
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol., 31 October 2024
Sec. Gastrointestinal Cancers: Colorectal Cancer
Volume 14 - 2024 | https://doi.org/10.3389/fonc.2024.1475270
 Fumiaki Tanino1
Fumiaki Tanino1 Ken Yamashita1*
Ken Yamashita1* Shin Morimoto1
Shin Morimoto1 Yudai Takehara1
Yudai Takehara1 Noriko Yamamoto1
Noriko Yamamoto1 Yuki Kamigaichi1
Yuki Kamigaichi1 Tomoyuki Nishimura1
Tomoyuki Nishimura1 Hidenori Tanaka1
Hidenori Tanaka1 Hidehiko Takigawa1
Hidehiko Takigawa1 Yuji Urabe1
Yuji Urabe1 Toshio Kuwai2
Toshio Kuwai2 Fumio Shimamoto3
Fumio Shimamoto3 Shiro Oka1
Shiro Oka1Background: Additional surgery with lymph node (LN) dissection is recommended for pT1 colorectal carcinoma (CRC) resected by endoscopy, based on pathological risk factors for LN metastasis (LNM), according to guidelines by the Japanese Society for Cancer of the Colon and Rectum (JSCCR), National Comprehensive Cancer Network (NCCN), and European Society for Medical Oncology (ESMO).
Methods: We retrospectively analyzed 560 consecutive patients with T1 CRC who underwent endoscopic resection alone (n=190) or initial or additional surgery with LN dissection (n=370) between 1992 and 2017 at Hiroshima University Hospital. Patients were classified into LNM low- and high-risk groups according to guidelines by the JSCCR, NCCN, and ESMO as follows. Patients without any specified pathological LNM risk factor were included in the LNM low-risk group, while the high-risk group comprised all other patients. We analyzed the LNM predictive ability of each guideline.
Results: The LNM high-risk rate, sensitivity, specificity, positive and negative predictive values, accuracy of LNM risk, and AUC for LNM predictive ability were 82%, 100%, 19%, 9%, 100%, 26% and 0.596 in the JSCCR guidelines; 52%, 98%, 52%, 15%, 99%, 56%, and 0.749 in the NCCN; and 54%, 98%, 50%, 15%, 99%, 54%, and 0.743 in the ESMO, respectively.
Conclusions: The JSCCR guidelines could diagnose LNM in all cases but had the highest false-positive rate. It is important to reduce unnecessary additional surgeries for pT1 CRCs after ER.
With the proliferation of population-based screening programs for colorectal cancer (CRC) and advancements in endoscopic diagnosis, CRC incidence detected using endoscopy has increased. The number of patients with CRC is 1.8 million and increasing (1). The mortality rate from CRC is high and is the third leading cause of malignancy death in Japan (2). Intramucosal carcinoma (Tis) does not metastasize to the lymph node (LN) and is a good indication for endoscopic resection (ER) (3). Conversely, T1 CRCs with submucosal (SM) deep invasion typically require surgery with LN dissection because of the presence of LN metastases (LNM) (4). Additional surgery with LN dissection after ER is recommended for patients with T1 CRC with pathological risk factors, according to the guidelines. However, the reported rate of LNM in T1 CRC is approximately 10%, meaning approximately 90% of patients who undergo additional surgery do not have LNM. Therefore, performing additional surgery in patients without LNM may be an overtreatment, which is a current issue (4, 5). With the aging of the population, surgery may be impossible for some patients with T1 CRC because of advanced age, complications, or patient preferences.
En bloc endoscopic submucosal dissection (ESD) as a total excisional biopsy for clinical T1 CRC is a highly effective and safe treatment and establishes a precise histological diagnosis (6). Prior ER does not affect the recurrence or prognosis of T1 CRC after additional surgery (7, 8). Therefore, ER usage as an initial treatment for T1 CRC has been increasing (9). Additional surgery with LN dissection is recommended for pT1 CRC resected by ER when there are pathologically elevated risks for LNM. The pathological risk factors for LNM in T1 CRC and indications for additional surgery vary according to the Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines in Japan, National Comprehensive Cancer Network (NCCN) guidelines in the USA, and European Society for Medical Oncology (ESMO) guidelines in Europe (4, 10, 11). The guidelines are based on evidence from the reported literature and are developed by experts in each field, considering the actual situation in each country. Guidelines provide a clear basis for explaining techniques, procedures, and essential treatment. Different guidelines use varying criteria to assess the risks for LNM and may predict the risks for LNM differently in the same case. However, there have been few studies comparing the efficacy of the JSCCR, NCCN, and ESMO guidelines in predicting LNM in T1 CRC. Moreover, previous reports on LMM have examined patients who only underwent surgery. This study aimed to investigate the ability of each guideline to predict the risk for LNM in T1 CRC, including patients who were treated with ER alone, in view of actual clinical practice.
Figure 1 shows the flow chart of enrolled patients. Among 783 patients with T1 CRC who underwent ER or surgery between February 1992 and September 2017 at Hiroshima University Hospital, we excluded 223 patients for the following reasons: diagnosis of familial adenomatous polyposis or lynch syndrome, inflammatory bowel disease, synchronous CRCs which invasion depth was deeper than submucosal (SM), synchronous advanced CRC, or cancer of other organs, insufficient data for pathologic features, and follow up <5 years in ER alone group. We finally analyzed data from 560 patients with 560 T1 CRCs. ER procedures included ESD, endoscopic mucosal resection, and polypectomy. Of these, 190 patients underwent ER alone, 220 patients underwent surgery after ER, and 150 patients underwent surgery alone.
We classified the patients into the LNM low-risk and high-risk groups according to each guideline (JSCCR, NCCN, and ESMO). Patients without any of the pathological LNM risk factors specified in each guideline, who could be followed up without additional surgery, were defined as the low-risk group, while the other patients were classified as the high-risk group. This study adhered to the ethical standards of the Declaration of Helsinki (2014). The Ethics Committees at Hiroshima University and its affiliated hospital approved the study protocol (Approval No. 0198).
Indications for ER in early CRC followed JSCCR guidelines. Tis and T1 CRC with SM invasion depth <1000 µm had a low risk for LNM and was a good indication for en bloc resection by ER. The SM invasion depth of the lesion was comprehensively determined using normal and magnifying endoscopy, EUS, and barium enema examination. The endoscopist determined the treatment methods, considering the tumor size and morphology. While obvious clinical T1b (SM invasion depth ≥1000 µm) CRC was typically treated surgically, ER was performed based on the patient’s preferences and status (age, comorbidity, performance status, tumor location, and risk of surgery). According to the JSCCR guidelines, patients with positive vertical margin (VM) required surgery after ER. Patients with negative VM were considered to necessitate additional surgery if they were in the high-risk group based on JSCCR guidelines. Generally, additional surgery should be performed within 3 months of ER, and D2 LN dissection (middle LN) was performed.
The resected specimens were pinned onto a board and fixed in 10% buffered formalin for 12–48 hours. Specimens resected by ER were cut into 2 mm thick sections. Those resected by surgery were subsequently cut into parallel 3–4 mm thick sections, and small lesions or lesions suspected of SM invasion were cut into parallel 2 mm thick sections. A single gastrointestinal pathologist evaluated and diagnosed pathological features in all cases. Pathological features, including SM invasion depth, histological grade, budding grade, and lymphovascular invasion, were evaluated using hematoxylin-eosin staining and special staining (Victoria blue, Elastica van Gieson, D2-40, and Desmin) as required. SM invasion depth was measured from the lower border of the muscularis mucosae (MM) when identification or estimation of the location of the MM was possible. If it was impossible to identify or estimate the location of MM, SM invasion depth was measured from the surface layer of the mucosa (4, 12). The histological grade was classified into two types: favorable (tubular or papillary adenocarcinoma) and unfavorable (poorly differentiated adenocarcinoma, signet-ring cell carcinoma, and mucinous carcinoma), specified by the JSCCR guidelines in Japan. Conversely, CRC was graded into well-differentiated (G1), moderately differentiated (G2), poorly differentiated (G3), and undifferentiated (G4) adenocarcinoma according to the World Health Organization criteria.
The budding grade was assessed per microscopic field at 200X magnification: low grade: grade 1, 0–4 buds; high grade: grade 2, 5–9 buds; and grade 3, 10 or more buds (13). Positive VM was defined as the presence of tumors or mucinous components at the VM. According to the JSCCR guidelines, the definition of pathological features allowing for follow-up without additional surgery includes negative VM, SM invasion depth <1000 µm, main histology grade of tubular or papillary adenocarcinoma, negative lymphovascular invasion, and budding grade 1 (4). According to NCCN, it includes: negative VM, G1/2 differentiation, and negative lymphovascular invasion (11). According to ESMO, it includes negative VM, G1/2 differentiation, negative lymphovascular invasion, and budding grade 1 (10). Histological differentiation in JSCCR guidelines was the predominant differentiation, whereas, in NCCN or ESMO guidelines, it was the most poorly differentiation in the specimens resected.
The follow-up period was extended beyond 5 years after ER. Patients underwent interviews, physical examinations, blood tests, and chest and abdominal computed tomography (CT) every 6 months for the first 3 years, followed by annual assessments for the next 2 years. Total colonoscopy was performed annually for 5 years. In the group with ER alone, the presence of LNM in regional LN was considered positive if detected by CT during surveillance. Patients were categorized as LNM-negative if their regional LNs remained metastasis-free for at least 5 years.
We analyzed the clinicopathological variables, including age, sex, location, tumor size, macroscopic type, treatment method, main histology, SM invasion depth, lymphovascular invasion, budding grade, LNM, and the rate of the high-risk group. We assessed the sensitivity, specificity, positive and negative predictive values, and the accuracy of LNM risk for each guideline. Moreover, we measured the areas under the receiver operating characteristic curves (AUC) for the ability of each guideline to identify patients with LNM.
Data are expressed as mean ± standard deviation. Fisher’s exact test was used to compare qualitative variables, and the Wilcoxon rank-sum test was used to compare quantitative variables. We evaluated the associations using multiple logistic regression analyses. The odds ratio (OR) and 95% confidence interval (95% Cl) were calculated for each variable to estimate the risk factors for LNM in each guideline. Values with p < 0.05 were considered statistically significant. The risk factors for LNM, as defined in each guideline, were assessed using the AUC. All data were statistically analyzed using JMP statistical software version. 16.2.0 (SAS Institute, Cary, NC, USA).
Table 1 shows the baseline characteristics of the 560 patients and lesions. The average age of those enrolled was 66 ± 11 years, with 357 (64%) being males. Regarding lesion location, 426 (76%) were located in the colon, and the mean tumor size was 24 mm. The protruded macroscopic type was observed in 293 (52%) cases. Regarding the main histology specified in the JSCCR guidelines, 551 (98%) cases were tubular or papillary adenocarcinomas. Regarding the main histology specified in the NCCN and ESMO guidelines, 449 (80%) cases were classified as G1/2 adenocarcinomas. SM depth of more than 1000 µm was observed in 394 (70%) cases. There were 194(35%)cases of lymphovascular invasion and 126 (23%) of budding grade 2/3. LNM was observed in 45 (8%) cases. The number of high-risk groups, according to the JSCCR, NCCN, and ESMO guidelines, was 461 (82%) cases, 291 (52%), and 303 (54%), respectively.
Table 2 shows the univariate and multivariate analyses of pathological risk factors for LNM according to each guideline. In the univariate analysis, main histology, SM invasion depth, lymphovascular invasion, and budding grade were significantly different in each guideline. Subsequently, we performed a multivariate analysis of LNM risk factors in each guideline. The independent risk factors for LNM in T1 CRC were SM invasion depth (OR, 7.70; 95%Cl, 1.48–143), lymphovascular invasion (OR, 6.78; 95%Cl, 3.05–17.2), and budding grade (OR, 2.89; 95%Cl, 1.47–5.75) according to the JSCCR guidelines, were main histology (OR, 8.62; 95%Cl, 4.35–17.8) and lymphovascular invasion (OR, 10.2; 95%Cl, 4.6–26.0) according to the NCCN guidelines, and were main histology (OR, 7.05; 95%Cl, 3.35–15.4) and lymphovascular invasion (OR, 8.75; 95%Cl, 3.81–22.8) according to the ESMO guidelines.
Figure 2 shows the AUC for the ability of each guideline to identify patients with LNM. The AUC values for the JSCCR, NCCN, and ESMO guidelines were 0.596, 0.749, and 0.743, respectively. Table 3 presents the comparison of the ability to predict LNM among the guidelines. The sensitivity, specificity, positive and negative predictive values, and accuracy of LNM risk were 100% (45/45), 19% (99/515), 10% (45/461), 100% (99/99), and 26% (144/560), respectively, for the JSCCR guidelines; 98% (44/45), 52% (268/515), 15% (44/291), 99% (268/269) and 56% (312/560), respectively, for the NCCN guidelines; and 98% (44/45), 50% (256/515), 15% (44/303), 99% (256/257) and 54% (300/560), respectively, for ESMO guidelines. The JSCCR guidelines exhibited higher sensitivity but lower specificity and accuracy for LNM than that of other guidelines. A case with LNM was categorized as high-risk according to the JSCCR guidelines, based solely on SM invasion depth. Therefore, the case did not meet the criteria for high-risk classification in the ESMO and NCCN guidelines but could be considered high-risk in the JSCCR guidelines.
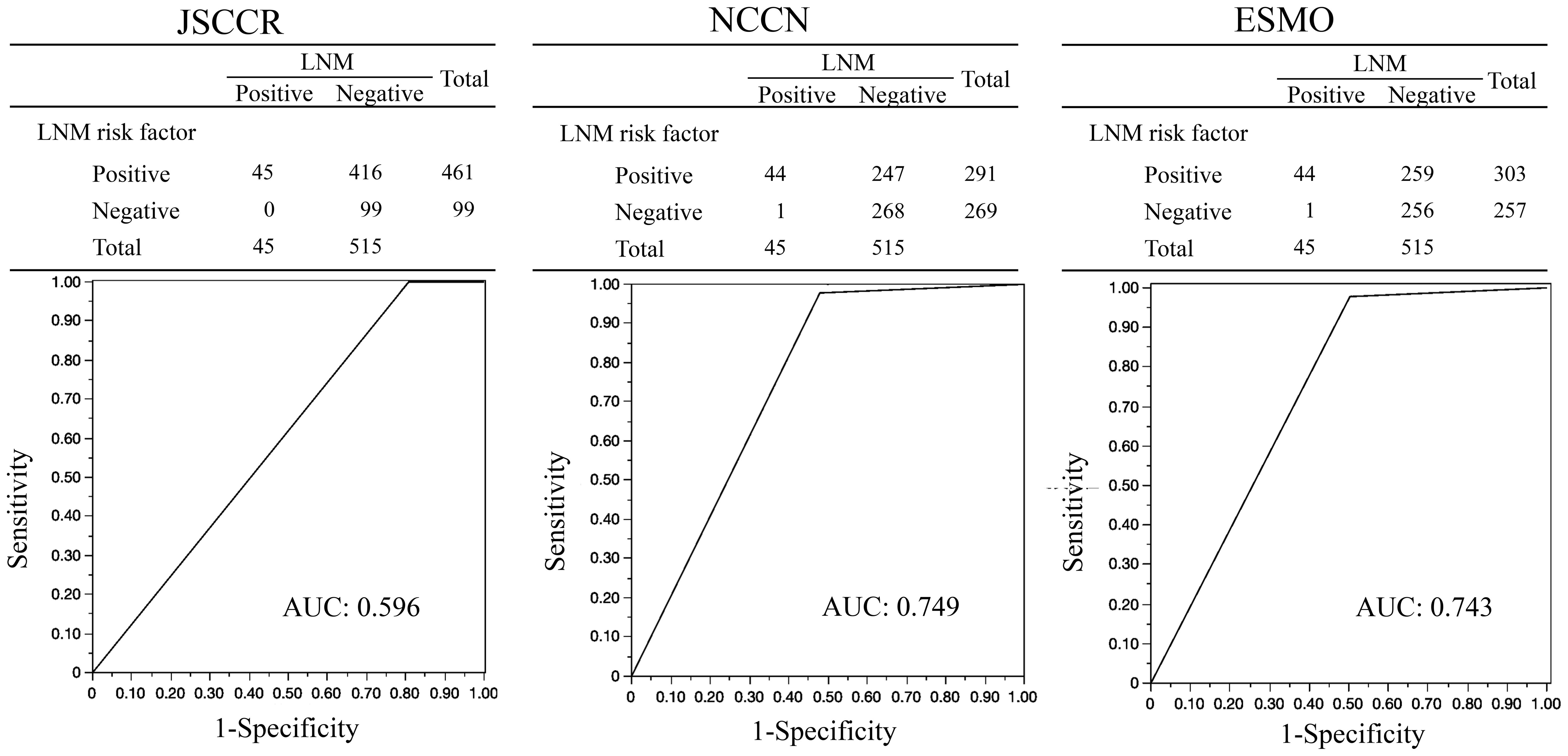
Figure 2. AUCs for the ability of each guideline to identify patients with LNM (n=560). AUC, Area Under the Curve; JSCCR, Japanese Society for Cancer of the Colon and Rectum; NCCN, National Comprehensive Cancer Network; ESMO, European Society for Medical Oncology; LNM, Lymph Node Metastasis.
We compared the predictive ability for LNM of each guideline in patients with T1 CRC treated by ER or surgery and observed that the JSCCR guidelines exhibited the highest sensitivity but the lowest specificity. Oka et al. demonstrated that the recurrence risk was negligible in patients at low risk of LNM according to the JSCCR guidelines as follows: 0.1% in patients with ER alone, 0% in patients with additional surgery after ER, and 3.1% in patients with surgery alone and revealed the validity of the JSCCR guidelines for pT1 CRC (14). Various risk factors for LNM have been reported in patients with T1 CRC beyond the JSCCR guidelines. Others have identified female sex, left-sided colorectal lesion, rectal lesion, completely disrupted MM, and histologic differentiation at the deepest invasive portion as risk factors for LNM (12, 15–20). Pathological risk factors for LNM in patients with T1 CRC differed significantly between the JSCCR and NCCN or ESMO guidelines in two respects. First, the pathological risk for LNM in only JSCCR guidelines includes an SM invasion depth of ≥1000 µm. Regarding the pathological risk for LNM, only SM invasion depth was predictable preoperatively using magnifying endoscopy and EUS. However, the measurement method of the SM invasion depth differed according to the gross type of lesion, the condition of MM, and the pathologists. Patients with T1 CRC whose pathological risk factor was only SM invasive depth were considered less likely to develop LNM, and the rate of LNM was 1.2–3.4% (5, 21–24). We previously reported among patients with T1 CRC in whom SM invasion depth was the only risk factor, there were no cases of LNM with SM invasion depth of <1,800 µm (21). In the absence of the other risk factors, SM invasion depth may be a weak risk factor for LNM, suggesting that surgery-related mortality may outweigh recurrence (25–27). Yoshii et al. reported that patients with the LNM risk factor of only SM invasion depth had a low cumulative risk of recurrence, with 2.3% in the surgery group after ER and 3.4% in the ER alone group (28). Conversely, although rare, patients with T1 CRC whose pathological risk factor for LNM was only SM invasion depth of ≥1000 µm had LNM in this study. Therefore, the indications for additional surgery in patients whose risk factor is SM invasion depth only should be carefully considered. Follow-up without additional surgery is considered acceptable in extremely older patients, patients with severe comorbidities, underlying diseases, or other primary advanced cancers leading to poor prognosis, and patients whose lesions are located in the rectum below the peritoneal reflection, which is at risk for colostomy.
Second, the evaluation methods for histological types differ between the JSCCR and NCCN or ESMO guidelines. The predominant histological grade defined in the JSCCR guidelines was not significantly different in the multivariate analysis. Conversely, the most poorly differentiated pathology defined in the NCCN and ESMO guidelines showed significant differences in the univariate and multivariate analysis. Some reports do not consider the predominant histological grade as an independent risk factor for LNM, (29–31) others have identified the most poorly differentiated pathology as such (19, 20, 30–32). Regarding the main histology, the most poorly differentiated pathology may have a greater impact as a risk factor for LNM than the predominant histology. In this study, in the multivariate analysis of risk factors for LNM defined in the ESMO guidelines, budding grade was not an independent risk factor. This may be attributed to the great influence of covariates, particularly the most poorly differentiated pathology. However, there is currently no definitive conclusion as to whether the predominant histological grade or most poorly differentiated pathology is more useful in predicting LNM. It is necessary to accumulate and verify cases in the near future.
The JSCCR guidelines exhibited the highest sensitivity, effectively identifying all patients with LNM, while its specificity was the lowest, resulting in identifying many patients without LNM as a high-risk group. This was primarily because SM invasion depth was included as a risk factor. Conversely, patients with false-positive LNM were also frequent, leading to the lowest accuracy of LNM among the three guidelines. Stratification of the risk for LNM is crucial to identifying patients who require additional surgery. Currently, there are various reports on predictors of the risk for LNM. To predict the risk for LNM, Kajiwara et al. performed a multivariate analysis of logistic regression analysis and developed a nomogram that incorporated SM invasion depth, lymphovascular invasion, main histology, sex, and location, which were found to be independent risk factors for LNM (33). The nomogram revealed that the SM invasion had the greatest impact on LNM. Moreover, they reported that adding an SM invasion depth of >2000 µm as a cutoff improved the ability to predict LNM. Yan et al. developed a nomogram that incorporated pathological features and imaging modalities (CT or MRI) and compared the ability to predict LNM in JSCCR guidelines. They reported that the AUC of the JSCCR was 0.75, whereas the AUC of the developed nomogram was 0.89, which had a high clinical application value (34). Ichimasa et al. developed an artificial intelligence (AI) model by analyzing 45 variables, including pathological risk factors and serum biomarkers for preoperative detection of LNM in patients with T1 CRC. Their model significantly reduced unnecessary additional surgery compared to following the JSCCR guidelines without missing the patients with LNM (35). Kudo et al. developed a machine-learning artificial neural network algorithm to predict LNM and reported that the AI showed higher discrimination power than the NCCN guidelines to predict LNM in patients with T1 CRCs. They concluded that AI could aid in decision-making regarding additional surgery performed after ER in patients with T1 CRCs (36). Moreover, Wada developed a predictive model for LNM by combining four miRNAs, five mRNAs, and pathological risk factors and reported that patients who underwent additional surgery were reduced to 18% (37). Further studies are needed to predict LNM in the future.
This study had some limitations. First, this study was a retrospective single-center study. Second, we did not reevaluate lymphovascular invasion using immunohistochemical staining in all cases. Some studies have reported that accurately assessing lymphovascular invasion may be challenging without using immunostaining (1, 38). Consequently, the assessment of lymphovascular invasion may be underestimated. Third, although ≥12 LNs should be dissected to diagnose advanced-stage colon cancer accurately according to NCCN guidelines, all the enrolled patients did not undergo dissection of 12 or more LNs. Thus, LNM may have been underestimated. Fourth, the exact number of LNs dissected in surgery was not known in all cases. Fifth, we included cases in which recurrence or metastasis was detected during follow-up after ER as LNM-positive cases, considering the clinical practice of opting for ER alone based on the patient’s preferences or circumstances even in situations where additional surgery may be necessary. On the other hand, there are pathological findings such as extramural cancer deposits without lymph node structure and skip lymphovascular invasion that cannot be accurately diagnosed without surgical resection (39). In the ER cases, accurate pathological diagnosis, including evaluation of these pathological findings and other potential risk factors, was not achieved, which may have also led to an underestimation. Finally, while the risk for LNM was assessed based on the macroscopic type of lesions in practice, the microscopic type was not included as a risk factor for LNM.
All guidelines exhibited high sensitivity for LNM, and only the JSCCR guidelines could diagnose LNM in all cases. However, the false-positive rate for LNM in the JSCCR guidelines was high. Reducing unnecessary additional surgery for pT1 CRCs after ER is an important issue.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
The studies involving humans were approved by The Ethics Committees at Hiroshima University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
FT: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. KY: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Writing – review & editing. SM: Conceptualization, Data curation, Writing – review & editing. YT: Conceptualization, Data curation, Writing – review & editing. NY: Conceptualization, Data curation, Writing – review & editing. YK: Conceptualization, Data curation, Writing – review & editing. TN: Conceptualization, Data curation, Writing – review & editing. HTan: Conceptualization, Data curation, Writing – review & editing. HTak: Conceptualization, Data curation, Writing – review & editing. YU: Conceptualization, Data curation, Writing – review & editing. TK: Conceptualization, Data curation, Writing – review & editing. FS: Data curation, Writing – review & editing. SO: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Cserni G, Sejben I, Bori R. Diagnosing vascular invasion in colorectal carcinomas: improving reproducibility and potential pitfalls. J Clin Pathol. (2013) 66:543–7. doi: 10.1136/jclinpath-2013-201587
2. Katanoda K, Hori M, Matsuda T, Shibata A, Nishino Y, Hattori M, et al. An updated report on the trends in cancer incidence and mortality in Japan, 1958–2013. Jpn J Clin Oncol. (2015) 45:390–401. doi: 10.1093/jjco/hyv002
3. Morson BC, Whiteway JE, Jones EA, Macrae FA, Williams CB. Histopathology and prognosis of Malignant colorectal polyps treated by endoscopic polypectomy. Gut. (1984) 25:437–44. doi: 10.1136/gut.25.5.437
4. Hashiguchi Y, Muro K, Saito Y, Ito Y, Ajioka Y, Hamaguchi T, et al. Japanese society for cancer of the colon and rectum (JSCCR) guidelines 2019 for the treatment of colorectal cancer. Int J Clin Oncol. (2020) 25:1–42. doi: 10.1007/s10147-019-01485-z
5. Yasue C, Chino A, Takamatsu M, Namikawa K, Ide D, Saito S, et al. Pathological risk factors and predictive endoscopic factors for lymph node metastasis of T1 colorectal cancer: a single-center study of 846 lesions. J Gastroenterol. (2019) 54:708–17. doi: 10.1007/s00535-019-01564-y
6. Asayama N, Oka S, Tanaka S, Hayashi N, Arihiro K, Chayama K. Endoscopic submucosal dissection as total excisional biopsy for clinical T1 colorectal carcinoma. Digestion. (2015) 91:64–9. doi: 10.1159/000368866
7. Rickert A, Aliyev R, Belle S, Post S, Kienle P, Kähler G. Oncologic colorectal resection after endoscopic treatment of Malignant polyps: does endoscopy have an adverse effect on oncologic and surgical outcomes? Gastrointest Endosc. (2014) 79:951–60. doi: 10.1016/j.gie.2013.11.014
8. Yamashita K, Oka S, Tanaka S, Nagata S, Hiraga Y, Kuwai T, et al. Preceding endoscopic submucosal dissection for T1 colorectal carcinoma does not affect the prognosis of patients who underwent additional surgery: a large multicenter propensity score-matched analysis. J Gastroenterol. (2019) 54:897–906. doi: 10.1007/s00535-019-01590-w
9. Urabe Y, Tanaka S, Saito Y, Igarashi M, Watanabe T, Sugihara K. Impact of revisions of the JSCCR guidelines on the treatment of T1 colorectal carcinomas in Japan. Z Gastroenterol. (2015) 53:291–301. doi: 10.1055/s-0034-1385764
10. Glynne-Jones R, Wyrwicz L, Tiret E, Brown G, Rödel C, Cervantes A, et al. Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. (2018) 29:iv263. doi: 10.1093/annonc/mdy161
11. Benson AB, Venook AP, Al-Hawary MM, Cederquist L, Chen YJ, Ciombor KK, et al. Rectal cancer, version 2.2018, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. (2018) 16:874–901. doi: 10.6004/jnccn.2018.0061
12. Nakadoi K, Oka S, Tanaka S, Hayashi N, Terasaki M, Arihiro K, et al. Condition of muscularis mucosae is a risk factor for lymph node metastasis in T1 colorectal carcinoma. Surg Endosc. (2014) 28:1269–76. doi: 10.1007/s00464-013-3321-9
13. Ueno H, Murphy J, Jass JR, Mochizuki H, Talbot IC. Tumour ‘budding’ as an index to estimate the potential of aggressiveness in rectal cancer. Histopathology. (2002) 40:127–32. doi: 10.1046/j.1365-2559.2002.01324.x
14. Oka S, Tanaka S, Kajiwara Y, Saito S, Fukunaga Y, Takamatsu M, et al. Treatment decision for locally resected T1 colorectal carcinoma-verification of the Japanese guideline criteria for additional surgery based on long-term clinical outcomes. Am J Gastroenterol. (2024) 119:2019–27. doi: 10.14309/ajg.0000000000002715
15. Bosch SL, Teerenstra S, de Wilt JH, Cunningham C, Nagtegaal ID. Predicting lymph node metastasis in pT1 colorectal cancer: a systematic review of risk factors providing rationale for therapy decisions. Endoscopy. (2013) 45:827–34. doi: 10.1055/s-0033-1344238
16. Kitajima K, Fujimori T, Fujii S, Takeda J, Ohkura Y, Kawamata H, et al. Correlations between lymph node metastasis and depth of submucosal invasion in submucosal invasive colorectal carcinoma: a Japanese collaborative study. J Gastroenterol. (2004) 39:534–43. doi: 10.1007/s00535-004-1339-4
17. Ichimasa K, Kudo SE, Miyachi H, Kouyama Y, Ishida F, Baba T, et al. Patient gender as a factor associated with lymph node metastasis in T1 colorectal cancer: A systematic review and meta-analysis. Mol Clin Oncol. (2017) 6:517–24. doi: 10.3892/mco.2017.1172
18. Naito A, Iwamoto K, Ohtsuka M, Imasato M, Nakahara Y, Mikamori M, et al. Risk factors for lymph node metastasis in pathological T1b colorectal cancer. In Vivo. (2021) 35:987–91. doi: 10.21873/invivo.12341
19. Tanaka S, Haruma K, Oh-E H, Nagata S, Hirota Y, Furudoi A, et al. Conditions of curability after endoscopic resection for colorectal carcinoma with submucosally massive invasion. Oncol Rep. (2000) 7:783–8. doi: 10.3892/or.7.4.783
20. Ueno H, Mochizuki H, Hashiguchi Y, Shimazaki H, Aida S, Hase K, et al. Risk factors for an adverse outcome in early invasive colorectal carcinoma. Gastroenterology. (2004) 127:385–94. doi: 10.1053/j.gastro.2004.04.022
21. Nakadoi K, Tanaka S, Kanao H, Terasaki M, Takata S, Oka S, et al. Management of T1 colorectal carcinoma with special reference to criteria for curative endoscopic resection. J Gastroenterol Hepatol. (2012) 27:1057–62. doi: 10.1111/j.1440-1746.2011.07041.x
22. Zwager LW, Bastiaansen BAJ, Montazeri NSM, Hompes R, Barresi V, Ichimasa K, et al. Deep submucosal invasion is not an independent risk factor for lymph node metastasis in T1 colorectal cancer: A meta-analysis. Gastroenterology. (2022) 163:174–89. doi: 10.1053/j.gastro.2022.04.010
23. Mochizuki K, Kudo SE, Ichimasa K, Kouyama Y, Matsudaira S, Takashina Y, et al. Left-sided location is a risk factor for lymph node metastasis of T1 colorectal cancer: a single-center retrospective study. Int J Colorectal Dis. (2020) 35:1911–9. doi: 10.1007/s00384-020-03668-x
24. Yamaoka Y, Shiomi A, Kagawa H, Hino H, Manabe S, Chen K, et al. Lymph node metastasis in T1 colorectal cancer with the only high-risk histology of submucosal invasion depth ≥ 1000 μm. Int J Colorectal Dis. (2022) 37:2387–95. doi: 10.1007/s00384-022-04269-6
25. Vermeer NCA, Backes Y, Snijders HS, Bastiaannet E, Liefers GJ, Moons LMG, et al. National cohort study on postoperative risks after surgery for submucosal invasive colorectal cancer. BJS Open. (2018) 3:210–7. doi: 10.1002/bjs5.50125
26. Choi YS, Kim WS, Hwang SW, Park SH, Yang DH, Ye BD, et al. Clinical outcomes of submucosal colorectal cancer diagnosed after endoscopic resection: a focus on the need for surgery. Intest Res. (2020) 18:96–106. doi: 10.5217/ir.2019.00092
27. Kobayashi H, Mochizuki H, Morita T, Kotake K, Teramoto T, Kameoka S, et al. Characteristics of recurrence after curative resection for T1 colorectal cancer: Japanese multicenter study. J Gastroenterol. (2011) 46:203–21. doi: 10.1007/s00535-010-0341-2
28. Yoshii S, Nojima M, Nosho K, Omori S, Kusumi T, Okuda H, et al. Factors associated with risk for colorectal cancer recurrence after endoscopic resection of T1 tumors. Clin Gastroenterol Hepatol. (2014) 12:292–302.e3. doi: 10.1016/j.cgh.2013.08.008
29. Bae HJ, Ju H, Lee HH, Kim J, Lee BI, Lee SH, et al. Long-term outcomes after endoscopic versus surgical resection of T1 colorectal carcinoma. Surg Endosc. (2023) 37:1231–41. doi: 10.1007/s00464-022-09649-1
30. Fujino S, Miyoshi N, Kitakaze M, Yasui M, Ohue M, Osawa H, et al. Lymph node metastasis in T1 colorectal cancer: Risk factors and prediction model. Oncol Lett. (2023) 25:191. doi: 10.3892/ol.2023.13776
31. Shiina O, Kudo SE, Ichimasa K, Takashina Y, Kouyama Y, Mochizuki K, et al. Differentiation grade as a risk factor for lymph node metastasis in T1 colorectal cancer. DEN Open. (2023) 4:e324. doi: 10.1002/deo2.324
32. Ichimasa K, Kudo SE, Miyachi H, Kouyama Y, Mochizuki K, Takashina Y, et al. Current problems and perspectives of pathological risk factors for lymph node metastasis in T1 colorectal cancer: Systematic review. Dig Endosc. (2022) 34:901–12. doi: 10.1111/den.14220
33. Kajiwara Y, Oka S, Tanaka S, Nakamura T, Saito S, Fukunaga Y, et al. Nomogram as a novel predictive tool for lymph node metastasis in T1 colorectal cancer treated with endoscopic resection: a nationwide, multicenter study. Gastrointest Endosc. (2023) 97:1119–1128.e5. doi: 10.1016/j.gie.2023.01.022
34. Yan S, Ding H, Zhao X, Wang J, Deng W. Development and validation of a nomogram for further decision of radical surgery in pT1 colorectal cancer after local resection. Int J Colorectal Dis. (2021) 36:1499–506. doi: 10.1007/s00384-021-03928-4
35. Ichimasa K, Kudo SE, Mori Y, Misawa M, Matsudaira S, Kouyama Y, et al. Artificial intelligence may help in predicting the need for additional surgery after endoscopic resection of T1 colorectal cancer. Endoscopy. (2018) 50:230–40. doi: 10.1055/s-0043-122385
36. Kudo SE, Ichimasa K, Villard B, Mori Y, Misawa M, Saito S, et al. Artificial intelligence system to determine risk of T1 colorectal cancer metastasis to lymph node. Gastroenterology. (2021) 160:1075–1084.e2. doi: 10.1053/j.gastro.2020.09.027
37. Wada Y, Shimada M, Murano T, Takamaru H, Morine Y, Ikemoto T, et al. A liquid biopsy assay for noninvasive identification of lymph node metastases in T1 colorectal cancer. Gastroenterology. (2021) 161:151–62. doi: 10.1053/j.gastro.2021.03.062
38. Suzuki A, Togashi K, Nokubi M, Koinuma K, Miyakura Y, Horie H, et al. Evaluation of venous invasion by Elastica van Gieson stain and tumor budding predicts local and distant metastases in patients with T1 stage colorectal cancer. Am J Surg Pathol. (2009) 33:1601–7. doi: 10.1097/PAS.0b013e3181ae29d6
Keywords: colorectal cancer, T1, guideline, lymph node metastasis, endoscopic resection
Citation: Tanino F, Yamashita K, Morimoto S, Takehara Y, Yamamoto N, Kamigaichi Y, Nishimura T, Tanaka H, Takigawa H, Urabe Y, Kuwai T, Shimamoto F and Oka S (2024) Comparative prediction of lymph node metastasis in pT1 colorectal cancer among Western and Japanese guidelines. Front. Oncol. 14:1475270. doi: 10.3389/fonc.2024.1475270
Received: 03 August 2024; Accepted: 04 October 2024;
Published: 31 October 2024.
Edited by:
Bjørn Steinar Nedrebø, Haukeland University Hospital, NorwayReviewed by:
Ali Bohlok, Université libre de Bruxelles, BelgiumCopyright © 2024 Tanino, Yamashita, Morimoto, Takehara, Yamamoto, Kamigaichi, Nishimura, Tanaka, Takigawa, Urabe, Kuwai, Shimamoto and Oka. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ken Yamashita, a2VueWFtYTVAaGlyb3NoaW1hLXUuYWMuanA=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.