- Department of Hematology and Rheumatology, The Second Affiliated Hospital of Xiamen Medical College, Xiamen, China
Background: Interstitial lung disease (ILD) is a rare clinical presentation of primary myelofibrosis (PMF).
Case presentation: We report a case of ILD as the main manifestation on admission. A 58-year-old man was diagnosed with PMF owing to worsening anemia following treatment failure for conventional interstitial pneumonia.
Results: Anemia and interstitial pneumonia both significantly improved following treatment with a Janus kinase 2 gene inhibitor. In this report, we discuss the possible mechanisms underlying PMF complicated with ILD.
1 Introduction
Primary myelofibrosis (PMF) is a hematologic malignancy characterized by clonal expansion of one or more myeloid lineages, resulting in excessive hematopoietic cell production (1). Interstitial lung disease (ILD), with its rapid progression and poor prognosis, causes respiratory failure and poses a serious threat to life and health (2). Here, we report a case of PMF with ILD as the main manifestation. Our patient was diagnosed with PMF following conventional interstitial pneumonia treatment. Following treatment with a Janus kinase 2 (JAK2) gene inhibitor, his blood test results and his interstitial pneumonia significantly improved.
2 Case description
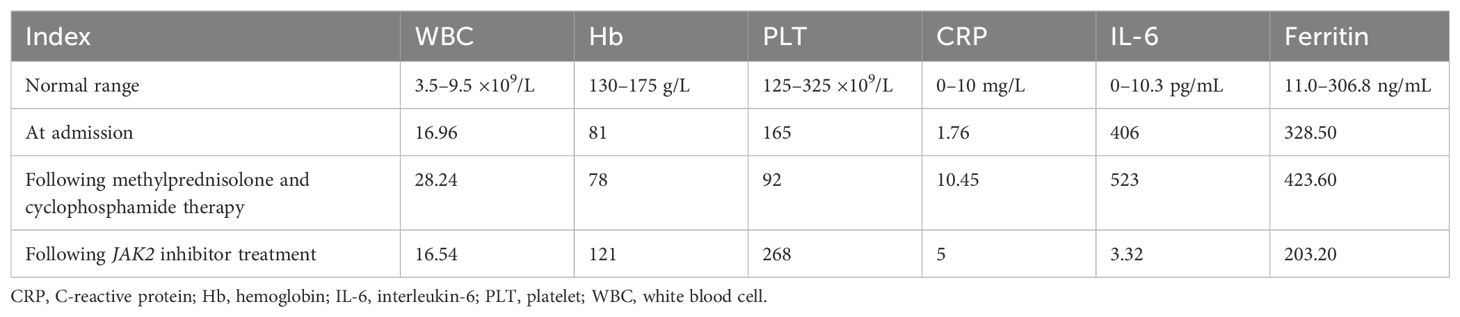
A 58-year-old man presented at our hospital with a 1-year history of cough that had worsened over the previous week. Physical examination findings were as follows: heart rate, 113 beats/min; respiratory rate, 28 breaths/min; blood pressure, 126/72 mmHg; and oxygen saturation (pulse oximetry), 92%. No clinical symptoms of enlarged superficial lymph nodes or liver or spleen symptoms were reported. He had no respiratory-relevant personal or familial medical history. His laboratory examination results were as follows: hemoglobin (Hb), 81.0 g/L [normal range (NR), 130–175 g/L]; white blood cell count, 16.96 × 109/L (NR, 3.50–9.50 × 109/L); platelet count, 165 × 109/L (NR, 125–325 × 109/L); reticulocyte count, 131.98 × 109/L (NR, 20–200 × 109/L); and reticulocyte ratio, 1.12% (NR, 0.3–3.0). The erythrocyte sedimentation rate was increased at 40 (NR, 0–15) mm/h. His serum glutamic oxaloacetic transaminase, serum glutamic pyruvic transaminase, serum γ-glutamyl transpeptidase, serum lactate dehydrogenase, and serum alkaline phosphatase levels were 16.90 IU/L (NR, 15.0–40.0 IU/L), 12.10 IU/L (NR, 9.0–50.0 IU/L), 78 IU/L (NR, 10.0–60.0 IU/L), 307.89 IU/L (NR, 100.0–240.0 IU/L), and 84 IU/L (NR, 45–125 IU/L), respectively. His creatine kinase and creatine kinase isoenzyme levels were within the NR. A sputum culture was negative; however, a sputum smear was positive for Mycobacterium tuberculosis. Serum tests for nine respiratory pathogens (Q fever rickettsial disease, influenza A virus, influenza B virus, respiratory syncytial virus, parainfluenza virus, Mycoplasma pneumoniae, Chlamydia pneumoniae, adenovirus, and Legionella pneumophila) yielded negative results. Polymerase chain reaction tests for coronavirus disease yielded negative results on three separate occasions, and his thyroid function test results were normal. Epstein–Barr virus DNA and cytomegalovirus (CMV) DNA levels were low (<4.0 × 102 copies/mL). Other test results were within the NR, as follows: arterial blood gas, pH 7.38 (NR, 7.35–7.45); partial pressure of oxygen, 59.40 (NR, 80–100) mmHg; partial pressure of carbon dioxide, 36.40 (NR, 35–48 mmHg) mmHg; and blood lactate level, 2.22 (0.5–2.0) mmol/L. Immunoglobulin and complement C4 levels were normal; however, the complement C3 level was reduced at 0.25 (NR, 0.9–1.8) g/L. Antinuclear antibodies, antiphospholipid antibodies, anti-extractable nuclear antibodies, anticyclic citrullinated peptide tests, myositis-specific autoantibodies, antineutrophil cytoplasmic antibodies, and rheumatoid factor were negative.
On admission, high-resolution computed tomography (CT) of the chest showed diffuse ground-glass opacities in the lungs and interstitial pneumonitis (Figures 1A–C). Metagenomic sequencing of the bronchoalveolar lavage fluid sample revealed no viral RNA, viral DNA, bacteria, fungi, mycoplasmas, chlamydia, or parasites. Pathology results in relation to bronchoalveolar lavage fluid showed the following: the color was light red, transparency was slightly turbid, 77% of the total cells were neutrophil cells, lymphocytes comprised 8%, macrophages comprised 9%, and ciliated epithelial cells comprised 6%, with no evidence of infection or malignant cells. A punch biopsy of the lung lesion was also performed, with results showing dilated alveolar spaces, alveolar epithelial hyperplasia, widened alveolar septum, fibroblastic proliferation fibrosis with collagen, and lymphocyte cells as infiltrating cells. With bronchiolar metaplasia, morphological characteristics conformed to interstitial pneumonia (Figure 2).
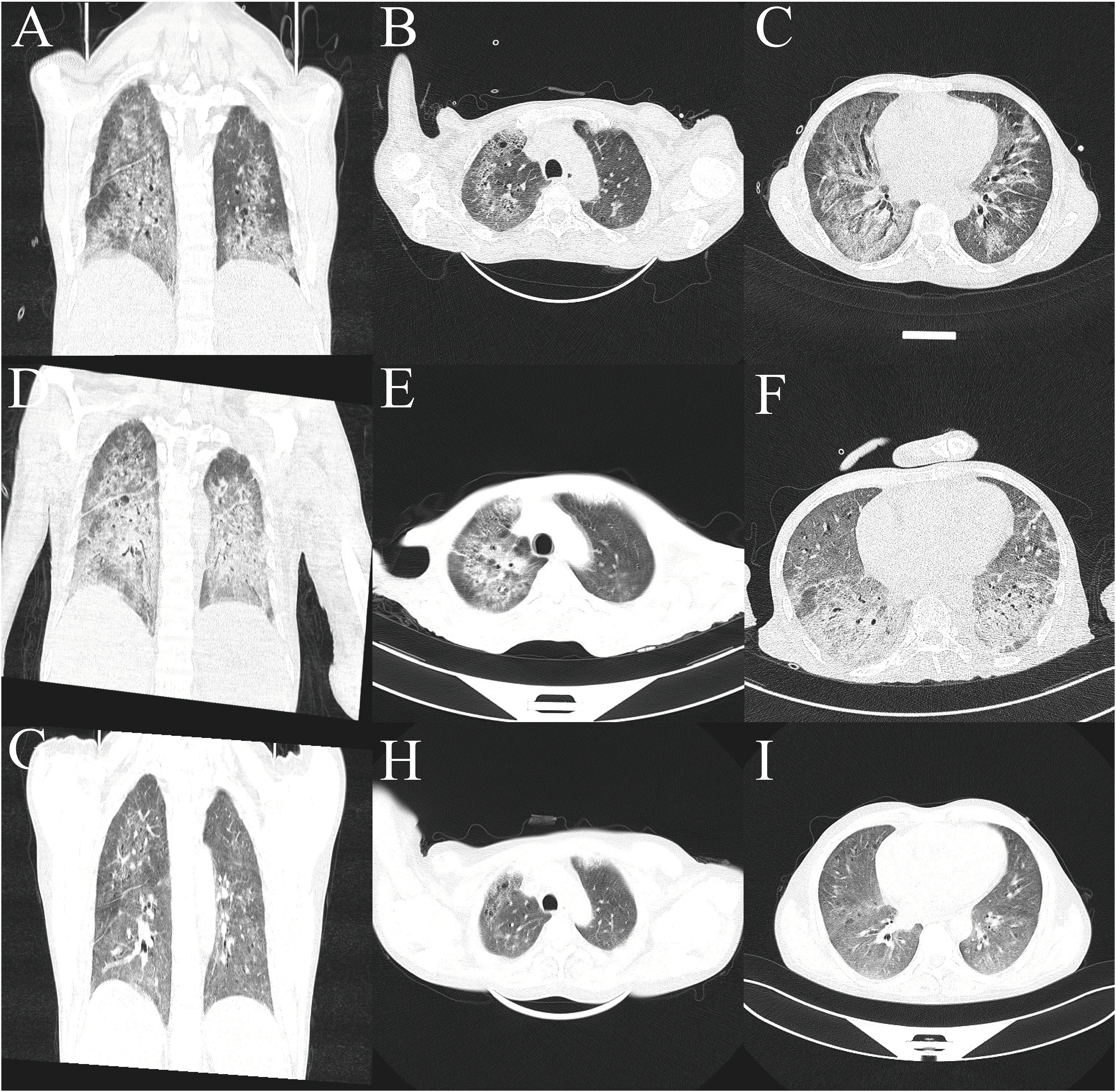
Figure 1. Lung computed tomography. (A–C) At admission. (D–F) Axial view after methylprednisolone and cyclophosphamide therapy. (G–I) After treatment using ruxolitinib, a JAK2 inhibitor.
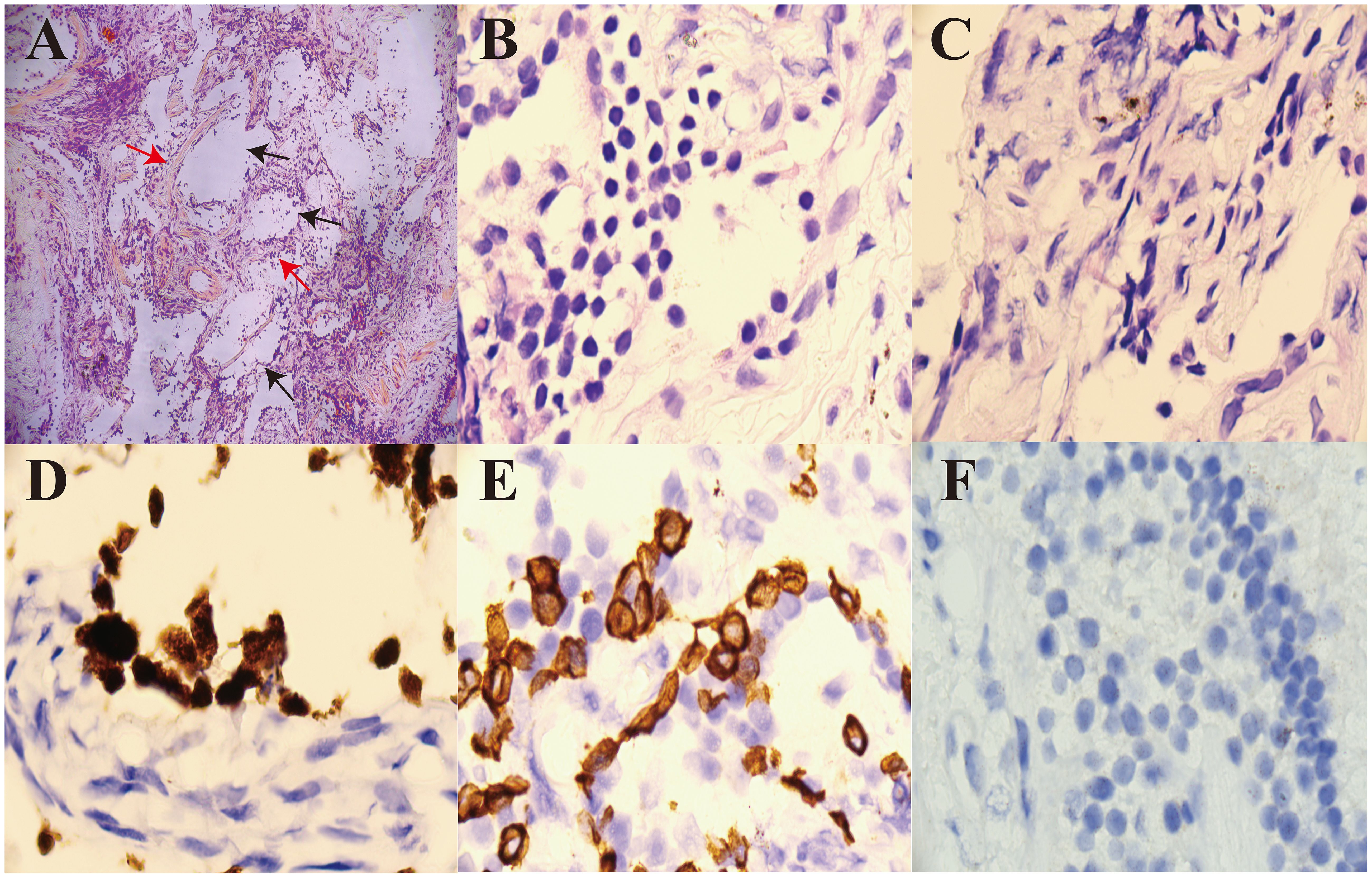
Figure 2. Pathological analysis of a percutaneous dorsal segment of the right lower lobe lung biopsy. (A) H&E staining shows dilated alveolar spaces (black arrow, ×100) and fibroblastic cell proliferation in the interstitium (red arrow, ×100). (B) H&E staining shows that the infiltrating cells were lymphocyte cells (×1,000). (C) H&E staining shows fibroblastic proliferation fibrosis with collagen (×1,000). (D) CK5/6 positive (×1,000). (E) TTF-1 positive (×1,000). (F) Acid-fast staining did not detect any acid-fast bacilli (×1,000). H&E, hematoxylin and eosin.
The patient was diagnosed with ILD and received methylprednisolone (500 mg/day for 3 days and tapering subsequently) and cyclophosphamide (0.4 g per week) combined with gamma globulin (0.4 g/kg per day for 5 days). However, the patient’s wheezing and cough did not improve, and a lung CT scan also indicated there was no improvement (Figures 1D–F). During treatment, his anemia worsened, and his anemia-related examination results were as follows: serum ferritin, 328.50 (NR, 11.0–306.8) ng/mL; serum iron, 32.27 (NR, 11–30) µmol/L; total iron-binding capacity, 38.27 (NR, 45–75) µmol/L; transferrin saturation, 84.32%; serum B12, 574.96 (NR, 180–914) pmol/L; serum folate, 18.76 (NR, 11.8–50.0) nmol/L; and reticulocyte percentage, 2.72% (NR, 0.3–3.0%). Direct and indirect antiglobulin test results were negative. A bone marrow puncture was performed. A peripheral blood smear revealed the presence of teardrop poikilocytes, occasional late erythroblasts, and late myelocytes (Figures 3A, B). Bone marrow morphology results revealed myeloid proliferation, megakaryocytic hyperplasia, and abnormal megakaryocytes (Figure 3C). Flow cytometry results indicated no developmental abnormalities in the bone marrow. Pathological findings from a bone marrow biopsy revealed the presence of myeloproliferative disease, namely, PMF. Immunohistology analysis findings were as follows: negative for CD2, CD5, CD20, PAX5, CD30, cyclin D1, CK, EMA, BCL2, Bcl6, C-myc, and EBER; and positive for CD10 and MUM1, Ki-67 (+50%–60%), and reticular fibers (2+) (Figures 3D–F). Bone marrow metaphase cytogenetics revealed a 46,XY karyotype. Positive JAK2/V617F and TET2 gene mutations were also identified, whereas the BCR-ABL mutation was absent.
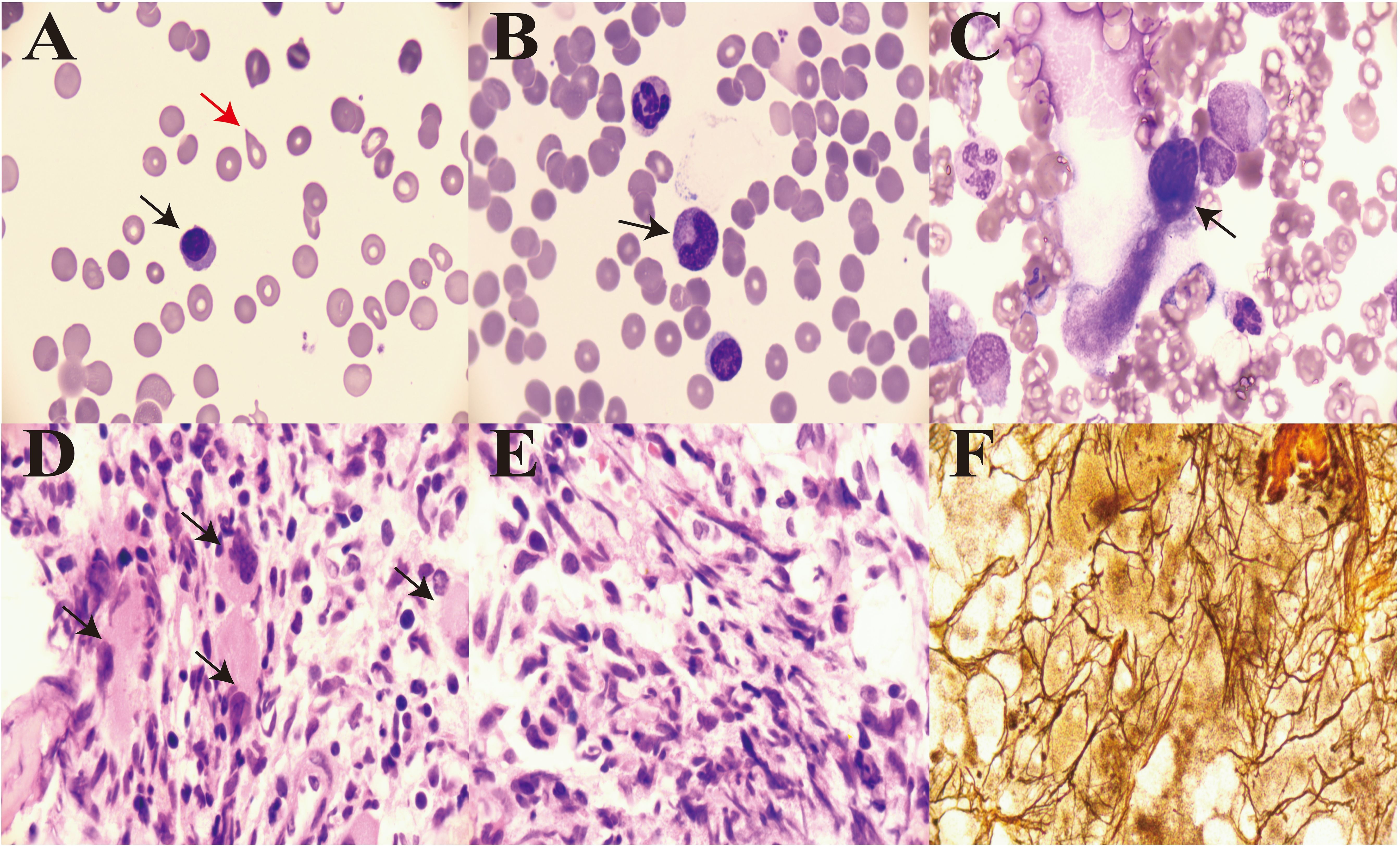
Figure 3. Bone morphology and pathology from a bone marrow biopsy. (A) Teardrop poikilocytes (red arrow) and occasional late erythroblasts in peripheral blood (×1,000, black arrow). (B) Late myelocytes in peripheral blood (×1,000, black arrow). (C) Abnormal megakaryocytes (×1,000, black arrow). (D) Cluster distribution of myeloblasts in marrow (×1,000, black arrow). (E) Diffuse increase in reticular fibers in marrow (×1,000). (F) Reticulin stain showing widespread positivity (×1,000).
Following the diagnosis of PMF, we administered a JAK2 inhibitor (ruxolitinib, 20 mg twice daily). One week later, the size of the lung lesions had improved significantly (Figures 1G–I). His Hb levels gradually increased, and interleukin (IL)-6 levels decreased (Table 1). His condition improved over 2 weeks, and he was discharged. At 12-month follow-up, the patient could engage in physical activities and showed no recurrence.
3 Discussion
This patient presented to the hospital with interstitial pneumonia as the main manifestation on admission. He received glucocorticoids, cyclophosphamide, anti-fibrotic agents, gamma globulin, and anti-infective therapy, but his condition did not improve and became progressively aggravated. Following a diagnosis of myelofibrosis and treatment with JAK2 inhibitors, his ILD improved significantly. This case report focused on the ILD, which is known to be mostly caused by secondary immune system disease, and its pathogenesis results from immune-related factors that cause lung lesions. Treatment typically targets the primary disease causing the ILD (3, 4). Our patient was diagnosed with ILD based on his clinical presentation and pathological lung lesion biopsy findings, as the primary cause was unclear. A diagnosis of ILD was made based on the etiological examination findings shown in Figure 2.
Owing to worsening anemia during treatment, a bone marrow aspiration and a pathological biopsy were performed, and he was diagnosed with PMF. Some cases of hematologic malignancy concomitant with ILD have previously been reported. Liu et al. reported the case of a male patient who had been diagnosed with myeloid neoplasm with secondary interstitial pneumonia (5). Li et al. reported on a patient who had been diagnosed with diffuse large B-cell lymphoma and ILD (6). Zou et al. reported on 321 cases of non-Hodgkin lymphoma presenting with ILD (7). However, reports concerning PMF present in patients with ILD are rare. The mechanism of action of JAK2 inhibitors in treating ILD requires further discussion, and a clear understanding is needed as to why patients with myelofibrosis develop ILD. We first focused on the possible mechanisms related to ILD in patients with myelofibrosis, which is divided into primary and secondary myelofibrosis. Myelofibrosis presents with routine blood abnormalities, abdominal distension owing to spleen enlargement, weight loss, and fatigue, with primary lung disease lesions rarely observed (8). In addition to JAK2/v617F gene positivity in this patient, we also detected TET2 Exon3 and TET2 Exon11 gene mutations. TET2 is essential for innate immune cell function. TET2 is most profoundly expressed during murine macrophage differentiation compared with other TET genes. TET2 is essential for controlling inflammation-inhibited IL-6 expression through recruiting histone deacetylase 2 (HDAC2). TET2 has various mechanisms in neoplastic and immune diseases and downregulates the expression of inflammatory cytokines IL-6 and IL-1β through recruiting HDAC enzymes for histone deacetylation in innate myeloid cells and macrophages (9). TET2-KO macrophages and dendritic cells produce more IL-6. Loss of TET2 in tumor-associated macrophages results in increased expression of inflammatory cytokines. Moreover, TET2 regulates the differentiation of regulatory T cells and smooth muscle cells. TET2 mutations counteract positive immune cell responses, including those of T cells and macrophages (10). The three primary IL-6 signaling pathways include classic signaling via the membrane-bound IL-6 receptor, trans-signaling using a soluble IL-6 receptor, and trans-presentation for antigen presentation (11). The relationship between IL-6 and ILD has previously been established (12). Stancil et al. identified the genetic or pharmacological targeting of the IL-6-related signaling pathway, leading to a reduction in fibrotic lung remodeling (13). From this perspective, the genetic mutations associated with PMF, especially TET2, may be one mechanism through which TET2 mutation induces increased IL-6, leading to the immune-mediated inflammatory syndrome of pulmonary ILD.
A JAK2 inhibitor (ruxolitinib) was initially used in the treatment of JAK2/V617F-positive myeloproliferative disorders. The treatment of ILD includes using JAK inhibitors such as tofacitinib (mostly JAK1/3 inhibitors), which has achieved a certain clinical efficacy. JAK2 inhibitors are considered to affect the release of IL-6 or inhibit its production, and a literature review was undertaken based on this consideration. Silva-Carmona reported that two patients with ILD were successfully treated using a JAK2 inhibitor, namely, ruxolitinib (14). Lescoat et al. demonstrated that JAK inhibitors had anti-inflammatory properties, in part, based on results showing the activation state of pro-inflammatory macrophages and the downregulation of interferon β and IL-6 expression (15). Mima et al. reported that IL-6 increased inflammatory cytokine production and hypertrophic marker expression in primary mouse chondrocytes by activating the JAK2/STAT3 pathway (16). Li et al. generated JAK2(V617F)-expressing murine macrophages and reported that p-STAT3 levels were associated with increased IL-6 production (17). Cheng et al. suggested that IL-6-induced vascular endothelial growth factor expression and angiogenesis were inhibited through the JAK2/STAT3 pathway in rheumatoid arthritis, providing novel insights into antiangiogenic activity in rheumatoid arthritis (18). We concluded that when JAK2 inhibitors are used to treat myelofibrosis, they also inhibit IL-6-induced JAK2-related signal transduction in the lungs to achieve therapeutic effects. Compared with other treatments for ILDs such as hormones and immunosuppressants, the curative effect of ruxolitinib was more significant than that of other treatments, including prednisone, cyclophosphamide, anti-fibrotic agents, and gamma globulin. Table 1 shows the results of the hemogram and inflammation index following treatment with ruxolitinib, which was consistent with our patient’s clinical manifestations.
We hypothesized that TET2 mutation would lead to an increased IL-6 level, activating the JAK signaling pathway and leading to an intense inflammatory reaction. This may explain interstitial pneumonia in myelofibrosis and how treatment with JAK2 inhibitors to block the IL-6-mediated JAK2 downstream signaling pathway also blocked the final inflammatory reaction in the lung.
The JAK2 inhibitor ruxolitinib, used to treat PMF, has been shown to be effective in alleviating symptoms, diminishing spleen size, and improving the survival rates of patients with JAK2 mutations (19). However, the variability in patient responses highlights the necessity for personalized treatment plans and for the exploration of combination therapies to ensure optimal results.
4 Conclusion
PMF can involve different systems other than the hematological system, as in our case, which involved the respiratory system. Treatment with a JAK2 inhibitor may act as a “two birds with one stone” approach to treat patients with PMF and ILD. A limitation of our case report is that only a single case is reported. Further case reports involving larger patient numbers are needed to validate our findings.
Data availability statement
The datasets presented in this article are not readily available because all data are in manuscript. Requests to access the datasets should be directed to eGlhb3BwMDAyNkAxNjMuY29t.
Ethics statement
The studies involving humans were approved by Ethical Review Approval of the Medical Ethics Committee of The Second Affiliated Hospital of Xiamen Medical College Approval No: 2024130. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
PX: Writing – original draft, Writing – review & editing, Conceptualization, Investigation, Supervision. ZD: Conceptualization, Writing – review & editing. QW: Investigation, Writing – review & editing. JS: Resources, Writing – review & editing. YC: Project administration, Writing – review & editing. YL: Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Natural Science Foundation of Fujian (approval number 2023J011640).
Acknowledgments
We would like to acknowledge the hard and dedicated work of all the staff. We appreciate the patient’s self-dedication.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Spivak JL. Are polycythemia vera, essential thrombocytosis, and primary myelofibrosis 1, 2, or 3 diseases? Leukemia. (2021) 35:1890–3. doi: 10.1038/s41375-021-01254-w
2. Guler SA, Scheschkowski T, Renner A, Kampf L, Gasser M, Maurer B. How we do it: interdisciplinary diagnosis and management of patients with interstitial lung disease and connective tissue disease. Chest. (2024) 166:352–61. doi: 10.1016/j.chest.2024.02.045
3. Maher TM. Interstitial lung disease: A review. JAMA. (2024) 331:1655–65. doi: 10.1001/jama.2024.3669
4. Damiani A, Orlandi M, Bruni C, Bandini G, Lepri G, Scaletti C, et al. The role of lung biopsy for diagnosis and prognosis of interstitial lung disease in systemic sclerosis: a systematic literature review. Respir Res. (2024) 25:138. doi: 10.1186/s12931-024-02725-1
5. Liu G, Ouyang R, Chen P, Liu T, Zhou L, Shen C. Myeloid neoplasm with the abnormality of PDGFRB gene secondary interstitial pneumonia: A case report. Zhong Nan Da Xue Xue Bao Yi Xue Ban. (2020) 45:345–8. doi: 10.11817/j.issn.1672-7347.2020.180709
6. Li S, Sun Y, Shao C, Xu K, Huang H. Diffuse large B-cell lymphoma in a patient with dermatomyositis-associated interstitial lung disease: A case report. Thorac Cancer. (2019) 10:2035–9. doi: 10.1111/1759-7714.13171
7. Zou W, Zhang J, Li Y, Zhang Z, Yang R, Yan Y, et al. Interstitial lung disease presents with varying characteristics in patients with non-Hodgkin lymphoma undergoing rituximab-containing therapies. Ann Hematol. (2024). doi: 10.1007/s00277-024-06013-2
8. Tefferi A. Primary myelofibrosis: 2023 update on diagnosis, risk-stratification, and management. Am J Hematol. (2023) 98:801–21. doi: 10.1002/ajh.26857
9. Cong B, Zhang Q, Cao X. The function and regulation of TET2 in innate immunity and inflammation. Protein Cell. (2021) 12:165–73. doi: 10.1007/s13238-020-00796-6
10. Jiang S. Tet2 at the interface between cancer and immunity. Commun Biol. (2020) 3:667. doi: 10.1038/s42003-020-01391-5
11. Swaroop AK, Negi P, Kar A, Mariappan E, Natarajan J, Namboori PKK, et al. Navigating IL-6: From molecular mechanisms to therapeutic breakthroughs. Cytokine Growth Factor Rev. (2024) 76:48–76. doi: 10.1016/j.cytogfr.2023.12.007
12. Jia G, Ramalingam TR, Heiden JV, Gao X, DePianto D, Morshead KB, et al. An interleukin 6 responsive plasma cell signature is associated with disease progression in systemic sclerosis interstitial lung disease. iScience. (2023) 26:108133. doi: 10.1016/j.isci.2023.108133
13. Stancil IT, Michalski JE, Hennessy CE, Hatakka KL, Yang IV, Kurche JS, et al. Interleukin-6-dependent epithelial fluidization initiates fibrotic lung remodeling. Sci Transl Med. (2022) 14:eabo5254. doi: 10.1126/scitranslmed.abo5254
14. Silva-Carmona M, Vogel TP, Marchal S, Guesmi M, Dubus JC, Leroy S, et al. Successful treatment of interstitial lung disease in STAT3 gain-of-function using JAK inhibitors. Am J Respir Crit Care Med. (2020) 202:893–7. doi: 10.1164/rccm.201906-1204LE
15. Lescoat A, Lelong M, Jeljeli M, Piquet-Pellorce C, Morzadec C, Ballerie A, et al. Combined anti-fibrotic and anti-inflammatory properties of JAK-inhibitors on macrophages in vitro and in vivo: Perspectives for scleroderma-associated interstitial lung disease. Biochem Pharmacol. (2020) 178:114103. doi: 10.1016/j.bcp.2020.114103
16. Mima Z, Wang K, Liang M, Wang Y, Liu C, Wei X, et al. Blockade of JAK2 retards cartilage degeneration and IL-6-induced pain amplification in osteoarthritis. Int Immunopharmacol. (2022) 113:109340. doi: 10.1016/j.intimp.2022.109340
17. Li R, Sun N, Chen X, Li X, Zhao J, Cheng W, et al. JAK2(V617F) mutation promoted IL-6 production and glycolysis via mediating PKM1 stabilization in macrophages. Front Immunol. (2020) 11:589048. doi: 10.3389/fimmu.2020.589048
18. Cheng WX, Huang H, Chen JH, Zhang TT, Zhu GY, Zheng ZT, et al. Genistein inhibits angiogenesis developed during rheumatoid arthritis through the IL-6/JAK2/STAT3/VEGF signalling pathway. J Orthop Translat. (2020) 22:92–100. doi: 10.1016/j.jot.2019.07.007
Keywords: interstitial pneumonia, Janus kinase 2, primary myelofibrosis, ruxolitinib, ten-eleven translocation 2
Citation: Xiao P, Dong Z, Wang Q, Su J, Chen Y and Lin Y (2024) Case report: Successful use of ruxolitinib to treat interstitial pneumonia as an unusual primary presentation in primary myelofibrosis—two birds with one stone. Front. Oncol. 14:1475036. doi: 10.3389/fonc.2024.1475036
Received: 05 August 2024; Accepted: 06 November 2024;
Published: 26 November 2024.
Edited by:
Shinobu Matsuura, Boston University, United StatesReviewed by:
Sunita Sharma, Lady Hardinge Medical College and Associated Hospitals, IndiaShiv Singh, Boston University, United States
Copyright © 2024 Xiao, Dong, Wang, Su, Chen and Lin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Pingping Xiao, eGlhb3BwMDAyNkAxNjMuY29t
 Pingping Xiao
Pingping Xiao Zhigao Dong
Zhigao Dong Qingqing Wang
Qingqing Wang Yongquan Chen
Yongquan Chen