- 1Department of Oncology, The Third Affiliated Hospital of Chongqing Medical University, Chongqing, China
- 2Department of Pathology, Daping Hospital, Army Medical University, Chongqing, China
Objective: To explore the efficacy of c in the multiline treatment of late-stage lung adenocarcinoma with Her-2 overexpression and epidermal growth factor receptor (EGFR) mutations.
Methods: We summarize the diagnosis and treatment of a female patient with EGFR 21L858R mutation combined with Her-2 overexpression in advanced lung adenocarcinoma, and analyze the effect of c in her treatment process.
Results: The patient was diagnosed with lung adenocarcinoma 8 years ago. After first-line treatment, the lung lesions enlarged. Following second-line treatment 5 years ago, intracranial metastasis occurred. After third-line treatment 3 years ago, intracranial and lung lesions enlarged. New lesions in the lungs, liver, and spleen appeared after fourth-line treatment 32 months ago. Lung progression occurred after fifth-line treatment 29 months ago. Liver and lung progression occurred after sixth-line treatment 22 months ago. Lung progression occurred after seventh-line treatment 19 months ago. The patient underwent eighth-line treatment with disitamab vedotin (RC48) + lung radiotherapy + liver intervention 13 months ago. Currently, the patient’s condition is stable, with a good quality of life, and the efficacy assessment is stable disease (SD). Conclusion: Her-2 overexpression can occur in late-stage EGFR-mutant lung adenocarcinoma after multiline treatment. RC48 can achieve sustained remission in these patients.
1 Introduction
Lung cancer is the most common malignant tumour, with non-small cell lung cancer (NSCLC) accounting for 80% to 85% of all lung cancers (1). Targeted therapies against specific molecular mutations, such as epidermal growth factor receptor (EGFR), anaplastic lymphoma kinase (ALK), and other driver genes, have rapidly advanced, improving the prognosis and quality of life of NSCLC patients (2). Compared to chemotherapy, targeted therapy has prolonged progression-free survival (PFS) and overall survival (OS) times. However, due to tumour heterogeneity and genomic instability, the widespread occurrence of secondary resistance, such as secondary gene mutations and activation of alternative signalling pathways, has greatly reduced the cure rate of non-small cell lung cancer, leading to the emergence of corresponding drug resistance issues (3). The emergence of immunotherapy has changed the treatment landscape for advanced NSCLC; however, research has mainly focused on NSCLC patients who are negative for driver genes (4, 5). For patients who are resistant to EGFR-tyrosine kinase inhibitors (TKIs) and have no standard targeted therapy, clinical studies have further explored the efficacy of immunotherapy in patients after EGFR-TKIs resistance, providing new treatment options for patients resistant to EGFR-TKIs (6, 7). Currently, immunotherapy combined with chemotherapy has shown initial effectiveness (8, 9), and the combination of immunotherapy with doublet chemotherapy and anti-angiogenesis quadruple therapy has shown remarkable efficacy (6). The frequency of Her-2 gene abnormalities in NSCLC is lower than that of EGFR, but its tumour driving mechanism is clear, and it is sensitive to some targeted drugs, making it a current research hotspot. This article summarizes the diagnosis and treatment of a patient with late-stage lung adenocarcinoma with Her-2 overexpression and EGFR mutation, aiming to explore the treatment efficacy of the antibody-drug conjugate (ADC) disitamab vedotin. The report is as follows.
2 Materials and methods
2.1 General information
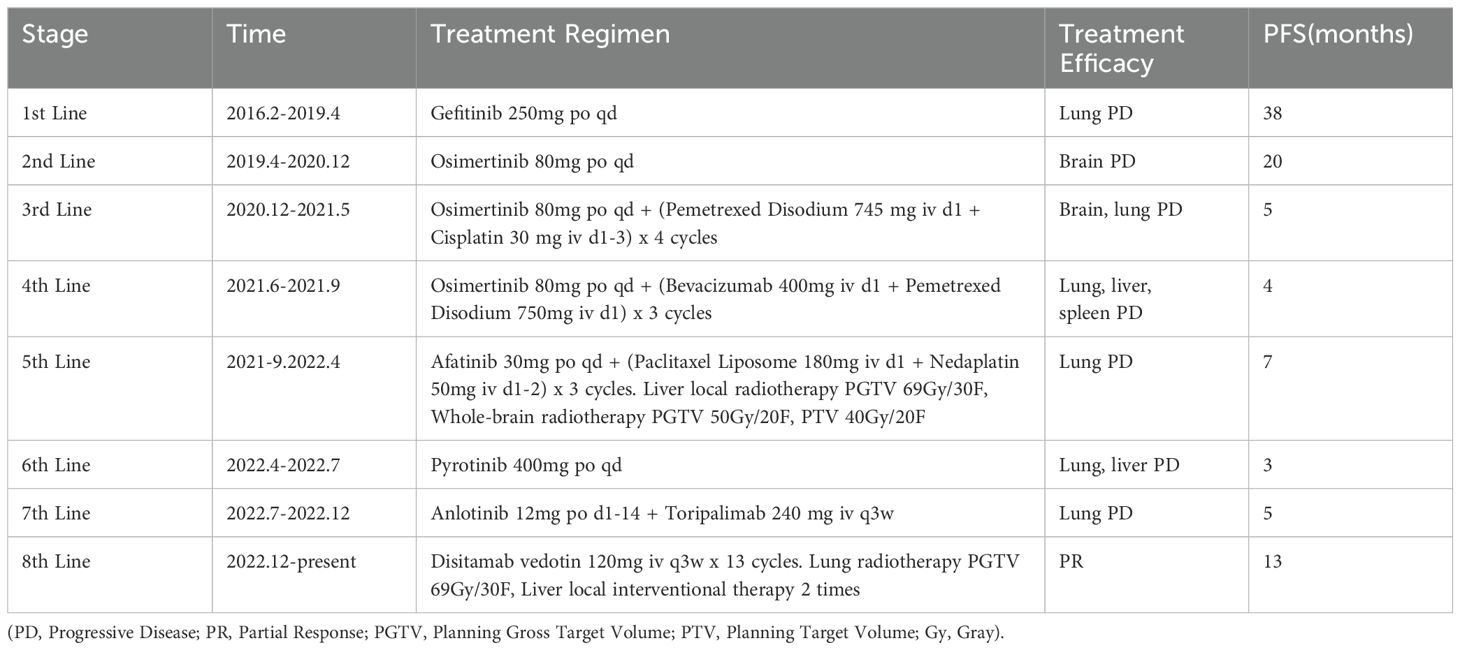
The patient, a 52-year-old female, was admitted to the hospital on September 8, 2021, due to “shortness of breath and increased fatigue after activity for 1 week.” The patient was diagnosed with lung adenocarcinoma at an outside hospital 8 years ago due to “persistent cough and shortness of breath for over 2 months.” After 38 months of first-line treatment, lung lesions enlarged. Twenty months into second-line treatment 5 years ago, intracranial metastasis occurred. Five months into third-line treatment 3 years ago, intracranial and lung lesions enlarged. Four months into fourth-line treatment 2.5 years ago, new lesions appeared in the lungs, liver, and spleen. Seven months into fifth-line treatment 2.2 years ago, lung progression occurred. Three months into sixth-line treatment, liver and lung progression occurred. Five months into seventh-line treatment, lung progression occurred. Thirteen months ago, the patient underwent eighth-line treatment with RC48, combined with lung radiotherapy and liver local interventional therapy. Currently, the patient’s condition is stable, with a good quality of life. This study was approved by the hospital’s ethics committee (Approval No: Coren Trial No. (4) of 2024), adhering to the principles of the Helsinki Declaration, and informed consent was obtained from the patient.
2.2 methods
2.2.1 Genetic testing
Pathological paraffin-embedded (FFPE) tissue and blood samples from the patient were used for genetic testing. DNA was extracted using the Magnetic Bead-based FFPE DNA Extraction Kit (Guangzhou Meiji Biotechnology Co., Ltd., Guangzhou, China) and the Magnetic Bead-based Blood Genomic DNA Extraction Kit-T5C (Tiangen Biochemical Technology (Beijing) Co., Ltd., Beijing, China). Next-generation sequencing (NGS) was performed using a gene capture panel to detect mutations in 122 genes related to solid tumors (Wuxi Zhenhe Biotechnology Co., Ltd.).
2.2.2 Immunohistochemistry
Specimens were fixed in 10% neutral buffered formalin for 24 hours, routinely dehydrated, embedded in paraffin, and sectioned at 3 μm. Hematoxylin and eosin (HE) staining was performed for microscopic observation. Immunohistochemistry staining was performed using an automated immunohistochemistry staining machine (Roche, Switzerland) and the UltraView Universal DAB Detection Kit (Ventana), purchased from Roche Diagnostics Products (Shanghai) Co., Ltd.
2.3 Statistical analysis
Count data were expressed as frequencies or percentages.
3 Results
3.1 Patient’s multiline treatment course
The patient’s multiline treatment course is shown in Table 1.
3.2 Genetic testing results
On 18 January 2016, genetic testing (tissue) showed an EGFR 21 L858R mutation. On 22 April 2019, genetic testing (peripheral blood) was negative for T790M mutation, with no relevant driver gene mutations detected. On 2 April 2020, genetic testing (peripheral blood) confirmed the EGFR 21 L858R mutation with a mutation abundance of 0.29%. On 14 September 2021, genetic testing (tissue and blood) showed an EGFR p.L858R mutation with a mutation frequency of 56.43%, and ERBB2 gene amplification with a copy number change of 26.07.
3.3 Pathology and Her-2 testing results
On 18 January 2016, a percutaneous lung biopsy (left upper lung) revealed adenocarcinoma. Immunohistochemistry did not detect Her-2. On 14 September 2021, a percutaneous lung biopsy (left lung) again showed adenocarcinoma. Immunohistochemistry did not detect Her-2. On 7 December 2022, a percutaneous lung biopsy (left lung tissue) confirmed adenocarcinoma. Immunohistochemistry showed Her-2 (3+).
3.4 Imaging results
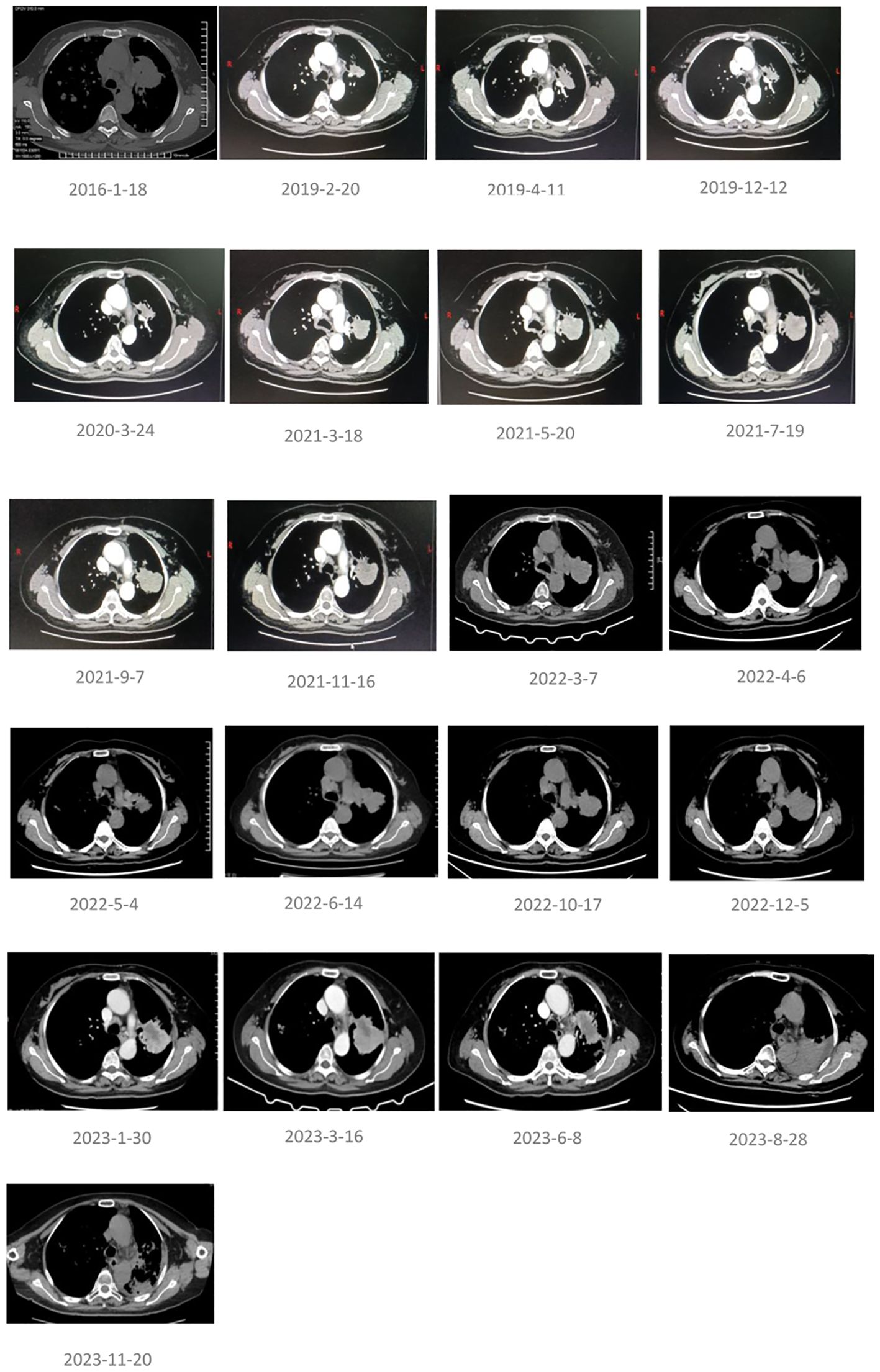
The pathology and imaging results at the initial diagnosis on 18 January 2016 are shown in Figure 1. The lung imaging results during the treatment period are shown in Figure 2. The imaging results of extracorporeal organs (brain, liver, spleen) before and most recently during RC48 treatment are shown in Figure 3.
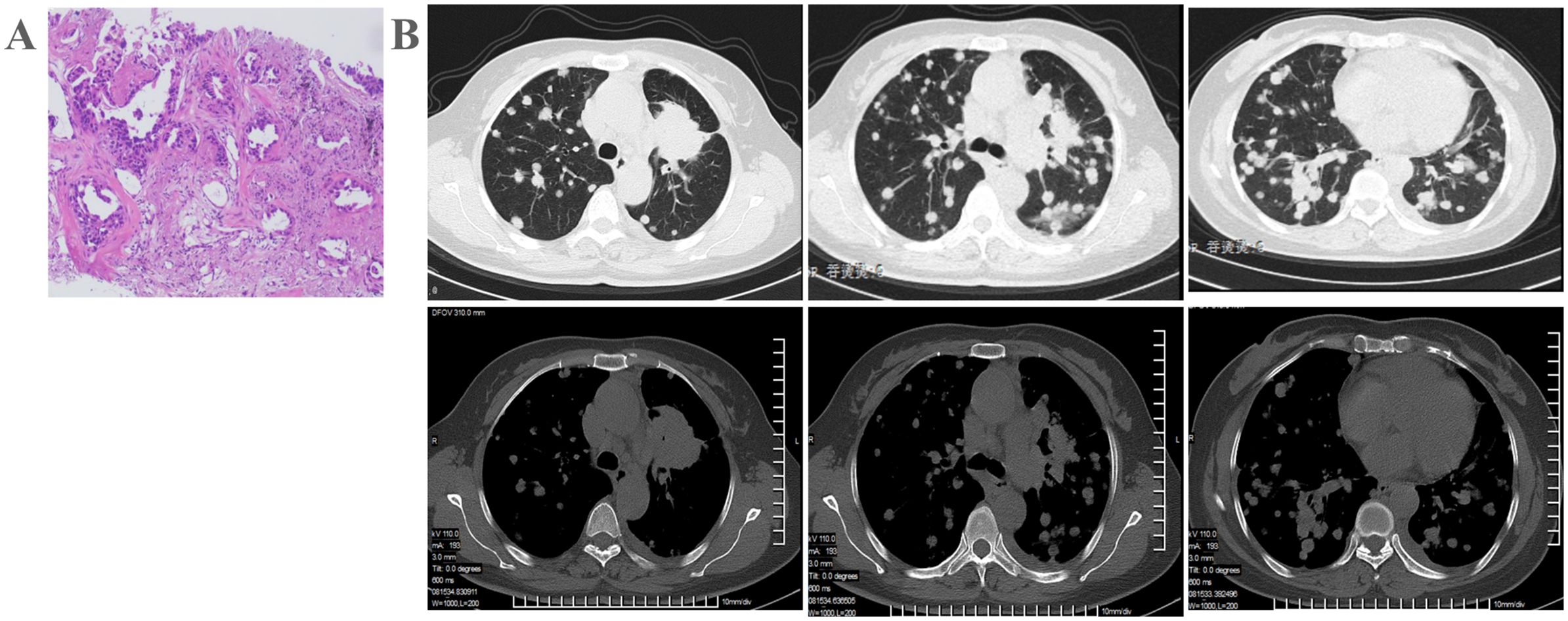
Figure 1. Patient examination before treatment. (A) Chest CT indicates left upper lung cancer with a high likelihood of bilateral lung metastasis, and multiple enlarged mediastinal lymph nodes. (B) Pathological biopsy confirms lung adenocarcinoma.
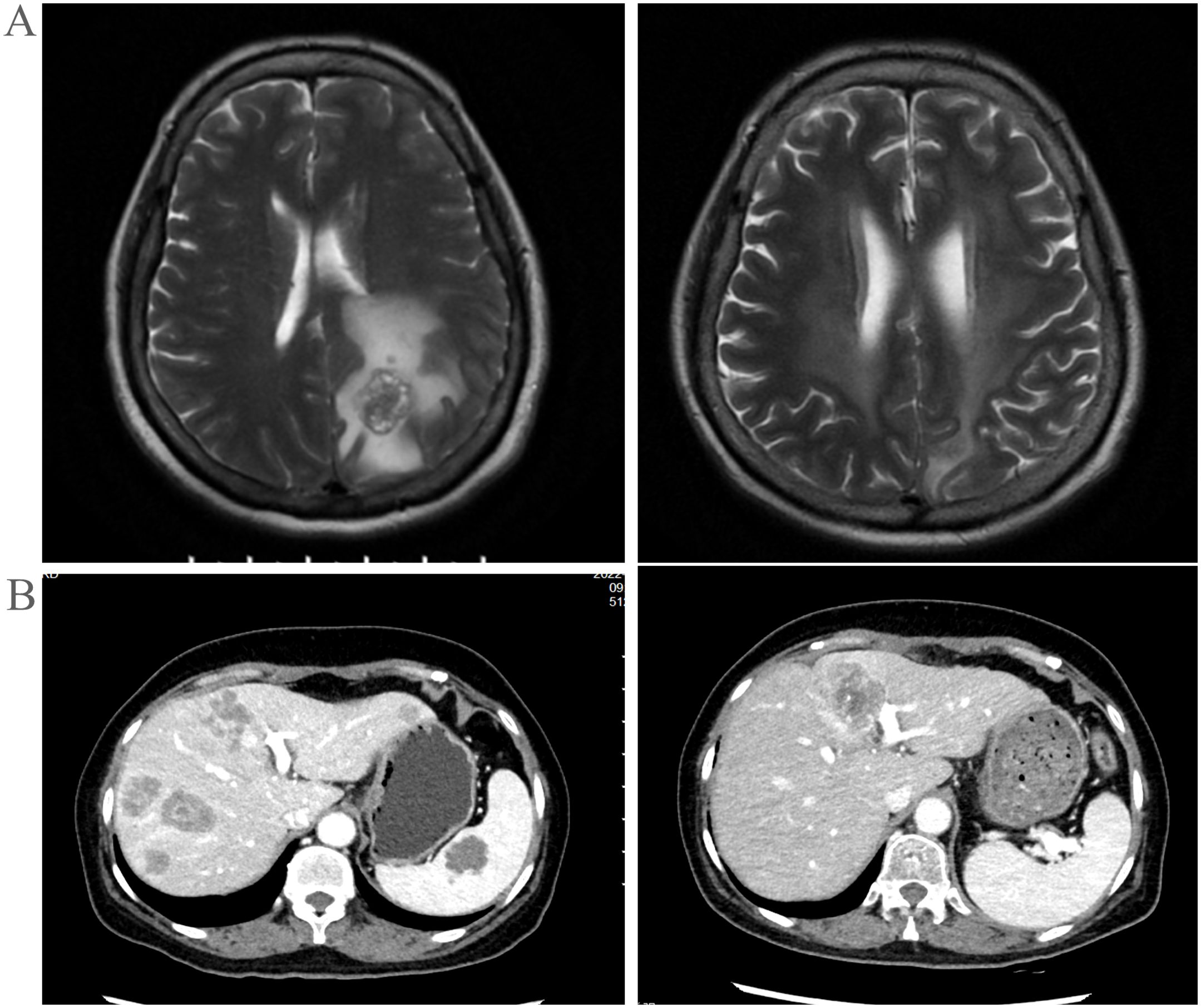
Figure 3. (A) Extra-pulmonary organs: head, MRI images before and after treatment. (B) Extra-pulmonary organs: liver, CT images at key points during the treatment process.
3.5 Efficacy
Since the start of eighth-line treatment, the patient has achieved 13 months of sustained remission. As of 20 January 2024, the patient’s overall survival (OS) time has reached 8 years.
4 Discussion
Most patients with non-small cell lung cancer (NSCLC) are diagnosed at an advanced stage and have a poor prognosis (10). In recent years, with the discovery of driver genes in NSCLC, especially adenocarcinoma, and advances in drug development, the survival of patients with advanced NSCLC has significantly improved, marking the advent of the targeted therapy era and providing new treatment options for NSCLC (11). Drugs such as gefitinib, dacomitinib, and osimertinib, EGFR-TKIs, have been approved by the FDA for the treatment of NSCLC with positive driver genes. However, the clinical efficacy of EGFR-TKIs is greatly limited by inevitable resistance, with resistance mechanisms including Her-2/Her-3/c-Met amplification and receptor tyrosine kinase-related bypass mechanisms, with Her-2 being the most representative (12).
Her-2 is a tyrosine kinase receptor in the ERBB/Her family and, together with other family members like EGFR, activates downstream signal transduction. Abnormalities in the Her-2 gene are closely related to the severity of many epithelial cell cancers, with tumours exhibiting strong metastatic and invasive capabilities, poor sensitivity to chemotherapy, and a high tendency for recurrence. Her-2 mutations and amplifications are associated with female gender, Asian ethnicity, non-smoking status, and poorly differentiated adenocarcinoma histology. In NSCLC, patients with Her-2 positivity have a shorter survival period compared to the general population (13). The forms of Her-2 variations in NSCLC primarily include mutations (2%–4%), amplifications (10%–20%), and overexpression (6%–35%). NSCLC resulting from Her-2 mutations, amplifications, or overexpression is referred to as Her-2 positive NSCLC (14).
In this study, the patient underwent a fourth genetic test (tissue and blood), which revealed an EGFR p.L858R mutation with a mutation frequency of 56.43% and ERBB2 gene amplification with a copy number change of 26.07. Afatinib, a pan-Her (EGFR/Her-1, Her-2, and Her-4) inhibitor, selectively and irreversibly binds to its HER family receptor targets, providing long-lasting inhibition. According to the LUX-Lung5 study (15), the progression-free survival (PFS) and objective response rate (ORR) in the afatinib plus paclitaxel group were significantly higher than those in the monotherapy chemotherapy group, with PFS (5.6 months vs. 2.8 months, HR=0.60, P=0.003) and ORR (32.1% vs. 13.2%, P=0.005) showing marked improvement. After multidisciplinary discussion and considering the patient’s financial situation, the fifth-line treatment was chosen as afatinib combined with paclitaxel liposome and nedaplatin for three cycles, followed by liver and brain radiotherapy for further tumour control. After seven months, the efficacy evaluation indicated disease progression.
Her-2 antibodies such as trastuzumab have not significantly improved efficacy compared to traditional chemotherapy, and pan-HER inhibitors like dacomitinib and afatinib have shown unsatisfactory results. However, some new TKIs have shown initial advantages. A phase II prospective clinical study (ChiCTR180000262) indicated that pyrotinib treatment for Her-2 amplified populations had an ORR of 22.2%, a median PFS of 6.3 months, and a median OS of 12.5 months. Additionally, 30.8% of cases with progression on EGFR-TKIs responded to pyrotinib, and the ORR for patients with brain metastases was 40% (16). After one month of pyrotinib treatment, the tumour size was significantly reduced, with the efficacy evaluated as partial response, but after three months, liver and lung progression recurred.
According to the 2022 Chinese Expert Consensus on Immunotherapy for Advanced NSCLC with Driver Genes, for patients with extensive progression after resistance to EGFR-TKIs and in the absence of effective targeted treatments, the use of immune checkpoint inhibitors (ICIs) is recommended. Among the recommended regimens, ICIs combined with chemotherapy and anti-angiogenesis treatment, and ICIs combined with platinum-based chemotherapy, have substantial clinical evidence.For patients similar to those in this study, who have undergone multiple lines of treatment and cannot tolerate high-intensity therapy, ICIs combined with anti-angiogenic treatment is recommended (16). A real-world study in China (17) demonstrated that in second-line and subsequent treatments for recurrent NSCLC patients, the combination of toripalimab and anlotinib showed synergistic effects, significantly prolonging PFS compared to immunotherapy alone or single-agent chemotherapy. Therefore, the seventh-line treatment employed toripalimab combined with anlotinib. After five months, the efficacy assessment indicated disease progression. A repeat lung biopsy and immunohistochemistry revealed Her-2 (3+) status.
Antibody-drug conjugates (ADCs) have emerged as one of the fastest-growing areas in lung cancer treatment in recent years. Combining tumour cell-specific monoclonal antibodies (mAbs) with cytotoxic drugs, ADCs achieve both tumour cell targeting and cell-killing capabilities, positioning them as a promising future direction in cancer therapy. For Her-2 mutant NSCLC, ADCs have shown outstanding performance. In a phase II basket trial of T-DM1 (18), the ORR was 44%, with a PFS of 5.0 months, although the sample size was relatively small. T-DM1 demonstrated some clinical efficacy in NSCLC patients with Her-2 mutations and amplifications, but its effectiveness in Her-2 overexpressing NSCLC did not meet expectations. Trastuzumab deruxtecan (T-DXd) has shown remarkable results in treating Her-2 mutant NSCLC. Initial data from a phase I study reported an ORR of 72.7% and a PFS of 11.3 months (19). The phase II study (DESTINY-Lung01) showed an ORR of 55% and a PFS of 8.2 months (20). In the DESTINY-Lung01 study, for the Her-2 overexpressing (3+ or 2+) cohort, results presented at the 2022 ESMO conference indicated that the ORR assessed by ICR was 26.5% (cohort 1, 6.4 mg/kg) and 34.1% (cohort 1a, 5.4 mg/kg); the median PFS was 5.7 and 6.7 months, respectively, and the median OS was 12.4 and 11.2 months, respectively (21). While T-DXd shows significant promise in Her-2 mutant NSCLC, its efficacy in Her-2 overexpressing NSCLC is limited.
RC48 is a novel humanised anti-Her-2 ADC. It uses a Her-2 antibody as a targeting carrier, covalently conjugated to a small molecule toxin (MMAE) via a cleavable linker. In a phase II study of third-line treatment for locally advanced or metastatic Her-2 overexpressing gastric cancer or gastroesophageal junction cancer (22), the results showed an ORR of 24.4%, a median PFS of 4.1 months, and a median OS of 7.6 months. In patients with previously failed chemotherapy for Her-2 overexpressing locally advanced or metastatic urothelial carcinoma, the ORR with RC48 treatment was 50.0% (23). RC48 has demonstrated clinical benefits in the treatment of gastric cancer and urothelial carcinoma.A real-world retrospective study (24) included 23 patients with advanced solid tumours such as breast cancer, gastric cancer, colorectal cancer, and bladder cancer, with at least Her-2 immunohistochemistry 1+ expression and failure after at least one systemic chemotherapy. All patients received RC48 treatment (as monotherapy, combined with immunotherapy, or combined with radiotherapy). The ORR was 43.5%, and the median PFS was 6.0 months. Further stratified analysis showed that the ORR for the HER-2 low/medium expression (1+ or 2+) group was 37.5%, with a median PFS of 5.75 months. For the HER-2 high expression (3+) group, the ORR was 57.1%, with a median PFS of 7 months. In the RC48 combined with PD-1 inhibitor group, the ORR was 53.8%, with a median PFS of 8 months. In the group combined with local radiotherapy, the ORR was 40.0%, with a median PFS of 6.0 months. Current phase II/III clinical studies of RC48-ADC are ongoing for indications such as breast cancer, lung cancer, and cholangiocarcinoma. Considering the availability of the drug, the patient opted for RC48 treatment, during which the lesion assessment indicated partial response.
Considering that radiotherapy can reduce the tumour burden of local lesions and release tumour antigens, RC48-ADC targets Her-2 antigens on the surface of tumour cells, precisely identifying and destroying tumour cells. This can also lead to extensive antigen release from other metastatic lesions, thereby activating T-cell immunity and forming a “point-to-surface” treatment strategy. Therefore, RC48-ADC and radiotherapy may have a synergistic effect, achieving better therapeutic outcomes (25). Consequently, to better reduce the tumour, the patient underwent palliative radiotherapy of the left lung and received two sessions of liver interventional therapy. During treatment, the patient experienced mild adverse events, including grade II leukopenia and grade I anaemia, which improved after symptomatic treatment. No other treatment-related adverse events such as skin toxicity, neurotoxicity, cardiotoxicity, pulmonary toxicity, gastrointestinal toxicity and hepatic toxicity occurred during treatment. Currently, the patient’s PFS is 13 months, with an OS of 8 years.
In summary, the patient achieved long-term survival through multiple lines of treatment, including targeted therapy, chemotherapy, radiotherapy, immunotherapy, and antibody-drug conjugates, indicating that lung cancer treatment is progressing towards a chronic disease management approach. This study highlights that Her-2 positivity in NSCLC presents a challenging therapeutic target, with current clinical needs not yet fully met. ADCs show great potential in the standard treatment pathway for Her-2 mutant NSCLC patients. However, further exploration is needed regarding the biological nature, treatment strategies, and diagnostic standards of Her-2 amplification/overexpression in lung cancer.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by the hospital’s ethics committee (Approval No: Coren Trial No. (4) of 2024), adhering to the principles of the Helsinki Declaration, and informed consent was obtained from the patient. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
ML: Data curation, Funding acquisition, Investigation, Project administration, Supervision, Writing – original draft. TW: Conceptualization, Data curation, Project administration, Visualization, Writing – review & editing. DL: Formal analysis, Visualization, Writing – original draft. YC: Methodology, Resources, Writing – original draft. WL: Conceptualization, Methodology, Project administration, Resources, Writing – original draft. RK: Conceptualization, Formal analysis, Writing – original draft. QX: Conceptualization, Data curation, Validation, Writing – original draft.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
2. Minguet J, Smith KH, Bramlage P. Targeted therapies for treatment of non-small cell lung cancer–Recent advances and future perspectives. Int J Cancer. (2016) 138:2549–61. doi: 10.1002/ijc.29915
3. Ettinger DS, Wood DE, Aisner DL, Akerley W, Bauman JR, Bharat A, et al. Non-small cell lung cancer, version 3.2022, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. (2022) 20:497–530. doi: 10.6004/jnccn.2022.0025
4. Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. (2016) 375:1823–33. doi: 10.1056/NEJMoa1606774
5. Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. (2015) 373:1627–39. doi: 10.1056/NEJMoa1507643
6. Reck M, Mok TSK, Nishio M, Jotte RM, Cappuzzo F, Orlandi F, et al. Atezolizumab plus bevacizumab and chemotherapy in non-small-cell lung cancer (IMpower150): key subgroup analyses of patients with EGFR mutations or baseline liver metastases in a randomised, open-label phase 3 trial. Lancet Respir Med. (2019) 7:387–401. doi: 10.1016/s2213-2600(19)30084-0
7. Garassino MC, Cho BC, Kim JH, Mazières J, Vansteenkiste J, Lena H, et al. Durvalumab as third-line or later treatment for advanced non-small-cell lung cancer (ATLANTIC): an open-label, single-arm, phase 2 study. Lancet Oncol. (2018) 19:521–36. doi: 10.1016/s1470-2045(18)30144-x
8. Jiang T, Wang P, Zhang J, Zhao Y, Zhou J, Fan Y, et al. Toripalimab plus chemotherapy as second-line treatment in previously EGFR-TKI treated patients with EGFR-mutant-advanced NSCLC: a multicenter phase-II trial. Signal Transduct Target Ther. (2021) 6:355. doi: 10.1038/s41392-021-00751-9
9. Han BTP, Zhao Y. A phase II study of tislelizumab plus chemotherapy in EGFR mutated advanced non-squamous NSCLC patients failed to EGFR TKI therapies: First analysis. Ann Oncol. (2021) 32:S1443–S4. doi: 10.1016/j.annonc.2021.10.167
10. Herbst RS, Morgensztern D, Boshoff C. The biology and management of non-small cell lung cancer. Nature. (2018) 553:446–54. doi: 10.1038/nature25183
11. Yang CY, Yang JC, Yang PC. Precision management of advanced non-small cell lung cancer. Annu Rev Med. (2020) 71:117–36. doi: 10.1146/annurev-med-051718-013524
12. Tan L, Zhang J, Wang Y, Wang X, Wang Y, Zhang Z, et al. Development of dual inhibitors targeting epidermal growth factor receptor in cancer therapy. J Med Chem. (2022) 65:5149–83. doi: 10.1021/acs.jmedchem.1c01714
13. Riudavets M, Sullivan I, Abdayem P, Planchard D. Targeting HER2 in non-small-cell lung cancer (NSCLC): a glimpse of hope? An updated review on therapeutic strategies in NSCLC harbouring HER2 alterations. ESMO Open. (2021) 6:100260. doi: 10.1016/j.esmoop.2021.100260
14. Mar N, Vredenburgh JJ, Wasser JS. Targeting Her-2 in the treatment of non-small cell lung cancer. Lung Cancer. (2015) 3):220–5. doi: 10.1016/j.lungcan.2014.12.018
15. Misra-Press A. LUX-lung 5: afatinib plus paclitaxel improves outcomes for metastatic NSCLC. MD Conf Express. (2015) 15:13–4. doi: 10.1177/1559897715588299
16. Song Z, Lv D, Chen SQ, Huang J, Li Y, Ying S, et al. Pyrotinib in patients with HER2-amplified advanced non-small cell lung cancer: A prospective, multicenter, single-arm trial. Clin Cancer Res. (2022) 28:461–7. doi: 10.1158/1078-0432.Ccr-21-2936
17. Zhang X, Zeng L, Li Y, Xu Q, Yang H, Lizaso A, et al. Anlotinib combined with PD-1 blockade for the treatment of lung cancer: a real-world retrospective study in China. Cancer Immunol Immunother. (2021) 70:2517–28. doi: 10.1007/s00262-021-02869-9
18. Li BT, Shen R, Buonocore D, Olah ZT, Ni A, Ginsberg MS, et al. Ado-trastuzumab emtansine for patients with HER2-mutant lung cancers: results from a phase II basket trial. J Clin Oncol. (2018) 36:2532–7. doi: 10.1200/jco.2018.77.9777
19. Tsurutani J, Iwata H, Krop I, Jänne PA, Doi T, Takahashi S, et al. Targeting HER2 with trastuzumab deruxtecan: A dose-expansion, phase I study in multiple advanced solid tumors. Cancer Discovery. (2020) 10:688–701. doi: 10.1158/2159-8290.Cd-19-1014
20. Li BT, Smit EF, Goto Y, Nakagawa K, Udagawa H, Mazières J, et al. Trastuzumab deruxtecan in HER2-mutant non-small-cell lung cancer. N Engl J Med. (2022) 386:241–51. doi: 10.1056/NEJMoa2112431
21. Smit EF, Felip E, Uprety D, Nagasaka M, Nakagawa K, Paz-Ares Rodríguez L, et al. Trastuzumab deruxtecan in patients with metastatic non-small-cell lung cancer (DESTINY-Lung01): primary results of the HER2-overexpressing cohorts from a single-arm, phase 2 trial. (2024) 5:439–54. doi: 10.1016/S1470-2045(24)00064-0
22. Peng Z, Liu T, Wei J, Wang A, He Y, Yang L, et al. Efficacy and safety of a novel anti-HER2 therapeutic antibody RC48 in patients with HER2-overexpressing, locally advanced or metastatic gastric or gastroesophageal junction cancer: a single-arm phase II study. Cancer Commun (Lond). (2021) 41:1173–82. doi: 10.1002/cac2.12214
23. Sheng X, Yan X, Wang L, Shi Y, Yao X, Luo H, et al. Open-label, multicenter, phase II study of RC48-ADC, a HER2-targeting antibody-drug conjugate, in patients with locally advanced or metastatic urothelial carcinoma. Clin Cancer Res. (2021) 27:43–51. doi: 10.1158/1078-0432.Ccr-20-2488
24. Wang P, Xia L. RC48-ADC treatment for patients with HER2-expressing locally advanced or metastatic solid tumors: a real-world study. BMC Cancer. (2023) 23:1083. doi: 10.1186/s12885-023-11593-9
25. Xu M, Chen R, Xing P, Kong Y, Zhao X, Zhang J, et al. A multicenter, phase II trial of RC48-ADC combined with radiotherapy, PD-1/PD-L1 inhibitor, GM-CSF, and sequential IL-2 (PRaG3.0 regimen) for salvage therapy in patients with HER2-expressing advanced solid tumors. J Clin Oncol. (2023) 1:e14614. doi: 10.1200/jco.2023.41.16_suppl.e14614
Keywords: HER-2 overexpression, epidermal growth factor receptor, lung adenocarcinoma, disitamab vedotin, efficacy, multiline treatment, conservative treatment
Citation: Lan M, Wang T, Luo D, Chen Y, Liang W, Kong R and Xie Q (2024) Application of disitamab vedotin in the multiline treatment of EGFR mutation-positive lung adenocarcinoma with Her-2 overexpression. Front. Oncol. 14:1472545. doi: 10.3389/fonc.2024.1472545
Received: 22 August 2024; Accepted: 06 November 2024;
Published: 12 December 2024.
Edited by:
Qinglin Shen, Jiangxi Provincial People’s Hospital, ChinaReviewed by:
Ruo Wang, Shanghai Jiao Tong University, ChinaJelena Stojsic, University of Belgrade, Serbia
Copyright © 2024 Lan, Wang, Luo, Chen, Liang, Kong and Xie. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rui Kong, NjUwNDkxQGhvc3BpdGFsLmNxbXUuZWR1LmNu; Qichao Xie, eGllcWljaGFvQGhvc3BpdGFsLmNxbXUuZWR1LmNu
 Meiling Lan1
Meiling Lan1 Yan Chen
Yan Chen Rui Kong
Rui Kong