
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 01 October 2024
Sec. Hematologic Malignancies
Volume 14 - 2024 | https://doi.org/10.3389/fonc.2024.1468948
Objectives: Granulocyte colony-stimulating factor (G-CSF) is routinely administered after autologous hematopoietic stem cell transplantation (auto-HSCT) to decrease the duration of neutropenia and diminish the incidence of febrile neutropenia. Nevertheless, the most advantageous timeframe for administering G-CSF in the transplantation setting remains elusive.
Material and Methods: We conducted a cross-sectional study of 200 patients diagnosed with hematological malignancies who underwent auto-HSCT between July 2017 and January 2022. Patients were divided into two groups of 100 individuals based on the timing of G-CSF administration after auto-HSCT. In the first group, G-CSF was administered on post-transplantation day +1, while in the second group, G-CSF was administered on post-transplantation day +5. Patient demographics and clinical outcomes, including time to neutrophil engraftment, time to platelet engraftment, length of hospital stay, duration of fever, and incidence of bacterial and fungal bloodstream infections, were compared between the two groups.
Results: We identified a significantly shorter platelet engraftment time in the day +5 group than in the day +1 group (P<0.001), though the groups were similar regarding neutrophil engraftment time. The total number of G-CSF injections differed significantly according to the administration schedule. The number of red blood cells and length of hospital stay was greater in the day +1 group (all P<0.001). The incidence of bacterial and fungal bloodstream infections and duration of fever did not differ between the groups.
Conclusion: Delayed administration of G-CSF on day +5 is as effective as early administration and can positively influence platelet engraftment, transfusion support, and hospitalization time.
Hematopoietic stem cell transplantation is a widely recognized sequential process aimed at substituting an individual’s blood and lymphoid systems with a fresh system obtained from hematopoietic stem cells (HSCs). These HSCs can be procured from a healthy donor (referred to as allogeneic transplantation) or from the patient (known as autologous transplantation). Over the past six decades, this procedure has been extensively employed to treat aggressive hematological malignancies, including leukemia and lymphoma. Autologous hematopoietic stem cell transplantation (auto-HSCT) is a widely used therapeutic option in managing patients with hematological malignancies and non-malignant hematologic and genetic disorders (1, 2).
As part of transplant preparation, high-dose chemotherapy can cause neutropenia, potentiating severe and potentially fatal complications such as infections (3, 4). The course of neutrophil recovery following auto-HSCT is influenced partly by the exogenous administration of myeloid growth factors such as granulocyte colony-stimulating factors (G-CSFs) (5). G-CSF is a glycoprotein and hematopoietic cytokine that stimulates the mobilization and activation of neutrophils and their precursors, thus attenuating the severity and duration of neutropenia and its clinical complications (6). Current guidelines recommend that G-CSF be started 1–5 days after high-dose chemotherapy and auto-HSCT administration and continued until neutrophil recovery (7). Myeloid engraftment is the first of three consecutive days with an absolute neutrophil count (ANC) of 0.5 × 109/L or more; growth factors usually continue until the ANC remains above this threshold for three consecutive days (8).
A growing body of interest is developing regarding the delayed initiation of G-CSF after auto-HSCT, mainly because of substantial economic benefits and drug side effects. Based on animal studies of bone marrow (BM) cell kinetics, most dividing myeloid progenitors/precursors are eliminated from the BM immediately after administering high-dose chemotherapy and do not reappear immediately after SC re-administration, making early G-CSF worthless (9). Studies that examined two practices of post-transplantation G-CSF starting points, early (day 0 to day +4) and late, reported inconsistent results regarding time to neutrophil and platelet engraftment, length of hospitalization, and incidence of febrile neutropenia (10–14). Most of these studies were conducted on small numbers of patients and varied significantly in patient characteristics and G-CSF regimen. More importantly, these studies had differences in the distribution of factors affecting transplant outcomes.
Overall, there needs to be a clear consensus about the optimum schedule of G-CSF administration post-transplant in clinical practice. This necessitates further evaluation to determine a reasonable starting point that would be clinically and financially beneficial, which was the aim of the present study. We hypothesized that delaying G-CSF administration to day +5 following auto-HSCT would achieve comparable or improved outcomes with reduced resource utilization compared to standard day +1 administration. We compared the auto-HSCT process between two groups of patients with different post-transplant G-CSF starting points in the Taleghani Bone Marrow Transplantation (BMT) center in Iran.
We performed a retrospective cross-sectional study to compare two schedules of G-CSF administration, early or delayed, during the post-transplant period. We evaluated the charts of 200 patients who underwent auto-HSCT for hematological malignancies or solid tumors and randomly received post-transplantation GCSF on day +1 or day +5 between July 2017 and January 2022 at Taleghani BMT Center. The Research Ethics Committee of Shahid Beheshti University of Medical Sciences (IR.SBMU.REC1390.568) approved the study protocol. Written informed consent was obtained from all patients. We divided the patients into two groups: 1) patients who received G-CSF (PDgrastim®, Pooyesh Darou, IR) 24 hours after the administration of the peripheral blood stem cells (day +1 group) and 2) patients who started G-CSF on the 5th day after transplantation (day +5 group). G-CSF at 5 µg/kg was intravenously administered in all cases and continued until day +10, and the dose was increased to 10 µg/kg until the ANC recovered to ≥ 0.5× 109/L for three consecutive days.
In the setting of auto-HSCT, 10 µg/kg of G-CSF was given subcutaneously for five days to collect a transplantable dose of CD34+ cells (>2×106 CD34+ cells/kg). Then, G-CSF-mobilized peripheral blood progenitor cells (PBPCs) were separated from the peripheral bloodstream using the Spectra Optia (Terumo BCT) apheresis machine system and measured by fluorescent-activated cell sorting. The underlying disease determined the typical preparative regimen for auto-HSCT in our hospital. It included a CEAM regimen (lomustine, etoposide, cytarabine, and melphalan) for patients with Hodgkin’s or non-Hodgkin’s lymphoma and high-dose melphalan for patients with myeloma. CD34+ cells were infused 12-24 hours after the last dose of chemotherapy (based on the drug’s half-life).
Based on our center’s protocols, patients with a hemoglobin (Hb) level below 7 g/dl received a blood transfusion, and if the platelet count was below 10,000, they received a platelet transfusion. Additionally, if the patient had a fever above 38.3°C or above 38°C on two consecutive measurements one hour apart, they were administered antibiotics.
Data collected included patient demographics, clinical and laboratory results, and patient transplantation outcomes. Clinical and laboratory variables included body mass index (BMI), diagnosis, conditioning regimens, previous history of local radiotherapy, BM pathologic status, and remission status. Patient transplantation outcomes included time to platelet and neutrophil engraftment (defined as platelet count > 20×109/L on three separate measurements without transfusion support or ANC > 0.5×109/L on three individual measurements), transfusion requirements during transplantation episode, fever, the incidence of bacterial and fungal bloodstream infections, and length of hospital stay.
Data were managed using SPSS statistical software (version 20.0, USA). Categorical variables are summarized as frequencies and percentages. Continuous variables are expressed as mean values and standard deviation (SD) or median and interquartile range depending on the data’s distribution and normality. Chi-squared, Mann-Whitney U, and Kruskal-Wallis tests were used where appropriate to assess differences between groups. The log-rank test was used to compare probabilities of engraftments, fever duration, and length of hospital stay between the groups. A P-value of less than 0.05 was considered significant.
The current study enrolled 200 patients with malignancies who underwent auto-HSCT from July 2017 to January 2022 at Taleghani BMT Center, Tehran, Iran. 114 (57%) patients were male, and 117 (58.5%) participants had complete remission at the time of transplantation. Among patients, 85 (42.5%) suffered from multiple myeloma, 73 (36.5%) from Hodgkin’s lymphoma, 35 (17.5%) from non-Hodgkin’s lymphoma, four (2%) from solid tumors, and three (1.5%) from acute myeloid leukemia. Regarding conditioning regimens, 110 (55%) received CEAM, 83 (41.5%) high-dose melphalan (200 mg/m2 in patients <55 years old and 140 mg/m2 in patients >55), three (1.5%) germ cell therapy (high dose ICE), three (1.5%) AML (melphalan busulfan), and one (0.5%) received the Ewing protocol. Notably, 67 (33.5%) participants had a history of radiation therapy. Regarding chemotherapy lines, 153 (76.5%) participants received less than two lines, mostly multiple myeloma patients (Table 1).
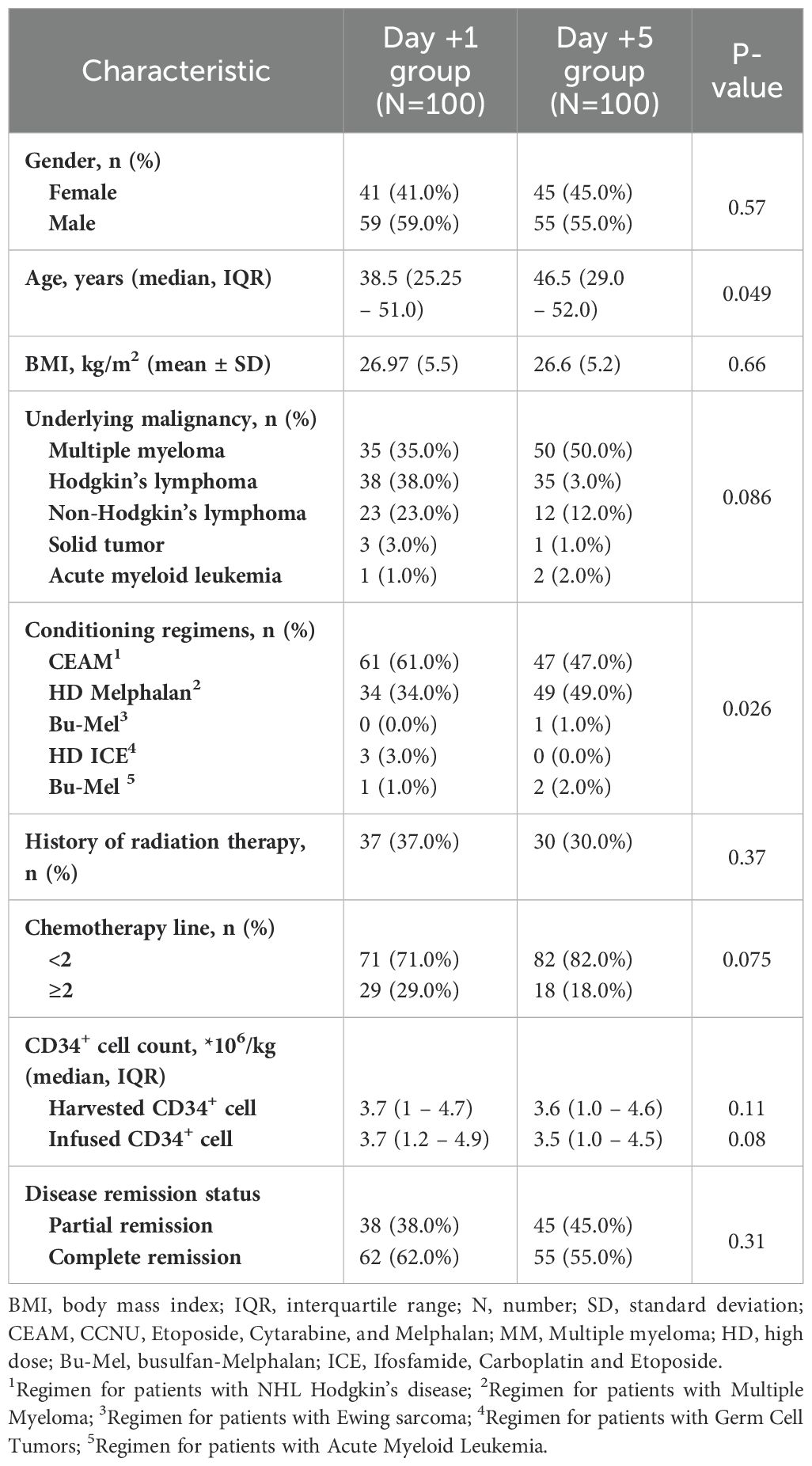
Table 1. Baseline characteristics of patients based on time of granulocyte colony-stimulating factor (GCSF) initiation.
The baseline characteristics of participants before auto-HSCT are listed in Table 1. Statistical analysis revealed no significant difference regarding gender, BMI, underlying malignancy, history of radiotherapy, chemotherapy lines, disease clinical remission status, and CD34+ cell count between patients who received GCSF one day after auto-HSCT and those who received GCSF five days after transplantation (P>0.05). However, some significant differences were seen in age and conditioning regimens (Table 1).
The outcomes of patients after receiving auto-HSCT are listed in Table 2. The total number of GCSF injections was significantly higher in the day +1 group compared with the day +5 group (P=0.005). The median time to platelet engraftment (Figure 1) was markedly higher among patients who received GCSF on day +1 vs. day +5 after transplantation (14 days vs. 12 days, P<0.001); the duration of hospitalization after transplantation (Figure 2) and the total length of hospital stay (Figure 3) were also significantly longer in the day +1 group (18 vs. 14 days, P<0.001 and 29 vs. 25 days, P<0.001, respectively). Time to neutrophil engraftment (Figure 4), duration of fever, bacteremia, fungal infection, and prophylactic drug use were comparable between the two groups (P>0.05) (Table 2).
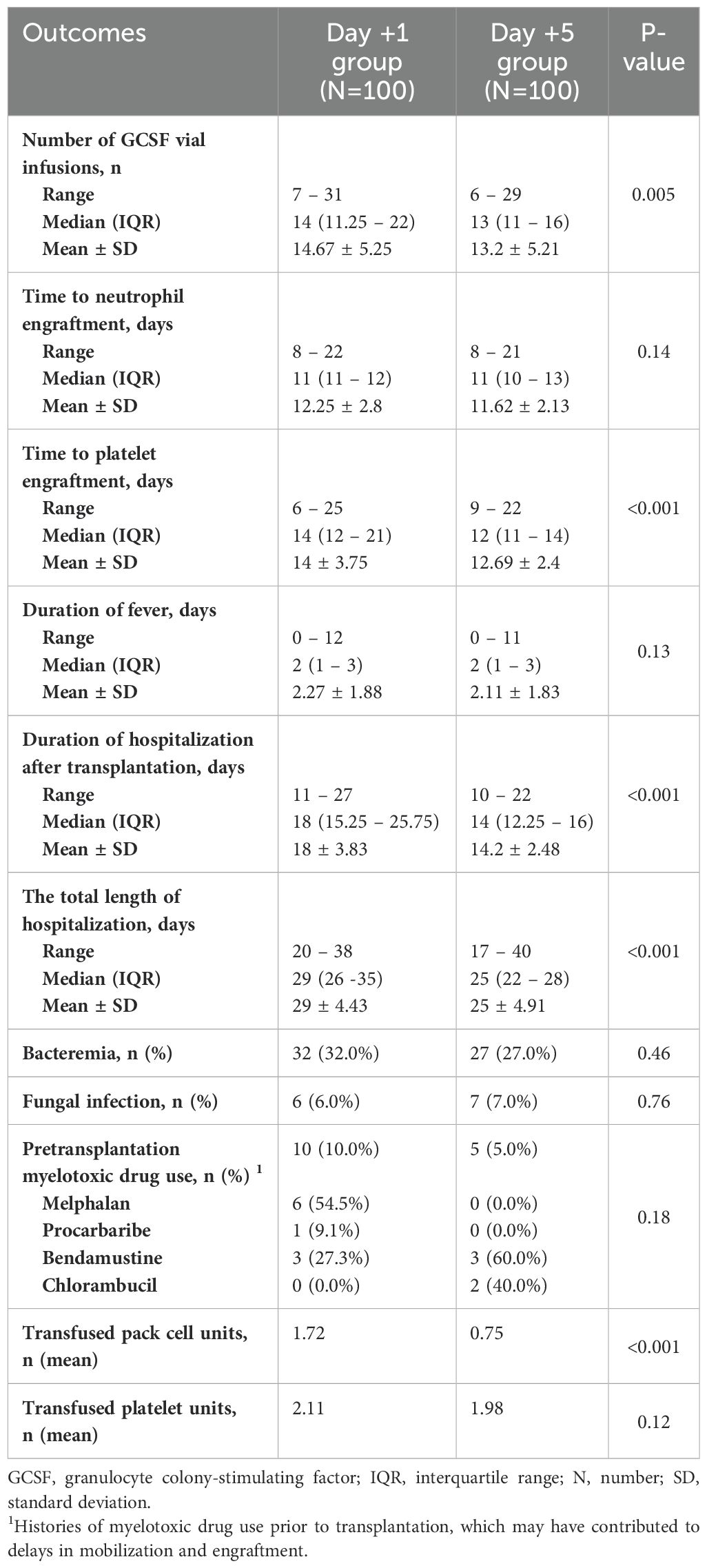
Table 2. Clinical outcomes of patients based on time of granulocyte colony-stimulating factor (GCSF) initiation.
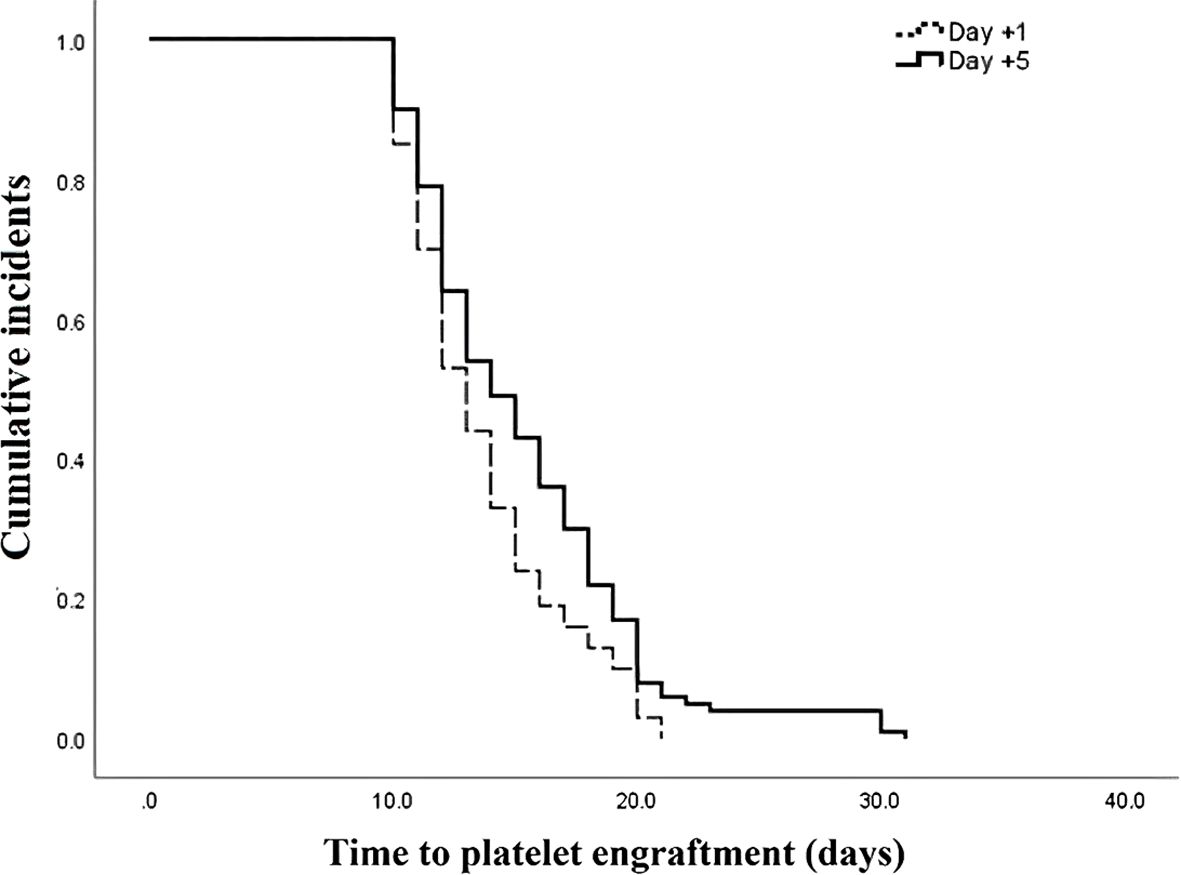
Figure 1. Time to platelet engraftment compared between patients with early (day +1) or delayed (day +5) initiation of granulocyte colony-stimulating factor (G-CSF) following autologous hematopoietic stem cell transplantation.
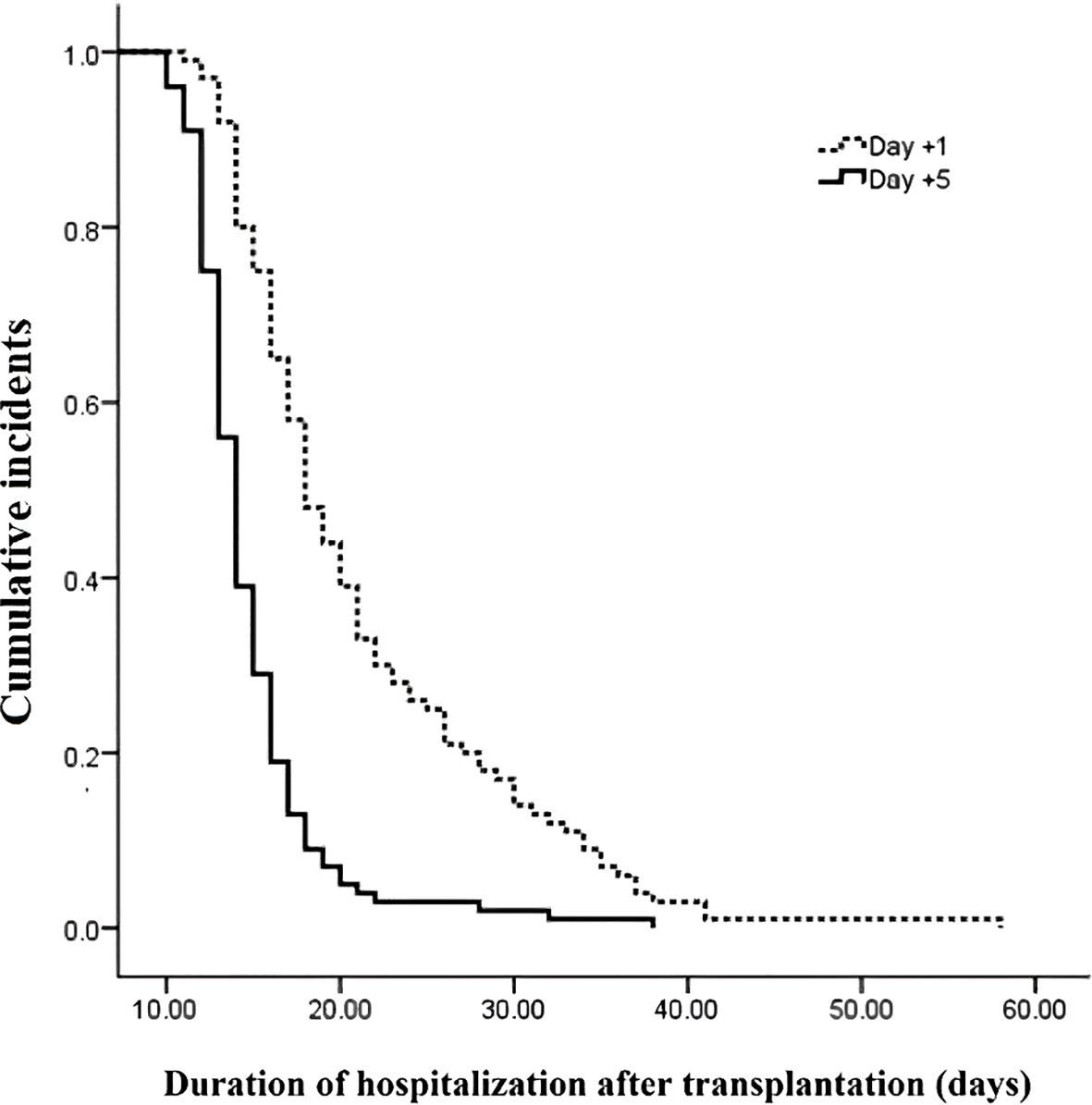
Figure 2. Duration of hospitalization after transplantation compared between patients with early (day +1) or delayed (day +5) initiation of granulocyte colony-stimulating factor (G-CSF) following autologous hematopoietic stem cell transplantation.
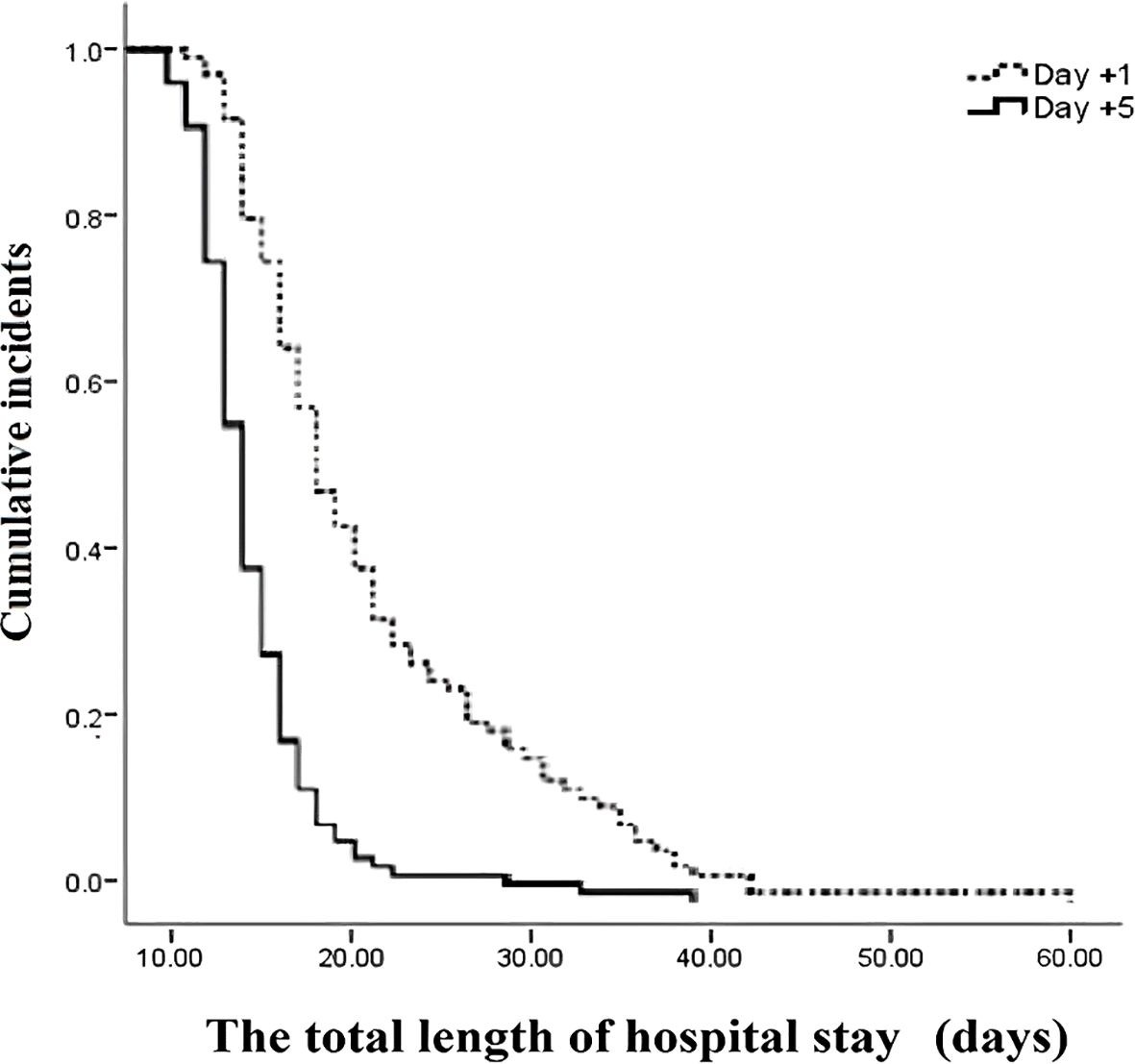
Figure 3. The total length of hospital stay compared between patients with early (day +1) or delayed (day +5) initiation of granulocyte colony-stimulating factor (G-CSF) following autologous hematopoietic stem cell transplantation.
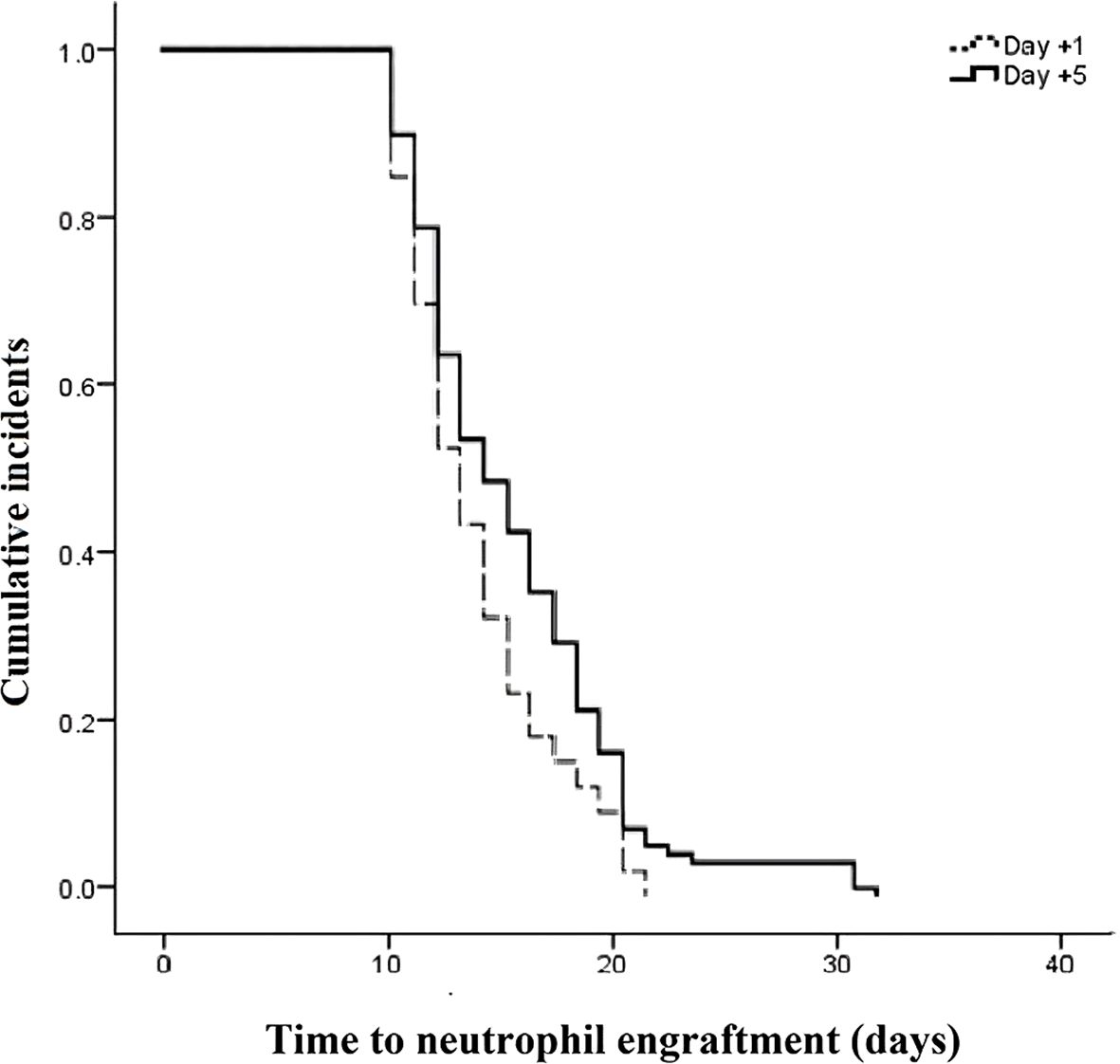
Figure 4. Time to neutrophil engraftment compared between patients with early (day +1) or delayed (day +5) initiation of granulocyte colony-stimulating factor (G-CSF) following autologous hematopoietic stem cell transplantation.
As a growing body of literature demonstrates, GCSF administration following hematopoietic stem cell transplantation is associated with reduced time for stem cell engraftment, decreased time of neutropenia, lower risk of infection, reduced length of hospital stay, and a lower mortality rate (12, 15–17). Although GCSF administration following HSCT is strongly recommended for better clinical outcomes, the optimal dosage and timing of GSCF initiation remain undetermined. We evaluated and compared clinical outcomes between patients who received early or delayed administration of GCSF following auto-HSCT; delayed initiation at day +5 after auto-HSCT was associated with earlier platelet engraftment, reduced packed cell, platelet, and GCSF administration, and reduced length of hospital stay compared to early initiation of GCSF treatment at day +1 post-transplantation. Moreover, the time interval for neutrophil engraftment, total days of febrile neutropenia, and infection rate were unaffected by delaying GCSF administration from day +1 to day +5 after auto-HSCT.
Several studies have been conducted to determine the optimal timing of GCSF initiation following stem cell transplantation (18–22); however, their results are conflicting. Following our findings, Demirer et al. revealed that time to neutrophil count recovery was not significantly different between patients who received GCSF at day 0 and those who received GCSF at day +5 following autologous stem cell transplantation (12). Moreover, delayed administration of GCSF from day 0 to day +5 following transplantation did not lead to an increased length of hospitalization, which is in line with our findings. Similarly, Janusek et al. (23) demonstrated that delaying the administration of GCSF until day +10 following autologous HSCT was not associated with deteriorated clinical outcomes relative to initiation at day +5 (23). Janusek et al. found that although delayed initiation of GCSF was associated with an increased time of neutrophil engraftment, the length of hospitalization was not affected (23). In our study, although treatment with GCSF at day +5 following autologous HSCT had no significant impact on the time of neutrophil engraftment, a decreased length of hospitalization was observed. These findings may indicate that the length of hospital stay is affected by neutrophil engraftment time, and other related factors like infections and platelet engraftment time may affect the clinical outcomes.
Our study revealed that patients who received GCSF at day +5 following auto-HSCT experienced faster platelet engraftment and a lower rate of transfusion of blood products than patients with an early administration of GCSF at day +1. In contrast to our findings, Demirer et al. showed that platelet recovery time, RBC transfusion, and platelet administration rate were comparable between patients who received GCSF at day 0 and day +5 following autologous stem cell transplantation (12). Ali et al. (24) also found that platelet recovery time was comparable between patients who received early GCSF at day 0 and those who underwent delayed GCSF administration at day +5 following auto-HSCT (24). Another recent study showed that time to platelet engraftment was significantly longer in patients who underwent delayed administration of GCSF at day +12 than those who experienced an early reception of GCSF at day +4 following transplantation (25). Considerable variations in study designs, participants, sample sizes, timings, and dosages of GCSF might be responsible for these differences.
A recent study found that delayed initiation of GCSF treatment at day +12 following stem cell transplantation was associated with lower total doses of GCSF (25), aligning with our finding that the total number of GCSF doses was significantly lower in patients who underwent delayed administration of GCSF at day +5 vs. day +1 after auto-HSCT. Similarly, Monge et al. (26) showed that in multiple myeloma patients undergoing autologous stem cell transplantation who received delayed GCSF administration on day +12 following transplantation, the total number of GCSF doses was lower relative to early treatment initiation at day +1 (26). Another study by Janusek et al. also found that significantly lower doses of GSCF were administered in patients who underwent delayed treatment of GCSF at day +5 following stem cell transplantation compared with those who received early GCSF administration (23). Moreover, in our study, the infection rate and the total number of febrile days were comparable between patients who received early and delayed GCSF, as observed in previous studies (12, 23–26). These findings indicate that delayed initiation of GCSF can be associated with reduced costs due to lower doses of injected GCSF, lower risk of infection, and decreased need to manage infectious diseases. Although our study did not evaluate hospitalization costs, we hypothesize that delayed administration of GCSF at day +5 following auto-HSCT might lead to a reduction in patient costs since the total doses of GCSF decreased, the infection rate remained unaffected, and the length of hospitalization fell, in line with prior studies (16, 19).
In contrast to our study, Sborov et al. (27) found that the duration of hospitalization and risk of infection was lower in multiple myeloma patients who underwent early GCSF administration at day +1 following autologous transplantation vs. those who received GCSF at day +5 or +7 after stem cell transplantation (27). Also, Hatch et al. (25) showed that patients undergoing auto-HSCT who received GCSF at day +4 following transplantation had faster neutrophil and platelet engraftment and shorter hospital stays when compared to those who received GCSF at day +12 after stem cell transplantation (25). Similarly, Monge et al. (26) demonstrated that delaying administration of GCSF from day +1 to day +12 after autologous stem cell transplantation in multiple myeloma patients led to increased neutrophil recovery time, neutropenia duration, and hospital stay (26), contrasting with our findings. One possible explanation for the existing differences is that in the earlier studies, patients underwent significantly delayed GCSF administration at day +12 following transplantation. Thus, although delayed administration of GCSF until day +5 following auto-HSCT may preserve the treatment efficacy, very delayed administration of GCSF is not recommended since it might be associated with an increased risk of developing unfavorable outcomes. Moreover, it is essential to note that our sample size was considerably larger than those of these two studies, each of which enrolled less than 100 patients. Nonetheless, further studies are needed to determine the optimal timing of GCSF initiation among patients undergoing auto-HSCT.
This study’s limitations include its single-center and cross-sectional design. We also enrolled patients with different underlying malignancies whose disease features might vary and affect the clinical outcomes. Finally, the potential effect of GCSF timing on hospitalization costs was not evaluated.
The present study has several strengths. First, a relatively large patient population was reviewed. Second, since the current study was performed at a single center, a uniform patient group was enrolled, lowering the risk of heterogeneity bias regarding the study population and treatment modalities. Third, data regarding patient characteristics and outcomes were all computerized which reduced the amount of missing data. However, the present study has also several limits which need to be acknowledged. The study was retrospective in design, introducing some degrees of restriction and bias risk. Moreover, the effect of various confounders was not excluded. Although several studies have been conducted, the optimal timing of GCSF initiation in cancer patients undergoing hematopoietic stem cell transplantation remains unclear since the literature is heterogeneous. Thus, multi-center studies with large sample sizes and randomized controlled designs are needed to elucidate the best timing of GCSF initiation among patients with malignancy who undergo auto-HSCT. In addition, evaluation of other clinical outcomes is strongly recommended.
The current study revealed that delayed administration of GCSF at day +5 following transplantation in patients with hematological or solid malignancies was associated with earlier platelet recovery, reduced need for blood product transfusion, reduced GCSF administration doses, and decreased hospitalization length. Moreover, no adverse effects regarding neutrophil engraftment time, febrile neutropenia duration, or rate of developing infectious diseases were found when delaying GCSF administration from day +1 to day +5 after auto-HSCT. The present study’s findings suggest that delayed administration of GCSF at day +5 following auto-HSCT can be associated with favorable clinical outcomes, equal treatment efficacy, and reduced hospitalization costs. However, the optimal timing of GCSF treatment initiation in patients receiving stem cell transplantation is yet to be understood. Hence, further studies are needed to examine the optimal timing of GCSF administration and related clinical outcomes in patients with malignancy who undergo stem cell transplantation.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
This study was approved by the Research Ethics Committee of Shahid Beheshti University of Medical Sciences (IR.SBMU.REC1390.568) and was conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained from the participants.
MM: Conceptualization, Methodology, Resources, Supervision, Writing – original draft. MK: Data curation, Formal analysis, Investigation, Resources, Writing – original draft, Writing – review & editing. ZT: Formal analysis, Investigation, Visualization, Writing – original draft, Writing – review & editing. VK: Data curation, Formal analysis, Investigation, Project administration, Writing – original draft, Writing – review & editing. SZ: Writing review and editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
We express sincere thanks to all those involved in this study, especially the nurses, Taleghani Bone Marrow Transplantation (BMT) Center, and the research coordinators.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
GCSF, Granulocyte Colony-Stimulating Factor; HSCT, Hematopoietic Stem Cell Transplantation; auto-HSCT, Autologous Hematopoietic Stem Cell Transplantation; CEAM, refers to a chemotherapy regimen (Cyclophosphamide, Etoposide, Cytarabine, Melphalan); BMI, Body Mass Index; AML, Acute Myeloid Leukemia; CD34+, Cluster of Differentiation 34 Positive (marker used to identify hematopoietic stem cells); BMT, Bone Marrow Transplant; PBPCs, Peripheral Blood Progenitor Cells; ANC, Absolute Neutrophil Count; Hb, Hemoglobin; IQR, interquartile range; SD, standard deviation; CEAM, CCNU, Etoposide, Cytarabine, and Melphalan; MM, Multiple myeloma; HD, high dose; Bu-Mel, busulfan-Melphalan; ICE, Ifosfamide, Carboplatin and Etoposide.
1. Snowden JA, Sánchez-Ortega I, Corbacioglu S, Basak GW, Chabannon C, de la Camara R, et al. Indications for haematopoietic cell transplantation for haematological diseases, solid tumours and immune disorders: current practice in Europe, 2022. Bone Marrow Transplant. (2022) 57:1217–39. doi: 10.1038/s41409-022-01691-w
2. Gorin NC. History and development of autologous stem cell transplantation for acute myeloid leukemia. Clin Hematol Int. (2021) 3:83–95. doi: 10.2991/chi.k.210703.002
3. Ferdjallah A, Young JH, MacMillan ML. A review of infections after hematopoietic cell transplantation requiring PICU care: transplant timeline is key. Front Pediatr. (2021) 9:634449. doi: 10.3389/fped.2021.634449
4. Espinoza JL, Wadasaki Y, Takami A. Infection complications in hematopoietic stem cells transplant recipients: do genetics really matter? Front Microbiol. (2018) 9:2317. doi: 10.3389/fmicb.2018.02317
5. Richard S, Schuster MW. Stem cell transplantation and hematopoietic growth factors. Curr Hematol Rep. (2002) 1:103–9.
6. Cetean S, Căinap C, Constantin AM, Căinap S, Gherman A, Oprean L, et al. The importance of the granulocyte-colony stimulating factor in oncology. Clujul Med. (2015) 88:468–72. doi: 10.15386/cjmed-531
7. Smith TJ, Bohlke K, Lyman GH, Carson KR, Crawford J, Cross SJ, et al. Recommendations for the use of WBC growth factors: american society of clinical oncology clinical practice guideline update. J Clin Oncol. (2015) 33:3199–212. doi: 10.1200/JCO.2015.62.3488
8. Ali MY, Oyama Y, Monreal J, Winter J, Tallman M, Gordon LI, et al. Reassessing the definition of myeloid engraftment after autotransplantation: it is not necessary to see 0.5 x 10(9)/l neutrophils on 3 consecutive days to define myeloid recovery. Bone Marrow Transplant. (2002) 30:749–52. doi: 10.1038/sj.bmt.1703741
9. Yankelevich M, Goodell MA, Kaplan J. Efficacy of delayed administration of post-chemotherapy granulocyte colony-stimulating factor: evidence from murine studies of bone marrow cell kinetics. Exp Hematol. (2008) 36:9–16. doi: 10.1016/j.exphem.2007.08.019
10. Singh AD, Parmar S, Patel K, Shah S, Shore T, Gergis U, et al. Granulocyte colony-stimulating factor use after autologous peripheral blood stem cell transplantation: comparison of two practices. Biol Blood Marrow Transplant. (2018) 24:288–93. doi: 10.1016/j.bbmt.2017.10.026
11. Yankelevich M, Hoogstra DJ, Abrams J, Chu R, Bhambhani K, Taub JW. Delayed Granulocyte Colony-Stimulating Factor (G-CSF) Administration after Chemotherapy Reduces Total G-CSF Doses without Affecting Neutrophil Recovery in a Randomized Clinical Study in Children with Solid Tumors. Pediatr Hematol Oncol. (2020) 37:665–75. doi: 10.1080/08880018.2020.1779885
12. Demirer T, Ayli M, Dagli M, Haznedar R, Genc Y, Fen T, et al. Influence of post-transplant recombinant human granulocyte colony-stimulating factor administration on peritransplant morbidity in patients undergoing autologous stem cell transplantation. Br J Haematol. (2002) 118:1104–11. doi: 10.1046/j.1365-2141.2002.03762.x
13. Ali N, Cooper B, Tomlinson B, Metheny L, Caimi P, Boughan K, et al. Treatment-related mortality following autologous hematopoietic stem cell transplantation is unaffected by timing of G-CSF administration. Bone Marrow Transplant. (2020) 55:1697–700. doi: 10.1038/s41409-020-0812-x
14. Thompson JM, Carlton P, Akard LP, Dugan MJ, Jansen J. Starting granulocyte-colony-stimulating factor (filgrastim) early after autologous peripheral blood progenitor cell transplantation leads to faster engraftment without increased resource utilization. Transfusion. (2009) 49:548–54. doi: 10.1111/j.1537-2995.2008.02006.x
15. Grosso D, Leiby B, Wilde L, Carabasi M, Filicko-O'Hara J, O'Hara W, et al. A prospective, randomized trial examining the use of G-CSF versus no G-CSF in patients post-autologous transplantation. Transplant Cell Ther. (2022) 28:831.e1–.e7. doi: 10.1016/j.jtct.2022.09.012
16. O'Rafferty C, O'Brien M, Smyth E, Keane S, Robinson H, Lynam P, et al. Administration of G-CSF from day +6 post-allogeneic hematopoietic stem cell transplantation in children and adolescents accelerates neutrophil engraftment but does not appear to have an impact on cost savings. Pediatr Transplant. (2016) 20:432–7. doi: 10.1111/petr.2016.20.issue-3
17. Schmitz N, Ljungman P, Cordonnier C, Kempf C, Linkesch W, Alegre A, et al. Lenograstim after autologous peripheral blood progenitor cell transplantation: results of a double-blind, randomized trial. Bone Marrow Transplant. (2004) 34:955–62. doi: 10.1038/sj.bmt.1704724
18. Vey N, Molnar S, Faucher C, Le Corroller AG, Stoppa AM, Viens P, et al. Delayed administration of granulocyte colony-stimulating factor after autologous bone marrow transplantation: effect on granulocyte recovery. Bone Marrow Transplant. (1994) 14:779–82.
19. Valteau-Couanet D, Faucher C, Aupérin A, Michon J, Milpied N, Boiron JM, et al. Cost effectiveness of day 5 G-CSF (Lenograstim) administration after PBSC transplantation: results of a SFGM-TC randomised trial. Bone Marrow Transplant. (2005) 36:547–52. doi: 10.1038/sj.bmt.1705097
20. Hornedo J, Solá C, Solano C, López JJ, Alonso S, Lluch A, et al. The role of granulocyte colony-stimulating factor (G-CSF) in the post-transplant period. Bone Marrow Transplant. (2002) 29:737–43. doi: 10.1038/sj.bmt.1703539
21. Osma MM, Ortuño F, Lozano ML, Gomez-Espuch J, Ayala F, Sanchez-Serrano I, et al. Administration of post-autologous PBSCT rhG-CSF is associated with long-term low concentrations of bone marrow hematopoietic progenitor cells. Bone Marrow Transplant. (2001) 27:1287–92. doi: 10.1038/sj.bmt.1703079
22. de Azevedo AM, Nucci M, Maiolino A, Vigorito AC, Simões BP, Aranha FJ, et al. A randomized, multicenter study of G-CSF starting on day +1 vs day +5 after autologous peripheral blood progenitor cell transplantation. Bone Marrow Transplant. (2002) 29:745–51. doi: 10.1038/sj.bmt.1703538
23. Neutropenia recovery linked to filgrastim initiation timing after autologous hematopoietic stem-cell transplantation. J Hematol Oncol Pharm (2016). 6:1
24. Ali N, Cooper B, Caimi P, El Jurdi N, Malek E, Metheny L, et al. 168 - early versus late initiation of granulocyte colony stimulating factor (G-CSF) following autologous hematopoietic stem cell transplantation in adult hematological Malignancies. Biol Blood Marrow Transplant. (2018) 24:S138–S9. doi: 10.1016/j.bbmt.2017.12.087
25. Hatch RV, Freyer CW, Carulli A, Redline G, Babushok DV, Frey NV, et al. Day 4 vs. day 12 G-CSF administration following reduced intensity peripheral blood allogeneic stem cell transplant. J Oncol Pharm Pract. (2022) 28:892–7. doi: 10.1177/10781552221080710
26. Monge J, Flicker K, Guarneri D, Solomon RS, Guo J, Contreras J, et al. Comparison of early versus delayed filgrastim (G-CSF) administration following autologous stem cell transplantation in patients with multiple myeloma - real-world data from a single-center institution. Blood. (2019) 134:5644. doi: 10.1182/blood-2019-130914
Keywords: hematopoietic stem cell transplantation, granulocyte colony-stimulating factor, delayed administration, early administration, engraftment
Citation: Mahdizadeh M, Karimi MA, Tajabadi Z, Kaveh V and Zamani S (2024) The impact of delayed versus early administration of granulocyte colony-stimulating factor following autologous hematopoietic stem cell transplantation on transplantation outcome. Front. Oncol. 14:1468948. doi: 10.3389/fonc.2024.1468948
Received: 22 July 2024; Accepted: 12 September 2024;
Published: 01 October 2024.
Edited by:
Jignesh D. Dalal, Case Western Reserve University, United StatesReviewed by:
Anant Vatsayan, Children’s National Hospital, United StatesCopyright © 2024 Mahdizadeh, Karimi, Tajabadi, Kaveh and Zamani. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Vahid Kaveh, a2F2ZWgudkBpdW1zLmFjLmly
†ORCID: Mahshid Mahdizadeh, orcid.org/0000-0002-8937-5495
Mohammad Amin Karimi, orcid.org/0000-0002-0638-1743
Zohreh Tajabadi, orcid.org/0000-0002-9854-7260
Vahid Kaveh, orcid.org/0000-0001-8363-9755
Shayan Zamani, orcid.org/0009-0007-1204-195X
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.