- 1Department of Ultrasound Medicine, The First Affiliated Hospital of Ningbo University, Ningbo, Zhejiang, China
- 2Department of Interventional Radiology, The First Affiliated Hospital of Ningbo University, Ningbo, Zhejiang, China
Background: Sub-pleural pulmonary lesions (SPLs) can be diagnosed by percutaneous needle biopsy (PNB) guided by both computed tomography (CT) and ultrasound (US). This investigation aims to compare the diagnostic accuracy and safety between US- and CT-guided PNB for SPLs.
Methods: This retrospective study analyzed SPL patients who underwent CT- or US-guided PNB in our hospital between January 2022 to January 2023. Furthermore, the technical success rates, duration of procedure, diagnostic yield, diagnostic accuracy, pneumothorax rates, and hemoptysis rates were compared between the 2 groups. Pneumothorax risk factors were assessed via the univariate and multivariate logistic regression tests.
Results: The data indicated that 213 patients who underwent CT- (n = 108) or US-guided (n = 105) PNB diagnosis had SPLs at the final diagnosis. Furthermore, both groups indicated similar operation times (20.1 ± 8.1 min vs. 19.9 ± 6.9 min, p = 0.793). The diagnostic accuracy and yield of the US group were 100% and 64.8%, respectively, whereas those of the CT group were 99.1% and 72.2%, respectively. Moreover, no significant differences were observed in diagnostic accuracy (p = 1.000) and diagnostic yield (p = 0.561) between the 2 groups. The CT group indicated markedly higher rates of chest tube insertion (6.5% vs. 0.0%, p = 0.014) and pneumothorax (24.1% vs. 1.9%, p = 0.001) than the US group. However, the hemoptysis rates were comparable between the 2 groups (2.7% vs. 2.9%, p = 1.000). In addition, CT guidance was the independent risk factor of pneumothorax (p = 0.003).
Conclusions: In summary, this research indicated that both US- and CT-guided PNB have high diagnostic accuracy for SPLs. However, US guidance may provide better safety than CT guidance.
Introduction
Peripheral pulmonary lesions are most frequently diagnosed via percutaneous needle biopsy (PNB) (1–3). Computed tomography (CT) is employed as guidance equipment because of the spatial resolution. It has been observed that CT-guided PNB is an efficient procedure with a high diagnostic rate (92 - 98%) (4); however, in addition to radiation exposure, it has also been linked with high complication rates. Moreover, 20 - 22% of cases suffer from the incidence of post-procedural pneumothorax (4).
Ultrasound (US)-guided PNB is usually used for diagnosing solid organ lesions, such as in the liver, prostate, thyroid, kidney, and breast (5–7). For pulmonary lesions, US-guided PNB is not commonly used because it is influenced by gas. However, the US-guided PNB can be used for the sub-pleural pulmonary lesions (SPLs) strickly adherent to pleural surface because these lesions are not or slightly influenced by the gas. The US-guided PNB has advantages over CT guidance, such as real-time biopsy needles and target lesion visualization during the procedure and the lack of radiation exposure (8).
Therefore, this investigation aimed to compare the diagnostic accuracy and safety between US- and CT-guided PNB for SPLs.
Materials and methods
Study design
This retrospective research was authorized by the ethical board of the First Affiliated Hospital of Ningbo University. Because of the retrospective nature of this study, the requirement of informed consent was waived. This investigation included SPL patients who underwent CT- or US-guided PNB in our hospital between January 2022 to January 2023. The inclusion criteria included patients (a) with SPLs strictly adherent to pleural surface, (b) with solid SPLs, and (c) with a high risk for lung cancer based on clinical/radiology characteristics (3). The exclusion parameters were: (a) patients who underwent PNB previously, (b) SPLs that reduced in size during follow-up, (c) SPLs that were completely blocked by the bone, and (d) patients with chronic cardiac, renal, pulmonary, or coagulatory disorders.
CT-guided PNB
The 16 Slice CT (Siemens, Berlin, Germany) was employed for CT-guided PNB. The CT-guided PNB was performed by 2 radiologists with more than 5 and 10 years of experience for lung biopsy. The set parameters were: 100 mA tube current, 120 kV tube voltage, 0.6 second gantry rotation time, 2 mm thickness, and 1.1 pitch. The patient’s position and needle paths were based on the lesion’s location (Figure 1A). During the procedure, the co-axial technique was employed. The lesion samples were harvested by inserting A 17G outer needle (DuoSmart™, Modena, Italy) into the lesions, followed by the insertion of an 18G inner semi-automatic core needle (Wego™, Weihai, China) via the outer needle (Figure 1B). From each lesion, more than 2 samples were acquired, which were then preserved in 10% formaldehyde for pathological assessment. In addition, the cell blocks were also prepared for cytology assessment. The biopsy procedure-related complications were assessed by a repeat CT scan. The histo-cytological diagnoses were made by two pathologists with 5 and 10 years expertise in lung cancer.
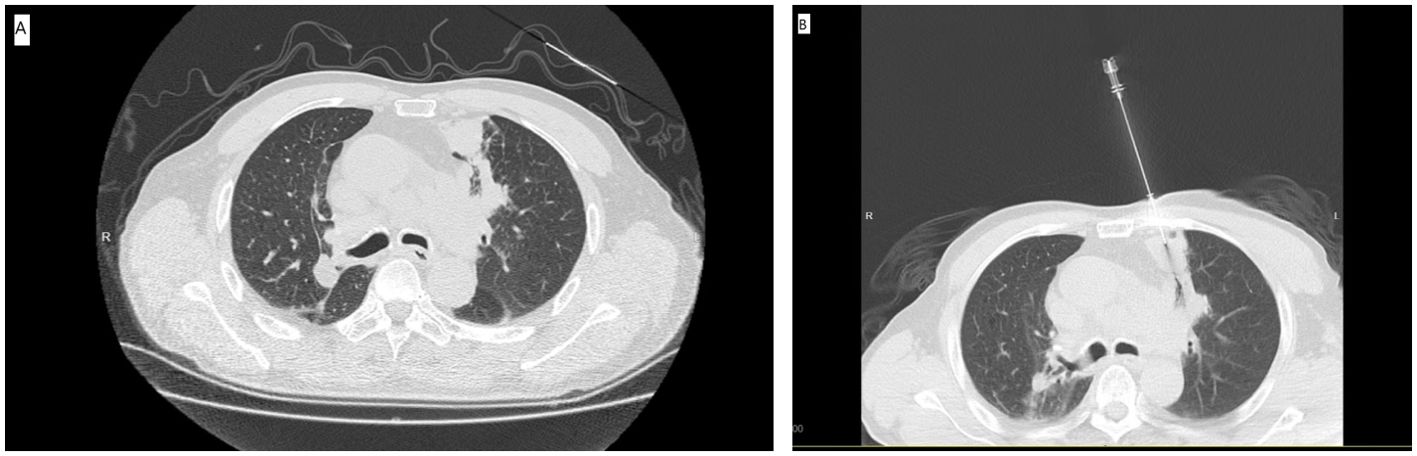
Figure 1. (A) The preoperative CT showed the SPL at left upper lobe. (B) The CT-guided PNB procedure.
Ultrasound-guided PNB
For the US-guided PNB, a US system (Philips, EPIQ7) with the convex array probe C5-1 was routinely employed. The US-guided PNB was performed by 2 radiologists with more than 5 and 10 years of experience for lung biopsy. The parameters of the US were: (a) depth: 10-13 cm; (b) time gain compensation: time gain compensation curve is adjusted for the shape of “|” or “”; (c) focus pointe: the focus pointe was set in the midfield zone. The tissue harmonic imaging was activated. The probe was equipped with a holed guide for needle insertion of various angle selections based on the lesions’ size and locations. For the small lesions (1-2 cm), we used a high frequency probe (eL18-4) to guide the PNB because the high frequency probe could provide a high image resolution. The patient’s position was decided in advance via the safest and shortest approach to visualize the US probe’s movement (Figure 2A). The biopsy needle path toward the lesion was visualized in real time (Figure 2B). The PNB procedure was same as the that for the CT group. The biopsy procedure-related complications were assessed by a chest CT scan. The histo-cytological diagnoses were made by two pathologists with 5 and 10 years expertise in lung cancer.
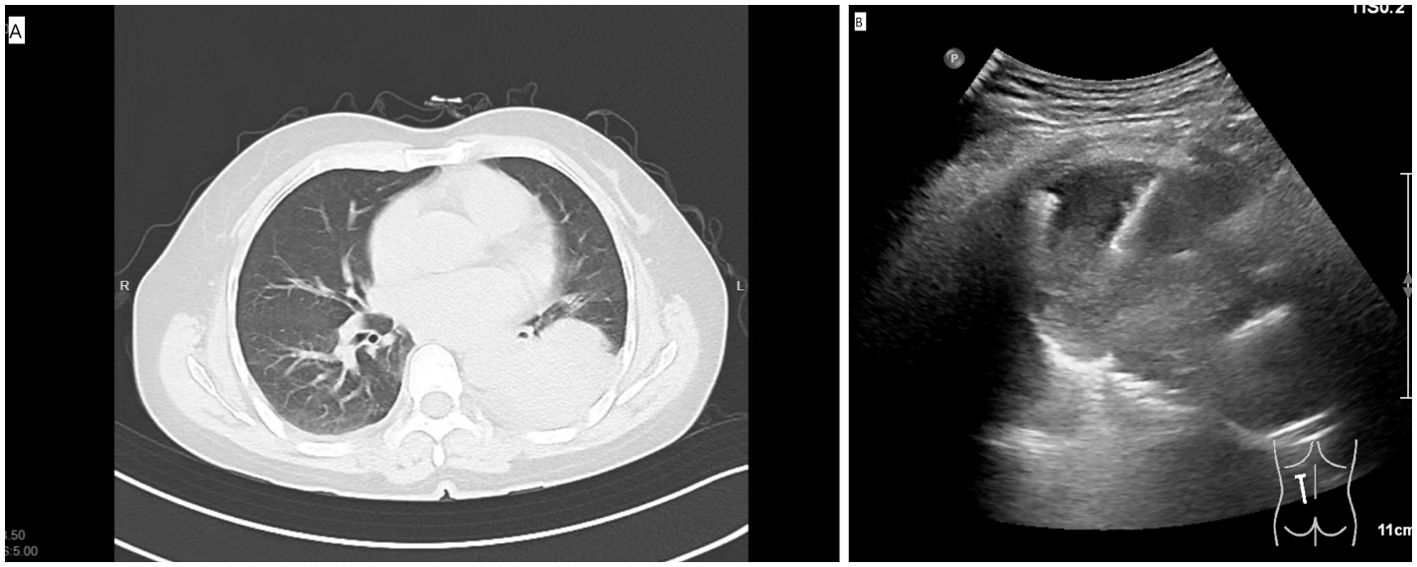
Figure 2. (A) The preoperative CT showed the SPL at left lower lobe. (B) The US-guided PNB procedure.
Assessment
The PNB-based diagnoses can be categorized into 4 types: (a) Suspected malignancy (described as atypical cells suspected of indicated malignancy) (9), (b) Malignancy, (c) Non-specific benignity (defined as benign pathology features that existed through and did not suffice for a formal diagnosis) (9), and (d) Specific benignity (described as lesions that were related to specific infectious diseases or benign tumors) (9). The PNB-based specific benignity and malignancy can be considered as the final diagnoses. However, the PNB-based non-specific benignity and suspected malignancy should be assessed further, such as by repeat biopsy, surgical resection, or CT follow-up. If the lesion size is reduced (≥ 20%) during the follow-up or is maintained (< 20%) for at least 1 year, it could be accepted as benignity (9).
At the final diagnosis, when PNB-based malignancy/suspected malignancy was confirmed as malignant the results were considered true positive (1). Whereas, when PNB-based benignity was confirmed as benignity at the final diagnosis the results were considered true negative (1). Diagnostic accuracy = (true positive + true negative)/all cases. Diagnostic yield = (PNB-based malignancy + PNB-based specific benignity)/all cases.
The PNB-related complications were classified as minor and major complications according to the Society of Cardiovascular & Interventional Radiology guidelines (10).
Statistical analyses
The not normally distributed and normally distributed continuous data were compared via the Mann-Whitney U test and the independent sample t-test, respectively. χ2/Fisher exact test was carried out to compare categorical data. Pneumothorax risk factors were assessed via univariate and multivariate logistic regression tests. The variables which exhibited a p-value < 0.1 in univariate analyses would be included in the multivariate analysis. The subgroup analyses were conducted based on the patients with lung nodules. SPSS 16.0 (SPSS, Chicago, Illinois, USA) was employed for all the statistical measurements and statistically, the p-value of < 0.05 was deemed as the significance threshold.
Results
Patients
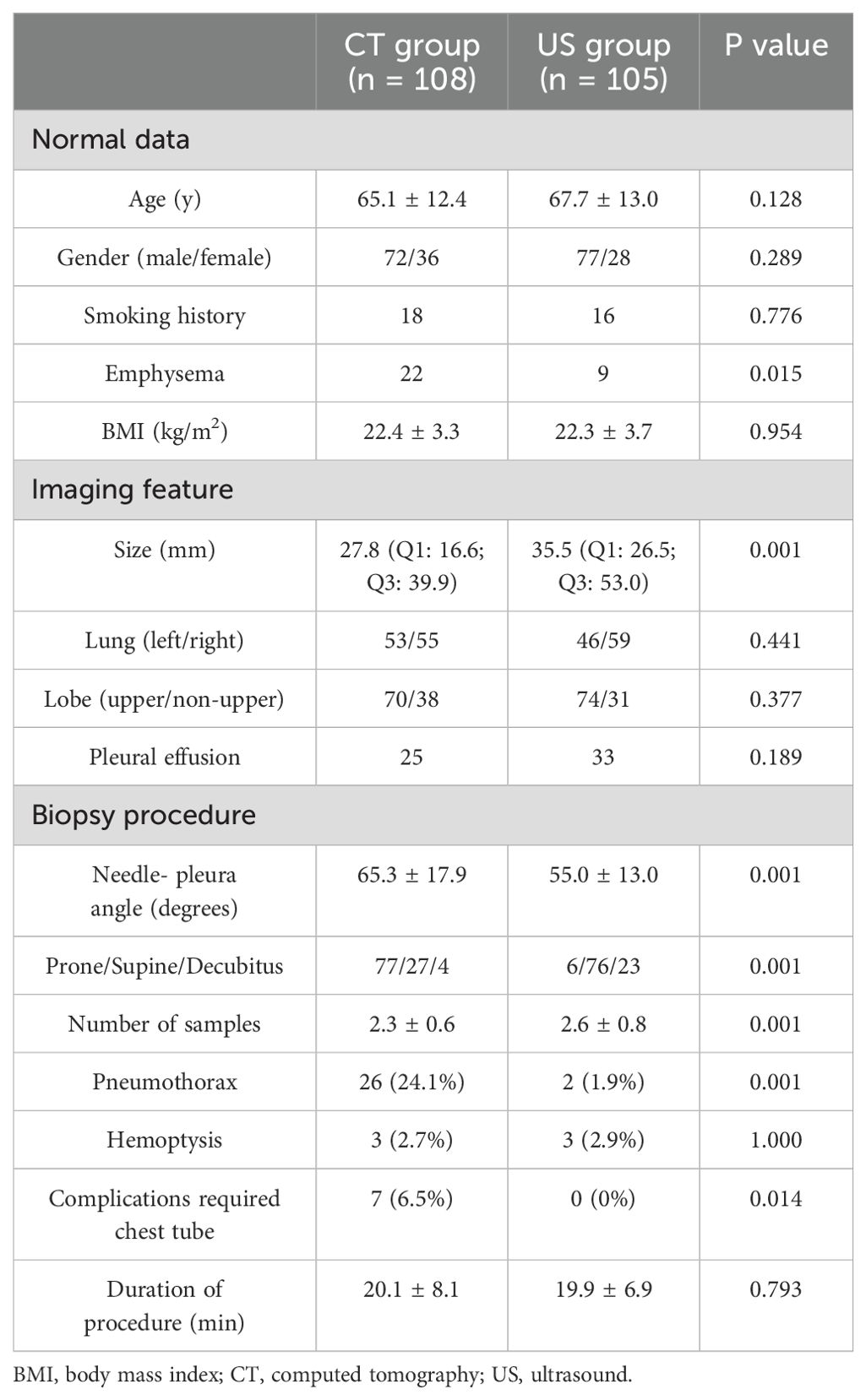
This research included 213 patients who underwent CT- (n = 108) or US-guided (n = 105) PNB diagnosis and had SPLs at the final diagnosis (Table 1). Furthermore, both groups indicated similar operation times (20.1 ± 8.1 min vs. 19.9 ± 6.9 min, p = 0.793), respectively.
Diagnostic performance
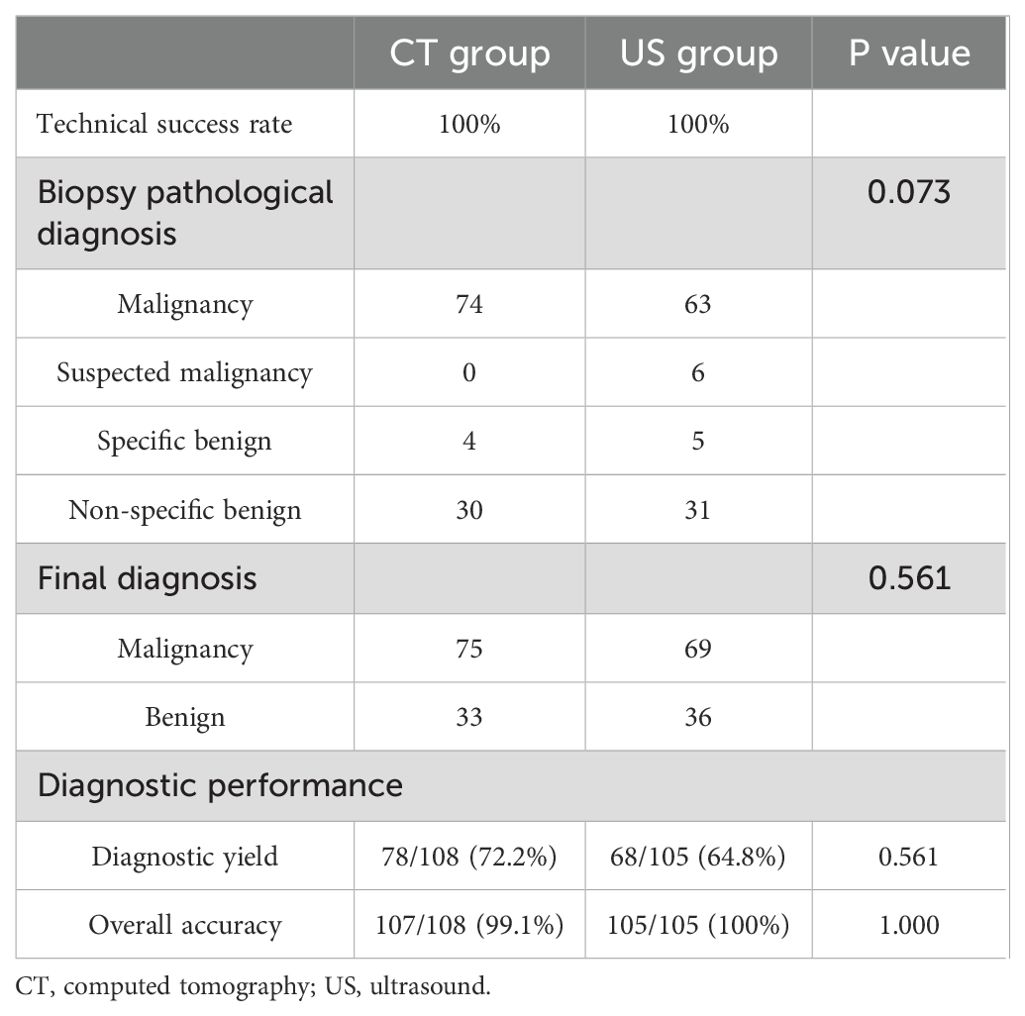
In the CT group, the PNB-based diagnoses included specific benignity (n = 4), malignancy (n = 74), and non-specific benignity (n = 30). Whereas the final diagnoses included benignity (n = 33) and malignancy (n = 75). Supplementary Table indicates the details of the final diagnoses. The diagnostic accuracy and yield were 99.1% and 72.2%, respectively (Table 2).
In the US group, the PNB-based diagnoses included specific benignity (n = 5), suspected malignancy (n = 6), malignancy (n = 63), and non-specific benignity (n = 31). The final diagnoses included benignity (n = 36) and malignancy (n = 69). Supplementary Table indicates the details of the final diagnoses. The diagnostic accuracy and yield were 100% and 64.8%, respectively (Table 2).
The rates of diagnostic accuracy and yield were comparable in both groups (p = 0.561 and 1.000).
Complications
In the CT group, 26 patients (24.1%) experienced pneumothorax, while 3 patients (2.7%) suffered hemoptysis. Furthermore, of 26 patients, 7 required chest tube insertion. In the US group, 2 patients (1.9%) experienced pneumothorax, 3 (2.9%) experienced hemoptysis, and none required chest tube insertion. In comparison with the US group, the CT group indicated markedly increased rates of chest tube insertion (p = 0.014) and pneumothorax (p = 0.001), whereas the hemoptysis rates were comparable in both groups (p = 1.000, Table 1). In the CT group, 19 patients were classified as minor complication and 7 patients were classified as major complication. In the US group, all of the 5 patients were classified as minor complication.
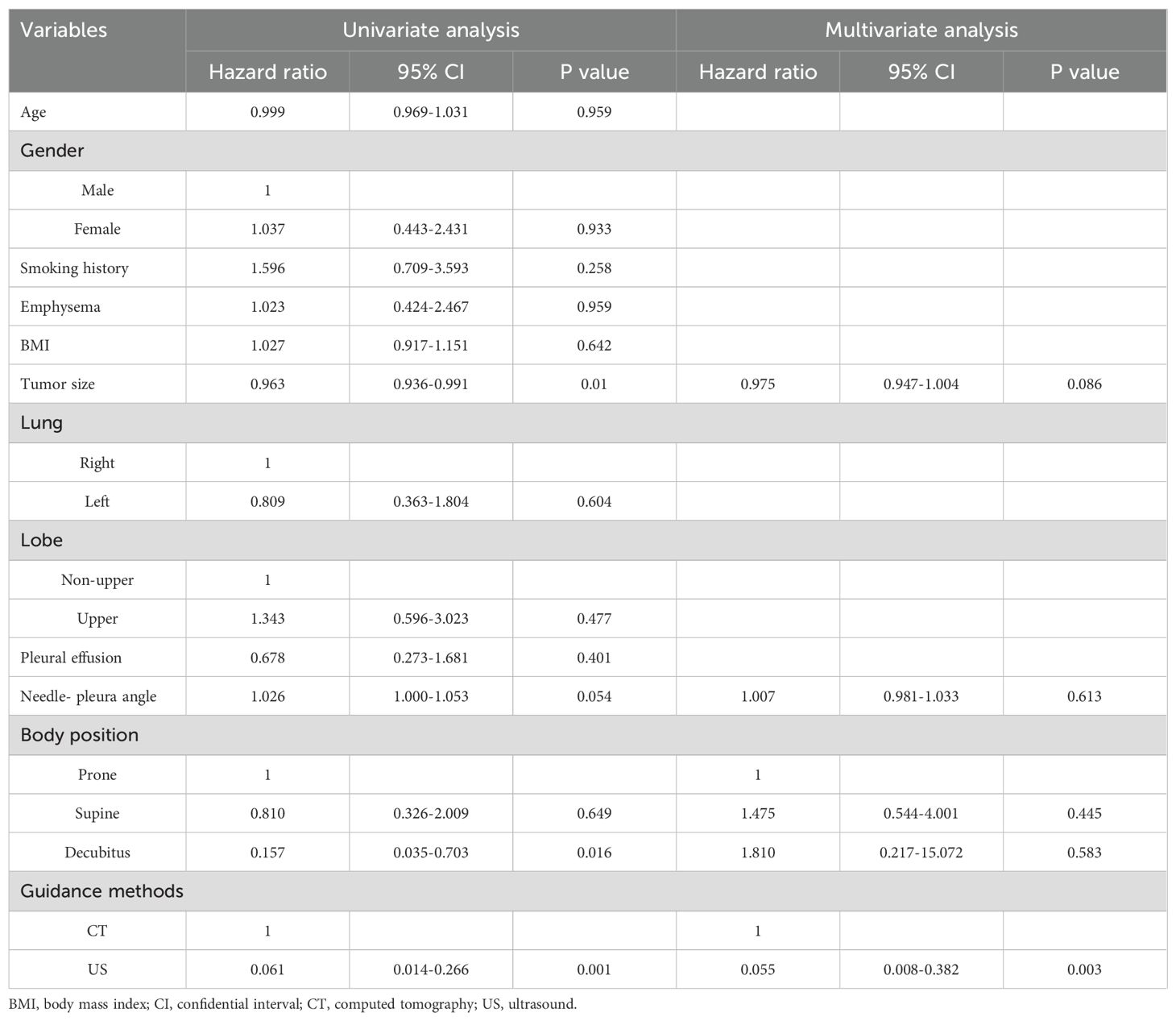
Table 3 shows the logistic analyses of pneumothorax. In the univariate analysis, the smaller lesion size (p = 0.01), larger needle-pleura angle (p = 0.054), decubitus position (p = 0.016), and CT guidance (p = 0.001). When these factors were included into the multivariate analysis, the independent risk factor was assessed to be CT guidance (p = 0.003).
Subgroup analyses
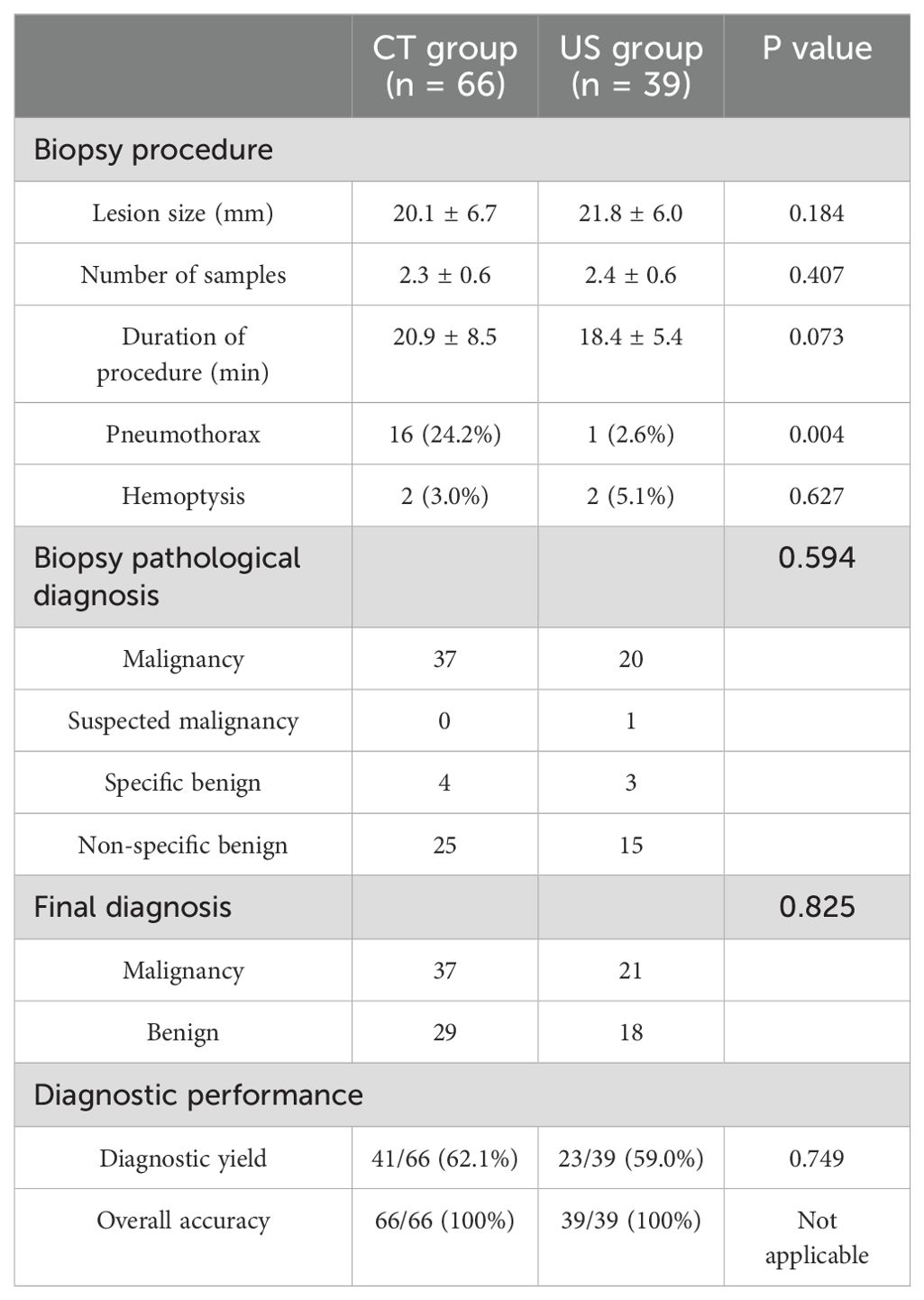
Table 4 shows the subgroup analyses based on the lung nodules. The results indicated comparable diagnostic yield (62.1% vs. 59.0%, p = 0.749) and accuracy (100% vs. 100%) between CT and US groups. Furthermore, the incident rate of pneumothorax was markedly higher in the CT group than in the US group (24.4% vs. 2.6%, p = 0.004), while that of the hemoptysis was comparable in both the groups (3.0% vs. 5.1%, p = 0.627).
Discussion
Currently, PNB is the primary diagnostic procedure for subpleural or peripheral lung lesions. PNB not only differentiates benign and malignant lesions but can also provide samples for molecular analyses, which can guide molecular target therapies (11–13). CT-guided PNB has been used for more than 30 years (14). However, CT-guided PNB has the limitation of lack of real-time monitoring. Some researchers used the C-arm cone-beam CT (CBCT) to achieve real-time guidance during the PNB procedure (15–17). Ren et al. (16) indicated that CBCT-guided PNB has better performance than CT fluoroscopy-guided PNB for small lung lesions radiation dose and complications. However, CBCT radiation affects both patients and operators.
US-guided PNB is also a commonly used method for diagnosing the peripheral lung adherent to pleural surface or pleural lesion without radiation (17–19). Furthermore, US has the advantage of real-time visualization of the target lesion. Jarmakani et al. (17) showed that US guidance could obtain more adequacy sample than CT guidance (98% vs. 87%, P = 0.02). However, Jarmakani study used both fine and core needles and this may cause the bias of the results. Yamamoto et al. (19) found that US guidance could obtain significantly higher diagnostic rate than CT guidance for lesions > 40 mm (94.1% vs. 70.6%, P = 0.009). However, for the smaller lesions (< 40 mm), the US-guided and CT-guided PNB showed similar diagnostic rates (90% vs. 86.8%) (19). However, Yamamoto study contained both pulmonary and pleural lesions.This study compared the clinical effect between US- and CT-guided PNB for SPLs. Compared to the previous studies, the present study only focused on the pulmonary lesions and only used the core needle for biopsy. Both groups indicated high and similar diagnostic yield (72.2% vs. 64.8%, p = 0.561) and accuracy (99.1% vs. 100%, p = 1.000). Furthermore, the rates of diagnostic accuracy in US- and CT-guided PNB were more than their previously reported rates (84 - 96%) and (77 - 96%), respectively for SPLs (20–22). The high diagnostic accuracy rates in this study might be because of the co-axial technique employed. The co-axial technique was utilized to acquire adequate samples to achieve a high diagnostic yield and accuracy.
This investigation also indicated notably higher pneumothorax and chest tube insertion rates of the CT group than the US group. Furthermore, the independent risk factor of pneumothorax was identified to be CT guidance. CT-guided PNB is performed blindly, while US-guided PNB of lesions can be performed with real-time visualization. US-guided PNB is advantageous as it can avoid normal lungs from the lesion with respiratory movement by visualization and vessels by color Doppler imaging. Yamamoto et al. (19) reported a substantially higher overall complication rate in the CT group than in the US group (24.3% vs. 3.3%, p < 0.001). These findings were in consistent with our findings. In addition, they also indicated that the US group pneumothorax rate was 0% (19). Overall, these data suggest that US-guided PNB is a safer technique than CT-guided PNB.
In this study, the CT group showed significantly higher rate of emphysema and smaller lesion size than US group. However, these factors were not associated with pneumothorax after univariate and multivariate logistic regression tests. Similarly, some previous studies also did not show that emphysema and lesion size were associated with pneumothorax (1, 2, 23).
Here, it was found that the hemoptysis incident rates in US- and CT-guided PNB were low and similar (2.7% vs. 2.9%, p = 1.000). This might be because the included lesions were SPLs and the needle seldom touches the intra-pulmonary vessels during PNB for SPLs.
The subgroup analyses indicated that both US- and CT-guided PNB provided high diagnostic accuracy for sub-pleural lung nodules. However, the US-guided PNB guidance indicated better safety than CT-guided PNB, suggesting that US guidance should be considered initially for performing PNB for sub-pleural lung nodules. In addition, to improve the technical reliability of the US-guided PNB for lung nodules, it is advisable the use of a dedicated probe, US transducers with a central hole for needle passage (24).
However, US-guided PNB for SPLs also has some limitations. Although US has the advantage of real-time visualization of the target lesion, US is not an accurate imaging method for characterizing peripheral lung lesions compared to CT scans. Only peripheral consolidations that are strictly adherent to the parietal pleural surface can be imaged because the interposition of even a few millimeters of air is able to block US signal, thus hiding even very big space-occupying lesions. In addition, the acoustic barrier represented by the bony structures of the thoracic cage reduces the visible pleural surface to 70%. As a result, US cannot replace chest CT in the examination of the whole lung.
There are certain limitations of this study. Firstly, this is a retrospective study; therefore, some baseline data, such as emphysema, lesion size, needle-pleura angle, position, and number of samples, were unbalanced between the 2 groups. Although these data did not interfere with the diagnostic accuracy and complication, the selection bias does exist. Secondly, it is single-center research. Further multi-center, randomized controlled trials are required. Thirdly, US-guided PNB is not suitable for central pulmonary lesions and peripheral lesions not adherent to pleural surface or lesions adherent to pleural surface but covered by the rib cage, therefore, its use is limited.
Conclusion
In summary, this research indicated that both US- and CT-guided PNB have high diagnostic accuracy for SPLs. However, US guidance may provide better safety than CT guidance.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by The First Affiliated Hospital of Ningbo University. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because this is a retrospective study.
Author contributions
YZ: Data curation, Writing – original draft, Writing – review & editing. XW: Methodology, Writing – original draft, Writing – review & editing. RH: Methodology, Writing – original draft, Writing – review & editing. SZ: Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2024.1474531/full#supplementary-material
References
1. Li EL, Ma AL, Wang T, Fu YF, Liu HY, Li GC. Low-dose versus standard-dose computed tomography-guided biopsy for pulmonary nodules: a randomized controlled trial. J Cardiothorac Surg. (2023) 18:86. doi: 10.1186/s13019-023-02183-8
2. Fu YF, Li GC, Xu QS, Shi YB, Wang C, Wang T. Computed tomography-guided lung biopsy: a randomized controlled trial of low-dose versus standard-dose protocol. Eur Radiol. (2020) 30:1584–92. doi: 10.1007/s00330-019-06464-6
3. MacMahon H, Naidich DP, Goo JM, Lee KS, Leung ANC, Mayo JR, et al. Guidelines for management of incidental pulmonary nodules detected on CT images: from the fleischner society 2017. Radiology. (2017) 284:228–43. doi: 10.1148/radiol.2017161659
4. Fu YF, Li GC, Cao W, Wang T, Shi YB. Computed tomography fluoroscopy-guided versus conventional computed tomography-guided lung biopsy: A systematic review and meta-analysis. J Comput Assist Tomogr. (2020) 44:571–7. doi: 10.1097/RCT.0000000000001044
5. Madhok IK, Parsa N, Nieto JM. Endoscopic ultrasound-guided liver biopsy. Clin Liver Dis. (2022) 26:127–38. doi: 10.1016/j.cld.2021.09.002
6. Lawrence EM, Lubner MG, Pickhardt PJ, Hartung MP. Ultrasound-guided biopsy of challenging abdominopelvic targets. Abdom Radiol (NY). (2022) 47:2567–83. doi: 10.1007/s00261-021-03223-4
7. Wu J, Kong R, Tian S, Li H, Liu JS, Xu Z, et al. Advances in ultrasound-guided vacuum-assisted biopsy of breast microcalcifications. Ultrasound Med Biol. (2021) 47:1172–81. doi: 10.1016/j.ultrasmedbio.2021.01.008
8. Park B, Park J, Shin KM, Lim JK, Hong J, Cha JG, et al. Ultrasound-guided lung biopsy for small (≤2 cm) subpleural lung lesions: comparison of diagnostic yield and safety with larger lesions. J Thorac Dis. (2023) 15:2485–96. doi: 10.21037/jtd-22-1546
9. Li C, Liu B, Meng H, Lv W, Jia H. Efficacy and Radiation Exposure of Ultra-Low-Dose Chest CT at 100 kVp with Tin Filtration in CT-Guided Percutaneous Core Needle Biopsy for Small Pulmonary Lesions Using a Third-Generation Dual-Source CT Scanner. J Vasc Interv Radiol. (2019) 30:95–102. doi: 10.1016/j.jvir.2018.06.013
10. Leoni CJ, Potter JE, Rosen MP, Brophy DP, Lang EV. Classifying complications of interventional procedures: a survey of practicing radiologists. J Vasc Interv Radiol. (2001) 12:55–9. doi: 10.1016/S1051-0443(07)61403-1
11. Zhang JH, Xia FF, Yang XS, Li Y. Computed tomography-guided lung biopsy for molecular tests: a meta-analysis. Kardiochir Torakochirurgia Pol. (2022) 19:96–101. doi: 10.5114/kitp.2022.117498
12. Cai G, Wong R, Chhieng D, Levy GH, Gettinger SN, Herbst RS, et al. Identification of EGFR mutation, KRAS mutation, and ALK gene rearrangement in cytological specimens of primary and metastatic lung adenocarcinoma. Cancer Cytopathol. (2013) 121:500–7. doi: 10.1002/cncy.v121.9
13. Ferretti GR, Busser B, de Fraipont F, Reymond E, McLeer-Florin A, Mescam-Mancini L, et al. Adequacy of CT-guided biopsies with histomolecular subtyping of pulmonary adenocarcinomas: influence of ATS/ERS/IASLC guidelines. Lung Cancer. (2013) 82:69–75. doi: 10.1016/j.lungcan.2013.07.010
14. Liu GS, Wang SQ, Liu HL, Liu Y, Fu YF, Shi YB. Computed tomography-guided biopsy for small (≤20 mm) lung nodules: A meta-analysis. J Comput Assist Tomogr. (2020) 44:841–6. doi: 10.1097/RCT.0000000000001071
15. Rotolo N, Floridi C, Imperatori A, Fontana F, Ierardi AM, Mangini M, et al. Comparison of cone-beam CT-guided and CT fluoroscopy-guided transthoracic needle biopsy of lung nodules. Eur Radiol. (2016) 26:381–9. doi: 10.1007/s00330-015-3861-6
16. Ren Q, Zhou Y, Yan M, Zheng C, Zhou G, Xia X. Imaging-guided percutaneous transthoracic needle biopsy of nodules in the lung base: fluoroscopy CT versus cone-beam CT. Clin Radiol. (2022) 77:e394–9. doi: 10.1016/j.crad.2022.02.005
17. Jarmakani M, Duguay S, Rust K, Conner K, Wagner JM. Ultrasound versus computed tomographic guidance for percutaneous biopsy of chest lesions. J Ultrasound Med. (2016) 35:1865–72. doi: 10.7863/ultra.15.10029
18. Sconfienza LM, Mauri G, Grossi F, Truini M, Serafini G, Sardanelli F, et al. Pleural and peripheral lung lesions: comparison of US- and CT-guided biopsy. Radiology. (2013) 266:930–5. doi: 10.1148/radiol.12112077
19. Yamamoto N, Watanabe T, Yamada K, Nakai T, Suzumura T, Sakagami K, et al. Efficacy and safety of ultrasound (US) guided percutaneous needle biopsy for peripheral lung or pleural lesion: comparison with computed tomography (CT) guided needle biopsy. J Thorac Dis. (2019) 11:936–43. doi: 10.21037/jtd.2019.01.88
20. Diacon AH, Schuurmans MM, Theron J, Schubert PT, Wright CA, Bolliger CT. Safety and yield of ultrasound-assisted transthoracic biopsy performed by pulmonologists. Respiration. (2004) 71:519–22. doi: 10.1159/000080638
21. Liao WY, Chen MZ, Chang YL, Wu HD, Yu CJ, Kuo PH, et al. US-guided transthoracic cutting biopsy for peripheral thoracic lesions less than 3 cm in diameter. Radiology. (2000) 217:685–91. doi: 10.1148/radiology.217.3.r00dc21685
22. Sheth S, Hamper UM, Stanley DB, Wheeler JH, Smith PA. US guidance for thoracic biopsy: a valuable alternative to CT. Radiology. (1999) 210:721–6. doi: 10.1148/radiology.210.3.r99mr23721
23. Portela-Oliveira E, Souza CA, Gupta A, Bayanati H, Inacio J, Rakhra K. Ultrasound-guided percutaneous biopsy of thoracic lesions: high diagnostic yield and low complication rate. Clin Radiol. (2021) 76:281–6. doi: 10.1016/j.crad.2020.12.004
Keywords: computed tomography, ultrasound, biopsy, sub-pleural lesions, pulmonary
Citation: Zhou Y, Wang X, Han R and Zhang S (2024) Ultrasound versus computed tomography guided percutaneous needle biopsy for subpleural pulmonary lesions. Front. Oncol. 14:1474531. doi: 10.3389/fonc.2024.1474531
Received: 01 August 2024; Accepted: 21 October 2024;
Published: 12 November 2024.
Edited by:
Rocco Trisolini, Agostino Gemelli University Polyclinic (IRCCS), ItalyReviewed by:
Marco Sperandeo, Home for Relief of Suffering (IRCCS), ItalyLeonello Fuso, Catholic University of the Sacred Heart, Italy
Copyright © 2024 Zhou, Wang, Han and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shengmin Zhang, Znl5emhhbmdzaGVuZ21pbkBuYnUuZWR1LmNu
 Yaoyao Zhou1
Yaoyao Zhou1 Shengmin Zhang
Shengmin Zhang