- 1Department of Obstetrics and Gynecology, West China Second University Hospital, Sichuan University, Chengdu, Sichuan, China
- 2Key Laboratory of Birth Defects and Related Diseases of Women and Children (Sichuan University), Ministry of Education, Sichuan, China
Background: The aim of this study was to determine the relationship between the lymph node ratio (LNR) and the prognostic values of gynecological cancer.
Materials and methods: PubMed, Web of Science, Embase, and the Central Cochrane Library were used to search for studies on LNR and gynecological cancer published before 18 April 2024. The effect measure for meta-analysis of primary outcomes was the hazard ratio (HR) for overall survival (OS), progression-free survival (PFS), and disease-free survival (DFS). Pooled HRs and 95% confidence intervals (CIs) were calculated using random- or fixed-effects models. Sensitivity analysis was applied to evaluate the robustness of the results. The I2 statistic was used to measure heterogeneity. Subgroup analysis and meta-regression were chosen to illustrate the potential heterogeneity of the risk factors for outcomes. Publication bias was assessed using Egger’s test and Begg’s funnel plots.
Results: A total of 34 studies with 23,202 cases were included in this meta-analysis. A meta-analysis found that higher LNR was associated with worse OS (HR = 2.42, 95% CI: 2.07–2.83; I2 = 77.4%, p < 0.05), PFS (HR = 1.97, 95% CI: 1.66-2.32; I2 = 0.00%, p > 0.05), and DFS (HR = 3.18, 95% CI: 2.12–4.76; I2 = 64.3%, p < 0.05). Moreover, meta-analysis revealed significant differences in the association between LNR and OS of cervical cancer (CC) (HR = 2.53, 95% CI: 1.94–3.31; I2 = 72.6%, p < 0.05), ovarian cancer (OC) (HR = 2.05, 95% CI: 1.66–2.54; I2 = 76.7%, p < 0.05), endometrial cancer (EC) (HR = 2.16, 95% CI: 1.48–3.16; I2 = 53.6%, p < 0.05), and vulvar cancer (VC) (HR = 8.13, 95% CI: 3.41–19.43; I2 = 57.2%, p < 0.05).
Conclusion: We observed a clear association between higher LNR and poorer prognosis in our study of patients with gynecological cancer. Further prospective studies are warranted to determine the optimal LNR and whether LNR can guide adjuvant therapy use in gynecological cancer. It is essential to conduct further prospective studies to establish the optimal LNR threshold, determine the minimum threshold of lymph node removal, and investigate whether LNR can serve as a reliable marker for guiding adjuvant therapy choices in gynecological cancer.
Systematic review registration: https://www.crd.york.ac.uk/PROSPERO/#recordDetails, CRD42024541187.
1 Introduction
Lymph node metastasis is a common occurrence in gynecological cancers and has a significant impact on patient prognosis. However, the number of positive nodes during pelvic lymphadenectomy can be influenced by surgical technique and the accuracy of pathological examination. To overcome potential confounding effects, the use of lymph node ratio (LNR) has been proposed. LNR calculates the ratio of positive lymph nodes to the total number of resected lymph nodes and provides a more accurate representation of pelvic lymph node metastasis status. It has been identified as an independent predictor of survival in various cancers, including colon cancer (1), oral cancer (2), pancreatic cancer (3), breast cancer (4), esophageal cancer (5), and lung cancer (6).
Recently, there has been interest in using LNR as a prognostic tool in gynecologic malignancies such as cervical cancer (CC), ovarian cancer (OC), endometrial cancer (EC), and vulvar cancer (VC). However, the conclusions of studies in this area are not consistent. To address this, we conducted a systematic search of scientific databases to identify relevant publications and to explore the relationship between lymph node ratio and overall survival (OS), progression-free survival (PFS), and disease-free survival (DFS) in gynecological cancers.
2 Materials and methods
2.1 Protocol registration
This meta-analysis was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (7). Before data extraction, the review was registered with the International Prospective Register of Systematic Reviews (PROSPERO, Registration Number CRD42024541187).
2.2 Data collection
PubMed, Web of Science, Embase, and the Central Cochrane Library were used to search for studies on LNR and gynecological cancer published before 18 April 2024. The following keywords were used for literature retrieval: (“lymph node ratio” or “Ratio, Lymph Node”) and (“Uterine Cervical Neoplasms” or “Neoplasm, Uterine Cervical “ or “Ovarian Neoplasms” or “Neoplasm, Ovary” or “Neoplasm, Endometrial” or “Endometrial Neoplasms” or “Vulvar Neoplasms” or “Neoplasm, Vulva”). Additionally, the references in the obtained papers were examined to find any other relevant research outside of these two key phrases in the query.
2.3 Eligibility criteria and exclusion criteria
Inclusion criteria were as follows: (1) studies that investigated the relationship between LNR and OS, PFS, or DFS; (2) studies with CC, OC, EC, and VC confirmed by pathology; (3) studies that reported a hazard ratio (HR) with 95% CI for OS, PFS, or DFS; and (4) full articles published in English.
Exclusion criteria were as follows: (1) useful data could not be extracted; (2) the survival data or 95% confidence interval (CI) were not reported; and (3) editorials, reviews, and comments. In addition, when the data of a patient were used in multiple studies, we selected the most recent study.
2.4 Data extraction of data and quality assessment
The data extracted mainly included the following: the first author, publication date, sample size, cancer type, country, average age, duration of follow-up, cutoff value for LNR, and patient outcomes, including OS, PFS, and DFS.
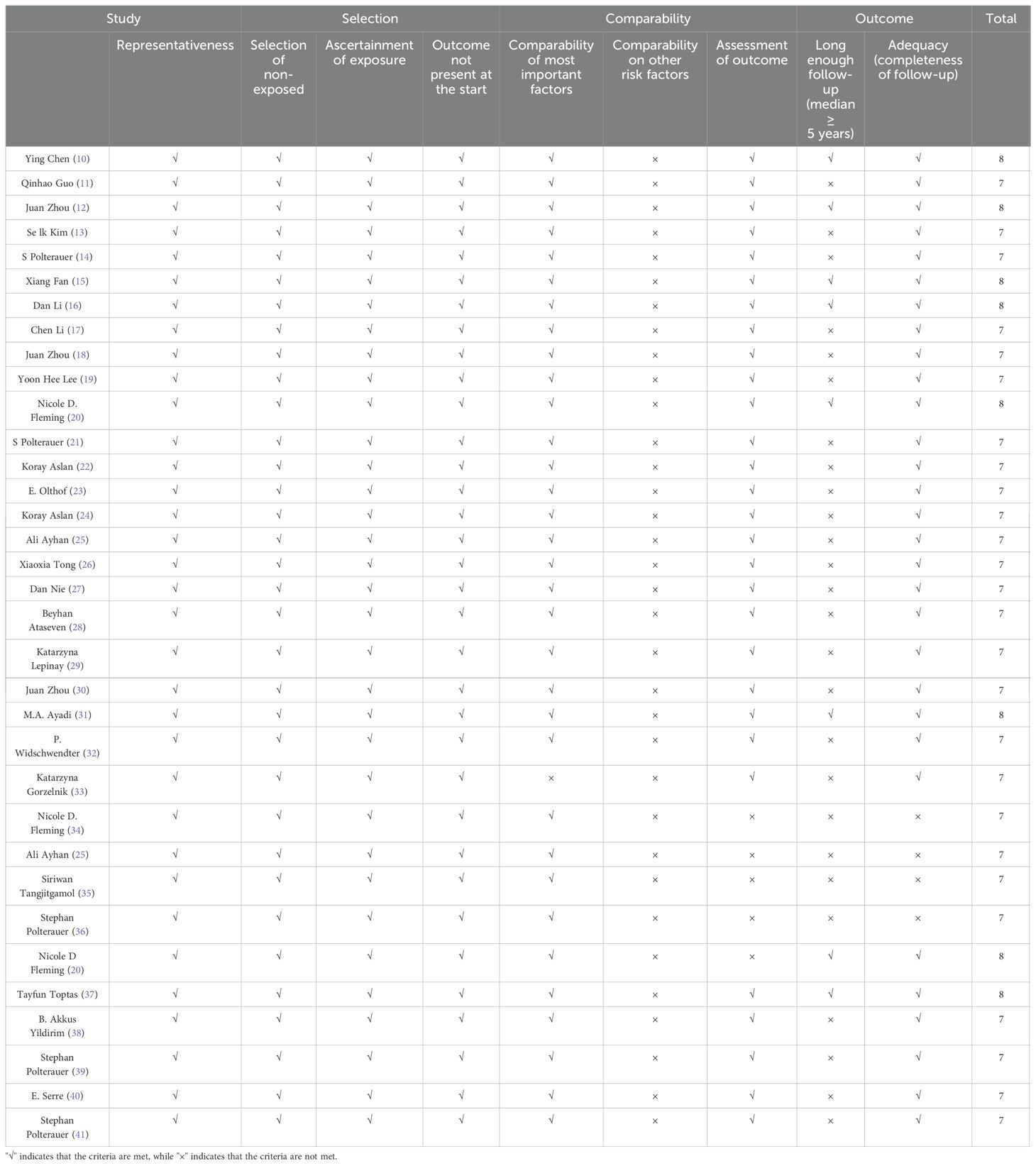
In this meta-analysis, the quality assessment for the non-randomized studies was evaluated by two reviewers independently based on the Newcastle–Ottawa quality assessment scale (NOS) (8). The NOS was based on three categories: selected cases, comparability between groups, and outcome assessment. A score ≥ 6 was considered high-quality literature, to be included in our study.
2.5 Main outcomes
OS refers to the period from the date of the initial therapy to the date of all-cause mortality. PFS refers to the time from the date of the initial therapy to the date of disease progression, which was the time following successful treatment without disease progression or symptoms. DFS refers to the time from surgery to the last follow-up with no evidence of recurrence or distant metastasis.
2.6 Statistical analysis
We used STATA 15.0 software (StataCorp LP, College Station, TX, United States) to pool the extracted data for this meta-analysis. Hazard ratios with 95% CI were collected from individual studies, then combined using a random- or fixed-effects model, and finally presented in forest plots. Statistical heterogeneity was quantified by I2 statistics. A random-effects model was used if there was prominent heterogeneity (p < 0.1 or I2 > 50%); otherwise, a fixed-effects model was adopted (p > 0.1 or I2 < 50%) (9). Sensitivity analysis was used to determine the robustness and stability of the results by calculating the heterogeneity in each situation in which a single study was removed in turn to evaluate the effect of a single study on the overall outcome. The risk of publication was assessed by visual inspection of Begg’s funnel plot and Egger’s linear regression test. In these two-tailed statistical tests, p < 0.05 (95% CI) was regarded as statistically significant. Meta-regression analysis, subgroup analysis, and publication bias were evaluated in analyses that included more than 10 studies.
3 Results
3.1 Study selection
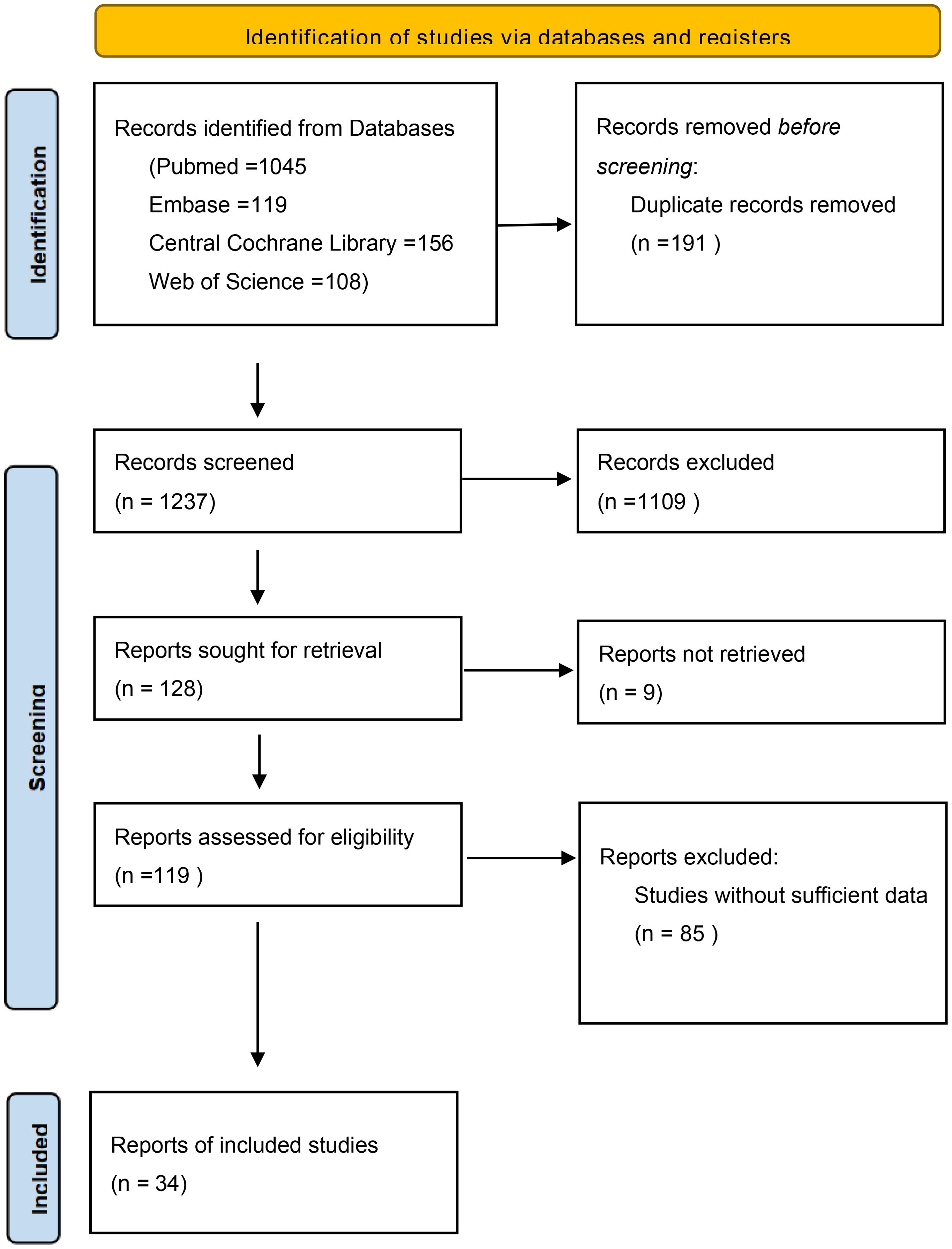
As shown in Figure 1, through electronic searching on PubMed, Web of Science, Embase, and the Central Cochrane Library, 1,428 potential articles were screened. After excluding duplicate studies there were 1,237 records. Then, the titles and abstracts were screened, and 1,109 publications were removed as irrelevant. Finally, 119 full-text articles were identified for qualification, and 85 ineligible papers were eliminated because they did not provide primary outcome measurements. In the end, a total of 34 studies with 23,202 cases were eligible for the current meta-analysis.
3.2 Characteristics of the included studies
The main characteristics of the included studies are presented in Table 1. In this study, all necessary data were extracted from 34 studies from different countries including China (n = 10), Türkiye (n = 6), USA (n = 3), Germany (n = 3), Poland (n = 3), Republic of Korea (n = 2), Austria (n = 2), Thailand (n = 1), Brazil (n = 1), Tunisia (n = 1), Netherlands (n = 1), and France (n = 1). All included studies were retrospective studies. Among these articles, 14 studies involved 6,289 patients diagnosed with CC, 9 studies involved 15,058 patients diagnosed with OC, 8 studies involved 1,017 patients diagnosed with EC, and 3 studies involved 838 patients diagnosed with VC. All studies were retrospective cases, and all were rated seven or more stars according to the NOS criteria (Table 2).
3.3 Meta-analysis
3.3.1 Primary outcomes
3.3.1.1 LNR and OS
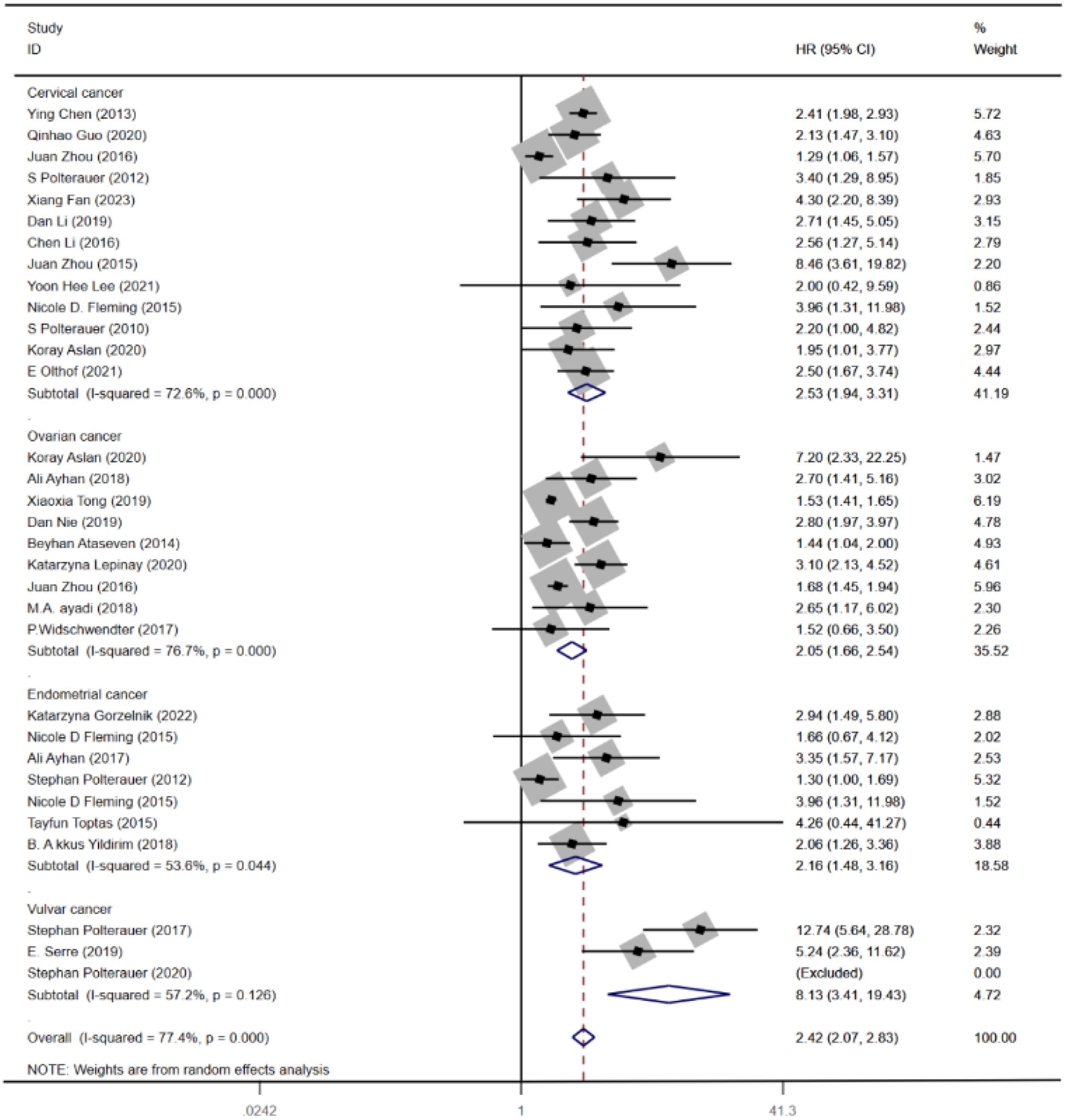
Out of the 34 (10–41) eligible studies, 32 (10–12, 14–34, 36–41) studies, namely, 13 (10–12, 14–23) studies with CC, 9 (24–32) studies with OC, 7 (20, 25, 33, 34, 36–38) studies with EC, and 3 (39–41) studies with VC, analyzed the association between LNR and OS. Using a random-effects model, the pooled results of HR and OS statistics from these 32 studies showed that higher levels of LNR were associated with worse OS (HR = 2.42, 95% CI: 2.07–2.83; I2 = 77.4%, p < 0.05), as shown in (Figure 2A). However, the results also indicated a high heterogeneity between studies (I2 = 77.4%, p < 0.05).
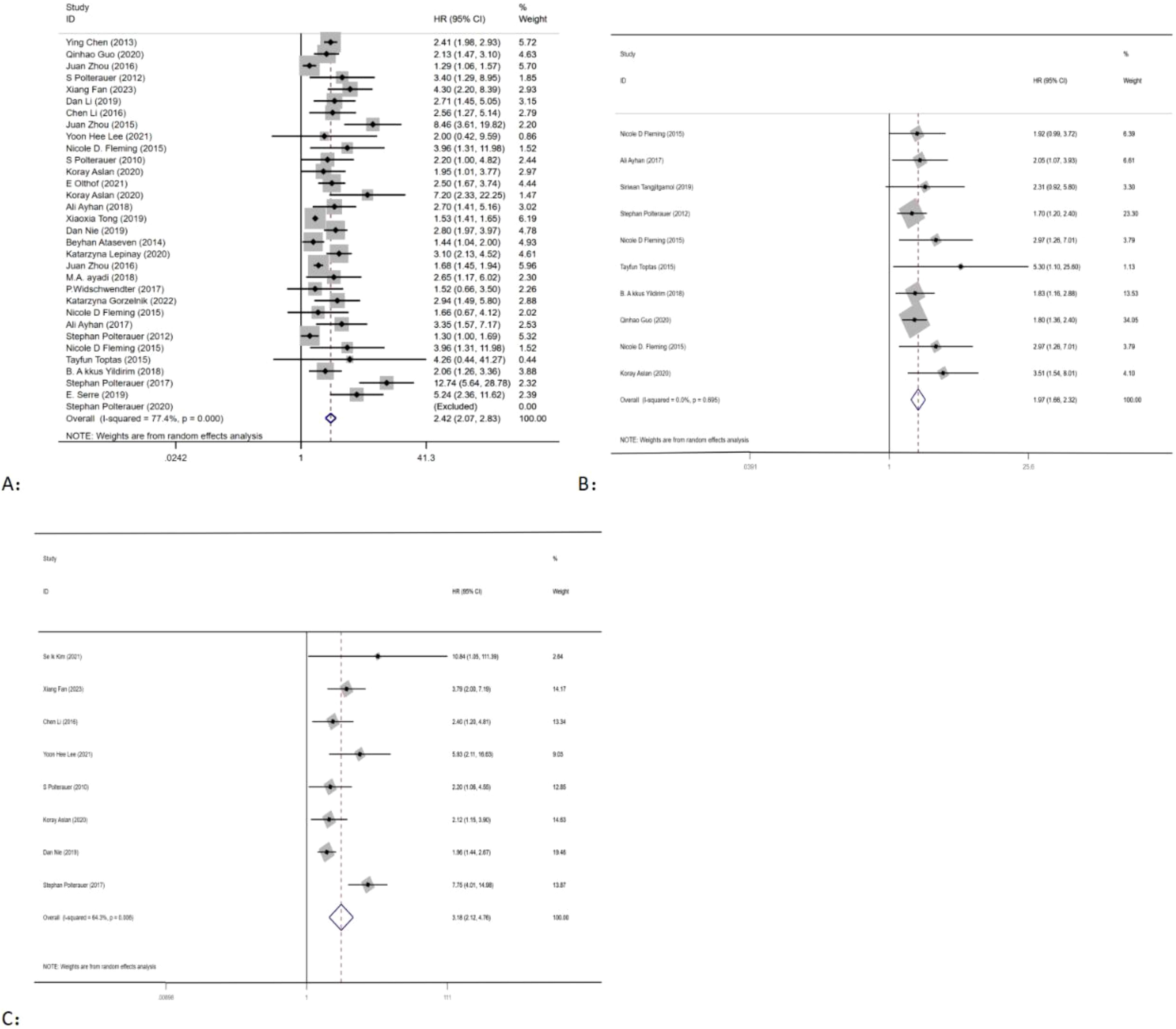
Figure 2. Forest plots and pooled estimates of the effect for meta-analysis of the association between LNR and OS (A), PFS (B), and DFS (C) in patients with gynecological cancer.
3.3.1.2 LNR and PFS
Seven studies (20, 25, 34–38) with EC, two studies (11, 15) with CC, and one study (24) with OC explored the association between LNR and PFS. Using a fixed-effects model, pooled results of HR and PFS statistics from 10 studies indicated that higher LNR levels were associated with worse PFS (HR = 1.97, 95% CI: 1.66–2.32; I2 < 50%, p > 0.05) (Figure 2B). The results showed that there was low heterogeneity between studies (I2 < 50%, p > 0.05).
3.3.1.3 LNR and DFS
Eight studies, consisting of six studies (13, 15, 17, 19, 21, 22) with CC, one study (24) with OC, and one study (39) with VC, were included in the analysis of the association between LNR and PFS. Using a random-effects model, the pooled results indicated that higher levels of LNR were associated with worse DFS (HR = 3.18, 95% CI: 2.12–4.76; I2 = 64.3%, p < 0.05) (Figure 2C). However, there was high heterogeneity between studies (I2 > 50%, p < 0.05).
3.3.2 Subgroup and meta-regression analysis
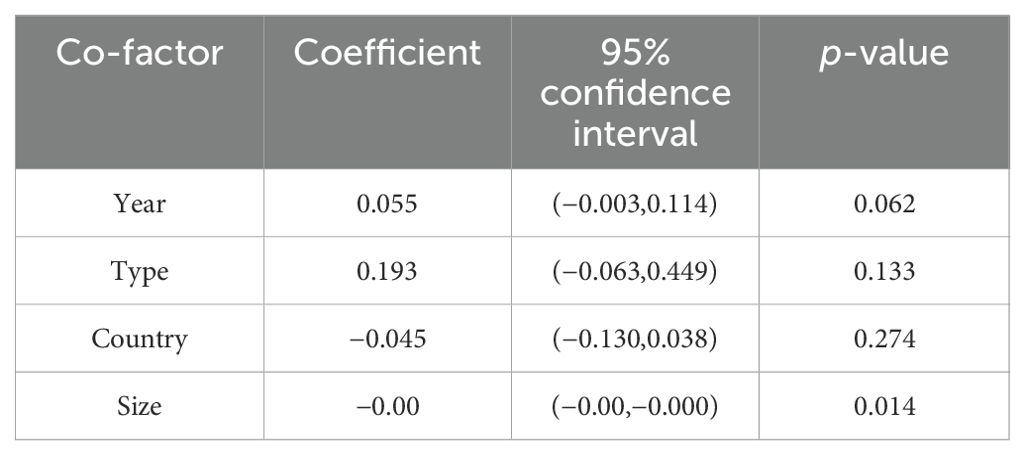
As the outcome of OS shows high heterogeneity, both meta-regression and subgroup analyses were conducted to explore the factors contributing to the high heterogeneity. Subgroup analysis was carried out based on the type of cancer. The analysis revealed significant differences in the association between LNR and OS of CC (HR = 2.53, 95% CI: 1.94–3.31; I2 = 72.6%, p < 0.05), OC (HR = 2.05, 95% CI: 1.66–2.54; I2 = 76.7%, p < 0.05), EC (HR = 2.16, 95% CI: 1.48–3.16; I2 = 53.6%, p < 0.05), and VC (HR = 8.13, 95% CI: 3.41–19.43; I2 = 57.2%, p < 0.05) (Figure 3). Furthermore, meta-regression analysis was carried out to investigate possible sources of heterogeneity. Single covariate regression was performed using variables such as country, sample size, type of gynecological cancer, and publication year. The results indicated that sample size was the main factor contributing to the heterogeneity, with a p-value of 0.014 (Table 3).
We did not conduct meta-analyses, and subgroup analyses for PFS and DFS, as they c.
3.3.3 Sensitivity analysis
In order to assess the stability of the models, a sensitivity analysis was conducted by excluding each individual study and calculating new HRs. The results showed that the HRs were relatively stable, as illustrated in Figure 4.
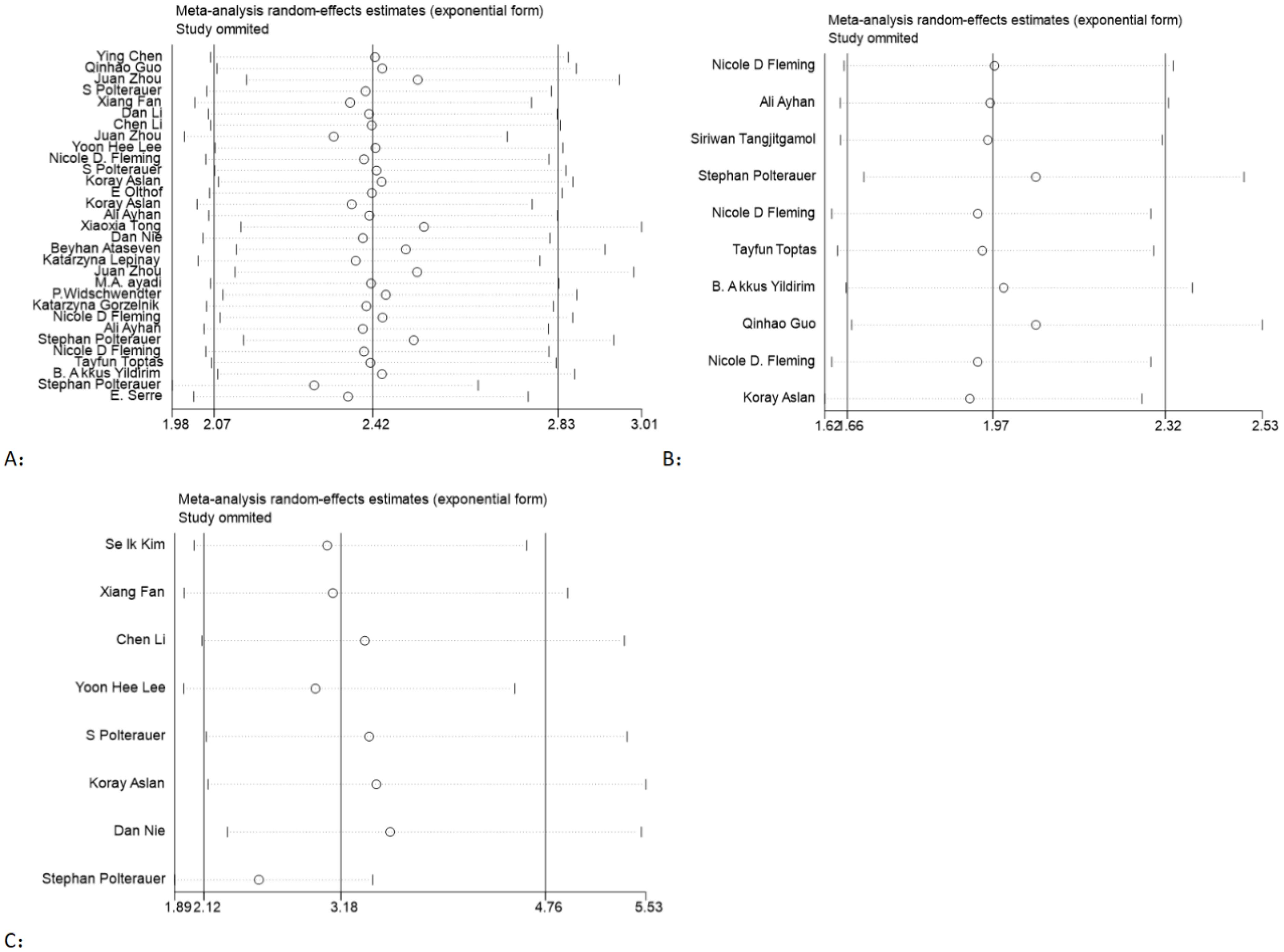
Figure 4. Sensitivity analysis of the association between LNR and OS (A), PFS (B), and DFS (C) in gynecological cancer.
3.3.4 Publication bias
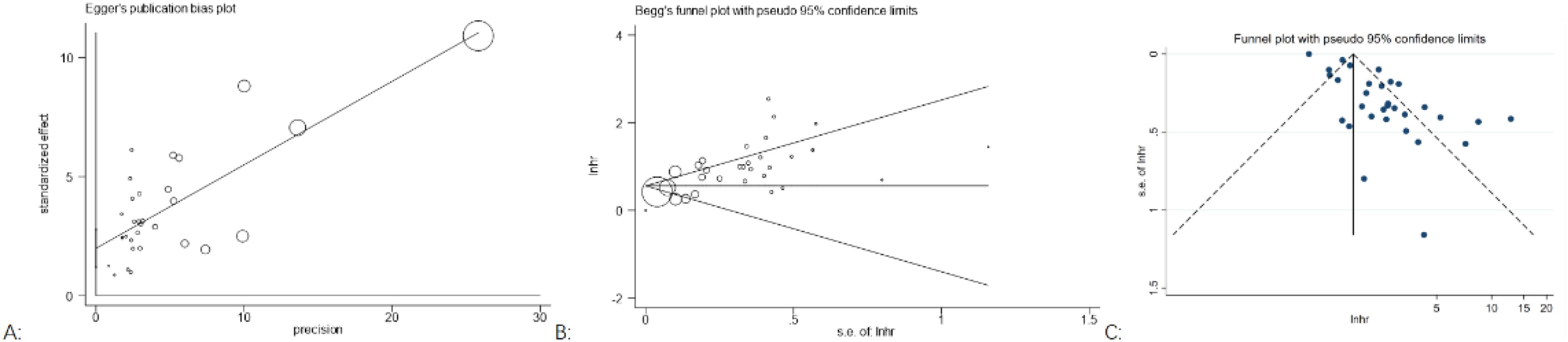
Publication bias was assessed by Begg’s funnel plots and Egger’s test. For OS, the p-values for Egger’s test (Figure 5A) and Begg’s test (Figure 5B) were p = 0.255 and p < 0.00, respectively. Visual inspection of Begger’s funnel plot (Figure 5C) was not symmetrical, suggesting evidence of publication bias. We did not conduct a publication bias analysis for PFS and DFS, as they did not include more than 10 studies.
4 Discussion
This meta-analysis revealed that higher LNR was significantly associated with poorer prognosis across multiple metrics. Specifically, it found that higher LNR was correlated with worse OS, PFS, and DFS, indicating that patients with higher LNR often have worse outcomes. Furthermore, the pooled HRs for OS across different types of cancers are 3.42 for CC, 2.05 for OC, 2.16 for EC, and 8.13 for VC, suggesting that higher LNR often correlates with worse survival outcomes in CC, OC, EC, and VC. These findings underscore the significant prognostic implications of LNR in gynecological cancer, highlighting its role as a critical factor in predicting patient outcomes.
For CC, based on the results of previous studies, the cutoff value of LNR in patients with CC ranges from 5% to 30% (9–22), with higher LNR consistently associated with poorer prognosis. A meta-analysis (42) conducted in 2017, comprising eight articles with 3,325 patients diagnosed with CC, confirmed that higher LNR was an unfavorable prognostic factor for OS and DFS, which is consistent with our findings. In 2018, the FIGO Committee added IIICI (pelvic lymph node metastasis only) and IIIC2 (para-aortic lymph node metastasis) to the FIGO staging system for CC (43). Nevertheless, this was solely based on anatomic location and did not consider other lymph node characteristics. Researchers have looked for a reliable lymph node measure in CC, yielding conflicting results. Yoon et al. (19) showed that LNR was the most robust biomarker for predicting tumor recurrence, while Qinhao et al. (11) found that positive lymph nodes had the best prognostic performance for OS and PFS. Therefore, further studies are warranted to explore this aspect and establish the most reliable measure.
In OC, previous studies have identified varying cutoff values for LNR ranging from 9% to 50% (23–31). Consistently, higher LNR was linked to poorer prognosis, which is in line with our study findings. It is worth noting that OC is a heterogeneous group of diseases with varying histology, molecular genetic analysis, and prognosis (44); some researchers have begun to conduct more in-depth research on the classification of epithelial OC. The prognostic value of lymph node ratio has been confirmed in high-grade serous ovarian carcinoma (45), low-grade serous ovarian carcinoma (22), clear cell ovarian carcinoma (27), and borderline ovarian tumors (46). Especially in borderline ovarian tumors (46), David controlled for age, histology, stage, tumor size, and adequate lymphadenectomy status, and LNR remained an independent factor for survival; qualitative assessment of lymph node involvement is not a prognostic factor for survival. Another study (30) based on the Surveillance, Epidemiology, and End Results database showed that there is a significant and independent correlation between higher LNR and poorer OS, and its prognostic value is superior to removed lymph nodes and positive lymph node counts. Despite this, a study conducted by Xiao et al. (26) found that LNR did not reach statistical significance for discriminating OS in stage IV patients, although it showed better performance than the number of positive lymph nodes. Therefore, future research should emphasize the prognostic utility of LNR in various stages of OC.
In EC, according to prior research (20, 25, 32–37), the cutoff values for LNR in EC patients span from 6.5% to 50%. These studies consistently indicated that higher LNR is associated with a worse prognosis, a trend observed in our study. Previous multi-center retrospective studies (20, 25, 32–37) have found a correlation between LNR and worse OS and PFS. Xi-Lin et al. (47) found that the lymph node ratio had a better predictive performance for these patients than the number of removed lymph nodes, the number of positive lymph nodes, and the number of negative lymph nodes. However, a study conducted by Fleming et al. (34) did not find a statistically significant association between LNR and OS, probably due to the small patient cohort in this single-institution study and the low median count of retrieved lymph nodes. This suggests that the prognostic value of LNR may be limited to patients who have undergone a minimum threshold of lymph node removal. Nevertheless, this threshold has not been universally adopted as a clinical standard. Future research should focus on identifying optimal lymph node removal strategies to mitigate the morbidity associated with systemic lymphadenectomy.
In VC, Kunos et al. (48) initially described LNR for prognostic assessment in patients with VC, and they found that patients with LNR > 20% had an increased likelihood of contralateral positive lymph nodes, recurrence, and cancer-specific death compared with patients with LNR < 20%. Moreover, some studies (38–40) stratified patients into three groups (LNR = 0%, 0% < LNR < 20%, and LNR > 20%), and the LNR > 20% group had the highest risk for OS and recurrence, which is consistent with our study. According to a study conducted by Polterauer et al., LNR appears to be a consistent, independent prognostic parameter for both OS and PFS, and its predictive value is superior to positive lymph node number. These studies support the predictive value of the LNR for VC.
Our research has certain strengths. First, this is the first complete meta-analysis to quantify the role of LNR in the prognosis of gynecological cancer. Second, this meta-analysis included a large number of primary studies (34 papers) and patients (23,202 patients with positive lymph nodes), which allowed for a more robust statistical analysis. Finally, our findings have demonstrated the importance of LNR in the prognosis of gynecological cancer. Therefore, we recommend LNR as a prognostic parameter that should be included in a future gynecological cancer staging system.
While our study has shown that LNR is of significant prognostic value in gynecologic cancers, it also has limitations. First, literature-based meta-analyses rely on published data and may be biased toward positive results, and we found that it does have bias in our study. In addition, the absence of data on tumor size, pathological stages, number of examined lymph nodes, number of metastasized lymph nodes, and surgical methods limited further subgroup analysis. Furthermore, the LNR cutoff in different studies was inconsistent. Finally, all included studies were retrospective, and this study type has intrinsic limitations. Thus, more prospective data are required to further ascertain the prognostic value of LNR in specific populations.
5 Conclusion
Higher LNR is linked to lower OS, PFS, and DFS in patients diagnosed with gynecological cancer. The prognostic value of LNR for OS is consistent across different types of gynecological cancer, including CC, OC, EC, and VC. Further prospective studies are essential to establish the optimal LNR threshold, determine the minimum threshold of lymph node removal, and evaluate whether LNR can effectively guide the use of adjuvant therapies in gynecological cancer.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Author contributions
MC: Writing – original draft. YW: Writing – original draft. YC: Writing – original draft. LH: Writing – review & editing. AZ: Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Ichhpuniani S, McKechnie T, Lee JY, Biro J, Lee Y, Park L, et al. Lymph node ratio as a predictor of survival for colon cancer: A systematic review and meta-analysis. Am Surgeon. (2024) 90:840–50. doi: 10.1177/00031348231209532
2. Gartagani Z, Doumas S, Kyriakopoulou A, Economopoulou P, Psaltopoulou T, Kotsantis I, et al. Lymph node ratio as a prognostic factor in neck dissection in oral cancer patients: A systematic review and meta-analysis. Cancers. (2022) 14(18). doi: 10.3390/cancers14184456
3. Karjol U, Chandranath A, Jonnada P, Cherukuru S, Annavarjula V, Morla SA, et al. Lymph node ratio as a prognostic marker in pancreatic cancer survival: A systematic review and meta-analysis. Cureus J Med Sci. (2020) 12(8). doi: 10.7759/cureus.9597
4. Liu JZ, Li YF, Zhang WF, Yang CH, Yang C, Chen L, et al. The prognostic role of lymph node ratio in breast cancer patients received neoadjuvant chemotherapy: A dose-response meta-analysis. Front Surg. (2022) 9. doi: 10.3389/fsurg.2022.971030
5. Song JN, Zhang H, Jian JL, Chen H, Zhu XD, Xie JF, et al. The prognostic significance of lymph node ratio for esophageal cancer: A meta-analysis. J Surg Res. (2023) 292:53–64. doi: 10.1016/j.jss.2023.07.027
6. Zhou J, Lin ZY, Lyu MY, Chen N, Liao H, Wang ZH, et al. Prognostic value of lymph node ratio in non-small-cell lung cancer: a meta-analysis. Japanese J Clin Oncol. (2020) 50:44–57. doi: 10.1093/jjco/hyz120
7. Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. PRISMA-P Group.Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. (2015) 4:1. doi: 10.1186/2046-4053-4-1
8. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. (2010). 65:603–5. doi: 10.1186/2046-4053-4-1
9. DerSimonian R, Laird N. Meta-analysis in clinical trials revisited. Contemp Clin Trials. (2015) 45:139–45. doi: 10.1016/j.cct.2015.09.002
10. Chen Y, Zhang L, Tian J, Ren XB, Hao Q. Combining the negative lymph nodes count with the ratio of positive and removed lymph nodes can better predict the postoperative survival in cervical cancer patients. Cancer Cell Int. (2013) 13:6. doi: 10.1186/1475-2867-13-6
11. Guo Q, Zhu J, Wu Y, Wen H, Xia LF, Yu M, et al. Comparison of different lymph node staging systems in patients with node-positive cervical squamous cell carcinoma following radical surgery. J Cancer. (2020) 11:7339–47. doi: 10.7150/jca.48085
12. Zhou J, Chen QH, Wu SG, He ZY, Sun JY, Li FY, et al. Lymph node ratio may predict the benefit of postoperative radiotherapy in node-positive cervical cancer. Oncotarget. (2016) 7:29420–28. doi: 10.18632/oncotarget.v7i20
13. Kim SI, Kim TH, Lee M, Kim HS, Chung HH, Lee TS, et al. Lymph node ratio is a strong prognostic factor in patients with early-stage cervical cancer undergoing minimally invasive radical hysterectomy. Yonsei Med J. (2021) 62:231–39. doi: 10.3349/ymj.2021.62.3.231
14. Polterauer S, Grimm C, Hofstetter G, Concin N, Natter C, Sturdza A, et al. Nomogram prediction for overall survival of patients diagnosed with cervical cancer. Br J Cancer. (2012) 107:918–24. doi: 10.1038/bjc.2012.340
15. Fan X, Wang Y, Yang N, Yang N, Zhu PF. Prognostic analysis of patients with stage IIIC1p cervical cancer treated by surgery. World J Surg Oncol. (2023) 21:186. doi: 10.1186/s12957-023-03076-9
16. Li D, Xu X, Yan D, Yuan SH, Ni J, Lou HM. Prognostic factors affecting survival and recurrence in patients with early cervical squamous cell cancer following radical hysterectomy. J Int Med Res. (2019) 48(4). doi: 10.1177/0300060519889741
17. Li C, Liu W, Cheng Y. Prognostic significance of metastatic lymph node ratio in Squamous cell carcinoma of the cervix. OncoTargets Ther. (2016) 9:3791–97. doi: 10.2147/OTT.S97702
18. Zhou J, Sun JY, Chen SY, Li FY, Lin HX, Wu SC, et al. Prognostic value of lymph node ratio in patients with small-cell carcinoma of the cervix based on data from a large national registry. OncoTargets Ther. (2015) 9:67–73. doi: 10.2147/OTT.S96206
19. Lee YH, Chong GO, Kim SJ, Hwang JH, Kim JM, Park NJY, et al. Prognostic value of lymph node characteristics in patients with cervical cancer treated with radical hysterectomy. Cancer Manag Res. (2021) 13:8137–45. doi: 10.2147/CMAR.S332612
20. Fleming ND, Frumovitz M, Schmeler KM, Dos Reis R, Munsell MF, Eifel PJ, et al. Significance of lymph node ratio in defining risk category in node-positive early stage cervical cancer. Gynecol Oncol. (2015) 136:48–53. doi: 10.1016/j.ygyno.2014.11.010
21. Polterauer S, Hefler L, Seebacher V, Rahhal J, Tempfer C, Horvat R, et al. The impact of lymph node density on survival of cervical cancer patients. Br J Cancer. (2010) 103:613–6. doi: 10.1038/sj.bjc.6605801
22. Aslan K, Meydanli MM, Oz M, Tohma YA, Haberal A, Ayhan A. The prognostic value of lymph node ratio in stage IIIC cervical cancer patients triaged to primary treatment by radical hysterectomy with systematic pelvic and para-aortic lymphadenectomy. J Gynecol Oncol. (2020) 31:e1. doi: 10.3802/jgo.2020.31.e1
23. Olthof E, Mom C, Wenzel H, Van Der Velden J, Van Der Aa M. The prognostic value of the number of positive lymph nodes and the lymph node ratio in early stage cervical cancer. Int J Gynecol Cancer. (2021) 31:A45. doi: 10.1136/ijgc-2021-IGCS.108
24. Aslan K, Meydanli MM, Akilli H, Durmus Y, Gokcu M, Kayikcioglu F, et al. Does lymph node ratio have any prognostic significance in maximally cytoreduced node-positive low-grade serous ovarian carcinoma? Arch Gynecol Obstet. (2020) 302:183–90. doi: 10.1007/s00404-020-05580-9
25. Ayhan A, Ozkan NT, Oz M, Comert GK, Cuylan ZF, Coban G, et al. Impact of lymph node ratio on survival in stage IIIC endometrioid endometrial cancer: a Turkish Gynecologic Oncology Group study. J Gynecol Oncol. (2018) 29(4). doi: 10.3802/jgo.2018.29.e48
26. Tong X, Li H, Chen H, Zhai D, Pang YY, Lin RY, et al. Prognostic significance of lymph node ratio in ovarian cancer. Open Med. (2019) 14:279–86. doi: 10.1515/med-2019-0024
27. Nie D, Mao X, Li Z. Prognostic value of lymph nodes ratio in patients with stage III ovarian clear cell carcinoma: A retrospective study of patients in Southwest China. J Cancer. (2019) 10:4689–94. doi: 10.7150/jca.29896
28. Ataseven B, Grimm C, Harter P, Prader S, Traut A, Heitz F, et al. Prognostic value of lymph node ratio in patients with advanced epithelial ovarian cancer. Gynecol Oncol. (2014) 135:435–40. doi: 10.1016/j.ygyno.2014.10.003
29. Lepinay K, Szubert S, Lewandowska A, et al. The association between lymph node metastases and long-term survival in patients with epithelial ovarian cancer. Contemp Oncol (Pozn). (2020) 24:163–71. doi: 10.5114/wo.2020.99029
30. Zhou J, He Z-Y, Li F-Y, Sun JY, Lin HX, Wu SG, et al. Prognostic value of lymph node ratio in stage IIIC epithelial ovarian cancer with node-positive in a SEER population-based study. Oncotarget. (2016) 7:7952–59. doi: 10.18632/oncotarget.6911
31. Ayadi MA, Masouri H, Ben safta I, Sakhri S, Hechiche M, Ben Hassouna J, et al. Impact of lymph node ratio on survival for epithelial ovarian cancer: A Tunisian study. Int J Gynecol Cancer. (2018) 28:648.
32. Widschwendter P, Schuh F, DeGregorio N, Friedl T, Scholz C, Bauer E. Lymph node ratio and outcome in ovarian cancer-a retrospective single center analysis. Int J Gynecol Cancer. (2017) 27:1007.
33. Gorzelnik K, Szubert S, Knafel A, Wojcikiewicz A, Nowakowski B, Koper K, et al. An analysis of the significance of the lymph node ratio and extracapsular involvement in the prognosis of endometrial cancer patients. Wspolczesna Onkol. (2022) 26:144–49. doi: 10.5114/wo.2022.118243
34. Fleming ND, Soliman PT, Westin SN, Dos Reis R, Munsell M, Klopp AH, et al. Impact of lymph node ratio and adjuvant therapy in node-positive endometrioid endometrial cancer. Int J Gynecol Cancer. (2015) 25:1437–44. doi: 10.1097/IGC.0000000000000510
35. Tangjitgamol S, Kittisiam T, Sriraumpuch J. Impact of metastatic lymph node to total lymph node ratio on survival of endometrial cancer patients. Gynecol Obstet Invest. (2019) 84:463–71. doi: 10.1159/000497484
36. Polterauer S, Khalil S, Zivanovic O, Abu Rustum NR, Hofstetter G, Concin N, et al. Prognostic value of lymph node ratio and clinicopathologic parameters in patients diagnosed with stage iiic endometrial cancer. Obstet Gynecol. (2012) 119:1210–18. doi: 10.1097/AOG.0b013e318255060c
37. Toptas T, Simsek T. Stage IIIC endometrial cancer: The need for novel subgrouping according to the ratio of metastatic lymph nodes. Arch Gynecol Obstet. (2015) 291:391–98. doi: 10.1007/s00404-014-3409-z
38. Yildirim BA, Onal C, Sari SY, Yavas G, Gultekin M, Guler OC, et al. The utility of dissected lymph node number and lymph node metastasis ratio in stage IIIC endometrium adenocarcinoma: A multicentric analysis. Int J Radiat Oncol Biol Phys. (2018) 102:E642–E43. doi: 10.1016/j.ijrobp.2018.07.1750
39. Polterauer S, Schwameis R, Grimm C, Macuks R, Iacoponi S, Zalewski K, et al. Prognostic value of lymph node ratio and number of positive inguinal nodes in patients with vulvar cancer. Gynecol Oncol. (2017) 147:92–7. doi: 10.1016/j.ygyno.2017.07.142
40. Serre E, Diguisto C, Body G, Raimond E, Bendifallah S, Touboul C, et al. Prognostic significance of groin lymph node ratio in vulvar squamous cell carcinoma. Gynecol Obstet Fertil Senol. (2020) 48:729–35.
41. Polterauer S, Schwameis R, Grimm C, Hillemannsc P, Jückstockd J, Hilpert F, et al. Lymph node ratio in inguinal lymphadenectomy for squamous cell vulvar cancer: Results from the AGO-CaRE-1 study. Gynecol Oncol. (2019) 153:286–91. doi: 10.1016/j.ygyno.2019.02.007
42. Cui H, Huang Y, Wen W, Li XD, Xu DY, Liu L. Prognostic value of lymph node ratio in cervical cancer: A meta-analysis. Med (United States). (2022) 101:E30745. doi: 10.1097/MD.0000000000030745
43. Wright JD, Matsuo K, Huang Y, Tergas AI, Hou JY, Khoury-Collado F, et al. Prognostic performance of the 2018 international federation of gynecology and obstetrics cervical cancer staging guidelines. Obstet Gynecol. (2019) 134:49–57. doi: 10.1097/AOG.0000000000003311
44. Prat J, D’Angelo E, Espinosa I. Ovarian carcinomas: at least five different diseases with distinct histological features and molecular genetics. Hum Pathol. (2018) 80:11–27. doi: 10.1016/j.humpath.2018.06.018
45. Ayhan A, Ozkan NT, Sari ME, Celik H, Dede M, Akbayir O, et al. Impact of lymph node ratio on survival in stage III ovarian high-grade serous cancer: a Turkish Gynecologic Oncology Group study. J Gynecol Oncol. (2018) 29(1). doi: 10.3802/jgo.2018.29.e12
46. Nusbaum DJ, Mandelbaum RS, Machida H, Matsuzaki S, Roman LD, Sood AK, et al. Significance of lymph node ratio on survival of women with borderline ovarian tumors. Arch Gynecol Obstet. (2020) 301:1289–98. doi: 10.1007/s00404-020-05535-0
47. Yang X-L, Huang N, Wang MM, Lai H, Wu D-J. Comparison of different lymph node staging schemes for predicting survival outcomes in node-positive endometrioid endometrial cancer patients. Front Med (Lausanne). (2021) 8:688535. doi: 10.3389/fmed.2021.688535
Keywords: lymph node ratio, gynecological cancer, prognosis, systematic review, meta-analysis
Citation: Chen M, Wang Y, Chen Y, Han L and Zheng A (2024) The prognostic values of lymph node ratio for gynecological cancer: a systematic review and meta-analysis. Front. Oncol. 14:1475348. doi: 10.3389/fonc.2024.1475348
Received: 03 August 2024; Accepted: 09 September 2024;
Published: 01 October 2024.
Edited by:
Sophia George, University of Miami, United StatesReviewed by:
Mikel Gorostidi, University of the Basque Country, SpainAngelo Finelli, ULSS2 Marca Trevigiana, Italy
Copyright © 2024 Chen, Wang, Chen, Han and Zheng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ling Han, aGFubGluZ2x1b2JvQHNpbmEuY29t; Ai Zheng, YWl6aGVuZzcxNkAxNjMuY29t
 Mengmeng Chen
Mengmeng Chen Yisi Wang
Yisi Wang Yali Chen1,2
Yali Chen1,2 Ling Han
Ling Han Ai Zheng
Ai Zheng