- 1Department of Scientific Research and Foreign Affairs, Henan Provincial People’s Hospital, Zhengzhou University People’s Hospital, Zhengzhou, Henan, China
- 2School of Computer Science and Technology, University of Science and Technology of China, Hefei, Anhui, China
- 3Department of Pathology, Henan Provincial People’s Hospital, Zhengzhou University People’s Hospital, Zhengzhou, Henan, China
- 4Department of Hepatobiliary and Pancreatic Surgery, Henan Provincial People’s Hospital, Zhengzhou University People’s Hospital, Zhengzhou, Henan, China
Background and aims: The levels of M2 macrophages are significantly associated with the prognosis of hepatocellular carcinoma (HCC), however, current detection methods in clinical settings remain challenging. Our study aims to develop a weakly supervised artificial intelligence model using globally labeled histological images, to predict M2 macrophage levels and forecast the prognosis of HCC patients by integrating clinical features.
Methods: CIBERSORTx was used to calculate M2 macrophage abundance. We developed a slide-level, weakly-supervised clustering method for Whole Slide Images (WSIs) by integrating Masked Autoencoders (MAE) with ResNet-32t to predict M2 macrophage abundance.
Results: We developed an MAE-ResNet model to predict M2 macrophage levels using WSIs. In the testing dataset, the area under the curve (AUC) (95% CI) was 0.73 (0.59-0.87). We constructed a Cox regression model showing that the predicted probabilities of M2 macrophage abundance were negatively associated with the prognosis of HCC (HR=1.89, p=0.031). Furthermore, we incorporated clinical data, screened variables using Lasso regression, and built the comprehensive prediction model that better predicted prognosis. (HR=2.359, p=0.001).
Conclusion: Our models effectively predicted M2 macrophage levels and HCC prognosis. The findings suggest that our models offer a novel method for determining biomarker levels and forecasting prognosis, eliminating additional clinical tests, thereby delivering substantial clinical benefits.
1 Introduction
According to the Global Cancer Statistics 2020, liver cancer is the third leading cause of cancer-related mortality, accounting for 8.3% of deaths globally, and the seventh most commonly diagnosed malignancy, comprising 4.7% of all cancer cases, causing serious public health problems and economic burden (1).
Tumor-associated macrophages (TAMs), which originate from bone marrow-derived blood monocytes (monocyte-derived macrophages) or yolk sac progenitors (tissue-resident macrophages), are a specialized subset of immune cells that are abundantly present in the tumor microenvironment of various solid tumors (2). There are two polarization types of TAMs. Under the stimulation of lipopolysaccharide (LPS), IFN-γ, etc., TAMs are activated into classically activated macrophages (M1 type). Conversely, under the stimulation of anti-inflammatory factors such as IL-10, TGF-β, etc., TAMs are activated into alternatively activated macrophages (M2 type) (3). Previous research indicates that M2 macrophages play a critical role in cancer-related inflammation and are involved in various aspects of tumor biology, including immunosuppression, tumor progression, invasion, and metastasis (2, 4–8).
CIBERSORTX, a bioinformatics tool developed by the Alizadeh Lab and Newman Lab, can be used to estimate the abundance of various cell types in a mixed cell population based on gene expression data (4). However, this approach relies on transcriptome sequencing, which is not a commonly performed clinical test and can be expensive. In recent years, image recognition technology has become increasingly prevalent in the medical field. Pathological tissues are routinely collected during the diagnosis and treatment of patients undergoing HCC surgery, and Whole Slide Images (WSIs) can provide valuable insights into tumor heterogeneity. Predicting the relative abundance of M2 macrophages directly from WSI images and subsequently forecasting patient survival would not only be more cost-effective and resource-efficient than the CIBERSORTx method but also highly convenient and promotable.
Implementing supervised learning in deep neural networks requires a substantial amount of labeled data. However, manual data labeling is both time-consuming and costly. Additionally, acquiring sufficient data in the medical field remains a significant challenge. WSIs typically contain more than 100,000 x 100,000 pixels (9), rendering them impractical for direct processing by convolutional neural networks (CNNs). The conventional approach involves dividing a WSI into patch-level images, often numbering over 10,000, and then individually annotating each image. However, this process also demands considerable human effort. Some studies have attempted to address this issue using weakly supervised clustering methods. The main strategies include increasing the sample size of the training set (10) and employing transfer learning techniques (11).
Therefore, the primary bottleneck in current computational pathology is the annotation of labels and the limited quantity of samples. The key technology employed in this study is the Masked Autoencoder (MAE), a self-supervised autoencoder that masks a portion of the input image and attempts to predict the masked tiles based on the visible tiles (12).
While self-supervised learning methods are widely used in natural language processing (NLP) (13, 14), the field of computer vision (CV) predominantly relies on supervised methods. Recent research by Kaiming He and colleagues has demonstrated that for visually dense images with high information redundancy, constructing a challenging task by masking a substantial proportion of patches can yield results comparable or even superior to those of supervised training (12).
In our study, we introduce a novel MAE-ResNet modeling method that integrates MAE with ResNet-32t, presenting a slide-level, weakly-supervised clustering approach for WSIs. Based on this, we developed and validated models to determine M2 macrophage levels and further predict patient prognosis.
2 Methods
2.1 Datasets
The data used in this study were sourced from Henan Provincial People’s Hospital and The Cancer Genome Atlas (TCGA) database, a comprehensive multi-center repository containing genomic data, clinical information, and pathological slides from various hospitals worldwide (15). The HCC cases from both sources contained information such as age, gender, stage, grade, T, N, M, surgery type, and overall survival. On this basis, the inclusion criteria for our study were: 1) pathologically diagnosed HCC, and 2) availability of at least one formalin-fixed and paraffin-embedded slide. The exclusion criteria were: 1) metastatic liver cancer, and 2) concurrent malignancies. For the TCGA dataset, in addition to the above criteria, RNA sequencing data were also required. Ultimately, the study included 132 HCC patients from the hospital and 353 from the TCGA database. This study aims to establish two models: one model to predict the relative abundance of M2 macrophages and another to forecast the prognosis of HCC patients using WSIs. The TCGA dataset is used to build and validate M2 macrophage level prediction model, while both the TCGA and hospital datasets are used for prognosis prediction.
2.2 Calculation of the relative abundance of M2 macrophages
We first obtained the RNA sequencing profile of HCC from TCGA database and downloaded the corresponding gff3 file (http://ftp.ebi.ac.uk/pub/databases/gencode/Gencode_human/release_22/gencode.v22.anno tation.gff3.gz) from GENCODE (https://www.gencodegenes.org/human/). Using gene length and gene count data, we calculated the Transcripts Per Million (TPM) for each gene. We employed CIBERSORTx to calculate the relative abundance of M2 macrophages. The tool is available at https://cibersortx.stanford.edu/. LM22 (22 immune cell types) was used as signature matrix file. We categorized the relative abundance of M2 macrophages into two groups: high and low. Subsequently, we developed a ResNet model, which was trained and validated to effectively distinguish between high and low relative abundances of M2 macrophages.
2.3 MAE-based weakly supervised learning method
MAE is a self-supervised autoencoder. We integrated MAE with ResNet-32t to introduce a slide-level, weakly-supervised clustering method for WSIs in order to predict the relative abundance of M2 macrophages. MAE demonstrates strong performance when a significant proportion of the input images are masked (12). Building on this, we utilized the encoder component of the MAE architecture to encode pathological images. These encoded images were then used to classify the relative abundance of M2 macrophages and to predict survival rates, using slide-level labels. In this M2 macrophage level prediction model, we randomly divided the TCGA dataset into training, validation, and testing sets in a 7:1.5:1.5 ratio. The overall training process was illustrated in Figure 1. The code for our study is available on GitHub (https://github.com/MinfanZhao/M2-HCC-Prognosis).
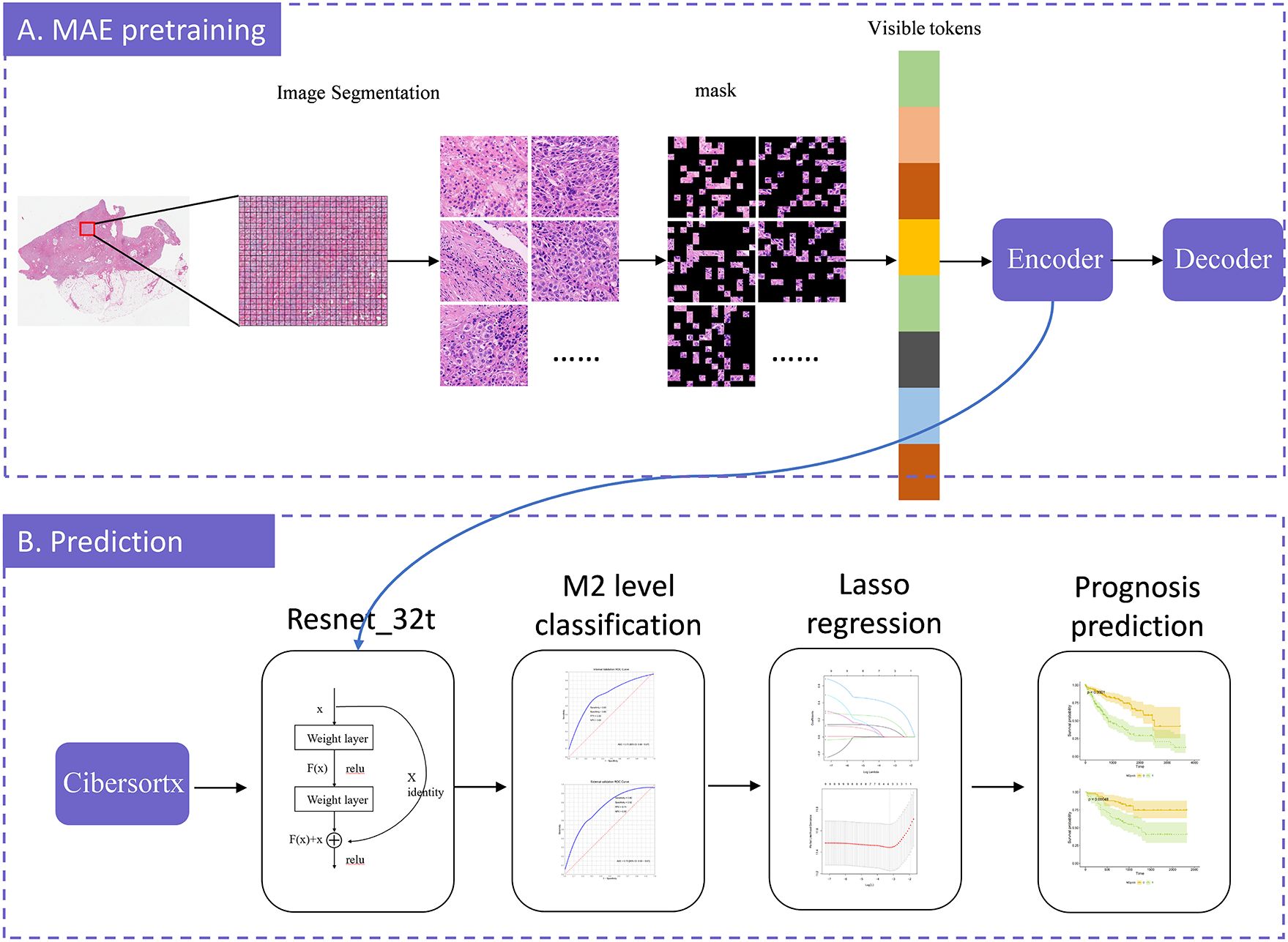
Figure 1. The model-training process illustration. The model construction consists of two stages: MAE-pretraining and prediction. In the MAE-pretraining phase, we first divided each slide into patches, and then split each patch into tiles. We randomly masked a portion of the tiles and predicted these masked tiles. The model was continuously optimized by minimizing the difference between the predicted tiles and the original image, iteratively improving prediction accuracy. Once the optimal prediction model was identified, we exported the encoder component to serve as the input for ResNet-32t. We employed CIBERSORTx to calculate the relative abundance of M2 macrophages and, based on these calculations, established a model to predict the relative abundance of M2 macrophages from WSI. We further employed LASSO regression to select potential prognostic factors and, by combining these factors with the predicted relative abundance of M2 macrophages, predicted overall survival. Finally, the model was validated using an external dataset.
2.3.1 Pre-processing
In the image preparation stage, we initially performed color standardization using histogram equalization. Due to the large size of WSI images and video memory limitations, they cannot be directly processed within the model. To address this issue, we segmented the images into smaller patches and resize them to 512 × 512 pixels. Additionally, we discarded patches containing more than 50% background areas.
2.3.2 Model training
The model training process comprises two components: MAE pre-training and ResNet-32t.
MAE pre-training can be divided into two asymmetrical components: an encoder and a decoder. The encoder was essentially a Visual Transformer (VIT) network, and in this study, the adopted model was ViT-large. In ViT-large, a random section of the input image was masked, and subsequently, the missing pixels were reconstructed. Each patch was further resized into tiles. We randomly shuffled these tiles, selected 25% for encoding, and masked the remaining 75%. Positional embeddings, along with 25% of the encoded tiles, are fed into the decoder to maintain the original order of the image blocks. The decoder then attempted to reconstruct the original image using the input information provided. Ultimately, the output image from the decoder was compared with the original image to compute the error. This error was backpropagated to update the model’s parameters. Through extensive training with WSI patches, the artificial intelligence model learned the information within the images and reconstructs them, thereby completing the MAE model training.
Subsequently, we retained only the encoder’s output, which consists of 1024-dimensional vectors, fed these into ResNet-32t to obtain the final classification results. This transformation enables the simultaneous loading of all WSI patches into GPU memory. Considering “Cluster High” as the positive class, our trained ResNet-32t predicted and categorized all slides as either “Cluster High” or “Cluster Low”.
2.3.3 Model testing
We used the validation dataset to assess the classification performance of the proposed method for weight selection during ResNet-32t training. We evaluated the AUC (Area Under the Curve) of our method by comparing the predicted levels of M2 macrophages in the testing dataset with the ground truth from liver histopathological slides.
Additionally, we calculated the ROC curves and AUC for classifying the relative abundance of M2 macrophages using the “pROC” package in R. We also computed the sensitivity, specificity, PPV (Positive Predictive Value), and NPV (Negative Predictive Value) for the optimal cut-off value and visualized the results using the ggplot2 package in R.
2.4 Prognosis prediction
Given the well-documented correlation between the relative abundance of M2 macrophages and tumor prognosis, we used the predicted probabilities of M2 macrophage levels (M2prob) from our MAE-ResNet model to predict the overall survival of HCC patients. Additionally, we aimed to develop a prognostic model using M2prob in conjunction with clinical data. In this prognosis prediction model, we used the TCGA dataset as the training set and the hospital data as the testing set. All statistical analyses were performed using R version 4.2.3.
2.4.1 Data cleaning and clinical features
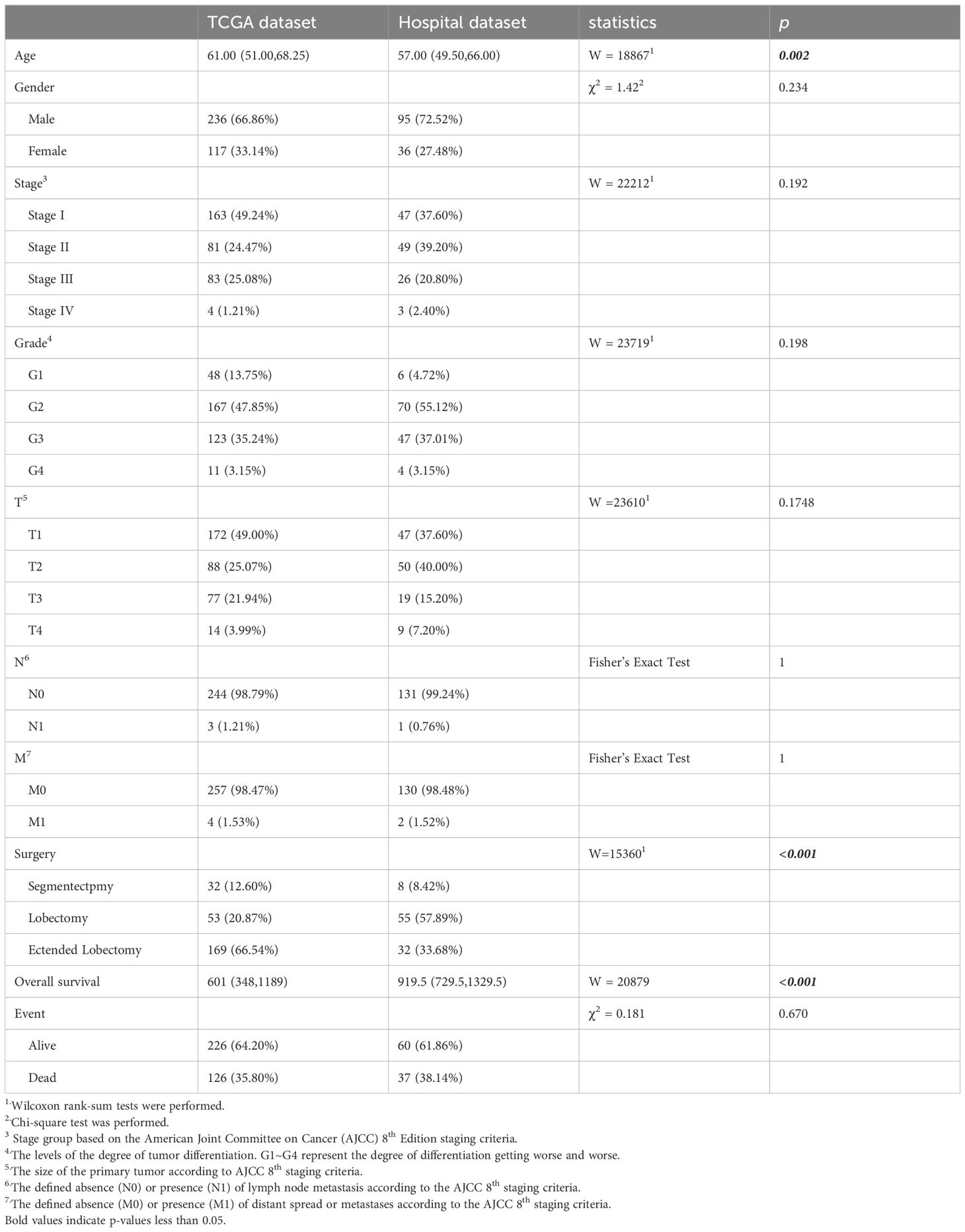
The independent variables included in the analysis were T (the range and size of the primary tumor), N (lymph node metastasis), M (the presence of metastasis), age, gender, stage, grade, surgery, overall survival, and event. General clinical information of the subjects and statistical tests for differences between the training and testing datasets are presented in Table 1. We performed t-tests, Wilcoxon rank-sum tests, chi-squared tests, or Fisher’s Exact Test, depending on the data type. Shapiro-Wilk tests were conducted for continuous variables to assess normality. For variables that met the criteria for normal distribution, t-tests were used; for those that did not, Wilcoxon rank-sum tests were employed. For categorical variables, Fisher’s exact test or chi-square tests were applied based on theoretical frequencies; for ordinal variables, the Wilcoxon rank-sum test was used. For missing values, multiple imputation was employed to fill the gaps. Table 1 displays the original data prior to imputation.
2.4.2 Relationship between M2prob and prognosis
We utilized a Cox regression model to assess the association between M2prob and the prognosis of HCC patients in the TCGA dataset, subsequently validating these findings in the hospital dataset. Cox regression analysis was conducted using the “survival” package in R.
2.4.3 Survival prediction model construction
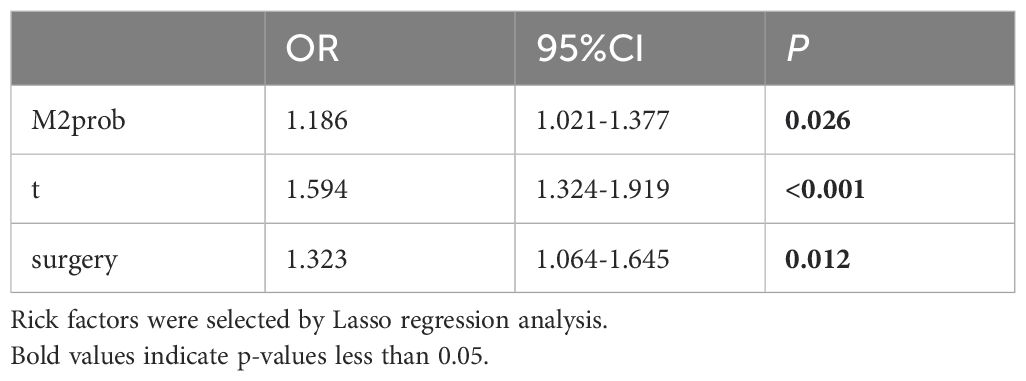
General clinical information is closely associated with the prognosis of HCC. We contemplated the integration of general clinical information and M2prob to establish a prediction model. Lasso regression was employed to preliminarily screen all variables presented in Table 1, and the model with the smallest mean squared error (MSE) was selected. Subsequently, we used this model to calculate the Mscore, predicted the prognosis of HCC, and verified it in the validation set.
2.5 Ethical declaration
Ethical approval for this study was granted by the Ethical Committee of Henan Provincial People’s Hospital in 2022. All procedures performed in this study adhered to the ethical standards of both the institutional and national research committees, as well as the Declarations of Helsinki and Istanbul.
3 Results
3.1 Clinical features
In the prognosis prediction section, TCGA patients were employed for model training, and patients from Henan Provincial People’s Hospital were used for validation. The following clinical variables were tested: age, gender, stage, grade, T (tumor size), N (lymph node involvement), M (metastasis presence), surgery, overall survival, and event status). The testing results are presented in Table 1. In the TCGA dataset, the median age of study subjects was 61 years (interquartile range: 51.00-68.25), with an median overall survival of 601 days (interquartile range: 348-1189) and an outcome event incidence rate of 35.80%. In the hospital dataset, the median age of the study subjects was 57 years (interquartile range: 49.50-66.00), with an median overall survival of 919.5 days (interquartile range: 729.5-1329.5) and an outcome event incidence rate of 38.14%. Age (p=0.002), surgery (p<0.001), and overall survival (p<0.001) showed significant differences between the two datasets. All other p-values from statistical tests were greater than 0.05.
3.2 TAM calculation
The relative abundance of M2 macrophages was determined using the CIBERSORTx algorithm. The median values (interquartile ranges) for M2 macrophages were 4.78 (3.86-5.58) in the training set, 4.70 (3.76-5.63) in the validation set, and 4.84 (4.05-5.86) in the testing set, respectively. HCC patients were categorized into groups with high and low M2 macrophage content (M2group).
3.3 WSI classification performance
Based on WSIs, we developed a model using the MAE approach to predict the relative proportion of M2 macrophages. In the validation dataset, the AUC (95% CI) was 0.73 (0.59-0.87), with sensitivity, specificity, PPV, and NPV values of 0.63, 0.83, 0.83, and 0.63, respectively. In the testing dataset, the AUC (95% CI) was 0.73 (0.59-0.87), with sensitivity, specificity, PPV, and NPV values of 0.90, 0.52, 0.71, and 0.80, respectively. ROC curves are shown in Figures 2A and B. The confusion matrices are presented in Figure 3.
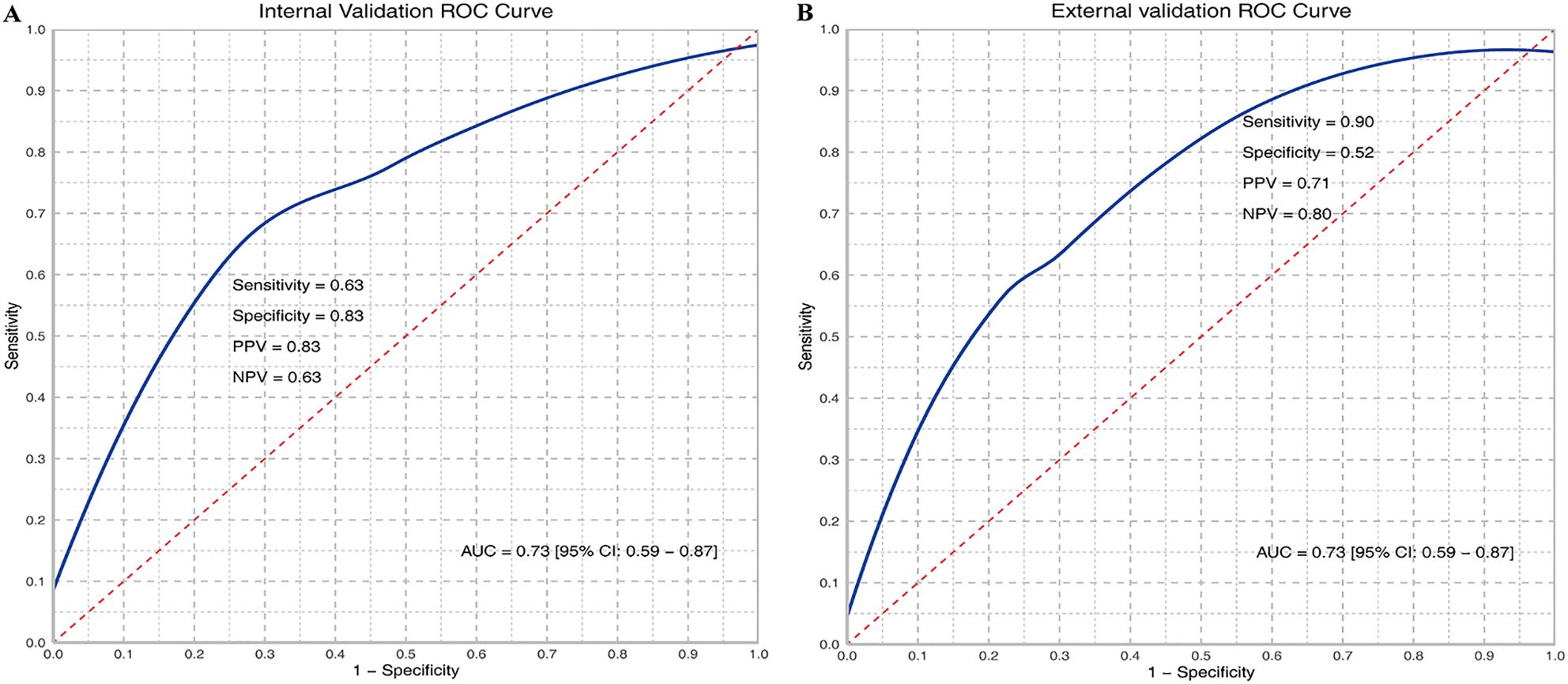
Figure 2. ROC curves of M2 relative abundance in internal (A) and external validation (B). TP, True Positive; TN, True Negative; FP, False Positive; FN, False Negative; Sensitivity = TP/(TP + FN); Specificity = TN/(TN + FP); PPV = TP/(TP + FP); NPV = TN/(TN + FN); AUC, Area under the curve.
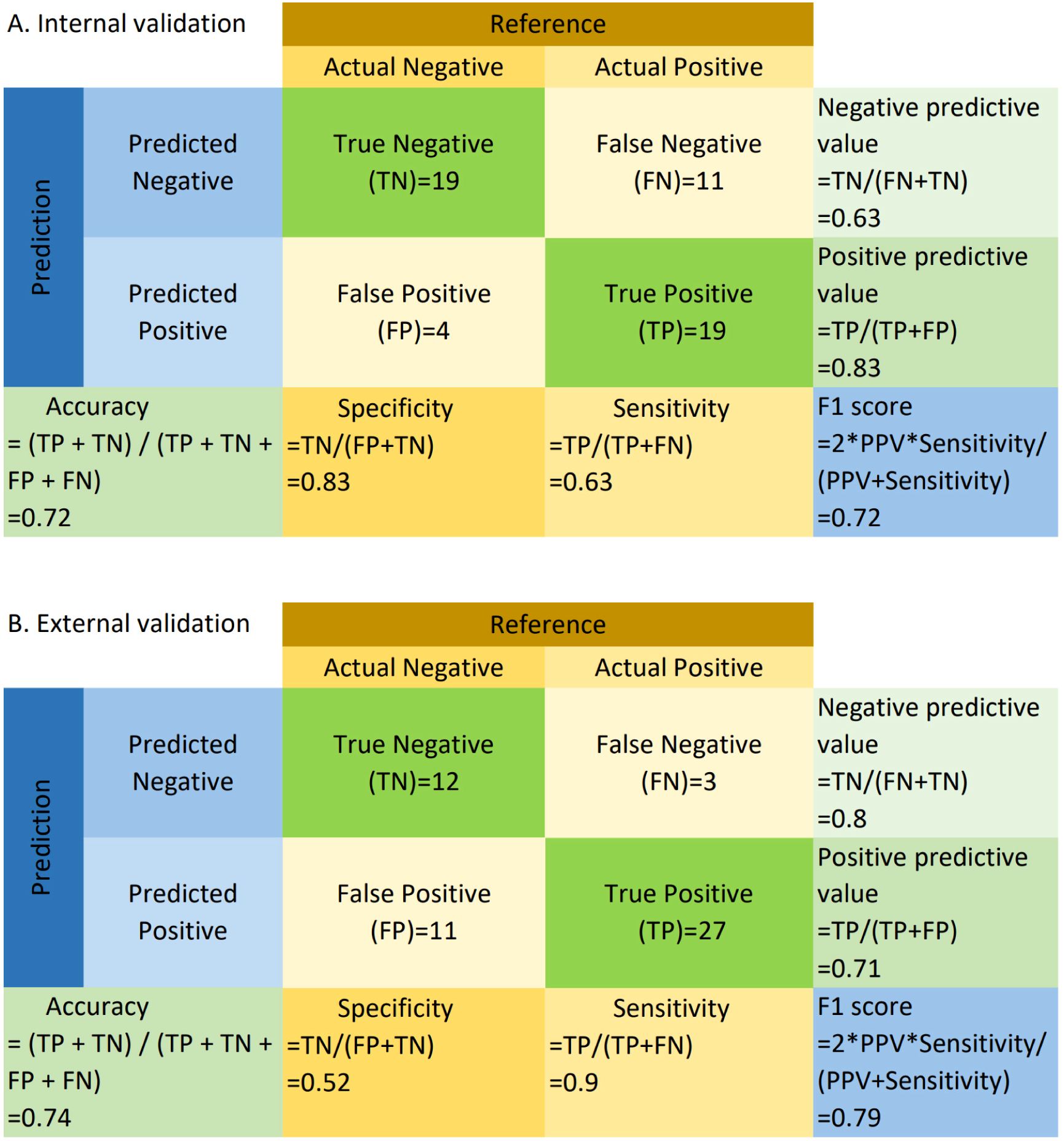
Figure 3. Confusion matrix for internal (A) and external validation (B). TP, True Positive; TN, True Negative; FP, False Positive; FN, False Negative; Sensitivity = TP/(TP + FN); Specificity = TN/(TN + FP); PPV = TP/(TP + FP); NPV = TN/(TN + FN); Accuracy = (TP + TN)/(TP + TN + FP + FN); F1 = 2 * (PPV * Sensitivity)/(PPV + Sensitivity). “Negative” refers to cases where the relative abundance of M2 macrophages is less than or equal to the median, and “positive” refers to cases where the relative abundance of M2 macrophages exceeds the median.
3.4 Prognostic prediction
3.4.1 M2prob and overall survival
We calculated predicted M2 macrophages proportion based on the WSIs. In the TCGA dataset, we included the M2 macrophages proportion, multiplied by 10 (M2prob), as an independent variable in the Cox regression equation to assess the impact of every 10% increase in M2 macrophages on prognosis. The results of the Cox regression analysis showed that M2 macrophage proportion was significantly associated with overall survival (HR=1.242, P=0.004), indicating statistical significance and suggesting that M2prob may influence the prognosis of liver cancer patients. Specifically, for every 10% increase in M2 macrophage proportion, the risk of death for patients increased by 24.2%. In the hospital dataset, M2prob remained significantly associated with prognosis (HR=1.89, P=0.031), with the risk of death for patients increasing by 89% for every 10% increase in M2 macrophage proportion. These results further supports its potential value as a prognostic factor.
3.4.2 Mscore and overall survival
Lasso regression was employed to identify risk factors. The coefficients of factors that finally enter the equation were shown in Table 2. Figure 4A showed the variation traits of the coefficient of factors. Ten-fold cross-validation method was used in the screening process, and a model with the smallest mean square error (MSE) were finally chosen (Figure 4B). Mscores were further calculated based on the screened risk factors and their coefficients in the Cox regression equation. The association between Mscore and survival prognosis in both the training and validation sets were calculated and Kaplan-Meier survival curves was constructed (Figures 5A, B).
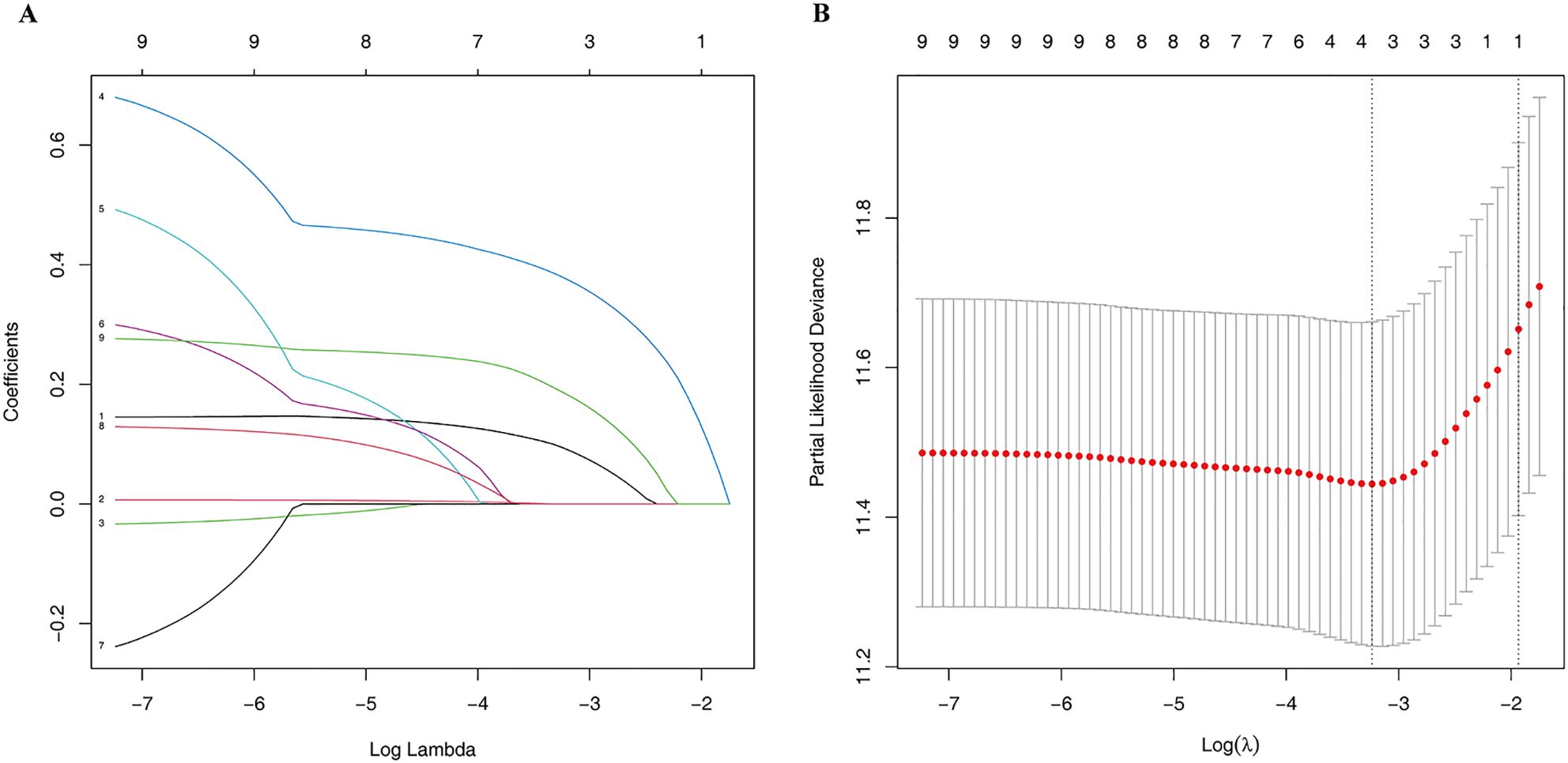
Figure 4. The Lasso regression process. The variation traits of the coefficient of factors (A) and the screening process of the Lasso regression model by 10-fold cross-validation (B).
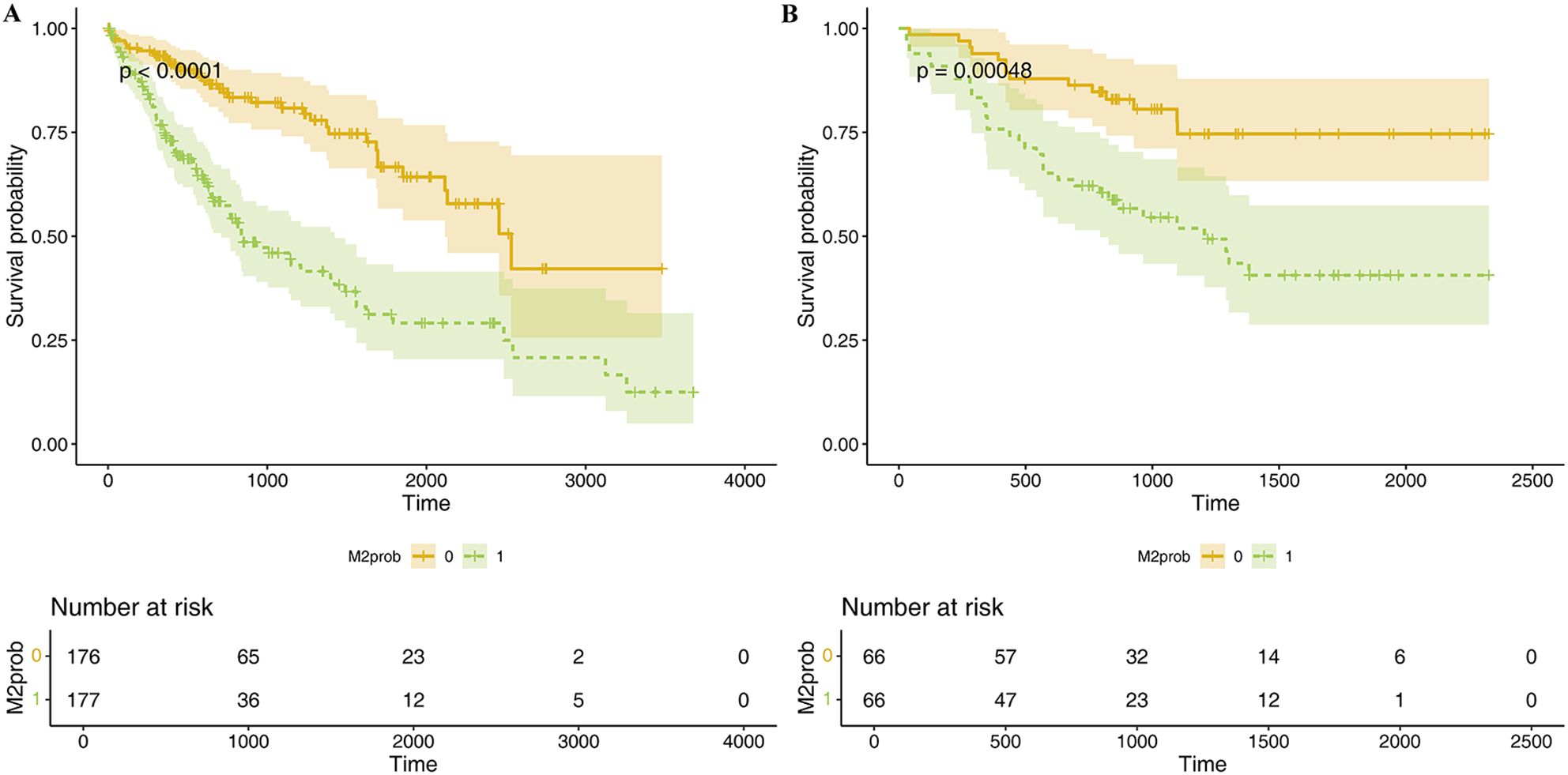
Figure 5. K-M survival curve of Mscore for predicting HCC prognosis in TCGA dataset (A) and hospital dataset (B). The curves illustrate the survival differences between high and low Mscore groups, with statistical significance assessed by the log-rank test. Below each curve, the "number at risk" table is displayed, indicating the number of patients remaining at each time point in both groups.
4 Discussion
This study predicts M2 macrophage levels from WSIs using only global labels, and forecasts prognosis based on M2prob. To our knowledge, this is the first report of cell-level WSI prediction using the MAE-ResNet model. This study shows that using a deep learning method on WSIs can predict M2 macrophage levels. This capacity enables the prognosis of HCC patients without the need for additional clinical tests. Although further validation is needed through prospective studies, constructing an MAE-ResNet model shows promise for extracting vital clinical information from WSIs.
Tumor microenvironment plays a crucial role in tumor progression, comprising a diverse array of cells including tumor-associated macrophages, cancer-associated fibroblasts, endothelial cells, dendritic cells, B cells, T cells, and lymphocytes, etc. (16, 17) Macrophages are the most abundant of these (18). M2 macrophages, often called anti-inflammatory macrophages, are characterized by their production of anti-inflammatory mediators such as IL-10, transforming growth factor-beta, and arginase 1 (19). M2 macrophages are induced through several key signaling pathways. TGF-β binds to its receptors on macrophages, activating the PI3K/Akt/mTOR and TGF-β/Smad pathways. The PI3K/Akt/mTOR pathway regulates protein translation and transcription, while the TGF-β/Smad pathway controls gene expression by activating Smad proteins. IL-4 and IL-6 binding to their receptors trigger the JAK/STAT pathway, activating STAT3 and STAT6, which induce M2 gene expression. Notch ligand-receptor interactions activate the Notch pathway, leading to the expression of M2-related genes like Arg-1 and IL-10. The Wnt/β-catenin pathway also contributes by stabilizing β-catenin, which regulates genes involved in inflammation resolution. Hypoxia-inducible factor-2α (HIF-2α) plays a role in both the Notch and Wnt/β-catenin pathways under low-oxygen conditions, promoting an anti-inflammatory phenotype. Together, these pathways drive M2 macrophage polarization and contribute to their pro-tumorigenic functions, such as immune suppression and tissue remodeling (20). Specifically, M2 macrophages suppress the host’s anti-tumor immunity by increasing the expression of immunosuppressive surface proteins, producing reactive oxidants, secreting T cell inhibitory cytokines, and releasing chemokines that attract regulatory T cells, thereby diminishing the anti-tumor activity of effector T cells. Moreover, M2 macrophages could promote angiogenesis and enhance tumor invasion and metastatic potential through mechanisms such as VEGF and MMP (21).
Previous studies have shown that M2 macrophages are closely related to the prognosis of tumors. Regulating the polarization level through certain molecules can affect tumor progression. Research by Z. He et al. showed that exosome-derived FGD5-AS1 can promote the malignant behaviors of pancreatic cancer cells by promoting M2 polarization (22). Furthermore, the study by Rui Xu et al. highlights the significant role of M2 macrophages in HCC prognosis by identifying M2 macrophage-related genes through weighted gene co-expression network analysis (WGCNA), and establishing a 5-gene signature associated with immune infiltration for reliable prognostic assessment (23). In terms of disease treatment, trying to promote M1-type polarization or inhibit M2-type polarization to achieve anti-tumor purposes has also become a prominent research focus. Researches have been conducted on various tumors, such as ovarian cancer (24), Colorectal Cancer (25), Glioblastoma (26), etc. The research results of Bufu Tang et al. showed that xCT-specific knockout can limit HCC metastasis risk in transgenic mouse models by inhibiting M2-type polarization (27). There are also studies showing that Cancer treatment strategies that reprogram M2-like TAMs into M1-like cells have also yielded encouraging results (28).
In general, it is widely acknowledged that M2 macrophages facilitate tumor growth and migration (5, 29–32). Therefore, a convenient and effective method to predict M2 macrophage levels in daily diagnosis and treatment would provide a solid foundation for clinical decision-making and prognosis prediction. However, conventional detection methods like flow cytometry (33) and transcriptome-based CIBERSORTx continue to pose challenges in routine clinical diagnosis and treatment. In recent years, with the development of computer vision, the use of WSIs has broadened beyond pathology teaching and remote diagnosis (34). An increasing number of studies focused on directly diagnosing or classifying tumors through WSI. This research field, known as “computational disease” (35), is expected to profoundly change cancer diagnosis methods. Previous studies have demonstrated that deep learning methods using WSI can predict gene-level features (36–40) and cell-level features (41–43), thus, we believe extracting biomedical information from WSI holds significant research potential. Unfortunately, existing methodologies fall short in addressing the significant challenges presented by the high resolution of WSIs (44), a situation exacerbated by the shortage of comprehensively annotated datasets (45). In order to address this problem, numerous studies have segmented WSIs into patches and utilized the Multiple Instance Learning paradigm pretrained on ImageNet (9, 46, 47). However, models pretrained on ImageNet might not effectively identify distinctive medical features. In 2022, He et al. introduced a self-supervised learning method for computer vision called MAE (12). This method, which includes an encoder and a decoder, randomly masks parts of the input image and then reconstructs it.
In our study, we present a new MAE-ResNet modeling method, which uses the encoder part of MAE to extract WSI features, thus avoiding patch-level manual annotation work and predicting the relative abundance of M2 macrophage based only on slide-level labels. Our model offering the following advantages: (1) Compared to traditional CNN models, it eliminates the need for extensive work of manual annotation. The pixel size of WSIs is too large to be directly processed by CNN models under the constraints of the current GPU memory conditions. Traditional CNN models address this by dividing a WSI into thousands of smaller patches for annotation. However, this task is often unfeasible for busy pathologists, limiting many valuable studies in practice. The MAE-ResNet model we use, through a self-supervised autoencoder, eliminates this large-scale annotation workload. It employs an unsupervised approach by masking parts of the image and continuously attempting to predict the masked portions, thereby extracting image features. This innovative solution to the large pixel size problem eliminates the need for extensive annotation efforts. By simplifying the workload, it also paves the way for future studies with larger sample sizes. (2) Compared to traditional biological methods, such as flow cytometry and transcriptome-based CIBERSORTx, the model used in our study is more cost-effective and easier to implement in clinical settings, as it requires only routine clinical pathology sections and does not add extra workload or costs, providing a significant advantage for future clinical translation.
After developing the MAE-ResNet model to predict M2 macrophage levels, we further explored whether M2 macrophage levels and clinical characteristics could predict the prognosis of HCC patients. At this stage, data from the TCGA database were used for training and internal validation, while data collected from our hospital were used for external validation. The internal validation set (TCGA dataset) was used to assess the reproducibility of the model, while data from our hospital were used to evaluate the transportability of the initial model’s predictions. The following cox regression analysis indicated that the predicted probabilities of M2 macrophages was negatively associated with the prognosis of HCC. To enhance the generalizability of the model, we incorporated common covariates related to HCC prognosis into the predictive model. CIBERSORT deconvolution algorithm, weighted gene co-expression network analysis (WGCNA), and the LASSO algorithm are frequently used for dimensionality reduction (48), in this study, we employed the LASSO regression algorithm. Based on TAM prediction probability and clinical data, the prognostic prediction model we constructed also achieved satisfactory results in internal and external validation data sets, indicating good reproducibility and transportability for this model.
Despite the promising results of our study, several limitations must be acknowledged. First, ensuring the consistency and quality of pathological slides presents a significant challenge. To address this, we employed color standardization through histogram equalization during the image preparation stage, which helped reduce variations in color intensity and brightness caused by differences in slide sources or laboratory conditions. Additionally, our external validation set, sourced from Henan Provincial People’s Hospital, showed strong predictive performance. This may be partly due to the fact that the TCGA database contains slides from different institutions, which allowed our training set to learn the technical differences between these institutions. Another limitation is that the number of pathological images used in this study is relatively small, and a larger dataset is necessary to obtain more robust and generalizable results.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by The Ethical Committee of Henan Provincial People’s Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
HT: Conceptualization, Methodology, Visualization, Writing – original draft. YT: Formal Analysis, Validation, Writing – original draft. DL: Data curation, Methodology, Writing – review & editing. MZ: Formal Analysis, Validation, Writing – review & editing. QL: Data curation, Writing – review & editing. LK: Supervision, Writing – review & editing. TQ: Conceptualization, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Henan Province Medical Science and Technology Research Plan Project (Project No. LHGJ20220052), which provided financial support for the publication fees.
Acknowledgments
During the preparation of this work the author(s) used ChatGPT by OpenAI in order to improve the text's readability. After using this tool/service, the author(s) reviewed and edited the content as needed and take(s) full responsibility for the content of the publication.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
AJCC, the American Joint Committee on Cancer; AUC, Area Under the Curve; CV, Computer Vision; HCC, Hepatocellular Carcinoma; M2prob, M2 macrophage levels; MAE, Masked Autoencoders; MSE, Mean Square Error; NLP, Natural Language Processing; NPV, Negative Predictive Value; PPV, Positive Predictive Value; TAMs, Tumor-associated macrophages; TCGA, The Cancer Genome Atlas; TPM, Transcripts Per Million; WSI, Whole Slide Images.
References
1. Sung H, Ferlay J, Siegel RL. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
2. Komohara Y, Fujiwara Y, Ohnishi K, Takeya M. Tumor-associated macrophages: Potential therapeutic targets for anti-cancer therapy. Adv Drug Delivery Rev. (2016) 99:180–5. doi: 10.1016/j.addr.2015.11.009
3. Qiu SQ, Waaijer SJH, Zwager MC, de Vries EGE, van der Vegt B, Schröder CP. Tumor-associated macrophages in breast cancer: Innocent bystander or important player? Cancer Treat Rev. (2018) 70:178–89. doi: 10.1016/j.ctrv.2018.08.010
4. Newman AM, Steen CB, Liu CL, Gentles AJ, Chaudhuri AA, Scherer F, et al. Determining cell type abundance and expression from bulk tissues with digital cytometry. Nat Biotechnol. (2019) 37:773–82. doi: 10.1038/s41587-019-0114-2
5. Zhao S, Mi Y, Guan B, Zheng B, Wei P, Gu Y, et al. Tumor-derived exosomal miR-934 induces macrophage M2 polarization to promote liver metastasis of colorectal cancer. J Hematol Oncol. (2020) 13:156. doi: 10.1186/s13045-020-00991-2
6. Xiao M, Bian Q, Lao Y, Yi J, Sun X, Sun X, et al. SENP3 loss promotes M2 macrophage polarization and breast cancer progression. Mol Oncol. (2022) 16:1026–44. doi: 10.1002/1878-0261.12967
7. Cheng Y, Zhong X, Nie X, Gu H, Wu X, Li R, et al. Glycyrrhetinic acid suppresses breast cancer metastasis by inhibiting M2-like macrophage polarization via activating JNK1/2 signaling. Phytomedicine. (2023) 114:154757. doi: 10.1016/j.phymed.2023.154757
8. Lu Y, Han G, Zhang Y, Zhang L, Li Z, Wang Q, et al. M2 macrophage-secreted exosomes promote metastasis and increase vascular permeability in hepatocellular carcinoma. Cell Commun Signal. (2023) 21:299. doi: 10.1186/s12964-022-00872-w
9. Sun C, Xu A, Liu D, Xiong Z, Zhao F, Ding W. Deep learning-based classification of liver cancer histopathology images using only global labels. IEEE J Biomed Health Inf. (2020) 24:1643–51. doi: 10.1109/JBHI.2019.2949837
10. Mahmood F, Borders D, Chen RJ, McKay GN, Salimian KJ, Baras A, et al. Deep adversarial training for multi-organ nuclei segmentation in histopathology images. IEEE Trans Med Imaging. (2020) 39:3257–67. doi: 10.1109/tmi.2019.2927182
11. Coudray N, Ocampo PS, Sakellaropoulos T, Narula N, Snuderl M, Fenyö D, et al. Classification and mutation prediction from non-small cell lung cancer histopathology images using deep learning. Nat Med. (2018) 24:1559–67. doi: 10.1038/s41591-018-0177-5
12. He K, Chen X, Xie S, Li Y, Dollár P, Girshick R. Masked autoencoders are scalable vision learners. In: Proceedings of the IEEE/CVF Conference on Computer Vision and Pattern Recognition (USA: IEEE) (2022) pp. 16000–9.
13. Lee J, Yoon W, Kim S, Kim D, Kim S, So CH, et al. BioBERT: a pre-trained biomedical language representation model for biomedical text mining. Bioinformatics. (2020) 36:1234–40. doi: 10.1093/bioinformatics/btz682
14. Yang Z, Dai Z, Yang Y, Carbonell J, Salakhutdinov RR, Le QV. Xlnet: Generalized autoregressive pretraining for language understanding. Adv Neural Inf Process Syst. (2019) 32.
15. Cancer Genome Atlas Research Network. Comprehensive and integrative genomic characterization of hepatocellular carcinoma. Cell. (2017) 169:1327–1341.e1323. doi: 10.1016/j.cell.2017.05.046
16. Zhang G, Gao Z, Guo X, Ma R, Wang X, Zhou P, et al. CAP2 promotes gastric cancer metastasis by mediating the interaction between tumor cells and tumor-associated macrophages. J Clin Invest. (2023) 133. doi: 10.1172/jci166224
17. Qin R, Ren W, Ya G, Wang B, He J, Ren S, et al. Role of chemokines in the crosstalk between tumor and tumor-associated macrophages. Clin Exp Med. (2023) 23:1359–73. doi: 10.1007/s10238-022-00888-z
18. Cassetta L, Pollard JW. A timeline of tumour-associated macrophage biology. Nat Rev Cancer. (2023) 23:238–57. doi: 10.1038/s41568-022-00547-1
19. Shapouri-Moghaddam A, Mohammadian S, Vazini H, Taghadosi M, Taghadosi SA, Mardani F, et al. Macrophage plasticity, polarization, and function in health and disease. J Cell Physiol. (2018) 233:6425–40. doi: 10.1002/jcp.26429
20. Toledo B, Zhu Chen L, Paniagua-Sancho M, Marchal JA, Perán M, Giovannetti E. Deciphering the performance of macrophages in tumour microenvironment: a call for precision immunotherapy. J Hematol Oncol. (2024) 17:44. doi: 10.1186/s13045-024-01559-0
21. Liu YT, Mao ZW, Ding Y, Wang WL. Macrophages as targets in hepatocellular carcinoma therapy. Mol Cancer Ther. (2024) 23:780–90. doi: 10.1158/1535-7163.Mct-23-0660
22. He B, Wang L, Zhu M, Xu JA, Chen M, Jiang E, et al. Exosome-derived FGD5-AS1 promotes tumor-associated macrophage M2 polarization-mediated pancreatic cancer cell proliferation and metastasis. Cancer Lett. (2022) 548:215751. doi: 10.1016/j.canlet.2022.215751
23. Xu R, Wu Q, Gong Y, Wu Y, Chi Q, Sun D, et al. A novel prognostic target-gene signature and nomogram based on an integrated bioinformatics analysis in hepatocellular carcinoma. Biocell. (2022) 46:1261–88. doi: 10.32604/biocell.2022.018427
24. An Y, Yang Q. Tumor-associated macrophage-targeted therapeutics in ovarian cancer. Int J Cancer. (2021) 149:21–30. doi: 10.1002/ijc.33408
25. Wang H, Tian T, Zhang J. Tumor-associated macrophages (TAMs) in colorectal cancer (CRC): from mechanism to therapy and prognosis. Int J Mol Sci. (2021) 22. doi: 10.3390/ijms22168470
26. Khan F, Pang L, Dunterman M, Lesniak MS, Heimberger AB, Chen P. Macrophages and microglia in glioblastoma: heterogeneity, plasticity, and therapy. J Clin Invest. (2023) 133. doi: 10.1172/jci163446
27. Tang B, Zhu J, Wang Y, Chen W, Fang S, Mao W, et al. Targeted xCT-mediated Ferroptosis and Protumoral Polarization of Macrophages Is Effective against HCC and Enhances the Efficacy of the Anti-PD-1/L1 Response. Adv Sci (Weinh). (2023) 10:e2203973. doi: 10.1002/advs.202203973
28. Zheng H, Peng X, Yang S, Li X, Huang M, Wei S, et al. Targeting tumor-associated macrophages in hepatocellular carcinoma: biology, strategy, and immunotherapy. Cell Death Discovery. (2023) 9:65. doi: 10.1038/s41420-023-01356-7
29. Wang C, Ma C, Gong L, Guo Y, Fu K, Zhang Y, et al. Macrophage polarization and its role in liver disease. Front Immunol. (2021) 12:803037. doi: 10.3389/fimmu.2021.803037
30. Lin SC, Liao YC, Chen PM, Yang YY, Wang YH, Tung SL, et al. Periostin promotes ovarian cancer metastasis by enhancing M2 macrophages and cancer-associated fibroblasts via integrin-mediated NF-κB and TGF-β2 signaling. J BioMed Sci. (2022) 29:109. doi: 10.1186/s12929-022-00888-x
31. Qiu S, Xie L, Lu C, Gu C, Xia Y, Lv J, et al. Gastric cancer-derived exosomal miR-519a-3p promotes liver metastasis by inducing intrahepatic M2-like macrophage-mediated angiogenesis. J Exp Clin Cancer Res. (2022) 41:296. doi: 10.1186/s13046-022-02499-8
32. Boutilier AJ, Elsawa SF. Macrophage polarization states in the tumor microenvironment. Int J Mol Sci. (2021) 22. doi: 10.3390/ijms22136995
33. Xu W, Wu Y, Liu W, Anwaier A, Tian X, Su J, et al. Tumor-associated macrophage-derived chemokine CCL5 facilitates the progression and immunosuppressive tumor microenvironment of clear cell renal cell carcinoma. Int J Biol Sci. (2022) 18:4884–900. doi: 10.7150/ijbs.74647
34. Dangott B, Parwani A. Whole slide imaging for teleconsultation and clinical use. J Pathol Inf. (2010) 1. doi: 10.4103/2153-3539.65342
35. Abels E, Pantanowitz L, Aeffner F, Zarella MD, van der Laak J, Bui MM, et al. Computational pathology definitions, best practices, and recommendations for regulatory guidance: a white paper from the Digital Pathology Association. J Pathol. (2019) 249:286–94. doi: 10.1002/path.5331
36. Zeng Q, Klein C, Caruso S, Maille P, Laleh NG, Sommacale D, et al. Artificial intelligence predicts immune and inflammatory gene signatures directly from hepatocellular carcinoma histology. J Hepatol. (2022) 77:116–27. doi: 10.1016/j.jhep.2022.01.018
37. Chen Z, Li X, Yang M, Zhang H, Xu XS. Optimization of deep learning models for the prediction of gene mutations using unsupervised clustering. J Pathol Clin Res. (2023) 9:3–17. doi: 10.1002/cjp2.302
38. Erak E, Oliveira LD, Mendes AA, Dairo O, Ertunc O, Kulac I, et al. Predicting prostate cancer molecular subtype with deep learning on histopathologic images. Modern pathology: an Off J United States Can Acad Pathology Inc. (2023) 36:100247. doi: 10.1016/j.modpat.2023.100247
39. Bourgade R, Rabilloud N, Perennec T, Pécot T, Garrec C, Guédon AF, et al. Deep learning for detecting BRCA mutations in high-grade ovarian cancer based on an innovative tumor segmentation method from whole slide images. Modern pathology: an Off J United States Can Acad Pathology Inc. (2023) 36:100304. doi: 10.1016/j.modpat.2023.100304
40. Pizurica M, Larmuseau M, Van der Eecken K, de Schaetzen van Briene L, Carrillo-Perez F, Isphordin S, et al. Whole slide imaging-based prediction of TP53 mutations identifies an aggressive disease phenotype in prostate cancer. Cancer Res. (2023) 83:2970–84. doi: 10.1158/0008-5472.Can-22-3113
41. Mu Y, Tizhoosh HR, Dehkharghanian T, Campbell CJV. Whole slide image representation in bone marrow cytology. Comput Biol Med. (2023) 166:107530. doi: 10.1016/j.compbiomed.2023.107530
42. Hu G, Wang B, Hu B, Chen D, Hu L, Li C, et al. From WSI-level to patch-level: Structure prior-guided binuclear cell fine-grained detection. Med image Anal. (2023) 89:102931. doi: 10.1016/j.media.2023.102931
43. Haq F, Bychkov A, Jung CK. A matched-pair analysis of nuclear morphologic features between core needle biopsy and surgical specimen in thyroid tumors using a deep learning model. Endocr Pathol. (2022) 33:472–83. doi: 10.1007/s12022-022-09733-1
44. Lin H, Chen H, Graham S, Dou Q, Rajpoot N, Heng PA, et al. Fast scanNet: fast and dense analysis of multi-gigapixel whole-slide images for cancer metastasis detection. IEEE Trans Med Imaging. (2019) 38:1948–58. doi: 10.1109/tmi.2019.2891305
45. Ben Hamida A, Devanne M, Webe J, Truntzer C, Derangère V, Ghiringhelli F, et al. Deep learning for colon cancer histopathological images analysis. Comput Biol Med. (2021) 136:104730. doi: 10.1016/j.compbiomed.2021.104730
46. Silva-Rodríguez J, Schmidt A, Sales MA, Molina R, Naranjo V. Proportion constrained weakly supervised histopathology image classification. Comput Biol Med. (2022) 147:105714. doi: 10.1016/j.compbiomed.2022.105714
47. Ghaffari Laleh N, Muti HS, Loeffler CML, Echle A, Saldanha OL, Mahmood F, et al. Benchmarking weakly-supervised deep learning pipelines for whole slide classification in computational pathology. Med image Anal. (2022) 79:102474. doi: 10.1016/j.media.2022.102474
Keywords: deep learning, masked autoencoders, computational pathology, liver cancer, tumor microenvironment
Citation: Tian H, Tian Y, Li D, Zhao M, Luo Q, Kong L and Qin T (2024) Artificial intelligence model predicts M2 macrophage levels and HCC prognosis with only globally labeled pathological images. Front. Oncol. 14:1474155. doi: 10.3389/fonc.2024.1474155
Received: 22 August 2024; Accepted: 09 December 2024;
Published: 20 December 2024.
Edited by:
Shuai Ren, Affiliated Hospital of Nanjing University of Chinese Medicine, ChinaCopyright © 2024 Tian, Tian, Li, Zhao, Luo, Kong and Qin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tao Qin, cXRnb29kZnJlZWNuQHp6dS5lZHUuY24=; Lingfei Kong, bGZrb25nOUAxNjMuY29t
†These authors have contributed equally to this work
 Huiyuan Tian1†
Huiyuan Tian1† Tao Qin
Tao Qin