- 1Department of Urology, Nanchong Central Hospital, The Second Clinical College, North Sichuan Medical College (University), Nanchong, Sichuan, China
- 2Department of Urology, School of Clinical Medicine, Southwest Medical University, Luzhou, Sichuan, China
Objective: This review assessed the prognostic significance of the systemic immune inflammation index (SII) in patients with urothelial carcinoma.
Methods: We performed a systematic review and cumulative meta-analysis of the primary outcomes according to the PRISMA criteria, and assessed study quality. Seven databases were searched: Embase, PubMed, Cochrane Library, Web of Science, China National Knowledge Infrastructure, Wanfang, and SinoMed, from the creation of each database until October 2024.
Results: The meta-analysis included 31 studies, including 14,437 patients with urothelial carcinoma. A low SII was significantly associated with better recurrence-free survival (RFS) (HR = 1.37, 95%CI (1.19, 1.56), P < 0.05), cancer-specific survival (CSS) (HR = 1.87, 95%CI (1.50, 2.34), P < 0.05), and overall survival (OS) (HR = 1.42, 95%CI (1.23, 1.64), P < 0.05). In addition, subgroup analysis found that higher SII was associated with poorer prognosis regardless of treatment regimen, tumor type, or SII cutoff, and that high SII was an important prognostic biomarker in the UC population.
Conclusion: A low SII may be associated with better RFS, CSS, and OS. The SII can be used as a is a potentially noninvasive and promising prognostic indicator for urothelial carcinoma; however, further studies with appropriate designs and larger sample sizes are needed to verify these findings.
1 Introduction
Urothelial carcinoma (UC) is one of the most prevalent malignant neoplasms of the genitourinary system and can arise in any segment of the transitional epithelium of the urinary tract, including the renal pelvis, ureter, bladder, and urethra. This cancer can be categorized into Upper Tract Urothelial Carcinoma (UTUC) and Bladder Urothelial Carcinoma (BC), based on its location. The global incidence and mortality rates for UC have been consistently rising annually (1). Take bladder cancer as an example, about 614,000 new cases and about 220,000 deaths were reported globally in 2022, of which about 90% were bladder urothelial carcinoma, making it one of the most lethal cancers in the world (2–4). With the aging of the global population, the incidence and mortality rates of UC are projected to continue to rise, and the prognosis for patients with UC remains generally unfavorable. Consequently, there is an urgent need to identify effective biomarkers that can enhance the diagnostic accuracy, therapeutic evaluation, and prognostic assessment of UC in clinical settings.
An increasing body of research has underscored the intricate relationship between inflammation and the initiation, progression, and spread of tumors, with chronic inflammation being implicated as a risk factor for tumorigenesis (5, 6). Currently, several inflammatory biomarkers are being considered as potential diagnostic and prognostic tools for cancer, including lymphocyte, neutrophil, and platelet counts, as well as C-reactive protein (CRP) and the neutrophil-lymphocyte ratio (NLR).While CRP and erythrocyte sedimentation rate (ESR) are accessible, cost-effective markers of systemic inflammation, their lack of specificity means they can be influenced by non-neoplastic conditions such as infections and autoimmune diseases (7, 8). The Systemic Immunoinflammatory Index (SII), a novel marker derived from neutrophil, lymphocyte, and platelet counts, has demonstrated its prognostic significance across a spectrum of cancers (9, 10). Numerous studies have indicated the potential utility of SII in predicting urothelial cancer outcomes, advocating for its incorporation into routine assessments for these patients. For instance, elevated SII levels have been shown to be an independent predictor of adverse prognosis and response to BCG therapy in patients with uroepithelial carcinoma (11). Furthermore, the role of immune checkpoint inhibitors (ICIs) in urothelial carcinoma treatment and their associated biomarkers for efficacy and prognosis have been a focus of recent research reviews (12). As an emerging biomarker, SII has demonstrated considerable promise in evaluating prognosis and monitoring treatment responses in urothelial carcinoma. Despite the exploration of SII’s prognostic significance in UC patients, findings have been inconsistent (13–15). Consequently, this meta-analysis seeks to evaluate the prognostic significance of SII in UC based on the extant evidence.
2 Methods
2.1 Search strategy
This study was registered in PROSPERO, and followed the PRISMA meta-analysis guidelines (16, 17) and AMSTAR guidelines (18) for quality assessment. Two researchers independently conducted systematic online literature retrieval and data extraction. Electronic science databases were searched, including PubMed, Embase, Cochrane Library, Web of Science, China National Knowledge Infrastructure (CNKI), Wanfang Database, and China Biomedical Database, from their inception to October 2024. The search terms were as follows: (“Systemic immune inflammation index” OR “SII”) AND (“Urothelial carcinoma” OR “Transitional Cell Carcinoma*”), and all searches were performed using a combination of MeSH words and free words. Additionally, relevant systematic reviews and references from the included studies were manually identified and retrieved for further analysis.
2.2 Eligibility criteria
The inclusion criteria were identified according to the PICOS (population, intervention, comparator, outcomes, and study) criteria. The inclusion criteria were formulated as shown below: (i)P (population): Patients whose UC was confirmed pathologically. (ii)I (intervention): The SII level was examined for UC patients, and studies identified a cutoff value of SII for stratifying patients as low/high SII. (iii)C (comparator): UC patients with high SII level. (iv) O (outcome): Studies report associations between SII and UC survival outcomes; During the defined follow-up period, patients had at least one of the following survival outcomes: cancer-specific survival (CSS), overall survival (OS), and relapse-free survival (RFS); and provided hazard ratios (HR) and corresponding 95% confidence intervals (CI)for survival outcomes or provided sufficient data to calculate them.(v) S (study design): Cohort studies, including prospective and retrospective cohorts published in English or Chinese.
The exclusion criteria were as follows: (i) studies on cell lines, tissues, or animals; (ii) studies without necessary data; (iii) duplicate articles; (iv) case series, review articles, letters, editorials, or reviews; and (v) studies involving patients without urothelial carcinoma.
2.3 Quality evaluation
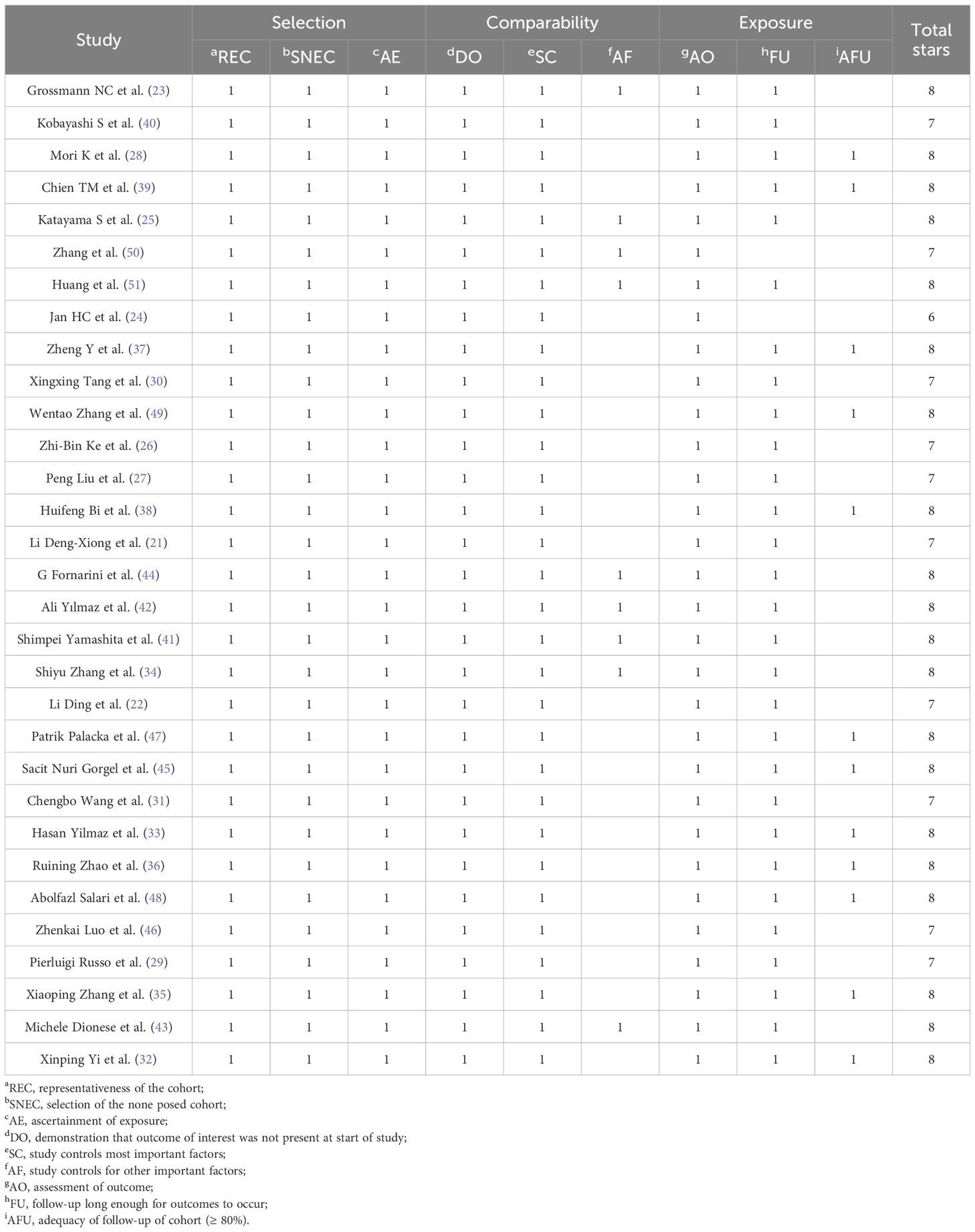
Based on the results of the identification process, we used the Newcastle–Ottawa Scale (NOS) to assess the quality of the included studies (19). This scale includes three areas: selection, comparability, and exposure. It assigns a score ranging from zero to nine stars, with studies receiving six or more stars being classified as high quality.
2.4 Data extraction
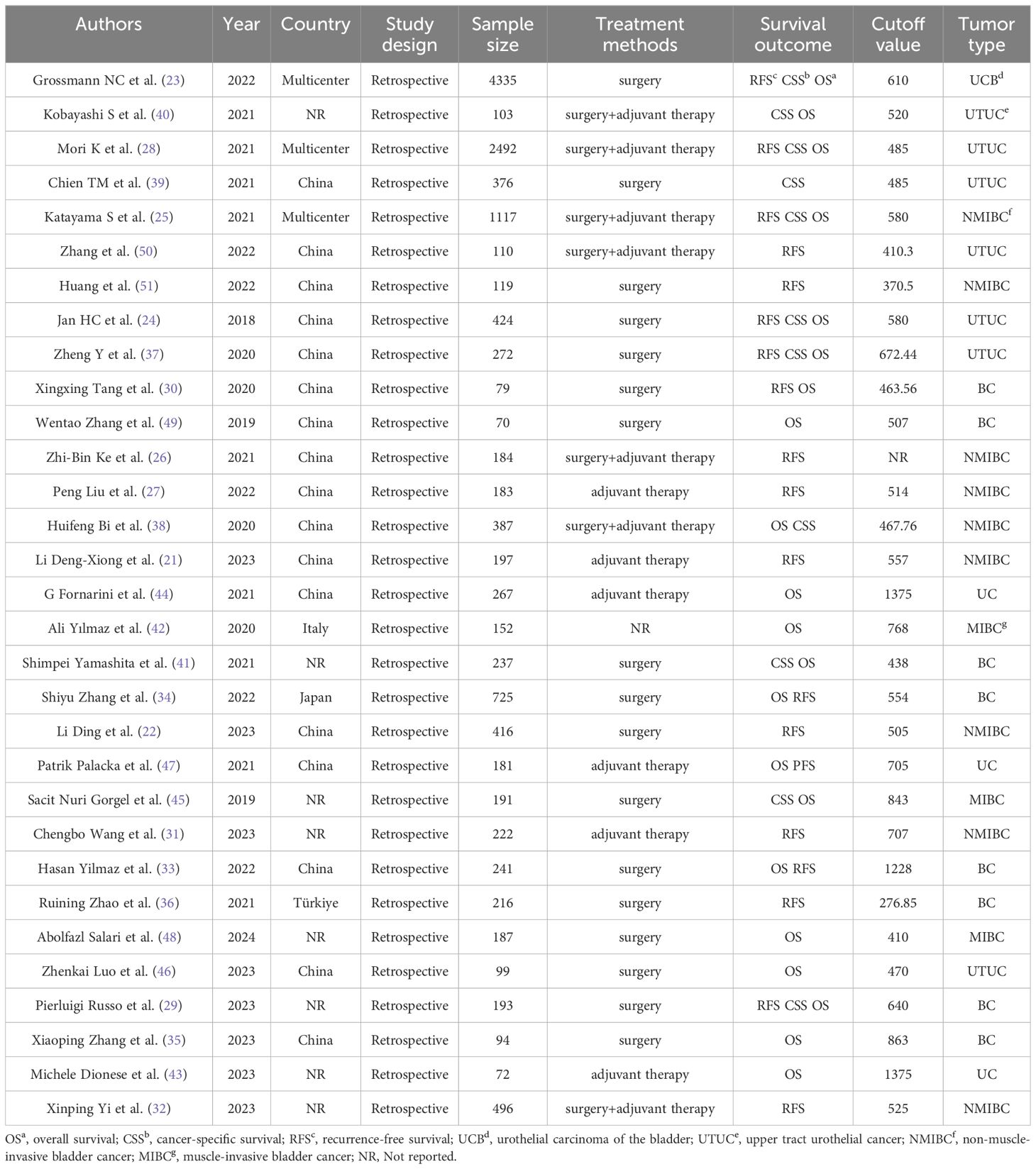
Two researchers employed a standardized data extraction form to meticulously collect the following details from the eligible studies: authors, year, country, study design, sample size, treatment methods, median follow-up, survival outcome, cutoff value, and tumor location. When continuous variables were reported as median and range in the main literature, we calculated the mean and SD (20).
2.5 Data analysis
Data analysis was conducted using Stata version 16. The HR and 95% CI of the multivariate analysis in each study was used to assess the importance of the SII in the prognosis of UC patients. In a meta-analysis, when the effect index is the HR, the risk ratio is usually taken as the logarithm of the effect value. Therefore, we used Stata 16 to find the logarithmic values of HR and the upper and lower limits of the 95% CI, and then performed a meta-analysis. The other parameters were extracted directly from the original study without conversion. We performed the Q and χ2 tests to value the heterogeneity between the included studies. If I2 > 50%, the differences between the studies were considered significant and random effect models were used. Otherwise, a fixed effects model was selected. In addition, sensitivity analyses were performed. The optimal cutoff value of the systemic inflammatory immune index was determined based on the receiver operating characteristic curve. Subgroup analyses were performed on tumor location, treatment modality, and SII cutoff values.
3 Results
3.1 Research description
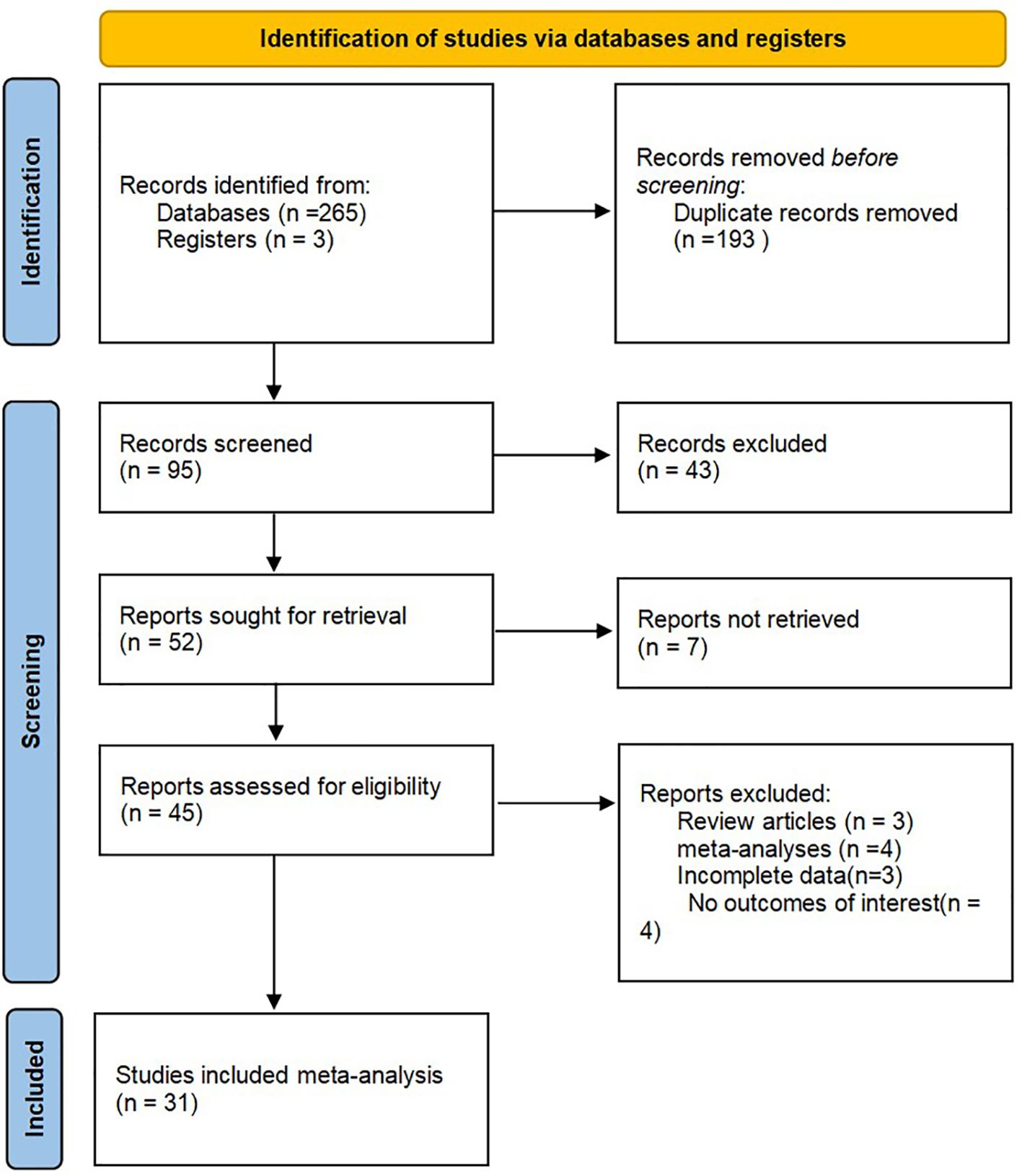
Through the search process, 268 studies were screened from the established databases, with an additional three studies discovered through manual searches. We utilized document management software to eliminate a total of 193 duplicate articles. After reviewing the titles and abstracts, 43 articles were excluded after reading titles and abstracts, seven were not retrieved, and 95 were included in careful reading, excluding four studies with no outcomes of interest, three systematic reviews, four meta-analyses, and three with incomplete data. Ultimately, 31 studies including 14,437 patients were included in the meta-analysis (21–51). A detailed systematic search process is presented in Figure 1. The baseline data of the included studies, including authors, year, country, study design, sample size, treatment methods, survival outcomes, cutoff values, and tumor locations are presented in Table 1.
3.2 Quality assessment
The quality assessment of the cohort studies was conducted using the modified Newcastle-Ottawa Scale (NOS), resulting in scores that ranged between 6 and 8, indicating a robust methodological quality across the studies (Table 2).
3.3 Recurrence-free survival
A total of 18 studies have reported the association between RFS and SII (21–37, 50, 51). The heterogeneity test showed high heterogeneity among studies (I2 = 81.2%, P < 0.05). The results of the meta-analysis showed that RFS was better in the low SII group than in the high SII group, indicating that patients with high SII had shorter RFS (HR = 1.37, 95%CI (1.19, 1.56), P < 0.05) (Figure 2A). At the same time, subgroup analysis conducted in this study showed that higher SII was associated with poorer RFS (p<0.05) regardless of treatment regimen, tumor type, or SII cut-off, and high SII was an important prognostic biomarker for poorer RFS in the UC population (Figures 2B–D).
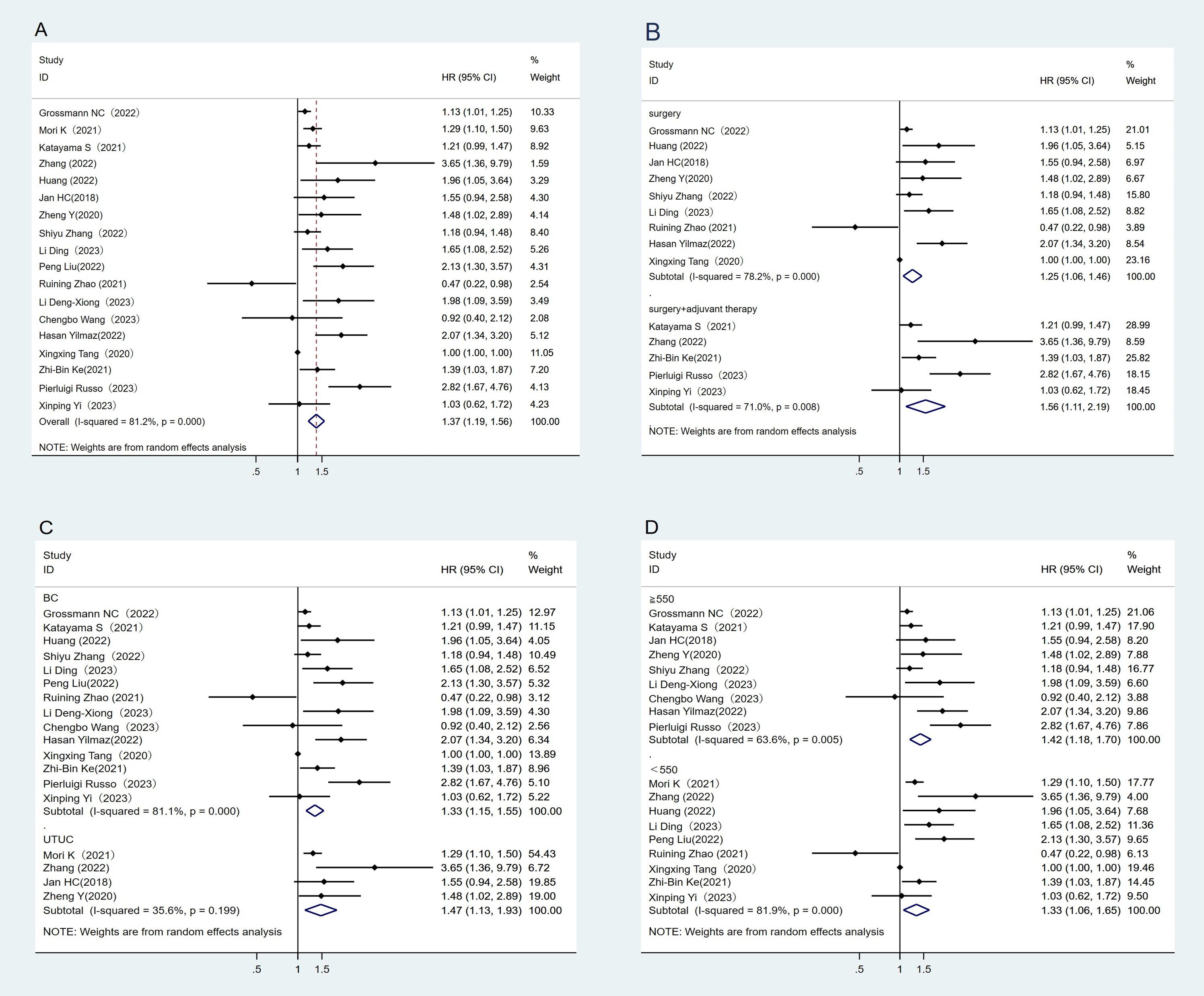
Figure 2. Forest plot and meta-analysis of the RFS between low and high SII. (A) Overall. (B) According to the treatment methods. (C) According to the tumor location. (D) According to the SII cutoff value.
3.4 Cancer-specific survival
A total of 12 studies have reported an association between CSS and SII (23–25, 28, 29, 35, 37–42). The heterogeneity test showed high heterogeneity among studies (I2 = 68.4%, P < 0.1). The results of meta-analysis showed that the lower SII group had better CSS than the higher SII group (HR = 1.87, 95%CI (1.50, 2.34), P < 0.05) (Figure 3A). We also performed subgroup analyses where higher SII was associated with poorer CSS, regardless of treatment regimen, tumor type, or SII cut-off (Figures 3B–D).
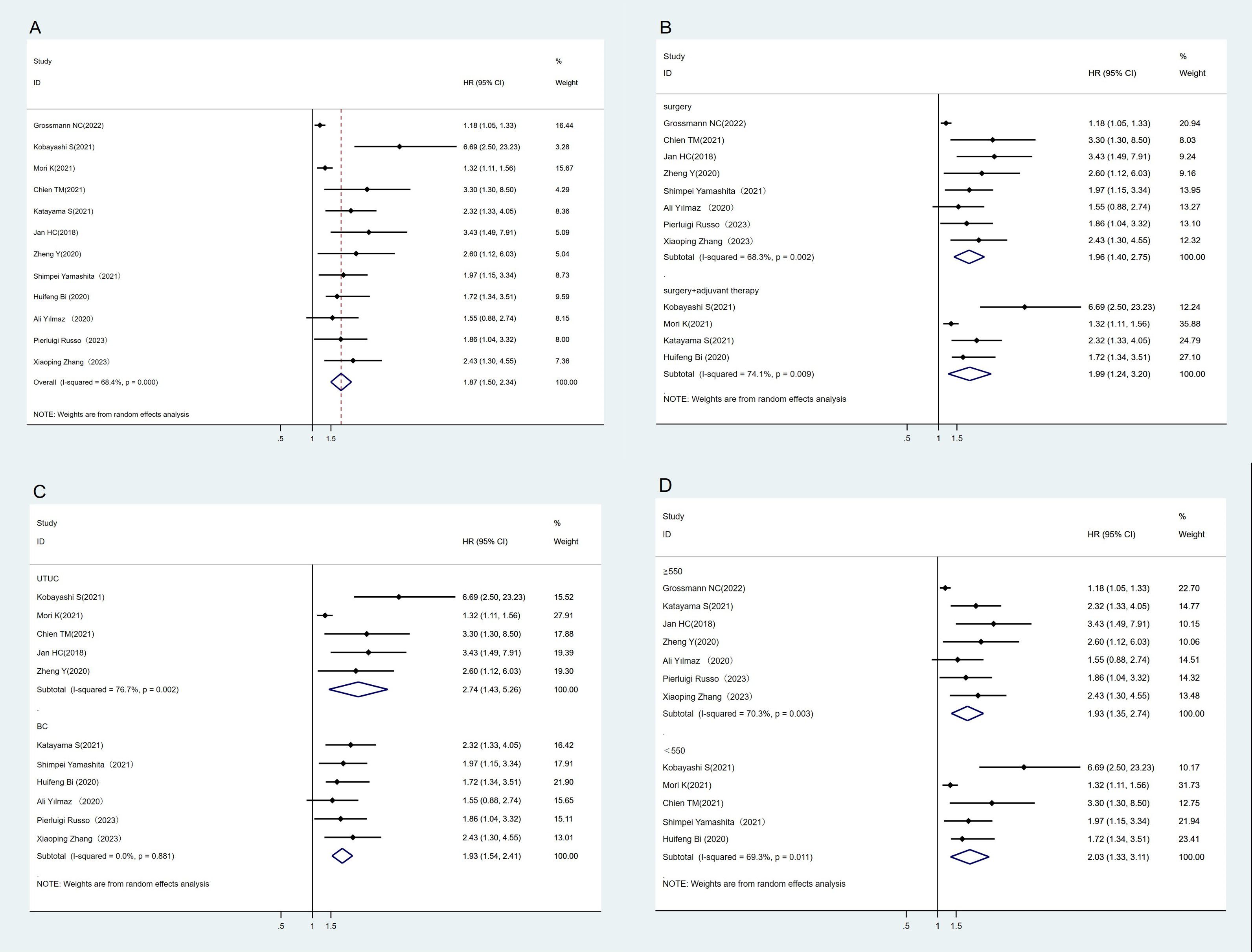
Figure 3. Forest plot and meta-analysis of the CSS between low and high SII. (A) Overall. (B) According to the treatment methods. (C) According to the tumor location. (D) According to the SII cutoff value.
3.5 Overall survival
A total of 20 studies have reported the association between OS and SII (23–25, 28–30, 33, 34, 37, 38, 40–49). The heterogeneity test showed high heterogeneity between studies (I2 = 84.9%, P < 0.1). The results of meta-analysis showed that the OS in the low SII group was better than that in the high SII group. (HR = 1.42, 95%CI (1.23, 1.64), P < 0.05) (Figure 4A). We also performed subgroup analyses where higher SII was associated with poorer OS, regardless of treatment regimen, tumor type, or SII cut-off (Figures 4B–D).

Figure 4. Forest plot and meta-analysis of the OS between low and high SII. (A) Overall. (B) According to the treatment methods. (C) According to the tumor location. (D) According to the SII cutoff value.
4 Sensitivity analysis and publication bias
Despite the inclusion of high-quality studies following stringent quality assessment, there was an inevitably high degree of heterogeneity between studies. We used sensitivity analysis to track the sources of heterogeneity for each outcome measure. The test showed no significant changes in the overall HR estimates for these survival outcomes, suggesting that the findings of the meta-analyses were robust and stable (Supplementary Figures S1–S3).
Begg’s test and funnel plots were used to assess publication bias in the included studies. Visual examination of the funnel plot revealed asymmetry, indicating a higher possibility of publication bias (Supplementary Figures S4–S6).
5 Discussion
Inflammation plays an important role in the biological behavior of tumors. Inflammatory cells in the tumor microenvironment participate in various proinflammatory responses. The number of immune cells and other components in the tumor microenvironment play an important role in the occurrence, malignant transformation, development, and metastasis of tumors (52). Immune cells in the tumor microenvironment, such as T cells, macrophages, and dendritic cells, as well as inflammatory cells, such as neutrophils and lymphocytes, are involved in tumor development and immune responses. The tumor microenvironment directly or indirectly affects tumor cell proliferation, migration, and angiogenesis by releasing various inflammatory mediators (53, 54). Long-term exposure to inflammatory cytokines can promote cell proliferation and angiogenesis (55), whereas DNA damage and excessive production of reactive oxygen species (ROS) stimulate tumor growth (56). Previous meta-analyses found that a high SII is independently associated with poor oncological outcomes in patients with renal cell carcinoma and colorectal cancer (57, 58). High SII values are associated with poor outcomes in patients with rectal cancer, including reduced OS and disease-free survival (59). An elevated SII is associated with poor OS in many solid tumors. The SII can act as a powerful prognostic indicator of poor outcomes in patients with solid tumors (60).
Research has established that chronic inflammation is widely involved in tumor occurrence and progression. Tumor-associated systemic inflammatory responses involve inflammatory cells and various inflammatory mediators (61). Our study revealed that a low SII was associated with better OS, RFS, and CSS, which is similar to the findings of previous studies. It has been suggested that this advantage can be explained by the function of neutrophils and lymphocytes and has been shown to be associated with oncological outcomes in several types of cancers (60, 62). The SII contains three types of peripheral blood inflammatory biomarkers based on platelet count (P), neutrophils (N), and lymphocytes (L) using the following formula: SII = P × N/L (63). SII can reflect the balance of inflammation and immune response better than a single marker (64) The combination of three blood components gives a more complete picture of the body’s immune and inflammatory state. For example, traditional inflammatory markers such as ESR (erythrocyte sedimentation rate) and CRP (C-reactive protein) may be interfered with by various factors such as malignant tumors and drugs, and have certain limitations. All data used for the calculation can be obtained from routine blood tests, which means that researchers can collect and analyze the SII data (58). It is a noninvasive measurement with the advantages of simplicity, ease of detection, low cost, and ease of analysis, and is suitable for promotion and use in primary medical institutions. Moreover, the calculation formula of SII is simple, easy to be quickly calculated and applied in clinical practice, does not increase the burden on patients, does not require additional laboratory tests or expensive reagents, and is highly cost-effective. Compared with some emerging serological assessment tools, such as liquid biopsy, although it has higher sensitivity and specificity, it has higher requirements for testing equipment and testing technology, and it is difficult to widely promote and apply in medical institutions in the short term (65).
SII can fluctuate based on a patient’s condition, tumor burden, and immunoinflammatory response status, thereby aiding in the monitoring of disease progression and treatment response. Research has demonstrated that SII serves as an independent prognostic factor across various tumors (including liver cancer, stomach cancer, colorectal cancer, etc.) (59, 66, 67), Its predictive capability surpasses that of conventional parameters such as the neutrophil to lymphocyte ratio (NLR) and the platelet to lymphocyte ratio (PLR). Additionally, SII is less influenced by a patient’s hydration status, rendering it more reliable for assessing the immunoinflammatory state of patients. Moreover, SII exhibits greater stability and reliability in diverse clinical scenarios due to its reduced sensitivity to fluid load compared to other indicators. Currently, SII is extensively utilized for predicting patient prognosis across multiple cancers-encompassing overall survival and disease-free survival—thus providing valuable prognostic evaluation metrics. Compared with some traditional tumor markers such as AFP (alpha-fetoprotein), which plays an important role in predicting prognosis, some tumor patients still show negative AFP, and the increase of AFP may also be related to other non-neoplastic diseases (68). Therefore, AFP alone has limitations in evaluating the prognosis of tumor patients.
Although the threshold at which SII predicts prognosis varies from study to study, the results show that the higher the SII, the worse the prognosis, which provides an important reference for clinical decision-making. A high SII score indicates an enhanced inflammatory response or a weakened immune response. An increase in the SII indicates an increase in the number of neutrophils and platelets, which leads to enhanced tumor cell growth, reproduction, and metastasis. Concurrently, a reduction in lymphocyte count results in a diminished capacity of the immune system to combat tumors. Lymphocytes, particularly T lymphocytes, are important weapons in antitumor immune responses (69). Lymphocytopenia is usually accompanied by leukocytosis and thrombocytosis, which may help tumor cells evade immune surveillance and prevent damage to the autoimmunity of cytotoxic T cells. A high SII reflects changes in the cancer microenvironment that are conducive to cancer occurrence, progression, and metastasis (70). SII is closely related to the prognosis of various tumors (such as hepatocellular carcinoma, colorectal cancer, and renal cell carcinoma) and can be used as an independent prognostic factor. Because of its simplicity, economy, noninvasiveness, and potential predictive value, the SII shows broad prospects for clinical applications. A high SII may indicate a strong inflammatory response and immune suppression, which are related to immune escape and tumor progression. Tumor cells alter the tumor microenvironment by secreting cytokines and chemokines to promote their growth, invasion, and metastasis.
To find out the accurate effect of SII on the prognosis of urothelial carcinoma, we conducted a meta-analysis including 31 articles and 14,437 patients to investigate the association between SII status and the prognosis of urothelial carcinoma. A high SII was an independent predictor of RFS, CSS, and OS in patients with urothelial carcinoma.
A high SII was associated with poor OS, RFS, and CSS in patients with urothelial carcinoma, and the clinical features indicated that the cancer was more malignant. This is in line with the results of another study: compared with the detailed subgroup analysis in this paper, patients with a low SII had better OS in UTUC based on the tumor location (12). SII may help predict how patients with cancer respond to treatments, including surgery, chemotherapy, radiation, and immunotherapy. In some cases, a high SII is associated with adverse reactions to certain treatments. These results suggest that the SII can play an important role as an effective factor for poor prognosis and guide the clinical treatment of patients with urothelial carcinoma. However, due to the limitations of this study, further high-quality studies are required to verify our results.
Our meta-analysis has several limitations. First, an optimal SII threshold was not determined. The included studies used different critical thresholds, which may have led to heterogeneity among the studies. There is no standard value; therefore, the conclusions may differ. Second, the included studies were retrospective rather than prospective. The original data inevitably have limitations and deviations that reduce the strength of the argument, which may lead to selection bias. Further prospective studies are required to confirm this. Third, our meta-analysis included only qualified published studies in English or Chinese and did not include relevant articles in other languages, which may also lead to inherent heterogeneity. Fourth, the SII cutoff values were inconsistent, which may have led to heterogeneity. Fifth, most of the studies were conducted in Asia, and the results may be more relevant to Asian patients. The sample sizes also varied significantly. The relatively small sample size led to the relatively low reliability of this study. The clinical application of the SII in urothelial carcinoma has shown a close relationship with tumor prognosis. Future studies should explore the role of the SII in different tumor types and validate its application in individualized treatment strategies.
6 Conclusion
This meta-analysis showed that elevated SII before treatment was associated with OS, RFS, and CSS in patients with urothelial carcinoma. Therefore, SII monitoring may be an effective method for improving the survival rate of patients with urothelial carcinoma. Well-designed, large-scale prospective studies should be conducted to evaluate and verify the correlation between SII and prognosis in patients with urothelial carcinoma.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author/s.
Author contributions
LZ: Formal analysis, Methodology, Writing – original draft, Data curation, Writing – review & editing. ZW: Conceptualization, Methodology, Writing – original draft, Writing – review & editing. YL: Data curation, Writing – review & editing. SG: Data curation, Writing – review & editing. ZZ: Data curation, Writing – review & editing. LG: Formal analysis, Writing – review & editing. CM: Validation, Writing – review & editing. KL: Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Sichuan Province Science and Technology Planning Project under Grant number 2020YFS0320.
Acknowledgments
The authors thank Professor Yunxiang Li for continuous support and encouragement.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer QW declared a past co-authorship with the authors to the handling editor.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2024.1469444/full#supplementary-material
Supplementary Figure 1 | Sensitivity analysis of the RFS between low and high SII.
Supplementary Figure 2 | Sensitivity analysis of the CSS between low and high SII.
Supplementary Figure 3 | Sensitivity analysis of the OS between low and high SII.
Supplementary Figure 4 | Publication bias funnel plot for RFS.
Supplementary Figure 5 | Publication bias funnel plot for CSS.
Supplementary Figure 6 | Publication bias funnel plot for OS.
References
1. Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. CA: Cancer J Clin. (2024) 74:12–49. doi: 10.3322/caac.21820
2. Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2024) 74:229–63. doi: 10.3322/caac.21834
3. Guo C, Trivedi R, Tripathi AK, Nandy RR, Wagner DC, Narra K, et al. Higher expression of annexin A2 in metastatic bladder urothelial carcinoma promotes migration and invasion. Cancers. (2022) 14. doi: 10.3390/cancers14225664
4. Lopez-Beltran A, Cookson MS, Guercio BJ, Cheng L. Advances in diagnosis and treatment of bladder cancer. BMJ (Clinical Res ed). (2024) 384:e076743. doi: 10.1136/bmj-2023-076743
5. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. (2011) 144:646–74. doi: 10.1016/j.cell.2011.02.013
6. Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. (2008) 454:436–44. doi: 10.1038/nature07205
7. Clough J, Colwill M, Poullis A, Pollok R, Patel K, Honap S. Biomarkers in inflammatory bowel disease: a practical guide. Ther Adv Gastroenterol. (2024) 17:17562848241251600. doi: 10.1177/17562848241251600
8. Watson J, Salisbury C, Banks J, Whiting P, Hamilton W. Predictive value of inflammatory markers for cancer diagnosis in primary care: a prospective cohort study using electronic health records. Br J Cancer. (2019) 120:1045–51. doi: 10.1038/s41416-019-0458-x
9. Chen L, Kong X, Wang Z, Wang X, Fang Y, Wang J. Pre-treatment systemic immune-inflammation index is a useful prognostic indicator in patients with breast cancer undergoing neoadjuvant chemotherapy. J Cell Mol Med. (2020) 24:2993–3021. doi: 10.1111/jcmm.14934
10. Guo D, Zhang J, Jing W, Liu J, Zhu H, Fu L, et al. Prognostic value of systemic immune-inflammation index in patients with advanced non-small-cell lung cancer. Future Oncol (London England). (2018) 14:2643–50. doi: 10.2217/fon-2018-0285
11. Takano S, Yoshitomi H, Kagawa S, Furukawa K, Takayashiki T, Kuboki S, et al. Long-term outcomes and significance of preoperative lymphocyte-to-monocyte ratio as a prognostic indicator in patients with invasive pancreatic neoplasms after repeat pancreatectomy. BMC Cancer. (2020) 20:111. doi: 10.1186/s12885-020-6602-4
12. Liu W, Zhang Y, Wang M, Wang M, Yang Q. High systemic immune-inflammation index predicts poor prognosis and response to intravesical BCG treatment in patients with urothelial carcinoma: a systematic review and meta-analysis. Front Oncol. (2023) 13:1229349. doi: 10.3389/fonc.2023.1229349
13. Claps F, Rossin G, van Rhijn BWG, Mir MC, Mertens LS, Ongaro L, et al. The Utility of Inflammatory Serum Markers in the Assessment of Perioperative Morbidity after Radical Cystectomy for Bladder Cancer. Medicina (Kaunas Lithuania). (2023) 59. doi: 10.3390/medicina59050926
14. Zheng J, Peng L, Zhang S, Liao H, Hao J, Wu S, et al. Preoperative systemic immune-inflammation index as a prognostic indicator for patients with urothelial carcinoma. Front Immunol. (2023) 14:1275033. doi: 10.3389/fimmu.2023.1275033
15. Cao W, Shao Y, Zou S, Wang N, Wang J. Prognostic significance of systemic immune-inflammation index in patients with bladder cancer: A systematic review and meta-analysis. Medicine. (2022) 101:e30380. doi: 10.1097/MD.0000000000030380
16. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ (Clinical Res ed). (2021) 372:n71. doi: 10.1136/bmj.n71
17. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. Updating guidance for reporting systematic reviews: development of the PRISMA 2020 statement. J Clin Epidemiol. (2021) 134:103–12. doi: 10.1016/j.jclinepi.2021.02.003
18. Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ (Clinical Res ed). (2017) 358:j4008. doi: 10.1136/bmj.j4008
19. Jiang XH, Chen XJ, Xie QQ, Feng YS, Chen S, Peng JS. Effects of art therapy in cancer care: A systematic review and meta-analysis. Eur J Cancer Care. (2020) 29:e13277. doi: 10.1111/ecc.13277
20. Luo D, Wan X, Liu J, Tong T. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat Methods Med Res. (2018) 27:1785–805. doi: 10.1177/0962280216669183
21. Deng-Xiong L, Qing-Xin Y, De-Chao F, Fa-Cai Z, Rui-Cheng W, Shi X, et al. Systemic immune-inflammation index (SII) during induction has higher predictive value than preoperative SII in non-muscle-invasive bladder cancer patients receiving intravesical bacillus calmette -guerin. Clin genitourinary Cancer. (2023) 21:e145–52. doi: 10.1016/j.clgc.2022.11.013
22. Ding L, Wang X, Deng X, Xia W, Wang K, Yu X, et al. Preoperative systemic immune-inflammation index as a significant prognostic factor after TURBT in patients with non-muscle-invasive bladder cancer: A retrospective study based on propensity score matching analysis. Cancer Med. (2023) 12:7019–28. doi: 10.1002/cam4.v12.6
23. Grossmann NC, Schuettfort VM, Pradere B, Rajwa P, Quhal F, Mostafaei H, et al. Impact of preoperative systemic immune-inflammation Index on oncologic outcomes in bladder cancer patients treated with radical cystectomy. Urologic Oncol. (2022) 40:106.e111–106.e119. doi: 10.1016/j.urolonc.2021.10.006
24. Jan HC, Yang WH, Ou CH. Combination of the preoperative systemic immune-inflammation index and monocyte-lymphocyte ratio as a novel prognostic factor in patients with upper-tract urothelial carcinoma. Ann Surg Oncol. (2019) 26:669–84. doi: 10.1245/s10434-018-6942-3
25. Katayama S, Mori K, Pradere B, Laukhtina E, Schuettfort VM, Quhal F, et al. Prognostic value of the systemic immune-inflammation index in non-muscle invasive bladder cancer. World J Urol. (2021) 39:4355–61. doi: 10.1007/s00345-021-03740-3
26. Ke ZB, Chen H, Chen JY, Cai H, Lin YZ, Sun XL, et al. Preoperative abdominal fat distribution and systemic immune inflammation were associated with response to intravesical Bacillus Calmette-Guerin immunotherapy in patients with non-muscle invasive bladder cancer. Clin Nutr (Edinburgh Scotland). (2021) 40:5792–801. doi: 10.1016/j.clnu.2021.10.019
27. Liu P, Chen S, Gao X, Liang H, Sun D, Shi B, et al. Preoperative sarcopenia and systemic immune-inflammation index can predict response to intravesical Bacillus Calmette-Guerin instillation in patients with non-muscle invasive bladder cancer. Front Immunol. (2022) 13:1032907. doi: 10.3389/fimmu.2022.1032907
28. Mori K, Resch I, Miura N, Laukhtina E, Schuettfort VM, Pradere B, et al. Prognostic role of the systemic immune-inflammation index in upper tract urothelial carcinoma treated with radical nephroureterectomy: results from a large multicenter international collaboration. Cancer immunol immunother: CII. (2021) 70:2641–50. doi: 10.1007/s00262-021-02884-w
29. Russo P, Marino F, Rossi F, Bizzarri FP, Ragonese M, Dibitetto F, et al. Is Systemic Immune-Inflammation Index a Real Non-Invasive Biomarker to Predict Oncological Outcomes in Patients Eligible for Radical Cystectomy? Medicina (Kaunas Lithuania). (2023) 59. doi: 10.3390/medicina59122063
30. Tang X, Cao Y, Liu J, Wang S, Yang Y, Du P. Diagnostic and predictive values of inflammatory factors in pathology and survival of patients undergoing total cystectomy. Mediators Inflammation. (2020) 2020:9234067. doi: 10.1155/2020/9234067
31. Wang C, Jin W, Ma X, Dong Z. The different predictive value of mean platelet volume-to-lymphocyte ratio for postoperative recurrence between non-muscular invasive bladder cancer patients treated with intravesical chemotherapy and intravesical chemohyperthermia. Front Oncol. (2022) 12:1101830. doi: 10.3389/fonc.2022.1101830
32. Yi X, Pi J, Liu C, Xiong Y, Liu J, Fu W, et al. The relationship between inflammatory response markers and the prognosis of non-muscle invasive bladder cancer and the development of a nomogram model. Front Oncol. (2023) 13:1189086. doi: 10.3389/fonc.2023.1189086
33. Yilmaz H, Cinar NB, Avci IE, Telli E, Uslubas AK, Teke K, et al. The systemic inflammation response index: An independent predictive factor for survival outcomes of bladder cancer stronger than other inflammatory markers. Urologic Oncol. (2023) 41:256.e251–256.e258. doi: 10.1016/j.urolonc.2022.11.011
34. Zhang S, Du J, Zhong X, Tan P, Xu H, Zhang J, et al. The prognostic value of the systemic immune-inflammation index for patients with bladder cancer after radical cystectomy. Front Immunol. (2022) 13:1072433. doi: 10.3389/fimmu.2022.1072433
35. Zhang X, Liu Q. Systemic immune inflammation index and T-staging predict prognosis in patients with muscle-invasive bladder cancer. Archivos espanoles urologia. (2023) 76:511–8. doi: 10.56434/j.arch.esp.urol.20237607.63
36. Zhao R, Shan J, Nie L, Yang X, Yuan Z, Xu H, et al. The predictive value of the ratio of the product of neutrophils and hemoglobin to lymphocytes in non-muscular invasive bladder cancer patients with postoperative recurrence. J Clin Lab Anal. (2021) 35:e23883. doi: 10.1002/jcla.23883
37. Zheng Y, Yu D, Yu Z, Zhao D, Chen Y, Chen W, et al. Association of preoperative systemic Immune-inflammation Index and Prognostic Nutritional Index with survival in patients with Upper Tract Urothelial Carcinoma. J Cancer. (2020) 11:5665–77. doi: 10.7150/jca.44915
38. Bi H, Shang Z, Jia C, Wu J, Cui B, Wang Q, et al. Predictive values of preoperative prognostic nutritional index and systemic immune-inflammation index for long-term survival in high-risk non-muscle-invasive bladder cancer patients: A single-centre retrospective study. Cancer Manage Res. (2020) 12:9471–83. doi: 10.2147/CMAR.S259117
39. Chien TM, Li CC, Lu YM, Chou YH, Chang HW, Wu WJ. The predictive value of systemic immune-inflammation index on bladder recurrence on upper tract urothelial carcinoma outcomes after radical nephroureterectomy. J Clin Med. (2021) 10. doi: 10.3390/jcm10225273
40. Kobayashi S, Ito M, Takemura K, Suzuki H, Yonese I, Koga F. Preoperative models incorporating the systemic immune-inflammation index for predicting prognosis and muscle invasion in patients with non-metastatic upper tract urothelial carcinoma. Int J Clin Oncol. (2022) 27:574–84. doi: 10.1007/s10147-021-02088-3
41. Yamashita S, Iwahashi Y, Miyai H, Matsumura N, Hagino K, Kikkawa K, et al. Usefulness of preoperative high systemic immune-inflammation index as a prognostic biomarker in patients who undergo radical cystectomy for bladder cancer: multicenter analysis. Diagnostics (Basel Switzerland). (2021) 11. doi: 10.3390/diagnostics11122194
42. Yılmaz A, Yılmaz H, Tekin SB, Bilici M. The prognostic significance of hemoglobin-to-red cell distribution width ratio in muscle-invasive bladder cancer. Biomarkers Med. (2020) 14:727–38. doi: 10.2217/bmm-2020-0045
43. Dionese M, Basso U, Pierantoni F, Lai E, Cavasin N, Erbetta E, et al. Prognostic role of systemic inflammation indexes in metastatic urothelial carcinoma treated with immunotherapy. Future Sci OA. (2023) 9:Fso878. doi: 10.2144/fsoa-2023-0049
44. Fornarini G, Rebuzzi SE, Banna GL, Calabrò F, Scandurra G, De Giorgi U, et al. Immune-inflammatory biomarkers as prognostic factors for immunotherapy in pretreated advanced urinary tract cancer patients: an analysis of the Italian SAUL cohort. ESMO Open. (2021) 6:100118. doi: 10.1016/j.esmoop.2021.100118
45. Gorgel SN, Akin Y, Koc EM, Kose O, Ozcan S, Yilmaz Y. Retrospective study of systemic immune-inflammation index in muscle invasive bladder cancer: initial results of single centre. Int Urol Nephrol. (2020) 52:469–73. doi: 10.1007/s11255-019-02325-9
46. Luo Z, Yan Y, Jiao B, Huang T, Liu Y, Chen H, et al. Prognostic value of the systemic immune-inflammation index in patients with upper tract urothelial carcinoma after radical nephroureterectomy. World J Surg Oncol. (2023) 21:337. doi: 10.1186/s12957-023-03225-0
47. Palacka P, Slopovsky J, Obertova J, Chovanec M, Rejlekova K, Sycova-Mila Z, et al. Survival prediction by baseline systemic immune-inflammation index (SII) and its changes during first-line platinum-based treatment in a Caucasian population of patients with metastatic urothelial carcinoma (MUC). Anticancer Res. (2021) 41:5749–59. doi: 10.21873/anticanres.15391
48. Salari A, Ghahari M, Bitaraf M, Fard ES, Haddad M, Momeni SA, et al. Prognostic value of NLR, PLR, SII, and dNLR in urothelial bladder cancer following radical cystectomy. Clin genitourinary Cancer. (2024) 22:102144. doi: 10.1016/j.clgc.2024.102144
49. Zhang W, Wang R, Ma W, Wu Y, Maskey N, Guo Y, et al. Systemic immune-inflammation index predicts prognosis of bladder cancer patients after radical cystectomy. Ann Trans Med. (2019) 7:431. doi: 10.21037/atm.2019.09.02
50. Zhang X, Gao Y, Xu H, Liu Y, Hu X, Lu G. Preoperative systemic immune-inflammation index to evaluate intravesical recurrence of upper urinary tract urothelial carcinoma treated with radical nephroureterectomy. J Modern Urol. (2022) 27:30–34.
51. Huang CN. Significance of systemic immune inflammatory index in diagnosis and treatment of patients with bladder urothelial carcinoma. Jilin University (2022). doi: 10.27162/d.cnki.gjlin.2022.005356
52. Stoiber D, Assinger A. Platelet-leukocyte interplay in cancer development and progression. Cells. (2020) 9. doi: 10.3390/cells9040855
53. Iyengar NM, Gucalp A, Dannenberg AJ, Hudis CA. Obesity and cancer mechanisms: tumor microenvironment and inflammation. J Clin Oncol. (2016) 34:4270–6. doi: 10.1200/JCO.2016.67.4283
54. Wislez M, Rabbe N, Marchal J, Milleron B, Crestani B, Mayaud C, et al. Hepatocyte growth factor production by neutrophils infiltrating bronchioloalveolar subtype pulmonary adenocarcinoma: role in tumor progression and death. Cancer Res. (2003) 63:1405–12. doi: 10.1002/cncr.11215
55. Greten FR, Grivennikov SI. Inflammation and cancer: triggers, mechanisms, and consequences. Immunity. (2019) 51:27–41. doi: 10.1016/j.immuni.2019.06.025
56. Brenner C, Galluzzi L, Kepp O, Kroemer G. Decoding cell death signals in liver inflammation. J Hepatol. (2013) 59:583–94. doi: 10.1016/j.jhep.2013.03.033
57. Dong M, Shi Y, Yang J, Zhou Q, Lian Y, Wang D, et al. Prognostic and clinicopathological significance of systemic immune-inflammation index in colorectal cancer: a meta-analysis. Ther Adv Med Oncol. (2020) 12:1758835920937425. doi: 10.1177/1758835920937425
58. Jin M, Yuan S, Yuan Y, Yi L. Prognostic and clinicopathological significance of the systemic immune-inflammation index in patients with renal cell carcinoma: A meta-analysis. Front Oncol. (2021) 11:735803. doi: 10.3389/fonc.2021.735803
59. Menyhart O, Fekete JT, Győrffy B. Inflammation and colorectal cancer: A meta-analysis of the prognostic significance of the systemic immune-inflammation index (SII) and the systemic inflammation response index (SIRI). Int J Mol Sci. (2024) 25. doi: 10.3390/ijms25158441
60. Zhong JH, Huang DH, Chen ZY. Prognostic role of systemic immune-inflammation index in solid tumors: a systematic review and meta-analysis. Oncotarget. (2017) 8:75381–8. doi: 10.18632/oncotarget.18856
61. Lasry A, Zinger A, Ben-Neriah Y. Inflammatory networks underlying colorectal cancer. Nat Immunol. (2016) 17:230–40. doi: 10.1038/ni.3384
62. Meng L, Yang Y, Hu X, Zhang R, Li X. Prognostic value of the pretreatment systemic immune-inflammation index in patients with prostate cancer: a systematic review and meta-analysis. J Trans Med. (2023) 21:79. doi: 10.1186/s12967-023-03924-y
63. Hirahara N, Tajima Y, Matsubara T, Fujii Y, Kaji S, Kawabata Y, et al. Systemic immune-inflammation index predicts overall survival in patients with gastric cancer: a propensity score-matched analysis. J gastrointestinal Surg. (2021) 25:1124–33. doi: 10.1007/s11605-020-04710-7
64. Wang Y, Ni Q. Prognostic and clinicopathological significance of Systemic Immune-Inflammation Index in cancer patients receiving immune checkpoint inhibitors: a meta-analysis. Ann Med. (2023) 55:808–19. doi: 10.1080/07853890.2023.2181983
65. Finkle JD, Boulos H, Driessen TM, Lo C, Blidner RA, Hafez A, et al. Validation of a liquid biopsy assay with molecular and clinical profiling of circulating tumor DNA. NPJ Precis Oncol. (2021) 5:63. doi: 10.1038/s41698-021-00202-2
66. Luo J, Qin X, Zhang X, Zhang Y, Yuan F, Shi W, et al. Prognostic implications of systemic immune-inflammation index in myocardial infarction patients with and without diabetes: insights from the NOAFCAMI-SH registry. Cardiovasc Diabetol. (2024) 23:41. doi: 10.1186/s12933-024-02129-x
67. Li M, Li Z, Wang Z, Yue C, Hu W, Lu H. Prognostic value of systemic immune-inflammation index in patients with pancreatic cancer: a meta-analysis. Clin Exp Med. (2022) 22:637–46. doi: 10.1007/s10238-021-00785-x
68. Qian L, Li C, Luo Y, Meng S. Research progress of AFP in the diagnosis and therapy of hepatocellular carcinoma. Sheng wu gong cheng xue bao = Chin J Biotechnol. (2021) 37:3042–60. doi: 10.13345/j.cjb.210235
69. Grisaru-Tal S, Dulberg S, Beck L, Zhang C, Itan M, Hediyeh-Zadeh S, et al. Metastasis-Entrained Eosinophils Enhance Lymphocyte-Mediated Antitumor Immunity. Cancer Res. (2021) 81:5555–71. doi: 10.1158/0008-5472.CAN-21-0839
Keywords: meta-analysis, urothelial carcinoma, prognosis, systemic immune inflammation index, SII
Citation: Zheng L, Wang Z, Li Y, Ge S, Zeng Z, Gan L, Meng C and Li K (2024) Prognostic significance of systemic immune inflammation index in patients with urothelial carcinoma: a systematic review and meta-analysis. Front. Oncol. 14:1469444. doi: 10.3389/fonc.2024.1469444
Received: 23 July 2024; Accepted: 09 December 2024;
Published: 23 December 2024.
Edited by:
Caixia Sun, Nanyang Technological University, SingaporeReviewed by:
Retnagowri Rajandram, University of Malaya, MalaysiaYongqing Lai, Peking University, China
Qiang Wei, Sichuan University, China
Bo Chen, University of California, Davis, United States
Copyright © 2024 Zheng, Wang, Li, Ge, Zeng, Gan, Meng and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yunxiang Li, bGl5dW54aWFuZzM2OUAxMjYuY29t
†These authors have contributed equally to this work
 Lei Zheng1†
Lei Zheng1† Yunxiang Li
Yunxiang Li Chunyang Meng
Chunyang Meng