- 1First Department of Propaedeutic Internal Medicine and Diabetes Center, School of Medicine, National and Kapodistrian University of Athens, Laiko General Hospital, Athens, Greece
- 2Department of Clinical Therapeutics, School of Medicine, National and Kapodistrian University of Athens, Alexandra General Hospital, Athens, Greece
Multiple myeloma (MM) is the second most common hematological malignancy, characterized by unregulated monoclonal proliferation in the bone marrow. Monoclonal gammopathy of undetermined significance (MGUS) and smoldering multiple myeloma (SMM) are premalignant conditions that can progress to MM. Identifying etiological risk factors for MM and its precursor diseases is crucial for prevention. Obesity, diet, vitamin D levels, and gut microbiota alterations have been identified as lifestyle factors affecting MM and MGUS risk. Upon disease onset, treatment strategies aim to reduce disease burden, enhance prognosis, and optimize patients’ quality of life. Nutrition and body weight have been shown to affect disease progression and treatment outcomes. MM patients often present with vitamin D, vitamin B12, and folate deficiencies, which worsen disease prognosis. High body mass index is linked to increased death rates among MM patients and an increased risk of MGUS transformation to MM. Gut microbiota has also been associated with disease progression and response to treatment. This literature review aims to summarize the available evidence regarding the impact of nutrition and nutritional status on MM patients beyond prevention, highlighting the significance of gut microbiome and dysbiosis in MM progression.
Introduction
Multiple Myeloma (MM) is the second most common hematological malignancy (1) which accounts for about 10% of all hematological malignancies (2, 3). The main characteristic of MM is the unregulated monoclonal proliferation in the bone marrow leading to the overproduction of nonfunctional intact immunoglobulins or immunoglobulin chains (3, 4). About 0.8% of men and women in the United States will be diagnosed with MM at some point in their life and the annual age-adjusted incidence is estimated 7.2 per 100.000 individuals between 2017 and 2021 (5).
Monoclonal gammopathy of undetermined significance (MGUS) is a premalignant asymptomatic condition where the presence of a M-protein in serum or an abnormal ratio between the free kappa and lambda light-chains (LC-MGUS) occurs, without evidence of MM or other lymphoproliferative (LP) diseases (6, 7). The prevalence of MGUS is approximately 5% in population >70 years old and increases with age (8). It is also estimated that the average annual risk of progression from MGUS to MM is about 1% (9). The prevalence of LC-MGUS, the precursor of light-chain MM, is 0.7-0.8% (10).
Identifying the etiological risk factors for MM or MGUS is of major importance for disease prevention. Several etiological risk factors related to lifestyle have been identified to alter the risk of MM or MGUS. These factors include obesity (11–17), diet (6, 18, 19), vitamin D levels (20–22) and alterations of gut microbiota (23); obesity being currently the only established modifiable risk factor (24, 25).
Upon the failure of preventive measures and the onset of disease, it becomes imperative to implement treatment strategies designed to reduce disease burden, enhance prognosis (26), and optimize patients’ quality of life (27). The introduction of new therapies has significantly enhanced the prognosis for patients with MM and have increased survival rates. However, disease progression in MM continues to result in significant morbidity rates, which becomes even more complicated by on-going treatment side-effects (28). This is closely affected by lifestyle factors such as nutrition and body weight that have been shown to have an impact on the risk of disease progression (27).
Although there are numerous studies linking nutrition and body weight with MM risk (18, 29–32), the available data on the effect of these aspects on disease progression is limited (27). Over the last decades a part of research has been focusing on how nutritional aspects and body weight modulate the active MM disease course as well as their effect on treatment (27). Studies have shown that MM patients present vitamin D, vitamin B12 (33) and folate (33, 34) deficiencies, worsening disease prognosis. Additionally, high body mass index (BMI) is linked with higher death rates among MM patients (35) as well as with a higher risk of progression of MGUS to MM (36). Gut microbiota has also been the subject of research with studies showing an association between microbiota and disease progression-response to treatment (37–39).
The purpose of this literature review is to summarize and elucidate the available evidence about the impact of nutrition and nutritional status on MM patients, beyond the context of prevention, in an effort to highlight its significance on disease prognosis and emphasize on the role of gut microbiome and dysbiosis in MM progression.
Epidemiology
Multiple Myeloma represents about 1% of all cancers and roughly 10% of all hematologic malignancies (40). Over the last decades, an increased number of global deaths has been reported due to MM, with limited information regarding epidemiology and disease burden especially in developing countries (41). It has been estimated that each year about 32.000 individuals are diagnosed with the disease and about 13.000 lose their life (42). Between 2017 and 2021, MM presented an annual age-adjusted incidence of 7.2 per 100.000 individuals (5). According to a study conducted by Cowan et al. (2018), MM incident cases from 1990 to 2016 increased by 126%, and deaths increased by 94%. Of the 126% increase in global incident cases, 40.4% was due to population growth, 52.9% was attributed to an aging population, and 32.6% resulted from higher age-specific incidence rates (41). MM appears to have different incident rates between sexes with men having slightly higher incident rates than women. Studies have also shown that there are racial disparities, with Afro-descendants presenting two times higher risk compared to their White counterparts (40) and Asians presenting lower incidence compared to Whites. MM incidence depends on age and reaches its maximum during the seventh decade of life (43). The median age of patients at the time the diagnosis is 65 years (44), with very few cases occurring under the age of 40 (43). Additionally, randomized controlled trials using modern therapy have found a median survival of MM patients of approximately 6 years (45).
Regarding the premalignant conditions, MGUS presents an overall prevalence of 2.4% (46) and 70 years mean age at the time of diagnosis, being predominantly a disease of the elderly (46, 47). Similarly to MM, MGUS is also associated with race and ethnicity (48). The highest age-adjusted prevalence of MGUS is reported in the black population (0.99%), followed by Mexican Americans (0.55%), while the lowest prevalence is observed in white populations (0.21%) (49). Concerning smoldering multiple myeloma (SMM), the prevalence of this premalignant condition is 0.5% in individuals over 40 years of age and increases with age while it is higher in men than in women (0.7% vs. 0.4%) (50). Similar to MGUS, SMM also presents race disparities with high prevalence in black individuals than other racial groups (51–53). However, it should be noted that because of the asymptomatic nature of SMM its diagnosis is rare, and it is estimated that only 3-6% of patients with MM are diagnosed at this precursor state (54, 55).
The impact of body composition on multiple myeloma progression
Obesity is a well-established and potentially modifiable risk factor linked to a higher incidence of MM (56, 57). However, the association of obesity with clinical outcomes after MM diagnosis is less clear.
Data from two pooled analyses of large prospective cohorts have indicated a relation between high BMI and mortality. However, it remains vague whether increased cancer incidence, decreased survival after diagnosis, or both may explain the increased mortality among patients with higher BMI. A pooled analysis of MM mortality involving 1.5 million participants (including 1,388 MM deaths) from 20 prospective cohorts within the National Cancer Institute Cohort Consortium observed an increased MM mortality associated with higher BMI in early adulthood, as well as higher BMI and waist circumference at cohort entry (16). Moreover, data from seven prospective cohorts including 239.597 African Americans adults indicated a steady increase in mortality with rising BMI. The hazard ratios reached 1.43 (95% CI: 1.03 to 1.97) for BMIs of 35 kg/m² or higher compared to those with normal BMIs (58).
Another study involving 2,968 MM patients in the Veterans Health Administration System found that underweight patients had increased mortality, while overweight and obese patients had lower mortality compared to those with normal weight. Additionally, patients with weight loss of 10% or more from baseline in the year prior to diagnosis indicated increased mortality (59).
In a subgroup of 108 patients who underwent a whole-body low-dose computed tomography before induction therapy, a significant inverse correlation was observed between adverse cytogenetics and both visceral adipose tissue (VAT) of the abdomen and pelvis. No correlation was found between visceral or subcutaneous adipose tissue and adverse events. However, a significant inverse correlation was observed between abdominal (p=0.03) and pelvic (p=0.035) VAT and treatment response. Abdominal VAT remained significant (p=0.034) independently of revised ISS stage and treatment. BMI did not show a significant correlation with treatment response or the investigated cytogenetics (60).
A very recent study examined the impact of BMI on progression-free survival (PFS) and overall survival (OS) in 1,142 newly diagnosed patients from the Multiple Myeloma Research Foundation CoMMpass registry. The results showed that underweight and severely obese patients had lower median PFS and OS compared to those who were normal weight, overweight or moderately obese. Multivariable models linking PFS and OS with BMI indicated that underweight patients had a significantly higher risk of death (HR: 2.32; 95% CI: 1.09, 4.97) (61).
In addition, apart from body weight per se, measures of muscle and adipose tissue mass have been associated with poor outcomes in various malignancies (62). A study examined the association between muscle and fat areas and radiodensity, and OS in people with newly diagnosed MM (62). Overall, 341 patients diagnosed with MM from 2010-2019 who had an 18F-fluorodeoxyglucose positron emission tomography/computed tomography within 3 months prior to diagnosis, or after diagnosis but within <1 month of starting treatment were included in the study. Median follow up was 5.7 years. Sarcopenia was defined as skeletal muscle index below the sex-specific median in the population. The prevalence of sarcopenia ranged from 46% to 56% depending on the cutoff used. Median PFS was 33.4 (95% CI 27.2-49.8) months in patients with sarcopenia compared to 45.0 (95% CI: 31.9-71.0) months in patients without sarcopenia (p=0.25). Median OS was 7.6 (95% CI: 5.8-not reached) years and 9.3 years (95%CI: 6.1-not reached) in patients with and without sarcopenia, respectively (p=0.77). Nevertheless, low muscle radiodensity was associated with higher disease stage, anemia, and renal failure. OS was 5.6 vs. 9.0 years in patients with muscle radiodensity in the lower vs. middle/upper tertiles, respectively (p=0.02). Authors conclude that body composition evaluating using routine low-dose CT images taken at diagnosis can offer valuable prognostic insights for patients with MM. Assessments of muscle and fat quality might be more effective in predicting disease outcomes than simply measuring muscle and fat quantity (62).
Another study also examined whether the presence of sarcopenia had prognostic value in patients with newly diagnosed MM (63). Sarcopenia was determined by utilizing a deep learning-based convolutional neural network algorithm on CT images of the abdomen. Subjects with newly diagnosed MM from January 2005 to July 2019 who had a standard dose CT scan that included the L3 vertebral level performed anytime within 6 months of diagnosis were enrolled in the study. A total of 322 participants were included in the analysis. The median age was 66 (range 37-95) and 20% were older than 75 years of age. The median BMI was 26.7 (range: 16.2-59.7) and 30% had a BMI greater than 30. Of the 200 patients with FISH cytogenetics information available, 49 (25%) were categorized as having high-risk cytogenetics. The median follow-up for this cohort was 72 months (95% CI: 63-83). Overall, 171 (53%) patients in this study cohort were categorized as sarcopenic. The study showed that the median OS for sarcopenic patients was 45 months compared to 90 months for those not sarcopenic (p=0.0005). Moreover, the 2-year mortality rate for sarcopenic patients was 40% compared to 18% for the non-sarcopenic patients (p<0.0001). In the multivariable model, the adverse prognostic impact of sarcopenia was independent of International Staging System (ISS) stage, age, and high-risk FISH cytogenetics (63).Therefore, body composition may be associated with poor outcomes in people with MM. However, further prospective studies are needed to examine whether sarcopenia is independently associated with MM outcomes.
Nutrition as a risk factor for multiple myeloma
The etiology of MGUS remains largely unknown and there have been no studies examining the impact of diet on MGUS or its progression to MM. Data from the population-based AGES Study (N=5,764) indicated that fruit consumption at least three times per week during adolescence led to lower risk of MGUS (OR=0.62, 95%CI 0.41-0.95) and fruit consumption at least three times per week during the late life led to decreased risk of progressing from MGUS to MM (HR=0.34, 95%CI 0.13-0.89) when compared to lower fruit consumption.
Additionally, limited studies have evaluated the relation between diet and MM, with the results being inconclusive. Regarding meat consumption, one older study found a significant increase in risk per portion of red meat daily (64), while another one found that meat consumption increased non-significantly the risk among Blacks and decreased non-significantly the risk among Whites (18). Three studies have found an inverse association of fish intake and risk of MM (18, 19, 65). Moreover, two studies have examined dairy product consumption: one reported increased risk for total dairy intake and eggs (18) while the other identified a significant increased risk linked to yogurt consumption (66). In another study, vegetables consumption was found to have an inverse association with MM and a non-significant increase in risk for fruit consumption (18). In a more recent population-based case-control study, inverse associations for cooked tomatoes, cruciferous vegetables, fresh fish, alcohol and vitamin A and positive associations for cream soups, jello, ice cream and pudding with MM have been observed (30).
In a very recent study, after analyzing data from prospective cohorts of 69751 women (Nurses’ Health Study, 1984-2014) and 47232 men (Health Professionals Follow-up Study, 1986–2014), the association between dietary pattern and risk of MM was examined. In men, empirical dietary inflammatory pattern, empirical dietary indices for insulin resistance and empirical dietary indices for hyperinsulinemia were statistically significantly associated with increase in MM risk (16%, 9% and 11%, respectively) (33).
The impact of nutrition on multiple myeloma and the nutritional status of patients
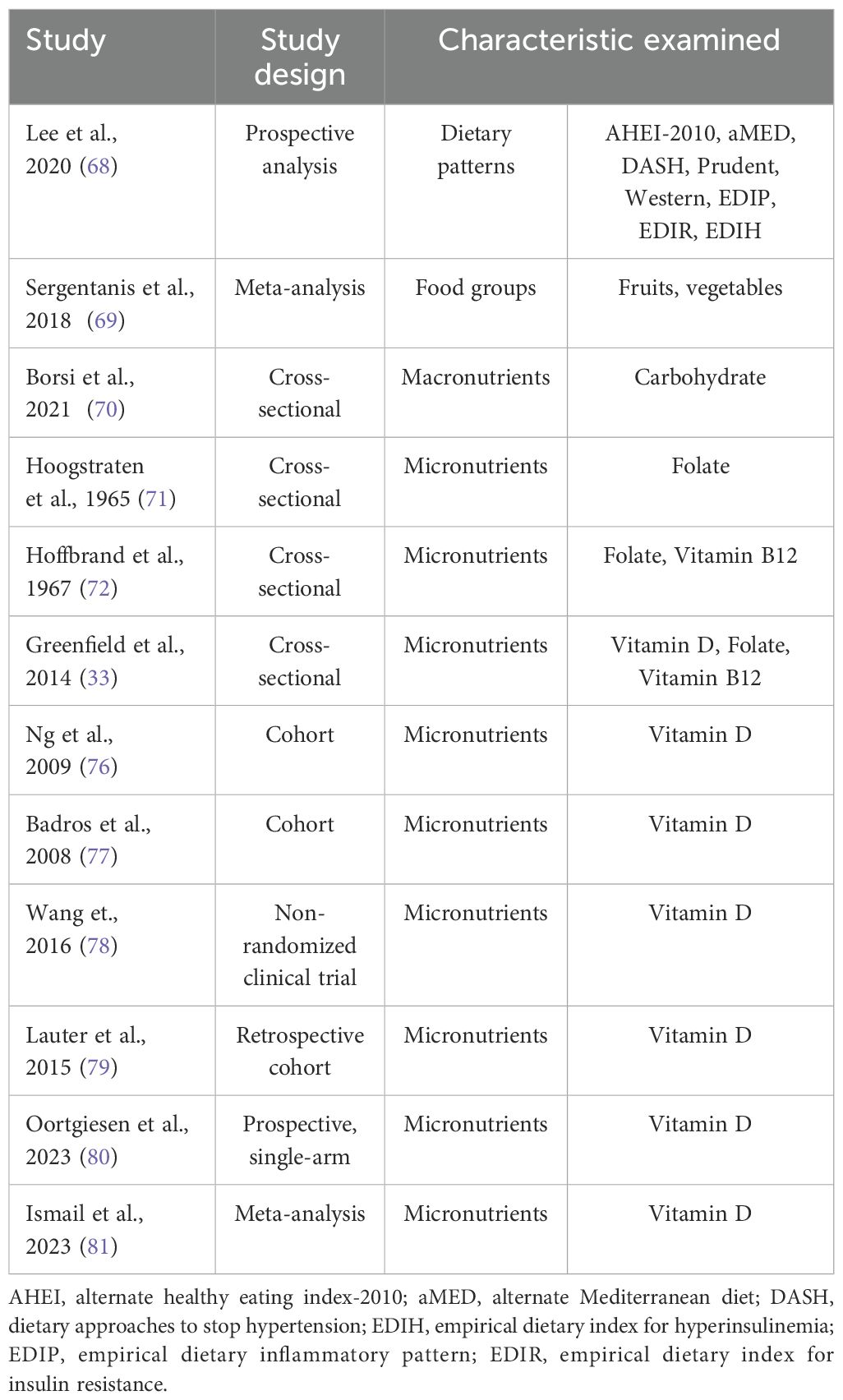
Nutrition is considered as a major modifiable risk factor for cancer and MM development with many available studies assessing the relation between nutrition and MM risk (18, 30, 61, 67). However, studies about the role of nutrition and nutritional status on MM patients and disease progression are limited (27) (Table 1).
Dietary patterns, food groups and macronutrient intake
A recent pooled prospective survival analyses of 423 MM patients derived from the Nurses’ Health Study (1986-2016) and the Health Professionals Follow-up Study (1988-2016) examined the association of dietary patterns (Alternate Healthy Eating Index (AHEI)-2010, Alternate Mediterranean Diet, Dietary Approaches to Stop Hypertension, Prudent, Western and empirical dietary inflammatory patterns and empirical dietary indices for insulin resistance and hyperinsulinemia) followed before diagnosis with MM survival. Data showed 295 deaths related to MM among 345 total deaths. Mortality was 15-24% lower for each one standard deviation (SD) increase in favorable dietary pattern scores and 16-24% higher per 1-SD increase in “unhealthy” diet scores. Researchers concluded that MM patients with healthier dietary habits before diagnosis may exhibit better survival than those who followed less healthy dietary patterns (68).
A recent systematic review examined the association between fruit and vegetable consumption with the risk of hematological malignancies. Neither fruit nor vegetable consumption was related to MM risk (69).
A very recent study assessed the knowledge about nutrition and the quality of diet in 61 patients with MM. Significant changes of clinical parameters (hemoglobin, uric acid, albumin, total proteins, beta-2 microglobulin, percentage of plasmacytes in the bone marrow and D-dimers) were observed in patients with high or low carbohydrate intake compared to patients with medium carbohydrate consumption. Moreover, their knowledge about nutrition was not associated either with clinical indicators of disease status nor with their nutrient intake (70).
Micronutrients
It has long been known that MM patients may present nutritional deficiencies especially on folate levels. Early enough, in 1965, a study of Hoogstraten et al. showed that patients with active MM presented lower serum folate levels (71). Another study of Hoffbrand et al. (1967) aiming to determine the incidence, severity, and cause of B12 deficiency in patients with MM showed that among 32 patients participating in the study, 26 patients had low serum folate levels and 5 had vitamin B12 deficiency (72). In a more recent study aiming to evaluate the body composition and the nutritional status, among other parameters, of 32 intensively treated patients, with advanced but stable MM, 25% of the patients had reduced serum folate, 6% had reduced vitamin B12 and 59% of patients were vitamin D insufficient/deficient (33). Vitamin D levels have been of concern to the scientific community for its involvement in various diseases (73). MM is the most common malignancy affecting bone and up to 90% of patients develop bone lesions (74). On that account, studies have examined the prevalence of vitamin D deficiency in MM individuals and its role in disease progression (33, 75, 76). In a study conducted in 148 newly diagnosed MM patients, a ‘‘step-wise’’ association between International Staging System (ISS) staging and vitamin D deficiency was found. Additionally, the prevalence of vitamin D deficiency increased in parallel with ISS and reached its peak in stage 3 with 37% of patients presenting deficiency (76). It is also of major importance that subjects presenting vitamin D deficiency also present higher serum CRP and creatinine levels, markers that have been shown to predict MM prognosis, with the authors suggesting that vitamin D deficiency may lead to lower MM prognosis due to its activity in both skeletal and non-skeletal level (76). Greenfield et al. confirmed these results showing that 59% of the patients participating in the study were vitamin D insufficient/deficient (33). The high incidence of low vitamin D levels in MM patients compared to the general population, is predominantly caused by lower levels of activity in MM patients resulting in low sunlight exposure and it is independent of age, sex and disease activity (77). Recently, studies have highlighted that treatments such as thalidomide or bortezomib may be additional factors contributing to vitamin D deficiency and this is also associated with peripheral neuropathy. Moreover, vitamin D supplementation may prevent the development of severe neuropathy or reduce its severity among patients with MM (78). A study aiming to evaluate serum 5-hydroxyvitamin D (25(OH)D) status and supplementation with vitamin D on MM patients found that supplementation led to significantly higher levels of (25(OH)D) (79). Also, hemoglobin, leukocyte, erythrocyte levels were higher after supplementation, while lower thrombocyte levels were found. However, the authors concluded that although significant serum (25(OH)D) increases were observed, sufficient levels were not achieved even with doses exceeding the 1000 IUs per day (79). Contrary to these results, in a more recent study vitamin D supplementation of MM patients using substantially higher doses than recommended in guidelines, and more specifically a 3-level dose escalation regimen with a loading dose of 200,000 IU given at the beginning of the study and a maintenance dose of 800 IU, has been proved to be an effective approach to achieving adequate vitamin D levels after 6 months of supplementation. It has to be noted that the low toxicity risk allowed the use of high doses of the vitamin D supplementation (80). However, it is evident that more studies are needed to assess the efficacy of vitamin D supplementation on serum 25(OH)D levels and clarify the efficacy of such approaches and establishing specific guidelines for the repletion of not only 25(OH)D levels but also other vitamins through supplementation in patients with MM appears to be a distant goal.
A very recent meta-analysis which included 18 studies found that the prevalence of serum vitamin D deficiency and insufficiency in patients with MM was 39.4% and 34.1%, respectively. It was also observed a greater proportion of newly diagnosed patients who exhibited vitamin D deficiency and insufficiency (43.0% and 41.6%, respectively), compared to patients who were under treatment (41.6% and 32.3%, respectively) (81).
The role of gut microbiota in the progression of multiple myeloma
The human microbiota is a distinct collection of microorganisms that inhabit and evolve within the human body from the onset of life. These microorganisms interact with the host’s immune system, aiding in the formation of defenses against pathogens. Gut microbiome alterations, namely intestinal dysbiosis, are closely related to a variety of diseases. However, the mechanisms through which commensal bacteria influence a broad range of mucosal and extramucosal human disorders have only been partially understood. So far, little is known about the role of the gut microbiome and alterations of its metabolic functions in the development of MM (82).
In a cohort aiming to investigate the potential relation between the gut microbiome and MM, researchers found that higher bacterial diversity was associated to significant differences in metagenomic composition in 19 patients with newly diagnosed MM. Eleven opportunistic pathogens (Raoultella ornithinolytica, Citrobacter freundii, Enterobacter cloacae, Klebsiella aerogenes, Klebsiella variicola, Klebsiella pneumoniae, Streptococcus salivarius, Streptococcus oralis, Streptococcus gordonii, Streptococcus mitis, and Streptococcus pneumoniae) were found to have higher relative abundance in MM patients; these bacteria are nitrogen-recycling and may result from the accumulated urea nitrogen in MM. Moreover, fecal microbiota transplantation into 5TGM1 mice propose that a possible mechanism for the interaction between MM-enriched bacteria and MM progression is recycling urea nitrogen. Furthermore, MM progression seems to be promoted by Klebsiella pneumoniae via de novo synthesis of glutamine in mice while progression of MM was found milder in the mice fed with glutamine-deficient diet (83).
In a very recent study, the association of sustained minimal residual disease (MRD) negativity with dietary factors, stool metabolites and the stool microbiome in MM patients on lenalidomide maintenance was examined. Patients with sustained MRD negativity had increased stool butyrate amount, higher relative abundance of predicted butyrate producers and higher alpha diversity of the fecal microbiome at 3 months. Additionally, dietary proteins from seafood and plants were associated with butyrate at 3 months and sustained MRD negativity. Consumption of anthocyanidins, flavones, and flavanols was also associated with stool butyrate concentration (84).
In a very recent study, the gut microbiome and dietary intake in 30 patients with MM undergoing autologous stem cell transplantation were evaluated. A loss of alpha-diversity at the timepoint of engraftment due to a decrease in Blautia, Ruminococcus, and Faecalibacterium genera as a consequence to intravenous antibiotic exposure was observed. Alpha-diversity is a numerical measure used in microbiome studies to summarize species abundance within a sample, taking into account both species richness (number) and evenness (distribution). Higher alpha-diversity often indicates a healthier microbiota. Moreover, patients with higher fiber intake indicated higher relative abundance of Blautia at the pre-transplant timepoint. Partial response to therapy compared with complete response or very good partial response was observed to patients with lower alpha-diversity at engraftment timepoint (85).
Overall, the results of the aforementioned studies reveal a new role of the altered gut microbiome in the progression of MM suggesting new treatments by influencing the gut microbiota of patients with MM.
Conclusion
While there is rising interest in the role of diet and the gut microbiota in the development of MGUS and SMM to symptomatic myeloma, it is critical to show caution when drawing causal inferences. Diet, microbiota and cancer progression exhibit complicated interactions that are regulated by a variety of factors, including genetics, lifestyle and environmental exposure. Most existing studies are observational, making it difficult to establish direct causation. Therefore, while dietary interventions may show promise, they should be considered as part of a broader, multidimensional approach to disease management. Further prospective research examining longitudinal outcomes is necessary to determine the precise impact of dietary patterns, the interplay with microbiota and the role of dietary interventions on myeloma progression.
Author contributions
PK: Writing – original draft. GB: Writing – original draft. AT: Writing – review & editing. MG: Writing – review & editing. IN-S: Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Mirzaei H, Bagheri H, Ghasemi F, Khoi JM, Pourhanifeh MH, Heyden YV, et al. Anti-cancer activity of curcumin on multiple myeloma. Anticancer Agents Med Chem. (2021) 21:575–86. doi: 10.2174/1871520620666200918113625
2. Rajkumar SV. Multiple myeloma: Every year a new standard? Hematol Oncol. (2019) 37:62–5. doi: 10.1002/hon.2586
3. Rajkumar SV, Dimopoulos MA, Palumbo A, Blade J, Merlini G, Mateos MV, et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. (2014) 15:e538–48. doi: 10.1016/S1470-2045(14)70442-5
4. Brigle K, Rogers B. Pathobiology and diagnosis of multiple myeloma. Semin Oncol Nurs. (2017) 33:225–36. doi: 10.1016/j.soncn.2017.05.012
5. NATIONAL CANCER INSTITUTE. Cancer stat facts: myeloma(2024). Available online at: https://seer.cancer.gov/statfacts/html/mulmy.html. (accessed October 05, 2024).
6. Thordardottir M, Lindqvist EK, Lund SH, Costello R, Burton D, Steingrimsdottir L, et al. Dietary intake is associated with risk of multiple myeloma and its precursor disease. PloS One. (2018) 13:e0206047. doi: 10.1371/journal.pone.0206047
7. Mouhieddine TH, Weeks LD, Ghobrial IM. Monoclonal gammopathy of undetermined significance. Blood. (2019) 133:2484–94. doi: 10.1182/blood.2019846782
8. Kyle RA, Therneau TM, Rajkumar SV, Larson DR, Plevak MF, Offord JR, et al. Prevalence of monoclonal gammopathy of undetermined significance. New Engl J Med. (2006) 354:1362–9. doi: 10.1056/NEJMoa054494
9. Turesson I, Kovalchik SA, Pfeiffer RM, Kristinsson SY, Goldin LR, Drayson MT, et al. Monoclonal gammopathy of undetermined significance and risk of lymphoid and myeloid Malignancies: 728 cases followed up to 30 years in Sweden. Blood. (2014) 123:338–45. doi: 10.1182/blood-2013-05-505487
10. Dispenzieri A, Katzmann JA, Kyle RA, Larson DR, Melton LJ, Colby CL, et al. Prevalence and risk of progression of light-chain monoclonal gammopathy of undetermined significance: a retrospective population-based cohort study. Lancet. (2010) 375:1721–8. doi: 10.1016/S0140-6736(10)60482-5
11. Tentolouris A, Ntanasis-Stathopoulos I, Terpos E. Obesity and multiple myeloma: Emerging mechanisms and perspectives. Semin Cancer Biol. (2023) 92:45–60. doi: 10.1016/j.semcancer.2023.04.003
12. Tentolouris A, Ntanasis-Stathopoulos I, Eleftheriadou I, Malandrakis P, Tzeravini E, Gavriatopoulou M. Diabetes mellitus and multiple myeloma; common features of two distinct entities. Diabetes Metab Res Rev. (2022) 38(5):e3535. doi: 10.1002/dmrr.v38.5
13. Carson KR, Bates ML, Tomasson MH. The skinny on obesity and plasma cell myeloma: a review of the literature. Bone Marrow Transplant. (2014) 49:1009–15. doi: 10.1038/bmt.2014.71
14. De Pergola G, Silvestris F. Obesity as a major risk factor for cancer. J Obes. (2013) 2013:1–11. doi: 10.1155/2013/291546
15. Lichtman MA. Obesity and the risk for a hematological Malignancy: leukemia, lymphoma, or myeloma. Oncologist. (2010) 15:1083–101. doi: 10.1634/theoncologist.2010-0206
16. Teras LR, Kitahara CM, Birmann BM, Hartge PA, Wang SS, Robien K, et al. Body size and multiple myeloma mortality: a pooled analysis of 20 prospective studies. Br J Haematol. (2014) 166:667–76. doi: 10.1111/bjh.2014.166.issue-5
17. Wallin A, Larsson SC. Body mass index and risk of multiple myeloma: A meta-analysis of prospective studies. Eur J Cancer. (2011) 47:1606–15. doi: 10.1016/j.ejca.2011.01.020
18. Brown LM, Gridley G, Pottern LM, Baris D, Swanson CA, Silverman DT, et al. Diet and nutrition as risk factors for multiple myeloma among blacks and whites in the United States. Cancer Causes Control. (2001) 12:117–25. doi: 10.1023/A:1008937901586
19. Fritschi L, Ambrosini GL, Kliewer EV, Johnson KC, Canadian Cancer Registries Epidemiologic Research Group. Dietary fish intake and risk of leukaemia, multiple myeloma, and non-Hodgkin lymphoma. Cancer Epidemiol Biomarkers Prev. (2004) 13:532–7. doi: 10.1158/1055-9965.532.13.4
20. Mirhosseini N, Psihogios A, McLaren MD, Seely D. Vitamin D and multiple myeloma: A scoping review. Curr Oncol. (2023) 30:3263–76. doi: 10.3390/curroncol30030248
21. Burwick N. Vitamin D. and plasma cell dyscrasias: reviewing the significance. Ann Hematol. (2017) 96:1271–7. doi: 10.1007/s00277-017-3016-8
22. Gascoyne DM, Lyne L, Spearman H, Buffa FM, Soilleux EJ, Banham AH. Vitamin D receptor expression in plasmablastic lymphoma and myeloma cells confers susceptibility to vitamin D. Endocrinology. (2017) 158:503–15. doi: 10.1210/en.2016-1802
23. Yang Q, Wei Y, Zhu Y, Guo J, Zhang J, He Y, et al. The interaction between gut microbiota and host amino acids metabolism in multiple myeloma. Cancers (Basel). (2023) 15:1942. doi: 10.3390/cancers15071942
24. Lauby-Secretan B, Scoccianti C, Loomis D, Grosse Y, Bianchini F, Straif K. Body fatness and cancer — Viewpoint of the IARC working group. New Engl J Med. (2016) 375:794–8. doi: 10.1056/NEJMsr1606602
25. Birmann BM, Giovannucci E, Rosner B, Anderson KC, Colditz GA. Body mass index, physical activity, and risk of multiple myeloma. Cancer Epidemiology Biomarkers Prev. (2007) 16:1474–8. doi: 10.1158/1055-9965.EPI-07-0143
26. Went M, Cornish AJ, Law PJ, Kinnersley B, van Duin M, Weinhold N, et al. Search for multiple myeloma risk factors using Mendelian randomization. Blood Adv. (2020) 4:2172–9. doi: 10.1182/bloodadvances.2020001502
27. Shapiro YN, Peppercorn JM, Yee AJ, Branagan AR, Raje NS, Donnell EKO. Lifestyle considerations in multiple myeloma. Blood Cancer J. (2021) 11:172. doi: 10.1038/s41408-021-00560-x
28. Hodge A, Sheean P, O’Connor P, Tyler K, Kerschner A, Williams A, et al. Exploring health behaviors and the feasibility of a lifestyle intervention for patients with multiple myeloma. Supportive Care Cancer. (2022) 30:9771–9. doi: 10.1007/s00520-022-07385-9
29. Lee DH, Fung TT, Tabung FK, Colditz GA, Ghobrial IM, Rosner BA, et al. Dietary pattern and risk of multiple myeloma in two large prospective US cohort studies. JNCI Cancer Spectr. (2019) 3. doi: 10.1093/jncics/pkz025
30. Hosgood HD, Baris D, Zahm SH, Zheng T, Cross AJ. Diet and risk of multiple myeloma in Connecticut women. Cancer Causes Control. (2007) 18:1065–76. doi: 10.1007/s10552-007-9047-z
31. Caini S, Masala G, Gnagnarella P, Ermini I, Russell-Edu W, Palli D, et al. Food of animal origin and risk of non-Hodgkin lymphoma and multiple myeloma: A review of the literature and meta-analysis. Crit Rev Oncol Hematol. (2016) 100:16–24. doi: 10.1016/j.critrevonc.2016.02.011
32. Hofmann JN, Moore SC, Lim U, Park Y, Baris D, Hollenbeck AR, et al. Body mass index and physical activity at different ages and risk of multiple myeloma in the NIH-AARP diet and health study. Am J Epidemiol. (2013) 177:776–86. doi: 10.1093/aje/kws295
33. Greenfield DM, Boland E, Ezaydi Y, Ross RJM, Ahmedzai SH, Snowden JA, et al. Endocrine, metabolic, nutritional and body composition abnormalities are common in advanced intensively-treated (transplanted) multiple myeloma. Bone Marrow Transplant. (2014) 49:907–12. doi: 10.1038/bmt.2014.63
34. Wongrakpanich S, George G, Chaiwatcharayut W, Candelario N, Mittal V, Pomerantz S, et al. Frequency of folate deficiency in multiple myeloma patients: a 10-year retrospective study. Int J Lab Hematol. (2016) 38:e19–22. doi: 10.1111/ijlh.2016.38.issue-2
35. Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. (2003) 348:1625–38. doi: 10.1056/NEJMoa021423
36. Chang SH, Luo S, Thomas TS, O’Brian KK, Colditz GA, Carlsson NP, et al. Obesity and the transformation of monoclonal gammopathy of undetermined significance to multiple myeloma: A population-based cohort study. J Natl Cancer Inst. (2017) 109:djw264. doi: 10.1093/jnci/djw264
37. Brevi A, Cogrossi LL, Grazia G, Masciovecchio D, Impellizzieri D, Lacanfora L, et al. Much more than IL-17A: cytokines of the IL-17 family between microbiota and cancer. Front Immunol. (2020) 11:565470. doi: 10.3389/fimmu.2020.565470
38. Ahmed N, Ghannoum M, Gallogly M, de Lima M, Malek E. Influence of gut microbiome on multiple myeloma: friend or foe? J Immunother Cancer. (2020) 8:e000576. doi: 10.1136/jitc-2020-000576
39. Alkharabsheh O, Sidiqi MH, Aljama MA, Gertz MA, Frankel AE. The human microbiota in multiple myeloma and proteasome inhibitors. Acta Haematol. (2020) 143:118–23. doi: 10.1159/000500976
40. Rajkumar SV. Multiple myeloma: 2020 update on diagnosis, risk-stratification and management. Am J Hematol. (2020) 95:548–67. doi: 10.1002/ajh.25791
41. Cowan AJ, Allen C, Barac A, Basaleem H, Bensenor I, Curado MP, et al. Global burden of multiple myeloma. JAMA Oncol. (2018) 4:1221. doi: 10.1001/jamaoncol.2018.2128
42. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. (2020) 70:7–30. doi: 10.3322/caac.21590
43. Guedes A, Becker RG, Teixeira LEM. Multiple Myeloma (Part 1) - update on epidemiology, diagnostic criteria, systemic treatment and prognosis. Rev Bras Ortop (Sao Paulo). (2023) 58:361–7. doi: 10.1055/s-0043-1770149
44. Kyle RA, Gertz MA, Witzig TE, Lust JA, Lacy MQ, Dispenzieri A, et al. Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin Proc. (2003) 78:21–33. doi: 10.4065/78.1.21
45. Durie BGM, Hoering A, Abidi MH, Rajkumar SV, Epstein J, Kahanic SP, et al. Bortezomib with lenalidomide and dexamethasone versus lenalidomide and dexamethasone alone in patients with newly diagnosed myeloma without intent for immediate autologous stem-cell transplant (SWOG S0777): a randomised, open-label, phase 3 trial. Lancet. (2017) 389:519–27. doi: 10.1016/S0140-6736(16)31594-X
46. Landgren O, Graubard BI, Katzmann JA, Kyle RA, Ahmadizadeh I, Clark R, et al. Racial disparities in the prevalence of monoclonal gammopathies: a population-based study of 12 482 persons from the National Health and Nutritional Examination Survey. Leukemia. (2014) 28:1537–42. doi: 10.1038/leu.2014.34
47. Wadhera RK, Rajkumar SV. Prevalence of monoclonal gammopathy of undetermined significance: A systematic review. Mayo Clin Proc. (2010) 85:933–42. doi: 10.4065/mcp.2010.0337
48. Thorsteinsdottir S, Kristinsson SY. The consultant’s guide to smoldering multiple myeloma. Hematology. (2022) 2022:551–9. doi: 10.1182/hematology.2022000355
49. Landgren O, Graubard BI, Kumar S, Kyle RA, Katzmann JA, Murata K, et al. Prevalence of myeloma precursor state monoclonal gammopathy of undetermined significance in 12372 individuals 10-49 years old: a population-based study from the National Health and Nutrition Examination Survey. Blood Cancer J. (2017) 7:e618. doi: 10.1038/bcj.2017.97
50. Thorsteinsdottir S, Gislason GK, Aspelund T, Rögnvaldsson S, Oskarsson JTT, Petursdottir I, et al. Prevalence of smoldering multiple myeloma: results from the Iceland screens, treats, or prevents multiple myeloma (iStopMM) study. Blood. (2021) 138:151–1. doi: 10.1182/blood-2021-148617
51. Landgren O, Katzmann JA, Hsing AW, Pfeiffer RM, Kyle RA, Yeboah ED, et al. Prevalence of monoclonal gammopathy of undetermined significance among men in Ghana. Mayo Clin Proc. (2007) 82:1468–73. doi: 10.1016/S0025-6196(11)61089-6
52. Iwanaga M, Tagawa M, Tsukasaki K, Kamihira S, Tomonaga M. Prevalence of monoclonal gammopathy of undetermined significance: study of 52,802 persons in Nagasaki City, Japan. Mayo Clin Proc. (2007) 82:1474–9. doi: 10.1016/S0025-6196(11)61090-2
53. Ravindran A, Bartley AC, Holton SJ, Gonsalves WI, Kapoor P, Siddiqui MA, et al. Prevalence, incidence and survival of smoldering multiple myeloma in the United States. Blood Cancer J. (2016) 6:e486–6. doi: 10.1038/bcj.2016.100
54. Go RS, Gundrum JD, Neuner JM. Determining the clinical significance of monoclonal gammopathy of undetermined significance: A SEER–medicare population analysis. Clin Lymphoma Myeloma Leuk. (2015) 15:177–186.e4. doi: 10.1016/j.clml.2014.09.004
55. Sigurdardottir EE, Turesson I, Lund SH, Lindqvist EK, Mailankody S, Korde N, et al. The role of diagnosis and clinical follow-up of monoclonal gammopathy of undetermined significance on survival in multiple myeloma. JAMA Oncol. (2015) 1:168. doi: 10.1001/jamaoncol.2015.23
56. Landgren O, Rajkumar SV, Pfeiffer RM, Kyle RA, Katzmann JA, Dispenzieri A, et al. Obesity is associated with an increased risk of monoclonal gammopathy of undetermined significance among black and white women. Blood. (2010) 116:1056–9. doi: 10.1182/blood-2010-01-262394
57. Marinac CR, Birmann BM, Lee IM, Rosner BA, Townsend MK, Giovannucci E, et al. Body mass index throughout adulthood, physical activity, and risk of multiple myeloma: a prospective analysis in three large cohorts. Br J Cancer. (2018) 118:1013–9. doi: 10.1038/s41416-018-0010-4
58. Sonderman JS, Bethea TN, Kitahara CM, Patel AV, Harvey C, Knutsen SF, et al. Multiple myeloma mortality in relation to obesity among african Americans. J Natl Cancer Inst. (2016) 108:djw120. doi: 10.1093/jnci/djw120
59. Beason TS, Chang SH, Sanfilippo KM, Luo S, Colditz GA, Vij R, et al. Influence of body mass index on survival in veterans with multiple myeloma. Oncologist. (2013) 18:1074–9. doi: 10.1634/theoncologist.2013-0015
60. Groß JP, Nattenmüller J, Hemmer S, Tichy D, Krzykalla J, Goldschmidt H, et al. Body fat composition as predictive factor for treatment response in patients with newly diagnosed multiple myeloma - subgroup analysis of the prospective GMMG MM5 trial. Oncotarget. (2017) 8:68460–71. doi: 10.18632/oncotarget.19536
61. Shah UA, Whiting K, Devlin S, Ershler R, Kanapuru B, Lee DJ, et al. Extreme body mass index and survival in newly diagnosed multiple myeloma patients. Blood Cancer J. (2023) 13:13. doi: 10.1038/s41408-022-00782-7
62. Abdallah NH, Nagayama H, Takahashi N, Gonsalves W, Fonder A, Dispenzieri A, et al. Muscle and fat composition in patients with newly diagnosed multiple myeloma. Blood Cancer J. (2023) 13. doi: 10.1038/s41408-023-00934-3
63. Nandakumar B, Baffour F, Abdallah NH, Kumar SK, Dispenzieri A, Buadi FK, et al. Sarcopenia identified by computed tomography imaging using a deep learning–based segmentation approach impacts survival in patients with newly diagnosed multiple myeloma. Cancer. (2023) 129. doi: 10.1002/cncr.v129.3
64. Tavani A, La Vecchia C, Gallus S, Lagiou P, Trichopoulos D, Levi F, et al. Red meat intake and cancer risk: A study in Italy. Int J Cancer. (2000) 89. doi: 10.1002/(SICI)1097-0215(20000501)86:3<425::AID-IJC19>3.0.CO;2-S
65. Fernandez E, Chatenoud L, La Vecchia C, Negri E, Franceschi S. Fish consumption and cancer risk. Am J Clin Nutr. (1999) 70:85–90. doi: 10.1093/ajcn/70.1.85
66. Vlajinac HD, Pekmezović TD, Adanja BJ, Marinković JM, Kanazir MS, Suvajdzić ND, et al. Case-control study of multiple myeloma with special reference to diet as risk factor. Neoplasma. (2003) 50:79–83.
67. Zhang FF, Cudhea F, Shan Z, Michaud DS, Imamura F, Eom H, et al. Preventable cancer burden associated with poor diet in the United States. JNCI Cancer Spectr. (2019) 3. doi: 10.1093/jncics/pkz034
68. Lee DH, Fung TT, Tabung FK, Marinac CR, Devore EE, Rosner BA, et al. Prediagnosis dietary pattern and survival in patients with multiple myeloma. Int J Cancer. (2020) 147:1823–30. doi: 10.1002/ijc.v147.7
69. Sergentanis TN, Psaltopoulou T, Ntanasis-Stathopoulos I, Liaskas A, Tzanninis IG, Dimopoulos MA. Consumption of fruits, vegetables, and risk of hematological Malignancies: a systematic review and meta-analysis of prospective studies. Leuk Lymphoma. (2018) 59:434–47. doi: 10.1080/10428194.2017.1339873
70. Borsi E, Serban CL, Potre C, Potre O, Putnoky S, Samfireag M, et al. High carbohydrate diet is associated with severe clinical indicators, but not with nutrition knowledge score in patients with multiple myeloma. Int J Environ Res Public Health. (2021) 18:5444. doi: 10.3390/ijerph18105444
71. Hoogstraten B, Baker H, Gilbert HS. Serum folate and serum vitamin B12 in patients with Malignant hematologie diseases. Cancer Res. (1965) 25:1933–8.
72. Hoffbrand AV, Hobbs JR, Kremenchuzky S, Mollin DL. Incidence and pathogenesis of megaloblastic erythropoiesis in multiple myeloma. J Clin Pathol. (1967) 20:699–705. doi: 10.1136/jcp.20.5.699
73. Álvarez-Mercado AI, Mesa MD, Gil Á. Vitamin D: Role in chronic and acute diseases. In: Encyclopedia of Human Nutrition. Elsevier (2023). p. 535–44. doi: 10.1016/B978-0-12-821848-8.00101-3
74. Roodman GD. Pathogenesis of myeloma bone disease. J Cell Biochem. (2010) 109:283–91. doi: 10.1002/jcb.v109:2
75. Maier GS, Horas K, Kurth AA, Lazovic D, Seeger JB, Maus U. Prevalence of vitamin D deficiency in patients with bone metastases and multiple myeloma. Anticancer Res. (2015) 35:6281–5.
76. Ng AC, Kumar SK, Rajkumar SV, Drake MT. Impact of vitamin D deficiency on the clinical presentation and prognosis of patients with newly diagnosed multiple myeloma. Am J Hematol. (2009) 84:397–400. doi: 10.1002/ajh.21412
77. Badros A, Goloubeva O, Terpos E, Milliron T, Baer MR, Streeten E. Prevalence and significance of vitamin D deficiency in multiple myeloma patients. Br J Haematol. (2008) 142:492–4. doi: 10.1111/j.1365-2141.2008.07214.x
78. Wang J, Udd KA, Vidisheva A, Swift RA, Spektor TM, Bravin E, et al. Low serum vitamin D occurs commonly among multiple myeloma patients treated with bortezomib and/or thalidomide and is associated with severe neuropathy. Supportive Care Cancer. (2016) 24(7):3105–10. doi: 10.1007/s00520-016-3126-1
79. Lauter B, Schmidt-Wolf IGH. Prevalence, supplementation, and impact of vitamin D deficiency in multiple myeloma patients. Cancer Invest. (2015) 33:505–9. doi: 10.3109/07357907.2015.1081690
80. Oortgiesen BE, Dekens M, Stapel R, Alheraky A, Dannenberg P de K, Siemes C, et al. Effectiveness of a vitamin D regimen in deficient multiple myeloma patients and its effect on peripheral neuropathy. Supportive Care Cancer. (2023) 31:138. doi: 10.1007/s00520-023-07574-0
81. Ismail NH, Mussa A, Al-Khreisat MJ, Mohamed Yusoff S, Husin A, Johan MF, et al. The global prevalence of vitamin D deficiency and insufficiency in patients with multiple myeloma: A systematic review and meta-analysis. Nutrients. (2023) 15:3227. doi: 10.3390/nu15143227
82. Brevi A, Cogrossi LL, Lorenzoni M, Mattorre B, Bellone M. The insider: impact of the gut microbiota on cancer immunity and response to therapies in multiple myeloma. Front Immunol. (2022) 13. doi: 10.3389/fimmu.2022.845422
83. Jian X, Zhu Y, Ouyang J, Wang Y, Lei Q, Xia J, et al. Alterations of gut microbiome accelerate multiple myeloma progression by increasing the relative abundances of nitrogen-recycling bacteria. Microbiome. (2020) 8:74. doi: 10.1186/s40168-020-00854-5
84. Shah UA, Maclachlan KH, Derkach A, Salcedo M, Barnett K, Caple J, et al. Sustained minimal residual disease negativity in multiple myeloma is associated with stool butyrate and healthier plant-based diets. Clin Cancer Res. (2022) 28:5149–55. doi: 10.1158/1078-0432.CCR-22-0723
Keywords: multiple myeloma, monoclonal gammopathy of undetermined significance, obesity, nutrition, gut microbiome
Citation: Kanellos PT, Baxevanis GK, Tentolouris A, Gavriatopoulou M and Ntanasis-Stathopoulos I (2024) The role of nutrition and gut microbiome in the progression of multiple myeloma and its precursor disease. Front. Oncol. 14:1461128. doi: 10.3389/fonc.2024.1461128
Received: 07 July 2024; Accepted: 27 September 2024;
Published: 14 October 2024.
Edited by:
Wilson Gonsalves, Mayo Clinic, United StatesReviewed by:
Joselle Cook, Mayo Clinic, United StatesCopyright © 2024 Kanellos, Baxevanis, Tentolouris, Gavriatopoulou and Ntanasis-Stathopoulos. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ioannis Ntanasis-Stathopoulos, am9obm50YW5hc2lzQG1lZC51b2EuZ3I=
 Panagiotis T. Kanellos
Panagiotis T. Kanellos Georgios K. Baxevanis
Georgios K. Baxevanis Anastasios Tentolouris
Anastasios Tentolouris Maria Gavriatopoulou
Maria Gavriatopoulou Ioannis Ntanasis-Stathopoulos
Ioannis Ntanasis-Stathopoulos