
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Oncol. , 04 September 2024
Sec. Hematologic Malignancies
Volume 14 - 2024 | https://doi.org/10.3389/fonc.2024.1460557
We report a case of acute myeloid leukemia (AML) with retinoic acid receptor gamma (RARG) rearrangement, exhibiting clinical, morphological, and immunophenotypic features similar to classic acute promyelocytic leukemia (APL). RNA sequencing analysis of the patient’s bone marrow samples revealed the presence of nucleoporin 98 (NUP98)-RARG caused by translocation. AML with RARG rearrangement is insensitive to all-trans retinoic acid (ATRA) and arsenic trioxide. The patient received azacitidine therapy after failing ATRA and standard 3 + 7 therapy (idarubicin and cytarabine) and achieved complete remission. Conclusively, this acute myeloid leukemia subtype may benefit from azacitidine.
AML is a highly heterogeneous clonal disease of malignant hematopoietic stem cells, and APL is a distinct AML subtype. Patients with APL carrying the promyelocytic leukemia-retinoic acid receptor alpha (PML::RARA) fusion gene caused by the chromosomal translocation t(15;17)(q22;q21) are sensitive to ATRA and ATO treatments (1).
AML with nucleoporin 98- retinoic acid receptor gamma (NUP98-RARG) is identical to APL in terms of morphology, immunophenotype, and clinical manifestations but lacks the t(15;17)(q22;q21)/PML-RARA fusion (2). RARG, a member of the nuclear receptor superfamily, shares high homology with the retinoic acid receptors RARA and RARB, which are involved in retinoid signaling (3). An increasing number of patients with NUP98::RARG, PML::RARG, CPSF6::RARG, NPM1::RARG, and HNRNPc::RARG fusions are being reported. Nearly all AML cases demonstrate resistance to ATRA, and some patients show sensitivity to an AML chemotherapy regimen. Although chemotherapy may be effective as an alternative therapy in some patients, the prognosis of NUP98-RARG AML remains inferior to that of typical APL (4). This is the first case in which azacitidine therapy after the failure of idarubicin and cytarabine (IA) and ATRA regimens resulted in favorable outcomes.
In May 2023, a 32-year-old man presented to a hospital in Jinan, Shandong Province, China with fever and cough. Laboratory blood tests showed hemoglobin level, 96 g/L (reference range, 130–175 g/L); platelets, 21 × 109/L (reference range, 125–350 × 109/L); white blood cell count, 72.06 × 109/L (reference range, 3.5–9.5 × 109/L); and D-dimer concentration, 76.34 mg/L (reference range, 20.00–40.00 mg/L). The bone marrow (BM) aspirate morphology showed hypercellular BM with 97% abnormal promyelocytic granulocytes. Auer bodies were observed in some cells. The reverse transcription-polymerase chain reaction (RT–PCR) and fluorescence in situ hybridization (FISH) failed to detect the PML::RARA transcript in the BM. The karyotype analysis revealed 46, XY, t(11;12) (p15;q14) (10)/45, idem, -Y (7)/46, idem, -Y, +?8 (3). Hence, the patient was diagnosed with AML and was administered a treatment regimen of 20 mg all-trans retinoic acid (ATRA) twice a day in combination with IA induction (idarubicin 10 mg/m2 on days 1–3, 100 mg/m2 cytarabine on days 1–7).
Three weeks later, the patient was transferred to our hospital with myelosuppression and persistent fever. At the time of admission, blood tests showed hemoglobin, 59 g/L (reference range, 130–175 g/L); platelets, 26 × 109/L (reference range, 125–350 × 109/L); white blood cell count, 3.41 × 109/L (reference range, 3.5–9.5 × 109/L); prothrombin time, 15.9 s (reference range, 11–14.5 s); activated partial thromboplastin, 43.5 s (reference range, 28–45 s), fibrin degradation products (FDP) >150.00 ug/mL (reference range, < 5.00 ug/mL); fibrinogen, 3.56 g/L (reference range, 2.00–4.00 g/L); and D-dimer concentration > 20.0 ug/mL (reference range, < 5.00 ug/mL) (Figure 1). BM cytomorphology still supported APL (Figure 2B). Karyotype analysis of the chromosomes was 46, XY, der (11) t (11;12) (p15;q13), -12, +mar [15] (Figure 2A). The leukemia cellular immunophenotype was positive for CD117, CD13, CD33, MPO, and CD45dim and negative for CD34, CD38, HLA-DR, CD11b, CD14, and other T- or B-lymphoid lineage markers (Figure 2D). Positive NUP98/RARG fusion gene (Figure 3) and mutations were detected in several genes at various frequencies including WT1 (variant allele fraction, VAF, 39%), TET2 (VAF, 5.1%), ARID1A (VAF, 45.9%), and KDM6A (VAF, 23.5%). The patient highest temperature on admission was 39.2°C, and the COVID-19 nucleic acid test was positive. The patient was administered paxlovid as antiviral treatment and other anti-infection treatments. One week later, the COVID-19 nucleic acid test result was negative and the patient still had an intermittent fever. Blood metagenomic next-generation sequencing (mNGS) and blood cultures were performed. Blood cultures were negative, and mNGS identified Escherichia coli. The patient developed abdominal pain, and an abdominal computed tomography (CT) scan showed acute pancreatitis. His pancreatic enzyme levels were elevated, with amylase and lipase levels 806 IU/L (reference range, 30–180 IU/L) and 2310 IU/L (reference range, 23–300 IU/L), respectively. The patient was treated to inhibit pancreatic enzymes. Given his recent infection and poor performance status, the patient was unable to tolerate intensive chemotherapy. On June 13, the patient was treated with 75 mg/m2 azacitidine. However, he developed sudden dizziness, fever (38.0 °C), and blood pressure drop (73/45 mmHg) after two days. He was empirically placed on imipenem/cilastatin and tigecycline, administered fluid resuscitation, and started noradrenaline. When the patient’s vital signs were stable and the infection was controlled, 135 mg of azacitidine was continued for five days.
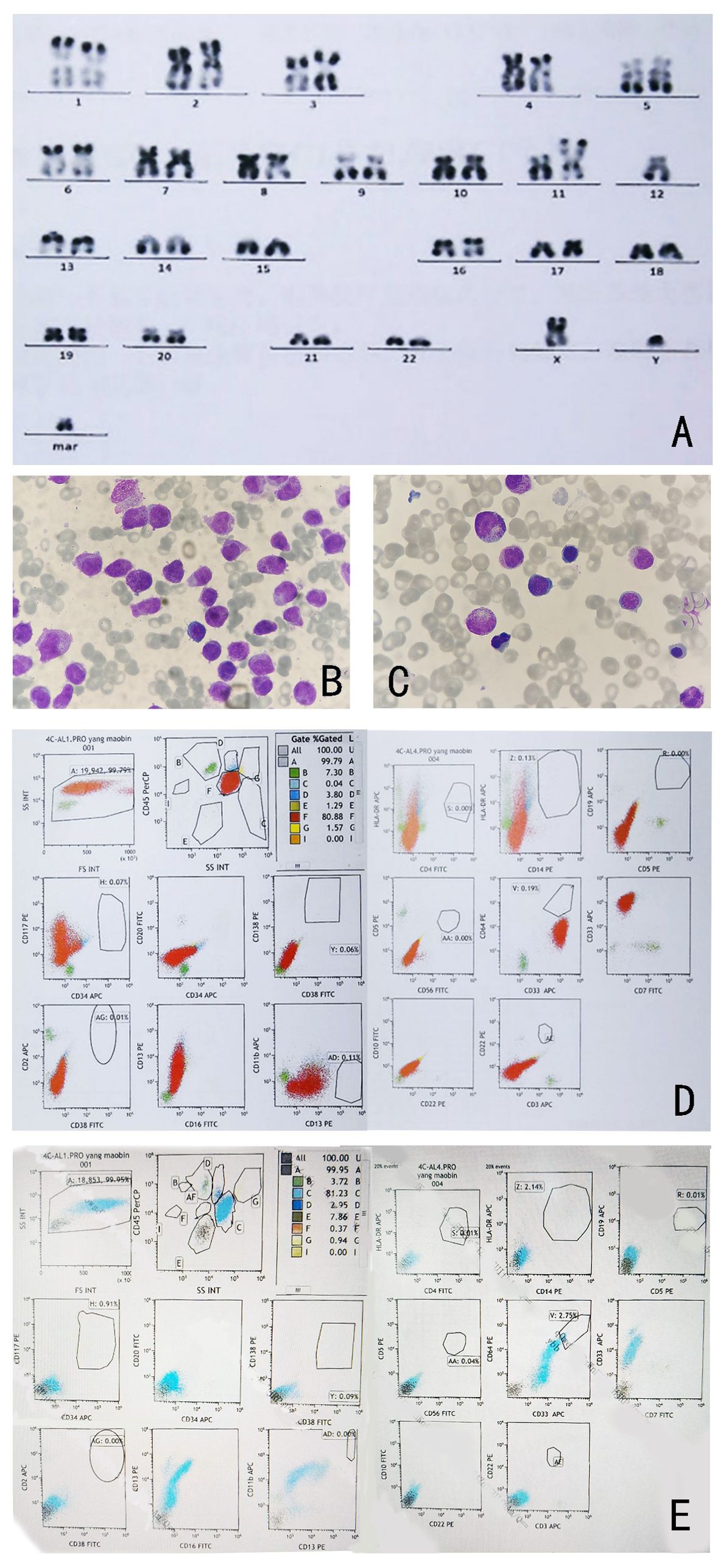
Figure 2. (A) Karyotype analysis shows 46, XY, t (11; 12) (p15; q13). (B) Morphology of bone marrow cells after IA and ATRA regimen. (C) Bone marrow morphology at complete remission after one cycle of azacitidine treatment (Wright-Giemsa-stained bone marrow smear, ×1000). (D) Immunophenotypic analysis results of bone marrow cells at diagnosis. (E): Immunophenotypic analysis results of bone marrow cells at complete remission.
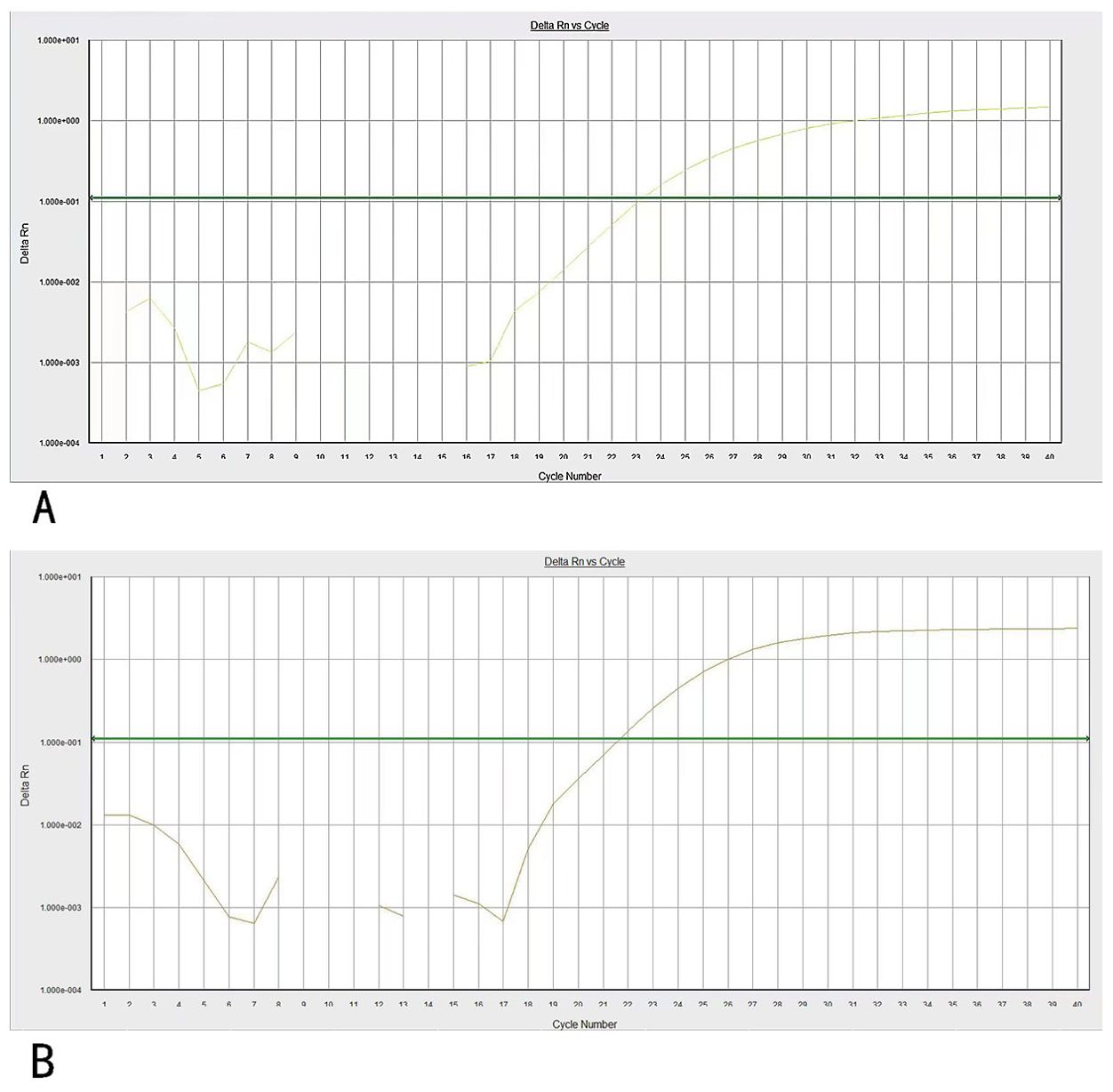
Figure 3. (A) PCR amplification curves of NUP98/RARG. (B) PCR amplification curves of ABL genes. Specific primers and probes were used for NUP98/RARG and ABL gene amplification.
Subsequently, the patient underwent craniocerebral + paranasal sinus CT for headache, which showed abnormal high-density foci in the brain and high density in the left nasal cavity with possible secretion (Figure 4). A consultation with the otolaryngology department suggested a diagnosis of rhinocerebral mucormycosis. The patient’s symptoms were relieved after antifungal administration and other treatments (the patient underwent sinonasal debridement at another hospital three months later; the postoperative pathomorphology was considered mucormycosis).
In July, BM aspirate morphology confirmed complete remission (CR) with negative minimal residual disease through flow cytometry (FCM) (Figures 2C, E). The positivity rate for NUP98 gene rearrangements dropped to 10%. Seven-day therapy with 75 mg/m2 azacitidine was continued. One month later, NUP98 rearrangement was negative. A seven-day treatment with 75 mg/m2 azacitidine was continued.
In January 2024, after four cycles of azacytidine treatment, the patient underwent allogeneic hematopoietic stem cell transplantation. Four months after transplantation, the patient remained in CR and was negative for the NUP98-RARG gene fusion (Table 1).
Such et al. first reported NUP98-RARG AML with APL morphology and features of the t(11;12)(p15;q13) translocation (2). When the patient relapsed and underwent induction therapy again, ATRA was administered in addition to chemotherapy. Although the patient achieved CR, the patient’s primary parental cells showed that AML with the NUP98-RARG rearrangement was insensitive to ATRA in vitro (5). In a global study of RARG, the 16 patients who received ATRA + ATO induction therapy (≥ 14 days) showed resistance to the drug combination and subsequently received AML induction therapy (6). However, another in vivo experiment conducted in a mouse model revealed that cells transformed with NUP98/RARG fusion were extremely sensitive to ATRA treatment (7), but the results of the murine model do not necessarily reproduce in humans. In the present case, the sensitivity of the patient could not be definitively assessed because he did not receive ATRA + ATO alone. It is unclear why the RARG rearrangements resemble the APL phenotype. A patient with a positive EZH2-D185H but negative PML-RARA fusion gene exhibited an APL phenotype by down regulating RARA and RARG expression; dysregulation of the RARA and RARG genes may be responsible for AML with an APL-like phenotype (8). Owing to NUP98-RARG AML resistance, when the patient does not show PML/RARA rearrangement, combined chemotherapy should be administered during induction therapy rather than ATRA + ATO.
The apparent paradox of ATRA producing resistance in vivo and responsiveness in vitro is due to several factors, including relatively short in vitro culture assays, different genetic backgrounds, and the acquisition of additional mutations. Most patients with RARG rearrangements have additional mutations in WT1, TP53, TET2, and KRAS. The acquisition of additional mutations, such as the WT1 mutation, may render patients with recombinant RARG resistant to ATRA. Approximately 10% of patients with AML have WT1 mutations, whereas WT1 mutations are present in over 50% of RARG-rearranged patients (6, 9). WT1 mutations are most closely associated with induction failure (10). NUP98-RARG gene rearrangements and multiple epigenetic-related gene mutations, such as TET2, ARID1A, and KDM6A, were detected in this patient. This may explain the effectiveness of the demethylated drugs. Azacitidine, a hypomethylating agent, incorporates into DNA and RNA, leading to hypomethylation and direct cytotoxicity to abnormal hematopoietic cells in the bone marrow. This mechanism may explain its effectiveness in treating AML with NUP98-RARG rearrangement, particularly regarding the presence of multiple epigenetic-related gene mutations in this specific patient. These mutations may increase the vulnerability of leukemic cells to demethylating agents, thereby enhancing treatment efficacy.
In one case, azacitidine was used for maintenance therapy after transplantation, and a patient with AML and NUP98-RARG gene fusion presenting as APL was treated with induction and consolidation chemotherapy using venetoclax in combination with chemotherapy, and then achieved CR followed by haploidentical hematopoietic stem cell transplantation. Post-transplantation maintenance therapy with venetoclax and azacitidine remained in CR after two cycles (11). The application of demethylation drugs for NUP98-RARG gene fusion in AML requires further investigation.
ATRA is a potent and highly specific inducer of CD38 expression in human promyelocytic leukemia cells. ATRA-induced CD38 expression in myeloid cells is mediated through RARA (12). In flow cytometry, CD33+, CD13+, CD38+, CD64+, CD34-, and HLA-DR- constitute the typical APL immunophenotype (13). In a global study on AML with RARG rearrangement, all patients were CD38- and the present patient was also CD38- (6). Most cases of this novel AML have morphological features similar to those of APL, making a timely diagnosis difficult. Therefore, some patients may receive ineffective ATRA therapy for long periods. Consequently, chemotherapy should be considered as soon as possible in CD38- and RARA-negative cases.
This is the first report of a patient with RARG rearrangement who achieved positive outcomes following demethylation therapy after the failure of ATRA and AML induction chemotherapy (AML-like regimens). Our case suggests that azacitidine may be a viable therapeutic option for patients with AML and NUP98-RARG fusion.
Due to ethical and privacy considerations, this case report involves only a single patient, and as such, detailed data cannot be shared. All data are kept confidential in accordance with ethical guidelines and privacy regulations.
The studies involving humans were approved by Shandong University Qilu Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was obtained from the patient. Written informed consent was obtained from the participant/patient(s) for the publication of this case report.
ZW: Data curation, Writing – original draft. LS: Writing – review & editing. SX: Writing – review & editing. XZ: Writing – review & editing. LW: Writing – review & editing. PQ: Writing – review & editing. QS: Writing – review & editing. MH: Writing – review & editing. YS: Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors thank Professor HongHu Zhu’s team at the Department of Hematology, Peking University People’s Hospital, for providing PCR analysis. We also acknowledge the support from the medical staff at Shandong University Qilu Hospital who assisted in the patient’s care.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Lo-Coco F, Avvisati G, Vignetti M, Mandelli F, Petti MC, De Padua C, et al. Retinoic acid and arsenic trioxide for acute promyelocytic leukemia. N Engl J Med. (2013) 369:111–21. doi: 10.1056/NEJMoa1300874
2. Such E, Cervera J, Valencia A, Pedro C, Martínez A, Díaz C, et al. A novel NUP98/RARG gene fusion in acute myeloid leukemia resembling acute promyelocytic leukemia. Blood. (2011) 117:242–5. doi: 10.1182/blood-2010-06-291658
3. Chambon P. A decade of molecular biology of retinoic acid receptors. FASEB J. (1996) 10:940–54. doi: 10.1096/fasebj.10.9.8801176
4. Zhang X, Sun J, Yu W, Jin J. Current views on the genetic landscape and management of variant acute promyelocytic leukemia. biomark Res. (2021) 9:33. doi: 10.1186/s40364-021-00284-x
5. Such E, Cordón L, Sempere A, Hernández J, Juncà J, González M, et al. In vitro all-trans retinoic acid sensitivity of acute myeloid leukemia blasts with NUP98/RARG fusion gene. Ann Hematol. (2014) 93:1931–3. doi: 10.1007/s00277-014-2073-5
6. Zhu HH, Qin YZ, Zhang ZL, Li X, Li Y, Huang J, et al. A global study for acute myeloid leukemia with RARG rearrangement. Blood Adv. (2023) 7:2972–82. doi: 10.1182/bloodadvances.2022008364
7. Qiu JJ, Zeisig BB, Li S, Ferrando A, Li H, Popplewell L, et al. Critical role of retinoid/rexinoid signaling in mediating transformation and therapeutic response of NUP98-RARG leukemia. Leukemia. (2015) 29:1153–62. doi: 10.1038/leu.2014.334
8. Coccaro N, Zagaria A, Orsini P, Cazzola M, Cuneo A, Montano N, et al. RARA and RARG gene downregulation associated with EZH2 mutation in acute promyelocytic-like morphology leukemia. Hum Pathol. (2018) 80:82–6. doi: 10.1016/j.humpath.2018.02.023
9. Madan V, Shyamsunder P, Han L, Yao J, Pabst T, Patel M, et al. Comprehensive mutational analysis of primary and relapse acute promyelocytic leukemia. Leukemia. (2016) 30:2430. doi: 10.1038/leu.2016.237
10. Niktoreh N, Weber L, Walter C, Thomas M, Wong T, Zhao M, et al. Understanding WT1 alterations and expression profiles in hematological Malignancies. Cancers (Basel). (2023) 15(13):3491. doi: 10.3390/cancers15133491
11. Yuan J, Pei R, Lu Y. Successful haploidentical hematopoietic stem cell transplantation with azacitidine and venetoclax maintenance therapy for acute myeloid leukemia with NUP98-RARG gene fusion. Turk J Hematol. (2023) 40:75–6. doi: 10.4274/tjh
12. Buteyn NJ, Fatehchand K, Santhanam R, et al. Anti-leukemic effects of all-trans retinoic acid in combination with Daratumumab in acute myeloid leukemia. Int Immunol. (2018) 30:375–83. doi: 10.1093/intimm/dxy040
Keywords: acute myeloid leukemia, acute promyelocytic leukemia, all-trans retinoic acid, nucleoporin 98-retinoic acid receptor gamma, azacitidine
Citation: Wei Z, Shao L, Xu S, Zhang X, Wang L, Qin P, Song Q, Hou M and Shi Y (2024) Case report: Successful therapy with azacitidine for acute myeloid leukemia with NUP98::RARG resembling acute promyelocytic leukemia. Front. Oncol. 14:1460557. doi: 10.3389/fonc.2024.1460557
Received: 06 July 2024; Accepted: 14 August 2024;
Published: 04 September 2024.
Edited by:
Nelida Ines Noguera, University of Rome Tor Vergata, ItalyReviewed by:
Nirmalya Saha, University of Michigan, United StatesCopyright © 2024 Wei, Shao, Xu, Zhang, Wang, Qin, Song, Hou and Shi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yan Shi, c2hpeWFuc2pqQDE2My5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.