Conceptualization, Data curation, Investigation, Methodology, Project administration, Writing – original draft, Writing – review & editing.
Funding
The authors declare financial support was received for the research and authorship. Publication of this article was supported by Italfarmaco SpA. The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article, or the decision to submit it for publication.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
2. Bhat GR, Hyole RG, Li J. Head and neck cancer: Current challenges and future perspectives. Adv Cancer Res. (2021) 152:67–102. doi: 10.1016/bs.acr.2021.05.002
3. Dos Santos ES, Pérez-de-Oliveira ME, Normando AGC, Gueiros LAM, Rogatto SR, Vargas PA, et al. Systemic conditions associated with increased risk to develop oral squamous cell carcinoma: Systematic review and meta-analysis. Head Neck. (2022) 44:2925–37. doi: 10.1002/hed.v44.12
4. Gatta G, Capocaccia R, Botta L. Descriptive epidemiology of the head and neck cancers in old patients. Front Oncol. (2023) 13:1102236. doi: 10.3389/fonc.2023.1102236
5. NCCN, guidelines version 2, head and neck cancers (2019). Available online at: https://www.nccn.org/professionals/physician_gls/pdf/head-and-neck.pdf (accessed 2024).
6. Grégoire V, Lefebvre JL, Licitra L, Felip E. EHNS-ESMO-ESTRO guidelines working group. Ann Oncol. (2010) 21(Suppl. 5):v184–6. doi: 10.1093/annonc/mdq185
7. Forastiere AA, Goepfert H, Maor M, Pajak TF, Weber R, Morrison W, et al. Concurrent chemotherapy and radiotherapy for organ preservation in advanced laryngeal cancer. N Engl J Med. (2003) 349:2091–8. doi: 10.1056/NEJMoa031317
8. Forastiere AA, Zhang Q, Weber RS, Maor MH, Goepfert H, Pajak TF, et al. Long-term results of RTOG 91-11: a comparison of three nonsurgical treatment strategies to preserve the larynx in patients with locally advanced larynx cancer. J Clin Oncol. (2013) 31:845–52. doi: 10.1200/JCO.2012.43.6097
9. Adelstein DJ, Li Y, Adams GL, Wagner H Jr, Kish JA, Ensley JF, et al. An intergroup phase III comparison of standard radiation therapy and two schedules of concurrent chemoradiotherapy in patients with unresectable squamous cell head and neck cancer. J Clin Oncol. (2003) 21:92–8. doi: 10.1200/JCO.2003.01.008
10. Descamps G, Karaca Y, Lechien JR, Kindt N, Decaestecker C, Remmelink M, et al. Classical risk factors, but not HPV status, predict survival after chemoradiotherapy in advanced head and neck cancer patients. J Cancer Res Clin Oncol. (2016) 142(10):2185–96. doi: 10.1007/s00432-016-2203-7
11. Bossola M, Antocicco M, Pepe G. Tube feeding in patients with head and neck cancer undergoing chemoradiotherapy: A systematic review. JPEN J Parenter Enteral Nutr. (2022) 46(6):1258–69. doi: 10.1002/jpen.2360
12. Muscaritoli M, Lucia S, Farcomeni A, Lorusso V, Saracino V, Barone C, et al. Prevalence of malnutrition in patients at first medical oncology visit: the PreMiO study. Oncotarget. (2017) 8(45):79884–96. doi: 10.18632/oncotarget.20168
13. Fakhry C, Zhang Q, Nguyen-Tân PF, Rosenthal DI, Weber RS, Lambert L, et al. Development and validation of nomograms predictive of overall and progression-free survival in patients with oropharyngeal cancer. J Clin Oncol. (2017) 35:4057–65. doi: 10.1200/JCO.2016.72.0748
14. Whitney RL, Bell JF, Tancredi DJ, Romano PS, Bold RJ, Wun T, et al. Unplanned hospitalization among individuals with cancer in the year after diagnosis. J Oncol Pract. (2019) 15(1):e20–9. doi: 10.1200/JOP.18.00254
15. Ackerman D, Laszlo M, Provisor A, Yu A. Nutrition management for the head and neck cancer patient. Cancer Treat Res. (2018) 174:187–208. doi: 10.1007/978-3-319-65421-8_11
16. Jager-Wittenaar H, Dijkstra PU, Dijkstra G, Bijzet J, Langendijk JA, van der Laan BFAM, et al. High prevalence of cachexia in newly diagnosed head and neck cancer patients: An exploratory study. Nutrition. (2017) 35:114–8. doi: 10.1016/j.nut.2016.11.008
17. Alshadwi A, Nadershah M, Carlson ER, Young LS, Burke PA, Daley BJ. Nutritional considerations for head and neck cancer patients: A review of the literature. J Oral Maxillofac Surg. (2013) 71(11):1853–60. doi: 10.1016/j.joms.2013.04.028
18. Pfister DG, Spencer S, Adelstein D, Adkins D, Anzai Y, Brizel DM, et al. Head and neck cancers, version 2.2020, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. (2020) 18(7):873–98. doi: 10.6004/jnccn.2020.0031
19. Martino R, Ringash J. Evaluation of quality of life and organ function in head and neck squamous cell carcinoma. Hematol Oncol Clin North Am. (2008) 22(6):1239–56. doi: 10.1016/j.hoc.2008.08.011
20. Rivelli TG, Mak MP, Martins RE, da Costa e Silva VT, de Castro G Jr.. Cisplatin based chemoradiation late toxicities in head and neck squamous cell carcinoma patients. Discov. Med. (2015) 20(108):57–66.
21. Muscaritoli M, Arends J, Bachmann P, Baracos V, Barthelemy N, Bertz H, et al. ESPEN practical guideline: Clinical nutrition in cancer. Clin Nutr. (2021) 40(5):2898–2913. doi: 10.1016/j.clnu.2021.02.005
22. Bernier J, Domenge C, Ozsahin M, Matuszewska K, Lefèbvre JL, Greiner RH, et al. Postoperative irradiation with or without concomitant chemotherapy for locally advanced head and neck cancer. N Engl J Med. (2004) 350:1945–52. doi: 10.1056/NEJMoa032641
23. Cooper JS, Pajak TF, Forastiere AA, Jacobs J, Campbell BH, Saxman SB, et al. Postoperative concurrent radiotherapy and chemotherapy for high-risk squamous-cell carcinoma of the head and neck. N Engl J Med. (2004) 350:1937–44. doi: 10.1056/NEJMoa032646
24. Noronha V, Joshi A, Patil VM, Agarwal J, Ghosh-Laskar S, Budrukkar A, et al. Once-a-week versus once-every-3-weeks cisplatin chemoradiation for locally advanced head and neck cancer: a phase III randomized noninferiority trial. J Clin Oncol. (2018) 36:1064–72. doi: 10.1200/JCO.2017.74.9457
25. Bachaud JM, Cohen-Jonathan E, Alzieu C, David JM, Serrano E, Daly-Schveitzer N. Combined postoperative radiotherapy and weekly cisplatin infusion for locally advanced head and neck carcinoma: final report of a randomized trial. Int J Radiat Oncol Biol Phys. (1996) 36:999–1004. doi: 10.1016/S0360-3016(96)00430-0
26. Orell-Kotikangas H, Österlund P, Saarilahti K, Ravasco P, Schwab U, Mäkitie AA. NRS-2002 for pre-treatment nutritional risk screening and nutritional status assessment in head and neck cancer patients. Support Care Cancer. (2015) 23(6):1495–502. doi: 10.1007/s00520-014-2500-0
27. Hung CY, Hsueh SW, Lu CH, Chang PH, Chen PT, Yeh KY, et al. A prospective nutritional assessment using mini nutritional assessment-short form among patients with head and neck cancer receiving concurrent chemoradiotherapy. Support Care Cancer. (2021) 29(3):1509–18. doi: 10.1007/s00520-020-05634-3
28. Nuttall FQ. Body mass index: Obesity, BMI, and health: A critical review. Nutr Today. (2015) 50(3):117–28. doi: 10.1097/NT.0000000000000092
29. Martínez-Herrera B-E, Gutiérrez-Rodríguez L-X, Trujillo-Hernández B, Muñoz-García M-G, Cervantes-González L-M, José Ochoa L-L, et al. Phase angle in head and neck cancer: A sex-differential analysis from biological and clinical behavior to health-related quality of life. Biomedicines. (2023) 11:1696. doi: 10.3390/biomedicines11061696
30. Axelsson L, Silander E, Bosaeus I, Hammerlid E. Bioelectrical phase angle at diagnosis as a prognostic factor for survival in advanced head and neck cancer. Eur Arch Otorhinolaryngol. (2018) 275(9):2379–86. doi: 10.1007/s00405-018-5069-2
31. Lupton-Smith A, Fourie K, Mazinyo A, Mokone M, Nxaba S, Morrow B. Measurement of hand grip strength: A cross-sectional study of two dynamometry devices. S Afr J Physiother. (2022) 78(1):1768. doi: 10.4102/sajp.v78i1.1768
32. Blauwhoff-Buskermolen S, Ruijgrok C, Ostelo RW, de Vet HCW, Verheul HMW, de van der Schueren MAE, et al. The assessment of anorexia in patients with cancer: cut-off values for the FAACT-A/CS and the VAS for appetite. Support Care Cancer. (2016) 24(2):661–6. doi: 10.1007/s00520-015-2826-2
33. Mehanna H, Carter B, Hartley A, Abou-Foul AK, Brooks J, Jones J, et al. Patient preference for commonly-used, head and neck cancer-specific quality of life questionnaires in the follow-up setting (Determin): A multi-centre randomised controlled trial and mixed methods study. Clin Otolaryngol. (2023) 48(4):613–22. doi: 10.1111/coa.14054
34. Benjamini Y A, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B (Methodological). (1995) 57(1):289–300. doi: 10.1111/j.2517-6161.1995.tb02031.x
35. Silander E, Nyman J, Bove M, Johansson L, Larsson S, Hammerlid E. Impact of prophylactic percutaneous endoscopic gastrostomy on malnutrition and quality of life in patients with head and neck cancer: a randomized study. Head Neck. (2012) 34:1–9. doi: 10.1002/hed.21700
36. Axelsson L, Silander E, Nyman J, Bove M, Johansson L, Hammerlid E. Effect of prophylactic percutaneous endoscopic gastrostomy tube on swallowing in advanced head and neck cancer: a randomized controlled study. Head Neck. (2017) 39:908–15. doi: 10.1002/hed.24707
37. McClelland S 3rd, Andrews JZ, Chaudhry H, Teckie S, Goenka A. Prophylactic versus reactive gastrostomy tube placement in advanced head and neck cancer treated with definitive chemoradiotherapy: a systematic reviews. Oral Oncol. (2018) 87:77–81. doi: 10.1016/j.oraloncology.2018.10.028
38. Wang J, Liu M, Liu C, Ye Y, Huang G. Percutaneous endoscopic gastrostomy versus nasogastric tube feeding for patients with head and neck cancer: a systematic review. J Rad Res. (2014) 55:559–67. doi: 10.1093/jrr/rrt144
39. Bossi P, Delrio P, Mascheroni A, Zanetti M. The spectrum of Malnutrition/Cachexia/Sarcopenia in oncology according to different cancer types and settings: A narrative review. Nutrients. (2021) 13(6):1980. doi: 10.3390/nu13061980
40. Mascarella MA, Ferdus J, Vendra V, Sridharan S, Sultanem K, Tsien C, et al. Sarcopenia predicts short-term treatment-related toxicity in patients undergoing curative-intent therapy for head and neck cancer: A systematic review and meta-analysis. Head Neck. (2024) 46(6). doi: 10.1002/hed.27688
41. Ganju RG, Morse R, Hoover A, TenNapel M, Lominska CE. The impact of sarcopenia on tolerance of radiation and outcome in patients with head and neck cancer receiving chemoradiation. Radiother Oncol. (2019) 137:117–24. doi: 10.1016/j.radonc.2019.04.023
42. Guglielmi G, Ponti F, Agostini M, Amadori M, Battista G, Bazzocchi A. The role of DXA in sarcopenia. Aging Clin Exp Res. (2016) 28(6):1047–60. doi: 10.1007/s40520-016-0589-3
43. Swartz JE, Pothen AJ, Wegner I, Smid EJ, Swart KM, de Bree R, et al. Feasibility of using head and neck CT imaging to assess skeletal muscle mass in head and neck cancer patients. Oral Oncol. (2016) 62:28–33. doi: 10.1016/j.oraloncology.2016.09.006
44. Van der Vorst MJDL, Neefjes ECW, Toffoli EC, Oosterling-Jansen JEW, Vergeer MR, Leemans CR, et al. Incidence and risk factors for acute kidney injury in head and neck cancer patients treated with concurrent chemoradiation with high-dose cisplatin. BMC Cancer. (2019) 19(1):1066. doi: 10.1186/s12885-019-6233-9
45. Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, et al. Sarcopenia: European consensus on definition and diagnosis: Report of the european working group on sarcopenia in older people. Age Ageing. (2010) 39(4):412–23. doi: 10.1093/ageing/afq034
46. Laurentani F, Russo C, Bandinelli S, et al. Age-associated changes in skeletal muscles and their effect on mobility: an operational diagnosis of sarcopenia. J Appl Physiol. (2003) 95:1851–60. doi: 10.1152/japplphysiol.00246.2003
47. Kitano M, Yasumatsu R. The impact of sarcopenia in the treatment for patients with head and neck cancer. Auris Nasus Larynx. (2024) 51(4):717–23. doi: 10.1016/j.anl.2024.05.004
48. Devries MC, Phillips SM. Supplemental protein in support of muscle mass and health: advantage whey. J Food Sci. (2015) 80(S1):A8–A15. doi: 10.1111/jfds.2015.80.issue-S1
49. Rivelli TG, Mak MP, Martins RE, da Costa e Silva VT, de Castro G Jr. Cisplatin based chemoradiation late toxicities in head and neck squamous cell carcinoma patients. Discovery Med. (2015) 20(108):57–66.
50. Zhang S, Wang C, Zhong W, Kemp AH, Guo M, Killpartrick A. Polymerized whey protein concentrate-based glutathione delivery system: Physicochemical characterization, bioavailability and sub-chronic toxicity evaluation. Molecules. (2021) 26(7):1824. doi: 10.3390/molecules26071824
51. Cereda E, Turri A, Klersy C, Cappello S, Ferrari A, Filippi AR, et al. Whey protein isolate supplementation improves body composition, muscle strength, and treatment tolerance in malnourished advanced cancer patients undergoing chemotherapy. Cancer Med. (2019) 8(16):6923–32. doi: 10.1002/cam4.2517
52. Crowder SL, Li Z, Sarma KP, Arthur AE. Chronic nutrition impact symptoms are associated with decreased functional status, quality of life, and diet quality in a pilot study of long-term post-radiation head and neck cancer survivors. Nutrients. (2021) 13(8):2886. doi: 10.3390/nu13082886
53. Ninfa A, Jansen F, Delle Fave A, Lissenberg-Witte BI, Pizzorni N, Baatenburg de Jong RJ, et al. The change in social eating over time in people with head and neck cancer treated with primary (Chemo) Radiotherapy: The role of swallowing, oral function, and nutritional status. Cancers (Basel). (2023) 15(5):1603. doi: 10.3390/cancers15051603
54. Bossola M. Nutritional interventions in head and neck cancer patients undergoing chemoradiotherapy: a narrative review. Nutrients. (2015) 7(1):265–76. doi: 10.3390/nu7010265
55. Van Wayenburg CA, Rasmussen-Conrad EL, van den Berg MG, Merkx MA, van Staveren WA, van Weel C, et al. Weight loss in head and neck cancer patients little noticed in general practice. J Prim Health Care. (2010) 2:16–21. doi: 10.1071/HC10016
56. Van Leeuwen PA, Kuik DJ, Klop WM, Sauerwein HP, Snow GB, Quak JJ. The impact of nutritional status on the prognoses of patients with advanced head and neck cancer. Cancer. (1999) 86:519–27. doi: 10.1002/(SICI)1097-0142(19990801)86:3<519::AID-CNCR22>3.0.CO;2-S
57. Evans DC, Corkins MR, Malone A, Miller S, Mogensen KM, Guenter P, et al. The use of visceral proteins as nutrition markers: An ASPEN position paper. Nutr Clin Pract. (2021) 36(1):22–8. doi: 10.1002/ncp.10588
Keywords: head and neck cancer, nutritional counselling, oral nutritional supplement, quality of life, body composition, high-dose cisplatin, chemotherapy, radiotherapy
Citation: Cardellini S, Deantoni CL, Paccagnella M, Casirati A, Pontara A, Marinosci A, Tresoldi M, Giordano L, Chiara A, Dell’Oca I, Di Muzio NG, Caccialanza R and Mirabile A (2024) The impact of nutritional intervention on quality of life and outcomes in patients with head and neck cancers undergoing chemoradiation. Front. Oncol. 14:1475930. doi: 10.3389/fonc.2024.1475930
Received: 04 August 2024; Accepted: 30 September 2024;
Published: 21 October 2024.
Edited by:
Guopei Zhu, Shanghai Jiao Tong University, ChinaCopyright © 2024 Cardellini, Deantoni, Paccagnella, Casirati, Pontara, Marinosci, Tresoldi, Giordano, Chiara, Dell’Oca, Di Muzio, Caccialanza and Mirabile. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Aurora Mirabile, bWlyYWJpbGUuYXVyb3JhQGhzci5pdA==
†ORCID: Riccardo Caccialanza, orcid.org/0000-0002-9379-3569
 Sara Cardellini
Sara Cardellini Chiara Lucrezia Deantoni
Chiara Lucrezia Deantoni Matteo Paccagnella
Matteo Paccagnella Amanda Casirati
Amanda Casirati Andrea Pontara1
Andrea Pontara1 Moreno Tresoldi
Moreno Tresoldi Nadia Gisella Di Muzio
Nadia Gisella Di Muzio Riccardo Caccialanza
Riccardo Caccialanza Aurora Mirabile
Aurora Mirabile