- 1Department of Obstetrics and Gynecology, West China Second University Hospital, Sichuan University, Chengdu, China
- 2Key Laboratory of Birth defects and Related Diseases of Women and Children (Sichuan University), Ministry of Education, Chengdu, China
Objective: Uterine inflammatory myofibroblastic tumor (UIMT) is a rare tumor of the female reproductive tract with uncertain malignant potential. Previous case series reports have limited our understanding of its diagnosis and treatment. Therefore, we conducted a retrospective analysis of patient files at West China Second University Hospital, Sichuan University to contribute valuable clinical insights to future treatment strategies for this disease.
Method: We comprehensively reviewed patient files of individuals diagnosed with UIMT from January 1st, 2013 to May 1st, 2023.
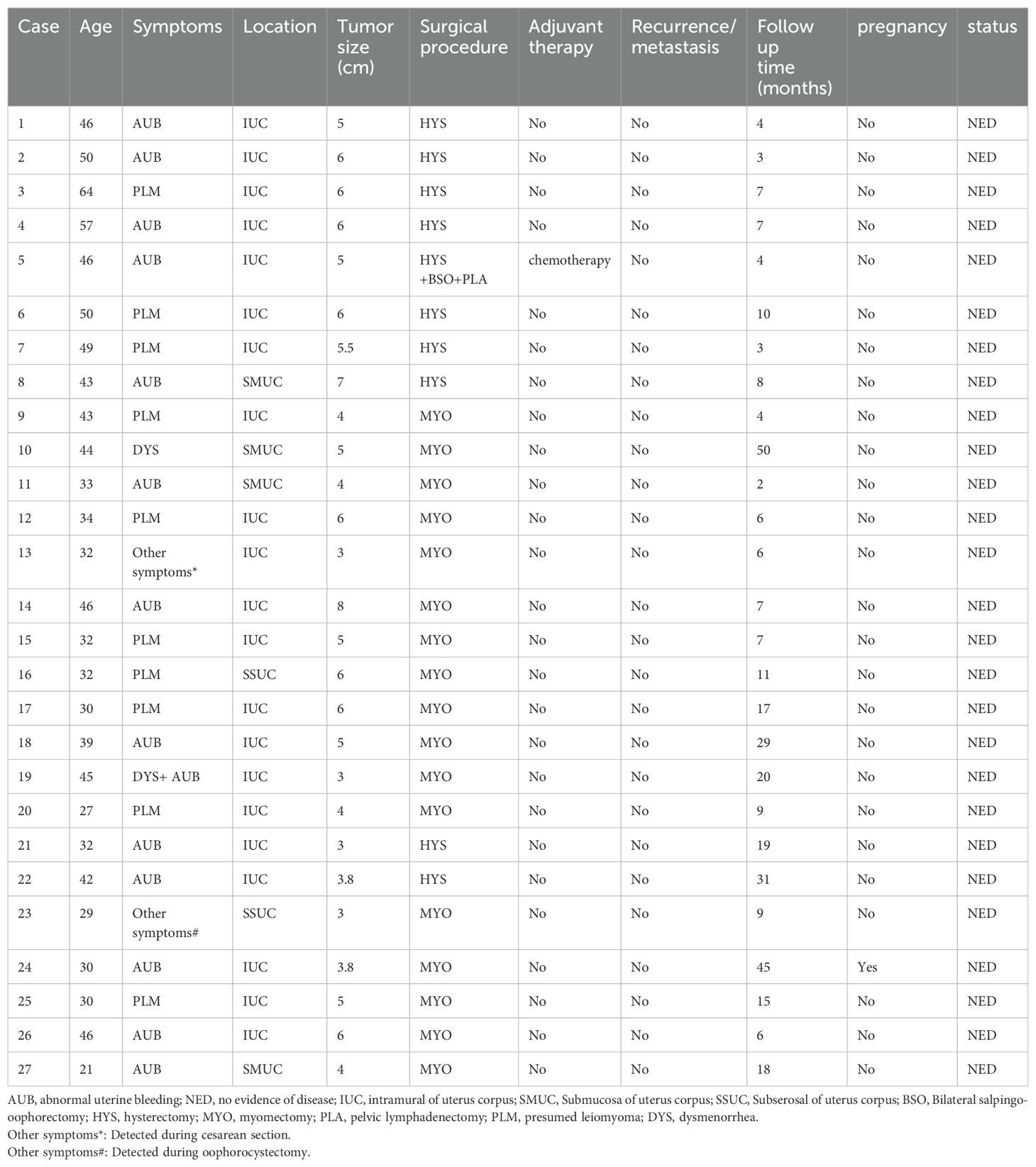
Results: We included twenty-seven cases of uterine inflammatory myofibroblastic tumor in our study. Of these, 51.85% (14 cases) were diagnosed with abnormal uterine bleeding, 2 cases had dysmenorrhea, and 12 were unexpectedly diagnosed with suspected uterine fibroids. Ten cases performed total hysterectomy, and 17 cases underwent lesion resection. The positive rate of anaplastic lymphoma kinase (ALK) immunohistochemistry reached 96.3%. After a median of 8 months follow-up time, all patients were disease-free and had survived.
Conclusion: Uterine inflammatory myofibroblastic tumor is easily misdiagnosed, making its diagnosis challenging. Histological features, immunohistochemical results, and molecular confirmation using fluorescence in situ hybridization (FISH) or Next-generation sequencing should be used to confirm the diagnosis. Positive ALK immunohistochemistry, ALK rearrangement, ALK fusion are helpful in diagnosis and ALK inhibitor therapy. Total hysterectomy is often performed for women who do not require fertility, while lesion resection and close follow-up may be considered for those who require fertility preservation.
Introduction
Inflammatory myofibroblastic tumor (IMT), a rare condition characterized by the presence of fibroblastic spindle cells with varying degrees of myxoid stroma and lymphoplasmacytic inflammation (1). Although initially discovered in the lungs, IMT can occur in various anatomical locations, including the female reproductive organs, particularly the uterus. In the uterus, IMT is responsible for only 0.1% of "leiomyomas", but its incidence rises to 10% in pregnant women and 14% in uterine smooth muscle tumors of uncertain malignant potential(STUMP) (2). Most cases of uterine inflammatory myofibroblastic tumor (UIMT) are benign, but the tumor can spread to surrounding tissues or recur after surgery. IMT often involves ALK gene rearrangements and has a low risk of metastasis. It is classified as a tumor of intermediate malignant potential, with recurrence and metastasis rates of approximately 25% and 2%, respectively (3).
Diagnosing IMT can be challenging due to its similarity to other conditions such as leiomyomas, leiomyosarcomas, endometrial stromal tumors, fibroleiomyomatosis, and ligamentous fibroma. The clinical overlap in morphology often leads to misdiagnosis, potentially resulting in a higher incidence than originally estimated. However, advancements in diagnostic tools, such as ALK immunohistochemical testing, have improved the ability to differentiate between STUMP and leiomyosarcoma. In fact, ALK immunohistochemistry, FISH, and RNA sequencing have proven to be highly specific in diagnosing UIMT, enabling its diagnosis with any positive result. Some ALK-negative cases can have other gene abnormalities, including RET, ROS1, or NTRK3 fusion, as well as PDGFRB 3’ fusion (4), THBS1, IGFBP5, DES, SEC31, TPM3, and TIMP3. DES-ALK, THBS1, FN1, DCTN1, and PPP1CB (5, 6). Additionally, certain fusion genes, such as TIMP3-ALK and THBS1-ALK, have been found to be highly enriched in pregnancy-related UIMT (7). However, the current diagnosis still relies on histological examination combined with immunohistochemistry, with ALK protein expression and ALK gene rearrangement serving as strong evidence for IMT diagnosis.
Currently, there is a lack of guidelines for the diagnosis and treatment of UIMT. Due to the rarity of the disease, previous literature has primarily consisted of individual case reports. Further research is needed to develop diagnostic and treatment methods for UIMT. The objective of this article is to conduct a retrospective analysis of UIMT patients in our hospital and contribute valuable clinical insights to future treatment strategies for this disease.
Materials and methods
Study population and data sources
This retrospective, observational, single-center study was conducted at West China Second University Hospital, Sichuan University, Chengdu, China, after receiving ethical approval from the hospital’s Ethics Committee. Written informed consent was obtained from all 27 patients who were diagnosed with uterine inflammatory myofibroblastic tumor and underwent surgery at the hospital between January 1, 2013, and May 11, 2023.
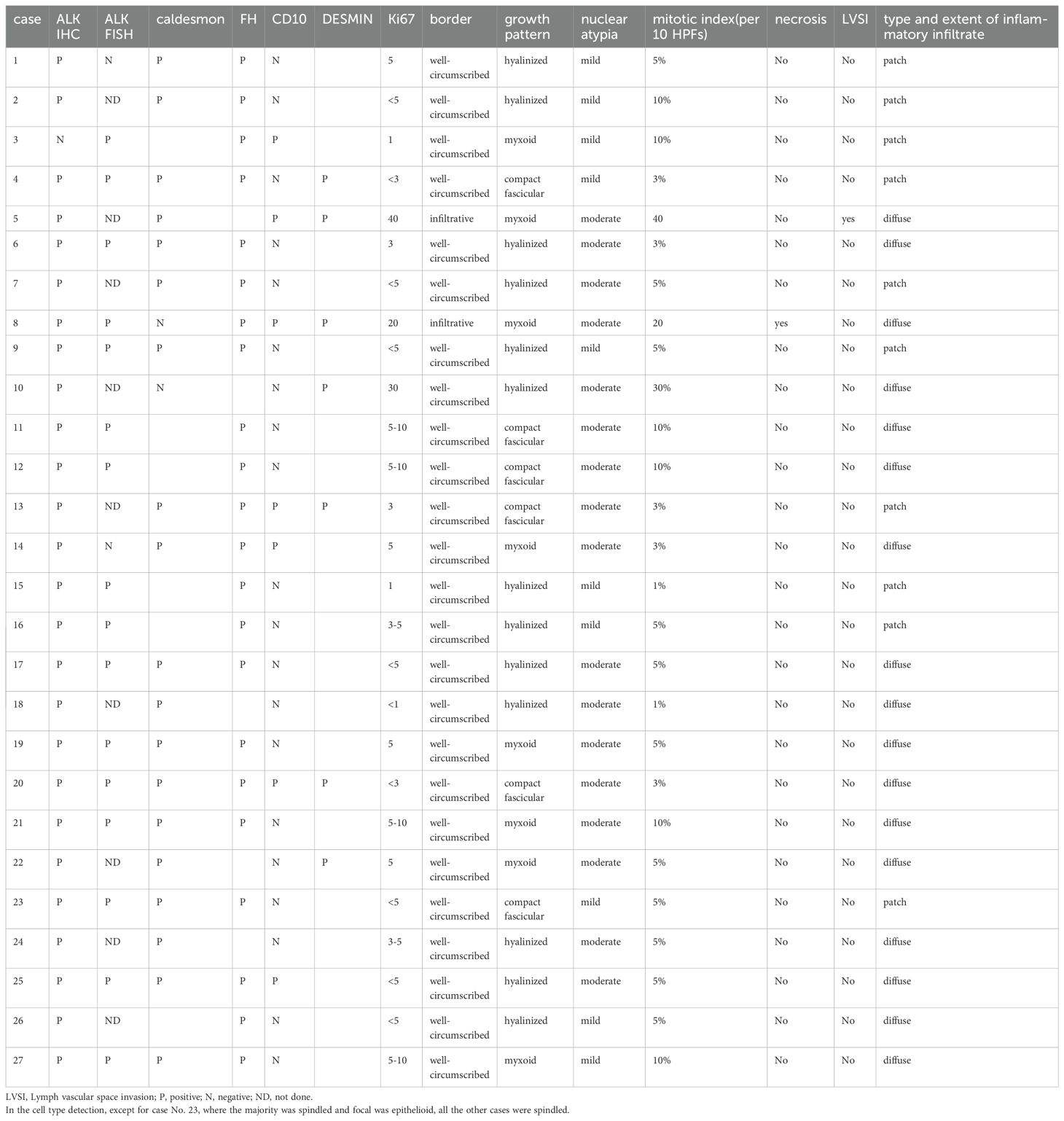
The study retrospectively examined patients’ clinical and pathological information. The pathological specimen was independently reviewed by two pathologists from West China Second University Hospital. Basic patient information, including age, symptoms, tumor characteristics (such as location and size), surgical procedures, comorbidities, adjuvant therapy, recurrence and metastasis rates, follow-up duration, and pregnancy status, was collected. Pathological features, including ALK immunohistochemistry, ALK fluorescence in situ hybridization, FH, CD10, caldesmon, desmin, Ki67, border, growth pattern, cell type, nuclear atypia, mitotic index, necrosis, lymphovascular invasion, type and extent of inflammatory infiltrates, as well as primary or metastatic status, were also collected. Oncological outcomes were assessed by following up with patients through outpatient visits and phone calls. Currently, all patients are disease-free, and no recurrence or metastasis has been reported.
Statistical analysis
For continuous normally distributed variables, mean ± standard deviation was used, and the t-test was used for analysis. The Levene’s test assessed variance homogeneity. For non-normally distributed continuous variables, medians (range) were used and analyzed using the Wilcoxon-Mann-Whitney U test. Pearson’s χ2 test or Fisher’s exact test was used for categorical variables. The likelihood ratio test was used to compare groups of categorical variables. SPSS version 25.0 (IBM Corp, Armonk, NY, USA) was used for statistical analysis. A two-sided p-value <0.05 was considered statistically significant. Descriptive analysis was used for non-continuous variables, and when the p-value was <0.05, the median and interquartile range were used.
Results
Clinical features
The study included 27 patients who were pathologically diagnosed with uterine inflammatory myofibroblastic tumor (UIMT). The patients' basic characteristics are presented in Tables 1–3. UIMTs have been documented in patients aged 21 to 64 years, with a median age of 42 years, and the tumors varied in size from 3 to 8 cm, with a median diameter of 5 cm. Abnormal uterine bleeding was the most common symptom, occurring in 51.85% of cases. Two cases had dysmenorrhea, and 12 cases were incidentally diagnosed due to suspected uterine fibroids. Notably, patient 19 had both dysmenorrhea and abnormal uterine bleeding, patient 13 was unexpectedly diagnosed during a cesarean section, and patient 23 during the removal of an ovarian cyst. The majority (21) of cases were located within the intramuscular layers of the uterus, while 3 were subserosal and 3 were submucosal. All cases of IMT occurred in the uterine body without any extrauterine lesions.
Pathological and molecular results
Macroscopically, most tumors were tan, pink, or white with no apparent capsule, and the sectioned surface of the tumor could have soft consistency, with a whorling appearance, hemorrhage, necrosis, myxoid features, and cyst formation (Figure 1). Tumor cells often comprised plump fusiform cells dispersed in a myxoid extracellular matrix with inflammatory infiltrates of varying amounts, while less commonly, the tumor cells may be epithelioid cell-like (Figure 2). 10 cases showed patchy infiltration, while 17 cases showed diffuse infiltration, and only 1 case exhibited lymphovascular space invasion (LVSI). Twenty-six cases tested positive for ALK immunohistochemistry, with only one case showing a negative result for ALK immunohistochemistry but a positive result for ALK FISH analysis. ALK FISH analysis was carried out on eighteen cases; sixteen cases showed positive results, two tested negative, and 9 cases did not undergo FISH testing.
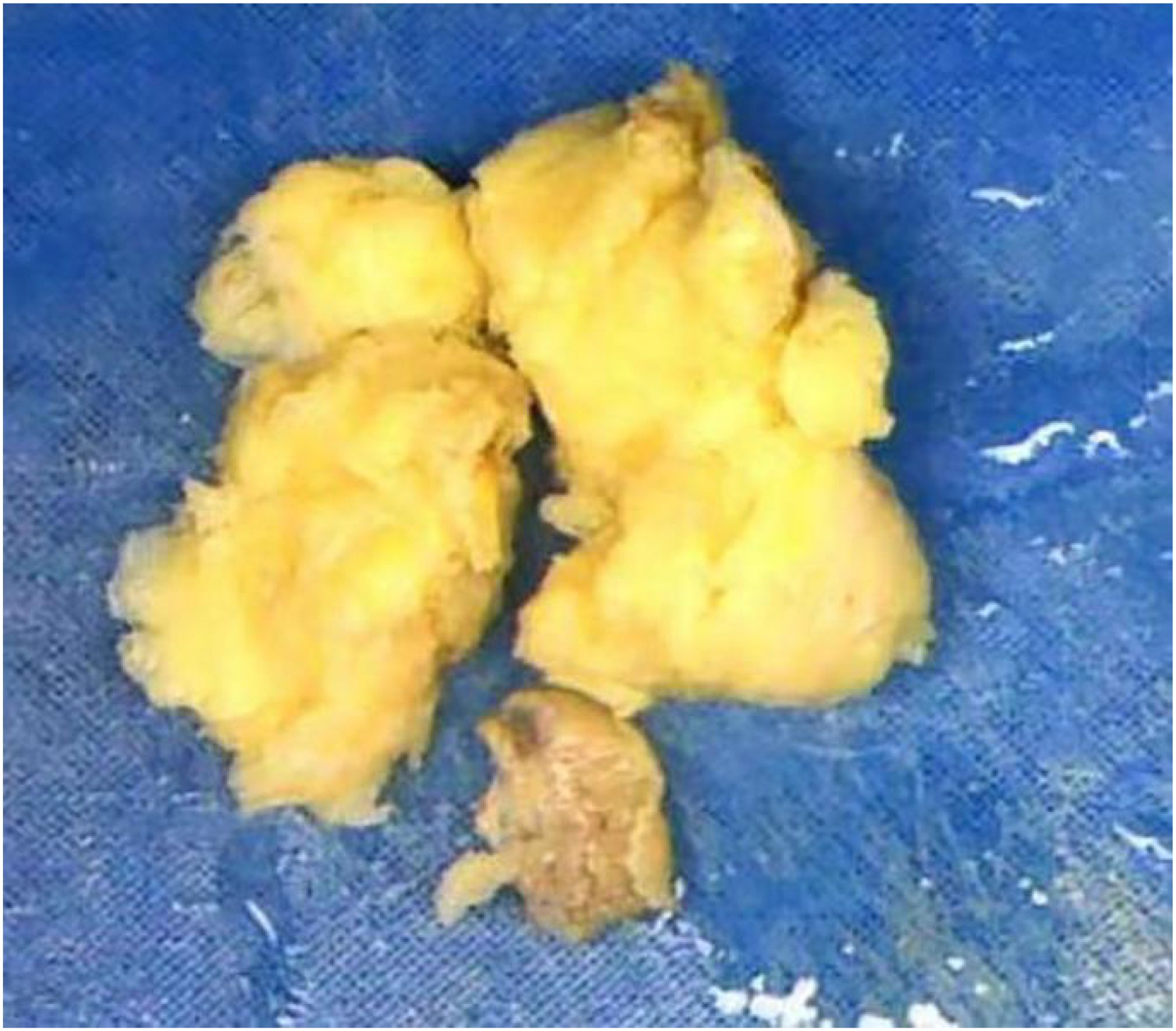
Figure 1. Uterine inflammatory myofibroblastic tumor. Macroscopically, most tumors were tan, pink, or white. The sectioned surface of the tumor can be of soft consistency, with a whorling appearance, hemorrhage, necrosis.
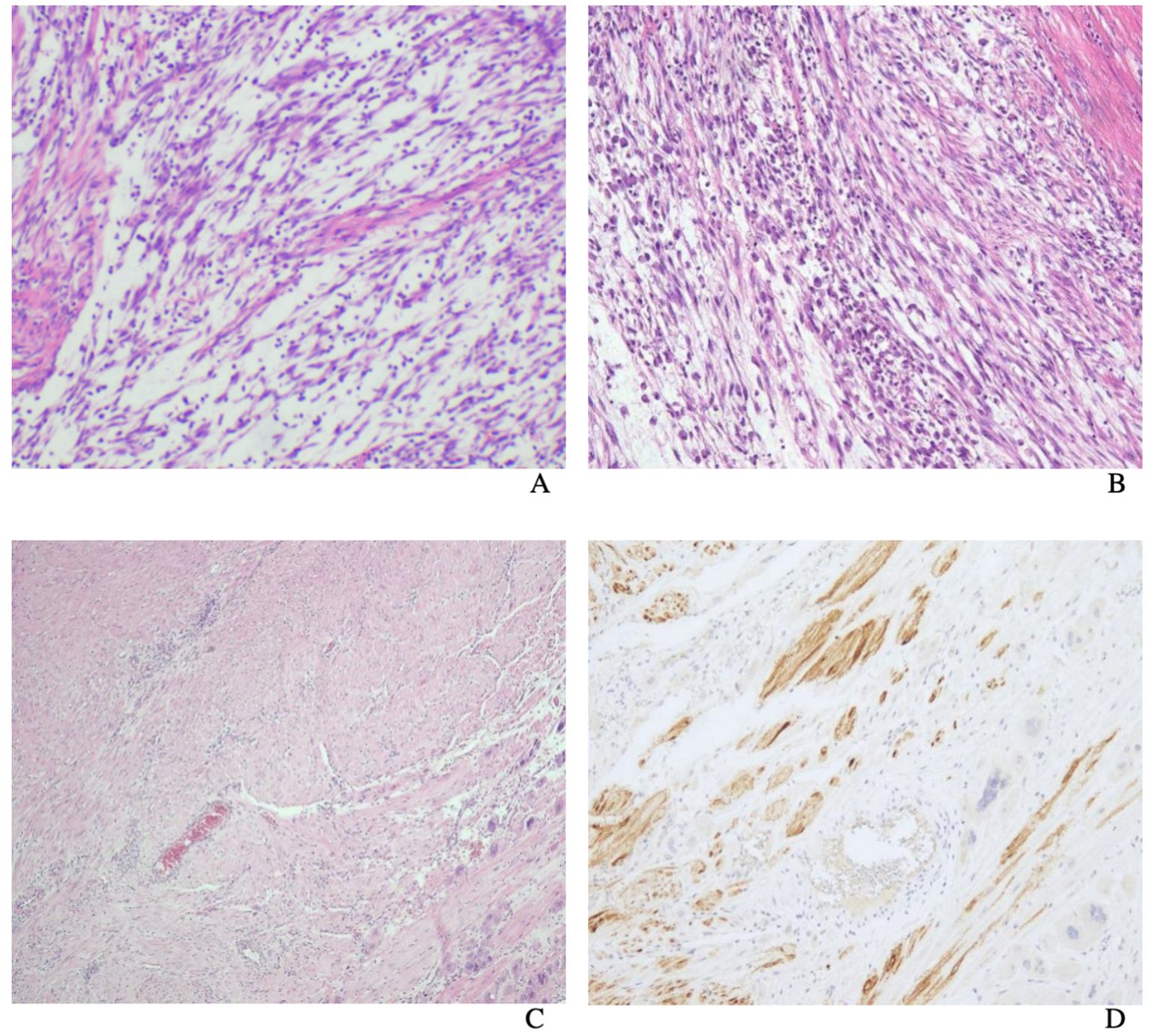
Figure 2. Uterine inflammatory myofibroblastic tumor. The tumor cells are usually fusiform cells dispersed in a myxoid extracellular matrix with varying degrees of inflammatory infiltrates (A). Tumor cells are composed of spindle and epithelioid cells admixed with lymphocytes (B). Uterine inflammatory myofibroblastic tumor. H&E (C) shows the appearance of tumor cells mimicked that of a smooth muscle tumor (smooth muscle-like) and ALK immunostain (D).
Treatment outcomes
Of the 27 patients, 10 underwent total hysterectomy, and 17 had lesion removal. Patient 5 underwent total hysterectomy, bilateral salpingectomy, and pelvic lymph node dissection, followed by adjuvant chemotherapy due to infiltrative growth, while patient 24 opted for fertility preservation and had a successful natural pregnancy after surgery, culminating in a term cesarean section. During the follow-up period, which ranged from 2 to 50 months, all patients remained disease-free. Additionally, the study found no significant difference in prognosis between tumor excision and hysterectomy, suggesting that alternative treatment options may be effective in managing UIMT.
Discussion
Our recent analysis of clinicopathological data at our hospital has provided new insights into the nature of uterine inflammatory myofibroblastic tumor (UIMT). The exact cause of IMT remains elusive, but it is believed that potential triggers may include traumatic injuries, bacterial or EB viral infections, chromosomal abnormalities, abnormal repair processes, severe surgeries, and inflammation spreading (8). According to the latest 2020 WHO definition, IMT is a distinctive and rare tumor characterized by a combination of spindle-shaped myofibroblast cells, fibroblast cells, plasma cells, eosinophils, lymphocytes, and other inflammatory cells, with minimal potential for metastasis (9).
Distinguishing UIMT from uterine leiomyoma based on morphology alone can be challenging due to the atypical clinical features commonly seen in UIMT. Recent research has shown that 0.3% of unselected leiomyomas were reclassified as IMTs based on positive anaplastic lymphoma kinase (ALK) immunohistochemistry (IHC) (4), even though routine ALK screening for the diagnosis of leiomyomas is not currently recommended. Our own study found that the median age of UIMT patients was 42 years, in line with previous reports by Karpathiou G. and Shukla PS (2, 10)., who observed a wide age range from 6 to 73 years, with a median age of 39 years. Abnormal uterine (51.85%) and the incidental discovery of uterine fibroids were the most common patient complaints. It is worth noting that many patients were asymptomatic, leading to a high rate of clinical underdiagnosis of UIMT. In other reports, IMT in other parts of the body, such as abdominal IMT, is more common in children and adolescents (11, 12). And the clinical manifestations of these IMTs are nonspecific and broad spectrum, ranging from asymptomatic to severe systemic symptoms (11, 12).
UIMTs are often mistaken for uterine leiomyomas, but they exhibit different texture and morphology, characterized by a softer, gelatinous consistency, and clear or irregular borders. They can be classified histologically into myxoid/vascular type, dense spindle cell type, or hypocellular fibrous type, with varying amounts of chronic inflammatory cells. They express ALK as well as smooth muscle markers (SMA, Desmin, Caldesmon), CD10, and others (6). ALK demonstrates high sensitivity and specificity in diagnosing IMT, with ALK gene rearrangement occurring at the 2p23 locus (13). Previous studies have reported ALK positivity rates of 87.5-100% (4), and in our study, the ALK IHC positivity rate reached 96.3%. Various ALK fusion partners have been identified in the literature on IMT (CARS, TPM4, TPM3 EML4, RANBP2, IGFBP4, ATIC, CLTC, etc.), while ALK-negative cases may show ROS1 and PDFGRB alterations, and a small proportion has ETV6-NTRK3 fusion (14). TIMP3 and THBS1 genes are more commonly identified fusion partners in pregnancy-related IMTs (15). Therefore, if ALK is negative, further RNA sequencing is needed to assist in the diagnosis. Nevertheless, it is crucial to note that ALK positivity is a key factor in diagnosing IMT. It is worth mentioning that no ALK fusion genes have been detected in uterine leiomyomas thus far. The current essential diagnostic criteria for IMT include the presence of spindle cell arrangement, infiltration of lymphocytes and plasma cells, expression of SMA, and frequent expression of ALK or ROS1. Notably, IMTs associated with pregnancy exhibit morphological features such as varying amounts of myxoid stroma and lymphoplasmacytic infiltrates (7).
The primary treatment for women diagnosed with noninvasive UIMT who do not require fertility preservation is total hysterectomy, while those who require fertility preservation may consider lesion resection with close follow-up. This approach is in line with previous studies. Prior to 2014, all reported cases of uterine IMT in the literature had a benign clinical course without evidence of recurrence or metastasis after surgery (1). However, research on UIMT invasiveness has been limited to the past decade. However, in patients with other IMT sites that have been reported, they still have a low recurrence rate after conservative surgery and treatment (11, 12)?.
In our case series, the invasive UIMT had a diameter of 5 cm, infiltrative borders, positive lymphovascular space invasion (LVSI), and 40 mitoses in 10 high-power fields. Malignant IMTs have been reported to have a lesion size ≥10.5 cm, severe nuclear atypia, ≥18 mitoses in 10 high-power fields, and features of lymphovascular invasion (5, 6). Studies on the invasiveness of IMT have found that myxoid dominance transitions to dense/spindle dominance in cases of multiple recurrences (16). Bennett’s study found that the dense/spindle type was associated with recurrence (5), while other studies have found that myxoid dominance is associated with a higher risk (1). The latest classification by the World Health Organization includes characteristics such as tumor diameter >7 cm, moderate to severe cytological atypia, increased mitotic index, necrosis, and lymphovascular infiltration. However, there are also reports of cases of metastasis and recurrence without these features.
Treatment for recurrent or invasive IMT usually involves a combination of chemotherapy, targeted therapy, or surgery. Tumors with larger diameters, typical nuclear atypia, lymphovascular infiltration, high mitotic count, and tumor necrosis are associated with poor outcomes (4). Understanding the genetics of IMT can facilitate targeted therapies. Approximately 10% of uterine IMTs are ALK-negative, and FISH or RNA sequencing is helpful for diagnosis (15). Confirmed ALK positivity has both diagnostic and therapeutic implications. ALK inhibitors, such as crizotinib, have shown effectiveness in treating recurrent and/or refractory invasive diseases (4, 17). Pregnancy appears to have a certain correlation with IMT, and targeted therapy using tyrosine kinase inhibitors may represent a new treatment option (17, 18).
Recent studies have indicated that minimally invasive procedures such as laparoscopy and hysteroscopy are preferred for treating UIMT, while careful monitoring and continued follow-up are effective strategies for women seeking to preserve fertility (17). However, UIMTs are rare tumors, and the understanding mostly comes from case reports with short follow-up periods, which can lead to selection bias in retrospective analyses.
Conclusion
Uterine inflammatory myofibroblastic tumor (UIMT) is a rare tumor that can be challenging to diagnose and treat. Total hysterectomy is often performed for women who do not require fertility, while lesion resection and close follow-up may be considered for those who require fertility preservation. ALK protein expression and gene rearrangements are used for diagnostic confirmation. Tumors with larger size, nuclear atypia, lymphovascular invasion, high mitotic count, and necrosis are associated with poorer outcomes. Improved understanding of the genetics and invasiveness of IMT is necessary for developing effective treatment strategies.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by The Medical Research Ethics Committee of West China Second University Hospital, Sichuan University belongs to Sichuan University, China. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
LB: Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Writing – original draft, Writing – review & editing. LH: Conceptualization, Investigation, Project administration, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. AZ: Data curation, Formal analysis, Resources, Supervision, Writing – original draft, Writing – review & editing. YC: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Parra-Herran C QC, Howitt BE, Dal Cin P, Quade BJ, Nucci MR. Inflammatory myofibroblastic tumor of the uterus: clinical and pathologic review of 10 cases including a subset with aggressive clinical course. Am J Surg Pathol. (2015) 39:157–68. doi: 10.1097/PAS.0000000000000330
2. Karpathiou G, Devouassoux-Shisheboran M, Stolnicu S, Chauleur C, Péoc’h M. Uterine inflammatory myofibroblastic tumor. Pathol Res Pract. (2023) 242. doi: 10.1016/j.prp.2023.154335
3. Etlinger P, Kuthi L, Kovács T. Inflammatory myofibroblastic tumors in the uterus: childhood-case report and review of the literature. Front Pediatr. (2020) 8. doi: 10.3389/fped.2020.00036
4. Kuisma H JV, Pasanen A, Heikinheimo O, Karhu A, Välimäki N, Aaltonen L, et al. Histopathologic and molecular characterization of uterine leiomyoma–like inflammatory myofibroblastic tumor comparison to molecular subtypes of uterine leiomyoma. Am J Surg Pathol. (2022) 46(8):1126–36. doi: 10.1097/PAS.0000000000001904
5. Bennett JA, Nardi V, Rouzbahman M, Morales-Oyarvide V, Nielsen GP, Oliva E. Inflammatory myofibroblastic tumor of the uterus: a clinicopathological, immunohistochemical, and molecular analysis of 13 cases highlighting their broad morphologic spectrum. Modern Pathol. (2017) 30:1489–503. doi: 10.1038/modpathol.2017.69
6. Devins KM, Samore W, Nielsen GP, Deshpande V, Oliva E. Leiomyoma-like morphology in metastatic uterine inflammatory myofibroblastic tumors. Modern Pathol. (2023) 36. doi: 10.1016/j.modpat.2023.100143
7. Devereaux KA FM, Hartinger S, Jones C, Kunder CA, Longacre TA. Pregnancy-associated inflammatory myofibroblastic tumors of the uterus are clinically distinct and highly enriched for TIMP3-ALK and THBS1-ALK fusions. Am J Surg Pathol. (2020) 44:970–81. doi: 10.1097/PAS.0000000000001481
8. Gleason BC, Hornick JL. Inflammatory myofibroblastic tumors: where are we now? J Clin Pathol. (2008) 61(4):428–37. doi: 10.1136/jcp.2007.049387
9. Kallen ME, Hornick JL. The 2020 WHO classification: what’s new in soft tissue tumor pathology? Am J Surg Pathol. (2021) 45(1):e1–e23. doi: 10.1097/PAS.0000000000001552
10. Shukla PS, Mittal K. Inflammatory myofibroblastic tumor in female genital tract. Arch Pathol Lab Med. (2018) 143:122–9. doi: 10.5858/arpa.2017-0575-RA
11. Estêvão-Costa J, Correia-Pinto J, Rodrigues FC, Carvalho JL, Campos M, Dias JA, et al. Gastric inflammatory myofibroblastic proliferation in children. Pediatr Surg Int. (1998) 13:95–9. doi: 10.1007/s003830050257
12. Fragoso AC, Eloy C, Estevão-Costa J, Campos M, Farinha N, Lopes JM. Abdominal inflammatory myofibroblastic tumor a clinicopathologic study with reappraisal of biologic behavior. J Pediatr Surg. (2011) 46:2076–82. doi: 10.1016/j.jpedsurg.2011.07.009
13. Ng ZY, Khaing CT, Aggarwal I. Inflammatory myofibroblastic tumor: an under recognized differential of uterine mesenchymal tumors. Int J Gynecol Cancer. (2022) 32:212–3. doi: 10.1136/ijgc-2021-003247
14. Zhang L, Luan L, Shen L, Xue R, Huang J, Su J, et al. Uterine inflammatory myofibroblastic tumor harboring novel NUDCD3-ROS1 and NRP2-ALK fusions: clinicopathologic features of 4 cases and literature review. Virchows Archiv. (2023) 482:567–80. doi: 10.1007/s00428-022-03457-7
15. Sim A, Devouassoux-Shisheboran M, Benmoulay-Rigollot C, Picot T, Péoc’h M, Karpathiou G. Uterine inflammatory myofibroblastic tumor with THBS1-INSR fusion. Pathol Res Pract. (2023) 246. doi: 10.1016/j.prp.2023.154500
16. Collins K RP, Euscher ED, Llanos AR, Garcia A, Malpica A. Uterine inflammatory myofibroblastic neoplasms with aggressive behavior, including an epithelioid inflammatory myofibroblastic sarcoma: a clinicopathologic study of 9 cases. Am J Surg Pathol. (2022) 46:105–17. doi: 10.1097/PAS.0000000000001756
17. Mandato VD, Valli R, Mastrofilippo V, Bisagni A, Aguzzoli L, La Sala GB. Uterine inflammatory myofibroblastic tumor. Medicine. (2017) 96. doi: 10.1097/MD.0000000000008974
Keywords: inflammatory myofibroblastic tumor, anaplastic lymphoma kinase, uterine leiomyoma, diagnosis, uterus, retrospective analysis
Citation: Bai L, Han L, Zheng A and Chen Y (2024) Uterine inflammatory myofibroblastic tumor: a retrospective analysis. Front. Oncol. 14:1461092. doi: 10.3389/fonc.2024.1461092
Received: 07 July 2024; Accepted: 26 August 2024;
Published: 20 September 2024.
Edited by:
Dragos Eugen Georgescu, Carol Davila University of Medicine and Pharmacy, RomaniaReviewed by:
José Estevão-Costa, University of Porto, PortugalKenji Nakano, Cancer Institute Hospital of Japanese Foundation for Cancer Research, Japan
Copyright © 2024 Bai, Han, Zheng and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yali Chen, WWFsaWNoZW4xODJAMTYzLmNvbQ==
 Liping Bai
Liping Bai Ling Han
Ling Han Ai Zheng
Ai Zheng Yali Chen
Yali Chen