
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Oncol., 08 January 2025
Sec. Gynecological Oncology
Volume 14 - 2024 | https://doi.org/10.3389/fonc.2024.1465987
This article is part of the Research TopicRecent Advancements and Developments in Targeted Drug Delivery Systems for Cancer Diagnosis and TherapyView all 4 articles
Background: Gynecological cancers are characterized by uncontrolled cell proliferation within the female reproductive organs. These cancers pose a significant threat to women’s health, impacting life expectancy, quality of life, and fertility. Nanoparticles, with their small size, large surface area, and high permeability, have become a key focus in targeted cancer therapy. The aim of this study is to review recent advancements in nanoparticles applied to gynecologic cancers, providing valuable insights for future research.
Methods: We retrieved all literature on nanoparticles in gynecologic cancers from the Web of Science Core Collection (WOSCC) database between January 1, 2004, and June 4, 2024. Data analysis and visualization were conducted using R software (version 4.4.0), VOSviewer (version 1.6.19.0), and CiteSpace (version 6.1).
Results: A total of 2,843 publications from January 1, 2004, to June 4, 2024 were searched. Over the past 20 years, there has been a significant increase in publications. The leading countries and institutions in terms of productivity are China and the Chinese Academy of Sciences. The most prolific author and the most co-cited author are Sood, A K and Siegel, Rl. The top journals are the International Journal of Nanomedicine (n=97), followed by ACS Applied Materials & Interfaces (n=72) and Journal of Materials Chemistry B (n=53). Keyword analysis shows current research focuses on two main areas: the application of nanoparticles for drug delivery and their broader applications in gynecologic cancers. Future research will likely focus on “silver nanoparticles,” “gold nanoparticles,” and “green synthesis.”
Conclusions: Over the past two decades, nanoparticles have rapidly advanced in the field of gynecologic cancers. Research has primarily focused on the applications of nanoparticles in drug delivery and applications. Future trends point toward optimizing synthesis techniques and advancing preclinical studies to clinical applications, particularly for silver and gold nanoparticles. These findings provide valuable scientific insights for researchers.
Gynecological cancers, characterized by uncontrolled cell growth in female reproductive organs, include five main types: cervical, ovarian, uterine, vaginal, and vulvar cancers (1). These malignancies pose significant threats to women’s health, impacting life expectancy, quality of life, and fertility (2). In 2024, statistics reported over 116,000 new cases of gynecological cancers (3). As one of the major cancers affecting women globally (4), gynecological malignancies not only have high incidence rates but also demand urgent improvement in prognosis (5–7). Current treatment modalities include surgery, chemotherapy, radiotherapy, and immunotherapy. Surgery is mainly applicable to early-stage solid tumors but may face risks of incomplete resection and potential tumor metastasis or implantation (8, 9). Chemotherapy is associated with cytotoxicity and low bioavailability, limiting its widespread application (10, 11). Immunotherapy has limited clinical applicability and is not applicable to tumor types with “immune suppression” or “immune exclusion.” (12).
In recent years, nanoparticles have shown potential to overcome limitations of traditional therapies and have rapidly progressed in biomedicine (13). Their advantages lie in their small size, large surface area, high permeability, and ability to effectively combine with various biomaterials. These characteristics give nanoparticles significant advantages in drug delivery and controlled release, making them increasingly favored in cancer treatment (14, 15). In the treatment of ovarian cancer, combining paclitaxel with other drugs in nanocarrier systems enables precise targeted delivery, reduces off-target toxicity, and effectively improves solubility issues (16). Furthermore, nanoparticles not only serve as drug carriers but also hold potential for direct anticancer applications. For instance, silica-coated gold (Au@SiO2) nanoparticles show promise in treating cervical cancer (17). Research has demonstrated that graphene oxide nanoparticles encapsulating chlorambucil can lower cellular toxicity and exhibit high drug loading efficiency and controlled release capabilities in treating cervical adenocarcinoma (18). Therefore, the application of nanoparticles in treating gynecological malignancies represents a frontier therapy with immense potential.
Bibliometrics is an academic discipline that employs statistical methods to provide an objective framework for tracking the evolution and structural composition of specific research fields (19, 20). It has been widely used to explore trends and hot topics across various publishing domains, including psychiatry, obstetrics, and gynecology (21, 22). To our knowledge, despite significant growth in publications related to nanoparticles in gynecologic cancers, there has been no bibliometric analysis conducted in this area. A bibliometric analysis of the application of nanoparticles in gynecologic malignancies can provide deeper insights into their role in the treatment of these cancers. This study undertook a bibliometric review and summary of literature from 2004 to 2024 pertaining to nanoparticles in gynecologic cancers. By exploring dimensions such as countries, institutions, journals, authors, and keywords associated with nanoparticles in gynecologic cancers, this analysis aims to offer valuable insights into potential gaps in the literature and guides future research directions, thereby contributing significantly to advancing the field.
On June 4, 2024, we conducted a comprehensive search in the Web of Science Core Collection (WOSCC) database. WOSCC, recognized as a premier academic information database, is distinguished by its selection of high-impact journals, which makes it particularly suitable for bibliometric analysis compared to other databases (23). In this study, we included only English-language publications categorized as “article” or “review”. Given the limited number of publications in the relevant field prior to 2004, the time span was set from January 1, 2004, to June 4, 2024. The specific literature selection process is depicted in Figure 1.
This study primarily utilized Microsoft Excel 2021, R software (version 4.4.0), VOSviewer (version 1.6.19.0), and CiteSpace (version 6.1) for visual analysis. The bibliometrix package in R software is specifically designed for bibliometric analysis (24). In this research, we utilized this package to create graphical representations of keyword counts, showcasing visual maps of hotspot development trends. VOSviewer utilizes probabilistic data standardization methods for visualizing data and constructing various network connections (25, 26). In this study, we used VOSviewer to analyze associations among countries, institutions, authors, references, and co-occurrence of keywords. CiteSpace, a robust exploration tool widely used in data visualization, employs standardized data aggregation and burst detection methods to track emerging research trends and future directions (27). In this manuscript, we employed CiteSpace to analyze centrality data of countries and institutions, overlapped journal co-citation networks, and conducted burst detection analysis of references and keywords, generating visualizations accordingly.
The data utilized in our study were obtained from publicly accessible databases containing published articles. As the study did not involve animal or human subjects, ethical approval from a committee was deemed unnecessary.
From January 1, 2004, to June 4, 2024, a total of 2,843 publications related to nanoparticles in gynecologic cancers were identified in the WOSCC database based on the search criteria. Among these, 2,661 articles (93.6%) were research articles and 182 articles (6.4%) were reviews. As shown in Figure 2, the number of publications steadily increased from 2004 to 2020. Although there was a slight decrease after 2020, the overall trend over the past 20 years has been upward. Moreover, from 2021 to 2023, the annual number of publications remained around 290, indicating sustained attention to nanoparticles in the field of gynecologic cancers research. Furthermore, there has been a notable increase in citations, rising from 4 in 2004 to 13,090 in 2023. This increase in citations further underscores the significant scientific impact and relevance of findings from this research.
Over the past 20 years, research on nanoparticles in gynecologic cancers has been conducted in 76 countries and regions. Table 1 lists the top 10 countries/regions by publication count. China leads with 1,042 publications, followed by the United States (n = 624) and India (n = 399). Figure 3A depicts the global distribution of publications in this field, showing concentrations in North America, Europe, and Asia. The collaboration network visualization for countries/regions, as shown in Figure 3B, depicts total link strength (TLS), which reflects the intensity of collaboration or co-occurrence between nodes. The width of the connecting lines indicates the levels of international collaboration. According to Table 1, the top three countries ranked by TLS are the United States, China, and India. Centrality, which assesses the importance of nodes within a network, exceeds 0.1, indicating pivotal nodes with significant influence (28). Notably, despite ranking second in publication count, the United States exhibits the highest TLS and centrality, underscoring its predominant influence in this field (Table 1).
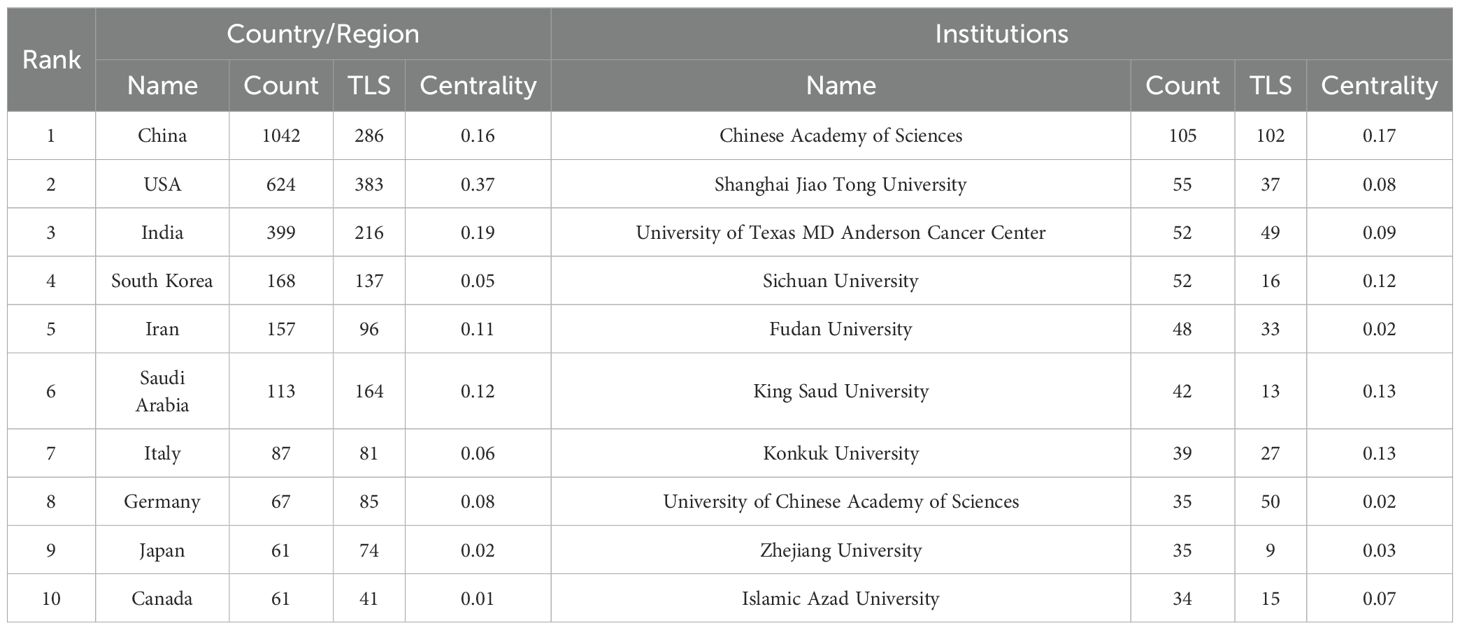
Table 1. Top 10 countries/regions and institutions in the field of nanoparticles in gynecologic cancers.
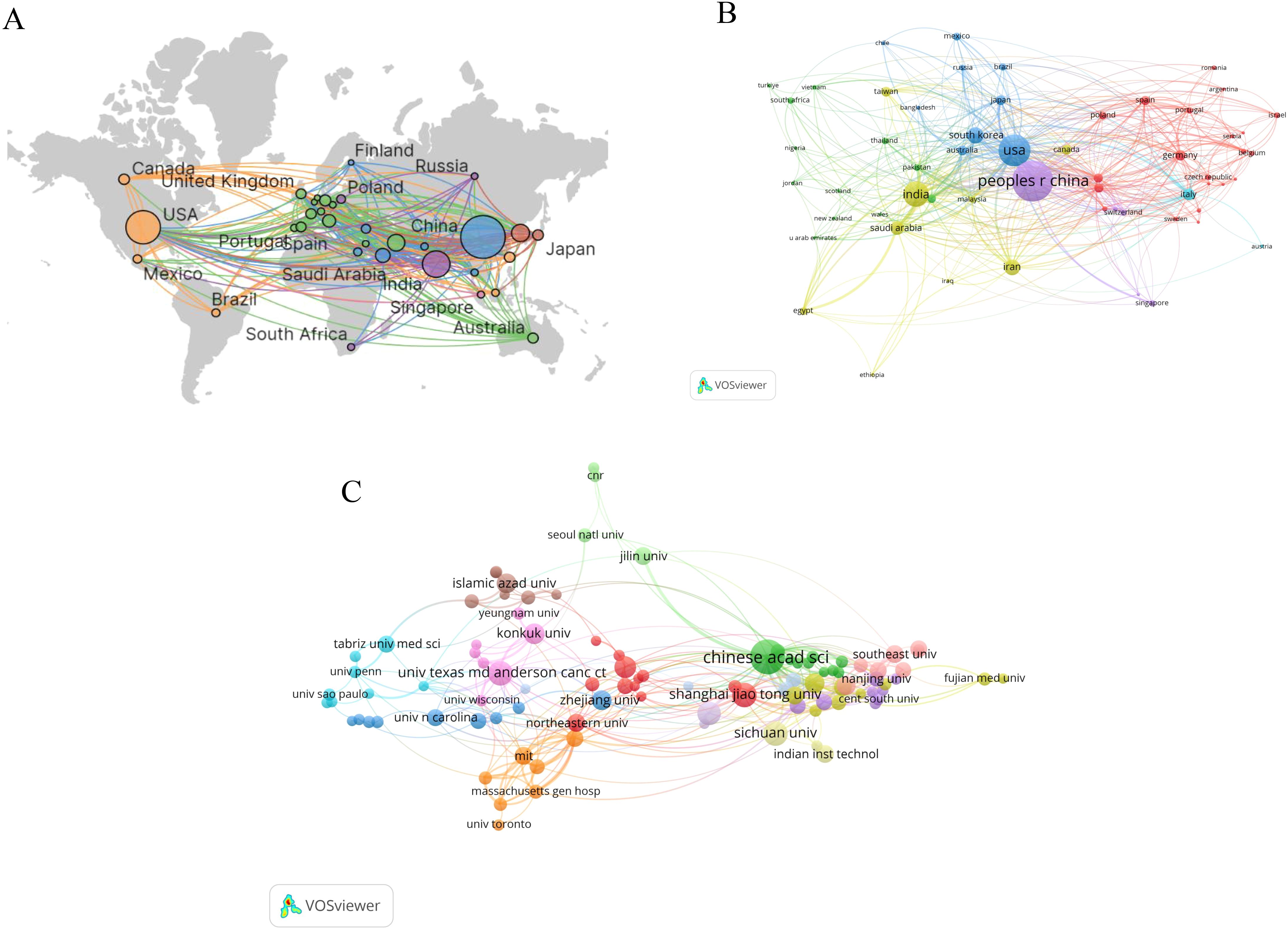
Figure 3. Visual map of countries/regions and institutions: (A) Geographic distribution map based on publication counts (B) Collaboration network visualization for countries/regions (C) Institutions with 10 or more publications.
With regard to institutions, Figure 3C shows the institutions with 10 or more publications. The top publishing institutions include the Chinese Academy of Sciences (n=105), Shanghai Jiao Tong University (n=55), University of Texas MD Anderson Cancer Center (n=52) and Sichuan University (n=52) (Table 1). Of significance, the Chinese Academy of Sciences not only leads in publication quantity but also demonstrates the highest TLS and centrality, highlighting its central role in this research landscape.
Over the past 20 years, 637 journals have published 2,843 articles on nanoparticles in gynecologic cancers. Among these, 15 journals have published 30 or more articles. Table 2 shows that the International Journal of Nanomedicine has the highest number of publications (n=97), followed by ACS Applied Materials & Interfaces (n=72) and Journal of Materials Chemistry B (n=53). The International Journal of Nanomedicine covers various aspects of nanotechnology applications in biomedicine, focusing on the potential clinical applications of nanoparticles in disease diagnosis, prevention, and treatment. In terms of impact factor, ACS Nano (n=32, IF=17.1) ranks highest among these journals. ACS Nano, as one of the top journals in the field of nanoscience, highlights research achievements in nanoscience and nanotechnology. The journal is interdisciplinary, spanning fields such as chemistry, physics, biology, and engineering.
Figure 4 presents a dual-map overlay visualization of academic journals, illustrating the relationships between journals. Citing journals are shown on the left side, and the cited journals are shown on the right side. Research published in journals focusing on chemistry/materials/physics and molecular/biology/genetics is frequently cited by journals focusing on physics/materials/chemistry and molecular/biology/immunology.
A total of 15,227 researchers have contributed articles in the field of nanoparticles in gynecologic cancers. Sood, A K has published the highest number of articles (n=30), followed by Lopez-Berestein G (n=22) and Steinmetz, NF (n=16). The collaboration network map among authors indicates close cooperation among them (Figure 5A). Co-cited authors are authors cited together in academic research, forming co-citation relationships (29). Figure 5B displays the network visualization map of co-cited authors. Siegel, RL ranks first in citation count (n=221), followed by Zhang, Y (n=213) and Gurunathan, S (n=191). Table 3 outlines the citation counts for the top 10 co-cited authors, totaling over 1,700 citations, highlighting their significant influence in the field of nanoparticles in gynecologic cancers. It is noteworthy that Gurunathan, S is among the top ten authors in the current field and is also one of the top ten co-cited authors. In recent years, this author has made significant contributions to the biomedical application of nanoparticles such as graphene and silver nanoparticles (30–32). Furthermore, the author has delved deeply into the biological functions of exosomes, particularly their potential as delivery vehicles in cancer therapy and as emerging nanoplatforms in biomedical applications (33).
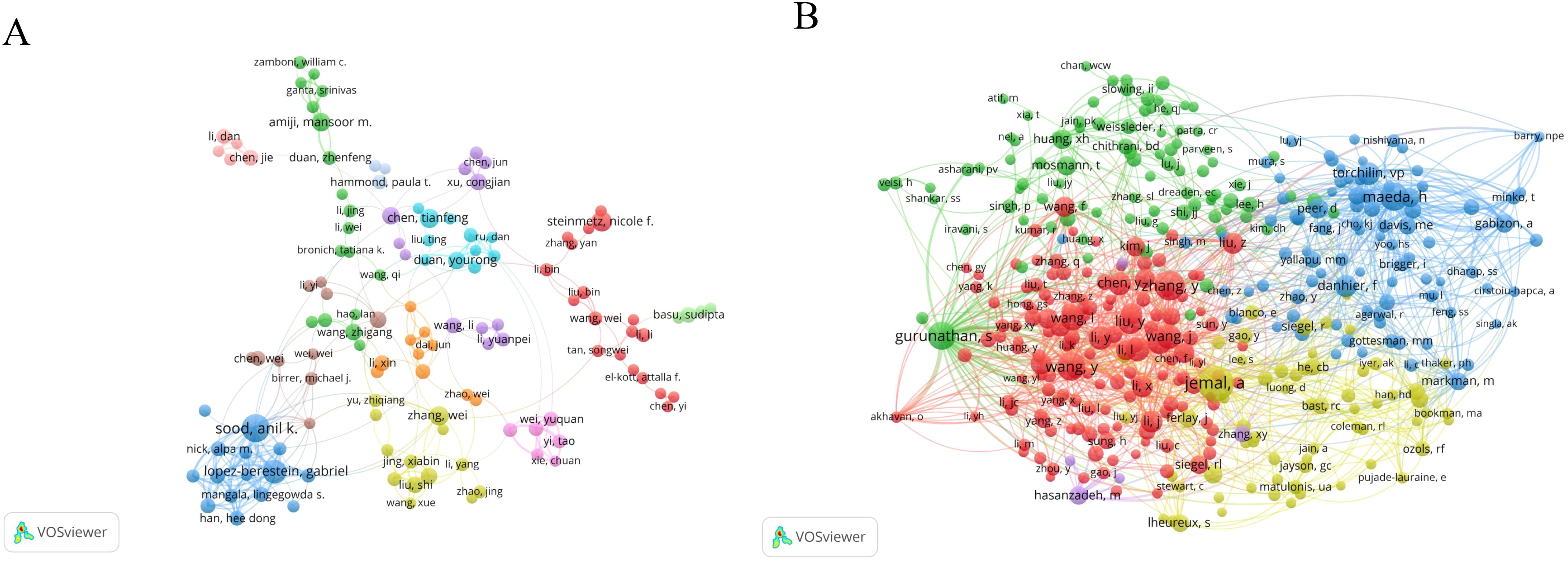
Figure 5. The network visualization map of authors: (A) Visualization analysis graph of author collaboration network; (B) The network visualization map of co-cited authors.
Table 4 outlines the top 10 co-cited references. These articles generally fall into three main themes: (1) cancer statistics, (2) nanoparticles in cancer therapy, and (3) research on cancer treatment methods and mechanisms. The two most co-cited articles are both from the prestigious journal “CA: A Cancer Journal for Clinicians,” focusing on global cancer statistics. The third most co-cited paper, published in Nature Nanotechnology, details a repository of nano-carriers and molecules for selective tumor targeting, emphasizing challenges in cancer treatment (34).
Figure 6A displays the co-citation network of references with 20 or more citations, divided into 5 clusters. Cluster 1 (red) primarily discusses nanotechnology applications in cancer therapy. Cluster 2 (green) focuses on the mechanisms of nanoparticle action at the cellular level. Cluster 3 (blue) centers on tumor data statistics. Cluster 4 (yellow) emphasizes drug delivery systems. Cluster 5 (purple) addresses challenges in clinical applications of nanomedicine. Figure 6B depicts a density visualization of references co-cited 20 times or more, with colors closer to yellow indicating higher cited volumes. It is evident that cluster1 (red) and cluster3 (blue) contribute significantly to the cited volumes.
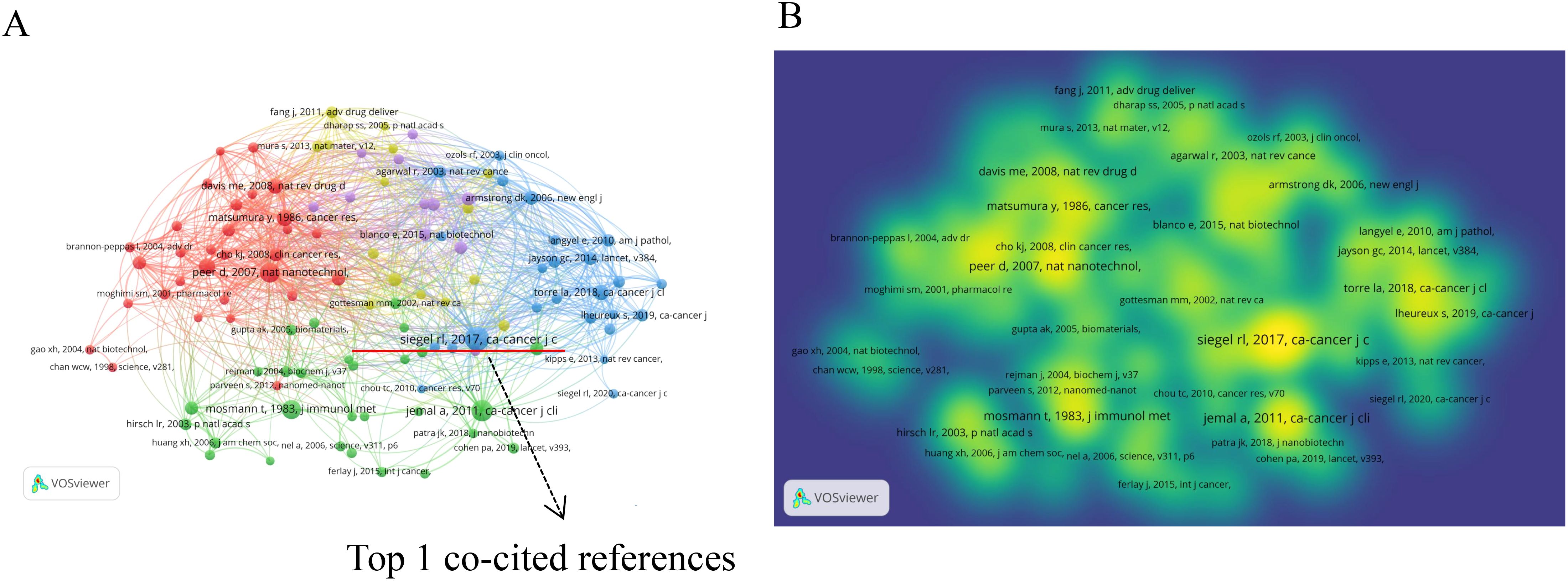
Figure 6. The network visualization map of references: (A) Co-citation network of references with 20 or more citations. (B) Density visualization of references co-cited 20 times or more.
Table 5 illustrates the top 25 references with the strongest citation bursts, with blue lines indicating the timeline and red segments representing burst intervals. Notably, 12 articles have experienced recent bursts, potentially indicating future trends in the field of nanoparticles in gynecologic cancers. Five articles focus on cancer data statistics, while four provide comprehensive reviews of two prominent types of gynecologic malignancies—ovarian and cervical cancers. The remaining three articles concentrate on cutting-edge therapeutic approaches for these diseases.
In the field of nanoparticles research related to gynecologic malignancies, Table 6 present the frequency distribution of the most commonly occurring 10 keywords. Among the top 10 keywords, excluding those directly related to the search terms, current popular topics include drug delivery, cells, therapy, apoptosis, expression, and release. Figure 7A presents the keyword co-occurrence visualization map with keywords appearing 10 times or more, comprising 484 nodes and 25,920 links. The size of each node representing a keyword is directly proportional to its frequency of occurrence. The graph can be divided into four major clusters based on different colors. The red cluster represents common gynecologic malignancies and treatment modalities, the green cluster includes commonly used nanoparticles in gynecologic malignancies, the blue cluster focuses on drug delivery systems, and the yellow cluster primarily discusses clinical mechanisms of nanoparticle action at the cellular level. Figure 7B displays an overlay visualization where keywords closer to yellow indicate recent significant impact, suggesting heightened attention on “silver nanoparticles”, “green synthesis” and “antibacterial” in recent years.
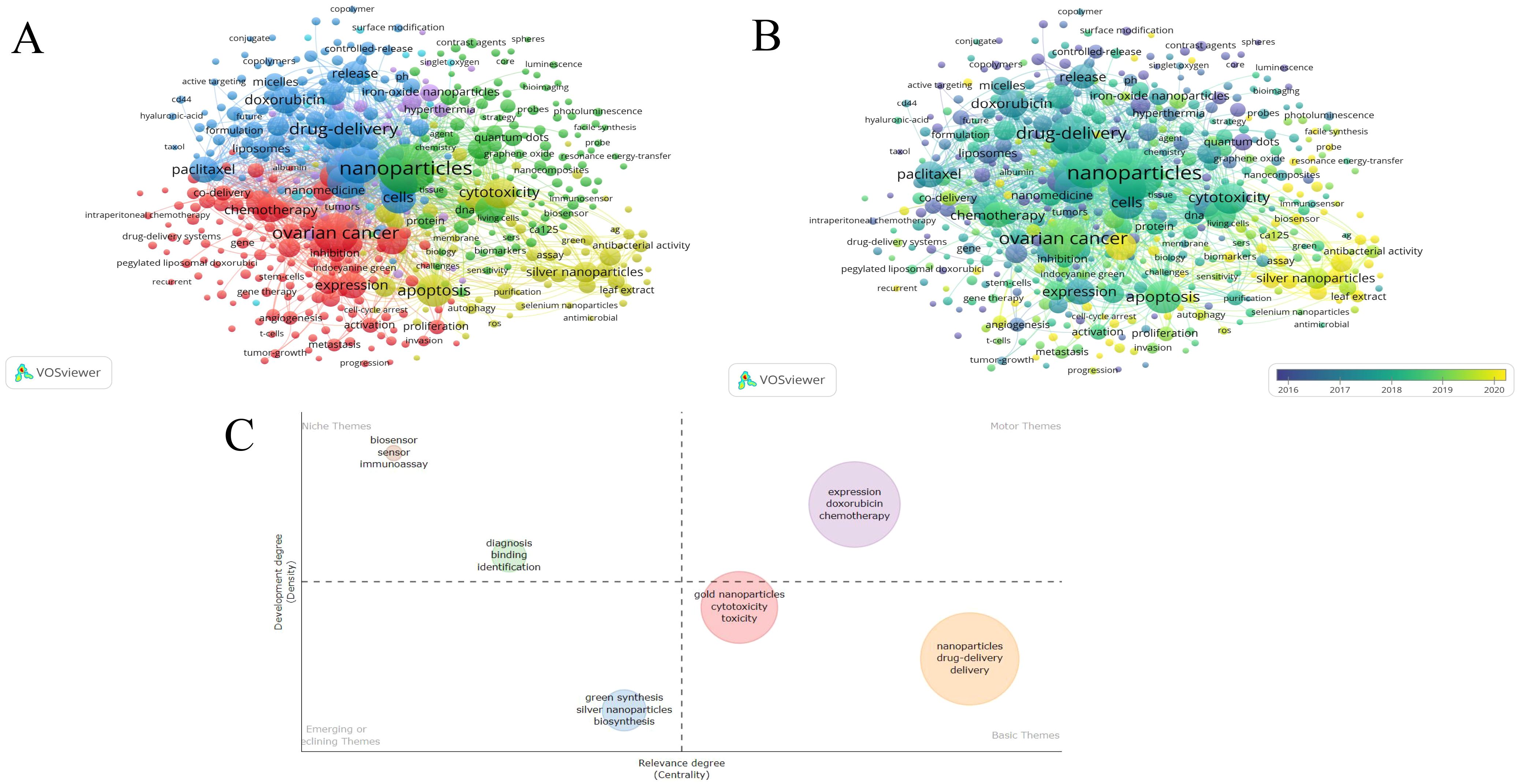
Figure 7. The network visualization map of keywords in the field of nanoparticles in gynecologic cancers: (A) Network visualization of keywords appearing 10 times or more. (B) Overlay visualization of keywords appearing 10 times or more. (C) Development trend and importance of hot spots.
In addition to current and past keywords, future potential hotspots and development trends are analyzed in Figure 7C. The first quadrant signifies crucial and well-developed issues, the second quadrant indicates well-developed but currently less significant topics, the third quadrant represents emerging or declining topics, and the fourth quadrant denotes important but less developed issues in the field. The first quadrant includes the keywords expression, doxorubicin, and chemotherapy, which signify advancements in nanotherapy offering new avenues for refining traditional chemotherapy methods. In the fourth quadrant, keywords such as gold nanoparticles, cytotoxicity, toxicity, nanoparticles, drug-delivery, and delivery underscore the application of nanoparticles in drug delivery, while emphasizing the critical need for further exploration in related cytotoxicity and toxicity assessment studies.
Table 7 illustrates the top 25 keywords with the strongest citation bursts. Keywords such as “antioxidant,” “cervical cancer,” “endometrial cancer,” “antibacterial,” “epithelial ovarian cancer,” “green synthesis,” “gold nanoparticles,” and “cisplatin resistance” have garnered significant attention in recent years and are likely to continue to attract interest. This highlights ongoing interests and signifies potential trends in the field of nanoparticles in gynecologic malignancies.
Publications are crucial indicators for assessing the academic vitality and developmental trends of research fields, providing an objective reflection of research dynamics in specific domains (35). Figure 2 shows there has been a significant increase in publications related to nanoparticles in gynecologic malignancies over the past two decades. Approximately 85.72% (n=2437) of these publications are concentrated in the last decade. Notably, while the United States ranks second in publication volume, accounting for only about 60% of China’s output, its centrality is more than twice that of China’s, indicating substantial influence in the field. This phenomenon can be attributed to factors such as the high burden of gynecologic malignancies in the United States and robust healthcare investments (36, 37). The United States is a global leader in the field of nanomedicine, accounting for 46% of the global market share in 2016 (38). In 2018, the National Institutes of Health (NIH) invested an estimated $445 million in nanomedicine research (39). This substantial financial investment has provided a solid foundation for the development of nanomedicines, particularly in the area of anticancer therapies. While nanotherapy may offer an efficient treatment option, the high research and production costs may restrict its widespread use in resource-limited countries. Developing countries could reduce production and supply costs for nanotherapy through supportive policies, such as tax incentives and patent protection adjustments. Furthermore, establishing international collaboration platforms to share key technologies and research outcomes can help lower research costs.
What’s more, the Chinese Academy of Sciences not only leads in terms of publication volume but also stands out as the institution with the greatest influence. Although it collaborates with institutions such as the Massachusetts Institute of Technology, University of California, Los Angeles, and Dana-Farber Cancer Institute, most collaborations remain within China, including Shanghai Jiao Tong University, Sichuan University, Fudan University, and Zhejiang University. Considering the serious health threat posed by gynecologic malignancies and the tremendous potential of nanoparticles in treating these cancers, strengthening international collaborations among institutions is imperative to advance research in this field.
A total of 637 academic journals have published research articles on nanoparticles in gynecologic malignancies, with the top 15 journals accounting for 23.29% of the publication output. The International Journal of Nanomedicine leads in publication volume; however, there remains a shortage of high-impact factor journals in this field. Sood, A K and Siegel, Rl are respectively the most prolific and most cited authors in this domain. Sood, A K’s research includes liposomal nanoparticles for delivering small interfering RNAs(siRNA) in cancer therapy and the application of RNA-targeted therapy in ovarian cancer treatment. Liposomal nanoparticles enhance stability and bioavailability, effectively delivering siRNA to tumor tissues for RNA-targeted therapy, which plays a crucial role in treating gynecologic malignancies (40, 41). And Siegel RL has made outstanding contributions to cancer statistics (42).
Table 5 lists the top 25 references with the strongest citation bursts. The three articles with the highest burst strengths are all from the prestigious journal CA: A Cancer Journal for Clinicians. The strongest burst is attributed to the publication by Sung H et al. on the 2020 global cancer statistics (43), followed by the research on the evolving management of epithelial ovarian cancer by Lheureux S et al. (44), and the relevant study on cancer data statistics from 2009 by Jemal A et al (45). Excluding literature solely focused on the current status of gynecologic tumors and considering both the strength and time of burst, two articles deserve particular attention. The first article, authored by Patra JK et al., comprehensively reviews the discovery and application of nanoparticles, emphasizing their crucial roles in cancer therapy and diagnosis. For instance, oleic acid-coated iron oxide nanoparticles can be employed for cancer diagnosis using near-infrared, while hyaluronic acid-coated iron oxide can be used for cancer treatment (46). The second article, authored by Barani M et al., focuses on nano-scale drug delivery systems in ovarian cancer treatment, providing robust scientific evidence for targeted therapy in ovarian cancer. The utilization of nanobiosensors to evaluate biomarkers has illuminated a novel path for guiding treatment in ovarian cancer patients (47).
Keywords condense the essence and core of a paper, representing the central themes and topics of research. Analyzing keyword frequency and burst detection can reflect research hotspots and future trends in a field. Based on keyword clustering, we elaborated on the following two main aspects in detail:
(1) Nanoparticles for drug delivery
Targeted drug delivery systems generally refer to the use of nanomedicine carriers, such as nanoparticles, administered via routes like vascular injection, to deliver drugs to specific sites of disease (48). Nanocarriers demonstrate potential in delivering various anticancer drugs and enhancing treatment efficacy (49), achieving active targeting from passive delivery, prolonging drug circulation in the body, and effectively eliminating cancer cells (50). Currently, common nanocarriers used for drug delivery in gynecologic malignancies include liposome nanoparticles, polymer nanoparticles, and inorganic nanoparticles. Ledezma-Gallegos et al. developed liposomal nanoparticles co-encapsulating cisplatin and mifepristone, demonstrating significant cytotoxic effects against cervical cancer cells, thereby enhancing chemotherapy efficacy (51). Wang et al. also found that liposomes loaded with miRNA-1284 and cisplatin enhance the treatment of cervical cancer (52). Li et al. improved the tumor microenvironment and enhanced the efficacy of drugs against gynecologic malignancies using cancer cell membrane-modified nanoparticles loaded with dexamethasone (53). Although research confirms that liposome nanoparticles can enhance the treatment of gynecologic cancers, either directly or indirectly, their effectiveness in encapsulating highly hydrophobic drugs, such as paclitaxel commonly used in ovarian cancer treatment, is limited. This limitation arises because the liposomal structure resembles that of biofilms, which complicates drug retention within the liposomes (54–56). Furthermore, Clinical translation still faces numerous challenges, such as low drug-loading capacity and high uptake rates in the liver and spleen, which may affect treatment safety (56, 57). Polymer nanoparticles exhibit characteristics such as water solubility, excellent stability, and biocompatibility. Researchers like Wang demonstrated the significance of folate-modified, pH-sensitive polymer nanoparticles co-delivering carboplatin and paclitaxel in both in vitro and in vivo models for treating cervical cancer (58). However, several unresolved challenges remain. First, the synthesis of polymer nanoparticles is complex and costly, potentially hindering large-scale clinical applications. Moreover, further investigation is required to ensure that polymer nanoparticles remain stable in vivo, evade immune recognition, and minimize potential toxicity (59, 60). Inorganic nanoparticles mainly include gold, silver, oxides, etc. Due to their magnetic, optoelectronic, antimicrobial, and plasmonic properties, they offer unique advantages in diagnostics, lymph node imaging, fluorescence tracking, etc (61, 62). For instance, silver nanoparticle-modified amine-functionalized mesoporous silica nanoparticles show significant potential in delivering doxorubicin for cervical cancer treatment (49). However, clinical application is limited due to inherent toxicity of some inorganic nanoparticles (63). Given the distinct advantages and drawbacks of different types of drug-delivery nanocarriers, the number of nano-drugs applied clinically for treating gynecologic malignancies remains limited. For example, Food and Drug Administration (FDA) approval of Doxil (liposomal doxorubicin) widely used for treating metastatic ovarian cancer represents a major breakthrough in the field. In summary, nanomedicine carriers for treating gynecologic cancers, including liposomes, polymers, and inorganic nanoparticles, hold immense potential and face significant challenges in enhancing drug delivery efficiency and treatment efficacy. Currently, their clinical application remains limited. Enhanced interdisciplinary collaboration and the accumulation of clinical data will help to more comprehensively evaluate the actual effectiveness of nanodrug carriers in treating gynecologic cancers.
(2) Nanoparticles in the research of gynecologic cancers
Through keyword clustering analysis, we found that magnetic, iron-oxide, silver, and gold nanoparticles are frequently mentioned in the context of gynecological cancers. Taking ovarian cancer, which has the highest mortality and poorest prognosis among gynecological malignancies, as an example, early-stage detection is challenging due to the absence of specific clinical symptoms and sensitive biomarkers. Traditional tissue biopsy is considered the gold standard for diagnosis, but it can lead to cancer cell dissemination into the peritoneum during early ovarian cancer tissue sampling (64, 65). Nanoparticles demonstrate promising performance in these aspects. For instance, Pan et al. developed a simple and cost-effective method using folate-modified fluorescent magnetic nanoparticles to capture and identify circulating tumor cells in ovarian cancer patients, aiding in metastasis diagnosis (66). Similarly, Liu et al. utilized folic acid conjugated magnetic iron oxide nanoparticles to bind to overexpressed folate receptor and thus stably attach to the surface of ovarian cancer cells, rendering the cells magnetic and thus enabling the possibility of early detection of metastatic ovarian cancer cells (67). In addition to the aforementioned nanoparticles, silver and gold nanoparticles also play crucial roles. These nanoparticles not only serve as popular keywords but also represent future research trends and hotspots (see Figure 7C, Table 7). In vitro experiments demonstrate that triangular silver nanoparticles significantly inhibit ovarian cancer cell proliferation and growth, suggesting their potential as a future therapeutic approach (68). Silver nanoparticles exert cytotoxic effects by activating the p53 gene, thereby suppressing cancer cell growth. Interactions between gemcitabine and silver nanoparticles exhibit cytotoxicity in ovarian cancer cells (69, 70). Curcumin-coated silver nanoparticles act as sensitizers for cisplatin chemotherapy, potentially reducing side effects and enhancing efficacy (70, 71). Gold nanoparticles, similar to silver nanoparticles, enhance sensitivity of ovarian cancer cells to cisplatin (72). Upon binding with cisplatin, gold nanoparticles not only augment cytotoxic effects against tumor cells but also mitigate toxicity towards normal cells (73). Apart from enhancing cisplatin’s functionality, gold nanoparticles arrest cancer cells in the radiosensitive cell cycle phase (G2/M), further sensitizing them to radiation therapy (74, 75).
Beyond ovarian cancer, superparamagnetic iron oxide nanoparticles demonstrate extensive potential in the treatment of other gynecological malignancies such as cervical, endometrial, and vulvar cancers. This novel magnetic resonance lymphography technique utilizes iron oxide nanoparticles, avoiding the use of radioactive isotopes, and enables detection by magnetic resonance imaging devices following lymphatic transport to lymph nodes. However, further clinical application and research are needed to fully develop this technology (76–78). Additionally, silver nanoparticles enhance camptothecin’s therapeutic efficacy against cervical cancer cells compared to monotherapy (79). L-histidine-terminated silver nanoparticles also show promise as a potential effective treatment for cervical cancer (80). Furthermore, silver nanoparticles bind with DNA for detection purposes in gynecological malignancies (81). Gold nanoparticles not only directly induce BAX gene expression to induce apoptosis in cervical cancer cells but also form complexes with antibodies as Au@SiO2 nanoparticles for potential therapeutic applications in cervical cancer (17, 82). Moreover, gold nanoparticles act as sensitizers in radiotherapy and ultrasound irradiation, exhibiting synergistic effects (83). Therefore, nanoparticles such as iron oxide, silver, and gold nanoparticles demonstrate multifaceted clinical applications in gynecological malignancies.
Overall, silver and gold nanoparticles show diverse clinical potential in the treatment of gynecologic malignancies. However, most studies remain confined to laboratory and in vitro research, with insufficient clinical trial data to fully support their safety and efficacy. Challenges to broader application include their in vivo stability, potential toxicity, and possible immune responses (84). Balancing their anticancer effects with potential side effects to facilitate safer and earlier clinical use remains a pressing issue.
Based on emerging keyword trends (see Table 7) and a hotspot analysis (Figure 7C), this study outlines three future trends in nanoparticles for gynecologic cancers as follows:
(1) Silver and gold nanoparticles remain pivotal in future research on nanoparticles in gynecologic cancers. These nanoparticles not only serve as sensitizers for radiotherapy and chemotherapy but also, due to their unique biological properties, exhibit effectiveness in improving wound healing processes post-surgery, with activities against bacteria, fungi, viruses, and parasites (85, 86). However, silver and gold nanoparticles may also exert toxicity on normal cells, particularly hepatocytes. Therefore, further research on these nanoparticles is essential.
(2) Transitioning nanoparticles from preclinical stages to clinical application remains a crucial challenge. Although silver and gold nanoparticles have shown promising results in both animal and cell experiments (87, 88), they have not yet been approved by the U.S. Food and Drug Administration for the treatment of gynecologic malignancies. Currently, the types of nanoparticles used in clinical trials are limited, with most studies focusing on approved products. Research on nanoparticles for clinical trials remains insufficient and requires further advancement (89). Future researches need further bridge the gap between the theoretical potential and practical clinical application of nanoparticles to facilitate their viability as treatments for gynecologic cancer.
(3) Green synthesis represents a crucial approach for synthesizing nanoparticles in the future. It is a method that uses natural resources and eco-friendly approaches to produce materials, aiming to minimize the impact of toxic chemicals. Compared to traditional methods, green synthesis utilizes the reducing potential of compounds found in biological organisms, offering advantages such as environmental friendliness, sustainability, and minimal toxicity of by-products (90). However, current green synthesis methods often operate under mild conditions and require further optimization regarding pH, temperature, and other chemical parameters (91). Moreover, green synthesis of nanomedicines still faces challenges in terms of reproducibility, relatively high costs, product standardization, and scalability (92). Therefore, further research is needed to optimize cost-effective methods for the green synthesis of nanomaterials.
Several limitations exist in this study. Firstly, we exclusively relied on the WoSCC database for our search. While WoSCC is commonly used for bibliometric analysis, it may not encompass relevant literature from other databases, which could lead to the omission of some pertinent studies. Secondly, our study only considered English-language articles, potentially overlooking non-English publications and thereby potentially reducing credibility. Lastly, articles included in our study spanned from January 1, 2004, to June 4, 2024, and the WoSCC database is continuously updated, suggesting that recently published literature may be underestimated.
This study provides a comprehensive and objective bibliometric analysis of the application of nanoparticles in the field of gynecologic malignancies. The results indicate that the application of nanoparticles in this area has broad prospects and is developing rapidly. In terms of the number of publications, China and the Chinese Academy of Sciences are leading, while the United States dominates in academic influence. Future research, whether at the individual, institutional, or national level, should strengthen international exchange and collaboration to promote scientific progress and technological innovation in this field. Meanwhile, it is found that silver nanoparticles and gold nanoparticles show great potential in therapy, while green synthesis has received high attention as a sustainable preparation method for nanoparticles. Research hotspots focus on the drug delivery and diagnostic applications of nanoparticles, with future trends pointing toward the optimization of synthesis technologies and the translation of preclinical research into clinical applications. Strengthening international cooperation and standardizing technical protocols will help accelerate the clinical application of nanotechnology in the treatment of gynecologic cancers.
The original contributions presented in the study are included in the article. Further inquiries can be directed to the corresponding authors.
YZ: Conceptualization, Data curation, Methodology, Writing – original draft. LC: Data curation, Supervision, Writing – review & editing. TW: Conceptualization, Supervision, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. CDC. Gynecologic Cancers Basics, in: Gynecologic cancers (2024). Available online at: https://www.cdc.gov/gynecologic-cancer/about/index.html (Accessed June 11, 2024).
2. Casey C, Chen LM, Rabow MW. Symptom management in gynecologic Malignancies. Expert Rev Anticancer Ther. (2011) 11:1077–89. doi: 10.1586/era.11.83
3. Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. CA Cancer J Clin. (2024) 74:12–49. doi: 10.3322/caac.21820
4. Suneja G, Viswanathan A. Gynecologic Malignancies. Hematol Oncol Clin North Am. (2020) 34:71–89. doi: 10.1016/j.hoc.2019.08.018
5. Yun BS, Park EH, Ha J, Lee JY, Lee KH, Lee TS, et al. Incidence and survival of gynecologic cancer including cervical, uterine, ovarian, vaginal, vulvar cancer and gestational trophoblastic neoplasia in Korea, 1999–2019: Korea Central Cancer Registry. Obstet Gynecol Sci. (2023) 66:545–61. doi: 10.5468/ogs.23208
6. Jelovac D, Armstrong DK. Recent progress in the diagnosis and treatment of ovarian cancer. CA Cancer J Clin. (2011) 61:183–203. doi: 10.3322/caac.20113
7. Lee YC, Lheureux S, Oza AM. Treatment strategies for endometrial cancer: current practice and perspective. Curr Opin Obstet Gynecol. (2017) 29:47–58. doi: 10.1097/GCO.0000000000000338
8. Surgery for cancer - NCI (2015). Available online at: https://www.cancer.gov/about-cancer/treatment/types/surgery (Accessed November 7, 2024).
9. Chen Z, Zhang P, Xu Y, Yan J, Liu Z, Lau WB, et al. Surgical stress and cancer progression: the twisted tango. Mol Cancer. (2019) 18:132. doi: 10.1186/s12943-019-1058-3
10. Akbarali HI, Muchhala KH, Jessup DK, Cheatham S. Chemotherapy induced gastrointestinal toxicities. Adv Cancer Res. (2022) 155:131–66. doi: 10.1016/bs.acr.2022.02.007
11. Pei Z, Chen S, Ding L, Liu J, Cui X, Li F, et al. Current perspectives and trend of nanomedicine in cancer: A review and bibliometric analysis. J Control Release. (2022) 352:211–41. doi: 10.1016/j.jconrel.2022.10.023
12. Tan S, Li D, Zhu X. Cancer immunotherapy: Pros, cons and beyond. BioMed Pharmacother. (2020) 124:109821. doi: 10.1016/j.biopha.2020.109821
13. Jiang Y, Pu K. Multimodal biophotonics of semiconducting polymer nanoparticles. Acc Chem Res. (2018) 51:1840–9. doi: 10.1021/acs.accounts.8b00242
14. Rai A, Noor S, Ahmad SI, Alajmi MF, Hussain A, Abbas H, et al. Recent advances and implication of bioengineered nanomaterials in cancer theranostics. Medicina (Kaunas). (2021) 57:91. doi: 10.3390/medicina57020091
15. Chen C, Wang S, Li L, Wang P, Chen C, Sun Z, et al. Bacterial magnetic nanoparticles for photothermal therapy of cancer under the guidance of MRI. Biomaterials. (2016) 104:352–60. doi: 10.1016/j.biomaterials.2016.07.030
16. Khalifa AM, Elsheikh MA, Khalifa AM, Elnaggar YSR. Current strategies for different paclitaxel-loaded Nano-delivery Systems towards therapeutic applications for ovarian carcinoma: A review article. J Control Release. (2019) 311–312:125–37. doi: 10.1016/j.jconrel.2019.08.034
17. Yu H, Zheng R, Lei F, Wang W, Guo W, Zhang L, et al. Antibody-conjugated silica-coated gold nanoparticles in targeted therapy of cervical cancer. Am J Transl Res. (2022) 14:1518–34.
18. Kumari S, Nehra A, Gupta K, Puri A, Kumar V, Singh KP, et al. Chlorambucil-loaded graphene-oxide-based nano-vesicles for cancer therapy. Pharmaceutics. (2023) 15:649. doi: 10.3390/pharmaceutics15020649
19. Ellegaard O, Wallin JA. The bibliometric analysis of scholarly production: How great is the impact? Scientometrics. (2015) 105:1809–31. doi: 10.1007/s11192-015-1645-z
20. Moed HF. New developments in the use of citation analysis in research evaluation. Arch Immunol Ther Exp (Warsz). (2009) 57:13–8. doi: 10.1007/s00005-009-0001-5
21. Wilson M, Sampson M, Barrowman N, Doja A. Bibliometric analysis of neurology articles published in general medicine journals. JAMA Netw Open. (2021) 4:e215840. doi: 10.1001/jamanetworkopen.2021.5840
22. Brandt JS, Hadaya O, Schuster M, Rosen T, Sauer MV, Ananth CV. A bibliometric analysis of top-cited journal articles in obstetrics and gynecology. JAMA Netw Open. (2019) 2:e1918007. doi: 10.1001/jamanetworkopen.2019.18007
23. Li K, Rollins J, Yan E. Web of Science use in published research and review papers 1997–2017: a selective, dynamic, cross-domain, content-based analysis. Scientometrics. (2018) 115:1–20. doi: 10.1007/s11192-017-2622-5
24. Campra M, Riva P, Oricchio G, Brescia V. Bibliometrix analysis of medical tourism. Health Serv Manage Res. (2022) 35:172–88. doi: 10.1177/09514848211011738
25. Ding X, Yang Z. Knowledge mapping of platform research: a visual analysis using VOSviewer and CiteSpace. Electron Commer Res. (2022) 22:787–809. doi: 10.1007/s10660-020-09410-7
26. Van Eck NJ, Waltman L. Software survey: VOSviewer, a computer program for bibliometric mapping. Scientometrics. (2010) 84:523–38. doi: 10.1007/s11192-009-0146-3
27. Chen C. CiteSpace II: Detecting and visualizing emerging trends and transient patterns in scientific literature. J Am Soc Inf Sci. (2006) 57:359–77. doi: 10.1002/asi.20317
28. Mu J, Zhong H, Zeng D, Fan J, Jiang M, Liu M, et al. Research trends and hotspots in the relationship between outdoor activities and myopia: A bibliometric analysis based on the web of science database from 2006 to 2021. Front Public Health. (2022) 10:1047116. doi: 10.3389/fpubh.2022.1047116
29. Zhang J, Song L, Xu L, Fan Y, Wang T, Tian W, et al. Knowledge domain and emerging trends in ferroptosis research: A bibliometric and knowledge-map analysis. Front Oncol. (2021) 11:686726. doi: 10.3389/fonc.2021.686726
30. Gurunathan S, Kim JH. Synthesis, toxicity, biocompatibility, and biomedical applications of graphene and graphene-related materials. Int J Nanomedicine. (2016) 11:1927–45. doi: 10.2147/IJN.S105264
31. Zhang XF, Liu ZG, Shen W, Gurunathan S. Silver nanoparticles: synthesis, characterization, properties, applications, and therapeutic approaches. Int J Mol Sci. (2016) 17:1534. doi: 10.3390/ijms17091534
32. Zhang XF, Shen W, Gurunathan S. Silver nanoparticle-mediated cellular responses in various cell lines: an in vitro model. Int J Mol Sci. (2016) 17:1603. doi: 10.3390/ijms17101603
33. Gurunathan S, Kang MH, Kim JH. A comprehensive review on factors influences biogenesis, functions, therapeutic and clinical implications of exosomes. Int J Nanomedicine. (2021) 16:1281–312. doi: 10.2147/IJN.S291956
34. Peer D, Karp JM, Hong S, Farokhzad OC, Margalit R, Langer R. Nanocarriers as an emerging platform for cancer therapy. Nat Nanotechnol. (2007) 2:751–60. doi: 10.1038/nnano.2007.387
35. Cheng K, Guo Q, Yang W, Wang Y, Sun Z, Wu H. Mapping knowledge landscapes and emerging trends of the links between bone metabolism and diabetes mellitus: A bibliometric analysis from 2000 to 2021. Front Public Health. (2022) 10:918483. doi: 10.3389/fpubh.2022.918483
36. Yue X, Pruemer JM, Hincapie AL, Almalki ZS, Guo JJ. Economic burden and treatment patterns of gynecologic cancers in the United States: evidence from the Medical Expenditure Panel Survey 2007-2014. J Gynecol Oncol. (2020) 31:e52. doi: 10.3802/jgo.2020.31.e52
37. Health expenditures in the U.S. Statista . Available online at: https://www.statista.com/topics/6701/health-expenditures-in-the-us/ (Accessed July 15, 2024).
38. Bowman DM, Gatof J. Reviewing the regulatory barriers for nanomedicine: global questions and challenges. Nanomedicine (Lond). (2015) 10:3275–86. doi: 10.2217/nnm.15.169
39. Access denied | National nanotechnology initiative . Available online at: https://www.nano.gov/about-nni/what/funding (Accessed November 13, 2024).
40. Ozpolat B, Sood AK, Lopez-Berestein G. Liposomal siRNA nanocarriers for cancer therapy. Adv Drug Delivery Rev. (2014) 66:110–6. doi: 10.1016/j.addr.2013.12.008
41. Barata P, Sood AK, Hong DS. RNA-targeted therapeutics in cancer clinical trials: Current status and future directions. Cancer Treat Rev. (2016) 50:35–47. doi: 10.1016/j.ctrv.2016.08.004
42. Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. (2023) 73:17–48. doi: 10.3322/caac.21763
43. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
44. Lheureux S, Braunstein M, Oza AM. Epithelial ovarian cancer: Evolution of management in the era of precision medicine. CA Cancer J Clin. (2019) 69:280–304. doi: 10.3322/caac.21559
45. Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. (2009) 59:225–49. doi: 10.3322/caac.20006
46. Patra JK, Das G, Fraceto LF, Campos EVR, Rodriguez-Torres MDP, Acosta-Torres LS, et al. Nano based drug delivery systems: recent developments and future prospects. J Nanobiotechnology. (2018) 16:71. doi: 10.1186/s12951-018-0392-8
47. Barani M, Bilal M, Sabir F, Rahdar A, Kyzas GZ. Nanotechnology in ovarian cancer: Diagnosis and treatment. Life Sci. (2021) 266:118914. doi: 10.1016/j.lfs.2020.118914
48. Torchilin VP. Drug targeting. Eur J Pharm Sci. (2000) 11:S81–91. doi: 10.1016/S0928-0987(00)00166-4
49. Ghobadi M, Salehi S, Ardestani MTS, Mousavi-Khattat M, Shakeran Z, Khosravi A, et al. Amine-functionalized mesoporous silica nanoparticles decorated by silver nanoparticles for delivery of doxorubicin in breast and cervical cancer cells. Eur J Pharm Biopharm. (2024) 201:114349. doi: 10.1016/j.ejpb.2024.114349
50. Jain V, Jain S, Mahajan SC. Nanomedicines based drug delivery systems for anti-cancer targeting and treatment. Curr Drug Deliv. (2015) 12:177–91. doi: 10.2174/1567201811666140822112516
51. Ledezma-Gallegos F, Jurado R, Mir R, Medina LA, Mondragon-Fuentes L, Garcia-Lopez P. Liposomes co-encapsulating cisplatin/mifepristone improve the effect on cervical cancer: in vitro and in vivo assessment. Pharmaceutics. (2020) 12:897. doi: 10.3390/pharmaceutics12090897
52. Wang L, Liang TT. CD59 receptor targeted delivery of miRNA-1284 and cisplatin-loaded liposomes for effective therapeutic efficacy against cervical cancer cells. AMB Express. (2020) 10:54. doi: 10.1186/s13568-020-00990-z
53. Li M, Wang Y, Zhang L, Liu Q, Jiang F, Hou W, et al. Cancer cell membrane-enveloped dexamethasone normalizes the tumor microenvironment and enhances gynecologic cancer chemotherapy. ACS Nano. (2023) 17:16703–14. doi: 10.1021/acsnano.3c03013
54. Bartoli MH, Boitard M, Fessi H, Beriel H, Devissaguet JP, Picot F, et al. In vitro and in vivo antitumoral activity of free, and encapsulated taxol. J Microencapsul. (1990) 7:191–7. doi: 10.3109/02652049009021832
55. Cabanes A, Briggs KE, Gokhale PC, Treat JA, Rahman A. Comparative in vivo studies with paclitaxel and liposome-encapsulated paclitaxel. Int J Oncol. (1998) 12:1035–40. doi: 10.3892/ijo.12.5.1035
56. Lee MK. Liposomes for enhanced bioavailability of water-insoluble drugs: in vivo evidence and recent approaches. Pharmaceutics. (2020) 12:264. doi: 10.3390/pharmaceutics12030264
57. Fenton OS, Olafson KN, Pillai PS, Mitchell MJ, Langer R. Advances in biomaterials for drug delivery. Adv Mater. (2018) 7:e1705328. doi: 10.1002/adma.201705328
58. Wang J. Combination treatment of cervical cancer using folate-decorated, pH-sensitive, carboplatin and paclitaxel co-loaded lipid-polymer hybrid nanoparticles. Drug Des Devel Ther. (2020) 14:823–32. doi: 10.2147/DDDT.S235098
59. Du X, Chen C, Yang L, Cui Y, Tan B. Bibliometric and visualized analysis of the application of nanotechnology in glioma. Front Pharmacol. (2022) 13:995512. doi: 10.3389/fphar.2022.995512
60. Palanikumar L, Al-Hosani S, Kalmouni M, Nguyen VP, Ali L, Pasricha R, et al. pH-responsive high stability polymeric nanoparticles for targeted delivery of anticancer therapeutics. Commun Biol. (2020) 3:95. doi: 10.1038/s42003-020-0817-4
61. Manshian BB, Jiménez J, Himmelreich U, Soenen SJ. Personalized medicine and follow-up of therapeutic delivery through exploitation of quantum dot toxicity. Biomaterials. (2017) 127:1–12. doi: 10.1016/j.biomaterials.2017.02.039
62. Arias LS, Pessan JP, Vieira APM, Lima TMT, Delbem ACB, Monteiro DR. Iron oxide nanoparticles for biomedical applications: A perspective on synthesis, drugs, antimicrobial activity, and toxicity. Antibiotics (Basel). (2018) 7:46. doi: 10.3390/antibiotics7020046
63. Soenen SJ, Rivera-Gil P, Montenegro J-M, Parak WJ, De Smedt SC, Braeckmans K. Cellular toxicity of inorganic nanoparticles: Common aspects and guidelines for improved nanotoxicity evaluation. Nano Today. (2011) 6:446–65. doi: 10.1016/j.nantod.2011.08.001
64. Xiao Y, Bi M, Guo H, Li M. Multi-omics approaches for biomarker discovery in early ovarian cancer diagnosis. EBioMedicine. (2022) 79:104001. doi: 10.1016/j.ebiom.2022.104001
65. Budiana ING, Angelina M, Pemayun TGA. Ovarian cancer: Pathogenesis and current recommendations for prophylactic surgery. J Turk Ger Gynecol Assoc. (2019) 20:47–54. doi: 10.4274/jtgga.galenos.2018.2018.0119
66. Pan Y, Wang Z, Ma J, Zhou T, Wu Z, Ding P, et al. Folic acid-modified fluorescent-magnetic nanoparticles for efficient isolation and identification of circulating tumor cells in ovarian cancer. Biosensors (Basel). (2022) 12:184. doi: 10.3390/bios12030184
67. Liu W, Nie L, Li F, Aguilar ZP, Xu H, Xiong Y, et al. Folic acid conjugated magnetic iron oxide nanoparticles for nondestructive separation and detection of ovarian cancer cells from whole blood. Biomater Sci. (2016) 4:159–66. doi: 10.1039/C5BM00207A
68. Yin M, Xu X, Han H, Dai J, Sun R, Yang L, et al. Preparation of triangular silver nanoparticles and their biological effects in the treatment of ovarian cancer. J Ovarian Res. (2022) 15:121. doi: 10.1186/s13048-022-01056-3
69. Yuan YG, Peng QL, Gurunathan S. Silver nanoparticles enhance the apoptotic potential of gemcitabine in human ovarian cancer cells: combination therapy for effective cancer treatment. Int J Nanomedicine. (2017) 12:6487–502. doi: 10.2147/IJN.S135482
70. Ramezani T, Nabiuni M, Baharara J, Parivar K, Namvar F. Sensitization of Resistance Ovarian Cancer Cells to Cisplatin by Biogenic Synthesized Silver Nanoparticles through p53 Activation. Iran J Pharm Res. (2019) 18:222–31.
71. Hussain Y, Islam L, Khan H, Filosa R, Aschner M, Javed S. Curcumin-cisplatin chemotherapy: A novel strategy in promoting chemotherapy efficacy and reducing side effects. Phytother Res. (2021) 35:6514–29. doi: 10.1002/ptr.7225
72. Xiong X, Arvizo RR, Saha S, Robertson DJ, McMeekin S, Bhattacharya R, et al. Sensitization of ovarian cancer cells to cisplatin by gold nanoparticles. Oncotarget. (2014) 5:6453–65. doi: 10.18632/oncotarget.220
73. Patra CR, Bhattacharya R, Mukherjee P. Fabrication and functional characterization of goldnanoconjugates for potential application in ovarian cancer. J Mater Chem. (2010) 20:547–54. doi: 10.1039/b913224d
74. Turner J, Koumenis C, Kute TE, Planalp RP, Brechbiel MW, Beardsley D, et al. Tachpyridine, a metal chelator, induces G2 cell-cycle arrest, activates checkpoint kinases, and sensitizes cells to ionizing radiation. Blood. (2005) 106:3191–9. doi: 10.1182/blood-2005-03-1263
75. Ojeda F, Diehl HA, Folch H. Radiation induced membrane changes and programmed cell death: possible interrelationships. Scanning Microsc. (1994) 8:645–51.
76. Talluri S, Malla RR. Superparamagnetic iron oxide nanoparticles (SPIONs) for diagnosis and treatment of breast, ovarian and cervical cancers. Curr Drug Metab. (2019) 20:942–5. doi: 10.2174/1389200220666191016124958
77. Gao Y, Qian H, Tang X, Du X, Wang G, Zhang H, et al. Superparamagnetic iron oxide nanoparticle-mediated expression of miR-326 inhibits human endometrial carcinoma stem cell growth. Int J Nanomedicine. (2019) 14:2719–31. doi: 10.2147/IJN.S200480
78. Jedryka MA, Klimczak P, Kryszpin M, Matkowski R. Superparamagnetic iron oxide: a novel tracer for sentinel lymph node detection in vulvar cancer. Int J Gynecol Cancer. (2020) 30:1280–84. doi: 10.1136/ijgc-2020-001458
79. Yuan YG, Zhang S, Hwang JY, Kong IK. Silver nanoparticles potentiates cytotoxicity and apoptotic potential of camptothecin in human cervical cancer cells. Oxid Med Cell Longev. (2018) 2018:6121328. doi: 10.1155/2018/6121328
80. Mohammed Asik R, Manikkaraja C, Tamil Surya K, Suganthy N, Priya Aarthy A, Mathe D, et al. Anticancer potential of L-histidine-capped silver nanoparticles against human cervical cancer cells (SiHA). Nanomaterials (Basel). (2021) 11:3154. doi: 10.3390/nano11113154
81. Li Z, Gopinath SCB, Lakshmipriya T, Anbu P, Perumal V, Wang X. Self-assembled silver nanoparticle-DNA on a dielectrode microdevice for determination of gynecologic tumors. BioMed Microdevices. (2020) 22:67. doi: 10.1007/s10544-020-00522-3
82. Kamil Shareef NA, Zandsalimi F, Tavoosidana G. Gold nanoparticles (AuNPs) decrease the viability of cervical cancer cells by inducing the BAX gene and activating antioxidant enzymes. Mol Biol Rep. (2024) 51:287. doi: 10.1007/s11033-024-09253-7
83. Shanei A, Akbari-Zadeh H. Investigating the sonodynamic-radiosensitivity effect of gold nanoparticles on heLa cervical cancer cells. J Korean Med Sci. (2019) 34:e243. doi: 10.3346/jkms.2019.34.e243
84. Talarska P, Boruczkowski M, Żurawski J. Current knowledge of silver and gold nanoparticles in laboratory research-application, toxicity, cellular uptake. Nanomaterials (Basel). (2021) 11:2454. doi: 10.3390/nano11092454
85. Sakthi Devi R, Girigoswami A, Siddharth M, Girigoswami K. Applications of gold and silver nanoparticles in theranostics. Appl Biochem Biotechnol. (2022) 194:4187–219. doi: 10.1007/s12010-022-03963-z
86. Toczek J, Sadłocha M, Major K, Stojko R. Benefit of silver and gold nanoparticles in wound healing process after endometrial cancer protocol. Biomedicines. (2022) 10:679. doi: 10.3390/biomedicines10030679
87. Al-Doaiss A, Jarrar Q, Moshawih S. Hepatic histopathological and ultrastructural alterations induced by 10 nm silver nanoparticles. IET Nanobiotechnol. (2020) 14:405–11. doi: 10.1049/iet-nbt.2020.0039
88. Niżnik Ł, Noga M, Kobylarz D, Frydrych A, Krośniak A, Kapka-Skrzypczak L, et al. Gold nanoparticles (AuNPs)-toxicity, safety and green synthesis: A critical review. Int J Mol Sci. (2024) 25:4057. doi: 10.3390/ijms25074057
89. Huang H, Feng W, Chen Y, Shi JL. Inorganic nanoparticles in clinical trials and translations. Nano Today. (2020) 35:1–24. doi: 10.1016/j.nantod.2020.100972
90. Miu BA, Dinischiotu A. New green approaches in nanoparticles synthesis: an overview. Molecules. (2022) 27:6472. doi: 10.3390/molecules27196472
91. Gupta D, Boora A, Thakur A, Gupta TK. Green and sustainable synthesis of nanomaterials: Recent advancements and limitations. Environ Res. (2023) 231:116316. doi: 10.1016/j.envres.2023.116316
Keywords: gynecologic cancers, nanoparticles, nanomaterials, bibliometric analysis, visualization analysis
Citation: Zhou Y, Chen L and Wang T (2025) Nanoparticles in gynecologic cancers: a bibliometric and visualization analysis. Front. Oncol. 14:1465987. doi: 10.3389/fonc.2024.1465987
Received: 17 July 2024; Accepted: 16 December 2024;
Published: 08 January 2025.
Edited by:
Sharon R. Pine, University of Colorado Anschutz Medical Campus, United StatesReviewed by:
Smrithi Padmakumar, Merck Sharp & Dohme Corp, United StatesCopyright © 2025 Zhou, Chen and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lizhang Chen, bGljaGU0MDA1QDEyNi5jb20=; Tingting Wang, d2FuZ3Rpbmc5MTEyM0AxMjYuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.