- The Gastrointestinal Surgery of Xingtai Central Hospital, Xingtai, Hebei, China
Objective: To investigate the expression and clinical significance of Notch-1 and Numb protein in colon cancer tissues and regional lymph node metastases.
Methods: Immunohistochemical method was used to detect the expression of Notch-1 protein and Numb protein in 110 cases of colon cancer tissues, along with tumor adjacent tissues and 56 cases of MLN tissues, and to analyze its role in colon cancer and MLN tissue.
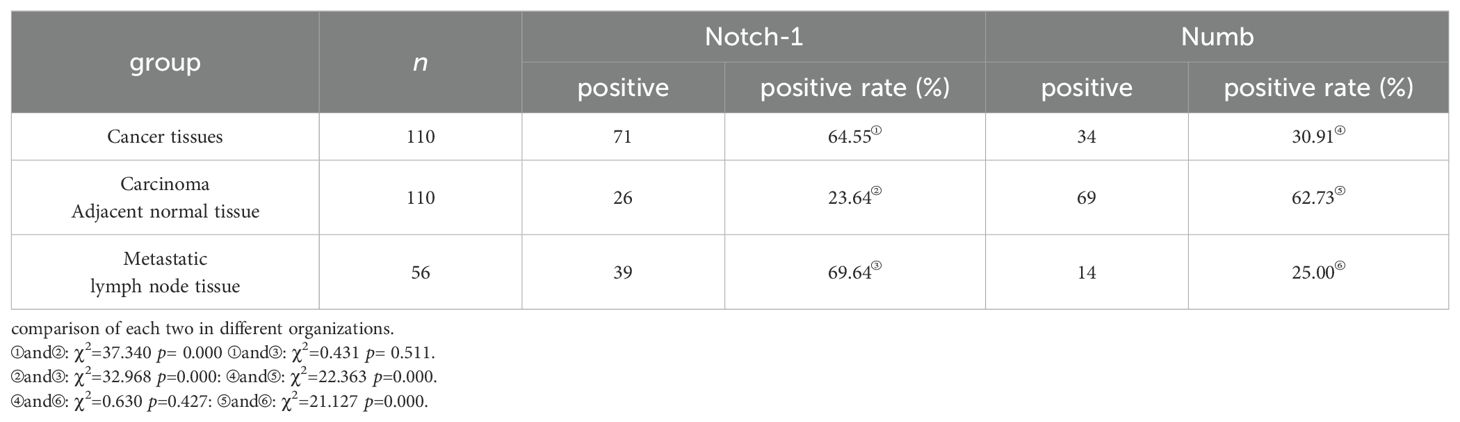
Results: Comparing colon cancer tissue or lymph node metastases with tumor adjacent tissue, the positive expression rate of Numb was significantly decreased, while the positive expression of Notch-1 was significantly increased in colon cancer tissue or lymph node metastases (both p<0.05). The expression of Notch-1 and Numb was correlated with the lymph node metastasis, TNM stage, and degree of differentiation (p<0.05). The expression between Numb and Notch-1 showed negative correlation in colon cancer tissues (r=−0.261, p<0.05). There was no relationship between the expression of Numb and Notch-1 protein in colon cancer and metastatic lymph node tissue (p>0.05).
Conclusion: Numb expression is decreased and Notch-1 expression is increased in colon cancer tissue and metastatic lymph node tissue, suggesting that the interaction between the two proteins may play a promote role in the development, invasion, and metastasis of colon cancer. There was no relationship between the expression of Numb and Notch-1 protein in colon cancer and metastatic lymph node tissue, suggesting that there is no obvious enhancement of the cancer cells; in the process of lymph node metastasis, the degree of malignant biological behavior remains relatively stable.
Introduction
In recent years, the incidence of colon cancer is increasing gradually, and colon cancer has heterogeneity, which requires individualized treatment plan, so personalized treatment has become a new trend in the field of cancer treatment. The key of personalized therapy lies in the high selectivity of tumor targets, and the selection of targeted drugs with good effect and light side effects has become a research hotspot. Molecular targeted therapy can effectively improve the prognosis of cancer patients, improve the survival rate of patients, and become another new method in addition to surgical resection, radiotherapy, and chemotherapy, providing a new treatment for colon cancer patients. Notch-1 protein is involved in cell proliferation, differentiation, apoptosis, etc. (1, 2). The abnormal transmission of Notch-1 signaling pathway may be related to the occurrence of tumors (3). Numb is known as the fate determinant of cell differentiation and can influence cell differentiation in a variety of ways. The role of Numb in the Notch signaling pathway has become the focus of current research (4, 5). It has been suggested that Numb may inhibit the invasion and metastasis of melanoma by regulating NOTCH-CCNE axis, and upregulation Numb inhibitors may play a role in the treatment of melanoma (6). By detecting the expression of Notch-1 protein and Numb protein in colon cancer and MLN, this study provides a possible theoretical basis for the occurrence, development, and metastasis of colon cancer. At the same time, Notch-1 and Numb are targeted to provide a possible molecular basis for the targeted therapy and prognosis of colon cancer.
Data and methods
General information
A total of 110 carcinoma tissues and adjacent carcinoma tissues of patients with colon cancer who had surgery in the gastrointestinal surgery of Xingtai Central Hospital in China during October 2019–October 2022 were selected. A total of 56 regional lymph node metastases tissues were selected as samples among the above cases. Of the 110 cases of colon cancer patients, 57 cases were men, 53 cases were women, ages were 41–83 years old, with an average age of 67 years old, and 44 cases were in stage I+II and 66 cases were in stage III+IV. There were 68 cases with tumor diameter ≤5 cm and 42 cases with tumor diameter >5 cm. None of the selected cases received neoadjuvant therapy before surgery and had no intestinal obstruction before surgery. All specimens were treated with radical resection of colon cancer and were pathologically diagnosed as colonic adenocarcinoma.
Reagent
Numb polyclonal antibody and Notch-1 polyclonal antibody are bought from Proteintech Group, Inc. (Wuhan, Hubei, China).; the kit and citric acid antigen repair reagents were bought from Beijing Zhong Shan -Golden Bridge Biological Technology CO.,LTD (Beijing, China).
Method
Using the immunohistochemical SP method, the specimens were made of biopsies that were 4 μm, fully hydrated after conventional xylene dewaxing, and repaired by citric acid antigen. Peroxidase is blocked by 3% hydrogen peroxide in each biopsy and incubated for 10 min at room temperature, and the serum was removed; Numb or Notch-1 primary polyclonal antibody is added to each biopsy as a fight and incubated for 90 min at room temperature. Each biopsy was incubated for 30 min at room temperature with the second antibody, added by streptomyces avidin peroxidase reagents, and colored in DAB microscope.
The result judgment standard
Numb is mainly expressed in the cytoplasm and cell membrane; the yellow particles mean positive cells. Notch-l protein is mainly expressed in the cytoplasm and the nucleus in tan particles; the color intensity of positive cell and the number of positive cells were judged based on the integral semi-quantitative method. Colorless, pale yellow, tan, and brown were marked by 0, 1, 2, and 3, respectively, according to positive staining intensity. Each biopsy was marked by the number of positive cells (0, 1, 2, 3, and 4 points mean <10%, 10%–10%, 26%–50%, 51%–70%, and >70%, respectively). Comprehensive score is equal to the product of two grades, 0 means negative, 1–4 means weakly positive, 5–8 means moderate positive, and >8 means strong positive. Negative and weakly positive scores are viewed as a negative expression; moderate positive and strong positive are viewed as positive expression.
Statistical processing
The SPSS 21.0 software was used for data analysis, where the significance of count data was compared by χ2 test, and the expression correlation of Numb and Notch-1 proteins was analyzed by Spearman rank correlation. p-value <0.05 was considered to be statistically significant.
Results
The Numb and Notch-1 expression in different tissues
As shown in Table 1, in colon cancer and metastasis lymph node tissues, the positive expression rate of Numb was significantly lower than those in the adjacent normal tissue; the positive expression rate of Notch-1 was significantly higher than that in the adjacent normal tissue (Figure 1). The difference was statistically significant (p < 0.05). The positive expression rate of Numb or Notch-1 has no statistically significant difference between cancer and metastasis lymph node tissues (p > 0.05).

Figure 1. Immunohistochemical detection of Notch-1 protein and Numb protein expression in different tissues (SP × 100). (A) Notch-1 is highly expressed in cancer tissues; (B) Notch-1 is highly expressed in lymph node metastatic tissues; (C) Notch-1 is lowly expressed in tumor adjacent tissues; (D) Numb is lowly expressed in cancer tissues; (E) Numb is lowly expressed in lymph node metastatic tissues; (F) Numb is highly expressed in tumor adjacent tissues.
Clinicopathological factors according to the expression of Numb and Notch-1 protein in 110 patients with colon cancer
As shown in Table 2, the expression of Numb was significantly associated in colon cancer with the lymph node metastasis, TNM stage, and degree of differentiation (p < 0.05), not with age, gender, serous membrane invasion, and tumor size (p > 0.05). The expression of Notch-1 was significantly associated in colon cancer with the lymph node metastasis, TNM stage, and degree of differentiation (p < 0.05), not with age, gender, serous membrane invasion,and tumor size (p > 0.05).
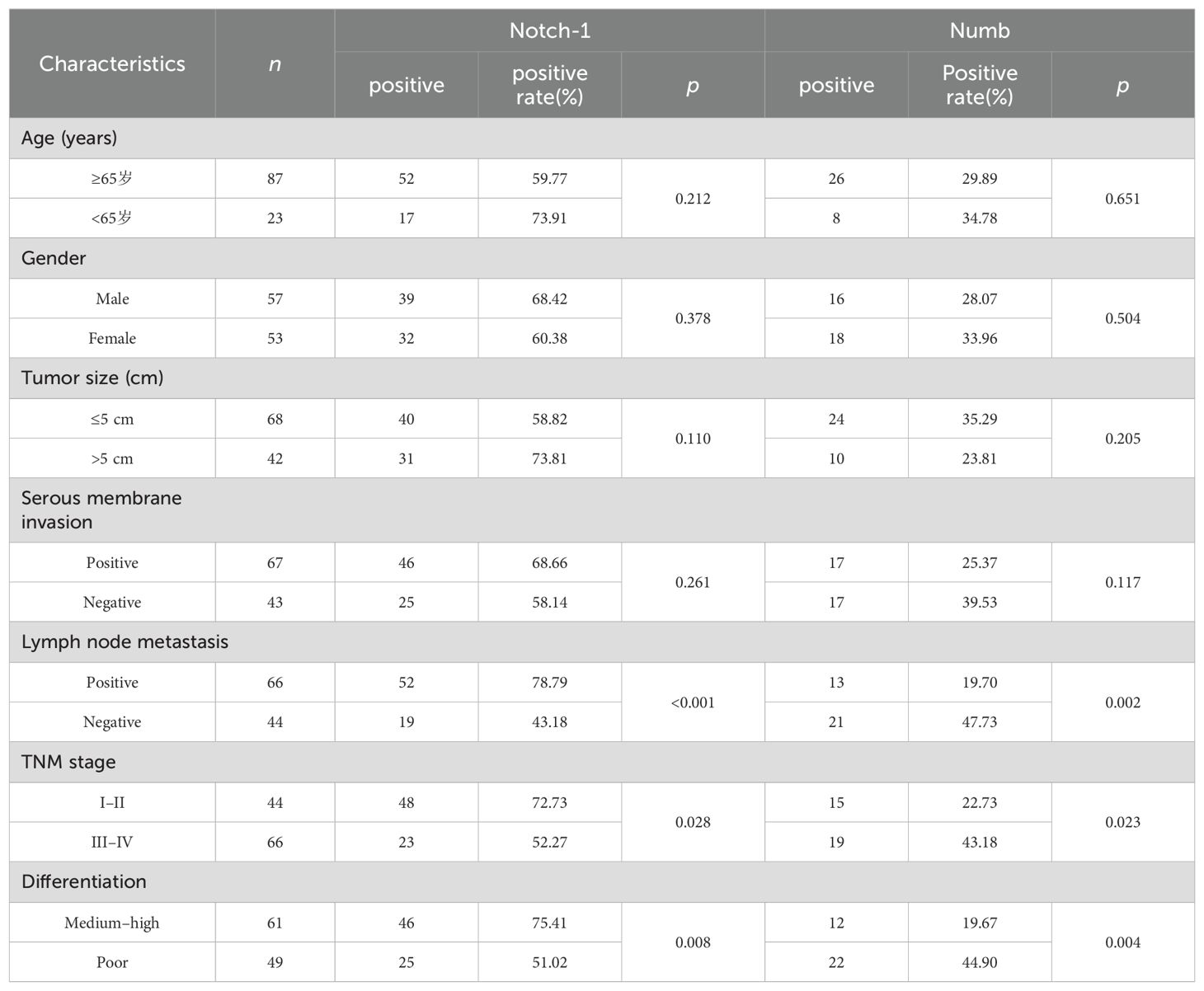
Table 2. Clinicopathological factors according to the expression of Numb and Notch-1 protein in 110 patients with colon cancer tissues.
Discussion
After the Notch ligand binds to corresponding receptors in the Notch signaling pathway, it causes the division and release of the Notch Intracellular Domain (NICD), acts on downstream target genes, and regulates cell development, proliferation, differentiation, and other activities (7, 8). Notch-1 signaling pathway is related to the occurrence, development, invasion, and metastasis of tumors. However, there are many unexplained factors that affect the Notch signaling pathway. Notch-1, as one of the important members of the Notch family, has gradually become a new trend in the study of colon cancer (9, 10). The most important feature of Numb is the regulation of cell differentiation through asymmetric allocation during mitosis and is therefore known as a determinant of cell fate, and these properties have important implications for the role of Numb in cell physiological development and various diseases (11, 12). Numb and Notch are antagonistic proteins in many literatures (13, 14). Studies have shown that in colorectal cancer, loss of Numb expression leads to abnormal activation of Notch signaling pathway, which is closely related to the occurrence and development of colon cancer (3). However, it has been suggested that different subtypes of Numb have different roles in tumors, with NUMB exon 12 (E12) hop isomer p65/p66 promoting epithelial-to-mesenchymal transformation (EMT) and cancer cell migration in vitro and promoting cancer metastasis in mice; the p71/p72 isomer acts as a negative regulator of Notch-1 by ubiquitinating the Notch-1 intracellular domain (N1ICD) and promoting its degradation, and the NUMB isoform is considered to be a key regulator of EMT and cancer cell migration (15). In this study, the expression of Notch-1 protein and Numb protein in colon cancer tissues and MLN tissues was detected to analyze the relationship between the two proteins and clinical case factors, so as to provide a possible theoretical basis for finding molecular targets for colon cancer and predicting lymph node metastasis.
Notch signaling pathway promotes the occurrence and development of tumors in most cases but inhibits tumors in some cases. This opposite result may be caused by the different expression levels of Notch-1 protein in different tumor cells and different stages of tumor development (16, 17). This study showed that the expression of Notch-1 protein in colon cancer tissues was significantly higher than that in adjacent tissues (p < 0.05), suggesting that the high expression of Notch-1 leads to abnormal activation of Notch signaling pathway, which may be involved in the occurrence and development of colon cancer and play a role in promoting cancer, which is consistent with Xu et al. (18, 19). Many downstream target genes regulated by Notch-1 (such as CyclinD1, Hes1, Bcl-2, NF-κB, and Hey-1) are closely related to cell proliferation cycle and self-renewal (20, 21). Some studies have found that in mouse intestinal epithelial cells, the lack of Golgi membrane protein 1 causes abnormal activation of Notch, thus affecting the differentiation and maturation of intestinal epithelial cells, leading to the occurrence and development of malignant tumors. Inhibition of Notch abnormal expression by drugs can inhibit the tumor progression of intestinal epithelial cells lacking Golgi membrane protein-1, suggesting that Golgi membrane protein-1 prevents colon tumorigenesis by regulating Notch signaling pathway (22). The Schmidt EM study found that blocking Notch and MAPK signaling pathways by targeted drugs plays a regulatory role in the proliferation and plasticity of different colon cancer cell subsets (23). Pu described the role of newly developed drugs in the regulation of colon cancer by affecting the Notch signaling pathway (24). Therefore, the abnormal activation of Notch may affect the occurrence and development of tumors and also provide a certain theoretical basis for the targeted therapy of colon cancer.
In many cancer types, Numb acts as a tumor suppressor, and its downregulation leads to the development of tumors (3, 25). In this study, it was found that the expression of Numb in para-cancerous tissues was significantly higher than that in cancerous tissues and metastatic lymph nodes (p<0.05) and was related to the degree of tissue differentiation, presence or absence of lymph node metastasis, and TNM stage (p<0.05), but not related to tumor size and presence or absence of envelope invasion (p>0.05). It is suggested that Numb plays a cancer-suppressing role in colon cancer, which is consistent with Zhang et al. (26). Cheng et al. (27) showed that Numb can negatively regulate epithelial–mesenchymal transformation through Wnt signaling pathway, thus inhibiting the development of colorectal cancer, which is consistent with the results of this study. However, the downregulation of Numb expression is negatively correlated with the depth of invasion and tumor size, which is different from the results of this study. It is considered that Numb may be affected by multiple factors or play a role in the development of tumor through multiple signaling pathways. In lung adenocarcinoma, Numb inhibits Notch pathway and epithelial–mesenchymal transformation, inhibiting tumor growth, while in lung squamous cell carcinoma, Numb may promote tumor proliferation (28). Saha et al. (29) found in colon cancer that NUMB may play an important role in the bias effect of Wnt/Notch signaling crosstalk through KRT19. Zhang analyzed the role of Numb in tumor by searching literature with various software, suggesting that in colorectal cancer, NUMBL inhibits Notch pathway in colorectal tumor with unchanged NUMB expression, and the decrease in NUMBL expression leads to increased malignancies and poor prognosis (30). Because Numb has different subtypes and contains different domains, it may play a different role in different tumor tissues and play a cancer suppressor role in colorectal cancer.
Abnormal expression of Notch signaling pathway can affect cell differentiation and induce undifferentiated cells to turn to malignant cells, resulting in the occurrence of tumors, and the degree of differentiation of malignant tumors affects the prognosis of patients (31). In this study, it was found that the positive expression rate of Notch-1 was higher in colon cancer tissues with poor differentiation types (p < 0.05), suggesting that the expression of Notch-1 is related to the differentiation types of colon cancer and may be involved in cell differentiation, which is closely related to the occurrence and development of colon cancer.
Lymphatic duct metastasis is the main way of colon cancer recurrence and metastasis and also the key factor of postoperative recurrence of colon cancer (32, 33). The presence of regional lymph node metastasis and the number of metastatic lymph nodes are important determinants of prognosis in patients with colon cancer, so preoperative evaluation of lymph node metastasis is crucial (34). Jepsen used pT1 colorectal cancer with regional lymph node metastasis to investigate the association between the presence of miR-17/92 cluster and LNM, and the results suggested that early regional lymph node metastasis of colon cancer was associated with the high expression level of miR-17/92 cluster members (miR-17-3p, miR-92a) (35). Jiang (36) established a new cell line FDOVL from metastatic lymph nodes of patients with primary platinum-resistant ovarian cancer and found that NOTCH1-pC702fs mutation was only highly expressed in FDOVL cell lines and metastatic lymph nodes, and this mutation promoted the migration and invasion of tumor cells. These effects were significantly inhibited by NOTCH inhibitor LY3039478, suggesting that NOTCH1 mutation may be a driver of lymph node metastasis in ovarian cancer. This study found that the expression of Notch-1 protein in colon cancer tissues with lymph node metastasis was higher than that in colon cancer tissues without lymph node metastasis, suggesting that the overexpression of Notch-1 protein may promote lymph node metastasis of colon cancer. Gonulcu et al. (6) found that the expression status and expression level of Numb and lymph node metastasis and stage are significantly correlated with the survival of colorectal cancer patients. Cox regression analysis showed that lymph node metastasis and downregulation of Numb are independent prognostic factors of colon cancer. In this study, the downregulation of Numb expression is associated with lymph node metastasis in colon cancer tissues, suggesting that the downregulation of Numb expression may promote lymph node metastasis in colon cancer. Yang et al. (37) suggest the following: Numb controls the migration of epithelial cells by regulating intercellular connectivity, and the inhibition of the expression of Numb can promote the migration and invasion of colon cancer cells induced by TGF-β, upregulate the expression of EMT-related molecule Snail, inhibit the expression of E-cadherin, resulting in the destruction of intercellular links, and participate in the invasion and metastasis of colon cancer cells. Ulintz et al. (38) found that both the primary tumor region and the corresponding lymph node metastasis were polyclonal, and the clonal population of each lymph node was different. In some patients, clusters of cancer cells in specific lymph nodes originate from multiple different regions of the tumor. However, there was no significant difference in the expression of Notch-1 protein and Numb protein between colon cancer tissues and MLN tissues (p>0.05), suggesting that the malignancy degree of cancer cells did not improve significantly during lymph node metastasis and the biological behavior remained relatively stable. The study also found that poorly differentiated colon cancer patients had a high rate of lymph node metastasis, suggesting that poorly differentiated cancer cells were at high risk of lymph node metastasis. Therefore, in patients with colon cancer, no definite metastatic lymph nodes were found on preoperative imaging, while patients with poor tissue differentiation indicated by preoperative colonoscopy biopsy may be predicted to have a greater potential risk of lymph node metastasis, providing a possible theoretical basis for clinical preoperative staging and follow-up treatment.
The abnormal expression of Notch-1 protein and Numb protein may affect the differentiation degree of tumor cells and lymph node metastasis, thus promoting the occurrence, development, recurrence, and metastasis of colon cancer, providing a possible theoretical basis for exploring the biological behavior of colon cancer cells and targeted therapy. However, the mechanism of Notch-1 and Numb in the lymph node metastasis of colon cancer needs further study.
Author contributions
JM: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. JZ: Data curation, Formal analysis, Resources, Writing – review & editing. NY: Conceptualization, Data curation, Resources, Writing – review & editing. CM: Data curation, Project administration, Resources, Software, Writing – original draft, Formal analysis, Writing – review & editing. YL: Funding acquisition, Investigation, Writing – review & editing, Project administration.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Xingtai City key research and development plan self-raised project (grant number: 2022ZC191).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Katoh M, Katoh M. Precision medicine for human cancers with Notch signaling dysregulation (Review). Int J Mol Med. (2020) 45:279–97. doi: 10.3892/ijmm.2019.4418
2. Pennarubia F, Ito A, Takeuchi M, Haltiwanger RS. Cancer-associated Notch receptor variants lead to O-fucosylation defects that deregulate Notch signaling. J Biol Chem. (2022) 298:102616. doi: 10.1016/j.jbc.2022.102616
3. Gonulcu SC, Unal B, Bassorgun IC, Ozcan M, Coskun HS, Elpek GO, et al. Expression of Notch pathway components (Numb, Itch, and Siah-1) in colorectal tumors: A clinicopathological study. World J Gastroenterol. (2020) 26:3814–33. doi: 10.3748/wjg.v26.i26.3814
4. Ortega-Campos SM, García-Heredia JM. The multitasker protein: A look at the multiple capabilities of NUMB. Cells. (2023) 12:333. doi: 10.3390/cells12020333
5. Zhu D, Xia J, Liu C, Fang C. Numb/Notch/PLK1 signaling pathway mediated hyperglycemic memory in pancreatic cancer cell radioresistance and the therapeutic effects of metformin. Cell Signal. (2022) 93:110268. doi: 10.1016/j.cellsig.2022.110268
6. Hristova DM, Fukumoto T, Takemori C, Gao L, Hua X, Wang JX, et al. NUMB as a therapeutic target for melanoma. J Invest Dermatol. (2022) 142:1882–92. doi: 10.1016/j.jid.2021.11.027
7. Shi F, Sun MH, Zhou Z, Wu L, Zhu Z, Xia SJ, et al. Tumor-associated macrophages in direct contact with prostate cancer cells promote Malignant proliferation and metastasis through NOTCH1 pathway. Int J Biol Sci. (2022) 18:5994–6007. doi: 10.7150/ijbs.73141
8. Mohamed SY, Kaf RM, Ahmed MM, Elwan A, Ashour HR, Ibrahim A, et al. The prognostic value of cancer stem cell markers (Notch1, ALDH1, and CD44) in primary colorectal carcinoma. J Gastrointest Cancer. (2019) 50:824–37. doi: 10.1007/s12029-018-0156-6
9. Dunkin D, Iuga AC, Mimouna S, Harris CL, Haure-Mirande JV, Bozec D, et al. Intestinal epithelial Notch-1 protects from colorectal mucinous adenocarcinoma. Oncotarget. (2018) 9:33536–48. doi: 10.18632/oncotarget.26086
10. Liao W, Li G, You Y, Wan H, Wu Q, Wang C, et al. Antitumor activity of Notch-1 inhibition in human colorectal carcinoma cells. Oncol Rep. (2018) 39:1063–71. doi: 10.3892/or.2017.6176
11. Choi HY, Seok J, Kang GH, Lim KM, Cho SG. The role of NUMB/NUMB isoforms in cancer stem cells. BMB Rep. (2021) 54:335–43. doi: 10.5483/BMBRep.2021.54.7.048
12. Huang C, Ji C, Wang J. Current thoughts on cellular functions of numb-associated kinases. Mol Biol Rep. (2023) 50:4645–52. doi: 10.1007/s11033-023-08372-x
13. Luo Z, Mu L, Zheng Y, Shen W, Li J, Xu L, et al. NUMB enhances Notch signaling by repressing ubiquitination of NOTCH1 intracellular domain. J Mol Cell Biol. (2020) 12:345–58. doi: 10.1093/jmcb/mjz088
14. Kim H, Ronai ZA. Rewired Notch/p53 by Numb'ing Mdm2. J Cell Biol. (2018) 217:445–6. doi: 10.1083/jcb.201712007
15. Zhan Z, Yuan N, You X, Meng K, Sha R, Wang Z, et al. Exclusion of NUMB exon12 controls cancer cell migration through regulation of notch1-SMAD3 crosstalk. Int J Mol Sci. (2022) 23:4363. doi: 10.3390/ijms23084363
16. Singh AK, Shuaib M, Prajapati KS, Prajapati KS, Kumar S. Rutin potentially binds the gamma secretase catalytic site, down regulates the notch signaling pathway and reduces sphere formation in colonospheres. Metabolites. (2022) 12:926. doi: 10.3390/metabo12100926
17. Aster JC, Pear WS, Blacklow SC. The varied roles of notch in cancer. Annu Rev Pathol. (2017) 12:245–75. doi: 10.1146/annurev-pathol-052016-100127
18. Xu K, Shen K, Liang X, Li Y, Nagao N, Li J, et al. MiR-139-5p reverses CD44+/CD133+-associated multidrug resistance by downregulating NOTCH1 in colorectal carcinoma cells. Oncotarget. (2016) 7:75118–29. doi: 10.18632/oncotarget.12611
19. Tyagi A, Sharma AK, Damodaran C. A review on notch signaling and colorectal cancer. Cells. (2020) 9:1549. doi: 10.3390/cells9061549
20. Emam O, Wasfey EF, Hamdy NM. Notch-associated lncRNAs profiling circuiting epigenetic modification in colorectal cancer. Cancer Cell Int. (2022) 22:316. doi: 10.1186/s12935-022-02736-2
21. Xia R, Xu M, Yang J, Ma X. The role of Hedgehog and Notch signaling pathway in cancer. Mol Biomed. (2022) 3:44. doi: 10.1186/s43556-022-00099-8
22. Pu Y, Song Y, Zhang M, Long CF, Li J, Wang Y, et al. GOLM1 restricts colitis and colon tumorigenesis by ensuring Notch signaling equilibrium in intestinal homeostasis. Signal Transduct Target Ther. (2021) 6:148. doi: 10.1038/s41392-021-00535-1
23. Schmidt EM, Lamprecht S, Blaj C, Schaaf C, Krebs S, Blum H, et al. Targeting tumor cell plasticity by combined inhibition of NOTCH and MAPK signaling in colon cancer. J Exp Med. (2018) 215:1693–708. doi: 10.1084/jem.20171455
24. Pu Z, Yang F, Wang L, Diao Y, Chen D. Advancements of compounds targeting Wnt and Notch signalling pathways in the treatment of inflammatory bowel disease and colon cancer. J Drug Targeting. (2021) 29:507–19. doi: 10.1080/1061186X.2020.1864741
25. Zhang H, Qi S, Liu Z, Li CY, Li MJ, Zhao XB. Melatonin inhibits 17β-estradiol-induced epithelial-mesenchymal transition in endometrial adenocarcinoma cells via upregulating numb expression. Gynecol Obstet Invest. (2022) 87:89–99. doi: 10.1159/000522170
26. Zhang H, Ye Y, Zhou J. Relationship between p53 gene mutation and Numb protein expression and clinicopathological features and prognosis of colorectal cancer. Chin J Gen Surg. (2022) 37:122–6. doi: 10.3760/cma.j.cn113855-20210920-00561
27. Cheng C, Huang Z, Zhou R, An H, Cao G, Ye J, et al. Numb negatively regulates the epithelial-to-mesenchymal transition in colorectal cancer through the Wnt signaling pathway. Am J Physiol Gastrointest Liver Physiol. (2020) 318:G841–53. doi: 10.1152/ajpgi.00178.2019
28. Kikuchi H, Sakakibara-Konishi J, Furuta M, Kikuchi E, Kikuchi J, Oizumi S, et al. Numb has distinct function in lung adenocarcinoma and squamous cell carcinoma. Oncotarget. (2018) 9:29379–91. doi: 10.18632/oncotarget.25585
29. Saha SK, Yin Y, Chae HS, Cho SG. Opposing regulation of cancer properties via KRT19-mediated differential modulation of wnt/β-catenin/notch signaling in breast and colon cancers. Cancers (Basel). (2019) 11:99. doi: 10.3390/cancers11010099
30. Zhang Y, Yang H, Liu W, Song Q, Li Y, Zhang JJ. Comprehensive pan-cancer analysis of expression profiles and prognostic significance for NUMB and NUMBL in human tumors. Med (Baltimore). (2023) 102:e34717. doi: 10.1097/MD.0000000000034717
31. Misiorek JO, Przybyszewska-Podstawka A, Kałafut J, Paziewska B, Rolle K, Rivero-Müller A, et al. Context matters: NOTCH signatures and pathway in cancer progression and metastasis. Cells. (2021) 10:94. doi: 10.3390/cells10010094
32. Kudo SE, Ichimasa K, Villard B, Mori Y, Misawa M, Saito S, et al. Artificial intelligence system to determine risk of T1 colorectal cancer metastasis to lymph node. Gastroenterology. (2021) 160:1075–84. doi: 10.1053/j.gastro.2020.09.027
33. Hartwig MF, Slumstrup L, Fiehn AK, Gögenur I. The risk of lymph node metastasis in patients with T2 colon cancer. Colorectal Dis. (2023) 25:853–60. doi: 10.1111/codi.16485
34. Polack M, Hagenaars SC, Couwenberg A, Kool W, Tollenaar RAEM, Vogel WV, et al. Characteristics of tumour stroma in regional lymph node metastases in colorectal cancer patients: a theoretical framework for future diagnostic imaging with FAPI PET/CT. Clin Transl Oncol. (2022) 24:1776–84. doi: 10.1007/s12094-022-02832-9
35. Jepsen RK, Novotny GW, Klarskov LL. et al.Early metastatic colorectal cancers show increased tissue expression of miR-17/92 cluster members in the invasive tumor front. Hum Pathol. (2018) 80:231–8. doi: 10.1016/j.humpath.2018.05.027
36. Jiang W, Ouyang X, Jiang C, Yin L, Yao Q, Pei X, et al. A NOTCH1 mutation found in a newly established ovarian cancer cell line (FDOVL) promotes lymph node metastasis in ovarian cancer. Int J Mol Sci. (2023) 24:5091. doi: 10.3390/ijms24065091
37. Yang Y, Li L, He H, Shi M, He L, Liang S, et al. Numb inhibits migration and promotes proliferation of colon cancer cells via RhoA/ROCK signaling pathway repression. Exp Cell Res. (2022) 411:113004. doi: 10.1016/j.yexcr.2021.113004
Keywords: colon cancer tissue, metastatic lymph node tissue, tumor adjacent tissue, Notch-1 protein, Numb protein
Citation: Ma J, Zhen J, Yang N, Meng C and Lian Y (2024) Expression and clinical significance of Numb and Notch-1 proteins between tissue of colon cancer and regional lymph node metastases. Front. Oncol. 14:1467517. doi: 10.3389/fonc.2024.1467517
Received: 20 July 2024; Accepted: 28 October 2024;
Published: 03 December 2024.
Edited by:
Qingyu Luo, Dana–Farber Cancer Institute, United StatesReviewed by:
Armel Hervé Nwabo Kamdje, Université de Garou, CameroonHong Yu, The University of Texas Health Science Center at San Antonio, United States
Copyright © 2024 Ma, Zhen, Yang, Meng and Lian. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yanjun Lian, bGlhbmFsaWFuQDE2My5jb20=
 Jingyou Ma
Jingyou Ma Jinpeng Zhen
Jinpeng Zhen