- Department of Thyroid Surgery, Ningbo No.2 Hospital, Ningbo, Zhejiang, China
Background: Papillary thyroid cancer (PTC) with lymph node metastasis (LNM) is associated with an increased risk of postoperative recurrence. Understanding the clinical characteristics of PTC patients can help surgeons assess the likelihood of LNM. This study aims to identify risk factors for LNM in PTC patients.
Methods: We retrospectively analyzed clinical data from 2,428 patients diagnosed with PTC who underwent initial thyroid surgery in our single center. Risk factors related to central lymph node metastasis (CLNM), lateral lymph node metastasis (LLNM), and skip metastasis were investigated by univariate and multivariate binary logistic regression analyses.
Results: Univariate analysis revealed that the following factors were associated with an increased likelihood of CLNM (P<0.05): male gender, age < 45 years, maximal axial diameter (MAD) > 1 cm, body mass index (BMI) ≥ 28 kg/m² and multifocality. Univariate analysis also revealed that patients with age < 45 years, MAD > 1 cm, BMI ≥ 28 kg/m², multifocality, and CLNM demonstrated a higher propensity for LLNM (P<0.05). Lower pole tumors were more likely to have CLNM than upper pole tumors, while upper pole tumors were more prone to present LLNM (P<0.05) and skip metastasis (P<0.05). Multivariable binary logistic regression analysis identified that gender (odds ratio [OR], 1.732; 95% CI, 2.113–2.577; P < 0.001), age (OR, 1.905; 95% CI, 1.596–2.273; P < 0.001), MAD (OR, 4.639; 95% CI, 3.639–5.913; P < 0.001), and multifocality (OR, 1.860; 95% CI, 1.453–2.381; P < 0.001) were independent risk factors for CLNM and MAD (OR, 5.289; 95% CI, 3.777–7.404; P<0.001), multifocality (OR, 1.858; 95% CI, 1.248–2.766; P=0.002), and CLNM (OR, 5.030; 95% CI, 3.347–7.561; P<0.001) for LLNM.
Conclusion: Despite the overall postoperative recurrence rate in PTC patients is low, identifying risk factors such as male gender, age < 45 years, MAD > 1 cm, multifocality, and CLNM can help predict LNM. In specific cases, selective lymphadenectomy in the central or lateral neck area may be warranted.
1 Introduction
In recent years, the global incidence of thyroid cancer has seen a steady increase, making it the most prevalent malignant tumor of the endocrine system (1). Notably, the number of new thyroid cancer cases worldwide rose from approximately 122,800 in 2000 to 586,202 by 2020 (2, 3). Among malignant thyroid tumors, over 90% are classified as papillary thyroid cancer (PTC), which primarily spreads through lymph node metastasis (LNM) (4, 5). LNM typically begins in the central region before progressing to the lateral neck area (6), although isolated cases of skip metastasis (lateral neck metastasis without central involvement) also occur (7, 8).Research has highlighted the correlation between LNM in the neck and an elevated risk of post-surgery recurrence (9). Additionally, LNM in the lateral neck region often indicates a poor prognosis and an increased likelihood of requiring additional surgical intervention (10, 11).
However, controversy surrounds the routine use of central lymph node dissection (CLND) during PTC surgery due to the associated risks, including laryngeal nerve injury and hypoparathyroidism (12, 13). The American Thyroid Association (ATA) recommends therapeutic CLND for patients with clinical evidence of lymph node involvement (14). Identifying risk factors associated with LNM is crucial for surgeons to accurately assess the likelihood of lymph node involvement in patients. This knowledge enables more precise surgical planning and informed decision-making regarding neck lymph node dissection (LND) (15, 16). Despite ongoing debate in clinical practice, this retrospective study specifically examines the correlations between various clinical characteristics and central lymph node metastasis (CLNM), lateral lymph node metastasis (LLNM), and skip metastasis. The findings aim to facilitate the identification of risk factors for LNM and provide guidance for clinical decision-making.
2 Materials and methods
2.1 Patients and clinical data collection
We conducted a retrospective analysis of 2,428 patients diagnosed with PTC who underwent their initial thyroid surgery at Ningbo NO. 2 Hospital between January 2023 and December 2023. The study included patients who met the following criteria:
● Underwent their first thyroid surgery
● Possessed comprehensive clinical data
● Received postoperative pathology confirmation of PTC without concurrent other types of thyroid cancer
Clinical and pathological characteristics, including gender, age, maximal axial diameter (MAD), body mass index (BMI), multifocality, Hashimoto’s thyroiditis (HT), CLNM, LLNM, and tumor location, were extracted from electronic medical records and the Ningbo Resident Health Big Data Archive. Continuous variables (age, MAD, BMI) were categorized based on predefined cut-off points:
● Age: ≥45 years and <45 years
● MAD: >1 cm and ≤1 cm
● BMI: ≥28 kg/m² (obese) and <28 kg/m² (non-obese)
We categorized tumor location as upper pole, middle pole, and lower pole (excluding scattered and isthmic tumors). The diagnosis of HT and LNM relied on postoperative histopathological examination of tissue samples.
2.2 Statistical analysis
Data management and statistical analyses were performed using IBM SPSS Statistics, Version 27.0 (SPSS Inc., Chicago, IL, USA). Categorical variables were presented as frequencies and percentages. Univariate analysis utilized the chi-square test, with P < 0.05 considered statistically significant. Variables identified through univariate analysis underwent multivariate binary logistic regression to assess the impact of potential risk factors. Results were reported as odds ratios (ORs) with 95% confidence intervals (CIs). A significance level of P<0.05 was considered statistically significant.
3 Results
This retrospective analysis included 2,428 patients diagnosed with PTC, of whom 944 (38.88%) exhibited CLNM and 177 (7.23%) presented with LLNM.
3.1 CLNM
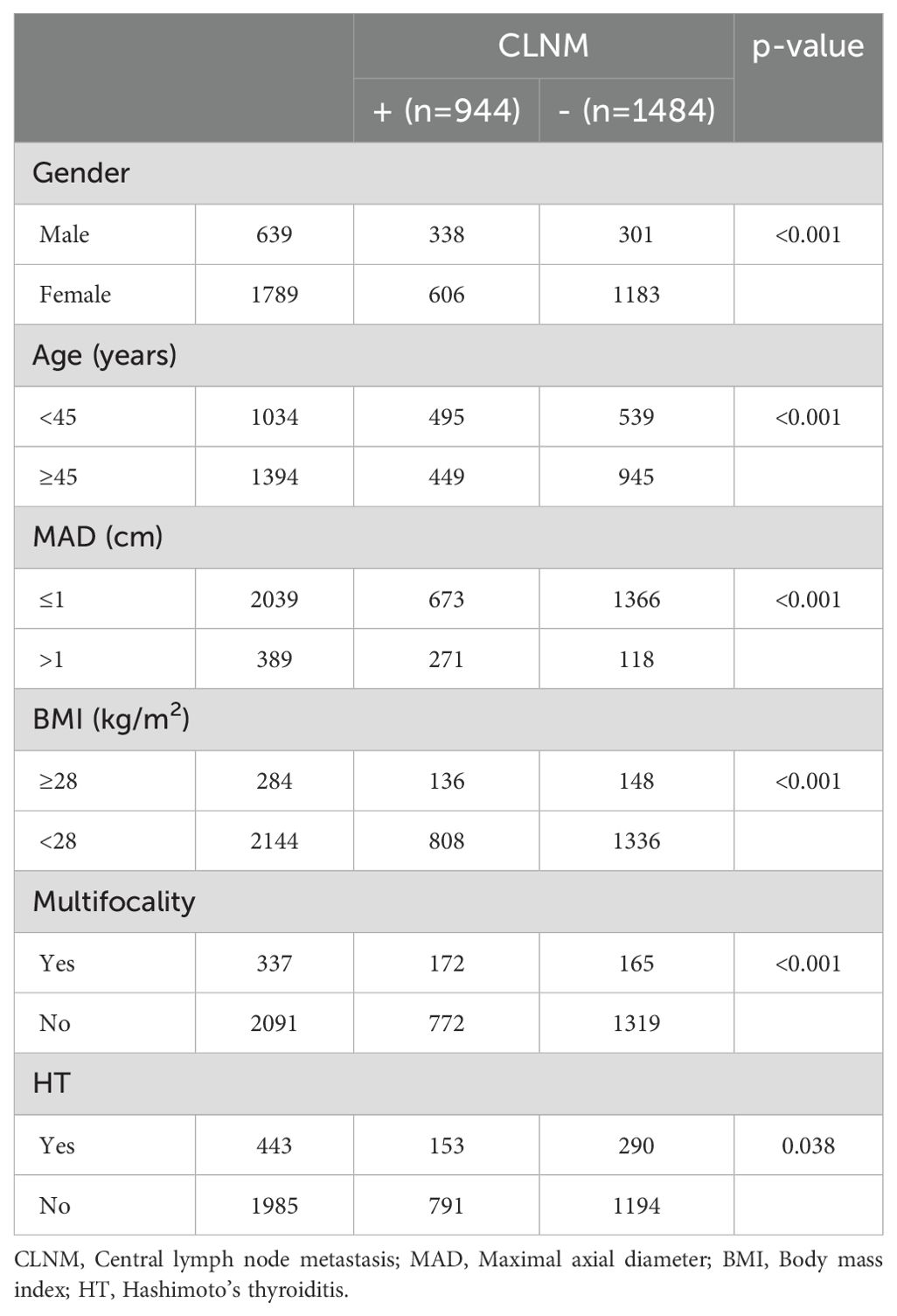
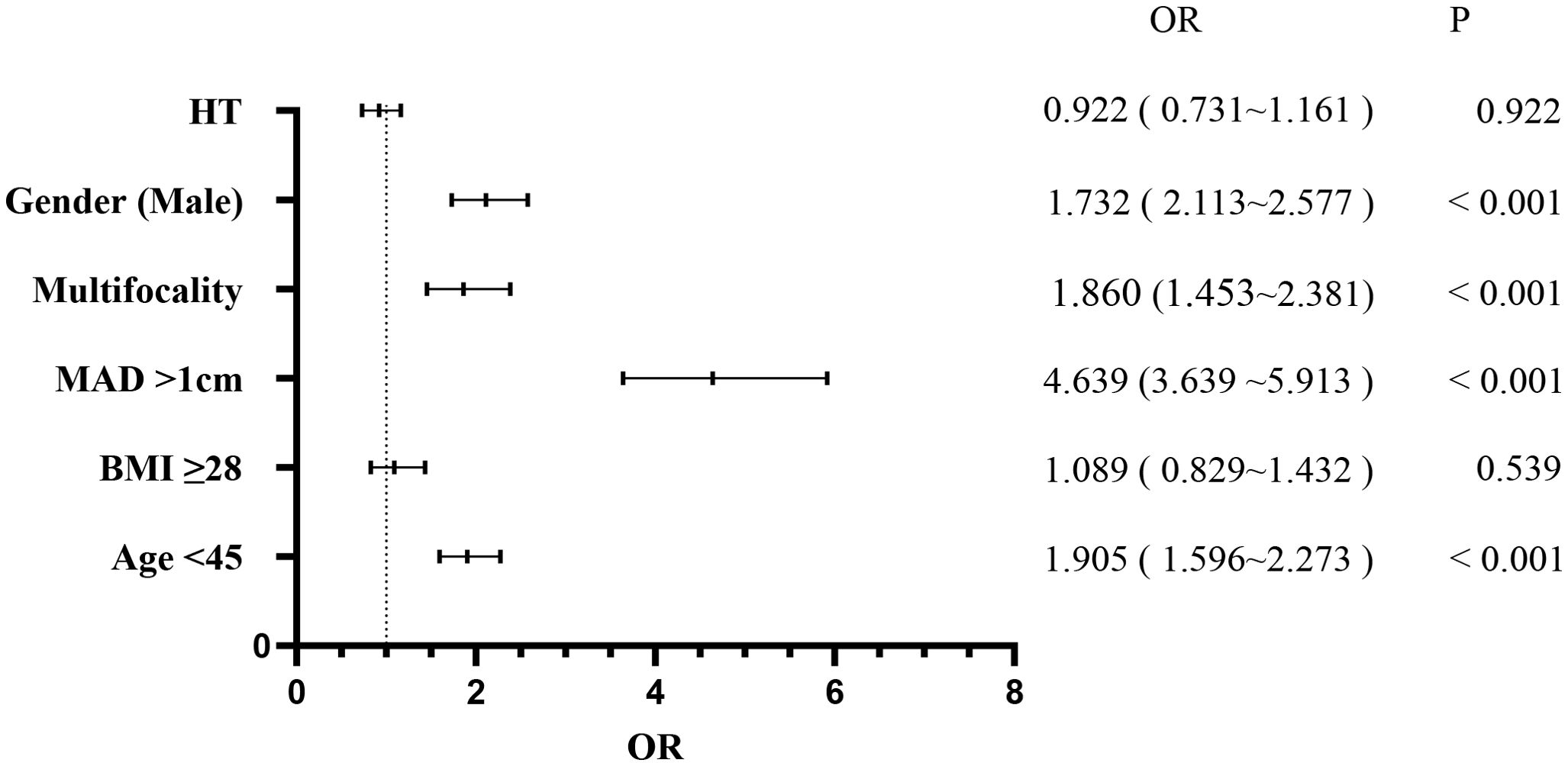
Patients were stratified into the CLNM+ group (944 patients) and the CLNM- group (1,484 patients). Univariate analysis revealed significant associations between CLNM and gender (P < 0.001), age (P < 0.001), MAD (P < 0.001), BMI (P < 0.001), multifocality (P < 0.001), and HT (P = 0.038) (Table 1). Male patients, patients younger than 45 years, patients with MAD > 1 cm, BMI ≥ 28 kg/m², and multifocality were more likely to have CLNM, whereas patients with HT had a lower risk. Multivariable binary logistic regression analysis showed that male gender (OR, 1.732; 95% CI, 2.113–2.577; P < 0.001), age < 45 years (OR, 1.905; 95% CI, 1.596–2.273; P < 0.001), MAD > 1 cm (OR, 4.639; 95% CI, 3.639–5.913; P < 0.001), and multifocality (OR, 1.860; 95% CI, 1.453–2.381; P < 0.001) were independent risk factors for CLNM (Figure 1). HT (OR, 0.922; 95% CI, 0.731–1.161; P = 0.922) and BMI (OR, 1.089; 95% CI, 0.829–1.432; P = 0.539) showed weaker associations with CLNM. The Hosmer and Lemeshow Test (P = 0.792) indicated a good fit for the regression model.
3.2 LLNM
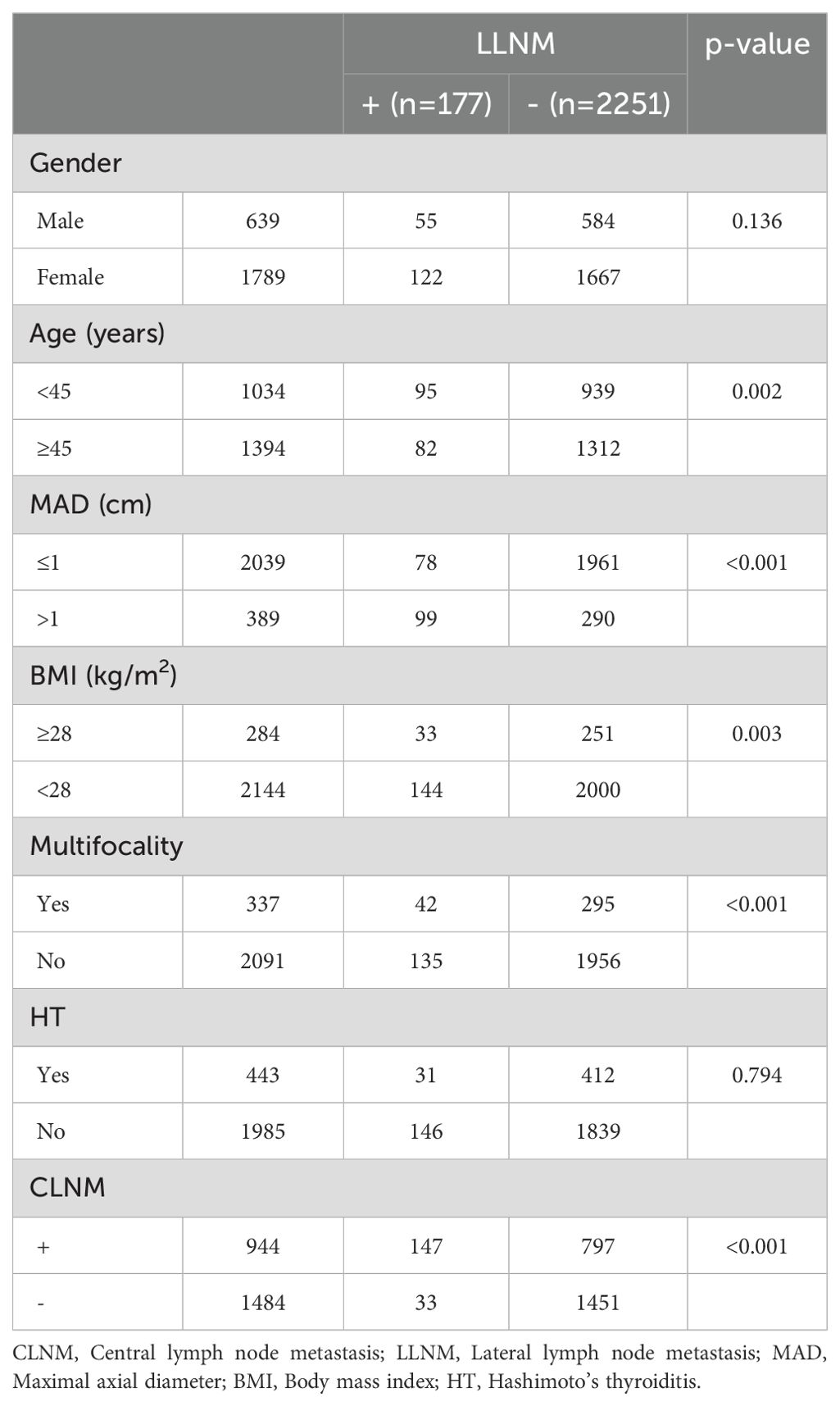
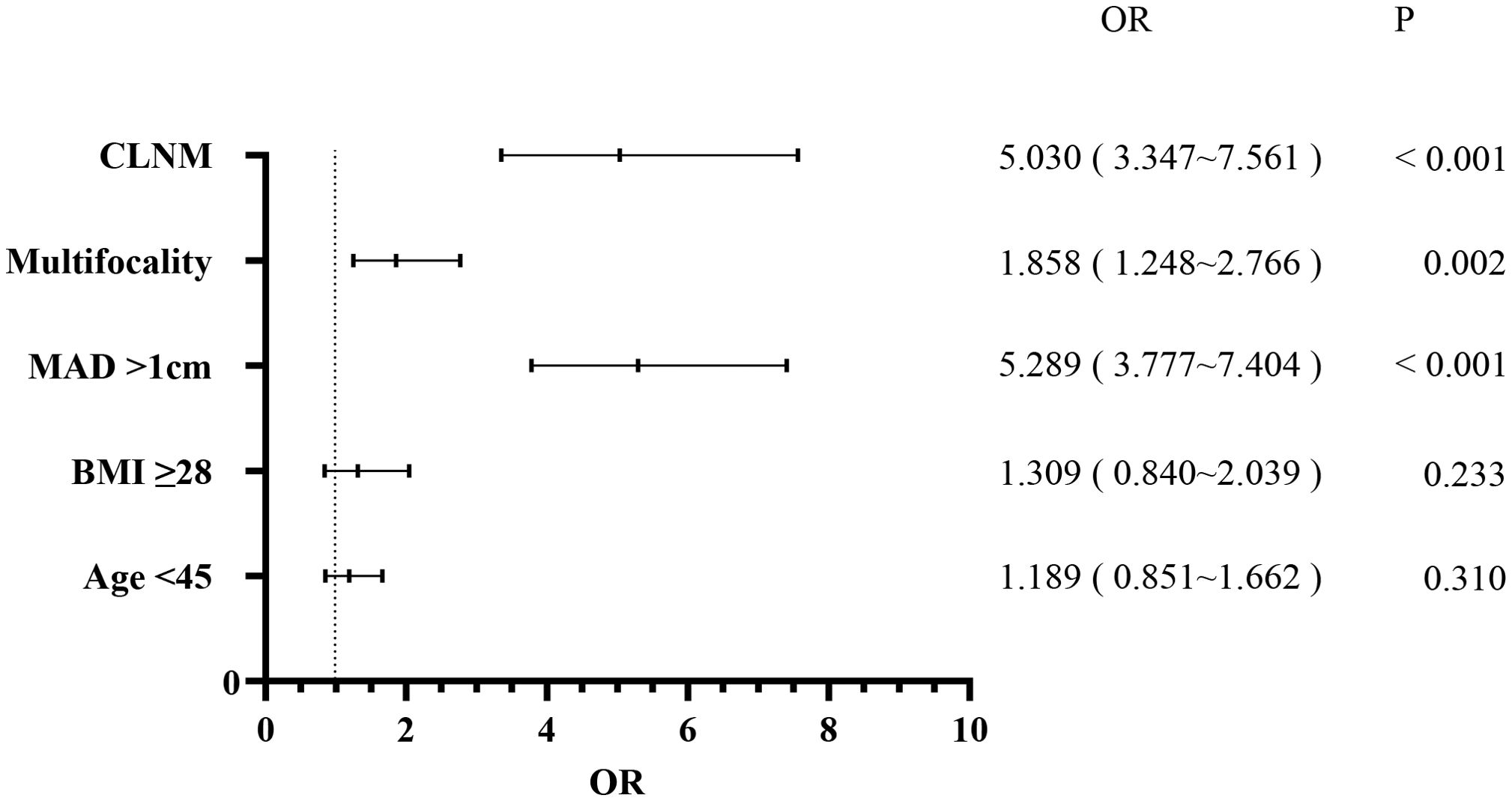
Patients were also categorized into the LLNM+ group (177 patients) and the LLNM- group (2,251 patients). Univariate analysis identified significant correlations between LLNM and age (P = 0.002), MAD (P < 0.001), BMI (P = 0.003), multifocality (P < 0.001), and CLNM (P < 0.001) (Table 2). HT (P = 0.794) and gender (P = 0.136) showed no association with LLNM. Patients younger than 45 years, with MAD > 1 cm, BMI ≥ 28 kg/m², multifocality, and CLNM exhibited a higher likelihood of]LLNM. Multivariate binary logistic regression analysis indicated that MAD > 1 cm (OR, 5.289; 95% CI, 3.777–7.404; P < 0.001), multifocality (OR, 1.858; 95% CI, 1.248–2.766; P = 0.002), and CLNM (OR, 5.030; 95% CI, 3.347–7.561; P < 0.001) were independent risk factors for LLNM (Figure 2). Age (OR, 1.189; 95% CI, 0.851–1.662; P = 0.310) and BMI (OR, 1.309; 95% CI, 0.840–2.039; P = 0.233) showed weaker associations with LLNM. The Hosmer and Lemeshow Test (P = 0.648) indicated a good fit for the regression model.
3.3 Tumor location and LNM
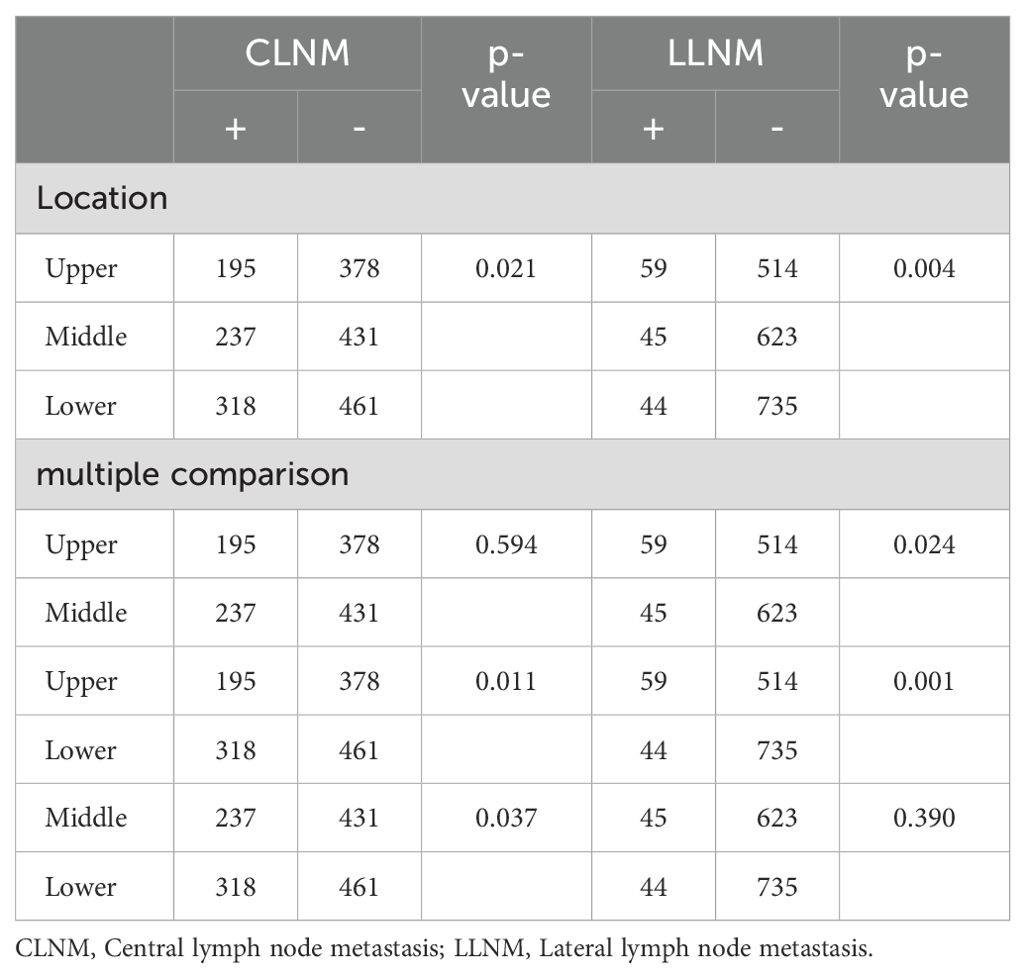
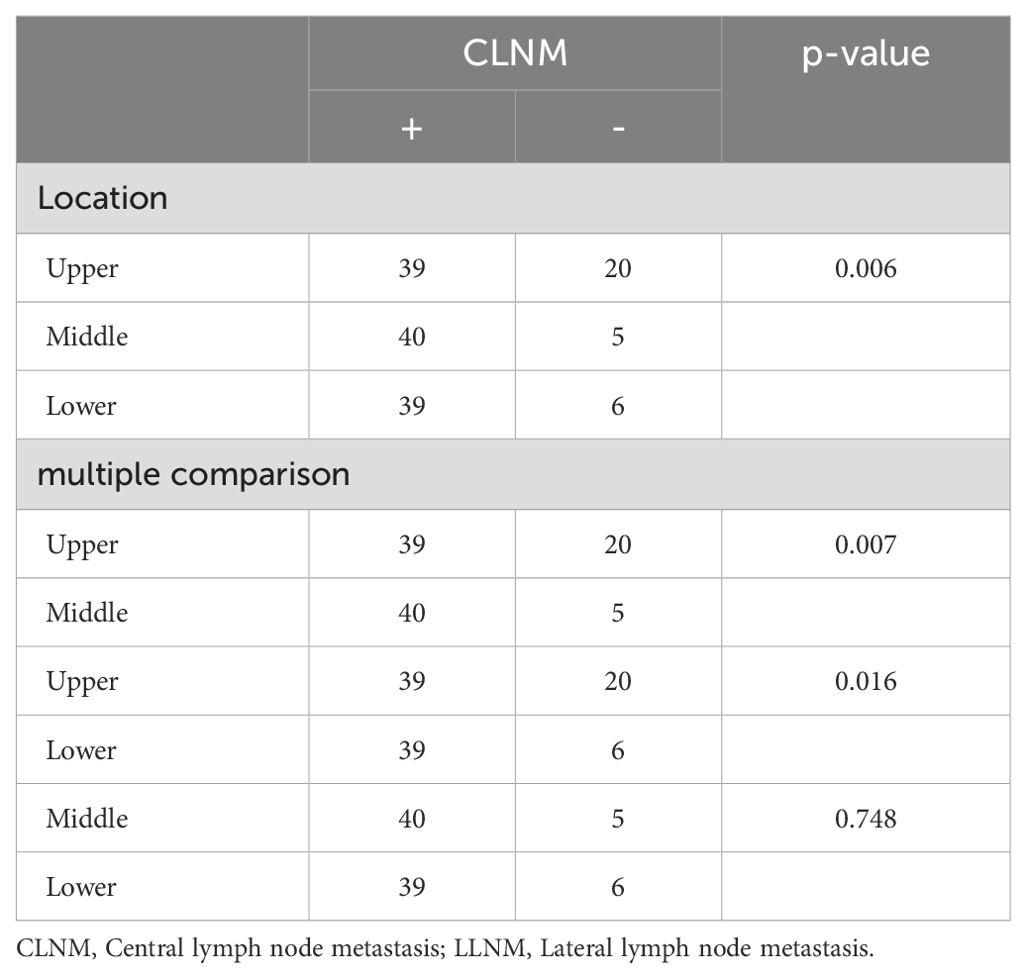
Tumor location was categorized as upper, middle, and lower (excluding multi-site distribution tumors and isthmic tumors, with a total of 2,020 patients included). Chi-square tests and multiple comparisons (Table 3) indicated that lower pole tumors were more likely to have CLNM compared to upper pole tumors (P = 0.011), while upper pole tumors were more prone to LLNM than lower pole tumors (P = 0.001). Due to multiple comparisons among the three groups, a P-value < 0.0167 was considered statistically significant. In a subset of 149 patients with LLNM (Table 4), upper pole tumors showed a higher propensity for skip metastasis (LLNM without CLNM) compared to those located in the middle (P = 0.007) or lower pole (P = 0.016).
4 Discussion
The prognosis for PTC patients is generally favorable, with a low recurrence rate. However, conflicting studies exist regarding the impact of LNM on survival. Some suggest that PTC patients with LNM at initial surgery or experiencing LNM recurrence have a lower survival rate (17–20), while others find no significant effect (21, 22). In most countries, routine lateral lymph node dissection (LLND) occurs only when clear evidence of LNM is present. Prophylactic lymphadenectomy is not recommended for patients without preoperatively detected cervical LNM. At our hospital, CLND is routinely performed for most PTC patients. LLND is only performed when LLNM is confirmed by preoperative or intraoperative pathological examination. Despite controversy over the prognostic significance of lymph node involvement at initial surgery, regional lymph node recurrence remains a concern (23, 24). Considering patients’ quality of life, the presence of LNM plays a crucial role in mitigating subsequent surgical interventions.
Preoperative assessment of LNM in PTC relies on color Doppler ultrasound (US) and neck computerized tomography (CT). However, US sensitivity for detecting LNM is only 60-70% (25, 26), leading to challenges in distinguishing reactive lymphadenopathy from true metastatic disease. Enhanced CT scans can identify calcified metastatic nodes but have limitations in detecting non-calcified nodes (27). Clinicians still struggle to determine the need for LND before PTC surgery.
Our study analyzed risk factors associated with CLNM, LLNM, and skip metastasis. We found that male gender, age <45 years, MAD >1 cm, BMI ≥28 kg/m², and multifocality correlated with CLNM. Interestingly, HT emerged as a protective factor for CLNM. Among these factors, male gender, age <45 years, and MAD >1cm emerged as independent risk factors. While previous studies also linked age <45, male gender, tumor size, and multifocality to CLNM (28–30). Some studies indicated HT as a risk factor for CLNM (28), but others suggested potential protective mechanisms of HT (31). The specific impact of HT remains unclear. Our study showed a reduction in the risk of CLNM occurrence in HT patients. Close monitoring for CLNM is recommended in patients with these independent risk factors, considering prophylactic CLND.
The variables associated with the incidence of LLNM included age < 45 years, MAD > 1 cm, BMI ≥ 28 kg/m², multifocality, and CLNM. Meanwhile, MAD > 1 cm, multifocality, and CLNM established as independent risk factors for LLNM. This study also revealed that tumors situated in the upper pole exhibited a higher propensity for developing LLNM and skip metastasis, while those located in the lower pole were more likely to develop CLNM. A meta-analysis suggested that CLNM, tumor size, multifocality, male gender, and tumor location were independent risk factors for LLNM (32), which aligns with the results of this study. Zhao et al. (33) also identified a tumor diameter > 1 cm and CLNM as independent risk factors for LLNM, and found that upper pole tumors had a higher propensity for skip metastasis, consistent with our findings. Upper pole tumors are more likely to demonstrate skip metastasis, potentially due to preferential dissemination of the tumor via the superior thyroid artery to the lateral lymph nodes rather than through the lymphatic vessels (34).We recommend that PTC patients with preoperative examinations indicating tumor size > 1 cm, multiple lesions, or evident CLNM found intraoperatively, should be carefully evaluated for the potential presence of LLNM. These individuals may undergo lateral lymph node (LLN) biopsy during surgery, and if deemed necessary, LLND may be performed. For tumors located at the upper pole, even without apparent CLNM, monitoring of LLN should still be conducted.
Furthermore, BMI was a key variable of interest in this investigation. Given the observed higher incidence of LNM among obese patients in clinical practice, we conducted a comparison between obese patients (BMI ≥ 28 kg/m²) and non-obese patients (BMI < 28 kg/m²). Univariate analysis revealed that obese patients exhibited a greater likelihood of CLNM and LLNM compared to non-obese patients. However, multivariate analysis indicated that BMI did not independently contribute to the risk of LNM. The relationship between obesity and LNM remains ambiguous, with limited relevant studies available (35). Inclusion of BMI in the analysis provided clinicians valuable insights.
Although numerous studies have investigated the risk factors for CLNM or LLNM, the conclusion is still controversial. Our study conducted a comprehensive statistical comparison of the risk factors associated with CLNM, LLNM, and skip metastasis, offering a more thorough analysis. LNM significantly contributes to postoperative recurrence and poor prognosis in patients with PTC, potentially necessitating secondary surgery and impacting patients’ psychological well-being and quality of life. However, performing LND on all patients may increase the incidence of unnecessary surgical complications such as recurrent laryngeal nerve injury, hypoparathyroidism, and chylothorax. Consequently, preoperatively evaluating LNM and determining whether to perform LND has remained a challenging issue for surgeons. Some studies have indicated that prophylactic CLND can reduce local recurrence rates in high-risk patients (36, 37), while therapeutic LLND should be considered for those with suspected or confirmed LLNM preoperatively (14). US and CT imaging have inherent limitations in distinguishing between different types of lymph nodes, and biopsy is not routinely performed as an examination method. When combined with an analysis of LNM risk factors, these imaging methods can enhance effectiveness and accuracy in evaluating lymph nodes, reducing the likelihood of misdiagnosis, ultimately diminishing the risk of recurrence, and enhancing patient quality of life.
Our study has certain limitations. Firstly, it is a retrospective study, and despite the large sample size, the results may be subject to bias. Secondly, there is a possibility of occult cancer or LNM that cannot be diagnosed through pathology. Thirdly, due to the limited number of skip metastasis cases, we did not conduct a multivariate analysis in this subgroup. Therefore, we hope that future research will involve a multi-center prospective study to more accurately analyze the risk factors for PTC patients.
5 Conclusion
Despite the overall postoperative recurrence rate in PTC patients is low, identifying risk factors such as male gender, age < 45 years, MAD > 1 cm, multifocality, and CLNM can help predict LNM. In specific cases, selective lymphadenectomy in the central or lateral neck area may be warranted.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Human Research Ethics Committee of Ningbo No.2 Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The retrospective nature of the study, approved by the Human Research Ethics Committee of Ningbo No.2 Hospital, obviated the need for informed consent from the subjects.
Author contributions
KY: Formal analysis, Methodology, Writing – original draft, Writing – review & editing, Conceptualization. XW: Funding acquisition, Supervision, Writing – review & editing, Methodology. LD: Supervision, Writing – review & editing, Visualization. QL: Data curation, Writing – review & editing. YX: Data curation, Writing – review & editing. YW: Data curation, Writing – review & editing. WZ: Formal analysis, Funding acquisition, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study received funds from Ningbo Leading Medical and Health Discipline (grant no. 2022-F18), Ningbo Public Service Technology Foundation (grant no. 2023S139) and Zhu Xiushan Talent Award Fund of Ningbo No.2 Hospital (grant no. 2023HMYQ04) to cover publication costs.
Acknowledgments
We gratefully acknowledge Xianjiang Wu, Director of the Thyroid Surgery Center, for providing us with the data platform, and Dr. Qiuda Zheng (Queensland Alliance for Environmental Health Sciences, The University of Queensland, Brisbane, Queensland, Australia) for offering valuable insights during the paper composition.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2024.1473858/full#supplementary-material
References
1. Cabanillas ME, McFadden DG, Durante C. Thyroid cancer. Lancet. (2016) 388:2783–95. doi: 10.1016/S0140-6736(16)30172-6
2. Parkin DM, Bray F, Ferlay J, Pisani P. Estimating the world cancer burden: Globocan 2000. Int J Cancer. (2001) 94:153–6. doi: 10.1002/(ISSN)1097-0215
3. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
4. Lee SH, Roh J-L, Gong G, Cho K-J, Choi S-H, Nam SY, et al. Risk factors for recurrence after treatment of N1b papillary thyroid carcinoma. Ann Surg. (2019) 269:966–71. doi: 10.1097/SLA.0000000000002710
5. Eskander A, Merdad M, Freeman JL, Witterick IJ. Pattern of spread to the lateral neck in metastatic well-differentiated thyroid cancer: A systematic review and meta-analysis. Thyroid. (2013) 23:583–92. doi: 10.1089/thy.2012.0493
6. Sivanandan R, Soo KC. Pattern of cervical lymph node metastases from papillary carcinoma of the thyroid. Br J Surg. (2001) 88:1241–4. doi: 10.1046/j.0007-1323.2001.01843.x
7. Lim YC, Koo BS. Predictive factors of skip metastases to lateral neck compartment leaping central neck compartment in papillary thyroid carcinoma. Oral Oncol. (2012) 48:262–5. doi: 10.1016/j.oraloncology.2011.10.006
8. Lei J, Zhong J, Jiang K, Li Z, Gong R, Zhu J. Skip lateral lymph node metastasis leaping over the central neck compartment in papillary thyroid carcinoma. Oncotarget. (2017) 8:27022–33. doi: 10.18632/oncotarget.15388
9. Leboulleux S, Rubino C, Baudin E, Caillou B, Hartl DM, Bidart J-M, et al. Prognostic Factors for Persistent or Recurrent Disease of Papillary Thyroid Carcinoma with Neck Lymph Node Metastases and/or Tumor Extension beyond the Thyroid Capsule at Initial Diagnosis. J Clin Endocrinol Metab. (2005) 90:5723–9. doi: 10.1210/jc.2005-0285
10. Ito Y, Kudo T, Takamura Y, Kobayashi K, Miya A, Miyauchi A. Lymph node recurrence in patients with N1b papillary thyroid carcinoma who underwent unilateral therapeutic modified radical neck dissection. World J Surg. (2011) 36:593–7. doi: 10.1007/s00268-011-1391-1
11. Baek SK, Jung KY, Kang SM, Kwon SY, Woo JS, Cho SH, et al. Clinical risk factors associated with cervical lymph node recurrence in papillary thyroid carcinoma. Thyroid. (2010) 20:147–52. doi: 10.1089/thy.2008.0243
12. Ahn S-H, Hong HJ, Kwon SY, Kwon KH, Roh J-L, Ryu J, et al. Guidelines for the surgical management of laryngeal cancer: Korean society of thyroid-head and neck surgery. Clin Exp Otorhinolaryngol. (2017) 10:1–43. doi: 10.21053/ceo.2016.01389
13. Calò PG, Pisano G, Medas F, Marcialis J, Gordini L, Erdas E, et al. Total thyroidectomy without prophylactic central neck dissection in clinically node-negative papillary thyroid cancer: is it an adequate treatment? World J Surg Oncol. (2014) 12:152. doi: 10.1186/1477-7819-12-152
14. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American thyroid association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American thyroid association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. (2016) 26:1–133. doi: 10.1089/thy.2015.0020
15. McDow AD, Pitt SC. Extent of surgery for low-risk differentiated thyroid cancer. Surg Clinics North America. (2019) 99:599–610. doi: 10.1016/j.suc.2019.04.003
16. Krajewska J, Kukulska A, Oczko-Wojciechowska M, Kotecka-Blicharz A, Drosik-Rutowicz K, Haras-Gil M, et al. Early diagnosis of low-risk papillary thyroid cancer results rather in overtreatment than a better survival. Front Endocrinol. (2020) 11. doi: 10.3389/fendo.2020.571421
17. Beasley NJ, Lee J, Eski S, Walfish P, Witterick I, Freeman JL. Impact of nodal metastases on prognosis in patients with well-differentiated thyroid cancer. Arch Otolaryngol Head Neck Surg. (2002) 128:825–8. doi: 10.1001/archotol.128.7.825
18. Sugitani I, Fujimoto Y, Yamamoto N. Papillary thyroid carcinoma with distant metastases: Survival predictors and the importance of local control. Surgery. (2008) 143:35–42. doi: 10.1016/j.surg.2007.06.011
19. Zhou L, Li Q, Chen S, Huang Y, Wei W, Zhang C, et al. Synergic effects of histology subtype, lymph node metastasis, and distant metastasis on prognosis in differentiated thyroid carcinoma using the SEER database. Gland Surg. (2020) 9:907–18. doi: 10.21037/gs-20-273
20. Adam MA, Pura J, Goffredo P, Dinan MA, Reed SD, Scheri RP, et al. Presence and number of lymph node metastases are associated with compromised survival for patients younger than age 45 years with papillary thyroid cancer. J Clin Oncol. (2015) 33:2370–5. doi: 10.1200/JCO.2014.59.8391
21. Sancho JJ, Lennard TW, Paunovic I, Triponez F, Sitges-Serra A. Prophylactic central neck disection in papillary thyroid cancer: a consensus report of the European Society of Endocrine Surgeons (ESES). Langenbecks Arch Surg. (2014) 399:155–63. doi: 10.1007/s00423-013-1152-8
22. Kim SK, Woo JW, Lee JH, Park I, Choe JH, Kim JH, et al. Prophylactic central neck dissection might not be necessary in papillary thyroid carcinoma: analysis of 11,569 cases from a single institution. J Am Coll Surg. (2016) 222:853–64. doi: 10.1016/j.jamcollsurg.2016.02.001
23. Lee J, Song Y, Soh EY. Central lymph node metastasis is an important prognostic factor in patients with papillary thyroid microcarcinoma. J Korean Med Sci. (2014) 29:48-52. doi: 10.3346/jkms.2014.29.1.48
24. Randolph GW, Duh QY, Heller KS, LiVolsi VA, Mandel SJ, Steward DL, et al. The prognostic significance of nodal metastases from papillary thyroid carcinoma can be stratified based on the size and number of metastatic lymph nodes, as well as the presence of extranodal extension. Thyroid. (2012) 22:1144–52. doi: 10.1089/thy.2012.0043
25. Eun NL, Son EJ, Kim JA, Gweon HM, Kang JH, Youk JH. Comparison of the diagnostic performances of ultrasonography, CT and fine needle aspiration cytology for the prediction of lymph node metastasis in patients with lymph node dissection of papillary thyroid carcinoma: A retrospective cohort study. Int J Surg. (2018) 51:145–50. doi: 10.1016/j.ijsu.2017.12.036
26. Wu LM, Gu HY, Qu XH, Zheng J, Zhang W, Yin Y, et al. The accuracy of ultrasonography in the preoperative diagnosis of cervical lymph node metastasis in patients with papillary thyroid carcinoma: A meta-analysis. Eur J Radiol. (2012) 81:1798–805. doi: 10.1016/j.ejrad.2011.04.028
27. Hoang JK, Vanka J, Ludwig BJ, Glastonbury CM. Evaluation of cervical lymph nodes in head and neck cancer with CT and MRI: tips, traps, and a systematic approach. AJR Am J Roentgenol. (2013) 200:W17–25. doi: 10.2214/AJR.12.8960
28. Zhang C, B-j L, Liu Z, Wang L-L, Cheng W. Predicting the factors associated with central lymph node metastasis in clinical node-negative (cN0) papillary thyroid microcarcinoma. Eur Arch Oto Rhino Laryngol. (2020) 277:1191–8. doi: 10.1007/s00405-020-05787-1
29. Park JP, Roh J-L, Lee JH, Baek JH, Gong G, Cho K-J, et al. Risk factors for central neck lymph node metastasis of clinically noninvasive, node-negative papillary thyroid microcarcinoma. Am J Surg. (2014) 208:412–8. doi: 10.1016/j.amjsurg.2013.10.032
30. Du J, Yang Q, Sun Y, Shi P, Xu H, Chen X, et al. Risk factors for central lymph node metastasis in patients with papillary thyroid carcinoma: a retrospective study. Front Endocrinol (Lausanne). (2023) 14:1288527. doi: 10.3389/fendo.2023.1288527
31. Zhu F, Shen YB, Li FQ, Fang Y, Hu L, Wu YJ. The effects of Hashimoto thyroiditis on lymph node metastases in unifocal and multifocal papillary thyroid carcinoma. Medicine. (2016) 95:e2674. doi: 10.1097/MD.0000000000002674
32. So YK, Kim M-J, Kim S, Son Y-I. Lateral lymph node metastasis in papillary thyroid carcinoma: A systematic review and meta-analysis for prevalence, risk factors, and location. Int J Surg. (2018) 50:94–103. doi: 10.1016/j.ijsu.2017.12.029
33. Zhao H, Huang T, Li H. Risk factors for skip metastasis and lateral lymph node metastasis of papillary thyroid cancer. Surgery. (2019) 166:55–60. doi: 10.1016/j.surg.2019.01.025
34. Shao L, Wang Z, Dong W, Sun W, Zhang H. Risk factors associated with preferential lateral lymph node metastasis in papillary thyroid carcinoma. Cancer Med. (2023) 12:20670–6. doi: 10.1002/cam4.v12.22
35. Wu C, Wang L, Chen W, Zou S, Yang A. Associations between body mass index and lymph node metastases of patients with papillary thyroid cancer. Medicine. (2017) 96:e6202. doi: 10.1097/MD.0000000000006202
36. Popadich A, Levin O, Lee JC, Smooke-Praw S, Ro K, Fazel M, et al. A multicenter cohort study of total thyroidectomy and routine central lymph node dissection for cN0 papillary thyroid cancer. Surgery. (2011) 150:1048–57. doi: 10.1016/j.surg.2011.09.003
Keywords: papillary thyroid cancer, central lymph node metastasis, lateral lymph node metastasis, skip metastasis, risk factor
Citation: Yu K, Wu X, Dai L, Le Q, Xie Y, Wang Y and Zhang W (2024) Risk factors associated with lymph node metastasis in papillary thyroid cancer: a retrospective analysis based on 2,428 cases. Front. Oncol. 14:1473858. doi: 10.3389/fonc.2024.1473858
Received: 31 July 2024; Accepted: 22 October 2024;
Published: 06 November 2024.
Edited by:
Erivelto Martinho Volpi, Hospital Alemão Oswaldo Cruz, BrazilReviewed by:
Khawla S. Al-Kuraya, King Faisal Specialist Hospital and Research Centre, Saudi ArabiaPeisong Wang, The 1st Hospital of Jilin University, China
Copyright © 2024 Yu, Wu, Dai, Le, Xie, Wang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Weidong Zhang, emhhbmd3ZWlkb25ncXExMUAxNjMuY29t
 Kejie Yu
Kejie Yu Xianjiang Wu
Xianjiang Wu Lei Dai
Lei Dai Qi Le
Qi Le