
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Oncol., 17 January 2025
Sec. Thoracic Oncology
Volume 14 - 2024 | https://doi.org/10.3389/fonc.2024.1469438
This article is part of the Research TopicPharmacogenomics for Improving Drug Safety and Efficacy in CancerView all 8 articles
Trastuzumab deruxtecan (DS-8201) is an antibody-drug conjugate (ADC) designed to target HER2 mutations. We reported a case study demonstrating a favorable response to DS-8201 in a patient with HER2 mutation-positive non-small cell lung cancer (NSCLC) who exhibited resistance to initial immunotherapy, along with delayed immune-related events of hypoadrenocorticism occurring five months after discontinuation of immune checkpoint inhibitors. After reviewing the relevant literature, we discussed the mechanism of ADC agents underlying their anti-tumor activity and the potential impact of DS-8201 on the tumor microenvironment. This case highlights the efficacy of DS-8201 in NSCLC, particularly in individuals who have failed first-line immunotherapy, and provide valuable insights for clinicians exploring innovative strategies for the management of patients with lung cancer.
Human epidermal growth factor receptor 2 (HER2, also known as ERBB2) alterations, encompassing gene amplification, mutation, and protein overexpression, are identified in 2-3% of nonsquamous non-small cell lung cancer (NSCLC) (1). Unlike mature targeted therapies for oncogene-driven NSCLC such as epidermal growth factor receptor (EGFR) and anaplastic lymphoma kinase (ALK), there is currently no approved standard targeted therapy for NSCLC with HER2 alterations (2). The predominant treatment is platinum-based chemotherapy, either alone or in combination with immunotherapy or antiangiogenic therapy, referring to the treatment of stage IV patients without driver mutations, with an objective response rate (ORR) remaining below 30% (3).
Inspired by research in other solid tumors and gene mutation contexts, other anti-tumor agents have been explored in the landscape of HER2-positive NSCLC. The combination of chemotherapy with trastuzumab, a HER2-targeting monoclonal antibody, did not yield significant benefits in phase II trials (4, 5). The combination of trastuzumab and pertuzumab also demonstrated an ORR of less than 30% in a limited sample size (6). The non-selective tyrosine kinase inhibitor (TKI) afatinib failed to achieve satisfactory efficacy in 13 pretreated HER2-positive patients in the Phase II NICHE trial (7); while poziotinib exhibited ORRs of 27.8% and 39% in two cohorts of the ZENITH20 trial, with median progression-free survival (PFS) of 5.5 months and 5.6 months (8, 9), respectively. Furthermore, pyrotinib demonstrated a 30% ORR in second-line monotherapy (10), and its combination with apatinib exhibited promising therapeutic efficacy in the PATHER2 trial (ORR 51.5%, median PFS 6.9 months) (11). Trastuzumab emtansine (T-DM1), a classic representative of antibody-drug conjugates (ADCs), demonstrated limited efficacy when guided by HER2 protein expression levels, with treatment responses observed in less than 20% of patients with immunohistochemically confirmed HER2-positive status (12). In conclusion, the current treatment requirements of the HER2-positive NSCLC population remain unmet, with several agents not exhibiting as satisfactory efficacy as in gastric and breast cancers or in oncogene-negative NSCLC. This underscores the urgent need for alternative therapeutic strategies (3).
The emergence of trastuzumab deruxtecan (T-DXd, also known as DS-8201) represents a potential paradigm shift. DS-8201 is a HER2-targeted ADC that has shown promising results in breast and gastric cancer (13, 14). Recent studies suggest that DS-8201 may also hold therapeutic potential in lung cancer patients. In particular, the open-label, multicenter phase II DESTINY-Lung 01 trial (NCT03505710) (15) in 2022 evaluated the efficacy and safety of 6.4 mg/kg DS-8201 in 91 patients with metastatic HER2-mutant NSCLC refractory to standard treatment. The study demonstrated an impressive ORR of 54.9%, with a median progression-free survival (PFS) of 8.2 months and a median overall survival (OS) of 17.8 months. The most common adverse event observed was interstitial lung disease (ILD), which occurred in 26% of patients. DESTINY-Lung 01 led to FDA approval of DS-8201 for HER2-positive NSCLC. Subsequently, the DESTINY-Lung 02 trial (NCT04644237) (16), a blinded phase II study, compared the efficacy and safety of DS-8201 at doses of 5.4 mg/kg and 6.4 mg/kg in 152 platinum-treated patients with HER2-mutant NSCLC, more than 70% of whom had received prior anti-PD-(L)1 therapy. ORRs and median PFS in the two dose arms were 50.0%, 10.0 months vs. 56.0%, 12.9 months, respectively, with a differential incidence of grade ≥ 3 adverse events favoring the 5.4mg/kg dose. Recently, the phase II DESTINY-Lung 05 study (NCT05246514) further confirmed the efficacy and safety of DS-8201 in the Chinese population. These findings may pave the way for improved therapeutic options in this challenging cancer subtype. However, given that DS-8201 has not yet been included in standard treatment regimens, there are currently few real-world observational studies on its efficacy and safety in lung cancer patients.
Here we report a case focusing on the use of DS-8201 in a patient with metastatic NSCLC who has demonstrated resistance to first-line immunotherapy. By delving into the mechanism of action of ADC drugs and reviewing previous research on DS-8201, this report sheds light on the potential efficacy and safety of DS-8201 as a viable treatment option for these patients.
The adult patient, over 60 years of age, who had smoked 40 cigarettes per day for over 20 years and had quit smoking 20 years ago, presented with symptoms of cough and breathlessness and underwent a chest enhanced computed tomography (CT) on April 17, 2023. Imaging revealed a 35mm×24mm×27mm lobulated soft tissue mass with heterogeneous enhancement in the dorsal segment of the right lower lobe of the lung along with stretched adjacent interlobar pleura. Further evaluation of radionuclide bone imaging revealed no abnormalities. Tumor marker detection revealed elevated levels of Cyfra 21-1 (7.1ng/mL), SCC (35.0ng/mL), and PROGRP (69.90 pg/mL), while CEA (2.71ng/mL) level was within normal limits (Figure 1). Subsequent needle biopsy of the right lung mass confirmed the diagnosis of invasive lung adenocarcinoma with low programmed cell death ligand 1 (PD-L1) expression (tumor proportion score (TPS) < 1%) and harboring mutations in ERBB2 exon20 p.Y772_A775 dup with frequency of 79.98% and TP53 IVS9 c.993 + 2T with frequency of 69.52%. The patient first presented to our hospital and underwent positron emission tomography (PET)-CT on May 22, which showed the primary lung lesion (3.5 cm, SUVmax 11.0), bilateral hilar and mediastinal lymph node metastases (2R, 4R, 7, 8), and a 1.4 cm metastatic lesion in the left hepatic lobe (SUVmax 4.7) (Figure 1). The patient was staged as cT2aN3M1b and IVA. Four cycles of immunochemistry treatments with sintilimab in combination with pemetrexed and cisplatin were initiated on June 7, with a 10% reduction in chemotherapy dose due to the patient’s poor pulmonary function and Cancer and Aging Research Group (CARG) score of 4. Despite an initial response of stable disease (SD) according to the Response Evaluation Criteria in Solid Tumors (RECIST, version 1.1), disease progression was observed after two subsequent cycles of sintilimab maintenance therapy. On November 9, an enhanced CT scan revealed enlargement of the lung lesion to 6.2 cm×2.7 cm, metastatic lymph nodes, the liver lesion to 5.4 cm×5.2 cm, and a newly discovered right pleural effusion. In addition, head-enhanced magnetic resonance imaging (MRI) revealed the presence of a new brain metastasis. Of note, the patient also developed hypothyroidism, probably related to ICIs therapy, which was treated with hormone supplementation.
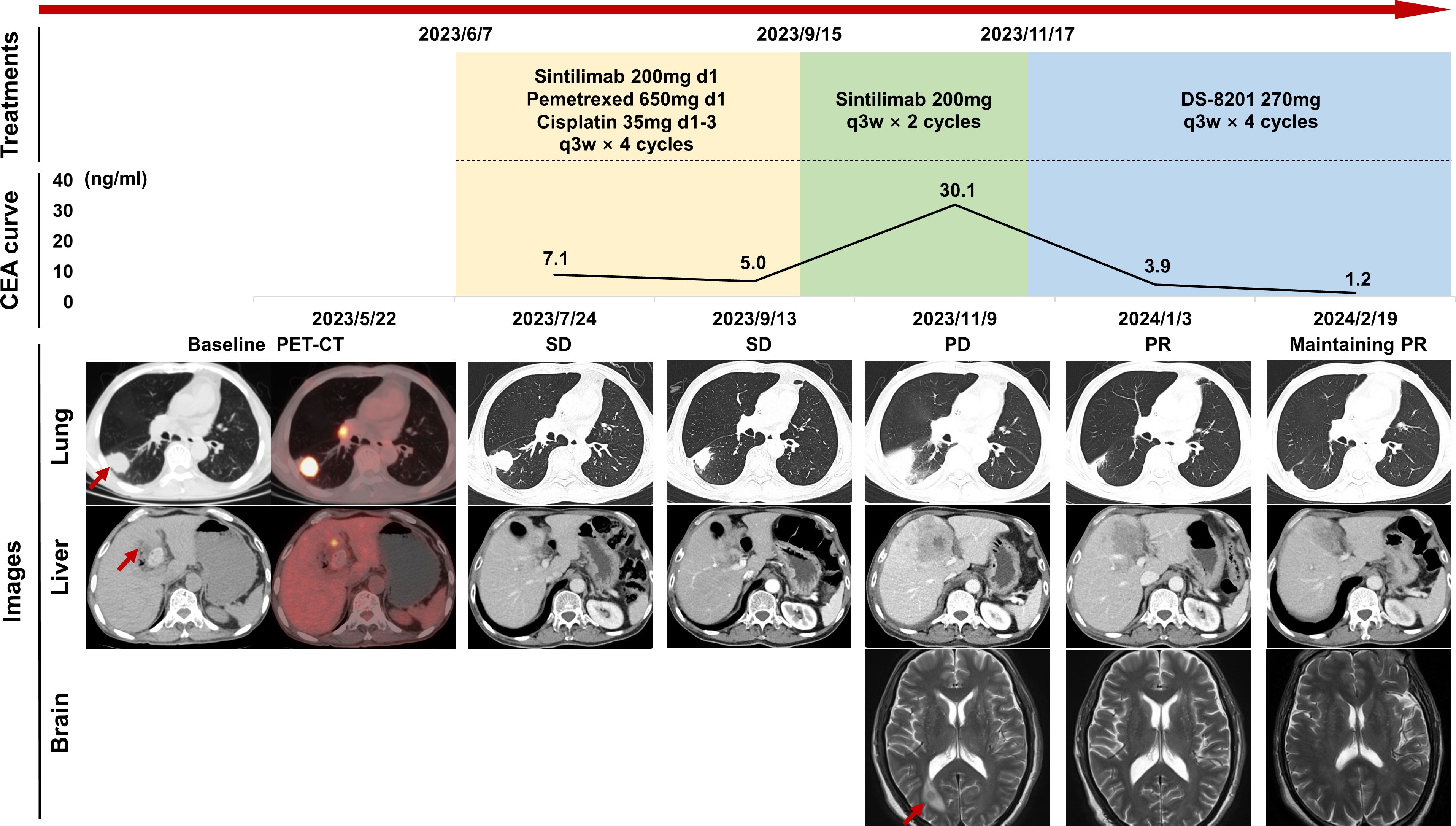
Figure 1. Timeline of patient treatment and efficacy assessment. The “Treatments” section shows the time course of the treatment regimens, including immunochemistry, immune maintenance, and attempt of DS-8201. The “CEA curve” section illustrates the dynamic changes in tumor marker levels over time. The “Images” section displays chest and abdominal CT scans and brain MRI images at different stages of disease development, with efficacy assessments based on RECIST criteria. Red arrows indicate tumor lesions. CEA, carcinoembryonic antigen; CT, computed tomography; MRI, magnetic resonance imaging; PD, progression disease; PET, positron emission tomography; PR, partial response; q3w, quaque 3 weeks; RECIST, response evaluation criteria in solid tumors; SD, stable disease.
Due to the disease progression, the patient was subsequently treated with four cycles of DS-8201 (270 mg, every 3 weeks), which was started on November 17, after exclusion of contraindications. Surprisingly, the lung lesion was reduced by 50% to 3.1 cm×1.7 cm after two cycles, achieving a partial response (PR) and maintaining PR along after four cycles.
On February 23, the patient developed a transient fever of 39.5°C and improved with antibiotics. Upon returning to our hospital on March 6 for the next treatment cycle, the patient complained of severe anorexia, fatigue, and shoulder pain, with an ECOG score of 2, height of 162cm, weight of 42kg, and hyponatremia (Na+ 132mmol/L). Blood analysis revealed an elevated level of lymphocytes proportion of 41.5% (March 6) to 50.7% (March 11) and a declined neutrophil-to-lymphocyte ratio (NLR) of 0.89 (March 6) to 0.81 (March 11). Further investigations revealed low serum total cortisol (<0.50μg/dL) and adrenocorticotropic hormone (ACTH) (<3.00 pg/mL). Due to a history of immune-related adverse event (irAE) and a 5-month cessation of ICI therapy, delayed ICI-associated adrenal insufficiency was considered. Prompt treatment with prednisone resulted in symptomatic improvement together with normalization of lymphocytes and NLR, and further MRI evaluation ruled out pituitary lesions or enlargement.
In this case report, we present an elderly patient with HER2-positive NSCLC who progressed after first-line immunochemical therapy but demonstrated a remarkable response upon switching to DS-8201 treatment. The dose of DS-8201 administered was 270 mg (equivalent to 6.4 mg/kg). As of the current report, the patient has not experienced any severe adverse events related to DS-8201, such as ILD or neutropenia, and has achieved a PFS of 8 months. Therefore, our plan is to continue treatment with DS-8201 and reduce the dose to 240 mg (approximately 5.4 mg/kg), while closely monitoring the patient for efficacy and safety during follow-up visits.
The ADC agent was designed to consist of a monoclonal antibody, a cytotoxic agent (payload), and a linker to facilitate their conjugation (17). The antibody targets specific tumor cell surface proteins to ensure high tumor specificity, while the Fc region can bind to effector cells with Fcγ receptors, including NK cells, monocyte-macrophages, and neutrophils, thereby activating the antibody-dependent cellular cytotoxicity (ADCC). In addition, the cytotoxic payload can diffuse from the targeted tumor cells to neighboring cells with limited or absent target expression, eliciting an anti-tumor effect known as the “bystander effect”, which is critical to effectively combat heterogeneous tumors.
The breakthrough of DS-8201 can be attributed to its innovative drug design. The payload of DS-8201 functions as a potent topoisomerase I inhibitor with superior membrane permeability compared to trastuzumab emtansine. Extensive in vitro and in vivo experiments have demonstrated its potent anti-tumor activity through the bystander effect (18). Notably, in vivo testing has shown that the bystander effect of DS-8201 does not affect distant cells from the inoculated HER-2 target, providing evidence of the drug’s safety profile (18).
In 2019, DS-8201 made a spectacular appearance by achieving durable anti-tumor activity in previously treated HER2-positive breast cancer in the DESTINY-Breast 01 trial (14). The subsequent DESTINY-Breast series confirmed that DS-8201 can delay disease progression in patients with HER2-positive metastatic breast cancer, even including those with low HER2 expression (19). During the same period, DS-8201 also demonstrated satisfactory efficacy in the second-/third-line treatment of advanced gastric cancer in the DESTINY-Gastric series trials (13, 20). Along with the researches in the pan-cancer field (21), research on the application of DS-8201 in lung cancer is still in its nascent stages, with limited reports, mostly isolated cases (22–27). A literature search was conducted in May, 2024, and relevant case reports were summarized in Table 1. Most of these real-world case reports administered DS-8201 to patients after multiple lines of treatment, and achieved good efficacy with most patients experiencing PFS of more than 6 months. However, adverse events such as myocarditis, myelosuppression, and ILD were reported in some cases.
Pending further clinical trials in large populations, DS-8201 has not yet been recommended for the first-line treatment of HER2-positive NSCLC. However, some ongoing studies are exploring its potential in combination with immunotherapy, hormone receptor modulators, DNA damage repair inhibitors, and other agents (28). Preclinical models have demonstrated the ability of DS-8201 to modulate the tumor microenvironment (TME) towards an inflammatory phenotype, resulting in increased infiltration of dendritic cells (DCs) and CT8+T cells, as well as enhanced expression of PD-L1 and MHC-I molecules on tumor cells (29). Research in HER2-positive gastric cancer cell lines has also indicated that DS-8201 has the capacity to modulate immune-related pathways, particularly the cGAS-STING pathway, enhance the gene expression signature of tumor immunogenicity, promote IFN-1 response, and induce DCs activation, which in turn leads to increased tumor cell cytotoxicity (30). From a safety standpoint, while ICIs kill tumors by regulating the immune response, there is a risk of autoimmune damage occurring because of over-activated immune cells, leading to irAEs. This may occur via mechanisms whereby cytotoxic T lymphocytes erroneously target normal tissues that express homologous antigens in a manner analogous to tumor cells, and whereby the upregulation of inflammatory cytokines mediates tissue injury. It is worth noting that some studies have suggested that the occurrence of irAEs may be associated with better immunotherapy efficacy and positive survival outcomes (31, 32). Therefore, it is reasonable to speculate that any factors that facilitate the function of ICI agents may increase the risk of irAEs.
In the case presented, the patient exhibited indications of thyroid toxicity during treatment with sintilimab, followed by a delayed onset of adrenal insufficiency. Adrenal insufficiency is recognized as a rare endocrine irAE. Previous studies have reported the concurrent occurrence of thyroid and adrenal dysfunction, as well as other multiple endocrine irAEs in patients receiving ICIs (33, 34), which may be attributed to the response of T cells within the highly vascularized endocrine system and the complex interactions among various hormonal axes (35). Additionally, the patient experienced several transient episodes of mild neutropenia during the treatment course, while leukocyte, platelet, and hemoglobin levels remained within normal ranges. Each episode of neutropenia was promptly resolved after administration of granulocyte colony-stimulating factor (G-CSF). The patient had no significant prior history of pulmonary or cardiovascular disease, suggesting a favorable safety profile when evaluating for other irAEs such as myocarditis and ILD. Considering the hypothesis that DS-8201 may enhance the efficacy of ICIs through a synergistic effect, we propose that the provocation of delayed irAE in this patient after immunotherapy withdrawal may be potentially related to the activation of TME by DS-8201. This prompts the synergistic potential of combining immunotherapy with DS-8201 to mutually enhance treatment efficacy. However, the lack of evaluation of the status of inflammatory cytokines (such as interleukins and TNF-α), anti-drug antibodies against DS-8201, or specific flow cytometry analysis of lymphocyte subtypes during the March 6 visit, when the patient presented symptoms of adrenal insufficiency, makes it challenging to discriminate whether the observed elevation in lymphocytes was attributed to the recent infection on February 23 or related to an irAE induced by DS-8201. It is essential to monitor these assessments in subsequent patients to determine whether the onset of immune reactions following treatment with DS-8201 is due to acute events, such as infections, or represents a delayed irAE. Furthermore, while our case presents intriguing possibilities, it is critical to acknowledge that a phase Ib trial evaluating the efficacy of DS-8201 in combination with nivolumab versus monotherapy (NCT03523572) presented at ESMO Breast Cancer 2022 did not show a significant improvement, indicating ongoing controversy regarding the benefit of combination regimens.
Moving forward, a deeper understanding of the mechanisms underlying the interactions of DS-8201 and its clinical implications are essential to guide the management of HER2-positive cancers. Further mechanistic and clinical research is warranted to elucidate the full potential of DS-8201 in the treatment landscape of HER2-positive malignancies.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
LW: Conceptualization, Investigation, Software, Writing – original draft, Data curation, Methodology, Project administration. SW: Writing – original draft, Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software. WZ: Data curation, Formal analysis, Resources, Software, Validation, Visualization, Writing – review & editing. ZZ: Conceptualization, Funding acquisition, Investigation, Project administration, Resources, Validation, Writing – review & editing. YC: Funding acquisition, Methodology, Project administration, Resources, Supervision, Validation, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The study was supported by the National High Level Hospital Clinical Research Funding (2022-PUMCH-C-003).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Jebbink M, de Langen AJ, Boelens MC, Monkhorst K, Smit EF. The force of HER2 - A druggable target in NSCLC? Cancer Treat Rev. (2020) 86:101996. doi: 10.1016/j.ctrv.2020.101996
2. Yu Y, Yang Y, Li H, Fan Y. Targeting HER2 alterations in non-small cell lung cancer: Therapeutic breakthrough and challenges. Cancer Treat Rev. (2023) 114:102520. doi: 10.1016/j.ctrv.2023.102520
3. Passaro A, Peters S. Targeting HER2-mutant NSCLC - the light is on. New Engl J Med. (2022) 386:286–9. doi: 10.1056/NEJMe2119442
4. Gatzemeier U, Groth G, Butts C, Van Zandwijk N, Shepherd F, Ardizzoni A, et al. Randomized phase II trial of gemcitabine-cisplatin with or without trastuzumab in HER2-positive non-small-cell lung cancer. Ann Oncol. (2004) 15:19–27. doi: 10.1093/annonc/mdh031
5. Lara PN Jr., Laptalo L, Longmate J, Lau DH, Gandour-Edwards R, Gumerlock PH, et al. Trastuzumab plus docetaxel in HER2/neu-positive non-small-cell lung cancer: a California Cancer Consortium screening and phase II trial. Clin Lung Cancer. (2004) 5:231–6. doi: 10.3816/clc.2004.n.004
6. Hainsworth JD, Meric-Bernstam F, Swanton C, Hurwitz H, Spigel DR, Sweeney C, et al. Targeted therapy for advanced solid tumors on the basis of molecular profiles: results from myPathway, an open-label, phase IIa multiple basket study. J Clin Oncol. (2018) 36:536–42. doi: 10.1200/JCO.2017.75.3780
7. Dziadziuszko R, Smit EF, Dafni U, Wolf J, Wasag B, Biernat W, et al. Afatinib in NSCLC with HER2 mutations: results of the prospective, open-label phase II NICHE trial of European thoracic oncology platform (ETOP). J Thorac Oncol. (2019) 14:1086–94. doi: 10.1016/j.jtho.2019.02.017
8. Le X, Cornelissen R, Garassino M, Clarke JM, Tchekmedyian N, Goldman JW, et al. Poziotinib in non-small-cell lung cancer harboring HER2 exon 20 insertion mutations after prior therapies: ZENITH20-2 trial. J Clin Oncol. (2022) 40:710–8. doi: 10.1200/jco.21.01323
9. Cornelissen R, Prelaj A, Sun S, Baik C, Wollner M, Haura EB, et al. Poziotinib in treatment-naive NSCLC harboring HER2 exon 20 mutations: ZENITH20-4, A multicenter, multicohort, open-label, phase 2 trial (Cohort 4). J Thorac Oncol. (2023) 18:1031–41. doi: 10.1016/j.jtho.2023.03.016
10. Zhou C, Li X, Wang Q, Gao G, Zhang Y, Chen J, et al. Pyrotinib in HER2-mutant advanced lung adenocarcinoma after platinum-based chemotherapy: A multicenter, open-label, single-arm, phase II study. J Clin Oncol. (2020) 38:2753–61. doi: 10.1200/jco.20.00297
11. Yang G, Xu H, Yang Y, Zhang S, Xu F, Hao X, et al. Pyrotinib combined with apatinib for targeting metastatic non-small cell lung cancer with HER2 alterations: a prospective, open-label, single-arm phase 2 study (PATHER2). BMC Med. (2022) 20:277. doi: 10.1186/s12916-022-02470-6
12. Hotta K, Aoe K, Kozuki T, Ohashi K, Ninomiya K, Ichihara E, et al. A phase II study of trastuzumab emtansine in HER2-positive non-small cell lung cancer. J Thorac Oncol. (2018) 13:273–9. doi: 10.1016/j.jtho.2017.10.032
13. Shitara K, Bang YJ, Iwasa S, Sugimoto N, Ryu MH, Sakai D, et al. Trastuzumab deruxtecan in previously treated HER2-positive gastric cancer. New Engl J Med. (2020) 382:2419–30. doi: 10.1056/NEJMoa2004413
14. Modi S, Saura C, Yamashita T, Park YH, Kim SB, Tamura K, et al. Trastuzumab deruxtecan in previously treated HER2-positive breast cancer. New Engl J Med. (2020) 382:610–21. doi: 10.1056/NEJMoa1914510
15. Li BT, Smit EF, Goto Y, Nakagawa K, Udagawa H, Mazières J, et al. Trastuzumab deruxtecan in HER2-mutant non-small-cell lung cancer. New Engl J Med. (2022) 386:241–51. doi: 10.1056/NEJMoa2112431
16. Goto K, Goto Y, Kubo T, Ninomiya K, Kim SW, Planchard D, et al. Trastuzumab deruxtecan in patients with HER2-mutant metastatic non-small-cell lung cancer: primary results from the randomized, phase II DESTINY-lung02 trial. J Clin Oncol. (2023) 41:4852–63. doi: 10.1200/jco.23.01361
17. Tarantino P, Carmagnani Pestana R, Corti C, Modi S, Bardia A, Tolaney SM, et al. Antibody-drug conjugates: Smart chemotherapy delivery across tumor histologies. CA Cancer J Clin. (2022) 72:165–82. doi: 10.3322/caac.21705
18. Ogitani Y, Hagihara K, Oitate M, Naito H, Agatsuma T. Bystander killing effect of DS-8201a, a novel anti-human epidermal growth factor receptor 2 antibody-drug conjugate, in tumors with human epidermal growth factor receptor 2 heterogeneity. Cancer Sci. (2016) 107:1039–46. doi: 10.1111/cas.12966
19. Modi S, Jacot W, Yamashita T, Sohn J, Vidal M, Tokunaga E, et al. Trastuzumab deruxtecan in previously treated HER2-low advanced breast cancer. New Engl J Med. (2022) 387:9–20. doi: 10.1056/NEJMoa2203690
20. Van Cutsem E, di Bartolomeo M, Smyth E, Chau I, Park H, Siena S, et al. Trastuzumab deruxtecan in patients in the USA and Europe with HER2-positive advanced gastric or gastroesophageal junction cancer with disease progression on or after a trastuzumab-containing regimen (DESTINY-Gastric02): primary and updated analyses from a single-arm, phase 2 study. Lancet Oncol. (2023) 24:744–56. doi: 10.1016/s1470-2045(23)00215-2
21. Meric-Bernstam F, Makker V, Oaknin A, Oh DY, Banerjee S, González-Martín A, et al. Efficacy and safety of trastuzumab deruxtecan in patients with HER2-expressing solid tumors: primary results from the DESTINY-panTumor02 phase II trial. J Clin Oncol. (2024) 42:47–58. doi: 10.1200/jco.23.02005
22. Kato Y, Kato Y, Minegishi Y, Suzuki T, Nakamichi S, Matsumoto M, et al. Efficacy with trastuzumab deruxtecan for non-small-cell lung cancer harboring HER2 exon 20 insertion mutation in a patient with a poor performance status: A case report. Onco Targets Ther. (2021) 14:5315–9. doi: 10.2147/ott.S341290
23. Riudavets M, Azarine A, Smaali S, Kim YW, Thomas de Montpréville V, Grecea AM, et al. Unexpected cardiotoxicity in patients with HER2-mutant NSCLC treated with trastuzumab deruxtecan: A case report. JTO Clin Res Rep. (2022) 3:100432. doi: 10.1016/j.jtocrr.2022.100432
24. Xu J, He B, Wang Y, Wu M, Lu Y, Su Z, et al. Positive response to trastuzumab deruxtecan in a patient with HER2-mutant NSCLC after multiple lines therapy, including T-DM1: a case report. Front Oncol. (2023) 13:1268260. doi: 10.3389/fonc.2023.1268260
25. Wang B, Song Y, Yang X, Chen C. HER2 exon 20 insertion mutations and myelosuppression in lung adenocarcinoma patient: a case report and response to trastuzumab deruxtecan. J Cardiothorac Surg. (2023) 18:97. doi: 10.1186/s13019-023-02181-w
26. He X, Hou L, Bai J, Sun C, Wang D, An G. Trastuzumab deruxtecan (DS8201) for advanced non-small cell lung cancer with HER2 exon 20 insertion mutation: a case report. Anti-Cancer Drugs. (2024) 35:101–8. doi: 10.1097/cad.0000000000001535
27. Nam S, Lim SM, Cho BC, Lee JB. Successful rechallenge of trastuzumab deruxtecan after drug-induced interstitial lung disease in a NSCLC with HER2 mutation: A case report. JTO Clin Res Rep. (2024) 5:100628. doi: 10.1016/j.jtocrr.2023.100628
28. Li Y, Li L, Fu H, Yao Q, Wang L, Lou L. Combined inhibition of PARP and ATR synergistically potentiates the antitumor activity of HER2-targeting antibody-drug conjugate in HER2-positive cancers. Am J Cancer Res. (2023) 13:161–75.
29. Iwata TN, Ishii C, Ishida S, Ogitani Y, Wada T, Agatsuma T. A HER2-targeting antibody-drug conjugate, trastuzumab deruxtecan (DS-8201a), enhances antitumor immunity in a mouse model. Mol Cancer Ther. (2018) 17:1494–503. doi: 10.1158/1535-7163.Mct-17-0749
30. Oh KS, Nam AR, Bang JH, Jeong Y, Choo SY, Kim HJ, et al. Immunomodulatory effects of trastuzumab deruxtecan through the cGAS-STING pathway in gastric cancer cells. Cell Commun Signal. (2024) 22:518. doi: 10.1186/s12964-024-01893-3
31. Haratani K, Hayashi H, Chiba Y, Kudo K, Yonesaka K, Kato R, et al. Association of immune-related adverse events with nivolumab efficacy in non-small-cell lung cancer. JAMA Oncol. (2018) 4:374–8. doi: 10.1001/jamaoncol.2017.2925
32. Eggermont AMM, Kicinski M, Blank CU, Mandala M, Long GV, Atkinson V, et al. Association between immune-related adverse events and recurrence-free survival among patients with stage III melanoma randomized to receive pembrolizumab or placebo: A secondary analysis of a randomized clinical trial. JAMA Oncol. (2020) 6:519–27. doi: 10.1001/jamaoncol.2019.5570
33. Fukushima K, Kitayama S, Sazuka M, Kodera R, Oba K, Toyoshima K, et al. Adrenal insufficiency and thyrotoxicosis following combined immune checkpoint inhibitor use: A case report and literature review. Cureus. (2024) 16:e60850. doi: 10.7759/cureus.60850
34. Grouthier V, Lebrun-Vignes B, Moey M, Johnson DB, Moslehi JJ, Salem JE, et al. Immune checkpoint inhibitor-associated primary adrenal insufficiency: WHO vigiBase report analysis. Oncologist. (2020) 25:696–701. doi: 10.1634/theoncologist.2019-0555
Keywords: antibody-drug conjugate, trastuzumab deruxtecan, immune-related adverse events, lung cancer, case report
Citation: Wang L, Wen S, Zhu W, Zhang Z and Cheng Y (2025) Response to trastuzumab deruxtecan and delayed immune-related events in a patient with metastatic HER2-positive NSCLC: a case report and literature review. Front. Oncol. 14:1469438. doi: 10.3389/fonc.2024.1469438
Received: 23 July 2024; Accepted: 24 December 2024;
Published: 17 January 2025.
Edited by:
Zhi-Yao He, Sichuan University, ChinaReviewed by:
Massimo Fantini, Precision Biologics, Inc., United StatesCopyright © 2025 Wang, Wen, Zhu, Zhang and Cheng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhiyang Zhang, emhhbmd6aGl5YW5nMTExQDE2My5jb20=; Yuejuan Cheng, Y2hlbmd5dWVqdWFucHVtY2hAMTYzLmNvbQ==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.