- 1Graduate Institute, Shandong University of Traditional Chinese Medicine, Jinan, Shandong, China
- 2Department of Oncology, Weifang Hospital of Traditional Chinese Medicine, Weifang, Shandong, China
- 3Department of Pathology, Weifang Hospital of Traditional Chinese Medicine, Weifang, Shandong, China
Ductal carcinoma in situ (DCIS), a noninvasive breast cancer, rarely metastasises to distant locations. When the initial lesion is stable, bone marrow metastasis (BMM) and bone marrow necrosis (BMN) are even less common. Here, we report the case of a 47-year-old female patient who underwent localized surgery and radiotherapy for right-sided DCIS. The patient also had a mutation in the breast cancer susceptibility gene 1 (BRCA1, OMIM: 113705) and tested positive for the progesterone and estrogen receptors. After 11 years of disease-free survival, the patient developed severe thrombocytopenia, anemia, fever, malaise, generalized multifocal pain, and irregular vaginal bleeding. A nodule was later found in the right axilla, and a postoperative biopsy revealed tumor cells from the breast. After three bone marrow biopsies, Positron Emission Tomography, 18F-fluorodeoxyglucose, positron emission tomography, computed tomography (18F-FDG PET/CT) scans, and other examinations, she was finally diagnosed with breast cancer BMM and BMN (stable primary lesion without bone metastasis). Despite symptomatic supportive treatment, the patient ultimately died rapidly as her condition deteriorated. In this case, we explored the possible mechanisms of BMM in this patient with DCIS by reviewing the literature related to this case and discussing the heterogeneous clinical presentation and pathologic phenotype. The diagnostic and therapeutic course of this case was extremely challenging. This suggests to clinicians that regular checkups and monitoring are necessary, even if the rate of distant metastasis from DCIS is low.
1 Introduction
Breast ductal carcinoma in situ (DCIS) is characterized by abnormal epithelial cells restricted within the mammary ducts, surrounded by intact myoepithelial cells and basement membrane (1). It accounts for about 25% of new breast cancer diagnoses and is considered non-invasive as long as it remains within the ducts (2–4). However, low-grade DCIS has the potential to progress into invasive cancer, with rare occurrences of distant metastasis reported at only 0.14% (5, 6). The most common sites of distant metastasis in breast cancer are the lungs, bones, liver, and brain (7–9). Symptomatic bone marrow metastasis (BMM) is an exceptionally rare complication in DCIS, with most cases arising in the context of invasive breast cancer (10). In addition, asymptomatic bone marrow metastases are reported in 20-30% of patients with early-stage breast cancer and are usually caused by disseminated tumor cells (DTCs) that are clinically insignificant (10, 11). Under specific conditions (e.g., changes in the tumor microenvironment caused by systemic inflammation), these dormant cells may be reactivated. When circulating tumor cells (CTCs) invade the bone marrow, replacing normal tissue and causing symptoms like anemia, thrombocytopenia, and coagulation abnormalities, it’s called symptomatic bone marrow involvement (10, 12). Bone marrow necrosis (BMN) is a rare clinicopathologic condition, often overlooked in living patients, characterized by extensive necrosis of hematopoietic tissues and stroma, with symptoms including bone pain, fever, and hematologic abnormalities such as anemia and thrombocytopenia (13, 14). Common symptoms of bone pain are due to inflammation and increased pressure within the bone caused by the metastasis or necrosis of bone marrow, which activates the peripheral sensory nerve endings within the bone marrow by releasing inflammatory mediators and mechanical compression or deformation (15). Malignant tumors are the main cause of BMN, accounting for approximately 90% of BMN cases, with malignant diseases of the hematopoietic system accounting for 60%, and extensive BMN secondary to solid tumors are rare and are usually an end-stage manifestation of symptomatic BMM (14). This report describes a rare case of a female patient who developed symptomatic BMM and eventually BMN 11 years after breast-conserving surgery and radiotherapy. By reviewing the relevant literature, we attempted to analyze the pathological mechanism, clinical characteristics, and treatment options to provide a reference for diagnosing and managing similar cases.
2 Case description
A 47-year-old woman presented to our hospital on February 24, 2024, with fatigue and neck, shoulder, and back pain for the past month. She had been admitted to the hospital in January 2013 for “bloody right nipple discharge for two months.” At that time, she underwent a segmental excision of the right breast lesion and a biopsy of the anterior lymph nodes. The lesion was completely excised with negative margins. Pathologic findings showed non-invasive right breast ductal carcinoma in situ (Figures 1A–C). Immunohistochemistry detected carcinoma in situ estrogen receptor (ER) (+90%), progesterone receptor (PgR) (+80%), human epidermal growth factor receptor 2 (HER2) (+), E-cad (+), 34βE12 (+), P120 (membrane +), Ki-67 (<10%), P53 (+<10%), and SMA (+) (Figure 2A). The breast cancer was graded as pTisN0M0. Genetic testing showed a positive result for the S1 locus of the BRCA1 gene. The patient refused further right mastectomy. Eight weeks after surgery, the patient underwent conformal radiotherapy to the right breast at a dose of DT: 4800cGy/25f, with enhanced DT to the right breast surgical area: 800cGy/4f. The patient was discharged with a good recovery. Despite being advised to take tamoxifen (20 mg daily) due to her hormone receptor-positive (HR+) breast cancer, the patient stopped the medication a few days after discharge and did not attend regular follow-up examinations.
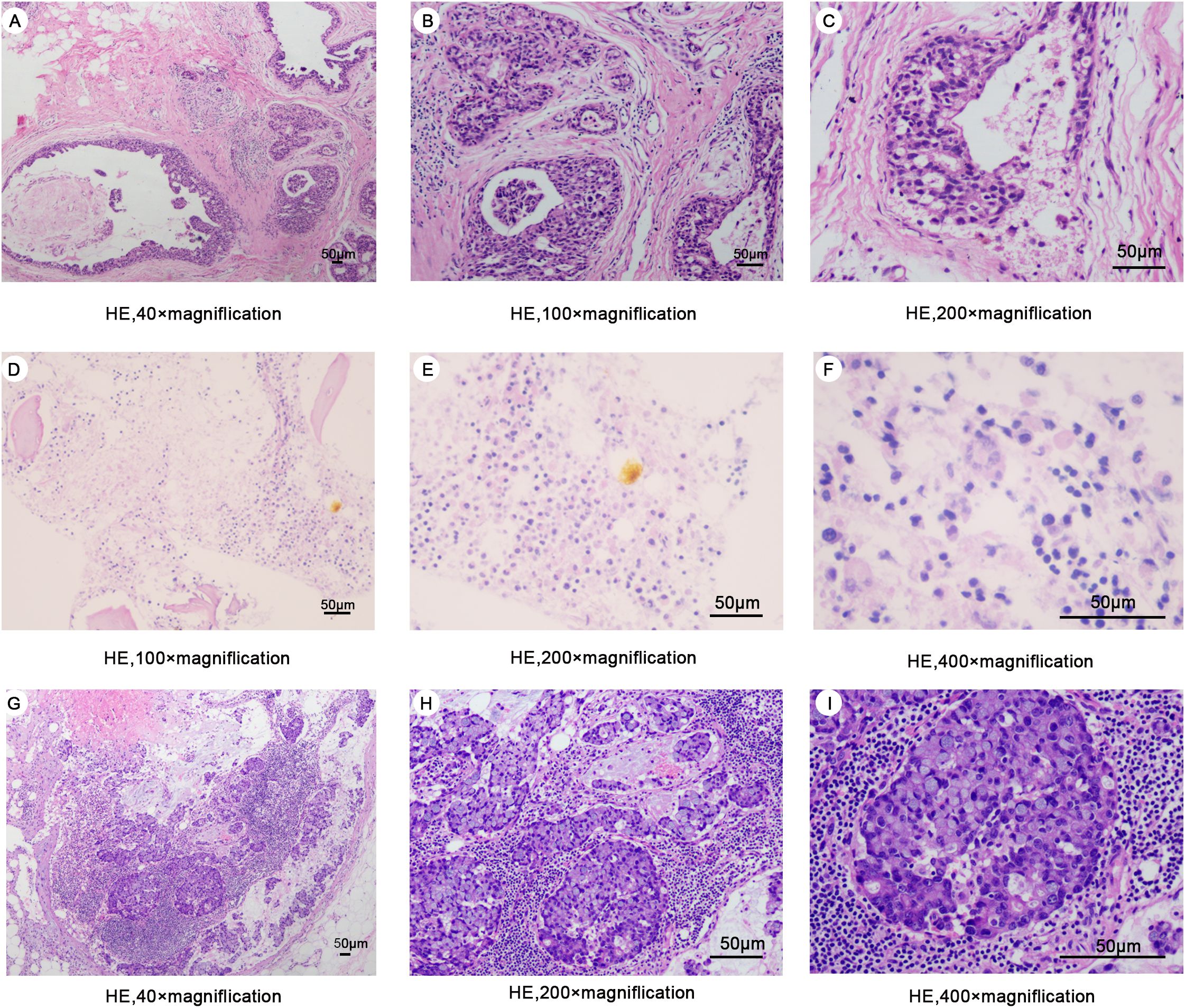
Figure 1. (A–C) Hematoxylin-eosin staining of surgical excision specimens of the right breast in 2013 (D–F) Hematoxylin-eosin staining of bone marrow biopsy specimens in 2024. (G–I) Hematoxylin-eosin staining of a specimen of the right axillary mass in 2024.
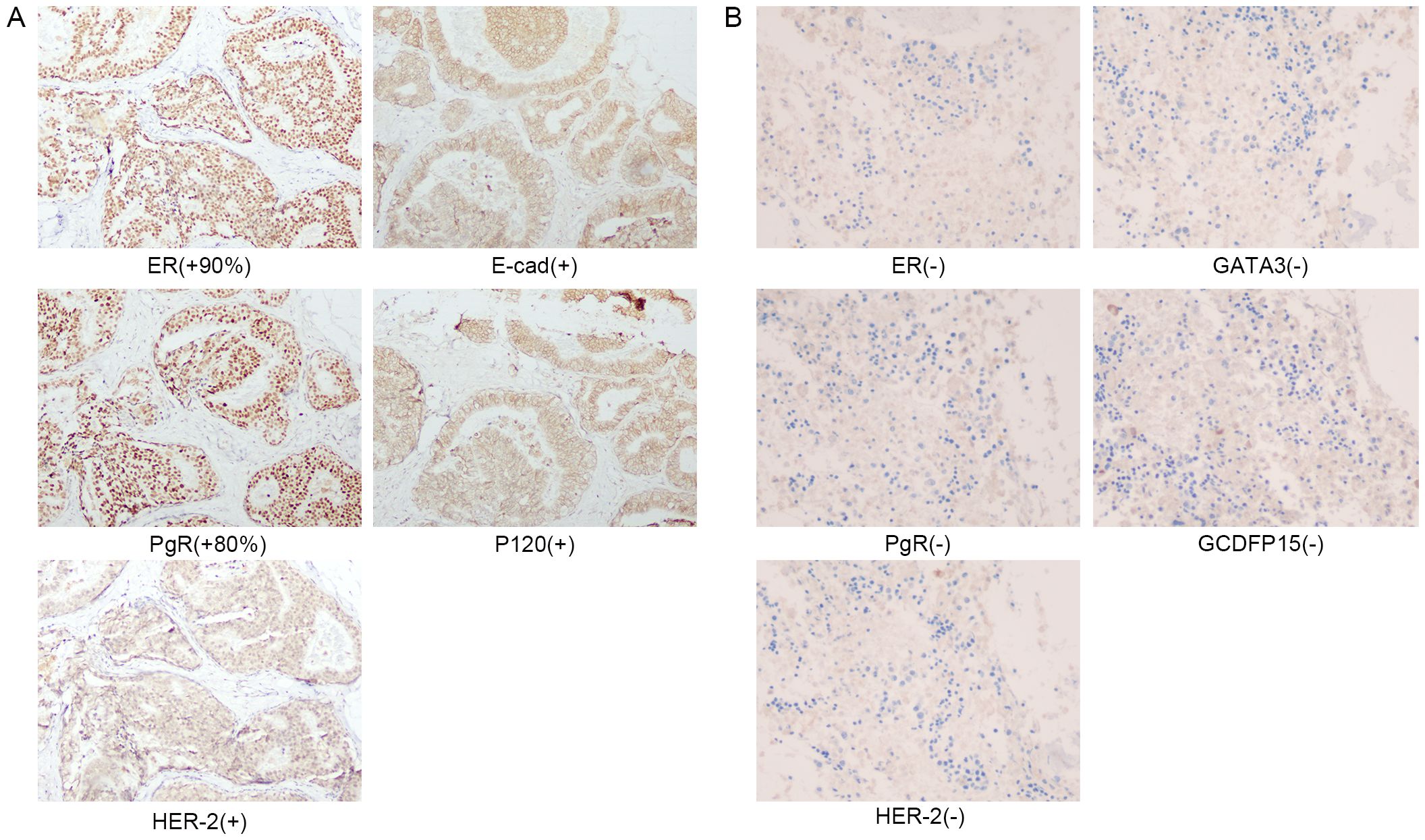
Figure 2. (A) Immunohistochemical staining of right breast surgical excision specimen in 2013. (B) Immunohistochemical staining of the second bone marrow biopsy in March 2024.
On this admission, the patient reported heavy and prolonged menstrual flow for the last two months. There was a history of Coronavirus disease 2019 (COVID-19) infection in previous months. The patient denied any family history of genetic disorders or malignant tumors. The breast examination was unremarkable, showing no changes in the right breast or the scar area from the right axillary surgery. Routine blood tests showed platelets (16 × 109/L) and hemoglobin 51 g/L. Tumor markers showed CA153 at 45 U/L, while CEA and CA125 were within normal ranges. On March 2, the patient developed a fever accompanied by vaginal bleeding of about 40 ml, and platelets continued to fall (12 × 109/L). Novel Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Nucleic Acid Test Returns Positive. Two days later, her plasma D-dimer increased to 55.29 mg/L. By March 7, her alkaline phosphatase had abnormally increased to 2398 U/L. Computed tomography (CT) scans of the craniocerebral, thoracic, abdominal, and pelvic regions, along with breast MRI, did not reveal any recurrence or other metastatic foci of breast cancer. Subsequent 18F-FDG PET/CT examination showed no abnormal metabolism in the area of the right breast surgery or elsewhere in the body (Figures 3A, B), diffuse hyperdensity in the bone marrow cavity, mainly located in the medial skeleton, suggestive of BMM, and no destruction of the bone cortex. (Figures 3C, D). Serum immunofixation electrophoresis revealed normal serum immunoglobulin G, A, and M levels, reducing the likelihood of multiple myeloma.
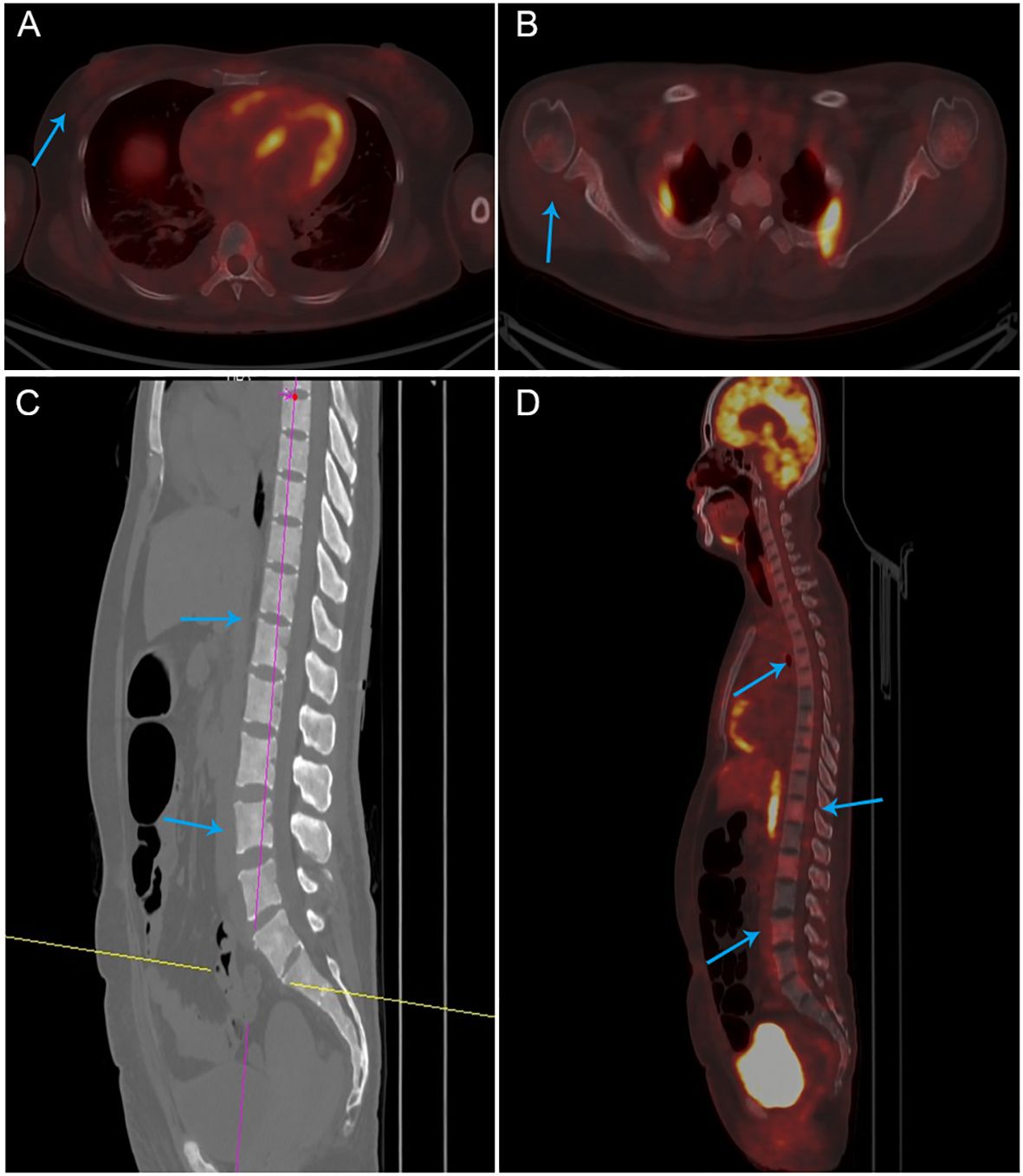
Figure 3. PET-CT images (A, B) There was no abnormal metabolism in the area of the right breast surgery or the axilla. (C, D) Diffuse hyperdensity in the medullary cavity of the bone, no destruction of the bone cortex.
Upon the patient’s admission to the hospital, we performed the first bone marrow aspiration biopsy. The biopsy results indicated metastatic cancer in the marrow. Immunohistochemistry results showed ER (-), PgR (-), and HER2 (-). We conducted another bone marrow aspiration biopsy two weeks later to clarify the diagnosis. We examined two pieces of bone marrow tissue obtained from different puncture sites in the patient’s iliac bone, and both results confirmed BMN (Figures 1D–F). The immunohistochemistry results of bone marrow samples showed ER (-), PgR (-), HER2 (-), GATA3 (-), and GCDFP (-) (Figure 2B). We transfused red blood cells, platelets, and plasma and provided antiviral therapy, symptomatic hemostasis, analgesia, and herbal adjuvant therapy until the definitive diagnosis was achieved.
Surprisingly, a mass was found near the patient’s right axilla during a physical examination one month later. The mass measured approximately 1.5 cm × 2 cm with no redness, ulceration, or tenderness. A puncture biopsy revealed tumor cells. Subsequently, the patient underwent tumor resection, and the pathology showed invasive adenocarcinoma (Figures 1G–I). Immunohistochemistry showed ER (-), PgR (+5%), c-erbB-2 (1+), E-cad (+), GATA-3 (±), GCDFP-15 (-), Ki67 (+ 50%), and p120 (membrane +) (Figure 4). Based on all examinations and laboratory results, we diagnosed the patient with invasive ductal carcinoma of the breast with bone marrow metastasis and axillary metastasis. The patient had an Eastern Cooperative Oncology Group (ECOG) physical status score 4. After assessing the patient’s physical condition, we decided on conservative supportive treatment. On April 19, the patient presented with a gradual worsening of pain in the right upper extremity. One week later, the patient once more presented with heavy vaginal bleeding, malaise, fever, and dyspnea following exertion. The treatment was terminated based on the patient’s expressed wishes. She subsequently succumbed to her illness the following day.
3 Discussion
DCIS is usually not considered metastatic. However, our patient developed BMM and BMN 11 years after the diagnosis of DCIS, a rare occurrence (16). Clinicopathologic parameters associated with aggressive recurrence and distant metastasis include patient age (<40 years), DCIS size, nuclear grade, presence or absence of consolidated necrosis, histologic type, Ki-67 staining, multifocality, surgical margins, and mode of detection (asymptomatic versus screening test) (17, 18). The patient was 35 at diagnosis, prompted by bloody nipple discharge. No other regular risk factors for distant metastasis were present. Because distant metastases after DCIS are rare and mostly reported as case studies, none of these risk factors were statistically significant. Generally, the mechanisms of recurrence and metastasis in early-stage breast cancer are linked to the completeness of initial treatment, the dormancy and activation of tumor cells, genetic factors, and changes in the tumor microenvironment (19, 20).
Standard treatment for DCIS includes radiotherapy (RT) following breast-conserving surgery (BCS) (21). The NSABP protocol B-17 showed a 12-year local recurrence rate of 32% for DCIS patients treated with resection only and 16% for resection plus radiotherapy (22). Tamoxifen is a selective estrogen receptor modulator (SERM) that is widely used to treat early-stage breast cancer that is hormone receptor-positive (ER+/PgR+) and can significantly reduce the risk of breast cancer recurrence and death (23). Studies have shown that adding tamoxifen to local excision and radiation therapy in ER-positive DCIS patients can reduce the 10-year risk of recurrence (24). However, noncompliance with tamoxifen therapy, especially early discontinuation, may lead to increased recurrence and mortality in breast cancer patients (20, 25). Patients did not take tamoxifen regularly, potentially expanding the recurrence risk.
The tumor microenvironment (TME) plays a crucial role in BMM, promoting tumor cells’ survival, dormancy, and eventual reactivation. Breast cancer cells can reprogram the bone marrow microenvironment, which promotes tumor cell adhesion, angiogenesis, and remodeling of the bone marrow stroma (26). Dormant tumor cells typically survive the successful treatment of the primary tumor and enter a clinically asymptomatic state (27). In the bone marrow, immune cells help form pre-metastatic niches, creating an environment favorable for the survival of DTCs (28, 29). These immune cells secrete pro-inflammatory cytokines that enhance breast cancer tumor cell migration and colonization and mediate the reactivation of dormant DTCs, leading to significant metastasis after long-term dormancy (30). Emerging evidence suggests that COVID-19 can reactivate dormant tumor cells in response to microenvironmental cues, such as inflammatory or immune-mediated signals, leading to tumor progression and metastasis, systemic inflammation, widespread coagulation dysfunction, and multi-organ dysfunction (31). Upon admission, this patient was infected with COVID-19 but was not systematically treated. After this admission, the patient was re-infected with COVID-19, accompanied by fever, elevated D-dimer, and falling platelets. The patient tested positive for the novel coronavirus and had a fever, which conformed to the typical presentation of COVID-19 (32). Subsequently, the patient’s condition deteriorated dramatically. We speculate that the patient’s BMM after 11 years of asymptomatic survival and the rapid development of BMN may be due to immune activation by COVID-19, resulting in a transformation from asymptomatic BMM to symptomatic BMM.
Distant metastasis of breast DCIS in the absence of local lesion recurrence is rare. Previous studies have reported two cases of DCIS where patients developed metastases to the liver, lung, bone, and colon after breast-conserving surgery and endocrine therapy despite the stability of the primary lesion (33, 34). Unlike them, the patient in this case had hardly received any endocrine treatment, and the site of distant metastasis was the bone marrow, which is much more aggressive, has a worse prognosis, and is much rarer. Notably, this patient initially experienced abnormal symptoms, including irregular vaginal bleeding, heavy and prolonged menstrual periods, and later bone pain. As the condition progressed, severe pain (NRS score 8) developed in multiple areas. An increase in vaginal bleeding accompanied each worsening of clinical symptoms. The patient’s fever gradually returned to normal after antiviral treatment, which complicated the diagnosis. Laboratory findings associated with BMN typically reveal anemia, thrombocytopenia, elevated alkaline phosphatase levels, decreased blood calcium, and increased plasma D-dimer. However, these manifestations do not necessarily occur simultaneously (35). Upon admission, the patient presented with anemia and low platelet counts. Subsequent laboratory tests revealed a progressive increase in plasma D-dimer, elevated alkaline phosphatase, decreased blood calcium, and abnormal liver function. Nonetheless, these indicators lack specificity.
Symptomatic bone marrow metastasis (BMM) with a stable primary lesion is rare and prone to misdiagnosis. The patient was admitted to the hospital, and peripheral blood tests showed anemia and a persistent drop in platelets. The initial chest CT examination showed no recurrence or metastasis in the breast, while the PET-CT examination showed diffuse high density in the bone marrow cavity and no metabolic abnormalities in the right breast, armpit, or other areas. These findings made the diagnosis more difficult. In this case, the first step was to rule out hematologic disorders. Bone marrow examination is a high-yield test for identifying BMM from solid tumors (36, 37). In some cases where the primary tumor is occult, immunohistochemical results of bone marrow aspiration and bone marrow biopsy can help identify an unknown primary tumor (36). The first bone marrow biopsy confirmed metastatic cancer of undetermined origin, while further tests ruled out blood disorders. Although BMM is generally straightforward to detect, pinpointing the exact primary site can be challenging. Mammary BMM is commonly associated with bone metastases, and its occurrence without bone metastasis is extremely rare (34). Whole-body CT scans showed no bone metastases, and no cortical bone destruction was observed in the results of the PET-CT. A bone marrow biopsy confirmed metastatic cancer in the bone marrow, yet systemic examination findings remained inconclusive, making the diagnostic process more challenging. The eventual identification of a painless right axillary mass, absent on earlier imaging, was pivotal for diagnosis. By integrating findings from three bone marrow biopsies, PET-CT scans, and the axillary mass, it was concluded that the patient exhibited invasive carcinoma of the breast with BMM and BMN, accompanied by axillary metastasis. Extensive BMN is rare in solid tumors and is highly susceptible to underdiagnosis and misdiagnosis. A retrospective analysis showed that out of 16,651 bone marrow biopsy specimens, only 2 cases of BMN were associated with breast cancer (38). In this case, with an insidious primary disease and nonspecific symptoms, an aggressive multisite bone marrow aspiration biopsy and PET-CT were performed, which provided the prerequisites for the final definitive diagnosis.
Management of bone marrow metastasis remains a clinical challenge due to the lack of established guidelines (39). Due to the rarity of bone marrow metastases from breast cancer, there is a lack of systematic treatment guidelines, and the published literature consists mainly of case reports and studies with small sample sizes. Personalized treatment plans based on the molecular profile of the tumor and the patient’s clinical condition are essential for optimizing outcomes. Chemotherapy is the mainstay of treatment, and weekly paclitaxel therapy is stable and much less toxic. However, bone marrow toxicity of cytotoxic drugs remains an unavoidable problem. Fluorouracil analogs have shown promising efficacy in clinical application and have been reported to be effective for bone marrow metastasis and DIC in gastric cancer (40). Interestingly, Chan, B. reported a case of bone marrow metastasis from triple-negative breast cancer in which the patient developed liver failure after first-line application of albumin-bound paclitaxel, which was then stabilized and controlled by continuous application of 5-FU infusion, followed by oral capecitabine (41). For HER-positive breast cancer bone marrow metastases, adding antibody-drug couplings targeting HER2 prolongs patient survival (42). By searching the literature, we have seen that immunotherapy has shown promising efficacy in bone marrow metastasis of other types of tumors (43). Still, for triple-negative breast cancer, the application has only been reported for metastasis to other sites (44, 45). No studies are related to the application of immune checkpoint inhibitors for BMM. Further studies are expected to address this issue. It has been found that BMM patients’ OS strongly correlates with platelet levels and ECOG scores (34, 46). In this case, the patient was ultimately diagnosed with BMN and had significant suppression of bone marrow hematopoiesis, with symptoms such as grade IV thrombocytopenia (12 × 109/L) and vaginal bleeding. Moreover, the patient was fragile (ECOG 4), and chemotherapy could not be applied in this case. The patient and family agreed to conservative supportive care. Although the patient’s condition improved slightly with plasma transfusion, platelet transfusion, analgesics, anti-infection measures, and herbal medicine, the lack of effective interventions for BMN and the patient’s critical condition led to a deterioration in her condition, and she died the day after being discharged from the hospital. This case highlights the urgent need for further research into effective treatment strategies for BMN associated with breast cancer, especially for patients with severe comorbidities and impaired bone marrow function.
Breast cancer is a remarkably heterogeneous malignant tumor, and its temporal heterogeneity (dynamic changes in molecular characteristics between primary and recurrent foci) and spatial heterogeneity (differences between different metastatic sites) pose significant diagnostic and therapeutic challenges (44, 45). In this case, the patient’s breast cancer progressed from ductal carcinoma in situ (ER+/PgR+/HER2+) to invasive ductal carcinoma (ER-/PgR-/HER2-) and showed further molecular phenotypic changes in bone marrow metastasis and axillary metastasis. The first bone marrow biopsy showed metastatic carcinoma and subsequent bone marrow biopsies showed bone marrow necrosis, suggesting a dynamic change in molecular phenotype, culminating in a triple-negative phenotype. The evolution of breast cancer from ductal carcinoma in situ to invasive ductal carcinoma is characterized by changes in hormone receptor and HER2 status reflecting the heterogeneity of its molecular phenotype, and mutations in the BRCA1 gene in the genetic background may play an important role in this process. Elevated expression of the BRCA1 gene, an essential gene involved in DNA damage repair, cell cycle regulation, and genome stability, is closely associated with an increased risk of early distant metastasis in ER+ breast cancer patients (19, 47). BRCA1 gene mutations are strongly associated with genomic instability, epithelial mesenchymal transition (EMT), and immune microenvironmental interactions, leading to significant inter- and intratumoral heterogeneity, which affects clinical outcomes and drug resistance (48). Our patient carries a BRCA1 mutation, and she declined the recommendation for prophylactic mastectomy. The exact time point of the molecular typing change remains uncertain due to the lack of adequate follow-up review in this case, which constitutes a significant limitation.
4 Conclusion
We describe a rare case of a female patient who developed symptomatic bone marrow metastasis (BMM) and bone marrow necrosis (BMN) 11 years after the diagnosis of DCIS. DCIS may develop into a tumor that is highly aggressive under certain circumstances. Regular follow-up examinations after systemic therapy are necessary. In breast cancer patients presenting with unexplained anemia, fatigue, fever, bone pain, or abnormal vaginal bleeding while the primary lesion remains stable, the possibility of bone marrow metastasis or bone marrow necrosis should be considered. A bone marrow biopsy should be performed actively, and a PET-CT examination should be performed if necessary to confirm the diagnosis as soon as possible. Early treatment can benefit patient survival.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
SZ: Investigation, Writing – original draft, Writing – review & editing. ZD: Investigation, Supervision, Writing – review & editing. JW: Writing – review & editing, Supervision. XZ: Writing – review & editing, Supervision. WD: Writing – review & editing, Supervision.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Allred DC. Ductal carcinoma in situ: terminology, classification, and natural history. J Natl Cancer Inst Monogr. (2010) 2010:134–8. doi: 10.1093/jncimonographs/lgq035
2. Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2024) 74:229–63. doi: 10.3322/caac.21834
3. Coleman WB. Breast ductal carcinoma in situ: precursor to invasive breast cancer. Am J Pathol. (2019) 189:942–5. doi: 10.1016/j.ajpath.2019.03.002
4. Ward EM, DeSantis CE, Lin CC, Kramer JL, Jemal A, Kohler B, et al. Cancer statistics: Breast cancer in situ. CA Cancer J Clin. (2015) 65:481–95. doi: 10.3322/caac.21321
5. Koh VCY, Lim JCT, Thike AA, Cheok PY, Thu MMM, Li H, et al. Behaviour and characteristics of low-grade ductal carcinoma in situ of the breast: literature review and single-centre retrospective series. Histopathology. (2019) 74:970–87. doi: 10.1111/his.2019.74.issue-7
6. Roses RE, Arun BK, Lari SA, Mittendorf EA, Lucci A, Hunt KK, et al. Ductal carcinoma-in-situ of the breast with subsequent distant metastasis and death. Ann Surg Oncol. (2011) 18:2873–8. doi: 10.1245/s10434-011-1707-2
7. Chen W, Hoffmann AD, Liu H, Liu X. Organotropism: new insights into molecular mechanisms of breast cancer metastasis. NPJ Precis Oncol. (2018) 2:4. doi: 10.1038/s41698-018-0047-0
8. Yates LR, Knappskog S, Wedge D, Farmery JHR, Gonzalez S, Martincorena I, et al. Genomic evolution of breast cancer metastasis and relapse. Cancer Cell. (2017) 32:169–84.e7. doi: 10.1016/j.ccell.2017.07.005
9. Largillier R, Ferrero JM, Doyen J, Barriere J, Namer M, Mari V, et al. Prognostic factors in 1,038 women with metastatic breast cancer. Ann Oncol. (2008) 19:2012–9. doi: 10.1093/annonc/mdn424
10. Hartkopf AD, Brucker SY, Taran F-A, Harbeck N, von Au A, Naume B, et al. Disseminated tumour cells from the bone marrow of early breast cancer patients: Results from an international pooled analysis. Eur J Cancer. (2021) 154:128–37. doi: 10.1016/j.ejca.2021.06.028
11. Braun S, Vogl FD, Naume B, Janni W, Osborne MP, Coombes RC, et al. A pooled analysis of bone marrow micrometastasis in breast cancer. N Engl J Med. (2005) 353:793–802. doi: 10.1056/NEJMoa050434
12. Yang R, Jia L, Cui J. Mechanism and clinical progression of solid tumors bone marrow metastasis. Front Pharmacol. (2024) 15:1390361. doi: 10.3389/fphar.2024.1390361
13. Janssens AM, Offner FC, Van Hove WZ. Bone marrow necrosis. Cancer: Interdiscip Int J Am Cancer Society. (2000) 88:1769–80. doi: 10.1002/(SICI)1097-0142(20000415)88:8<1769::AID-CNCR3>3.0.CO;2-H
14. Paydas S, Ergin M, Baslamisli F, Yavuz S, Zorludemir S, Sahin B, et al. Bone marrow necrosis: clinicopathologic analysis of 20 cases and review of the literature. Am J Hematol. (2002) 70:300–5. doi: 10.1002/ajh.10114
15. Ivanusic JJ. Molecular mechanisms that contribute to bone marrow pain. Front Neurol. (2017) 8:458. doi: 10.3389/fneur.2017.00458
16. Udayasiri RI, Luo T, Gorringe KL, Fox SB. Identifying recurrences and metastasis after ductal carcinoma in situ (DCIS) of the breast. Histopathology. (2023) 82:106–18. doi: 10.1111/his.14804
17. Bannani S, Rouquette S, Bendavid-Athias C, Tas P, Levêque J. The locoregional recurrence post-mastectomy for ductal carcinoma in situ: Incidence and risk factors. Breast. (2015) 24(5):608–12. doi: 10.1016/j.breast.2015.06.005
18. Ekatah GE, Turnbull AK, Arthur LM, Thomas J, Dodds C, Dixon JM. Margin width and local recurrence after breast conserving surgery for ductal carcinoma in situ. Eur J Surg Oncol. (2017) 43:2029–35. doi: 10.1016/j.ejso.2017.08.003
19. Chang HJ, Yang UC, Lai MY, Chen CH, Fann YC. High BRCA1 gene expression increases the risk of early distant metastasis in ER(+) breast cancers. Sci Rep. (2022) 12:77. doi: 10.1038/s41598-021-03471-w
20. Hershman DL, Shao T, Kushi LH, Buono D, Tsai WY, Fehrenbacher L, et al. Early discontinuation and non-adherence to adjuvant hormonal therapy are associated with increased mortality in women with breast cancer. Breast Cancer Res Treat. (2011) 126:529–37. doi: 10.1007/s10549-010-1132-4
21. Sagara Y, Freedman RA, Vaz-Luis I, Mallory MA, Wong SM, Aydogan F, et al. Patient prognostic score and associations with survival improvement offered by radiotherapy after breast-conserving surgery for ductal carcinoma in situ: A population-based longitudinal cohort study. J Clin Oncol. (2016) 34:1190–6. doi: 10.1200/JCO.2015.65.1869
22. Wehner P, Lagios MD, Silverstein MJ. DCIS treated with excision alone using the National Comprehensive Cancer Network (NCCN) guidelines. Ann Surg Oncol. (2013) 20:3175–9. doi: 10.1245/s10434-013-3176-2
23. Bryce CJ. Tamoxifen in early breast cancer. Lancet. (1998) 352:403. doi: 10.1016/S0140-6736(05)60500-4
24. Allred DC, Anderson SJ, Paik S, Wickerham DL, Nagtegaal ID, Swain SM, et al. Adjuvant tamoxifen reduces subsequent breast cancer in women with estrogen receptor-positive ductal carcinoma in situ: a study based on NSABP protocol B-24. J Clin Oncol. (2012) 30:1268–73. doi: 10.1200/JCO.2010.34.0141
25. Barron TI, Connolly R, Bennett K, Feely J, Kennedy MJ. Early discontinuation of tamoxifen: a lesson for oncologists. Cancer. (2007) 109:832–9. doi: 10.1002/cncr.v109:5
26. Yip RKH, Rimes JS, Capaldo BD, Vaillant F, Mouchemore KA, Pal B, et al. Mammary tumour cells remodel the bone marrow vascular microenvironment to support metastasis. Nat Commun. (2021) 12:6920. doi: 10.1038/s41467-021-26556-6
27. Gomis RR, Gawrzak S. Tumor cell dormancy. Mol Oncol. (2017) 11:62–78. doi: 10.1016/j.molonc.2016.09.009
28. Ridge SM, Sullivan FJ, Glynn SA. Mesenchymal stem cells: key players in cancer progression. Mol Cancer. (2017) 16:31. doi: 10.1186/s12943-017-0597-8
29. Pittet MJ, Michielin O, Migliorini D. Clinical relevance of tumour-associated macrophages. Nat Rev Clin Oncol. (2022) 19:402–21. doi: 10.1038/s41571-022-00620-6
30. Baram T, Rubinstein-Achiasaf L, Ben-Yaakov H, Ben-Baruch A. Inflammation-driven breast tumor cell plasticity: stemness/EMT, therapy resistance and dormancy. Front Oncol. (2020) 10:614468. doi: 10.3389/fonc.2020.614468
31. Francescangeli F, De Angelis ML, Zeuner A. COVID-19: a potential driver of immune-mediated breast cancer recurrence? Breast Cancer Res. (2020) 22:117. doi: 10.1186/s13058-020-01360-0
32. Abobaker A, Raba AA, Alzwi A. Extrapulmonary and atypical clinical presentations of COVID-19. J Med Virol. (2020) 92:2458–64. doi: 10.1002/jmv.v92.11
33. Wang Z, Zhang X, Ren H, Zhang L, Chen B. Multiple metastases of the liver and lung after breast-conserving surgery for ductal carcinoma in situ without microinvasion of the breast: A case report and literature review. Front Oncol. (2022) 12:855899. doi: 10.3389/fonc.2022.855899
34. Yang R, Jia L, Lu G, Lv Z, Cui J. Symptomatic bone marrow metastases in breast cancer: A retrospective cohort study. Front Oncol. (2022) 12:1042773. doi: 10.3389/fonc.2022.1042773
35. Chen R, Wu J, Yang J, Wei C, Liang D, Du J, et al. Analysis and clinical characteristics of 23 cases of bone marrow necrosis. Clin Lymphoma Myeloma Leukemia. (2021) 21:e356–e64. doi: 10.1016/j.clml.2020.12.001
36. Khan S, Awan SA, Jahangir S, Kamran S, Ahmad IN. Bone marrow metastasis in clear cell renal cell carcinoma: A case study. Cureus. (2019) 11:e4181. doi: 10.7759/cureus.4181
37. Rani HS, Hui M, Manasa PL, Uppin SG, Uppin MS, Paul TR, et al. Bone marrow metastasis of solid tumors: A study of 174 cases over 2 decades from a single institution in India. Indian J Hematol Blood Transfus. (2022) 38:8–14. doi: 10.1007/s12288-021-01418-9
38. Wool GD, Deucher A. Bone marrow necrosis: ten-year retrospective review of bone marrow biopsy specimens. Am J Clin Pathol. (2015) 143:201–13. doi: 10.1309/AJCP0TN1MCMOLMPK
39. Akagi H, Shimada A, Chin K, Domoto H. Successful stabilization of symptomatic bone marrow metastasis two times in a breast cancer patient. Anticancer Res. (2021) 41:3139–44. doi: 10.21873/anticanres.15099
40. Suto H, Inui Y, Okamura A. Long-term survival of a patient with gastric cancer with bone marrow metastasis receiving S-1 plus oxaliplatin beyond three years: a case report and literature review. Front Oncol. (2024) 14:1449212. doi: 10.3389/fonc.2024.1449212
41. Chan B, Lee JS, Yuan Y. Case report: An exceptional responder of low-dose continuous 5-FU in a patient with de-novo stage IV triple-negative breast cancer with liver and bone marrow failure. Front Oncol. (2023) 13:1305584. doi: 10.3389/fonc.2023.1305584
42. Wu Q, He L, Luo J, Jin W, Xu Y, Wang C. Long-term remission under Disitamab Vedotin (RC48) in HR-positive/HER2-positive metastatic breast cancer with brain meningeal, and bone marrow involvement: A case report. Oncol Lett. (2022) 24:339. doi: 10.3892/ol.2022.13459
43. Huang RZ, Chen N, Hu Y, Hu WM, Wang FH, Chen DL. Case report: PD-1 inhibitor-based treatment strategies in gastric cancer complicated by bone marrow metastasis and disseminated intravascular coagulation: A report of two cases. Front Oncol. (2023) 13:1019702. doi: 10.3389/fonc.2023.1019702
44. Andrade de Oliveira K, Sengupta S, Yadav AK, Clarke R. The complex nature of heterogeneity and its roles in breast cancer biology and therapeutic responsiveness. Front Endocrinol (Lausanne). (2023) 14:1083048. doi: 10.3389/fendo.2023.1083048
45. Zardavas D, Irrthum A, Swanton C, Piccart M. Clinical management of breast cancer heterogeneity. Nat Rev Clin Oncol. (2015) 12:381–94. doi: 10.1038/nrclinonc.2015.73
46. Niu L, Lv H, Zhang M, Zeng H, Fu S, Cui S, et al. Clinicopathological features and prognosis of breast cancer combined with symptomatic bone marrow metastases: A 10-year, single-center, real-world study of 67 cases. Cancer Med. (2023) 12:10672–83. doi: 10.1002/cam4.v12.9
47. Fu X, Tan W, Song Q, Pei H, Li J. BRCA1 and breast cancer: molecular mechanisms and therapeutic strategies. Front Cell Dev Biol. (2022) 10:813457. doi: 10.3389/fcell.2022.813457
Keywords: ductal carcinoma in situ, bone marrow metastasis, bone marrow necrosis, bone marrow biopsy, 18F-FDG PET/CT, BRCA1, triple-negative breast cancer
Citation: Zhang S, Du Z, Wu J, Zhang X and Dong W (2024) Case report: Bone marrow metastasis and bone marrow necrosis occurring 11 years after ductal carcinoma in situ of the breast. Front. Oncol. 14:1473896. doi: 10.3389/fonc.2024.1473896
Received: 31 July 2024; Accepted: 09 December 2024;
Published: 23 December 2024.
Edited by:
Frederick Dirbas, Stanford University, United StatesReviewed by:
Hirotaka Suto, Hyogo Cancer Center, JapanWenxuan Zhang, Stony Brook Medicine, United States
Copyright © 2024 Zhang, Du, Wu, Zhang and Dong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhonghai Du, MTM1NzM2MDMwODdAMTYzLmNvbQ==
 Shuting Zhang
Shuting Zhang Zhonghai Du
Zhonghai Du Jun Wu2
Jun Wu2