- 1The First School of Clinical Medicine, Lanzhou University, Lanzhou, China
- 2Department of Neurosurgery, The First Hospital of Lanzhou University, Lanzhou, China
Introduction and importance: Intracranial dermoid cysts are rare, constituting 0.04% to 0.6% of all intracranial tumors. They often arise from ectodermal cells trapped during neural tube formation. We report a case of spontaneous rupture of a large tentorial epithelioid cyst, which caused massive dissemination of liquid cholesterol into the subarachnoid cisterns and ventricles.
Presentation of case: A 28-year-old male presented with a two-week history of headache and memory decline. CT and MRI revealed a 9x6 cm lesion in the left frontotemporal region with widespread dissemination of lipid droplets. Surgical resection was performed using a microscope combined with a neuroendoscope. Pathology confirmed a dermoid cyst.
Clinical discussion: Ruptured dermoid cysts can cause significant symptoms due to the dissemination of cyst contents. Imaging is crucial for diagnosis and surgical planning. The combined microscopic and neuroendoscopic approach minimized blind spots and allowed thorough tumor exposure, facilitating complete resection with minimal residual complications. Postoperative outcomes were favorable, with imaging confirming substantial tumor removal and restored cerebrospinal fluid circulation.
Conclusion: Prompt diagnosis and comprehensive surgical intervention are essential for managing ruptured intracranial dermoid cysts. Combined microscopic and neuroendoscopic techniques are effective in achieving extensive resection and reducing complications.
1 Introduction
Intracranial dermoid cysts are rare benign space-occupying lesions usually located in the midline, posterior fossa, suprasellar, frontonasal, or temporal-basal regions (1). The cyst wall is a stratified squamous epithelium containing dermal components, including sebaceous glands, sweat glands, and hair follicles (1, 2). These cysts typically accumulate exfoliated epithelium, sebaceous secretions, fat, and hair, leading to slow growth. Common symptoms included headache, seizures, focal neurological dysfunction, and so on. The rupture of dermoid cyst contents can lead to serious complications, including meningitis, hydrocephalus, endocrine disease, and even blindness (3, 4). We report a case of spontaneous rupture of a large tentorial epithelioid cyst in a male patient, which caused massive dissemination of liquid cholesterol into the subarachnoid cisterns and ventricles, blocking cerebrospinal fluid circulation.
This case report has been reported in line with the Surgical CAse REport (SCARE) Criteria (5).
2 Case report
A 28-year-old male was admitted to our hospital with a two-week history of headaches mainly located in the left frontotemporal region and memory decline. He denied any history of head trauma, fever, or seizures. Neurological examination revealed a slight decrease in short-term memory but no other focal neurological deficits.
Cranial computed tomography (CT) scan showed an approximately 9x6 cm hypodense lesion in the left front temporoparietal lobe (Figure 1A), without enhancement after contrast injection (Figure 1B). In another section, calcification was observed at the margins of the lesion, and multiple hypodensities were noted in the cerebral sulci and bilateral Sylvian fissures (Figure 1C). Magnetic resonance imaging (MRI) confirmed the diffusion of lipids after rupture, which were hyperintense on both T1 and T2-weighted images (Figures 1D, E). MRI also revealed a large heterogeneous cystic lesion, which was hypointense mixed with hyperintense on T1-weighted images and hyperintense on T2-weighted images (Figures 1F, G), and irregular hyperintense signals on diffusion weighted imaging (DWI) (Figure 1H). On sagittal images, lipid droplets were also seen in the fourth ventricle, interpeduncular cistern, ambient cistern, quadrigeminal cistern, and even within the spinal canal (Figures 1I, J). The entire subarachnoid space shows diffusely scattered hyperintense fat droplets. These imaging findings are consistent with the characteristics of a ruptured dermoid cyst, with extremely extensive dissemination of its contents, which is quite rare.
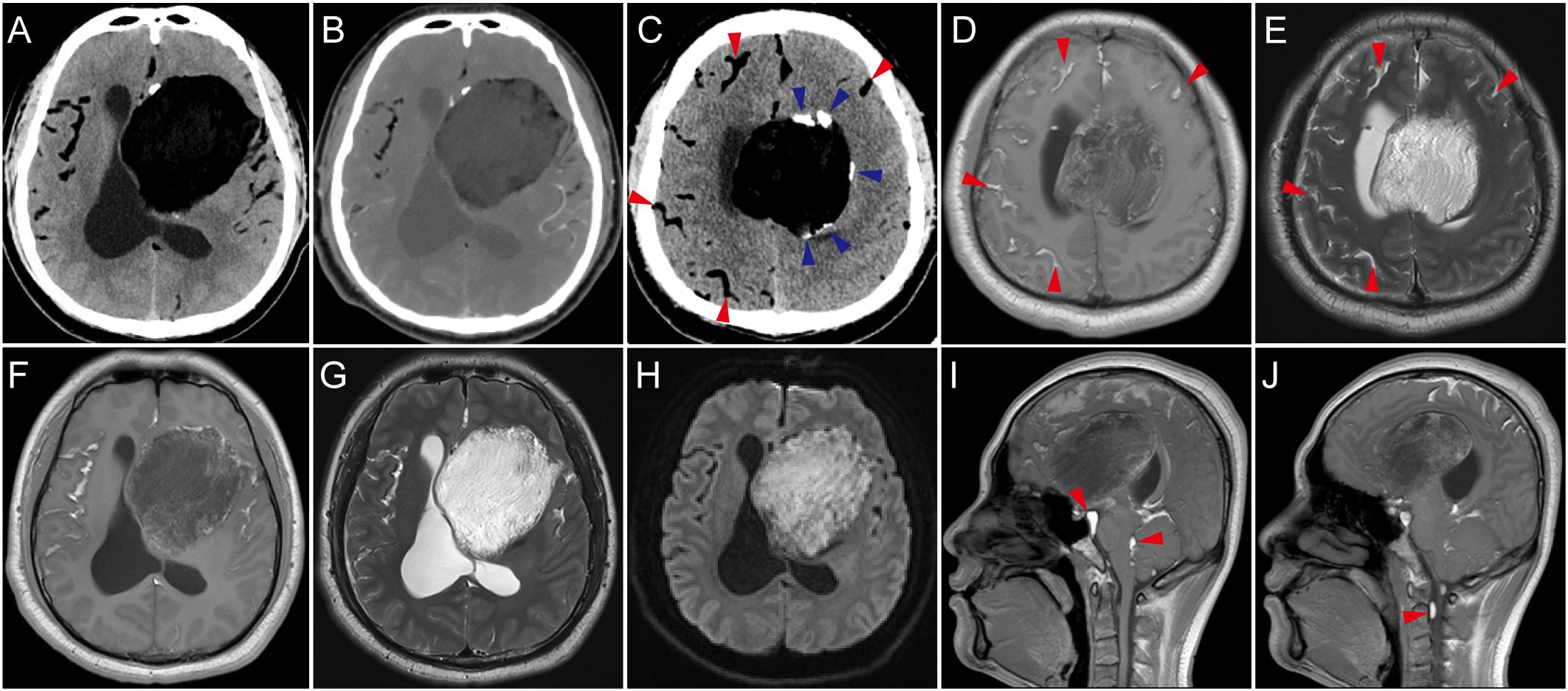
Figure 1. Imaging manifestations. (A) Computed tomography (CT) scan showed left frontal temporal there is a huge irregular low-density mass. (B) Contrast-enhanced CT showed no enhancement of the mass. (C) Calcification of the cyst wall (blue arrow) and hypodense droplets in the subarachnoid spaces (red arrow). (D, E) In the axial plane corresponding to (C), the disseminated material in the subarachnoid space showed high signal intensity on both T1-weighted and T2-weighted images. (F–H) MRI scans showed the lesion had a heterogeneous signal, predominantly low signal on T1-weighted images with intermixed high signal areas, primarily high signal on T2-weighted images, and irregular hyperintense signals on DWI. Dissemination of cyst contents was visible in the Sylvian fissures, exhibiting high signal on both T1-weighted and T2-weighted images. (I, J) On sagittal T1-weighted images, the dissemination is also observed in the fourth ventricle, interpeduncular cistern, ambient cistern, quadrigeminal cistern, and even within the spinal canal, obstructing the cerebrospinal fluid circulation.
The patient underwent a combined microscopic and neuroendoscopic surgical resection of the lesion. After opening the dura mater, small fat droplets were observed in the subarachnoid spaces, similar to the preoperative scan. A large amount of yellowish-white material and pilous tissue was found within the cyst (Figures 2A, B). During the surgery, cottonoids were used to cover the surrounding brain tissue, and the subarachnoid space was irrigated to wash out as much cyst dissemination as possible. The cyst wall was carefully dissected from the surrounding brain tissue and removed. Histopathological examination confirmed the diagnosis of a dermoid cyst (Figures 2C, D). Postoperative CT showed a significant reduction of the lesion (Figures 3A, B), and MRI demonstrated clear cerebrospinal fluid circulation, although some fat droplets persisted in the cerebral sulci (Figures 3C, D). Post surgery, the patient’s headache had significantly improved, and he had no other symptoms. At the three-month and one-year follow-ups, the patient reported no discomfort. CT scans at three months and MRI at one year showed no signs of recurrence, and the majority of the fat droplets had been reduced, some residual fat droplets remain (Figures 3E, F).
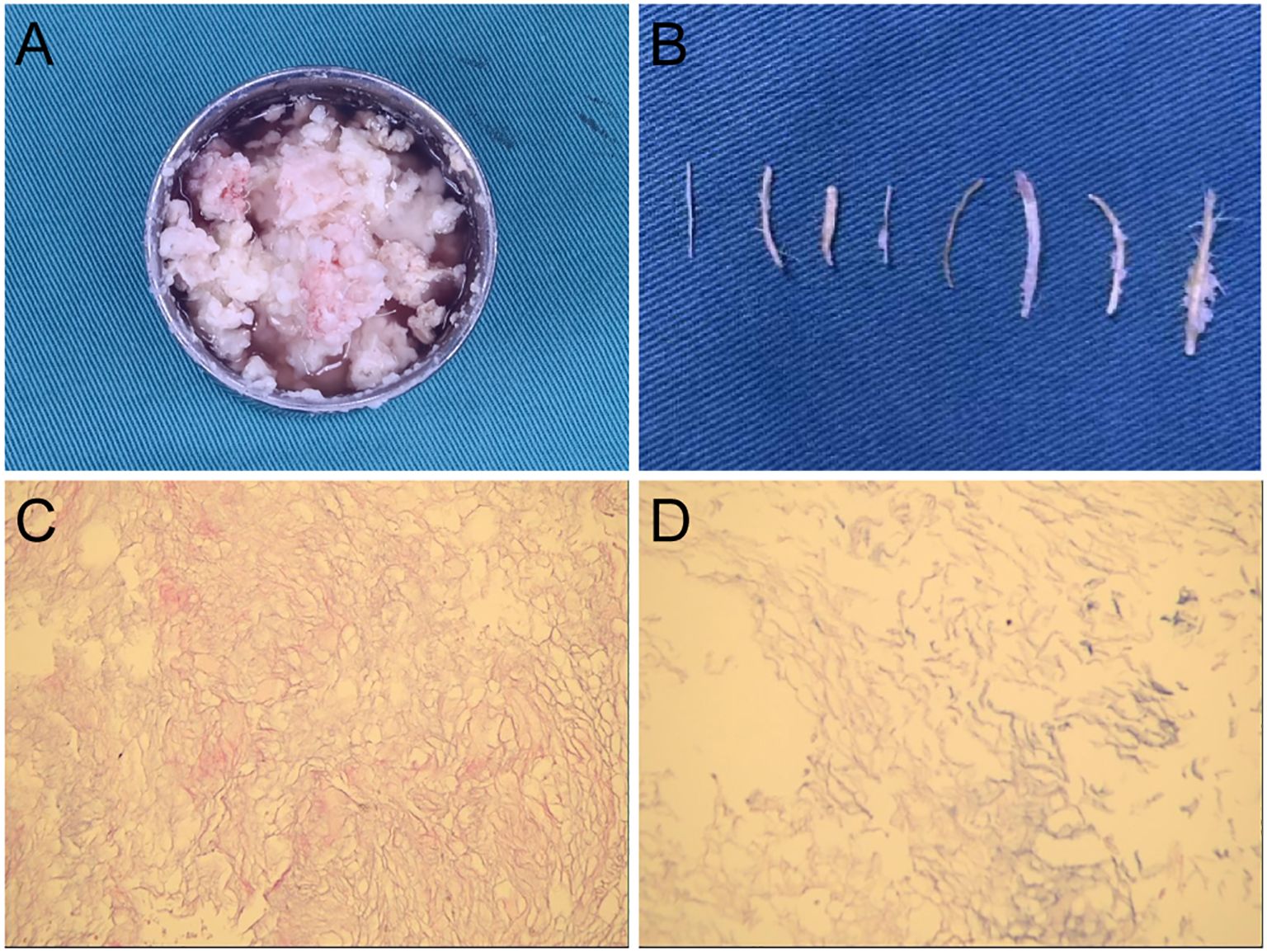
Figure 2. Intraoperative specimen and pathology results. (A, B) Yellowish-white material and pilous tissue was found within the cyst. (C, D) Histopathological examination confirmed the diagnosis of a dermoid cyst.
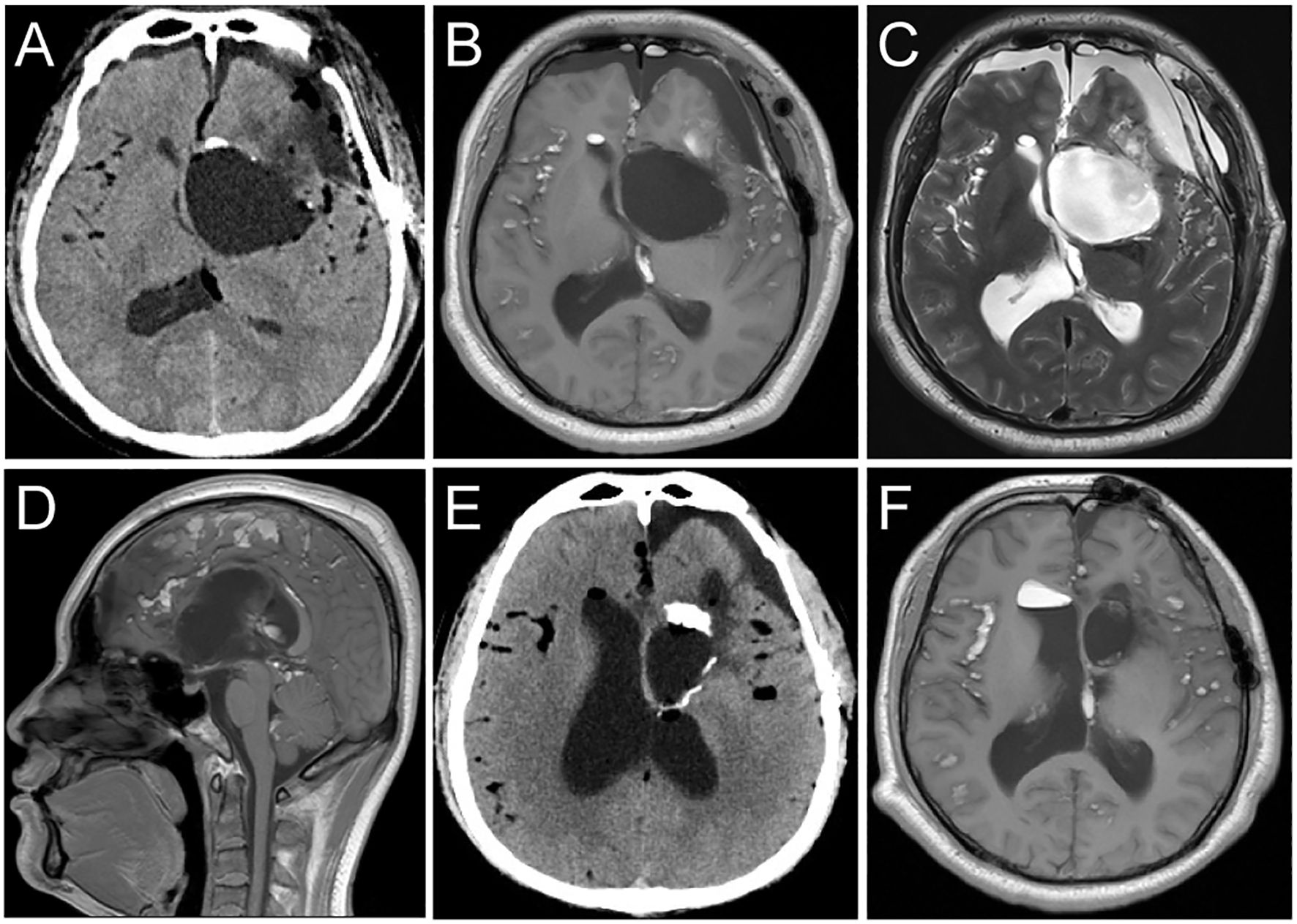
Figure 3. Postoperative follow-up imaging studies. (A) CT imaging post-surgery showed that the majority of the dermoid cyst had been completely resected. (B, C) T1 and T2 weighted MRI axial images show that the dermoid cyst has been resected. The residual disseminated material in the Sylvian fissure and subarachnoid space has decreased compared to the preoperative images. (D) The sagittal T1-weighted image shows restoration of normal cerebrospinal fluid circulation. (E, F) CT scans at three months and MRI at one year showed no signs of recurrence, and the majority of the fat droplets had been reduced, some residual fat droplets remain.
3 Discussion
Intracranial dermoid cysts account for 0.04% to 0.6% of all intracranial tumors (1). They typically arise due to incomplete separation of surface ectodermal cells from the neural tube between the third and fifth weeks of gestation, leading to entrapment of these cells within the neural tube. This mechanism explains why dermoid cysts are commonly located in the infratentorial and midline regions (6). However, some lesions are located in supratentorial and in the lateral ventricles due to the displacement of ectodermal cells by the developing neurovascular system, leading to ectopic positioning (7). In this case, the dermoid cyst occurred in the supratentorial front-temporoparietal region, which is relatively rare. The outer capsule of a dermoid cyst consists of dense fibrous connective tissue lined by stratified squamous epithelium. It contains various ectodermal derivatives, including sweat glands, sebaceous glands, hair follicles, squamous epithelium, teeth, and nails (8). The growth of dermoid cysts depends on the accumulation of desquamated keratin and sebaceous gland secretions rather than cell division, thus growing extremely slowly (4). Patients are typically asymptomatic, and symptoms only manifest when the cyst enlarges sufficiently to cause a mass effect, presenting symptoms such as headache, seizures, and focal neurological deficits (9). However, when a dermoid cyst ruptures, either spontaneously or due to external factors, its characteristics differ significantly from those of an unruptured dermoid cyst.
Dermoid cyst rupture is extremely rare and is currently categorized into two main types: spontaneous and non-spontaneous rupture. Spontaneous rupture is the most common type. One proposed explanation for spontaneous rupture involves rapid cyst enlargement due to age-related hormonal changes. However, a retrospective study on dermoid cyst rupture found that ruptures did not predominantly occur among hormonally active adolescents; the average age at rupture was 32 years (10, 11). Non-spontaneous rupture most commonly results from trauma, followed by factors such as cerebral pulsation and head movement (9). In this case, the patient experienced spontaneous rupture without external factors.
After dermoid cyst rupture, keratin and cholesterol breakdown products diffuse into the subarachnoid space and ventricles. The dissemination of cyst contents can cause a spectrum of symptoms such as headache, seizures, hydrocephalus, meningitis, cognitive dysfunction, focal neurological deficits, vasospasm leading to stroke, and even death (1). It is currently unclear whether these clinical manifestations arise immediately after cyst rupture or develop acutely following chronic spread of dermoid contents and inflammation (3). Headache, a primary symptom of dermoid cyst rupture, can manifest in various forms, ranging from intermittent to persistent, with severity from mild to severe (12). Depending on the location and size of the cyst, headaches can occur anywhere and may or may not be associated with meningeal signs. In this case, the patient’s complaint was moderate distending pain on the affected side lasting for two weeks, with no signs of meningeal irritation. We attribute this to the cerebrospinal fluid circulation disorder caused by the widespread dissemination of cyst contents into the subarachnoid space after the rupture or mass effect of the dermoid cyst.
The radiological appearance of dermoid cysts depends on their internal components and whether they have ruptured. On CT, dermoid cysts typically appear as low-density lesions due to their fatty content, occasionally with calcifications visible at the cyst wall and generally without surrounding edema. Unruptured dermoid cysts on MRI containing liquid cholesterol exhibit imaging characteristics similar to fat: high signal on T1-weighted images and variable signal intensity on T2-weighted images due to different contents. DWI often shows high or equal signal intensity (11). A ruptured dermoid cyst may show scattered high signal intensity within the subarachnoid space or ventricles on T1 and T2, primarily attributed to lipid substances, which typically appear as low signal on fat-suppressed sequences (1).
In this case, CT imaging of the dermoid cyst demonstrated typical features, with significant deformation and compression of the ipsilateral ventricle, midline shifts to the right, and shallower sulci and fissures with extensive low-density areas visible inside the cyst, indicating disseminated cyst contents, more prominent in the less compressed sulci of the right hemisphere. MRI revealed high signal intensity in the sulci and fissures on T1 and T2, with liquid fat diffusing outside the cyst, explaining the low signal on T1 within the lesion. These disseminated substances typically can persist for several years without absorption. Previous studies suggested that cyst contents do not further migrate or cause new neurological deterioration after spread. Similar findings were noted during postoperative follow-up in this case. CT at three months and MRI at one year revealed scattered fat droplets without significant repositioning.
However, a recent case report on traumatic dermoid cyst rupture highlighted significant ongoing migration of fat in the subarachnoid space over two years post-rupture (13). Another case report linked repetitive golf swings to dermoid cyst rupture, revealing gravitational migration of fat on MRI due to positional factors (14). Therefore, further follow-up is required to determine whether there will be further displacement of the fat droplets and whether it will result in related symptoms.
Regular imaging surveillance may be a reasonable management for small and asymptomatic lesions, whereas larger lesions causing mass effects typically warrant surgical intervention (15). During surgical treatment of ruptured dermoid cysts, decompression of the lesion is usually necessary to prevent cyst contents from entering the subarachnoid space. Extensive irrigation of the surrounding subarachnoid space is performed to wash out as much cystic material as possible, thereby reducing the incidence of postoperative fat dissemination and aseptic meningitis (1, 2). The residual cyst wall is the most common reason for tumor recurrence; therefore, complete removal of the cyst wall is necessary. If the cyst capsule is tightly adhered to surrounding neurovascular structures, subtotal resection with preservation of adherent tissue should be considered to avoid damaging critical structures and minimize complications. Subtotal resection of the cyst is rarely reported recurrence and progression; most patients have a favorable prognosis (1). Lipid droplets are diffusely distributed in the subarachnoid space after cyst rupture, and total removal of these droplets is difficult. It remains uncertain whether the symptoms caused by effluxion can be relieved by local lesion resection. However, glucocorticoid therapy may be beneficial for symptoms caused by spillage stimulation of surrounding structures (4, 16).
As shown in Table 1, we have reviewed research on ruptured epidermoid cysts with documented treatment and follow-up records (1, 17–35). Our findings indicate that most patients experienced symptom relief following surgical resection of the cyst. Notably, there was only one case of recurrence and one case of death, which might be attributed to the surgery being performed in a much earlier time period (17, 25). This underscores that surgical intervention remains the primary treatment for ruptured dermoid cysts. For patients presenting with meningitis as the main symptom, steroid therapy can be an effective alternative if surgery is not performed (36, 37). In cases where patients present with post-rupture hydrocephalus as the predominant symptom, cerebroventricular shunting can provide significant symptomatic relief (21, 28).
Radiotherapy is also a treatment for dermoid cysts, though it is typically reserved for cases of recurrence or malignant transformation. The clinical response of any tumor to radiotherapy depends on the inherent radiosensitivity of the tumor cells during treatment. Radiotherapy may reduce or eliminate the proliferative activity of the cells lining the dermoid cyst, thereby decreasing the rate of cyst content accumulation. Malignant transformation of dermoid cysts into squamous cell carcinoma (SCC) is extremely rare (38), primary intracranial SCC has a poor prognosis, and its management remains controversial. However, studies have shown that when complete tumor resection is possible, followed by radiotherapy, it is the optimal approach for improving patient outcomes (39–41). Radiotherapy, either alone or in combination with subtotal resection, can help reduce recurrence rates associated with incomplete tumor removal, especially in patients where the risks of additional neurosurgical procedures are elevated due to comorbidities (42). There are also reports of radiotherapy being used for intraspinal dermoid cysts (43), as well as for other benign intracranial tumors, such as epidermoid tumors (44), where the combination of surgery and adjuvant therapy has significantly improved survival rates (42).
In this case, due to the large size and deep location of the cyst, we adopted a combined microscopic and neuroendoscopic surgical treatment. Microscopic surgery is renowned for its high precision, offering excellent magnification and clarity, which facilitates the detailed visualization and differentiation of anatomical structures. This high magnification assists surgeons in accurately identifying and isolating pathological tissues. However, the microscope’s field of view is relatively limited, which may result in blind spots when addressing large or irregular lesions, potentially making it difficult to fully observe all aspects of the lesion. Additionally, the fixed perspective of the microscope restricts the ability to view hidden lesions or structures that lie outside its immediate field. In contrast, neuroendoscopy provides a broader field of view and superior illumination, enabling a more comprehensive observation of the lesion and its surrounding neurovascular structures. The close-range visualization offered by neuroendoscopy is advantageous for managing complex cases. Importantly, the use of angled endoscopes allows surgeons to navigate around obstacles and examine areas not visible with a microscope, thereby reducing blind spots. Nevertheless, neuroendoscopy involves a more complex operational technique and generally offers lower magnification compared to microscopy, which can impact the precision of handling fine structures. Combining microscopy and neuroendoscopy optimizes the reduction of blind spots and ensures thorough exposure of tumor regions obstructed by anatomical structures. This integrated approach significantly enhances the likelihood of achieving complete tumor resection. Postoperatively, the patients experienced significant pain relief, and CT imaging showed that the dermoid cyst was mostly resected without complications. One week later, an MRI showed restored patency of the cerebrospinal fluid circulation path and symmetrical ventricular shape, indicating a favorable surgical outcome. While most intracerebral fat deposits in brain sulci persisted, at three-month follow-up, the patient reported minimal discomfort with essentially resolved pain symptoms. CT shows a decrease in lipid deposition compared to before. One year later, the patient reported no specific discomfort. Follow-up MRI showed no recurrence, but lipid deposits were still present.
4 Conclusion
Reports of dermoid cysts are uncommon, and ruptured dermoid cysts are rare. This case presents an atypically located, large supratentorial dermoid cyst that ruptured spontaneously, causing extensive lipid dissemination within the subarachnoid space and resulting in symptoms such as headaches and memory impairment. Surgical intervention using a combined microscopic and neuroendoscopic approach proved effective, facilitating thorough resection and minimizing blind spots. Postoperative outcomes were favorable, with significant pain relief and restored cerebrospinal fluid circulation, with follow-up imaging showing no recurrence and decreased lipid deposition. This case underscores the importance of considering surgical resection for large dermoid cysts with mass effect and highlights the utility of combined microscopy and neuroendoscopy for complete cyst removal.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
YZ: Conceptualization, Methodology, Writing – review & editing, Data curation, Formal analysis, Software, Visualization, Writing – original draft. TD: Conceptualization, Data curation, Formal analysis, Methodology, Software, Visualization, Writing – original draft, Writing – review & editing. ZW: Conceptualization, Methodology, Writing – review & editing, Resources, Validation. HY: Data curation, Writing – review & editing. XM: Writing – review & editing. YW: Writing – review & editing. RD: Writing – review & editing. HL: Writing – review & editing. DW: Writing – review & editing. MZ: Conceptualization, Methodology, Resources, Writing – review & editing, Funding acquisition, Project administration, Supervision.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Medical Innovation and Development Project of Lanzhou University (lzuyxcx-2022-132), the Natural Science Foundation of Gansu Province of China (21JR7RA356), and the Science and Technology Program of Lanzhou (2022-ZD-93). The funders did not participate in the design or conduct of the study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Liu JK, Gottfried ON, Salzman KL, Schmidt RH, Couldwell WT. Ruptured intracranial dermoid cysts: clinical, radiographic, and surgical features. Neurosurgery. (2008) 62:377–84:384. doi: 10.1227/01.neu.0000316004.88517.29
2. Blitz SE, Bernstock JD, Dmytriw AA, Ditoro DF, Kappel AD, Gormley WB, et al. Ruptured suprasellar dermoid cyst treated with lumbar drain to prevent postoperative hydrocephalus: case report and focused review of literature. Front Surgery. (2021) 8:714771. doi: 10.3389/fsurg.2021.714771
3. Xin WQ, Lei Y, Chen D, Chen ZJ, Yang XY, Zhang N. Patient with epilepsy caused by the spontaneous rupture of an intracerebral dermoid cyst. World Neurosurg. (2020) 136:140–5. doi: 10.1016/j.wneu.2020.01.069
4. Borni M, Abdelhedi A, Kammoun B, Kolsi F, Boudawara MZ. Ruptured central nervous system dermoid cyst of suprasellar region manifesting as unusual epileptic seizure. World Neurosurg. (2019) 122:150–4. doi: 10.1016/j.wneu.2018.10.153
5. Sohrabi C, Mathew G, Maria N, Kerwan A, Franchi T, Agha RA, et al. The SCARE 2023 guideline: updating consensus Surgical CAse REport (SCARE) guidelines. Int J Surgery. (2023) 109:1136. doi: 10.1097/JS9.0000000000000373
6. Bishnoi I, Bishnoi S, Gahlawat N, Bhardwaj L, Duggal G, Singal G, et al. Management of a rare case of intraventricular ruptured dermoid cyst and chemical meningitis. Br J Neurosurg. (2023) 37:630–3. doi: 10.1080/02688697.2018.1530728
7. Garces J, Mathkour M, Beard B, Sulaiman OAR, Ware ML. Insular and sylvian fissure dermoid cyst with giant cell reactivity: case report and review of literature. World Neurosurg. (2016) 93:93:491.e1–5. doi: 10.1016/j.wneu.2016.05.037
8. Hatano K, Fujimoto A, Inenaga C, Otsuki Y, Enoki H, Okanishi T. Non-ruptured temporal lobe dermoid cyst concomitant with focal cortical dysplasia causing temporal lobe epilepsy-A case report and literature review. Brain Sci. (2021) 11:1136. doi: 10.3390/brainsci11091136
9. Park SK, Cho KG. Recurrent intracranial dermoid cyst after subtotal removal of traumatic rupture. Clin Neurol Neurosurg. (2012) 114:421–4. doi: 10.1016/j.clineuro.2011.11.006
10. Indulkar S, Hsich GE. Spontaneous rupture of intracranial dermoid cyst in a child. Neurology. (2011) 77:2070. doi: 10.1212/WNL.0b013e31823b479f
11. Shashidhar A, Sadashiva N, Prabhuraj AR, Narasingha Rao K, Tiwari S, Saini J, et al. Ruptured intracranial dermoid cysts: A retrospective institutional review. J Clin Neuroscience: Off J Neurosurg Soc Australasia. (2019), 67:172–7. doi: 10.1016/j.jocn.2019.04.025
12. Tolebeyan AS, Kuruvilla DE. Headache in ruptured intracranial dermoid cysts. Curr Pain Headache Rep. (2020) 24:31. doi: 10.1007/s11916-020-00863-x
13. Aktham A, Morita S, Takeuchi S, Ismail M, Hoz SS, Numazawa S, et al. Traumatic rupture of intracranial dermoid cyst with continuous fat droplet migration. Surg Neurol Int. (2023) 14:39. doi: 10.25259/SNI_801_2022
14. Obled L, Peciu-Florianu I, Perbet R, Vannod-Michel Q, Reyns N. Rare case of giant supratentorial dermoid cyst. World Neurosurg. (2020) 135:72–5. doi: 10.1016/j.wneu.2019.12.007
15. Singla N, Kapoor A. Ruptured intracranial dermoid: Is surgery indispensible: 11-year follow-up of a rare entity. World Neurosurg. (2016) 88:693.e23–693.e24. doi: 10.1016/j.wneu.2015.12.049
16. Kosuge Y, Onodera H, Sase T, Uchida M, Takasuna H, Ito H, et al. Ruptured dermoid cyst of the lateral cavernous sinus wall with temporary symptoms: a case report. J Med Case Rep. (2016) 10:224. doi: 10.1186/s13256-016-1007-3
17. Miller D. Case report; dermoid cyst of the frontal lobe with intraventricular rupture. J Neurology Neurosurg Psychiatry. (1950) 13:63–5. doi: 10.1136/jnnp.13.1.63
18. Maravilla KR. Intraventricular fat-fluid level secondary to rupture of an intracranial dermoid cyst. AJR Am J Roentgenology. (1977) 128:500–1. doi: 10.2214/ajr.128.3.500
19. Ford K, Drayer B, Osborne D, Dubois P. Case report. Transient cerebral ischemia as a manifestation of ruptured intracranial dermoid cyst. J Comput Assisted Tomogr. (1981) 5:895–7. doi: 10.1097/00004728-198112000-00021
20. Mikhael MA. Transient spasm of carotid siphon complicating ruptured cranial dermoid cyst. Radiology. (1982) 144:824. doi: 10.1148/radiology.144.4.7111731
21. Martin R, Knone A, Schuknecht B, Kuhn W. Rapid development of occlusion hydrocephalus by intraventricular fat possibly derived from a ruptured dermoid cyst. J Neurology Neurosurgery Psychiatry. (1989) 52:134–5. doi: 10.1136/jnnp.52.1.134-a
22. Takeuchi H, Kubota T, Kabuto M, Izaki K. Ruptured suprasellar dermoid cyst presenting olfactory delusion (Eigengeruchs erlebnis). Neurosurgery. (1993) 33:97–9. doi: 10.1227/00006123-199307000-00015
23. Nakamura M, Mizuguchi M, Momoi MY, Chou H, Masuzawa T. Transient cheiro-oral syndrome due to a ruptured intracranial dermoid cyst. Brain Dev-jpn. (2001) 23:261–3. doi: 10.1016/S0387-7604(01)00210-8
24. Zheng K, yong MB, Ma L, Jiang S. Ruptured intracranial dermoid cyst with infarction in the basal ganglia–case report. Neurol Med Chir (tokyo). (2010) 50:254–6. doi: 10.2176/nmc.50.254
25. Park SK, Cho KG. Recurrent intracranial dermoid cyst after subtotal removal of traumatic rupture. Clin Neurol Neurosurg. (2012) 114:421–4. doi: 10.1016/j.clineuro.2011.11.006
26. Fs E, Öztürk S, Çakin H, Mm A. Spontaneous rupture of intracranial dermoid cyst mimicking a primary psychiatric disorder. Noro Psikiyatr Ars. (2014) 51(2):181–3. doi: 10.4274/npa.y6962
27. Paik SC, Kim CH, Cheong JH, Kim JM. A ruptured dermoid cyst of the cavernous sinus extending into the posterior fossa. J Korean Neurosurg S. (2015) 57:364–6. doi: 10.3340/jkns.2015.57.5.364
28. Wani A A, Raswan US, Malik NK, Ramzan AU. Posterior fossa ruptured dermoid cyst presenting with hydrocephalus. Neurosciences. (2016) 21:358–60. doi: 10.17712/nsj.2016.4.20160280
29. Jin H, Guo ZN, Luo Y, Zhao R, Sun MS, Yang Y. Intracranial dermoid cyst rupture-related brain ischemia: Case report and hemodynamic study. Med (baltimore). (2017) 96:e5631. doi: 10.1097/MD.0000000000005631
30. Akbari SHA, Somasundaram A, Ferguson CJ, Roland JL, Smyth MD, Strahle JM. Focal traumatic rupture of a dermoid cyst in a pediatric patient: Case report and literature review. Child’s Nervous Syst: ChNS: Off J Int Soc Pediatr Neurosurg. (2018) 34:2485–90. doi: 10.1007/s00381-018-3879-6
31. Borni M, Abdelhedi A, Kammoun B, Kolsi F, Boudawara MZ. Ruptured central nervous system dermoid cyst of suprasellar region manifesting as unusual epileptic seizure. World Neurosurg. (2019) 122:150–4. doi: 10.1016/j.wneu.2018.10.153
32. Ochoa A, Saenz A, Argañaraz R, Mantese B. Ruptured dermoid cyst in the Meckel’s cave presenting with trigeminal neuralgia in a pediatric patient: a case report. Child’s Nervous Syst: ChNS: Off J Int Soc Pediatr Neurosurg. (2020) 36:3141–6. doi: 10.1007/s00381-020-04646-y
33. Xin WQ, Lei Y, Chen D, Chen ZJ, Yang XY, Zhang N. Patient with epilepsy caused by the spontaneous rupture of an intracerebral dermoid cyst. World Neurosurg. (2020) 136:140–5. doi: 10.1016/j.wneu.2020.01.069
34. Blitz SE, Bernstock JD, Dmytriw AA, Ditoro DF, Kappel AD, Gormley WB, et al. Ruptured suprasellar dermoid cyst treated with lumbar drain to prevent postoperative hydrocephalus: case report and focused review of literature. Front Surgery. (2021) 8:714771. doi: 10.3389/fsurg.2021.714771
35. Baraya N, Van Stavern GP, Stunkel L. Pearls & oy-sters: Case report of a ruptured suprasellar dermoid cyst presenting with junctional scotoma of traquair. Neurology. (2024) 103:e209559. doi: 10.1212/WNL.0000000000209559
36. Sha A AS, Cj F, Jl R, Md S, Jm S. Focal traumatic rupture of a dermoid cyst in a pediatric patient: case report and literature review. Child’s nervous system : ChNS : Off J Int Soc Pediatr Neurosurg. (2018) 34(12):2485–90. https://pubmed.ncbi.nlm.nih.gov/29961083/.
37. Wang YM, Chang TP, Lo CP, Tu MC. Spontaneous rupture of intracranial dermoid cyst with chemical meningitis. J Emerg Med. (2013) 44:e275–6. doi: 10.1016/j.jemermed.2012.06.023
38. Kim MS, Kim OL. Primary intracranial squamous cell carcinoma in the brain stem with a cerebellopontine angle epidermoid cyst. J Korean Neurosurg S. (2008) 44:401–4. doi: 10.3340/jkns.2008.44.6.401
39. Hamlat A, Hua ZF, Saikali S, Laurent JF, Gedouin D, Ben-Hassel M, et al. Malignant transformation of intra-cranial epithelial cysts: Systematic article review. J Neurooncol. (2005) 74:187–94. doi: 10.1007/s11060-004-5175-4
40. Kwon SM, Kim JH, Kim YH, Hong SH, Kim CJ. Treatment and survival outcomes of primary intracranial squamous cell carcinoma. World Neurosurgery. (2019) 125:e1–9. doi: 10.1016/j.wneu.2018.11.252
41. Mallick S, Biswas A, Kumar N, Sharma MC, Kumar R, Reddy RM, et al. Primary intracranial basaloid squamous cell carcinoma: An enigma. Neurologia I Neurochirurgia Polska. (2012) 46:489–95. doi: 10.5114/ninp.2012.31361
42. Shaikh MY, Sharif S, Rafay M. Primary intracranial squamous cell carcinoma arising in dermoid cyst. Asian J neurosurgery. (2019) 14:904–6. doi: 10.4103/ajns.AJNS_200_18
43. Bristow RG, Laperriere NJ, Tator C, Milosevic M, Wong CS. Post-operative radiotherapy for recurrent dermoid cysts of the spine: A report of 3 cases. J Neurooncol. (1997) 33:251–6. doi: 10.1023/A:1005739606895
Keywords: dermoid cyst, ruptured, microscopic surgery, neuroendoscopy, case report
Citation: Zhang Y, Deng T, Wu Z, Yang H, Ma X, Wang Y, Ding R, Li H, Wang D and Zheng M (2024) Microscopic and neuroendoscopic treatment of a large ruptured supratentorial dermoid cyst with extensive dissemination: a case report and literature review. Front. Oncol. 14:1468622. doi: 10.3389/fonc.2024.1468622
Received: 22 July 2024; Accepted: 25 September 2024;
Published: 14 October 2024.
Edited by:
Zhenyu Xiong, The State University of New Jersey, United StatesReviewed by:
Kaida Yang, Mount Sinai Hospital, United StatesXinxin Zhang, The State University of New Jersey, United States
Copyright © 2024 Zhang, Deng, Wu, Yang, Ma, Wang, Ding, Li, Wang and Zheng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Maohua Zheng, MjAwMm1hb2h1YUAxNjMuY29t
†These authors have contributed equally to this work and share first authorship
 Yuhang Zhang
Yuhang Zhang Tingzhen Deng
Tingzhen Deng Zhi Wu1,2
Zhi Wu1,2 Maohua Zheng
Maohua Zheng