- 1Department of Thoracic Surgery, The Second Hospital of Shandong University, Jinan, China
- 2Department of Thoracic Surgery, People’s Hospital of Laoling, Laoling of Dezhou, China
Purpose: This study was conducted to evaluate the postoperative short-term outcomes of patients undergoing video-assisted thoracoscopic surgery (VATS) for lung resection with the enhanced recovery after surgery (ERAS) protocol.
Methods: A single-institution, prospective randomized controlled study was conducted. The primary outcome measures were postoperative pulmonary complications (PPCs) and postoperative short-term effects.
Results: Among the 611 patients, 305 were assigned to the ERAS group, and 306 were assigned to the routine group. The ERAS group achieved earlier oral feeding, earlier mobilization, a shorter duration of drainage (2.0 vs. 5.0 days, P<0.001), and a shorter hospital stay (3.0 vs. 7.0 days, P<0.001). The biological impacts were confirmed to be significantly better for the ERAS group. Furthermore, the ERAS group also had a lower incidence of PPCs (11.5% vs. 22.9%, P<0.001) than did the routine group. Multivariate logistic regression analysis revealed the following predictors of drainage tube removal on the 1st day after surgery without pneumonia during hospitalization: comorbidity (P=0.029), surgical procedure (P=0.001), and operation time (P=0.039).
Conclusions: Implementation of the ERAS protocol led to a decreased incidence of PPCs, suggesting that the ERAS protocol has a better biological impact on patients undergoing VATS for lung resection. Multigradient individual ERAS protocols are recommended at different institutions according to the individual conditions of patients.
Clinical Trial Registration: https://register.clinicaltrials.gov/prs/app/action/SelectProtocol?sid=S0009ZT9&selectaction=Edit&uid=U0002ZGN&ts=3&cx=ks7hrg, identifier NCT04451473.
Introduction
Enhanced recovery after surgery (ERAS) was first reported in the late 1990s (1). ERAS strategies involve all aspects of perioperative care, aiming to improve patient prognosis, reduce complications, shorten hospital stays, and lower costs (2–6). The concept of ERAS has been applied in open or minimally invasive surgeries, including colorectal surgery (7), gynecological surgery (8), liver surgery (9), breast surgery (10), urological surgery (11), and spinal surgery (12).
Thoracic surgery is considered an invasive and traumatic procedure for patients (13). Minimally invasive surgeries, including video-assisted thoracoscopic surgery (VATS), have been widely used for surgical treatment (14, 15). Thoracic surgery has undergone two major advancements: one is the switch from open surgery to minimally invasive surgery, and the other is the use of structured clinical pathways based on ERAS guidelines (16, 17). Studies have also focused on ERAS strategies for thoracic surgery in recent years (18), especially in VATS for lung resection (19, 20), indicating that ERAS can efficiently minimize surgical trauma, improve the quality of postoperative recovery, and decrease financial burdens. However, the outcome data of ERAS programs applied for lung resection are limited, and more diverse regions of study are needed to determine the safety and effectiveness of ERAS strategies. The aim of this study was to determine the impact of the ERAS pathway on the short-term outcomes of patients who underwent lung resection.
Patients and methods
Patient eligibility
The inclusion criteria for patients were as follows (1): had lung lesions suitable for VATS, including benign and malignant lesions diagnosed by CT (enhanced or nonenhanced) or pathological results (2); were aged between 18 and 85 years (3); had a Karnofsky score ≥80 along with cardiopulmonary function, liver function, and renal function indicating the ability to tolerate minimally invasive surgery (4); had normal cognitive function and was able to cooperate with the rehabilitation training (21, 22); and (5) agreed on the protocol of the clinical trial and signed the consent form.
The exclusion criteria for patients were as follows (1): refused randomization (2); was unable to cooperate with the rehabilitation training or tolerate minimally invasive surgery due to cognitive or physical dysfunctions; and (3) participated in other clinical trials or had received treatment with anticancer drugs in other clinical trials.
Pretreatment workup
The study was approved by the Ethics Committee of the Second Hospital of Shandong University (SDDXDEYY-KYB-2020053). The study’s registration information is as follows: Clinical Trials. Gov ID: NCT04451473 (30/06/2020). Informed consent was obtained from all the patients. Our work was fully compliant with the CONSORT criteria, and this study is reported in line with the CONSORT criteria (23). We confirmed that all methods were performed in accordance with the relevant guidelines and regulations.
Randomization and allocation
This study was designed as a single-center, randomized, unblinded control trial. Random assignment was performed using the envelope method by a statistician at the Evidence-based Medicine Center of the Second Hospital of Shandong University. Once an informed consent form was signed, the patient was assigned to one group by opening the sequentially numbered envelope (24).
Study interventions
Control group (routine group)
The patients were randomly assigned to the control group (routine group) and received standard VATS; however, the perioperative management was mainly traditional without systemic ERAS protocol guidance. The traditional management methods were as follows: 1. the patient received no systemic physical pulmonary training before surgery; 2. the patient received sedatives to reduce anxiety preoperatively; 3. the patient fasted from solids for at least 8 hours and from liquids for at least 6 hours; 4. a transurethral catheter was routinely placed and then removed on the second day after surgery; 5. the patient remained completely supine for 6 hours after surgery; 6. the patient fasted from solids and liquids for 6 hours after surgery; 7. the patient achieved ambulation ≥24 hours after surgery; 8. two chest tubes were used when the upper lobe was moved; 9. the chest tube was removed when the highest volume did not exceed 100 ml/24 h; and 10. opioids were the most common drugs in the analgesic regime.
Intervention group (ERAS group)
The ERAS protocol was performed mainly according to two guidelines (16, 17), and physical pulmonary training in the general ward was recommended according to the protocol (25). The core items of ERAS application at our institution are shown as following:
1. Dedicated preoperative counseling and education should be given in the preoperative phase. Nutritional status screening and improvement, smoking cessation (2-4 weeks), alcohol dependency management, and even pulmonary prehabilitation (according to the patient’s cognitive ability and compliance) should also be accomplished in this phase.
2. Airway management during the preoperative examination after admission: climbing stairs or power-based cycling, which can enhance cardiopulmonary function, has often been used in the past few years; however, physical pulmonary training in the general ward is strongly recommended (25), especially during the COVID-19 pandemic, which has been ongoing since 2020. Education and training are better guided by a physiotherapist or by a charge nurse.
3. Aspirin withdrawal is not recommended for patients in the ERAS group unless VATS is complicated and accompanied by a high risk of bleeding. Low molecular weight heparin bridging treatment is used during aspirin withdrawal.
4. Sedatives for reducing anxiety preoperatively are prohibited.
5. Clear fluids are allowed up until 2-4 h before the induction of anesthesia, and oral carbohydrate loading can be used routinely.
6. A combination of regional and general anesthetic techniques should be used, and general anesthesia combined with nonintubated spontaneous breathing can be an alternative to double-lumen intubation if the anesthesiologist has mastered the technique.
7. Uniport VATS was routine for patients in the ERAS group. Wedge resection or segmentectomy should be meticulously planned through the scientific reading of thin-slice CT or 3D simulation before surgery. High-quality minimally invasive surgery is the foundation of ERAS protocol implementation.
8. A transurethral catheter is not routinely placed for the sole purpose of monitoring urine output without thoracic epidural anesthesia. Transurethral catheters can be used in one of the following conditions (1): the estimated operation time is more than 150 minutes or even 180 minutes (2); high risk of bleeding; and (3) critical patients with organ dysfunction. If the operation takes a longer time than expected without a urinary catheter, placing a disposable catheter is reasonable. The urinary catheter should be removed immediately after the operation if the patients’ respiratory and circulatory status is stable without prediction advanced life support.
9. The head of bed (by ≥30°) was immediately raised when the patient went back to the general ward. The patient could sit up straight with the help of the charge doctor or charge nurse 2 hours after returning to the ward, and the patient was asked to drink a little water if no postoperative nausea and vomiting (PONV) occurred; otherwise, PONV was treated using nonpharmacological control with or without pharmacological control [16] according to the degrees of discomfort. A small portion of a semifluid diet was encouraged if no PONV occurred. Then, the nutrition procedures could be conducted by specialized nutritionists from the Nutrition Department.
10. Early ambulation evaluation began when the patients could sit up straight 2 hours after returning to the ward. If the patient can keep the straight sitting position without obvious dizziness and weakness, he or she can try to leave the bed and stand beside the sickbed with electrocardiogram monitoring. Marching on the spot can be started if the vital signs and respiratory status remain stable, and then moderate ambulation can be tried with the accompaniment of medical staff and a family member.
11. A single tube was used for the patients in the ERAS group, and the chest tube was removed when the following conditions were met (1): no progressive bleeding or chylothorax (2); no persistent air leakage during continuous cough (3); no obvious atelectasis confirmed by physical examination or imaging examination; and (4) daily serous effusion less than 300 ml/24 h, with the highest volume not exceeding 450 ml/24 h.
12. A multimodal analgesic regimen is recommended for pain relief (16, 17). Opioids are inevitable in most cases, but they should be minimized. Patient education is important but not enough for anxious patients or patients suffering from severe pain, needing psychological counseling and needing additional techniques such as transcutaneous electrical nerve stimulation (TENS).
Withdrawal from the trial
Patients were withdrawn for one of the following reasons: (i) a withdrawal request was made by the patient or the family member; (ii) there was poor compliance with the training protocol; or (iii) severe complications, such as heart disease or stroke, occurred.
Sample size calculation
Several primary or secondary outcomes have been studied; however, postoperative pulmonary complications (PPCs) are an important and widely used index for evaluating short-term postoperative improvement (26). The criteria for PPCs were established according to the STS/ESTS definitions (27). It has been reported that the ERAS group has a lower incidence of PPC than the routine group (15.2% vs. 19.5%, P=0.022) (28). For this trial, a minimum 10% absolute risk reduction from a 19.5% PPC risk was set (25). A significant difference between groups was detected with a sample of 480 patients (p = 0.05, 80% power of test, two-sided test), considering a 20% inflation in the case of dropouts and a final sample size of 600.
Outcome measures and statistical analysis
The primary outcome measures were PPCs. The secondary outcome measures were some other postoperative short-term effects, postoperative long-term respiratory function, health-related quality of life (HRQoL), progression-free survival (PFS) and overall survival (OS) for patients who underwent VATS for lung cancer surgery. Continuous variables are expressed herein as means and standard deviations (SDs); data that were not normally distributed are presented as medians and ranges; and binary variables are presented as proportions. The data were evaluated through Student’s t test (data matching a normal distribution), nonparametric statistics, the chi-square test, or Fisher’s exact test, as appropriate. Multivariate logistic regression analysis was used to reveal the predictors of drainage removal on the first day after surgery without pneumonia during hospitalization. Statistical analysis and graph generation were performed with Stata 12.0 (StataCorp LP, College Station, TX, USA) at a significance level of 0.05. Multivariate logistic regression analysis was performed with SPSS statistics software (version 25; SPSS, Inc., Chicago, IL).
Results
Patient characteristics
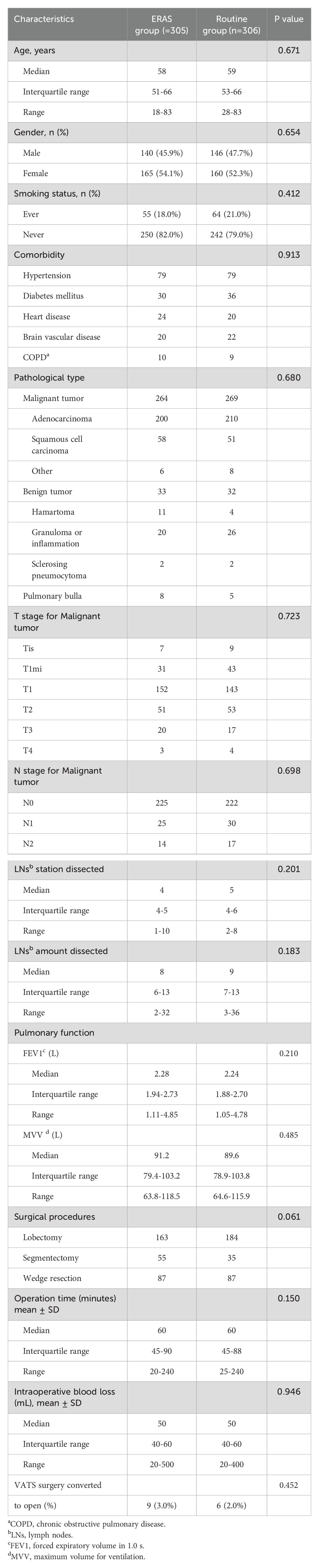
From July 2020 to June 2022, 611 patients were enrolled in the study; 305 patients were assigned to the ERAS group, and 306 were assigned to the routine group. The treatment protocol was completed for the enrolled patients. The patient characteristics were balanced between the two groups (Table 1).
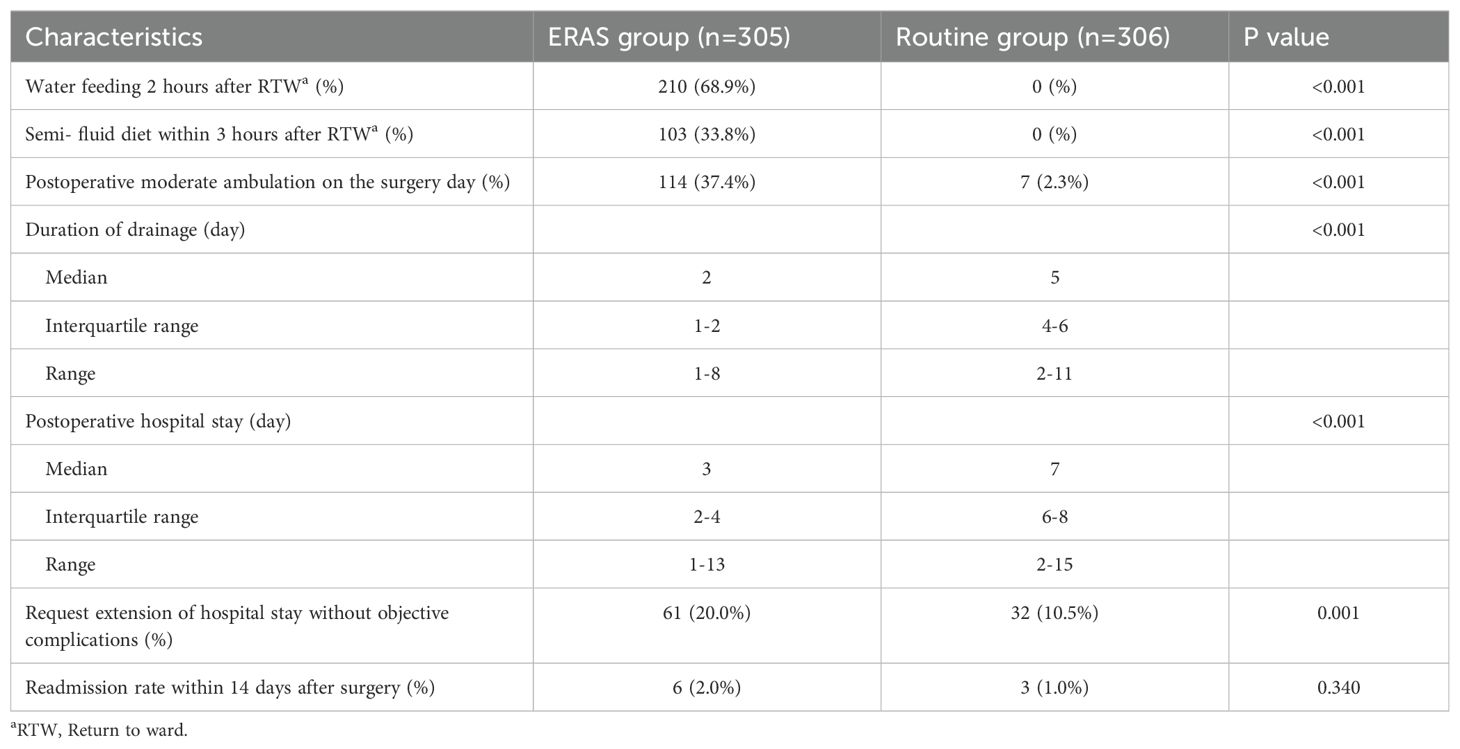
As shown in Table 2, 210 patients (68.9%) were able to drink water 2 hours after returning to the general ward in the ERAS group, 103 patients (33.8%) had a semifluid diet within 3 hours after returning to the general ward in the ERAS group, and 114 patients (37.4%) had moderate postoperative ambulation on the surgery day with guidance and an accompanying medical staff member and family member. The following three characteristics were significantly different between the ERAS group and the routine group: The duration of drainage in the ERAS group was significantly shorter than that in the routine group (median, 2 days with IQR 1-2 days vs. median, 5 days with IQR 4-6 days). The median postoperative hospital stay was 3 days (IQR, 2-4 days) in the ERAS group, and the median postoperative hospital stay was significantly longer (7 days, IQR, 6-8 days; P <0.001) in the routine group. The readmission rate within 14 days after surgery was similar: 2.0% (6 patients) in the ERAS group and 1.0% (3 patients) in the routine group (P=0.340). No mortality within 30 days occurred in both groups.
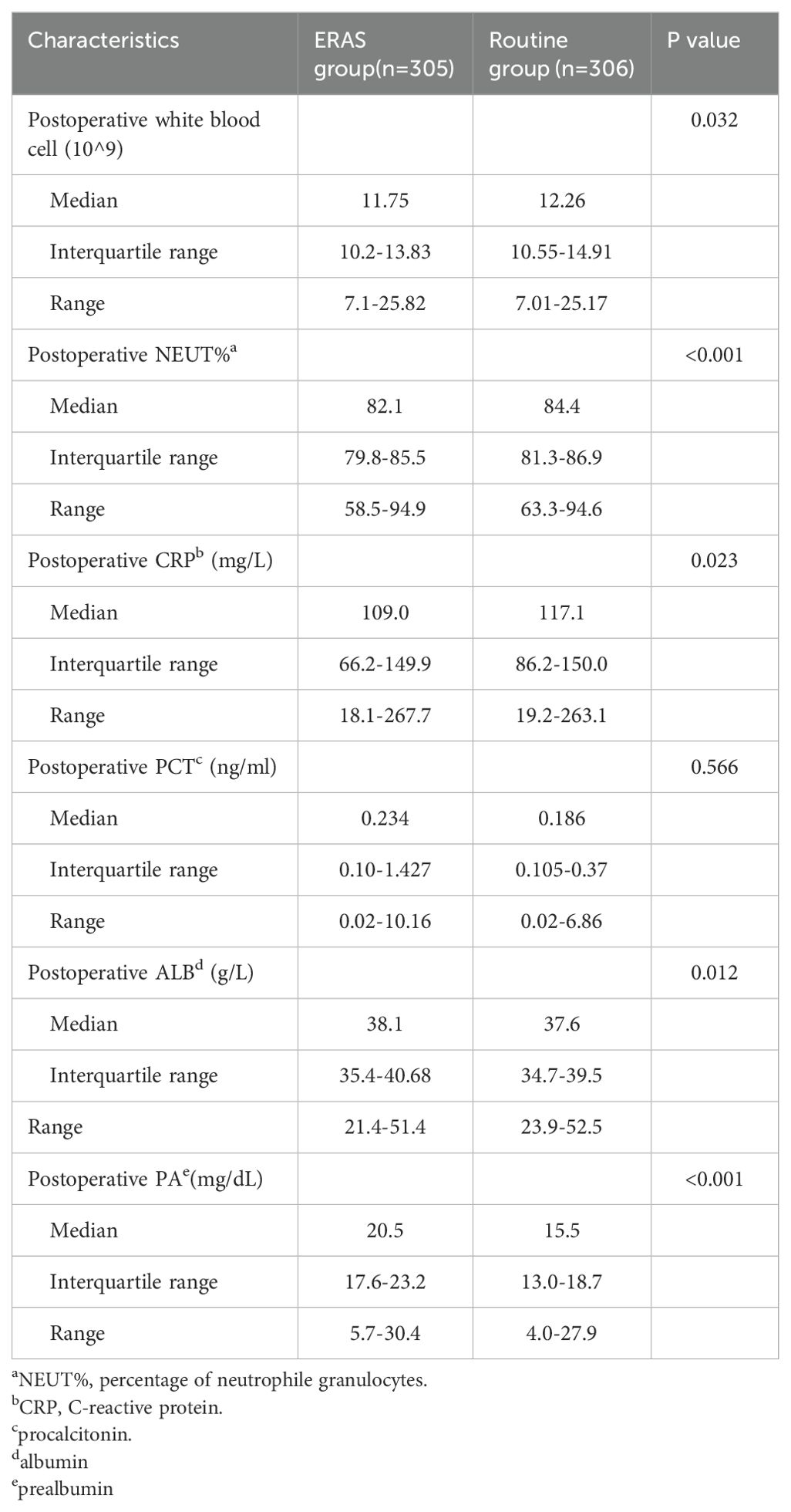
Postoperative blood samples were collected and tested two days after surgery (Table 3) unless the patients were discharged from the hospital the first day after surgery (10 patients in the ERAS group). The postoperative white blood cell (WBC) count, percentage of neutrophil granulocytes (NEUT%), and postoperative C-reactive protein (CRP) level were significantly different between the ERAS group and the routine group (p = 0.032; p <0.001; p = 0.023, respectively). Postoperative procalcitonin (PCT) was not significantly different between the two groups (P=0.566). The median albumin (ALB) level was 38.1 in the ERAS group (IQR, 35.4-40.7 g/L) and 37.6 in the routine group (IQR, 34.7-39.5 g/L; P=0.010). The median prealbumin (PA) level was 20.5 in the ERAS group (IQR, 17.6-23.2 mg/dL) and 15.5 in the routine group (IQR, 13.0-18.7 mg/dL; P<0.001).
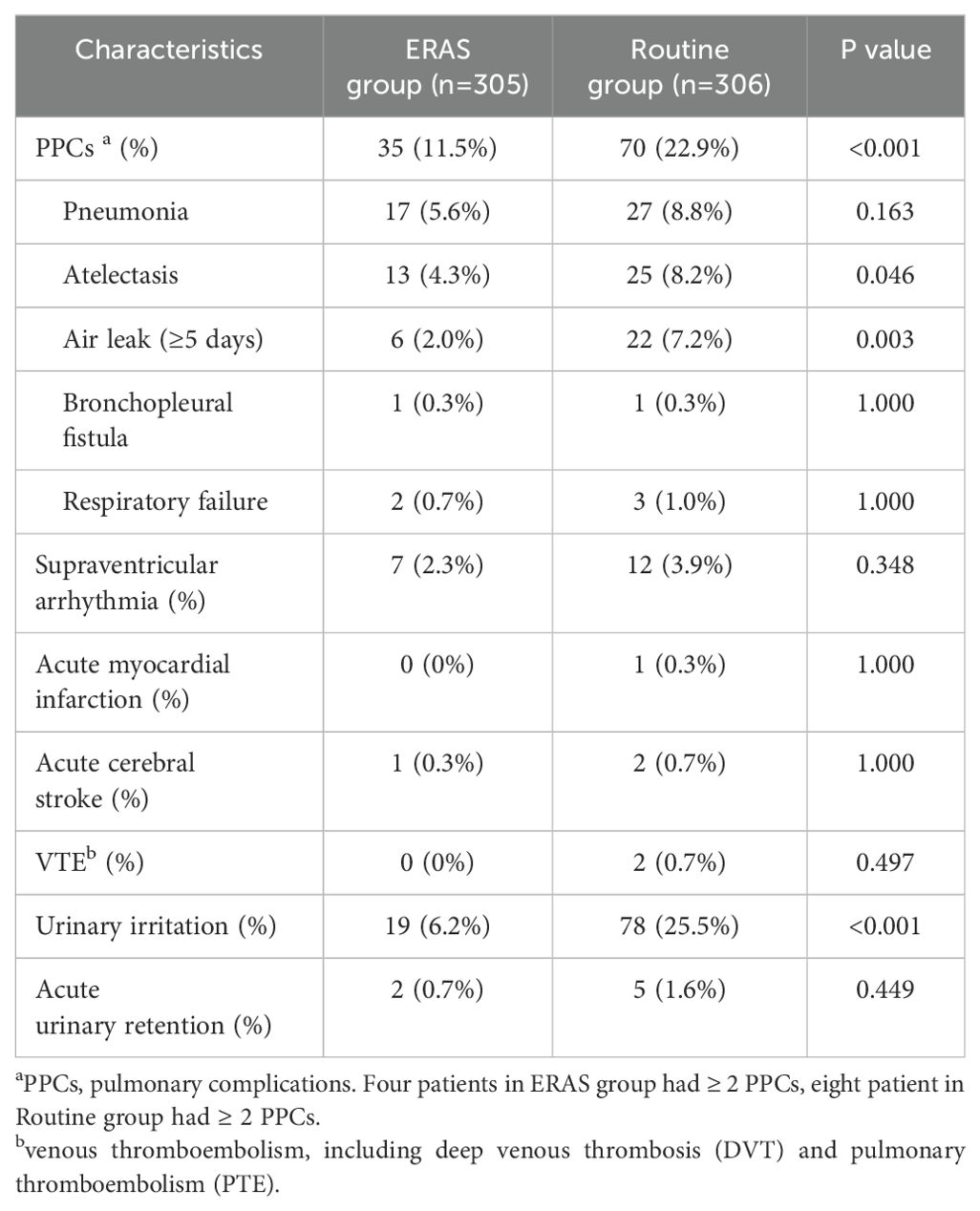
The postoperative early complications are shown in Table 4. For pulmonary complications (PPCs), 11.5% of the patients in the ERAS group had pulmonary complications, 22.9% of the patients in the routine group had pulmonary complications, and the difference was significant (P<0.001). There were greater incidences of atelectasis and air leakage in the routine group (P=0.046; P=0.003). Bronchopleural fistula and respiratory failure rarely occurred in either group. The occurrence of supraventricular arrhythmia, acute myocardial infarction, acute cerebral stroke, and venous thromboembolism was similar in both groups. Significantly more urinary irritation was observed in the routine group (ERAS group: 6.2%, routine group: 25.5%; P<0.001), and the frequency of acute urinary retention was similar between the two groups (ERAS group: 0.7%, routine group: 1.6%; P=0.449).
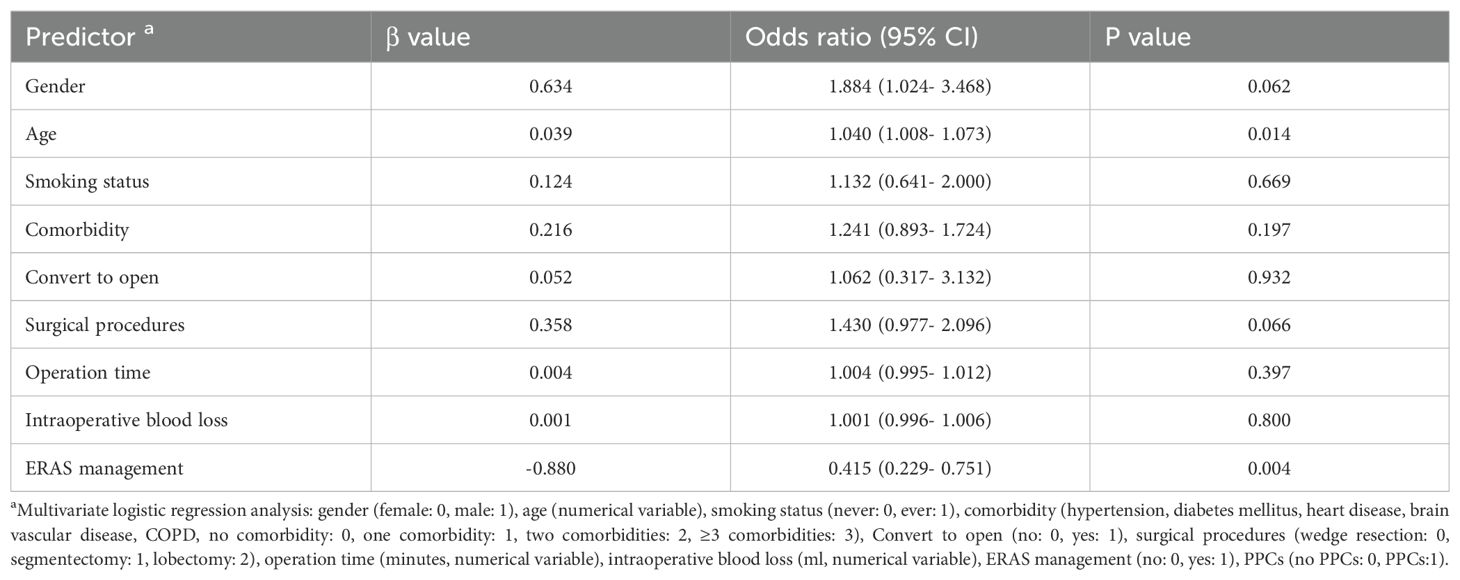
Multivariate logistic regression analysis was used to reveal the predictors of PPCs. Table 5 shows that age (P=0.014), ERAS management (P=0.004) were significant predictors. Gender and surgical procedures seemed to have the trend, but neither of them showed the significant statistical difference (P=0.062, P=0.066 respectively).
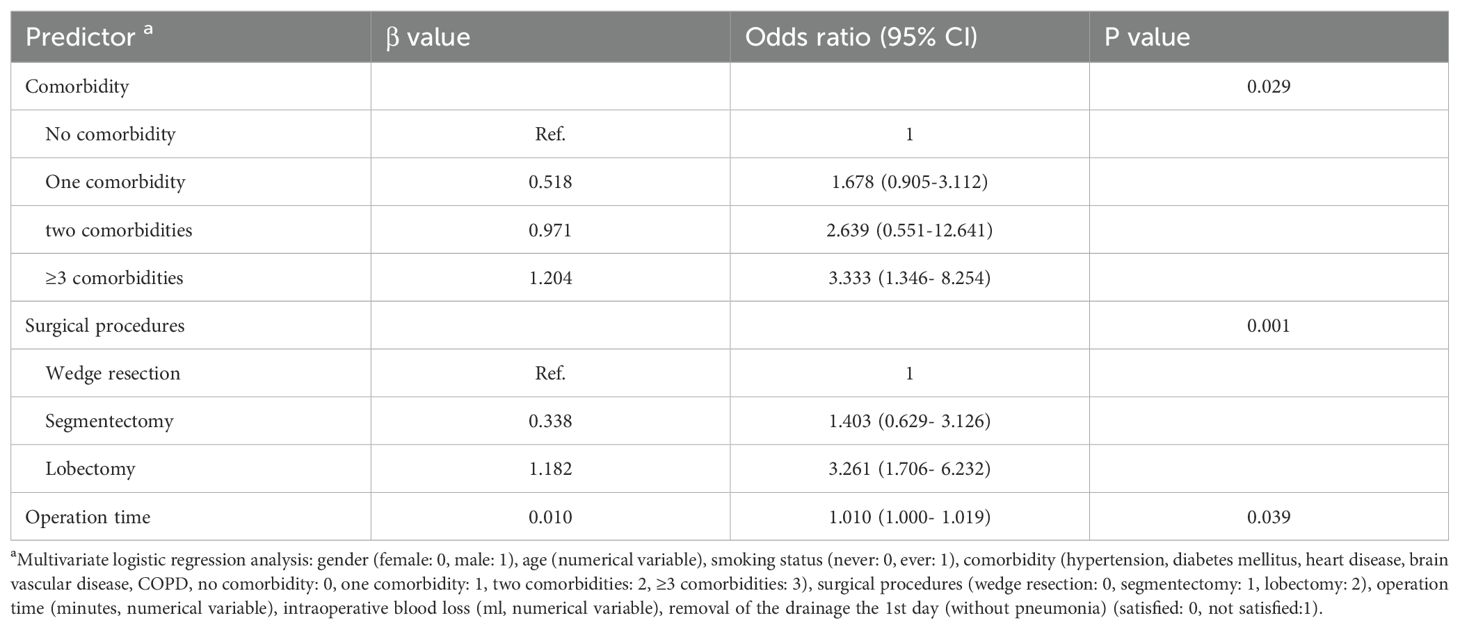
Multivariate logistic regression analysis was also used to reveal the predictors of drainage tube removal on the 1st day after surgery without causing pneumonia during hospitalization. Table 6 shows that comorbidity (P=0.029), surgical procedure (P=0.001) and operation time (P=0.039) were significant predictors. Rapid removal of chest drainage tubes and recovery without pneumonia may constitute the foundation of day surgery (from admission to discharge ≤48 hours).
Discussion
In recent years, increasing attention has been given to the implementation of ERAS protocols for various tumors (29–31). The ERAS program for lung surgery (16, 17) was described relatively late and was aimed to decrease postoperative morbidity and mortality.
In the traditional postoperative management mode, patients were asked to stay in the supine posture without drinking or eating for at least 6 hours. Patients were not required to remain in the supine position or fast for a long period when the combination of regional and general anesthetic techniques was used. Patients in the ERAS group were allowed to drink water and have a semifluid diet so that specialized nutritionists could use an earlier intervention. Even more than one-third of the patients in the ERAS group achieved moderate ambulation on the day of surgery, and this approach has been confirmed to be feasible and safe (32). Although contrary to the ERAS protocol, the chest drainage tube could be removed when the volume was less than 100 ml in the traditional mode. However, we suggest careful consideration of early removal of the drainage system, as this approach is relied on successful high-quality minimally invasive surgery, early oral feeding and ambulation. In this study, the ERAS group had a median postoperative hospital stay of 3 days (interquartile range: 2-4 days), which was still shortened gradually.
The biological impact, which was also investigated in our study, has been investigated in several other organ surgeries within the ERAS program (33–35). Blood laboratory examinations, including measurements of biomarker levels indicating the magnitude of surgical stress (36–38), were used in our study. In ERAS group, the postoperative white blood cell count, percentage of neutrophil granulocytes (NEUT%), C-reactive protein (CRP), were significantly lower than those in the routine group, and the levels of albumin (ALB), prealbumin (PA) were significantly higher for patients in the ERAS group. Similar results were obtained in liver surgery (35). Procalcitonin (PCT) increases during severe generalized bacterial, parasitic, or fungal infections (39). Moreover, there was no significant difference in postoperative PCT between the two groups, perhaps due to the similar incidence rate of pneumonia (ERAS group: 5.6%, routine group: 8.8%, P=0.163).
In our study, we found lower incidences of PPCs in the ERAS group than in the control group, and the same results were observed for atelectasis and air leakage (≥5 days). We suppose that early mobilization is crucial for reducing morbidity, promoting lung recruitment as soon as possible, promoting quick recovery of gastrointestinal function, and preventing deep venous thrombosis. Rogers LJ (19) also regarded early mobilization as the most important predictor. ERAS protocols tended to decrease the occurrence of pneumonia, although the difference was not significant. Urinary irritation was significantly lower in the ERAS group (6.2% vs. 25.5%, P<0.001), and acute urinary retention rarely occurred in the ERAS group, even for patients without perioperative transurethral catheters. Of the two patients with acute urinary retention, one man had urinary retention due to prostatitis, and the other patient, a woman, had urinary retention due to unknown reasons; she was cured after urinary catheterization.
We recommend that patients with fewer comorbidities, a smaller resection range, or less operation time be candidates for day surgery (from admission to discharge ≤48 hours), although the management details still need exploration and optimization.
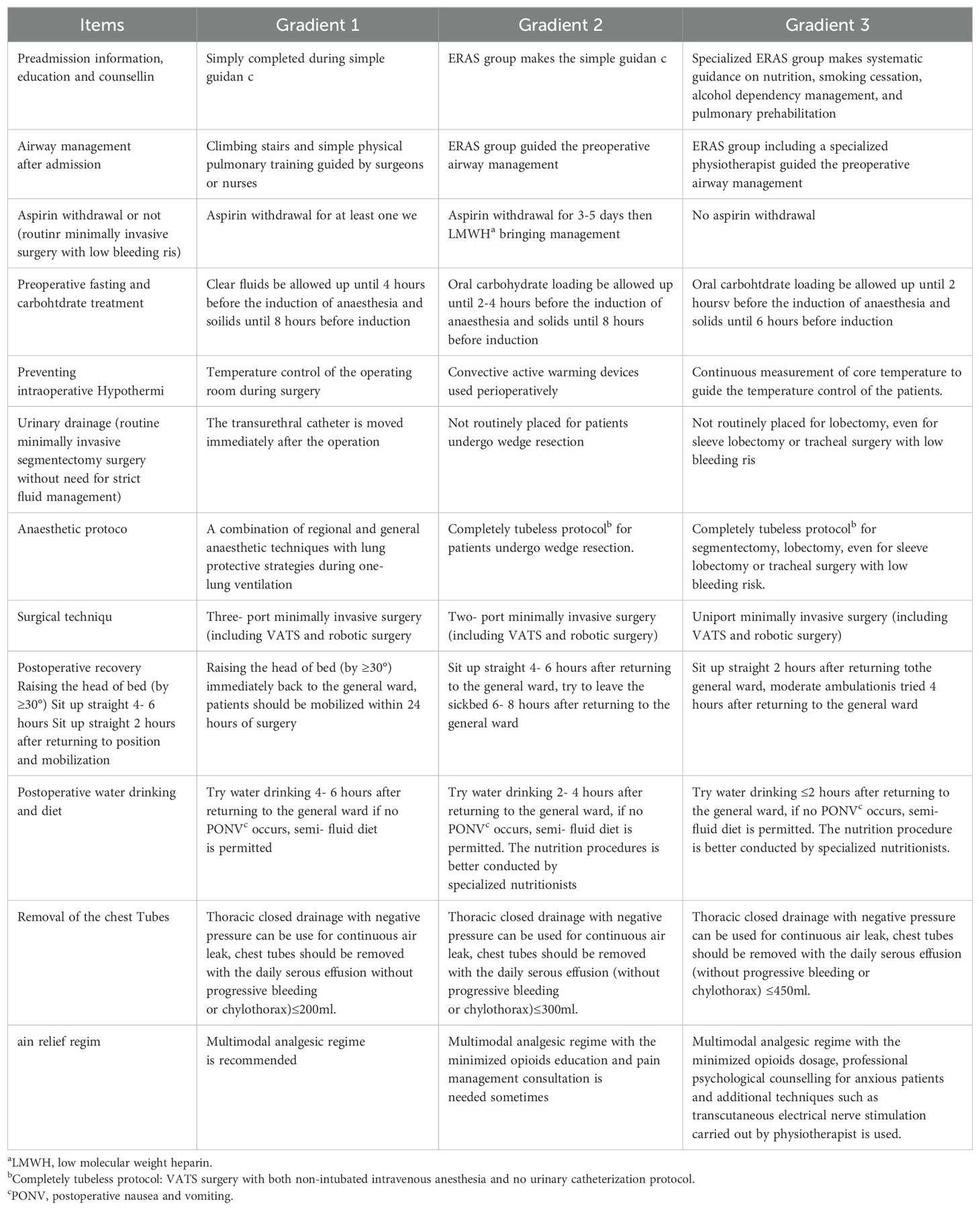
The results of this study were similar to some other studies (19, 28), and for postoperative hospital stay, PPCs like pneumonia and atelectasis, even better results were got in our study. The data couldn’t be used to compare directly due to the difference of enrolled population and implementation details among these studies, but the positive effect of ERAS was confirmed. Furthermore, we propose the concept of multi-gradient individual ERAS (MGI-ERAS). This means that the ERAS protocol is comprehensively formulated and performed in a gradient manner according to the individual conditions of the institutions, anesthetists, surgeons, nurses and patients. This approach may be convenient for medical centers attempting to gradually implement the ERAS protocol. The suggested practice elements of the MGI-ERAS are shown in Table 7. The recommendations of Gradient 1 are relatively easier to follow, and the recommendations of Gradient 3 may be the ultimate ERAS practice at present.
There are several limitations that should not be ignored. First, this was a single-center study. Second, the patients were enrolled more than 2 years previously, and gains in ERAS experience are inevitable, which may have led to bias. Third, a blinded method could not be used in this study, and the timing of return to diet, mobilization, removal of the chest tube, and discharge from the hospital might have been biased or even affected by the patients or their relatives. Moreover, pain control, psychological variables, postoperative functional recovery efficacy and quality of life were not presented. Finally, the economic outcomes of the ERAS program were not explored in this study.
Conclusion
In conclusion, the implementation of the ERAS protocol led to earlier return to diet and mobilization, lower incidences of PPCs and urinary irritation without acute urinary retention, and shorter durations of drainage and postoperative hospital stay, thus providing better biological impacts for patients undergoing VATS lung resection. MGI-ERAS is recommended for the implementation of the ERAS protocol at different institutions with respect to the individual conditions of the patients.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by the Ethics Committee of the Second Hospital of Shandong University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
YD: Data curation, Formal Analysis, Writing – original draft, Writing – review & editing. LZ: Data curation, Formal Analysis, Writing – review & editing. LS: Data curation, Formal Analysis, Methodology, Supervision, Writing – review & editing. WZ: Data curation, Formal Analysis, Methodology, Writing – review & editing. PL: Data curation, Supervision, Writing – review & editing. BC: Data curation, Supervision, Writing – review & editing. ZT: Data curation, Formal Analysis, Writing – review & editing. YZ: Data curation, Formal Analysis, Funding acquisition, Methodology, Project administration, Software, Supervision, Writing – original draft, Writing – review & editing. XZ: Conceptualization, Formal Analysis, Funding acquisition, Investigation, Project administration, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. The study is funded by Taishan Scholar Project and Key Laboratory of Thoracic Cancer, Shandong University.
Acknowledgments
We thank doctor Ling Li from Cheeloo Hospital of Shandong University, Ning Li, He Zhang from The Second Hospital of Shandong University, for discussion, guidance or practice of anesthesia and the recovery after that.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Kehlet H. Multimodal approach to control postoperative pathophysiology and rehabilitation. Br J Anaesth. (1997) 78:606–17. doi: 10.1093/bja/78.5.606
2. Kehlet H, Wilmore DW. Evidence-based surgical care and the evolution of fast-track surgery. Ann Surg. (2008) 248:189–98. doi: 10.1097/SLA.0b013e31817f2c1a
3. Roulin D, Donadini A, Gander S, Griesser A-C, Blanc C, Hübner M, et al. Cost-efectiveness of the implementation of an enhanced recovery protocol for colorectal surgery. Br J Surg. (2013) 100:1108–14. doi: 10.1002/bjs.9184
4. Joliat GR, Labgaa I, Petermann D, Hübner M, Griesser A-C, Demartines N, et al. Cost beneft analysis of an enhanced recovery protocol for pancreaticoduodenectomy. Br J Surg. (2015) 102:1676–83. doi: 10.1002/bjs.9957
5. Lee L, Mata J, Ghitulescu GA, Boutros M, Charlebois P, Stein B, et al. Cost-efectiveness of enhanced recovery versus conventional perioperative management for colorectal surgery. Ann Surg. (2015) 262:1026–33. doi: 10.1097/SLA.0000000000001019
6. Senturk JC, Kristo G, Gold J, Bleday R, Whang E. The development of enhanced recovery after surgery across surgical specialties. J Laparoendosc Adv Surg Tech A. (2017) 27:863–70. doi: 10.1089/lap.2017.0317
7. Ni X, Jia D, Chen Y, Wang L, Suo J. Is the enhanced recovery after surgery (ERAS) program effective and safe in laparoscopic colorectal cancer surgery? A meta-analysis of randomized controlled trials. J Gastrointest Surg. (2019) 23:1502–12. doi: 10.1007/s11605-019-04170-8
8. Keil DS, Schiff LD, Carey ET, Moulder JK, Goetzinger AM, Patidar SM, et al. Predictors of admission after the implementation of an enhanced recovery after surgery pathway for minimally invasive gynecologic surgery. Anesth Analg. (2019) 129:776–83. doi: 10.1213/ANE.0000000000003339
9. Damania R, Cocieru A. Impact of enhanced recovery after surgery protocols on postoperative morbidity and mortality in patients undergoing routine hepatectomy: review of the current evidence. Ann Transl Med. (2017) 5:341. doi: 10.21037/atm.2017.07.04
10. Sharif-Askary B, Hompe E, Broadwater G, Anolik R, Hollenbeck ST. The effect of enhanced recovery after surgery pathway implementation on abdominal-based microvascular breast reconstruction. J Surg Res. (2019) 242:276–85. doi: 10.1016/j.jss.2019.04.062
11. Lin C, Wan F, Lu Y, Li G, Yu L, Wang M. Enhanced recovery after surgery protocol for prostate cancer patients undergoing laparoscopic radical prostatectomy. J Int Med Res. (2019) 47:114–21. doi: 10.1177/0300060518796758
12. Angus M, Jackson K, Smurthwaite G, Carrasco R, Mohammad S, Verma R, et al. The implementation of enhanced recovery after surgery (ERAS) in complex spinal surgery. J Spine Surg. (2019) 5:116–23. doi: 10.21037/jss.2019.01.07
13. Boffa DJ, Allen MS, Grab JD, Gaissert HA, Harpole DH, Wright. CD. Data from The Society of Thoracic Surgeons General Thoracic Surgery database: the surgical management of primary lung tumors. J Thorac Cardiovasc Surg. (2008) 135:247–54. doi: 10.1016/j.jtcvs.2007.07.060
14. Boffa DJ, Kosinski AS, Furnary AP, Kim S, Onaitis MW, Tong BC, et al. Minimally invasive lung cancer surgery performed by thoracic surgeons as effective as thoracotomy. J Clin Oncol. (2018) 36:2378–85. doi: 10.1200/JCO.2018.77.8977
15. Caso R, Watson TJ, Khaitan PG, Marshall MB. Outcomes of minimally invasive sleeve resection. J Thorac Dis. (2018) 10:6653–9. doi: 10.21037/jtd.2018.10.97
16. Batchelor TJP, Rasburn NJ, Abdelnour-Berchtold E, Brunelli A, Cerfolio RJ, Gonzalez M, et al. Guidelines for enhanced recovery after lung surgery: recommendations of the Enhanced Recovery After Surgery (ERAS®) Society and the European Society of Thoracic Surgeons (ESTS). Eur J Cardiothorac Surg. (2019) 55:91–115. doi: 10.1093/ejcts/ezy301
17. Gao S, Barello S, Chen L, Chen C, Che G, Cai K, et al. Clinical guidelines on perioperative management strategies for enhanced recovery after lung surgery. Transl Lung Cancer Res. (2019) 8:1174–87. doi: 10.21037/tlcr.2019.12.25
18. Brown JK, Singh K, Dumitru R, Chan E, Kim MP. The benefits of enhanced recovery after surgery programs and their application in cardiothoracic surgery. Methodist Debakey. Cardiovasc J. (2018) 14:77–88. doi: 10.14797/mdcj-14-2-77
19. Rogers LJ, Bleetman D, Messenger DE, Joshi NA, Wood L, Rasburn NJ, et al. The impact of enhanced recovery after surgery (ERAS) protocol compliance on morbidity from resection for primary lung cancer. J Thorac Cardiovasc Surg. (2018) 155:1843–52. doi: 10.1016/j.jtcvs.2017.10.151
20. Gonzalez M, Abdelnour-Berchtold E, Perentes JY, Doucet Valérie, Zellweger M, Marcucci C, et al. An enhanced recovery after surgery program for video- assisted thoracoscopic surgery anatomical lung resections is cost-effective. J Thorac Dis. (2018) 10:5879–88. doi: 10.21037/jtd.2018.09.100
21. Licker M, Karenovics W, Diaper J, Frésard I, Triponez Frédéric, Ellenberger C, et al. Short-term preoperative high-intensity interval training in patients awaiting lung Cancer surgery: a randomized controlled trial. J Thorac Oncol. (2017) 12:323–33. doi: 10.1016/j.jtho.2016.09.125
22. Brocki BC, Andreasen JJ, Langer D, Souza DSR, Westerdahl E. Postoperative inspiratory muscle training in addition to breathing exercises and early mobilization improves oxygenation in high-risk patients after lung cancer surgery: a randomized controlled trial. Eur J Cardio-thorac Surg. (2016) 49:1483–91. doi: 10.1093/ejcts/ezv359
23. Schulz KF, Altman DG, Moher D, CONSORT Group. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. Ann Intern Med. (2010) 152(1):726–32. doi: 10.7326/0003-4819-152-11-201006010-00232
24. Schulz KF, Grimes DA. Allocation concealment in randomised trials: defending against deciphering. Lancet (London England). (2002) 359:614–8. doi: 10.1016/S0140-6736(02)07750-4
25. Zheng Yu, Mao M, Ji M, Zheng Q, Liu L, Zhao Z, et al. Does a pulmonary rehabilitation based ERAS program (PREP) affect pulmonary complication incidence, pulmonary function and quality of life after lung cancer surgery? Study protocol for a multicenter randomized controlled trial. BMC Pulm Med. (2020) 20:44. doi: 10.1186/s12890-020-1073-6
26. Bailey KL, Merchant N, Seo Y-J, Elashoff D, Benharash P, Yanagawa J. Short- term readmissions after open, thoracoscopic, and robotic lobectomy for lung Cancer based on the Nationwide readmissions database. World J Surg. (2019) 43:1377–84. doi: 10.1007/s00268-018-04900-0
27. Seder CW, Salati M, Kozower BD, Wright CD, Falcoz P-E, Brunelli A, et al. Variation in pulmonary resection practices between the society of thoracic surgeons and the European society of thoracic surgeons general thoracic surgery databases. Ann Thorac Surg. (2016) 101:2077–84. doi: 10.1016/j.athoracsur.2015.12.073
28. Wang C, Lai Y, Li P, Su J, Che G. Infuence of enhanced recovery after surgery (ERAS) on patients receiving lung resection: a retrospective study of 1749 cases. BMC Surg. (2021) 21:115. doi: 10.1186/s12893-020-00960-z
29. Lassen K, Coolsen MME, Slim K, Carli F, Aguilar-Nascimento JoséEde, Schäfer M, et al. Guidelines for perioperative care for pancreaticoduodenectomy: enhanced recovery after surgery (ERAS) Society recommendations. Clin Nutr. (2012) 31:817–30. doi: 10.1016/j.clnu.2012.08.011
30. Melloul E, Hübner M, Scott M, Snowden C, Prentis J, Dejong CHC, et al. Guidelines for perioperative care for liver surgery: Enhanced Recovery After Surgery (ERAS) Society recommendations. World J Surg. (2016) 40:2425–40. doi: 10.1007/s00268-016-3700-1
31. Mortensen K, Nilsson M, Slim K, Schäfer M, Mariette C, Braga M, et al. Consensus guidelines for enhanced recovery after gastrectomy: Enhanced Recovery After Surgery (ERASVR) Society recommendations. Br J Surg. (2014) 101:1209–29. doi: 10.1002/bjs.9582
32. Nakada T, Shirai S, Oya Y, Takahashi Y, Sakakura N, Ohtsuka T, et al. Four hours postoperative mobilization is feasible after thoracoscopic anatomical pulmonary resection. World J Surg. (2021) 45:631–7. doi: 10.1007/s00268-020-05836-0
33. Ruiz-Tovar J, Muñoz JL, Gonzalez J, Garcia A, Ferrigni C, Jimenez M, et al. C-reactive protein, fibrinogen, and procalcitonin levels as early markers of staple line leak after laparoscopic sleeve gastrectomy in morbidly obese patients within an Enhanced Recovery After Surgery (ERAS) program. Surg Endoscopy. (2017) 31:5283–5288. doi: 10.1007/s00464-017-5602-1
34. Liu X, Wang Y, Fu Z. Impact of enhanced recovery after surgery on postoperative neutrophil–lymphocyte ratio in patients with colorectal cancer. J Int Med Res. (2020) 48:30006052092594. doi: 10.1177/0300060520925941
35. Gonvers S, Jurt J, Joliat G-R, Halkic N, Melloul E, Hübner M, et al. Biological impact of an enhanced recovery after surgery programme in liver surgery. BJS Open. (2021) 5:zraa015. doi: 10.1093/bjsopen/zraa015
36. Nishiguchi K, Okuda J, Toyoda M, Tanaka K, Tanigawa N. Comparative evaluation of surgical stress of laparoscopic and open surgeries for colorectal carcinoma. Dis Colon Rectum. (2001) 44:223–30. doi: 10.1007/BF02234297
37. Warschkow R, Steffen T, Beutner U, Müller SA, Schmied BM, Tarantino I. Diagnostic accuracy of C-reactive protein and white blood cell counts in the early detection of inflammatory complications after open resection of colorectal cancer: a retrospective study of 1187 patients. Int J Colorectal Dis. (2011) 26:1405–13. doi: 10.1007/s00384-011-1262-0
38. Labgaa I, Joliat GR, Demartines N, Hubner M. Serum albumin is an early predictor of complications after liver surgery. Dig Liver Dis. (2016) 48:559–61. doi: 10.1016/j.dld.2016.01.004
Keywords: enhanced recovery after surgery, lung resection, thoracic surgery, postoperative pulmonary complications, day surgery
Citation: Ding Y, Zhou L, Shan L, Zhang W, Li P, Cong B, Tian Z, Zhao Y and Zhao X (2024) Video- assisted thoracoscopic lung resection with or without enhanced recovery after surgery: a single institution, prospective randomized controlled study. Front. Oncol. 14:1474438. doi: 10.3389/fonc.2024.1474438
Received: 01 August 2024; Accepted: 22 October 2024;
Published: 08 November 2024.
Edited by:
Han-Yu Deng, Sichuan University, ChinaReviewed by:
Massimo Baudo, Lankenau Institute for Medical Research, United StatesJia Huang, Shanghai Jiao Tong University, China
Copyright © 2024 Ding, Zhou, Shan, Zhang, Li, Cong, Tian, Zhao and Zhao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yunpeng Zhao, emhhb3l1bnBlbmdzZGR4QHNkdS5lZHUuY24=; Xiaogang Zhao, emhhb3hpYW9nYW5nQHNkdS5lZHUuY24=
†These authors have contributed equally to this work
 Yi Ding
Yi Ding Leiming Zhou2
Leiming Zhou2 Weiquan Zhang
Weiquan Zhang Yunpeng Zhao
Yunpeng Zhao Xiaogang Zhao
Xiaogang Zhao