- 1Department of Neurosurgery, University Hospital Leipzig, Leipzig, Germany
- 2Comprehensive Cancer Center Central Germany, University Hospital Leipzig, Leipzig, Germany
Objective: Cranial meningioma surgeries often involve significant blood loss and transfusions. Tranexamic acid (TXA) has been used to reduce blood loss in various surgeries. This meta-analysis of randomized placebo-controlled trials (RCTs) evaluates the impact of TXA in cranial meningioma surgery.
Methods: Pubmed, Web of Science, and Cochrane Library were searched for RCTs. Studies were compared for: Blood loss, operative time, hospital stay, reoperation rates, allogeneic and autologous transfusion, and incidence of complications.
Results: Seven RCTs with 490 patients receiving TXA and 491 receiving placebos were included. TXA significantly shortened operative time (Mean Difference (MD): -20.95; 95%CI: -39.94 to -1.95; p=0.03). Blood loss was lower with TXA (MD: -262.7 ml; 95%CI: -397.6 to -127.8; p=0.0001). Odds of reoperation were not significantly different (OR: 0.44; 95%CI: 0.13-1.45; p=0.18). TXA significantly reduced the need for RBC transfusions (OR: 0.47; 95%CI: 0.22-0.99; p<0.05). No significant differences were observed regarding postoperative seizures (OR: 1.06; 95%CI: 0.56-2.03; p=0.85), hydrocephalus (OR: 0.25; 95%CI: 0.03-2.29; p=0.22), or hematoma (OR: 0.52; 95%CI: 0.22-1.28; p=0.16). Hospital stay was shortened in the TXA group (MD: -1.23; 95%CI: -2.41 to -0.05; p=0.04).
Conclusion: This meta-analysis suggests that a single intraoperative dose of TXA reduces blood loss, allogeneic blood transfusions and shortens surgery time.
1 Introduction
Tranexamic acid (TXA) is an antifibrinolytic agent developed by Japanese research couple Shosuke and Utako Okamoto shortly after Second World (1). They aimed to control postpartum hemorrhage, which was one of the main causes of maternal death in post-war Japan. Since then, TXA has been adopted in several surgical and non-surgical disciplines as an effective and safe method to enhance hemostasis.
TXA is a lysin derivate that binds to plasminogen, preventing enzymatic degradation of formed fibrin meshwork and stabilizing the formed clot (2). However, up until 2010, controlled studies on TXA use were scarce. The WOMAN study confirmed the reduction of bleeding-associated complications nearly 50 years after the clinical introduction of TXA (3). Subsequently, the CRASH-2 trial proved a significant reduction in bleeding-associated deaths in patient after traumatic injury (4). Blood loss, and subsequent RBC transfusion are known risk factors for intra- and postoperative complications during the treatment course for intracranial meningiomas (5, 6). Furthermore, increasing age and use of anticoagulants presents an additional risk factor, for which the perioperative or even prophylactic use of tranexamic acid might be the solution (7). The resection of meningiomas is mostly performed in an elective surgical setting, which has to aim for most strict and tailored safety profile for the individual operation. To date, several retrospective and cohort studies report on use of TXA in meningioma surgery, usually with mixed results (8). This might be due to strong limitation in contemporary studies, including wide differences in dosing scheme of the TXA, ranging from weight-adjusted doses to standardized single doses of 1g TXA intravenously (9, 10). One of the best data sources on administration scheme comes from the CRASH-2 trial, which suggests that the time between bleeding and application of TXA acid should not overreach 3 hours (4). Apart from venous thromboembolism, TXA is reported to increase the risk of postoperative seizures, and might be a major drawback for the usage of TXA” (11, 12). The present meta-analysis of 981 patients from seven randomized placebo-controlled, and double-blinded studies aims to evaluate the exact risk-benefit ratio of TXA use in cranial meningioma surgery.
2 Methods
2.1 Search strategy and screening
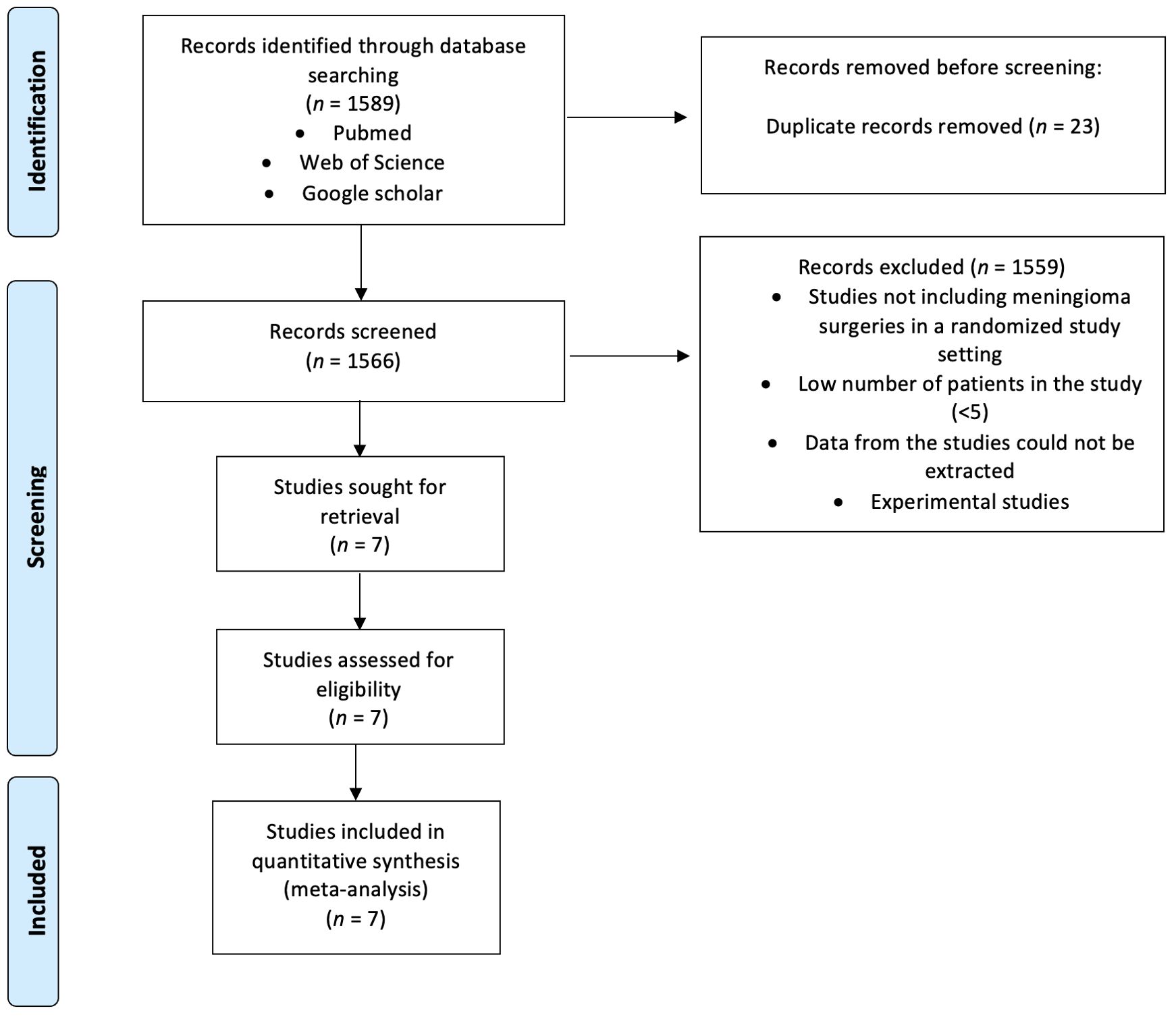
Following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines and the Cochrane Handbook for systematic Review of Interventions Version 6.4, we queried the PubMed, Google Scholar, and Web of Science databases from their inception to May 30, 2024 (13, 14). The search focused on English-language, full-text randomized controlled trials (RCTs) related to tranexamic acid (TXA) usage during meningioma resection using the mesh terms (“tranexamic acid” OR “TXA”) AND (“meningioma”) (see Figure 1). After duplicate removal, titles and abstracts were screened using the Rayyan Intelligent Systematic Review web application to identify studies that met the inclusion/exclusion criteria. Two reviewers (J.W. and M.V.) independently screened all studies, with disagreements resolved by a third author (E.G.).
2.2 Inclusion criteria
The inclusion criteria were determined according to the PICOS (population, intervention, comparator, outcomes, and study design) framework (15). These criteria were formulated as follows: patients had undergone surgical treatment for cranial meningioma; perioperative tranexamic acid therapies were performed; results were compared to a placebo control; all results of the prespecified endpoints were reported; and the studies were defined as prospective randomized, placebo-controlled, and double-blinded studies.
2.3 Data extraction and quality evaluation
Data extracted from the selected studies included publication year, author institution and country, study design, sample size, patient demographics (sex and age), extent of resection, complications (thromboembolic events, seizures, new onset neurological deficits, hematoma, reoperation, hydrocephalus), operative duration, TXA dosage, route and timing of TXA administration, estimated blood loss (EBL), cell saver use, need for blood transfusions [Fresh frozen plasma (FFP) transfusion, Red blood cell transfusion (RBC transfusion), platelet transfusion (PT)], complications (hematoma, hydrocephalus, seizure, revision surgery, venous thromboembolism) and length of hospital stay. Patients’ physical status at discharge was assessed using the Extended Glasgow Outcome Scale (GOSE) and categorized as good recovery (GOSE 7-8), moderate disability (GOSE 5-6), and severe disability (GOSE 1-4) (16). The Cochrane Bias Risk Tool was used to analyze the risk of bias in the included trials using the software Review Manager Web (RevMan Web Version 5.4.1 from The Cochrane Collaboration). The following five characteristics regarding risk of bias evaluation were considered in the analysis: selection bias, performance bias, detection bias, attrition bias, and reporting bias. Finally, a risk of bias summary chart and plot were generated. The National Institutes of Health Quality Assessment Tool for interventional studies (NIH-QAT) was used for the assessment of quality and risk of bias of included studies (17).
2.4 Statistical analysis
Statistical analyses were performed on operative duration, complications, transfusion requirements, estimated blood loss (EBL), postoperative seizures, postoperative hematomas, postoperative hydrocephalus, postoperative physical status, and length of hospital stay using Cochrane’s RevMan 5.4 (The Nordic Cochrane Center, Cochrane Collaboration). To assess statistical heterogeneity and inconsistency, χ² and I² statistics were employed, with an I² value of 50% or more indicating substantial heterogeneity (18). The relative contribution of individual studies, based on sample size, was considered in the estimation of treatment effects. To assess publication bias, we created Funnel plots for a visual examination of publication bias in the included studies. Effect sizes of categorical data were expressed as pooled odds ratio (OR) estimates. The relative contribution of individual studies, determined by sample size, was considered in estimating treatment effects. Random effects models were used to create forest plots showing the pooled estimates. This statistical stepwise workflow was applied to investigate the following endpoints: Seizure, need for transfusion (FFP, platelet concentrate, red blood cell concentrates), estimated blood loss, cell saver use, length of hospital stays, operative duration, hematoma, reoperation, hydrocephalus, and GOSE status at discharge.
3 Results
3.1 Study selection
An initial search of PubMed, Web of Science, and Google Scholar databases identified 1,589 records. After removing 23 duplicate records, 1,566 unique records were screened by title and abstract. Of these, 1,559 records were excluded for reasons such as not including meningioma surgeries in a randomized study setting, having a low number of patients in the study (<5), being unable to extract data from the studies, or being experimental studies. This left 7 studies sought for retrieval and assessed for eligibility. Ultimately, all 7 studies met the inclusion criteria and were included in the quantitative synthesis for the meta-analysis.
The selected studies were randomized, blinded, and placebo-controlled trials, providing primary clinical data on the use of tranexamic acid (TXA) during resection of intracranial meningiomas. The trials were conducted in various institutions and countries, offering a comprehensive overview of TXA’s efficacy and safety in this context. The detailed flowchart of the literature review process is presented in Figure 1.
3.2 Surgical characteristics of studies included in this meta-analysis
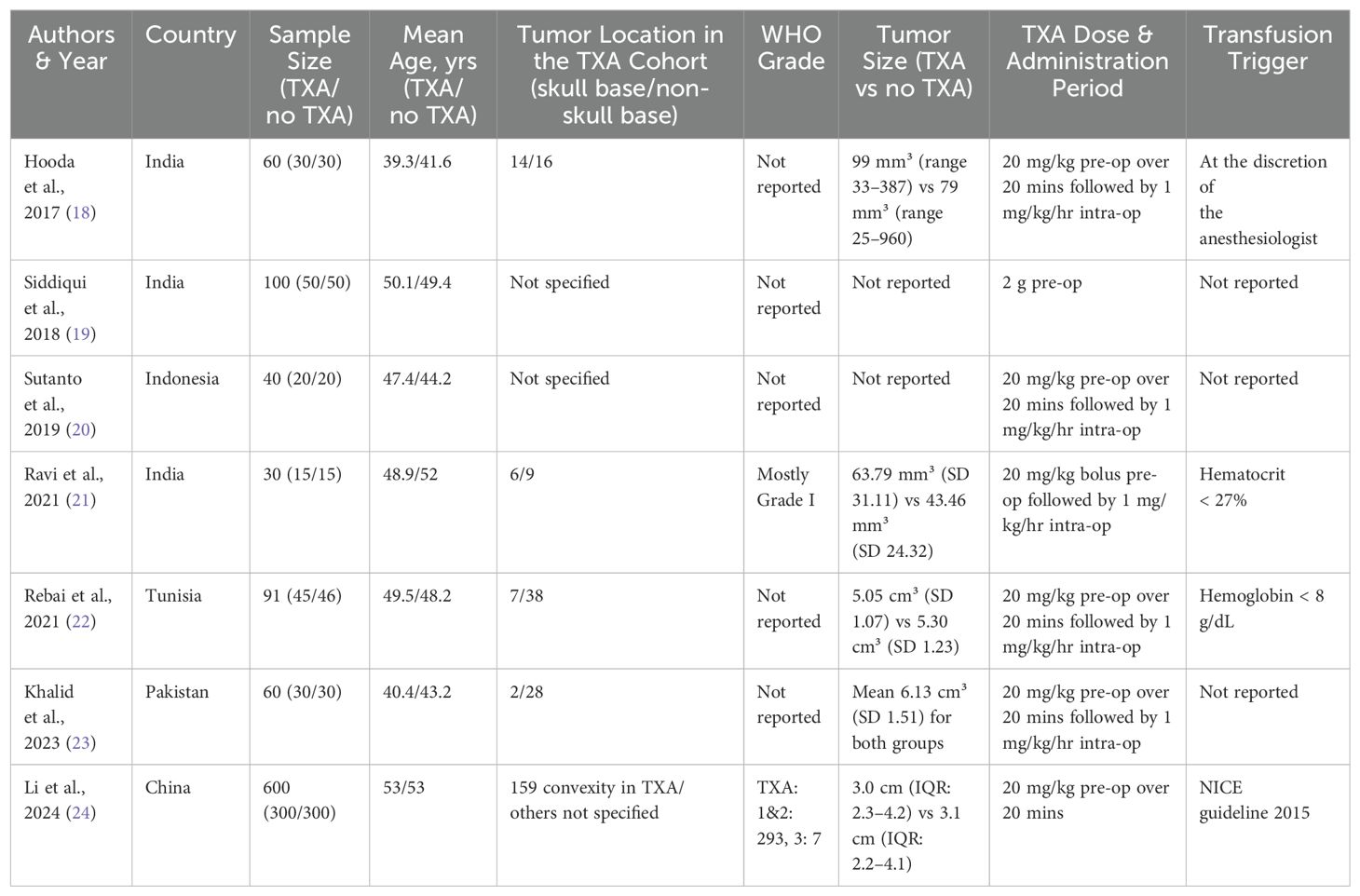
Table 1 summarizes the characteristics of seven studies included in the meta-analysis on the use of tranexamic acid (TXA) in meningioma surgeries, encompassing a total of 981 patients (19–25). The studies span across various countries including India, Indonesia, Tunisia, Pakistan, and China, with sample sizes ranging from 30 to 600 patients. The mean ages of participants in the TXA and control groups are fairly similar within each study, generally ranging from the late 30s to early 50s. Tumor location data is variably reported, with some studies specifying the proportion of skull base and non-skull base tumors. WHO grades are not consistently reported across the studies. Tumor sizes are provided in five of the seven studies (19, 22–25). The administration of TXA commonly involved a 20 mg/kg preoperative dose over 20 minutes, followed by a maintenance dose of 1 mg/kg per hour intraoperatively, except for Siddiqui et al. (20) who administered a single 2 g preoperative dose. Transfusion triggers vary, with one study specifying criteria such as hematocrit below 27% or hemoglobin below 8 g/dL, while others did not report this information (22, 23). This comprehensive table highlights the diverse methodologies and patient demographics in studies examining TXA’s efficacy and safety in meningioma surgeries.
3.3 Risk of bias and quality assessment
The risk of bias summary assessed the seven included studies for various biases (see Figure 2). All studies were evaluated for random sequence generation, allocation concealment, blinding of participants/personnel, blinding of outcome assessment, incomplete outcome data, and selective reporting. Most studies demonstrated a low risk of bias, indicated by green circles with plus signs. Some studies had unclear risks (yellow diamonds with question marks) or high risks (red circles with minus signs). Specifically, Siddiqui et al. (20) and Sutanto et al. (21) showed high risk or unclear risk in several domains, notably in blinding and random sequence generation. Furthermore, NIH-QAT tool was used to evaluate quality of the included trials (see Supplementary Figure S1).
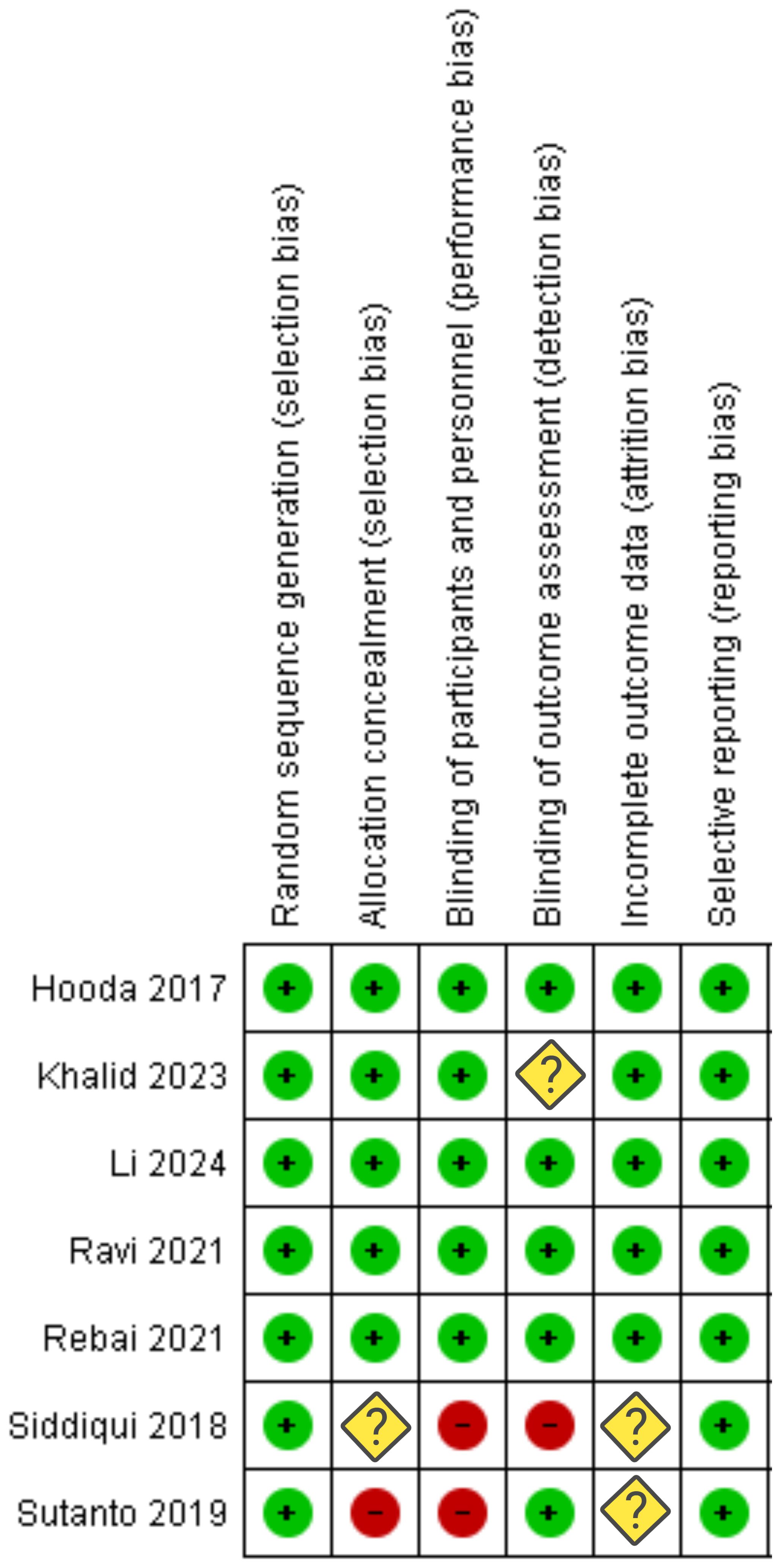
Figure 2. Risk of bias assessment for each category of bias (“+” constitutes low risk; “?” constitutes unclear risk; “-”constitutes high risk).
3.4 Blood Loss, allogeneic transfusion, and autotransfusion via cell saver
Data from 981 patients out of seven studies were included in the investigation of blood loss (19–25) (see Figure 3A). The pooled mean difference in blood loss between the TXA and placebo groups was -262.70 mL (95% CI: -397.58 to -127.82 mL), favoring TXA with a statistically significant reduction in blood loss (p = 0.0001). The effect of TXA on the need for blood transfusions was investigated (see Figure 3B). Data from six studies were included, comprising a total of 470 patients in the TXA group and 471 in the placebo group (19, 20, 22–25). The combined OR was 0.47 (95% CI: 0.22-0.99), indicating a significant reduction in transfusion requirements with tranexamic acid (p = 0.05). The heterogeneity among the studies was moderate (I² = 48%, p = 0.09). Furthermore, the impact of TXA on the administration of fresh frozen plasma (FFP) was analyzed (see Figure 3C). Data from three studies were included, with 375 patients in TXA group and 376 in the placebo group (19, 23, 25). The combined OR was 0.96 (95% CI: 0.52-1.78), showing no significant effect of tranexamic acid on FFP use (p = 0.90). Heterogeneity was low (I² = 0%, p = 0.81). The need for platelet transfusion was investigated based on two studies, including 330 patients in the TXA group and 330 the placebo group (19, 25) (see Figure 3D). The pooled OR was 1.84 (95% CI: 0.37-9.22), indicating no significant difference in platelet transfusion requirements between the TXA and placebo groups (p = 0.46). Heterogeneity was low (I² = 0%, p = 0.73). The influence of TXA on the use of cell saver was analyzed from data of two studies (19, 25), with a total of 330 patients in the TXA group and 330 in the placebo group (see Figure 3E). The combined OR was 0.85 (95% CI: 0.58-1.26), suggesting nonsignificant reduction in cell saver use with tranexamic acid (p = 0.43). Heterogeneity was low (I² = 0%, p = 0.55).
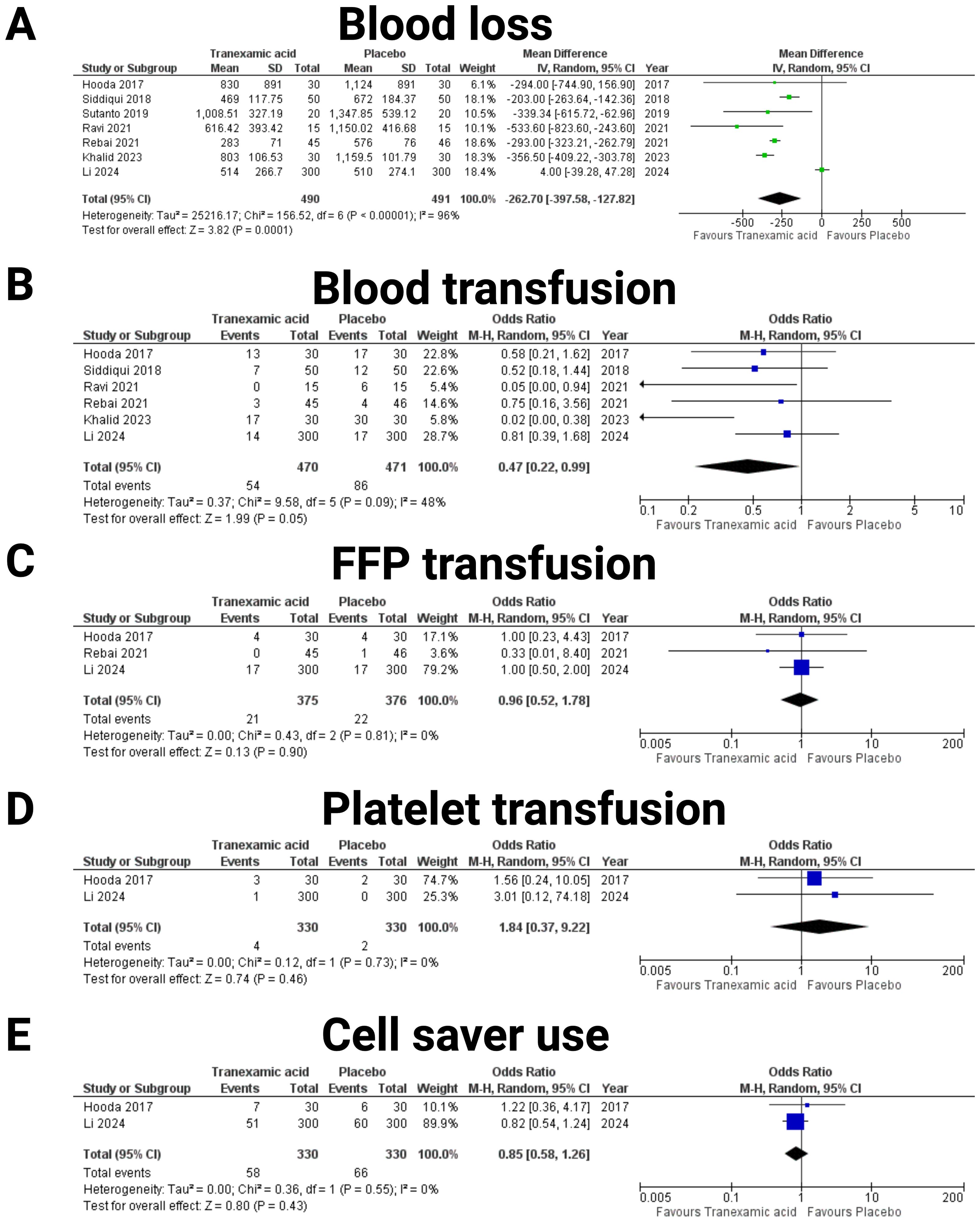
Figure 3. Forest plots illustrating the effect of tranexamic acid versus placebo on various blood loos, allogeneic and autotransfusion. Each panel displays the odds ratio (OR) or mean difference (MD) with 95% confidence intervals (CIs) for individual studies and the combined estimate. The following endpoints were investigated: (A) Blood loss, (B) Blood transfusion, (C) Fresh frozen plasma (FFP) transfusion, (D) Platelet transfusion, and (E) Cell saver use.
3.5 Operative time and length of hospital stay
The analysis of operative time included data of 981 patients from seven studies (18–24) (see Figure 4A). The combined mean difference was -20.95 minutes (95% CI: -39.94 to -1.95 minutes), indicating a significant reduction in operative time with tranexamic acid compared to placebo (p = 0.03). The heterogeneity was moderate to high (I² = 79%, p < 0.0001). Furthermore, data of 851 patients from four studies were analyzed regarding length of hospital stay (19, 20, 23, 25) (see Figure 4B). The pooled mean difference was -1.23 days (95% CI: -2.41 to -0.05 days), showing a significant reduction in the length of hospital stay for patients treated with tranexamic acid (p = 0.04). The heterogeneity was high (I² = 86%, p < 0.0001).

Figure 4. Forest plots illustrating the effect of tranexamic acid versus placebo operative time (A) and length of hospital stay (B). Each panel displays mean difference (MD) with 95% confidence intervals (CIs) for individual studies and the combined estimate.
3.6 Postoperative complications
The risk of postoperative hematoma was examined through data from three studies (19, 23, 25). The analysis involved 375 patients in the tranexamic acid group and 376 patients in the placebo group (see Figure 5A). The pooled odds ratio was 0.52 (95% CI: 0.21 to 1.28), suggesting a potential reduction in hematoma risk with tranexamic acid treatment, though this finding was not statistically significant (p = 0.16). Substantial heterogeneity was not found (I2 = 0%, p = 0.75). The effect of tranexamic acid on the incidence of hydrocephalus was assessed through two studies (19, 25) (see Figure 5B). The analysis included a total of 330 patients in the tranexamic acid group and 330 patients in the placebo group. The pooled analysis yielded an odds ratio (OR) of 0.25 (95% CI: 0.03 to 2.29), suggesting a trend towards a reduction in hydrocephalus among patients treated with tranexamic acid compared to placebo. However, this effect was not statistically significant (p = 0.22). The individual study estimates showed considerable variability, with wide confidence intervals crossing the line of no effect, indicating substantial uncertainty in the effect estimate but no statistical heterogeneity was observed (I2 = 0%, p = 0.22). The incidence of seizures was assessed in four studies (19, 20, 23, 25) (see Figure 5C). This analysis included 425 patients in the tranexamic acid group and 426 patients in the placebo group. The meta-analysis resulted in an odds ratio of 1.06 (95% CI: 0.56 to 2.03, I2 = 0%, p = 0.75), indicating no significant difference in seizure risk between the tranexamic acid and placebo groups (p = 0.85). The need for reoperation was evaluated in studies by Hooda et al. (19) and Li et al. (25) (see Figure 5D). The total number of patients analyzed included 330 in the tranexamic acid group and 330 in the placebo group. The combined odds ratio was 0.44 (95% CI: 0.13 to 1.45), indicating a non-significant trend towards fewer reoperations in the tranexamic acid group compared to placebo (p = 0.18). Significant heterogeneity was not found (I2 = 0%, p = 0.18). Figure 5 summarizes the results of postoperative complications. Events of venous thromboembolic complications were only observed in the study by Li et al. (25), which found 26 (26/300, 8.7%) instances in the TXA group, and 22 (22/300, 7.3%), respectively. The other studies by Hooda et al. (19), Ravi et al. (22), Rebai et al. (23), and Siddiqui et al. (20) observed no venous thromboembolic complications in any arm. In the pooled analysis of these five studies with 881 (440 in TXA, 441 in Placebo) patients, TXA was not associated with an increased risk of venous thromboembolic complications in meningioma surgery (OR: 1.18, 95% CI: 0.67- 2.07, I2 = 0%, p = 0.99).
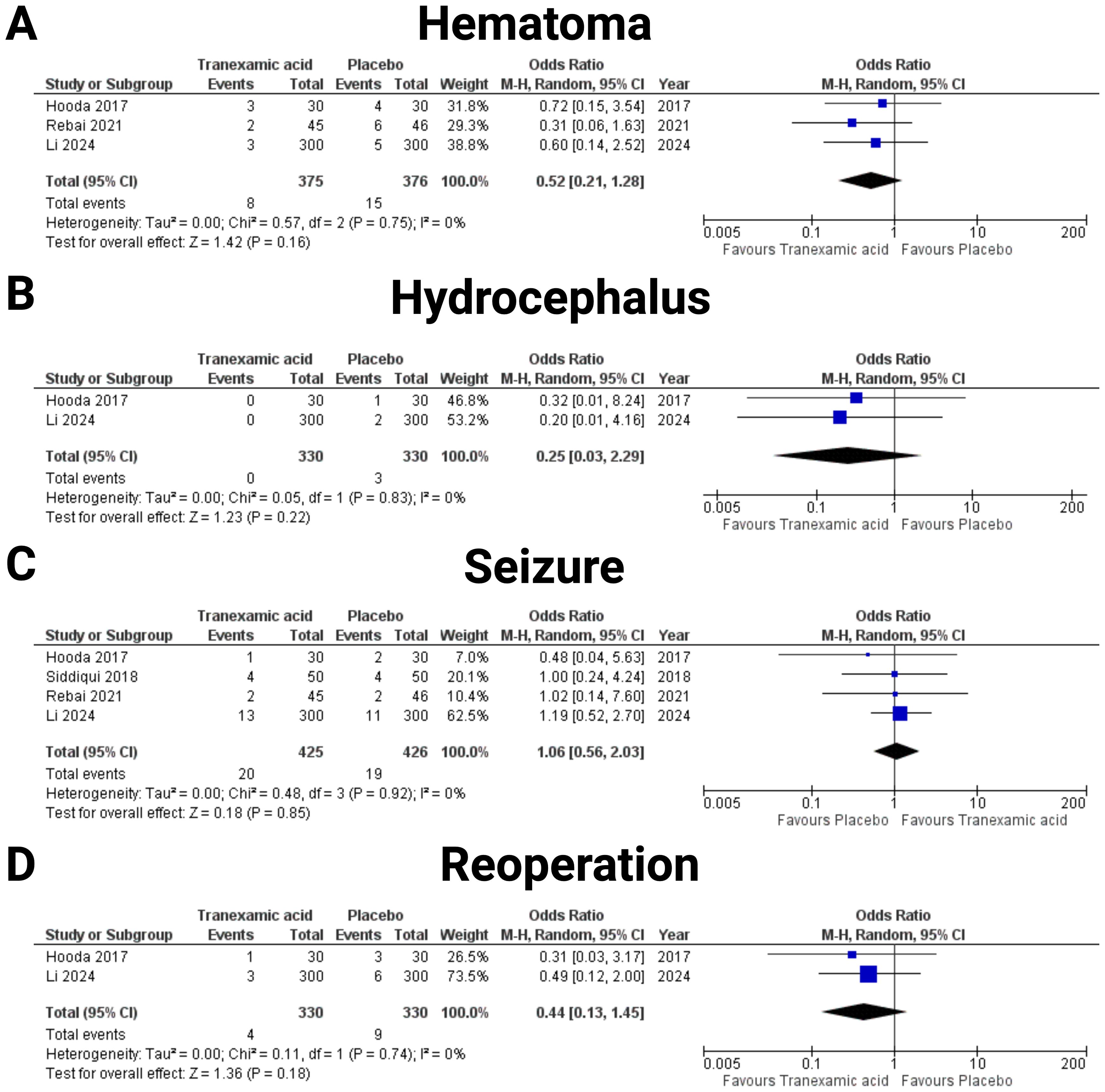
Figure 5. Forest plots illustrating the effect of tranexamic acid versus placebo on various Postoperative complications. Each panel displays the odds ratio (OR) with 95% confidence intervals (CIs) for individual studies and the combined estimate. The following endpoints were investigated: (A) Hematoma, (B) Hydrocephalus, (C) Seizure, and (D) Reoperation.
3.7 Physical status at discharge
A meta-analysis of the effect of TXA on the proportion of patients with good recovery, defined as a Glasgow Outcome Scale Extended (GOSE) score of 7-8 at discharge was performed. Two studies were included in this analysis (19, 23). The pooled analysis included 75 patients in the tranexamic acid group and 76 patients in the placebo group. The combined OR for achieving good recovery with tranexamic acid was 1.84 (95% CI: 0.87-3.89) compared to placebo, indicating a non-significant trend towards improved outcomes with tranexamic acid (p = 0.11) (see Figure 6). Heterogeneity among the studies was minimal, with an I² of 0%, suggesting consistent findings across the included studies (p = 0.77).
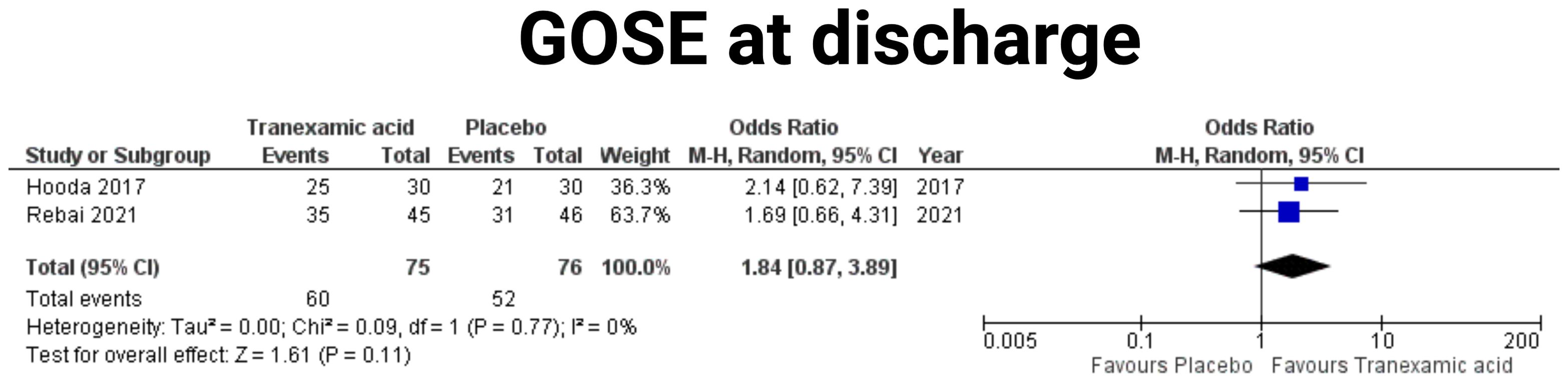
Figure 6. Forest plots illustrating the effect of tranexamic acid versus placebo on GOSE outcome at discharge. Each panel displays the odds ratio (OR) with 95% confidence intervals (CIs) for individual studies and the combined estimate.
3.8 Publication bias
To ensure acceptable reliability, we implemented three measures to investigate potential publication bias, we employed an extensive literature search strategy. Second, the trials included in this meta-analysis were meticulously selected according to strict inclusion and exclusion criteria. Finally, publication bias was assessed using funnel plots (see Figure 7). The majority of the plots exhibited symmetry, with data points scattered evenly around the central line, suggesting minimal publication bias for the majority of outcomes. However, blood loss (Figure 7A) and operative time (Figure 7F) show slightly asymmetry, suggesting potential publication bias in these outcomes.
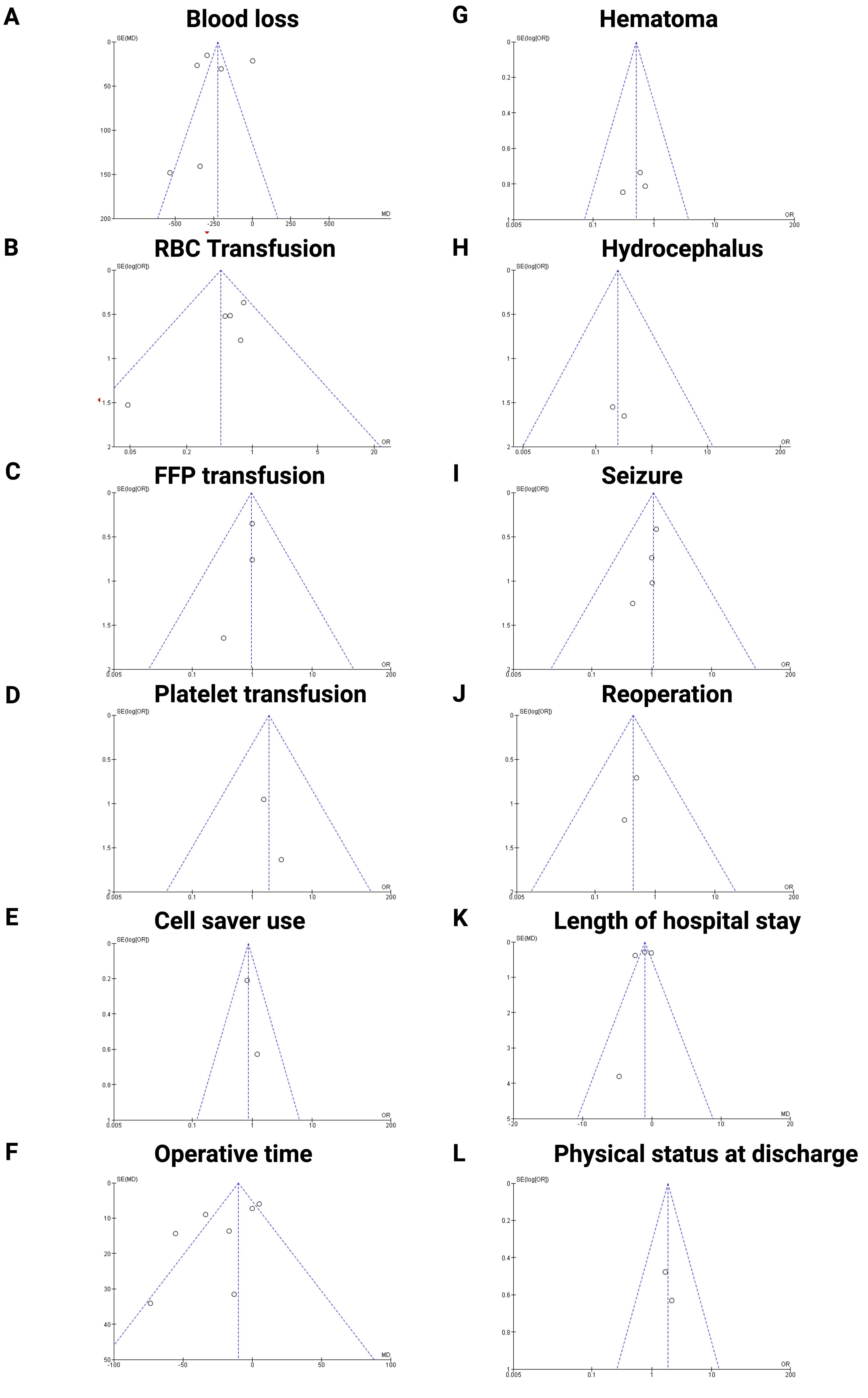
Figure 7. Funnel plots assessing publication bias for the following outcomes: (A) Blood loss, (B) RBC transfusion, € FFP transfusion, (D) Platelet transfusion, (E) Cell saver use, (F) Operative time, (G) Hematoma, (H) Hydrocephalus, (I) Seizure, (J) Reoperation, (K) Length of hospital stay, (L) Physical status at discharge, respectively. The plots indicate overall symmetry, suggesting minimal publication bias, except for operative time and blood loss which show slight asymmetry.
4 Discussion
We performed a meta-analysis of TXA administration in patients who underwent cranial meningioma surgery. To date, we publish the largest pooled patient collective with 981 patients and 7 eligible prospective randomized and double-blinded controlled trials. The findings of the present meta-analysis can be summarized as follows (see Supplementary Figure S1 (Graphical abstract)): (1) TXA therapy appears to be associated with reduced blood loss in surgically treated cranial meningiomas; (2) TXA treatment seems to lower the risk of blood transfusions during cranial meningioma surgery; (3) Operative time was significantly enhanced by TXA application; (4) Length of hospital stay was significantly shortened by intraoperative TXA therapy.
4.1 Blood loss and transfusion
The present study found a significantly reduced blood loss in cranial meningioma surgery by the use of TXA (MD: -262.7 ml (95% CI: -397.6- -127.8, p = 0.0001). Furthermore, TXA use was associated with significantly less blood product transfusions (OR: 0.47; 95% CI: 0.22-0.99; p < 0.05). Retrospective cohort of Rajagopalan et al. (5) showed that in the context of elective brain tumor surgery, meningiomas present a significant a risk factor for higher blood loss, which can even overreach the preoperative blood volume. The median of the published blood loss varied between 300 and 1100 ml (26). This very large difference might be due to non-standardized reporting of the underlying pathology, where different types of meningiomas are reported on together without differentiating between WHO grades, meningioma size skull-base vs. non-skull base localization, and vascularization (27, 28). This was also one of the main limitations of the included RCTs, where the subgroup analysis of the meningiomas is not stratified by these parameters regarding blood loss. Despite these limitations, the studies consistently highlight that blood loss can be a significant issue in meningioma surgery and may predict postoperative complications. Although there are inaccuracies in study designs, TXA appears to be a useful tool that can reduce bleeding and shorten operative time when used carefully. The debate around the risks of allogeneic blood transfusions, such as transfusion-related acute lung injury (TRALI), transfusion-associated circulatory overload (TACO), and acute kidney injury, underscores the need to consider patient-specific factors when administering TXA (29). Additionally, the high costs of collecting and administering allogeneic blood products further incentivize the use of effective intraoperative hemostasis strategies.
4.2 Complications
While a retrospective study suggested that TXA application increases the risk of venous thromboembolism in surgery for spinal tumors or other high risk patients with active malignancy, this risk profile seems to be not transferrable to TXA application in cranial meningioma surgery (29). The present meta-analysis synthesized 881 individual patient data and found no significant association between TXA and venous thromboembolism in cranial meningioma surgery. This finding might be explained by the mechanism of TXA as a synthetic lysine analog that competitively inhibits lysine-binding sites on fibrin clots to prevent clot lysis, which differs from regular clotting disorders caused by elevated systemic procoagulants relative to anticoagulants, which increases the risk of venous thromboembolism (20).
Irl et al. (30) described the effect of TXA on seizures. In this animal study it has been found that the mechanism of action is inhibition of GABA-receptors in hippocampus, which should lower the epilepsy-threshold and therefore have a strong pro-convulsive effect. The adverse-event database study FAERS also reports on several patients with epileptic symptomatic after TXA administration (31). The present meta-analysis showed that TXA administration is not associated with an increased risk of postoperative seizures (OR: 1.06 (95% CI: 0.56 to 2.03, I2 = 0%, p = 0.75). However, tumor locations were not stratified in the studies, and it is known that meningiomas at the convexity harbor higher risks of postoperative epilepsy risk compared to skull-base meningiomas (32). Further studies should primarily focus on this anatomical subgroup of meningioma patients.
Other postoperative safety indicators such as hematoma, hydrocephalus, physical status and revision surgery were not significantly influenced by the application of TXA. Nevertheless, it has to be considered that tumor location significantly influences the neurological functioning and postoperative outcome. Meling et al. (33) analyzed 1,148 patients and found that skull base meningiomas (SBMs) are linked to more complex surgeries and a higher risk of postoperative neurological decline, as well as preoperative neurological deficits (RR 1.4; p < 0.0001). Additionally, non-skull base meningiomas (NSBMs) had a higher incidence of seizures (RR 2.2; p < 0.0001) and a greater proportion of WHO grade II and III tumors (10% vs. 4%; p < 0.0001).
4.3 Vascularization and hemostasis of meningiomas
Intraoperative blood loss during meningioma surgery varies largely. Against this backdrop, several ongoing research strives to identify predictors of blood loss and management strategies to reduce blood loss. A recent retrospective study of 503 meningioma patients identified tumor area, preoperative albumin concentration, and preoperative platelet count as independent predictors for higher intraoperative blood loss (26). On the other side, other studies also focused on radiological assessment of meningiomas’ vascularity and the meningioma vascularity index based on flow-void volume in T2-weighted sequences has been established to predict blood loss and need for blood transfusion in preoperatively non-embolized meningiomas (34).
Maintaining hemostasis and reducing intraoperative blood loss is crucial for achieving safe maximal resection. Preoperative embolization is another option to reduce blood-loss, which aims to devascularize the lesion. Despite a meta-analysis of matched cohort studies with 434 patients found no significant difference in intraoperative blood loss between those being preoperatively embolized or non-embolized, preoperative embolization resulted in lower odds ratios of major surgically related complications (35). Hence, this procedure might be dedicated for some selective cases and identification of these might be facilitated by the use of arterial spin labeling perfusion MRI (36). TXA as an antifibrinolytic pharmacological means of preventing blood loss demonstrated efficacy in reducing blood loss and blood product usage across various other fields of surgery (37–41). The present meta-analysis investigated blood transfusion requirements in six studies with a total of 841 patients. The studies by Hooda et al. (19), Rebai et al. (23) and Li et al. (25) reported nonsignificant reductions in transfusions, while the studies by Khalid et al. (24), Ravi et al. (22), and Siddiqui et al. (20) showed significant decrease in transfusion requirements among TXA recipients. The reduced blood loss reduces the need for allogeneic blood transfusions, shortens the operative time and length of hospital stay. Hence, TXA administration might also reduce the costs of treatment as suggested in a matched cohort study with patients undergoing primary total hip and knee replacement surgery with or without TXA (42). Further studies will also have to address the economic burden of cranial meningioma surgery with or without TXA application.
4.4 Dosing
The current investigation found that TXA use is safe and effective in terms of reducing blood loss, blood transfusions, and facilitating operative time. Despite a high variability with regard to loading doses from 10 to 50 mg/kg, six of the seven included studies used the dose of 20 mg/kg before skin incision (43). This dosage is higher than those reported in orthopedic procedures but within safe limits as established by studies from coronary-artery surgery (44). Against this backdrop, the low complication profile observed in this analysis of 981 patients suggests TXA application as safe and effective.
4.5 Limitations & future directions
While the present investigation provides the largest meta-analysis of seven randomized placebo-controlled, and double-blinded trials with 981 patients regarding the use of TXA in cranial meningioma surgery, it is essential to consider some major limitations. The present meta-analysis includes seven studies, which implicates the risk of publication bias. Methods like funnel plot asymmetry might be unreliable for seven studies, making it difficult to draw definitive conclusions (45, 46). All studies in the meta-analysis were conducted outside the US and EU, which may represent a limitation due to geographical concentration. Furthermore, subgroup meta-analysis of outcome parameters by important factors such as age were not possible because the included studies did not stratify into elderly and non-elderly meningioma patients. Range of 95% confidence interval in terms of the analysis of reoperation rates was 1.32 which implicates that the results regarding this endpoint should be interpreted with caution. The indication to perform reoperations in terms of hydrocephalus or postoperative hematoma might also vary between the institutions and influence the outcome analysis. Future research should prioritize refining TXA dosage and administration protocols to enhance efficacy while minimizing risks. Analyzing clinical endpoints in future trials could benefit from focusing on a homogeneous anatomical subgroup of meningiomas, such as non-skull base tumors in order to identify potential functional outcome relevant differences (e.g. GOSE grading, seizure frequency). Additionally, investigating the use of TXA in combination with other hemostatic strategies, like preoperative embolization, could provide valuable insights into optimizing surgical outcomes. By comparing these approaches, researchers can develop more effective treatment protocols that improve patient safety and surgical efficiency in meningioma surgery.
5 Conclusion
This meta-analysis demonstrates that intraoperative administration of tranexamic acid (TXA) in cranial meningioma surgery significantly reduces intraoperative blood loss and the need for red blood cell transfusions, thereby shortening operative times and hospital stays. Despite these benefits, TXA does not significantly affect other clinical outcomes such as postoperative seizures, reoperation rates, neurological outcomes, or the incidence of hydrocephalus and hematomas. The safety profile of TXA remains favorable, with no significant increase in the risk of thromboembolic events or other severe complications.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
MV: Conceptualization, Data curation, Project administration, Visualization, Writing – original draft, Writing – review & editing. FA: Writing – review & editing. EG: Writing – review & editing. JW: Conceptualization, Data curation, Methodology, Software, Supervision, Validation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
The graphical abstract in this article was created using BioRender. The author(s) acknowledge support from the German Research Foundation (DFG) and Leipzig University within the program of Open Access Publishing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2024.1464671/full#supplementary-material
References
1. Relke N, Chornenki NLJ, Sholzberg M. Tranexamic acid evidence and controversies: An illustrated review. Res Pract Thromb Haemost. (2021) 5:e12546. doi: 10.1002/rth2.12546
2. Cai J, Ribkoff J, Olson S, Raghunathan V, Al-Samkari H, DeLoughery TG, et al. The many roles of tranexamic acid: An overview of the clinical indications for TXA in medical and surgical patients. Eur J Hematol. (2020) 104:79–87. doi: 10.1111/ejh.13348
3. Shakur H, Elbourne D, Gülmezoglu M, Alfirevic Z, Ronsmans C, Allen E, et al. The WOMAN Trial (World Maternal Antifibrinolytic Trial): tranexamic acid for the treatment of postpartum hemorrhage: an international randomized, double blind placebo controlled trial. Trials. (2010) 11:40. doi: 10.1186/1745-6215-11-40
4. Roberts I, Shakur H, Coats T, Hunt B, Balogun E, Barnetson L, et al. The CRASH-2 trial: a randomized controlled trial and economic evaluation of the effects of tranexamic acid on death, vascular occlusive events and transfusion requirement in bleeding trauma patients. Health Technol Assess. (2013) 17:1–79. doi: 10.3310/hta17100
5. Rajagopalan V, Chouhan RS, Pandia MP, Lamsal R, Rath GP. Effect of intraoperative blood loss on perioperative complications and neurological outcome in adult patients undergoing elective brain tumor surgery. J Neurosci Rural Pract. (2019) 10:631–40. doi: 10.1055/s-0039-3399487
6. Zuo MR, Liang RF, Li M, Xiang YF, Zhang SX, Yang Y, et al. A comprehensive study of risk factors for post-operative pneumonia following resection of meningioma. BMC Cancer. (2019) 19:100. doi: 10.1186/s12885-019-5271-7
7. Ogasawara C, Philbrick BD, Adamson DC. Meningioma: A review of epidemiology, pathology, diagnosis, treatment, and future directions. Biomedicines. (2021) 9:319. doi: 10.3390/biomedicines9030319
8. Clynch AL, Gillespie CS, Richardson GE, Mustafa MA, Islim AI, Keshwara SM, et al. Tranexamic acid use in meningioma surgery - A systematic review and meta-analysis. J Clin Neurosci. (2023) 110:53–60. doi: 10.1016/j.jocn.2023.01.012
9. Grassin-Delyle S, Couturier R, Abe E, Alvarez JC, Devillier P, Urien S. A practical tranexamic acid dosing scheme based on population pharmacokinetics in children undergoing cardiac surgery. Anesthesiology. (2013) 118:853–62. doi: 10.1097/ALN.0b013e318283c83a
10. Patel PA, Wyrobek JA, Butwick AJ, Pivalizza EG, Hare GMT, Mazer CD, et al. Update on applications and limitations of perioperative tranexamic acid. Anesth Analg. (2022) 135:460–73. doi: 10.1213/ANE.0000000000006039
11. Faraoni D, Levy JH. Optimal tranexamic acid dosing regimen in cardiac surgery: what are the missing pieces? Anesthesiology. (2021) 134:143–6. doi: 10.1097/ALN.0000000000003637
12. Islim AI, Ali A, Bagchi A, Ahmad MU, Mills SJ, Chavredakis E, et al. Postoperative seizures in meningioma patients: improving patient selection for antiepileptic drug therapy. J Neurooncol. (2018) 140:123–34. doi: 10.1007/s11060-018-2941-2
13. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
14. Higgins JPT, Thomas J, Chandler J, et al. Cochrane Handbook for Systematic Reviews of Interventions version 6.4 (updated August 2023). Cochrane (2023). Available online at: www.training.cochrane.org/handbook (Accessed 2nd April 2024).
15. Schardt C, Adams MB, Owens T, Keitz S, Fontelo P. Utilization of the PICO framework to improve searching PubMed for clinical questions. BMC Med Inform Decis Mak. (2007) 7:16. doi: 10.1186/1472-6947-7-16
16. Wilson JT, Pettigrew LE, Teasdale GM. Structured interviews for the Glasgow Outcome Scale and the extended Glasgow Outcome Scale: guidelines for their use. J Neurotrauma. (1998) 15:573–85. doi: 10.1089/neu.1998.15.573
17. Ma LL, Wang YY, Yang ZH, Huang D, Weng H, Zeng XT. Methodological quality (risk of bias) assessment tools for primary and secondary medical studies: what are they and which is better? Mil Med Res. (2020) 7:7. doi: 10.1186/s40779-020-00238-8
18. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. (2003) 327:557–60. doi: 10.1136/bmj.327.7414.557
19. Hooda B, Chouhan RS, Rath GP, Bithal PK, Suri A, Lamsal R. Effect of tranexamic acid on intraoperative blood loss and transfusion requirements in patients undergoing excision of intracranial meningioma. J Clin Neurosci. (2017) 41:132–8. doi: 10.1016/j.jocn.2017.02.053
20. Siddiqui AK, Raman R, Arshad Z, Hemlata SV, Hashmi AS. Use of tranexamic acid to reduce intraoperative bleeding in craniotomy for meningioma patients. Asian Arch Anesthesiol Resusc. (2018) 85:2621–9.
21. Sutanto S, Bisri DY, Bisri T. Effects of intravenous tranexamic acid on blood loss and transfusion requirements in tumor removal surgery of suspected meningioma. J Neuroanestesi Indones. (2019) 8:8–16. doi: 10.24244/jni.vol8i1.200
22. Ravi GK, Panda N, Ahluwalia J, Mahajan S, Chauhan R, Singla N. Effect of tranexamic acid on blood loss, coagulation profile, and quality of surgical field in intracranial meningioma resection: A prospective randomized, double-blind, placebo-controlled study. Surg Neurol Int. (2021) 12:272. doi: 10.25259/SNI_296_2021
23. Rebai L, Mahfoudhi N, Fitouhi N, Daghmouri MA, Bahri K. Intraoperative tranexamic acid use in patients undergoing excision of intracranial meningioma: Randomized, placebo-controlled trial. Surg Neurol Int. (2021) 12:289. doi: 10.25259/SNI_177_2021
24. Khalid A, Nazir U, Raza N, Aslam S, Majeed MN, Hassan Z. A comparison of the use of tranexamic acid versus placebo in patients undergoing excision of intracranial meningioma. Pak J Neurol Surg. (2023) 26:654–66. doi: 10.36552/pjns.v26i4.822
25. Li S, Liu M, Yang J, Yan X, Wu Y, Zhang L, et al. Intravenous tranexamic acid for intracerebral meningioma resections: A randomized, parallel-group, non-inferiority trial. J Clin Anesth. (2024) 92:111285. doi: 10.1016/j.jclinane.2023.111285
26. Wang C, Li P. Risk factors for intraoperative blood loss in resection of intracranial meningioma: Analysis of 530 cases. PloS One. (2023) 18:e0291171. doi: 10.1371/journal.pone.0291171
27. Tabibkhooei A, Azar M, Alagha A, Jahandideh J, Ebrahimnia F. Investigating effective factors on estimated hemorrhage intraoperative in brain meningioma surgery. Basic Clin Neurosci. (2020) 11:631–8. doi: 10.32598/bcn.9.10.370
28. Lü J. Correlation between preoperative imaging features and intraoperative blood loss of meningioma: a new scoring system for predicting intraoperative blood loss. J Neurosurg Sci. (2013) 57:153–61.
29. Pennington Z, Ehresman J, Schilling A, Feghali J, Hersh AM, Hung B, et al. Influence of tranexamic acid use on venous thromboembolism risk in patients undergoing surgery for spine tumors. J Neurosurg Spine. (2021) 35:663–73. doi: 10.3171/2021.1.SPINE201935
30. Irl H, Kratzer S, Schwerin S, Kochs E, Blobner M, Schneider G, et al. Tranexamic acid impairs hippocampal synaptic transmission mediated by gamma aminobutyric acid receptor type A. Eur J Pharmacol. (2017) 815:49–55. doi: 10.1016/j.ejphar.2017.10.001
31. Tian N, Sun Y, Liu Y, Jin J, Chen S, Han H, et al. Safety assessment of tranexamic acid: real-world adverse event analysis from the FAERS database. Front Pharmacol. (2024) 15:1388138. doi: 10.3389/fphar.2024.1388138
32. Wach J, Lampmann T, Güresir Á, Vatter H, Herrlinger U, Becker A, et al. Proliferative potential, and inflammatory tumor microenvironment in meningioma correlate with neurological function at presentation and anatomical location-from convexity to skull base and spine. Cancers (Basel). (2022) 14:1033. doi: 10.3390/cancers14041033
33. Meling TR, Da Broi M, Scheie D, Helseth E. Meningiomas: skull base versus non-skull base. Neurosurg Rev. (2019) 42:163–73. doi: 10.1007/s10143-018-0976-7
34. Lagman C, Ong V, Nguyen T, Alkhalid Y, Sheppard JP, Romiyo P, et al. The Meningioma Vascularity Index: a volumetric analysis of flow voids to predict intraoperative blood loss in nonembolized meningiomas. J Neurosurg. (2018) 130:1547–52. doi: 10.3171/2018.1.JNS172724
35. Schartz D, Furst T, Ellens N, Kohli GS, Rahmani R, Akkipeddi SMK, et al. Preoperative embolization of meningiomas facilitates reduced surgical complications and improved clinical outcomes: A meta-analysis of matched cohort studies. Clin Neuroradiol. (2023) 33:755–62. doi: 10.1007/s00062-023-01272-4
36. Yoo RE, Yun TJ, Cho YD, Rhim JH, Kang KM, Choi SH, et al. Utility of arterial spin labeling perfusion magnetic resonance imaging in prediction of angiographic vascularity of meningiomas. J Neurosurg. (2016) 125:536–43. doi: 10.3171/2015.8.JNS151211
37. Zhu J, Zhu Y, Lei P, Zeng M, Su W, Hu Y. Efficacy and safety of tranexamic acid in total hip replacement: a PRISMA- compliant meta-analysis of 25 randomized controlled trials. Med (Baltimore). (2017) 96:e9552. doi: 10.1097/MD.0000000000009552
38. Zhang LK, Ma JX, Kuang MJ, Zhao J, Lu B, Wang Y, et al. The efficacy of tranexamic acid using oral administration in total knee arthroplasty: a systematic review and meta-analysis. J Orthop Surg Res. (2017) 12:159. doi: 10.1186/s13018-017-0660-6
39. Mei A, Qiu L. The efficacy of tranexamic acid for orthognathic surgery: a meta-analysis of randomized controlled trials. Int J Oral Maxillofac Surg. (2019) 48:1323–8. doi: 10.1016/j.ijom.2018.07.027
40. Joseph J, Martinez-Devesa P, Bellorini J, Burton MJ. Tranexamic acid for patients with nasal hemorrhage (epistaxis). Cochrane Database Syst Rev. (2018) 12:CD004328. doi: 10.1002/14651858.CD004328.pub3
41. Liu ZG, Yang F, Zhu YH, Liu GC, Zhu QS, Zhang BY. Is tranexamic acid beneficial in open spine surgery? and its effects vary by dosage, age, sites, and locations: a meta-analysis of randomized controlled trials. World Neurosurg. (2022) 166:141–52. doi: 10.1016/j.wneu.2022.07.044
42. Irisson E, Hémon Y, Pauly V, Parratte S, Argenson JN, Kerbaul F. Tranexamic acid reduces blood loss and financial cost in primary total hip and knee replacement surgery. Orthop Traumatol Surg Res. (2012) 98:477–83. doi: 10.1016/j.otsr.2012.05.002
43. Brown NJ, Wilson B, Ong V, Gendreau JL, Yang CY, Himstead AS, et al. Use of tranexamic acid for elective resection of intracranial neoplasms: A systematic review. World Neurosurg. (2022) 160:e209–19. doi: 10.1016/j.wneu.2021.12.117
44. Myles PS, Smith JA, Painter T. Tranexamic acid in patients undergoing coronary-artery surgery. N Engl J Med. (2017) 376:1893. doi: 10.1056/NEJMc1703369
45. Von Hippel PT. The heterogeneity statistic I(2) can be biased in small meta-analyses. BMC Med Res Methodol. (2015) 15:35. doi: 10.1186/s12874-015-0024-z
Keywords: blood loss, complications, meningioma, Tranexamic Acid, transfusion
Citation: Vychopen M, Arlt F, Güresir E and Wach J (2024) Intraoperative tranexamic acid administration in cranial meningioma surgery: a meta-analysis of prospective randomized, double-blinded, and placebo-controlled trials. Front. Oncol. 14:1464671. doi: 10.3389/fonc.2024.1464671
Received: 14 July 2024; Accepted: 14 August 2024;
Published: 29 August 2024.
Edited by:
Terry Lichtor, Rush University Medical Center, United StatesReviewed by:
Daniel Dubinski, University Hospital Rostock, GermanyZhijie Zhao, Shanghai Jiao Tong University, China
Copyright © 2024 Vychopen, Arlt, Güresir and Wach. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Johannes Wach, am9oYW5uZXMud2FjaEBtZWRpemluLnVuaS1sZWlwemlnLmRl
 Martin Vychopen
Martin Vychopen Felix Arlt
Felix Arlt Erdem Güresir
Erdem Güresir Johannes Wach
Johannes Wach