- 1Department of Biotherapy, Cancer Center, West China Hospital, Sichuan University, Chengdu, China
- 2West China School of Medicine, Sichuan University, Chengdu, China
- 3West China School of Nursing, Sichuan University, Chengdu, China
- 4Department of Oncology and Hematology, Air Force Hospital of Western Theater Command, Chengdu, China
With the development of comprehensive treatment, locoregional transarterial chemotherapy has become an alternative conversion therapy, palliative therapy, and neoadjuvant therapy for many solid malignant tumors. Locoregional transarterial chemotherapy, which is most frequently used for treating liver cancer, has the characteristics of high regional efficacy and few systemic adverse reactions. In recent years, the number of relevant reports of locoregional chemotherapy for treating initially inoperable colorectal cancer (CRC), including non-metastatic and metastatic CRC, has gradually increased. However, the specific treatment options for such locoregional therapy are not the same, and its indications, medication regimens and combined treatments have not reached any consensus. In this review, the application status of locoregional transarterial chemotherapy in primary and metastatic CRC patients has been reviewed and summarized to provide a reference for future clinical work and scientific research.
1 Introduction
Colorectal cancer (CRC) is a common disease of the human digestive system with difficulties in the diagnosis and treatment (1). According to the global cancer statistics released, the current incidence and mortality of CRC rank third and second among all malignant tumors, respectively (2). With the global improvement of economies and living conditions, the number of newly diagnosed CRC patients is showing a rising trend, especially in developing countries. As the largest developing country, China is facing a more severe cancer burden due to backward cancer screening (3). The incidence of CRC in China has increased rapidly in the past twenty years and has reached 17.81/100,000 (4). If early detection and treatment are not available, such CRC patients may already be in the moderate or advanced stage when clinical symptoms appear. Patients with local invasion or distant organ metastasis (clinical stage III-IV) always have an extremely shorter survival time owing to the rapid progression of the primary disease and distant metastasis (DM). The five-year survival rate of CRC patients with DM is approximately about 10%, while in CRCs with localized lesions, the figure exceeds 90% (5–7).
At present, the radical cure for most CRC patients is surgical resection of the primary lesion with adjacent lymph nodes. Patients treated with surgery have obvious advantages in terms of quality of life and prognosis, compared to those treated with only drugs or palliative care. Once the tumor is evaluated as stage I or II, direct tumor excision is mostly regarded as the first choice without any neoadjuvant treatment (8). Due to the dominant position of surgical principles in CRC, the purpose of most preoperative treatments is to create operable conditions for surgeons. However, only a small number of CRC patients can undergo surgery in the initial stage because of local tumor infiltration, lymph node metastases and DM, etc. In the process of CRC treatment, the use of multidisciplinary team (MDT) is widespread, and long-term benefits have been confirmed after MDT discussions (9, 10). The satisfactory effect of MDT should be attributed to the rational application of various systemic and regional strategies in those patients, such as chemotherapy, radiotherapy, hormone therapy, biotherapy, ethnomedicine therapy, and interventional therapy, etc. (11) As a result, the concept of CRC treatment has been transformed into a pattern of combining multiple modes under the guidance of MDT with surgical treatment at the core.
Since CRC is a local lesion at the macro level, studies on locoregional therapies for CRC have never stopped. With the progress of interventional medicine, minimally invasive interventional chemotherapy has been included into the comprehensive treatment of various advanced solid tumors, and has achieved good results (12–16). Among them, liver cancer especially hepatocellular carcinoma (HCC) is the most widely applied kind of malignant tumors. In the 1970s, transcatheter arterial chemoembolization (TACE) was first proposed for liver cancer treatment using Seldinger technology and has been actively spread clinically (17). With more than 40 years of development of new drugs and materials, hepatic arterial infusion chemotherapy (HAIC) and TACE combined with other therapies have been used throughout the treatment of both primary and secondary liver cancer (18). Currently, TACE has become the first-line method for advanced HCC; indeed, it is considered the best choice for inoperable primary HCC (19). For secondary liver cancer, which is liver metastatic tumor, HAIC and TACE are also optional methods when surgical operation is not applicable for the liver lesion. Liver is the most common metastatic organ of CRC, and colorectal liver metastasis (CRLM) is also the main cause of cancer-related death (20). Statistics indicate that approximately 15% to 25% of CRC patients have liver metastasis at the time of initial diagnosis, and about 10% to 25% of CRC patients will ultimately develop CRLM after the primary lesion is resected (21–23). Although DM is often regarded as a contraindication to surgery, the best treatment for CRLM is resection of both the primary lesions and liver lesions if possible, as well as liver transplantation (24, 25). For CRLM patients with liver lesions already resected, the 5-year survival rate can be greater than 30%, while for patients without liver lesions resected, the 5-year survival rate can be less than 20%; however, there are only about 20% of patients with CRLM whose liver metastases can be surgically treated after the initial evaluation (26, 27). If conversion therapies for CRLM patients with unresectable liver metastases are successfully applied to reduce metastases to potentially resectable status, followed by surgery, the 5-year survival rate can be improved to the same level as that of CRLM with initially liver lesion resectable (28, 29). Therefore, multiple conversion therapies must be used to convert liver lesions to a resectable state, so as to improve the prognosis of CRLM. Normal hepatic tissue is mainly supplied by the portal vein, while the hepatic artery accounts for only approximately one-quarter of hepatic tissue; however, after the formation of liver metastases, the blood supply from the hepatic artery increases to 90% or more, which is also the anatomical basis for locoregional transarterial chemotherapy (HAIC and TACE) of hepatic lesions (30–32). The 5-year survival of CRLM has gradually increased in recent decades due to locoregional chemotherapeutic treatments.
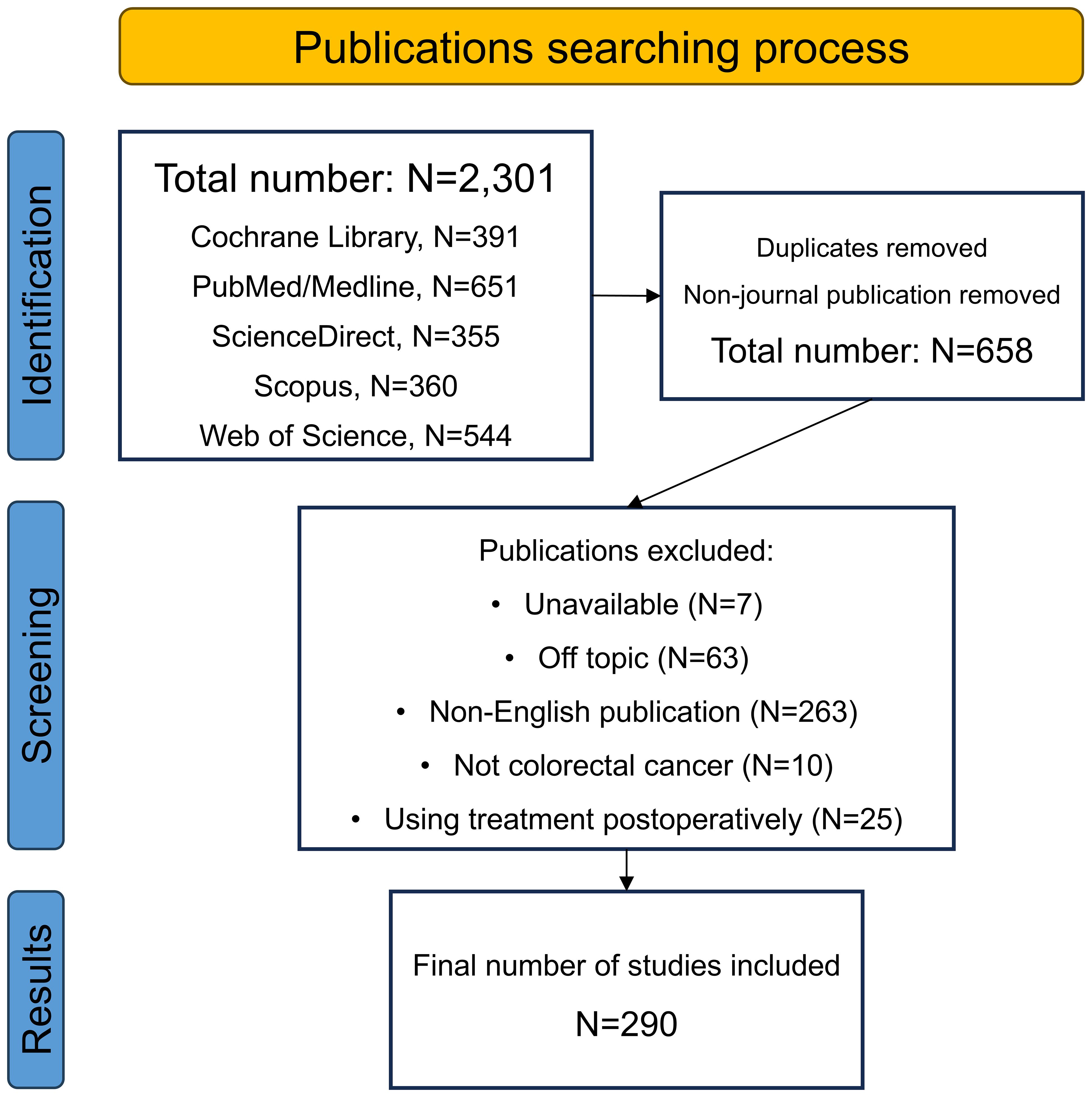
Apart from its universal use in liver cancer, interventional chemotherapy has also been actively applied in many other gastrointestinal cancers. For most advanced patients with gastric cancer (GC) or pancreatic cancer (PC), radical surgery is not applicable generally due to vast vascular involvement. Thus, conversion therapy including interventional chemotherapy must initially be used to improve the operation rate and R0 resection rate (33). Previous studies have demonstrated that interventional chemotherapy is an effective approach as both conversion and palliative treatment for advanced GC patients, and has a lower toxicity and complications than systemic chemotherapy (34, 35). Besides, when HAIC is used jointly with systemic chemotherapy in advanced PC, the response rate and survival are also improved (36, 37). However, reports of locoregional chemotherapies for primary CRC lesion are still rare. The good results in GC, PC and liver tumors have stimulated related studies in the CRC field. Recently, several researchers have performed large-scale investigations on the use of locoregional chemotherapy in CRC patients. To summarize recent updates of the locoregional transarterial chemotherapy and chemoembolization used for treating CRC in both localized and liver-metastatic lesions, we conducted this review by searching for data in electronic databases of Cochrane Library, PubMed/Medline, ScienceDirect, Scopus, and Web of Science. Figure 1 displays the flowchart of the searching strategy and process.
2 Hepatic arterial infusion chemotherapy for unresectable colorectal liver metastases
Serving as a palliative, conversion and adjuvant therapy, and using Seldinger technology and digital subtraction angiography (DSA), HAIC has become a common method for locoregional chemotherapy in patients with CRLM (38–41). During this process, chemotherapeutic drugs are precisely delivered to the supply arteries of liver metastatic lesions via catheters and microcatheters, after which the tumor can be specifically attacked. Through local puncture via the femoral or radial artery, the tumor-feeding arteries are superselected. Then, chemotherapeutic drugs at high concentrations directly reach the feeding artery without any metabolism and fully contact the tumor tissue, and the targeted cells are killed immediately. This method can increase the concentration of locoregional drugs by dozens of times and has lower systemic toxicity (42–44). Since tumors are very sensitive to the concentration of chemotherapeutic drugs, the multiplication of drug concentration around tumors will correspondingly improve the effect of chemotherapy. A pharmacokinetics study also indicated that when the concentration of local administration is doubled, the killing intensity of the targeted tumor cells can increase by more than ten times (45).
However, the use of HAIC alone in patients with CRLM remains controversial. For example, a case-control study showed that the median overall survival (OS) of CRLM patients treated with fluorouracil-based HAIC combined with systemic chemotherapy was extended to 32.8 months, while the median OS of patients treated with systemic chemotherapy alone was only 15.3 months, indicating a significant statistical difference (46). Additionally, a recent meta-analysis indicated that HAIC as a palliative method achieved a greater tumor remission rate for liver metastatic lesions in CRLM than did systemic chemotherapy, but there was no significant improvement in either progression-free survival (PFS) or OS (47). Therefore, most clinical institutions around the world have abandoned the use of HAIC alone in CRLM patients, and the combination of HAIC and systemic chemotherapy as well as targeted therapy has gradually become one of the directions of current applications in order to maximize its efficacy. A completed phase 2 study using HAIC with floxuridine plus systemic oxaliplatin and irinotecan reached an overall response rate (ORR) of 92% in 49 CRLM patients, with a median OS of 39.8 months (48). Another recent phase 2 study reported that HAIC using oxaliplatin plus systemic 5-fluorouracil (5-Fu) with cetuximab achieved an ORR of 88% in patients with 35 frontline CRLM patients; and the median PFS and OS were 17.9 and 46.3 months, respectively (49). However, other large-scale prospective trials on HAIC plus systemic therapy are still in the initial stage, so there is a lack of studies on the standard use of HAIC plus systemic therapy for CRLM (50).
As shown above, the drug selection is another controversy. Traditional chemotherapeutic agents, such as fluorouracils, oxaliplatin, and raltitrexed etc., are the most commonly used drugs for arterial infusion with their monotherapy or combination (51–53). In the United States, fluorouracils [5-Fu, irinotecan, and fluorodeoxyuridine (FUDR), etc.] are considered the most widely used drugs for HAIC (54). However, 5-Fu might be replaced because it has a longer arterial infusion time with a similar efficacy compared to other drugs (52, 55). One of the first-line systemic chemotherapy drugs for treating CRLM is oxaliplatin, which is the most commonly used drug in European countries, and it requires only approximately 2 hours for infusion; furthermore, oxaliplatin has shown efficacy as a HAIC drug in patients who are resistant to oxaliplatin-based systemic chemotherapy (56, 57). Recently, the anti-angiogenic agent bevacizumab was also reported to be useful for hepatic arterial infusion, and may be an interesting option for the second-line treatment of CRLM patients (51, 58, 59).
3 Transcatheter arterial chemoembolization for unresectable colorectal liver metastases
The addition of embolization after infusion chemotherapy is a characteristic of TACE compared to HAIC. After the completion of drug infusion, the tumor feeding arteries are subsequently embolized with commonly used embolic agents, such as gelatin sponges, lipiodol, or drug-eluting beads (DEBs), etc. in the end (18, 60–62). According to different embolizing materials used, the TACE technique mainly includes conventional TACE (cTACE) and DEB-TACE (63). Additionally, new materials have developed new strategies such as balloon-occluded TACE (B-TACE), which expand the range of TACE technique and show promising results in primary HCC (64). Figure 2 shows different type of locoregional transarterial chemotherapy in primary and liver metastatic CRC patients. The embolizing procedure of TACE can increase the drug contact time and drug concentration in the tumor tissue, resulting in more powerful cytotoxicity and ischemic necrosis of the tumor tissue; meanwhile, the drug concentration in surrounding normal tissue can be correspondingly decreased (18, 25, 60, 65–67).
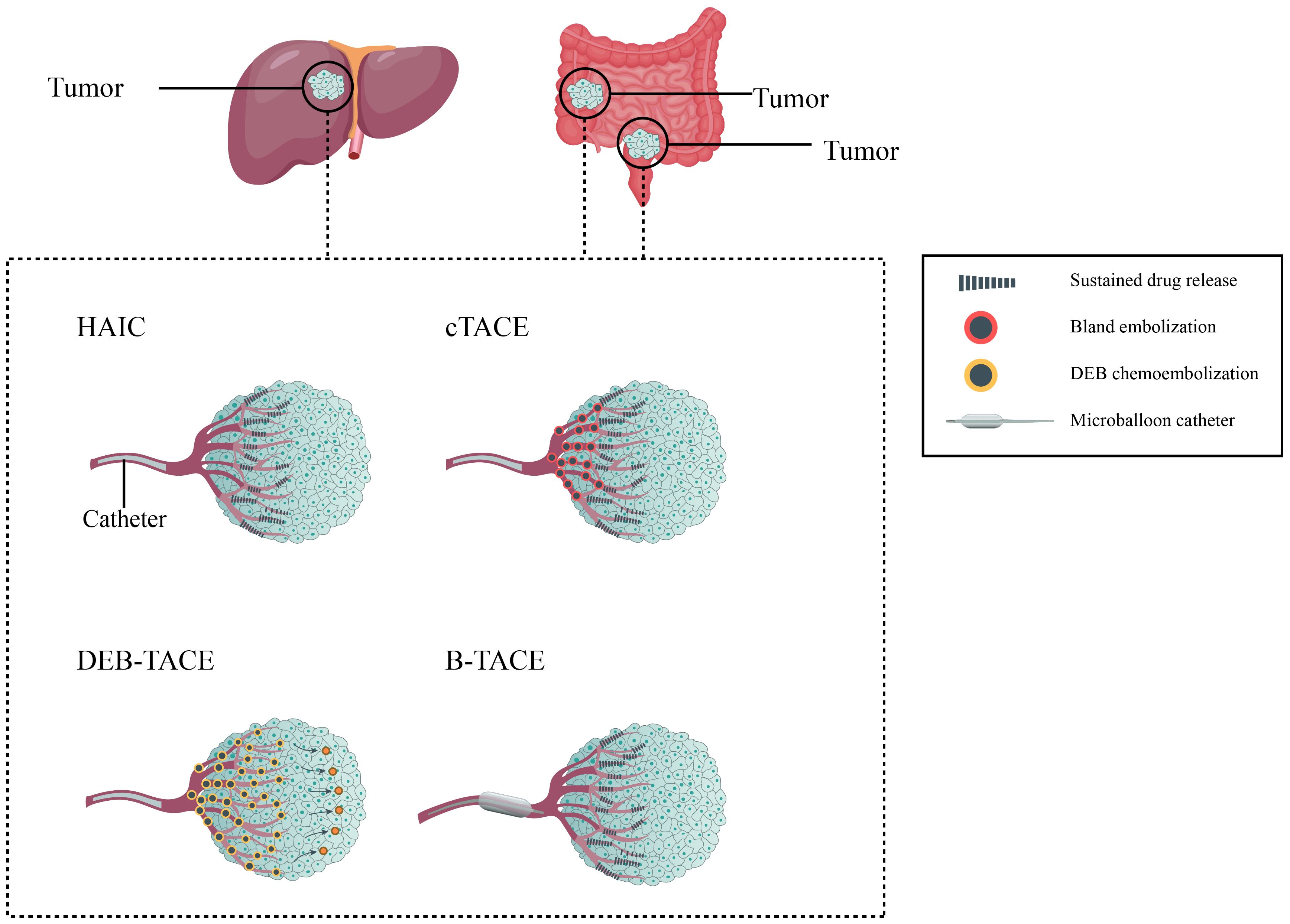
Figure 2. Different type of locoregional transarterial chemotherapy in primary and metastatic colorectal cancer patients.
However, no evidence has shown that TACE has better benefits in CRLM compared with HAIC, although TACE is a standard therapy. Indeed, there are reports on the combination of TACE and HAIC in treating liver tumors of CRLM, which may be another option when first-line therapy fails (68–70). Due to the controversy regarding the choice of cTACE or HAIC, DEB-TACE has become a promising star (71). DEB allows for the sustained and targeted release of chemotherapeutic agents directly to the tumor site, potentially enhancing the drug concentration within the tumor. DEB also prolongs the drug release time and enhances the effectiveness of the embolization procedure. Several small-scale studies have shown that the addition of DEB-TACE is reliable for treating liver lesions of CRLM, and the incidence of adverse events was in a low level even if more than three cycles of DEB-TACE were administered (72, 73). Although DEB-TACE has been widely carried out, there is no unified standard for the selection of loading drugs, embolic agents or catheter specifications, and its treatment endpoints are still controversial (74). The most commonly used loading drugs contain irinotecan and doxorubicin, and raltitrexed-loaded DEB has also been reported (75). However, no large studies have been finished yet, resulting in the starving for a direct comparison between DEB-TACE and cTACE in CRLM patients. Recently, Li et al. retrospectively analyzed a small sample of CRLM patients after multi-line therapies (76). DEB-TACE and cTACE were applied in a total of 22 patients, and the results indicated that 11 patients who received 300–500μm DEB-TACE had significantly longer PFS and OS than the other 11 patients who underwent cTACE. While DEB-TACE has shown benefits, there is limited evidence, especially large-scale studies, demonstrating a clear survival advantage of DEB-TACE compared to cTACE. Moreover, DEB is more expensive than conventional embolic agents, contributing to higher overall procedural costs.
Compared with the commonly accepted DEB specifications of 100–300μm or 300–500μm in diameter, recent studies have suggested that smaller specifications of 30–75μm could increase the drug concentration loaded in the tumor, thus exert stronger lethality (77–79). Hepatic lesions in CRLM are less hypervascular in comparison with primary HCC, thus DEB with smaller sizes may have the ability to deliver the drug deeper into the tumor tissue (80).
In addition, there is no standard system for evaluating the efficacy of DEB-TACE. Using different radiological evaluation criteria, such as the Response Evaluation Criteria in Solid Tumors (RECIST) criteria and the Choi criteria, might yield different prognostic results (81). This is because the RECIST criteria only take the long diameter of the tumor but not the density into account. After the TACE procedure, the change in tumor density is greater than the change in the long diameter because the supplying arteries are damaged. Although the RECIST criteria is currently the most commonly used objective method for assessing tumor response, it apparently has shortages compared to the Choi criteria.
Due to the above lack of consensus in both HAIC and TACE, large-scale clinical trials are urgent to provide basis for these treatment approaches of CRLM patients. Herein, we summarized completed and ongoing clinical trials regarding HAIC and TACE for CRLM in Table 1. These clinical trials may answer crucial issues and further assist clinical efforts.
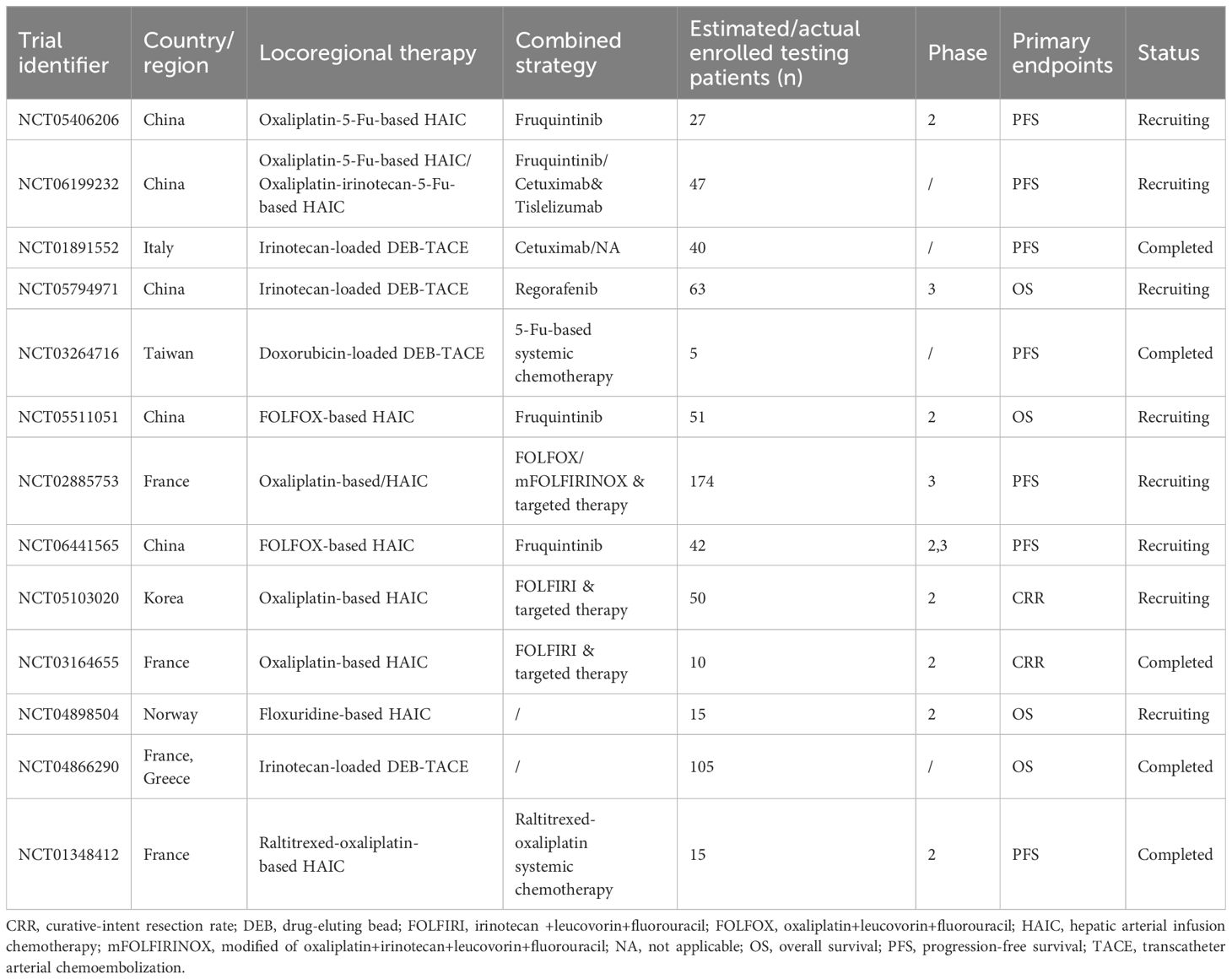
Table 1. Completed and ongoing clinical trials regarding locoregional interventional chemotherapy for colorectal liver metastasis.
4 Transarterial chemotherapy for non-metastatic colorectal cancer
To date, there are only a small amount of related data on locoregional interventional chemotherapy (infusion chemotherapy or chemoembolization) for primary non-metastatic CRC lesions (12, 60, 82–93). Due to the lack of large-scale clinical researches, consensus, and guidelines, this method has been implemented in only a few countries and hospitals (94). Currently, locoregional interventional chemotherapy is mainly applied for locally advanced CRC patients whose basic condition is poor and cannot tolerate the side effects of systemic intravenous chemotherapy. In addition, it can also be used for neoadjuvant therapy, inoperable situations, or postoperative local recurrence. In more than 30% advanced CRC patients, intestinal malignant obstruction is a common complication with high fatality (95). When malignant obstruction occurs, palliative surgery or metal stents imbedding are the most widely used methods. However, they cannot be suitable for patients with poor condition, as well as prevent tumor recurrence. Transarterial chemotherapy combined embolization has been applied in those patients as a safe, effective and feasible approach to prolong the non-obstruction time as well as survival time (93).
Adjuvant chemotherapy can prevent postoperative DM. In those CRC cases treated with surgery and adjuvant chemotherapy but suffered from DM, the resistance of systemic chemotherapy is the main cause. As the DM develops, chemoresistance may have already initiated. Given the high concentration and toxicity of chemotherapeutic drugs, locoregional interventional chemotherapy has the potential to partially reverse chemoresistance. Locally advanced CRC carries a higher risk of DM, so current studies on locoregional interventional chemotherapy in non-metastatic CRC predominantly focus on cases classified as locally advanced CRC.
The current published literature related to locoregional interventional chemotherapy for non-metastatic CRC is summarized in Table 2. To our knowledge, the largest-scale study to date is a finished multi-center randomized controlled trial (RCT) by Zhu et al. (NCT00643877) which was led by Zhongshan Hospital of Fudan University, Shanghai, China (90). It concluded that in CRC with stage II-III, the combination of hepatic and regional arterial chemotherapy using FUDR and oxaliplatin greatly decreased the 5-year liver metastasis rate (7% vs. 16%, P<0.001), thus increased the disease-free survival (DFS; 77% vs. 65%, P=0.001) and OS (84% vs. 76%, P=0.005). But limitations exist in this study such as the lack of neoadjuvant therapy in the control group, which might lower the credibility of its results.
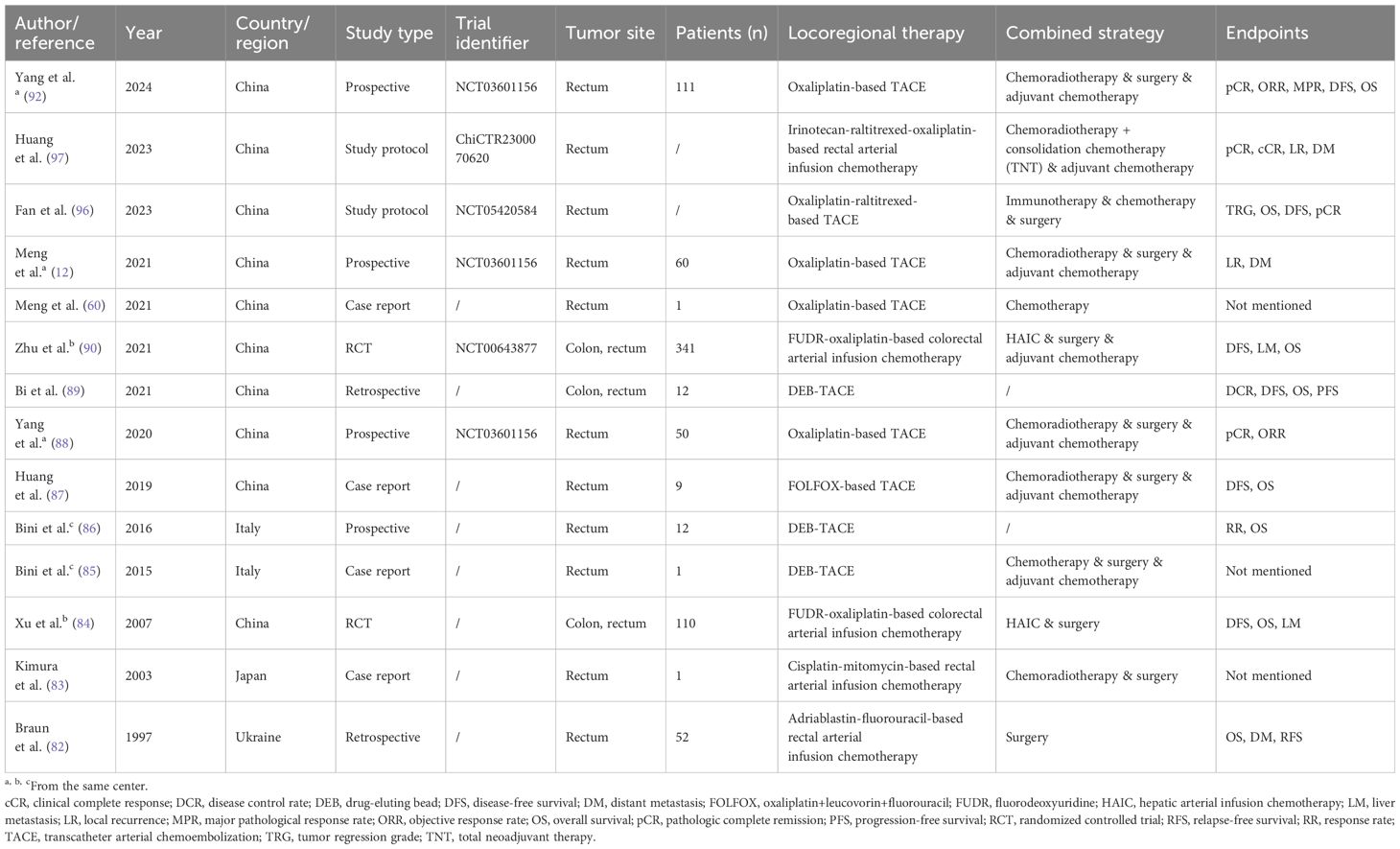
Table 2. Published literature regarding locoregional interventional chemotherapy for non-metastatic colorectal cancer.
According to the studies listed in the table, there is a trend that many new treatment methods are combined with locoregional chemotherapy to treat CRC. Recently, two phase 2 study protocols (NCT05420584 and NCT05957016) have been released regarding the combination of regional chemotherapy and multiple treatments, including systemic chemotherapy, radiotherapy, and immunotherapy for locally advanced rectal cancer (LARC) (96). Another study protocol (ChiCTR2300070620) designed by Huang et al. reported that total neoadjuvant therapy will also be applied concurrently with intra-arterial chemotherapy to LARC patients (97). However, all of these protocols are of single-arm studies, which may be a slight weakness in the study design. Still, the results will be full of expectation because preliminary results (NCT03601156) have proved the efficacy of regional chemotherapy in LARC, and its consecutive clinical trial is ongoing (NCT05957016) (88, 92).
5 Combine immunotherapy and locoregional transarterial chemotherapy in colorectal cancer
The potential mechanisms of combining locoregional transarterial chemotherapy with immunotherapy (such as immune checkpoint inhibitors) include enhancing tumor cell sensitivity to immunotherapy through high local concentrations of chemotherapeutic drugs, thereby improving therapeutic efficacy (98). Specific mechanisms involve chemotherapy-induced tumor antigen release and immunogenic cell death, which help enhance the efficacy of immune checkpoint inhibitors. TACE is shown to impact tumor angiogenesis as well as immune function within the tumor microenvironment (TME) (99). Nevertheless, the multifaceted physiological effects of TACE on the TME are uncertain, which encourages the exploration of potential synergies with different anti-tumor drugs to expand its application and improve patient outcomes. In recent years, several key clinical trials have evaluated the use of locoregional transarterial chemotherapy combined with immune checkpoint inhibitors, such as programmed cell death-1 (PD-1) and programmed cell death-ligand 1 (PD-L1) inhibitors, in liver cancer (98). Studies have shown that combined therapy results in significantly longer survival and greater tumor shrinkage compared to monotherapy. The combined therapy strategy has broad clinical practice prospects, including patient selection criteria, optimal drug combinations, and treatment regimens. In NCT05420584 and NCT05957016 trials, immunotherapy is applied both for LARC in combination with locoregional transarterial chemotherapy. Especially in NCT05957016 trial, Tislelizumab, a PD-1 inhibitor, is firstly reported in use for locoregional infusion.
However, some limitations in current researches and trials, such as better control of treatment side effects and optimization of individualized treatment plans, need further exploration. Several successful case studies demonstrated the specific clinical effects and patient outcomes of combining locoregional transarterial chemotherapy with immunotherapy, further proving the feasibility and efficacy of this combined therapy strategy in clinical practice.
6 Adverse effects of locoregional chemotherapies for colorectal cancer
Most studies have concluded that HAIC in treating CRLM is a safe method with side effects similar to those of conventional therapies. Symptoms from the digestive system are the most common complications after HAIC treatment with an incidence of up to one-third, including stomatitis, hyperbilirubinemia, elevation of liver enzymes, biliary sclerosis, nausea, vomiting, and diarrhea, etc. (55, 100, 101). Some studies reported that abdominal pain was the only specific side effect of HAIC, which might be due to the drug toxicity to blood vessels, especially when using oxaliplatin (102–104). But some complications related to catheterization, such as hepatic artery occlusion, thrombosis, bile duct necrosis and catheter-related infections, also need to be very highly alerted (47, 105). One example is that due to the long infusion time of approximately 44 hours, 5-Fu-based HAIC might cause a higher incidence of catheter-related thrombosis and infection (106). In summary, although most side effects of HAIC can be addressed by symptomatic treatments, it is necessary to strengthen the monitoring during the course of HAIC.
cTACE is less safe than HAIC in some aspects, although the case fatality rate of cTACE is less than 1% (107). Apart from having the same complications as HAIC, cTACE may result in more post-embolization complications thus prolonging hospital stays (104). Post-embolization syndrome, such as fever, pain, nausea, and vomiting, is a common and controllable reaction to the procedure. In rare cases, the embolization materials used during cTACE may inadvertently affect blood vessels in the surrounding area, leading to complications such as vessel injury or thrombosis (108). In addition, repeated TACE can lead to failure of liver function, thus affecting patient prognosis. The development of DEB-TACE has compensated for the shortcomings of traditional embolizing drugs in terms of action time and safety (109, 110). DEB can facilitate a more uniform distribution of chemotherapy drugs, thereby reducing systemic exposure and minimizing side effects associated with systemic drug administration (108). Therefore, systemic chemoresistance may also be postponed. But in a macro scope, there is no obvious difference in safety between DEB-TACE and cTACE. Several clinical trials and retrospective studies have assessed the safety of DEB-TACE for the treatment of HCC, but there is a lack of studies on the treatment of CRLM.
The application of TACE for primary lesions of CRC has also shown tolerable side effects in published reports. A study by Gao et al. indicated that TACE plus chemotherapy was safer than chemoradiotherapy for LARC patients, especially for reducing the anastomotic leakage (111). Since neoadjuvant radiation may increase the rate of anastomotic leakage after subsequent surgery, especially in rectal cancer patients, studies have confirmed that the single use of chemotherapy in neoadjuvant stage is safer with similar prognosis (112). This finding indicated that TACE may have the potential to replace the role of neoadjuvant radiotherapy in CRC.
Organ perforation is a potential severe adverse event, which is one of the reasons why locoregional chemoembolization of hollow organs is not as common as that of liver cancer. During the growth of tumor tissue, the blood supply of the surrounding tissue is relatively rich, and the central tissue is relatively ischemic. Coupled with the continuous compression and erosion of blood vessels by the tumor, ischemia and necrosis might occur in the center of the tumor. The tumor tissue infiltrates the whole layer of the gastric wall, and the muscle layer is broken, which can sometimes cause perforation. In published studies, no enterobrosis events were reported in patients who received locoregional interventional chemotherapy for primary CRC lesions. A rare syndrome reported in two case studies that delayed perforation occurred in the stomach and duodenum respectively after TACE in HCC patients (113, 114). The cause of such gastrointestinal tract perforation may be arterial ischemia due to embolization, especially after multiple TACEs. This syndrome can occur in such organs with enriched blood supply, so surgeons and nurses should pay more attention to the surgical procedure and post-TACE monitoring in CRC interventional chemotherapy.
In the context of locoregional therapies, the implementation of non-invasive monitoring (NIM) and follow-up is crucial. NIM techniques, such as imaging examinations, multi-model radiomics, and liquid biopsy, can regularly assess the treatment efficacy and promptly observe changes in the tumor (115–117). This is essential for evaluating the effectiveness of the treatment and formulating subsequent treatment plans, especially for those with combined treatments. Besides, NIM can early detect potential complications caused by the treatment, such as vascular injury, tumor bleeding, or local tissue necrosis. Timely intervention measures can be taken to reduce patient risk. Compared to invasive monitoring, NIM processes can improve patient compliance, reduce the psychological burden caused by the treatment, and promote overall health management of the patient. By performing NIM on cancer patients, doctors can adjust treatment plans based on the patient’s actual response, achieving better personalized treatment. This flexibility is important for enhancing treatment effectiveness and reducing side effects. Moreover, NIM plays an important role in patients’ follow-up. Through long-term observation, it is possible to evaluate patients’ safety, survival rates, recurrence rates, and other prognostic indicators, providing a basis for future treatment plans. The conventional NIM methods have intractable limitations, such as the exposure of imaging contrast agents and the limited specificity of serum protein. Advanced molecular detections, like DNA methylation in cell free circulating DNA (cfDNA) and circulating tumor-derived DNA (ctDNA), can be used to track response during metastatic CRC treatment, enabling NIM of tumor burden and drug resistance (115, 118). Furthermore, combining conventional NIM methods and repeated cfDNA or ctDNA assessments will be the directions for future researches to improve prognosis and quality of life in metastatic CRC patients.
7 Conclusions
Locoregional transarterial chemotherapy and chemoembolization have shown promising abilities to kill tumor cells, and relieve local symptoms for both primary lesions and liver metastases of CRC, especially when combined with systemic treatments. Meanwhile, the side effects can also be well controlled via various ways. As a palliative treatment, it can become a new addition for advanced patients and prolong survival. As a conversion or neoadjuvant therapy, it can create good conditions for subsequent radical surgery in both liver and primary lesions. On this basis, it is also expected to bring significant improvement to the prognosis. In the future, it is necessary to grasp the specific indications and contraindications, standardize the medication regimen, explore more accurate prognostic predictors, and carry out large-scale prospective studies in multiple centers to further verify the value of locoregional transarterial chemotherapy in CRC.
Author contributions
WM: Conceptualization, Data curation, Writing – original draft. LP: Data curation, Writing – original draft. LH: Data curation, Writing – original draft. QL: Funding acquisition, Resources, Supervision, Validation, Writing – review & editing. YS: Funding acquisition, Resources, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by (1) the National Natural Science Foundation of China (82203033); (2) the Sichuan Science and Technology Program (2023YFS0166; 2022YFQ0095); and (3) the National Clinical Research Center for Geriatrics, West China Hospital, Sichuan University (Z2023JC002).
Acknowledgments
All of the authors thank the funding agencies for their financial supporting. Thanks also go to our colleagues abroad, Didan Deng from The Hong Kong University of Science and Technology, and Sihan Li from University of Pittsburgh, for their professional English language editing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Li J-N, Li W, Cao L-Q, Liu N, Zhang K. Efficacy of mesenchymal stem cells in the treatment of gastrointestinal Malignancies. World J gastrointest Oncol. (2020) 12:365–82. doi: 10.4251/wjgo.v12.i4.365
2. Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, et al. Global cancer statistics 2022: globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2024) 74:229–63. doi: 10.3322/caac.21834
3. Jiang H, Zhang P, Gu K, Gong Y, Peng P, Shi Y, et al. Cost-effectiveness analysis of a community-based colorectal cancer screening program in Shanghai, China. Front Public Health. (2022) 10:986728. doi: 10.3389/fpubh.2022.986728
4. Zhang S, Sun K, Zheng R, Zeng H, Wang S, Chen R, et al. Cancer incidence and mortality in China, 2015. J Natl Cancer Center. (2021) 1:2–11. doi: 10.1016/j.jncc.2020.12.001
5. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. (2016) 66:7–30. doi: 10.3322/caac.21332
6. Bedri S, Sultan AA, Alkhalaf M, Al Moustafa AE, Vranic S. Epstein-barr virus (Ebv) status in colorectal cancer: A mini review. Hum Vaccin Immunother. (2019) 15:603–10. doi: 10.1080/21645515.2018.1543525
7. Ji Q, Zhou L, Sui H, Yang L, Wu X, Song Q, et al. Primary tumors release itgbl1-rich extracellular vesicles to promote distal metastatic tumor growth through fibroblast-niche formation. Nat Commun. (2020) 11:1211. doi: 10.1038/s41467-020-14869-x
8. Li Y, Wu M, Xu S, Huang H, Yan L, Gu Y. Colorectal cancer stem cell-derived exosomal long intergenic noncoding rna 01315 (Linc01315) promotes proliferation, migration, and stemness of colorectal cancer cells. Bioengineered. (2022) 13:10827–42. doi: 10.1080/21655979.2022.2065800
9. Przystupski D, Niemczura MJ, Górska A, Supplitt S, Kotowski K, Wawryka P, et al. In search of panacea-review of recent studies concerning nature-derived anticancer agents. Nutrients. (2019) 11:1426. doi: 10.3390/nu11061426
10. Zhang Q, Zhou Y, Song L, Fang W, Qiu M, Gu Y, et al. China special issue on gastrointestinal tumor-improved survival after multidisciplinary team decision for patients with advanced gastrointestinal cancer: A multicenter, noninterventional, controlled study. Int J Cancer. (2023) 153:1885–93. doi: 10.1002/ijc.34543
11. Khorsandi K, Kianmehr Z, Hosseinmardi Z, Hosseinzadeh R. Anti-cancer effect of gallic acid in presence of low level laser irradiation: ros production and induction of apoptosis and ferroptosis. Cancer Cell Int. (2020) 20:18. doi: 10.1186/s12935-020-1100-y
12. Meng W, Yang B, Huang B, Chen C, Zhu J, Jian D, et al. Impact of preoperative transcatheter rectal arterial chemoembolization with concurrent chemoradiotherapy on surgery and prognosis of patients with locally advanced rectal cancer. J Surg Oncol. (2021) 124:1451–8. doi: 10.1002/jso.26673
13. Sun Y, Li G, Hai P, Cao Y, Han P, Liu Y, et al. The comparative study for survival outcome of locally advanced cervical cancer treated by neoadjuvant arterial interventional chemotherapy or intravenous chemotherapy followed by surgery or concurrent chemoradiation. World J Surg Oncol. (2022) 20:389. doi: 10.1186/s12957-022-02859-w
14. Peng D, Zhang B, Yuan C, Tong Y, Zhang W. Gastric transcatheter chemoembolization can resolve advanced gastric cancer presenting with obstruction. Front Surg. (2022) 9:1004064. doi: 10.3389/fsurg.2022.1004064
15. Owen M, Makary MS, Beal EW. Locoregional therapy for intrahepatic cholangiocarcinoma. Cancers (Basel). (2023) 15:2384. doi: 10.3390/cancers15082384
16. Chevallier O, Zhao K, Marinelli B, Yarmohammadi H. Image-guided percutaneous locoregional therapies for hepatocellular carcinoma. Chin Clin Oncol. (2023) 12:17. doi: 10.21037/cco-22-119
17. Guan YS, He Q, Wang MQ. Transcatheter arterial chemoembolization: history for more than 30 years. ISRN Gastroenterol. (2012) 2012:480650. doi: 10.5402/2012/480650
18. Lewandowski R, Geschwind J, Liapi E, Salem R. Transcatheter intraarterial therapies: rationale and overview. Radiology. (2011) 259:641–57. doi: 10.1148/radiol.11081489
19. Chen C, Duan XT, Li GY, Hao XJ, Wang WL, Shen YF, et al. Evaluation of the efficacy of aparatinib and carrilizumab combined with transcatheter arterial chemoembolization in the treatment of primary hepatocellular carcinoma. Eur Rev Med Pharmacol Sci. (2023) 27:4135–44. doi: 10.26355/eurrev_202305_32322
20. Wang L, Sun Y, Luo X, Han H, Yin H, Zhao B, et al. Prophylactical low dose whole-liver irradiation inhibited colorectal liver metastasis by regulating hepatic niche in mice. Onco Targets Ther. (2020) 13:8451–62. doi: 10.2147/OTT.S263858
21. Xu J, Fan J, Qin X, Cai J, Gu J, Wang S, et al. Chinese guidelines for the diagnosis and comprehensive treatment of colorectal liver metastases (Version 2018). J Cancer Res Clin Oncol. (2019) 145:725–36. doi: 10.1007/s00432-018-2795-1
22. Valderrama-Treviño AI, Barrera-Mera B, Ceballos-Villalva JC, Montalvo-Javé EE. Hepatic metastasis from colorectal cancer. Euroasian J Hepatogastroenterol. (2017) 7:166–75. doi: 10.5005/jp-journals-10018-1241
23. Georgilis E, Gavriatopoulou M, Tsilimigras DI, Malandrakis P, Theodosopoulos T, Ntanasis-Stathopoulos I. Optimizing adjuvant therapy after surgery for colorectal cancer liver metastases: A systematic review. J Clin Med. (2023) 12:2401. doi: 10.3390/jcm12062401
24. Ferretti S, Tranchart H, Buell JF, Eretta C, Patriti A, Spampinato MG, et al. Laparoscopic simultaneous resection of colorectal primary tumor and liver metastases: results of a multicenter international study. World J Surg. (2015) 39:2052–60. doi: 10.1007/s00268-015-3034-4
25. Ren Y, Chen L, Huang S, Zhou C, Liu J, Shi Q, et al. Transarterial chemoembolization of unresectable systemic chemotherapy refractory liver metastases: A retrospective single-center analysis. Abdom Radiol (NY). (2020) 45:2862–70. doi: 10.1007/s00261-020-02584-6
26. Scherman P, Syk I, Holmberg E, Naredi P, Rizell M. Impact of patient, primary tumor and metastatic pattern including tumor location on survival in patients undergoing ablation or resection for colorectal liver metastases: A population-based national cohort study. Eur J Surg Oncol. (2021) 47:375–83. doi: 10.1016/j.ejso.2020.07.030
27. Rouyer M, François E, Cunha AS, Monnereau A, Noize P, Robinson P, et al. Effectiveness of cetuximab as first-line therapy for patients with wild-type kras and unresectable metastatic colorectal cancer in real-life practice: results of the erebus cohort. Clin Colorectal Cancer. (2018) 17:129–39. doi: 10.1016/j.clcc.2018.01.007
28. Adam R, Wicherts DA, de Haas RJ, Ciacio O, Lévi F, Paule B, et al. Patients with initially unresectable colorectal liver metastases: is there a possibility of cure? J Clin Oncol. (2009) 27:1829–35. doi: 10.1200/jco.2008.19.9273
29. Yasuno M, Uetake H, Ishiguro M, Mizunuma N, Komori T, Miyata G, et al. Mfolfox6 plus bevacizumab to treat liver-only metastases of colorectal cancer that are unsuitable for upfront resection (Tricc0808): A multicenter phase ii trial comprising the final analysis for survival. Int J Clin Oncol. (2019) 24:516–25. doi: 10.1007/s10147-018-01393-8
30. Tellez C, Benson AB 3rd, Lyster MT, Talamonti M, Shaw J, Braun MA, et al. Phase ii trial of chemoembolization for the treatment of metastatic colorectal carcinoma to the liver and review of the literature. Cancer. (1998) 82:1250–9. doi: 10.1002/(ISSN)1097-0142
32. Allen-Mersh TG, Earlam S, Fordy C, Abrams K, Houghton J. Quality of life and survival with continuous hepatic-artery floxuridine infusion for colorectal liver metastases. Lancet. (1994) 344:1255–60. doi: 10.1016/s0140-6736(94)90750-1
33. Hu C, Terashima M, Cheng X. Conversion therapy for stage iv gastric cancer. Sci Bull (Beijing). (2023) 68:653–6. doi: 10.1016/j.scib.2023.03.011
34. Xiang X, Guo F, Li G, Ma L, Zhu X, Abdulla Z, et al. Efficacy of intra-arterial chemotherapy with sequential anti-pd-1 antibody in unresectable gastric cancer: A retrospective real-world study. Front Oncol. (2022) 12:1015962. doi: 10.3389/fonc.2022.1015962
35. Aigner K, Vashist YK, Selak E, Gailhofer S, Aigner KR. Efficacy of regional chemotherapy approach in peritoneal metastatic gastric cancer. J Clin Med. (2021) 10:5322. doi: 10.3390/jcm10225322
36. Basra M, Patel H, Biglione A. Intra-arterial chemotherapy in patients with metastatic or locally aggressive pancreatic adenocarcinoma: A scoping review. Cureus. (2024) 16:e60696. doi: 10.7759/cureus.60696
37. Ouyang H, Ma W, Si T, Liu D, Chen P, Gerdtsson AS, et al. Systemic chemotherapy with or without hepatic arterial infusion chemotherapy for liver metastases from pancreatic cancer: A propensity score matching analysis. Clin Colorectal Cancer. (2023) 22:111–9. doi: 10.1016/j.clcc.2022.10.007
38. Randrian V, Pernot S, Sionneau B, Smith D, Lim A, Touchefeu Y, et al. Hepatic arterial infusion chemotherapy with folfirinox or oxaliplatin alone in metastatic colorectal cancer. Front Med (Lausanne). (2022) 9:830595. doi: 10.3389/fmed.2022.830595
39. Walker BS, Billingsley KG, Sutton TL, Kolbeck KJ, Korngold EK, Nabavizadeh N, et al. Hepatic arterial infusion pump chemotherapy combined with systemic therapy for patients with advanced colorectal liver metastases: outcomes in a newly established program. J Surg Oncol. (2022) 126:513–22. doi: 10.1002/jso.26911
40. Gholami S, Kemeny NE, Boucher TM, Gönen M, Cercek A, Kingham TP, et al. Adjuvant hepatic artery infusion chemotherapy is associated with improved survival regardless of kras mutation status in patients with resected colorectal liver metastases: A retrospective analysis of 674 patients. Ann Surg. (2020) 272:352–6. doi: 10.1097/SLA.0000000000003248
41. Groot Koerkamp B, Sadot E, Kemeny NE, Gönen M, Leal JN, Allen PJ, et al. Perioperative hepatic arterial infusion pump chemotherapy is associated with longer survival after resection of colorectal liver metastases: A propensity score analysis. J Clin Oncol. (2017) 35:1938–44. doi: 10.1200/jco.2016.71.8346
42. Gao L, Cai S, Cai A, Zhao Y, Xu T, Ma Y, et al. The improved antitumor efficacy of continuous intratumoral chemotherapy with cisplatin-loaded implants for the treatment of sarcoma 180 tumor-bearing mice. Drug Delivery. (2019) 26:208–15. doi: 10.1080/10717544.2019.1574938
43. Ammori JB, Kemeny NE. Regional hepatic chemotherapies in treatment of colorectal cancer metastases to the liver. Semin Oncol. (2010) 37:139–48. doi: 10.1053/j.seminoncol.2010.03.003
44. Long GB, Xiao CW, Zhao XY, Zhang J, Li X. Effects of hepatic arterial infusion chemotherapy in the treatment of hepatocellular carcinoma: A meta-analysis. Med (Baltimore). (2020) 99:e20745. doi: 10.1097/md.0000000000020745
45. Sakai H, Ikeda S, Harada T, Yonashiro S, Ozumi K, Ohe H, et al. Limitations of successive transradial approach in the same arm: the Japanese experience. Catheter Cardiovasc Interv. (2001) 54:204–8. doi: 10.1002/ccd.1268
46. Dhir M, Jones HL, Shuai Y, Clifford AK, Perkins S, Steve J, et al. Hepatic arterial infusion in combination with modern systemic chemotherapy is associated with improved survival compared with modern systemic chemotherapy alone in patients with isolated unresectable colorectal liver metastases: A case-control study. Ann Surg Oncol. (2017) 24:150–8. doi: 10.1245/s10434-016-5418-6
47. Zhang Y, Wang K, Yang T, Cao Y, Liang W, Yang X, et al. Meta-analysis of hepatic arterial infusion for liver metastases from colorectal cancer. Front Oncol. (2021) 11:628558. doi: 10.3389/fonc.2021.628558
48. Kemeny NE, Melendez FD, Capanu M, Paty PB, Fong Y, Schwartz LH, et al. Conversion to resectability using hepatic artery infusion plus systemic chemotherapy for the treatment of unresectable liver metastases from colorectal carcinoma. J Clin Oncol. (2009) 27:3465–71. doi: 10.1200/jco.2008.20.1301
49. Malka D, Verret B, Faron M, Guimbaud R, Caramella C, Edeline J, et al. Hepatic arterial oxaliplatin plus intravenous 5-fluorouracil and cetuximab for first-line treatment of colorectal liver metastases: A multicenter phase ii trial. Eur J Cancer. (2023) 195:113400. doi: 10.1016/j.ejca.2023.113400
50. Kim JS, Kim H, Lee SY, Han YD, Han K, Min BS, et al. Hepatic arterial infusion in combination with systemic chemotherapy in patients with hepatic metastasis from colorectal cancer: A randomized phase ii study - (Nct05103020) - study protocol. BMC Cancer. (2023) 23:691. doi: 10.1186/s12885-023-11085-w
51. Rigault E, Lacas B, Glehen O, Smith D, Dupont-Bierre E, Guimbaud R, et al. Intra-arterial hepatic bevacizumab and systemic chemotherapy in hepatic metastasis of colorectal cancer: A phase ii multicentric trial in second-line treatment. Cancer Treat Res Commun. (2023) 34:100674. doi: 10.1016/j.ctarc.2022.100674
52. Feng AW, Guo JH, Gao S, Kou FX, Liu SX, Liu P, et al. A randomized phase ii trial of hepatic arterial infusion of oxaliplatin plus raltitrexed versus oxaliplatin plus 5-fluorouracil for unresectable colorectal cancer liver metastases. Front Oncol. (2022) 12:913017. doi: 10.3389/fonc.2022.913017
53. Wei N, Zhang B, Wang Y, He XH, Xu LC, Li GD, et al. Transarterial chemoembolization with raltitrexed-based or floxuridine-based chemotherapy for unresectable colorectal cancer liver metastasis. Clin Transl Oncol. (2019) 21:443–50. doi: 10.1007/s12094-018-1942-0
54. Ensminger WD. Intrahepatic arterial infusion of chemotherapy: pharmacologic principles. Semin Oncol. (2002) 29:119–25. doi: 10.1053/sonc.2002.31679
55. Guo JH, Zhang HY, Gao S, Zhang PJ, Li XT, Chen H, et al. Hepatic artery infusion with raltitrexed or 5-fluorouracil for colorectal cancer liver metastasis. World J Gastroenterol. (2017) 23:1406–11. doi: 10.3748/wjg.v23.i8.1406
56. Boilève A, De Cuyper A, Larive A, Mahjoubi L, Najdawi M, Tazdait M, et al. Hepatic arterial infusion of oxaliplatin plus systemic chemotherapy and targeted therapy for unresectable colorectal liver metastases. Eur J Cancer. (2020) 138:89–98. doi: 10.1016/j.ejca.2020.07.022
57. Deschamps F, Elias D, Goere D, Malka D, Ducreux M, Boige V, et al. Intra-arterial hepatic chemotherapy: A comparison of percutaneous versus surgical implantation of port-catheters. Cardiovasc Intervent Radiol. (2011) 34:973–9. doi: 10.1007/s00270-010-9996-6
58. Sperling J, Schäfer T, Ziemann C, Benz-Weiber A, Kollmar O, Schilling MK, et al. Hepatic arterial infusion of bevacizumab in combination with oxaliplatin reduces tumor growth in a rat model of colorectal liver metastases. Clin Exp Metastasis. (2012) 29:91–9. doi: 10.1007/s10585-011-9432-6
59. Sperling J, Brandhorst D, Schäfer T, Ziemann C, Benz-Weißer A, Scheuer C, et al. Liver-directed chemotherapy of cetuximab and bevacizumab in combination with oxaliplatin is more effective to inhibit tumor growth of cc531 colorectal rat liver metastases than systemic chemotherapy. Clin Exp Metastasis. (2013) 30:447–55. doi: 10.1007/s10585-012-9550-9
60. Meng W, Gao Y, Xie J, Wu S, Jian B, Li Q, et al. Intra-arterial chemoembolization with chemotherapy for unresectable locally advanced rectal cancer: A case report and literature review. Ann Palliat Med. (2021) 10:9281–7. doi: 10.21037/apm-21-1881
61. Lewis AL, Holden RR. Dc bead embolic drug-eluting bead: clinical application in the locoregional treatment of tumours. Expert Opin Drug Delivery. (2011) 8:153–69. doi: 10.1517/17425247.2011.545388
62. Brown DB, Gould JE, Gervais DA, Goldberg SN, Murthy R, Millward SF, et al. Transcatheter therapy for hepatic Malignancy: standardization of terminology and reporting criteria. J Vasc Interv Radiol. (2007) 18:1469–78. doi: 10.1016/j.jvir.2007.08.027
63. Chang Y, Jeong SW, Young Jang J, Jae Kim Y. Recent updates of transarterial chemoembolilzation in hepatocellular carcinoma. Int J Mol Sci. (2020) 21:8165. doi: 10.3390/ijms21218165
64. Shirono T, Iwamoto H, Niizeki T, Shimose S, Kajiwara A, Suzuki H, et al. Durable complete response is achieved by balloon-occluded transcatheter arterial chemoembolization for hepatocellular carcinoma. Hepatol Commun. (2022) 6:2594–604. doi: 10.1002/hep4.2016
65. Poon RT, Fan ST, Tsang FH, Wong J. Locoregional therapies for hepatocellular carcinoma: A critical review from the surgeon’s perspective. Ann Surg. (2002) 235:466–86. doi: 10.1097/00000658-200204000-00004
66. Gao L, Li Q, Zhang J, Huang Y, Deng L, Li C, et al. Local penetration of doxorubicin via intrahepatic implantation of plga based doxorubicin-loaded implants. Drug Delivery. (2019) 26:1049–57. doi: 10.1080/10717544.2019.1676842
67. Fiorentini G, Sarti D, Nani R, Aliberti C, Fiorentini C, Guadagni S. Updates of colorectal cancer liver metastases therapy: review on debiri. Hepat Oncol. (2020) 7:Hep16. doi: 10.2217/hep-2019-0010
68. Irizato M, Nishiofuku H, Sato T, Maeda S, Toyoda S, Matsumoto T, et al. Transarterial chemoembolization with irinotecan-loaded beads followed by arterial infusion of 5-fluorouracil for metastatic liver tumors refractory to standard systemic chemotherapy. Interv Radiol (Higashimatsuyama). (2023) 8:92–6. doi: 10.22575/interventionalradiology.2022-0026
69. Zhang H, Guo J, Gao S, Zhang P, Chen H, Wang X, et al. Prognostic factors for transarterial chemoembolization combined with sustained oxaliplatin-based hepatic arterial infusion chemotherapy of colorectal cancer liver metastasis. Chin J Cancer Res. (2017) 29:36–44. doi: 10.21147/j.issn.1000-9604.2017.01.05
70. Wieners G, Pech M, Hildebrandt B, Peters N, Nicolaou A, Mohnike K, et al. Phase ii feasibility study on the combination of two different regional treatment approaches in patients with colorectal “Liver-only” Metastases: hepatic interstitial brachytherapy plus regional chemotherapy. Cardiovasc Intervent Radiol. (2009) 32:937–45. doi: 10.1007/s00270-009-9597-4
71. Fiorentini G, Aliberti C, Tilli M, Mulazzani L, Graziano F, Giordani P, et al. Intra-arterial infusion of irinotecan-loaded drug-eluting beads (Debiri) versus intravenous therapy (Folfiri) for hepatic metastases from colorectal cancer: final results of a phase iii study. Anticancer Res. (2012) 32:1387–95.
72. Cao F, Zheng J, Luo J, Zhang Z, Shao G. Treatment efficacy and safety of regorafenib plus drug-eluting beads-transarterial chemoembolization versus regorafenib monotherapy in colorectal cancer liver metastasis patients who fail standard treatment regimens. J Cancer Res Clin Oncol. (2021) 147:2993–3002. doi: 10.1007/s00432-021-03708-1
73. Szemitko M, Golubinska-Szemitko E, Wilk-Milczarek E, Falkowski A. Side effect/complication risk related to injection branch level of chemoembolization in treatment of metastatic liver lesions from colorectal cancer. J Clin Med. (2020) 10:121. doi: 10.3390/jcm10010121
74. Mikhail AS, Negussie AH, Mauda-Havakuk M, Owen JW, Pritchard WF, Lewis AL, et al. Drug-eluting embolic microspheres: state-of-the-art and emerging clinical applications. Expert Opin Drug Delivery. (2021) 18:383–98. doi: 10.1080/17425247.2021.1835858
75. Bi Y, Jiao D, Wang Y, Han X, Ren J. Preliminary outcomes of raltitrexed eluting bead-transarterial chemoembolization using callispheres® Beads for gastrointestinal adenocarcinoma liver metastasis. World J Surg Oncol. (2022) 20:229. doi: 10.1186/s12957-022-02696-x
76. Li H, Zhang X, Zhao W, Cai F, Qin J, Tian J. Efficacy of callispheres® Microspheres versus conventional transarterial chemoembolization in the treatment of refractory colorectal cancer liver metastasis. BMC Cancer. (2023) 23:970. doi: 10.1186/s12885-023-11350-y
77. Malagari K, Kiakidis T, Moschouris H, Charokopakis A, Vergadis C, Alevisopoulos N, et al. Prospective series of transarterial chemoembolization of metastatic colorectal cancer to the liver with 30-60 μm microspheres loaded with irinotecan. Cardiovasc Intervent Radiol. (2023) 46:880–90. doi: 10.1007/s00270-023-03446-6
78. Voizard N, Ni T, Kiss A, Pugash R, Raphael MJ, Coburn N, et al. Small particle debiri tace as salvage therapy in patients with liver dominant colorectal cancer metastasis: retrospective analysis of safety and outcomes. Curr Oncol. (2022) 29:209–20. doi: 10.3390/curroncol29010020
79. Mauri G, Varano GM, Della Vigna P, Bonomo G, Monfardini L, Zampino MG, et al. Transarterial embolization with small-size particles loaded with irinotecan for the treatment of colorectal liver metastases: results of the miracle iii study. Cardiovasc Intervent Radiol. (2018) 41:1708–15. doi: 10.1007/s00270-018-2017-x
80. Helmberger T, Lucatelli P, Pereira PL, Gjoreski A, Jovanoska I, Bansaghi Z, et al. Safety, feasibility and technical considerations from a prospective, observational study-cirel: irinotecan-tace for crlm in 152 patients. J Clin Med. (2022) 11:6178. doi: 10.3390/jcm11206178
81. Maraj T, Mirrahimi A, Dey C. Survival of patients with colorectal liver metastases after transarterial chemoembolization using irinotecan-eluting microspheres: A single-center retrospective analysis comparing recist 1.1 and choi criteria. J Vasc Interv Radiol. (2023) 34:983–90.e1. doi: 10.1016/j.jvir.2023.02.005
82. Braun EM, Kikot VA, Ugrinov OG, Lishchishina EM. Neoadjuvant intra-arterial polychemotherapy of locally advanced rectal cancer. Eur J Surg Oncol. (1997) 23:228–32. doi: 10.1016/s0748-7983(97)92412-4
83. Kimura H, Shima Y, Kinoshita S, Takahashi I. Successful resection of locally advanced rectal carcinoma combined with preoperative chemoradiation. Hepato-gastroenterology. (2003) 50:1393–5.
84. Xu J, Zhong Y, Weixin N, Xinyu Q, Yanhan L, Li R, et al. Preoperative hepatic and regional arterial chemotherapy in the prevention of liver metastasis after colorectal cancer surgery. Ann Surg. (2007) 245:583–90. doi: 10.1097/01.sla.0000250453.34507.d3
85. Bini R, Comelli S, Addeo A, Viora T, Vana F, Vaudano G, et al. Inferior mesenteric artery chemoembolization and chemotherapy for advanced rectal cancer: report of a clinical case. Tumori. (2015) 101:e82–4. doi: 10.5301/tj.5000270
86. Bini R, Comelli S, Leli R, Vaudano GP, Savio D, Viora T, et al. A novel approach to inoperable or recurrent rectal cancer by chemoembolization: A new arrow in our quiver? Oncotarget. (2016) 7:45275–82. doi: 10.18632/oncotarget.9940
87. Huang W, Wu J, Liu G, Lv Y, Huang J, Li W. Chemoradiotherapy with concurrent regional arterial chemotherapy for locally bulky unresectable rectal cancer: A case series. Oncol Res Treat. (2019) 42:678–83. doi: 10.1159/000502802
88. Yang B, Shan J, Feng Y, Dai N, Li M, Chen C, et al. Transcatheter rectal arterial chemoembolization with oxaliplatin plus S-1 concurrent chemoradiotherapy can improve the pathological remission rate in locally advanced rectal cancer: A comparative study. Radiat Oncol. (2020) 15:94. doi: 10.1186/s13014-020-01540-4
89. Bi Y, Shi X, Ren J, Yi M, Han X, Song M. Transarterial chemoembolization with doxorubicin-loaded beads for inoperable or recurrent colorectal cancer. Abdom Radiol (NY). (2021) 46:2833–8. doi: 10.1007/s00261-020-02877-w
90. Zhu D, Xia J, Gu Y, Lin J, Ding K, Zhou B, et al. Preoperative hepatic and regional arterial chemotherapy in patients who underwent curative colorectal cancer resection: A prospective, multi-center, randomized controlled trial. Ann Surg. (2021) 273:1066–75. doi: 10.1097/sla.0000000000004558
91. Meng WJ, Liu CH, Zheng RJ, Li CX. Regional transarterial chemoembolization combined with chemoradiotherapy for locally advanced rectal cancer: A retrospective study of a new combination. Front Oncol. (2023) 13:1201544. doi: 10.3389/fonc.2023.1201544
92. Yang W, Qian C, Luo J, Chen C, Feng Y, Dai N, et al. Efficacy and safety of preoperative transcatheter rectal arterial chemoembolisation in patients with locally advanced rectal cancer: results from a prospective, phase ii pcar trial. Clin Oncol (R Coll Radiol). (2024) 36:233–42. doi: 10.1016/j.clon.2024.01.015
93. Ding X, Ma Y, Yin M, Liu T, Jin S, Li C, et al. Transcatheter arterial perfusion chemotherapy combined with lipiodol chemoembolization for advanced colorectal cancer complicated by obstruction. Front Oncol. (2024) 14:1369829. doi: 10.3389/fonc.2024.1369829
94. China guideline for diagnosis and comprehensive treatment of colorectal liver metastases (Version 2023). Zhonghua Wei Chang Wai Ke Za Zhi. (2023) 26:1–15. doi: 10.3760/cma.j.cn441530-20221229-00542
95. Winner M, Mooney SJ, Hershman DL, Feingold DL, Allendorf JD, Wright JD, et al. Incidence and predictors of bowel obstruction in elderly patients with stage iv colon cancer: A population-based cohort study. JAMA Surg. (2013) 148:715–22. doi: 10.1001/jamasurg.2013.1
96. Fan Y, Zhu X, Xu C, Ding C, Hu J, Hong Q, et al. Neoadjuvant arterial embolization chemotherapy combined pd-1 inhibitor for locally advanced rectal cancer (Neci study): A protocol for a phase ii study. BMJ Open. (2023) 13:e069401. doi: 10.1136/bmjopen-2022-069401
97. Huang W, Huang P, Guo H, Huang Z, Wei M, Guo J, et al. Single-arm, phase ii study of intra-arterial chemotherapy plus total neoadjuvant therapy to optimise complete response in distal rectal cancer: A study protocol. BMJ Open. (2023) 13:e075023. doi: 10.1136/bmjopen-2023-075023
98. Pinato DJ, D’Alessio A, Fulgenzi CAM, Schlaak AE, Celsa C, Killmer S, et al. Safety and preliminary efficacy of pembrolizumab following transarterial chemoembolization for hepatocellular carcinoma: the petal phase ib study. Clin Cancer Res. (2024) 30:2433–43. doi: 10.1158/1078-0432.Ccr-24-0177
99. Liu H, Wang C, Wang R, Cao H, Cao Y, Huang T, et al. New insights into mechanisms and interventions of locoregional therapies for hepatocellular carcinoma. Chin J Cancer Res. (2024) 36:167–94. doi: 10.21147/j.issn.1000-9604.2024.02.06
100. Ito K, Ito H, Kemeny NE, Gonen M, Allen PJ, Paty PB, et al. Biliary sclerosis after hepatic arterial infusion pump chemotherapy for patients with colorectal cancer liver metastasis: incidence, clinical features, and risk factors. Ann Surg Oncol. (2012) 19:1609–17. doi: 10.1245/s10434-011-2102-8
101. Kanat O, Gewirtz A, Kemeny N. What is the potential role of hepatic arterial infusion chemo-therapy in the current armamentorium against colorectal cancer. J Gastrointest Oncol. (2012) 3:130–8. doi: 10.3978/j.issn.2078-6891.2011.025
102. Bouchahda M, Lévi F, Adam R, Rougier P. Modern insights into hepatic arterial infusion for liver metastases from colorectal cancer. Eur J Cancer. (2011) 47:2681–90. doi: 10.1016/j.ejca.2011.06.037
103. Ducreux M, Ychou M, Laplanche A, Gamelin E, Lasser P, Husseini F, et al. Hepatic arterial oxaliplatin infusion plus intravenous chemotherapy in colorectal cancer with inoperable hepatic metastases: A trial of the gastrointestinal group of the federation nationale des centres de lutte contre le cancer. J Clin Oncol. (2005) 23:4881–7. doi: 10.1200/jco.2005.05.120
104. He MK, Le Y, Li QJ, Yu ZS, Li SH, Wei W, et al. Hepatic artery infusion chemotherapy using mfolfox versus transarterial chemoembolization for massive unresectable hepatocellular carcinoma: A prospective non-randomized study. Chin J Cancer. (2017) 36:83. doi: 10.1186/s40880-017-0251-2
105. Kobayashi S, Kozaka K, Gabata T, Matsui O, Koda W, Okuda M, et al. Pathophysiology and imaging findings of bile duct necrosis: A rare but serious complication of transarterial therapy for liver tumors. Cancers (Basel). (2020) 12:2596. doi: 10.3390/cancers12092596
106. Kemeny NE, Niedzwiecki D, Hollis DR, Lenz HJ, Warren RS, Naughton MJ, et al. Hepatic arterial infusion versus systemic therapy for hepatic metastases from colorectal cancer: A randomized trial of efficacy, quality of life, and molecular markers (Calgb 9481). J Clin Oncol. (2006) 24:1395–403. doi: 10.1200/jco.2005.03.8166
107. Clark TW. Complications of hepatic chemoembolization. Semin Intervent Radiol. (2006) 23:119–25. doi: 10.1055/s-2006-941442
108. Gaba RC, Lokken RP, Hickey RM, Lipnik AJ, Lewandowski RJ, Salem R, et al. Quality improvement guidelines for transarterial chemoembolization and embolization of hepatic Malignancy. J Vasc Interv Radiol. (2017) 28:1210–23.e3. doi: 10.1016/j.jvir.2017.04.025
109. European Association for the Study of the Liver. Easl clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. (2018) 69:182–236. doi: 10.1016/j.jhep.2018.03.019
110. Qi X, Zhao Y, Li H, Guo X, Han G. Management of hepatocellular carcinoma: an overview of major findings from meta-analyses. Oncotarget. (2016) 7:34703–51. doi: 10.18632/oncotarget.9157
111. Gao YS, Qian GW, Zhang YF, Wu G, Li DY, Li WC, et al. Efficacy of preoperative systemic vein chemotherapy combined with regional intra-arterial chemoembolization in the management of locally advanced rectal cancer. Shijie Huaren Xiaohua Zazhi. (2013) 21:367–72. doi: 10.11569/wcjd.v21.i4.367
112. Lin H, Wang L, Zhong X, Zhang X, Shao L, Wu J. Meta-analysis of neoadjuvant chemotherapy versus neoadjuvant chemoradiotherapy for locally advanced rectal cancer. World J Surg Oncol. (2021) 19:141. doi: 10.1186/s12957-021-02251-0
113. Chennavasin P, Phoopat J, Udomchaisakul P, Gururatsakul M. Delayed gastric perforation following transarterial chemoembolization: case report. Int J Surg Case Rep. (2023) 104:107965. doi: 10.1016/j.ijscr.2023.107965
114. Kim SI, Jin YJ, Cho SG, Shin WY, Kim JM, Lee JW. Duodenal perforation and esophageal ischemia following transarterial chemoembolization for hepatocellular carcinoma: A case report. Med (Baltimore). (2016) 95:e3987. doi: 10.1097/md.0000000000003987
115. Barault L, Amatu A, Siravegna G, Ponzetti A, Moran S, Cassingena A, et al. Discovery of methylated circulating DNA biomarkers for comprehensive non-invasive monitoring of treatment response in metastatic colorectal cancer. Gut. (2018) 67:1995–2005. doi: 10.1136/gutjnl-2016-313372
116. Liu Y, Zhao L, Bao J, Hou J, Jing Z, Liu S, et al. Non-invasively identifying candidates of active surveillance for prostate cancer using magnetic resonance imaging radiomics. Vis Comput Ind BioMed Art. (2024) 7:16. doi: 10.1186/s42492-024-00167-6
117. Li ZY, Wang XD, Li M, Liu XJ, Ye Z, Song B, et al. Multi-modal radiomics model to predict treatment response to neoadjuvant chemotherapy for locally advanced rectal cancer. World J Gastroenterol. (2020) 26:2388–402. doi: 10.3748/wjg.v26.i19.2388
Keywords: transarterial chemotherapy, chemoembolization, colorectal cancer, neoadjuvant therapy, comprehensive treatment
Citation: Meng W, Pan L, Huang L, Li Q and Sun Y (2024) Applications of image-guided locoregional transarterial chemotherapy in patients with inoperable colorectal cancer: a review. Front. Oncol. 14:1464242. doi: 10.3389/fonc.2024.1464242
Received: 13 July 2024; Accepted: 08 August 2024;
Published: 23 August 2024.
Edited by:
Chen Liu, Army Medical University, ChinaReviewed by:
Tikam Chand Dakal, Mohanlal Sukhadia University, IndiaLi-na He, Sun Yat-sen University Cancer Center (SYSUCC), China
Yong Cheng, First Affiliated Hospital of Chongqing Medical University, China
Copyright © 2024 Meng, Pan, Huang, Li and Sun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yi Sun, eWlfc3VuMzA3QDE2My5jb20=; Qing Li, bGlxaW5nQHNjdS5lZHUuY24=
†These authors have contributed equally to this work and share first authorship
‡ORCID: Wenjun Meng, orcid.org/0000-0002-6780-8720
 Wenjun Meng
Wenjun Meng Lu Pan1,3†
Lu Pan1,3† Qing Li
Qing Li