- Department of Radiation Oncology, the Fourth Hospital of Hebei Medical University, Shijiazhuang, Hebei, China
Purpose: This study aims to optimize neoadjuvant radiotherapy target area for locally advanced rectal cancer (LARC) patients undergoing total neoadjuvant therapy (TNT) by examining local recurrence patterns.
Methods and materials: We retrospectively analyzed the clinical data of rectal cancer patients who undergone local recurrence after surgery. Recurrence sites were categorized and compared with initial diagnosis imaging, focusing on visible and suspicious lesions.
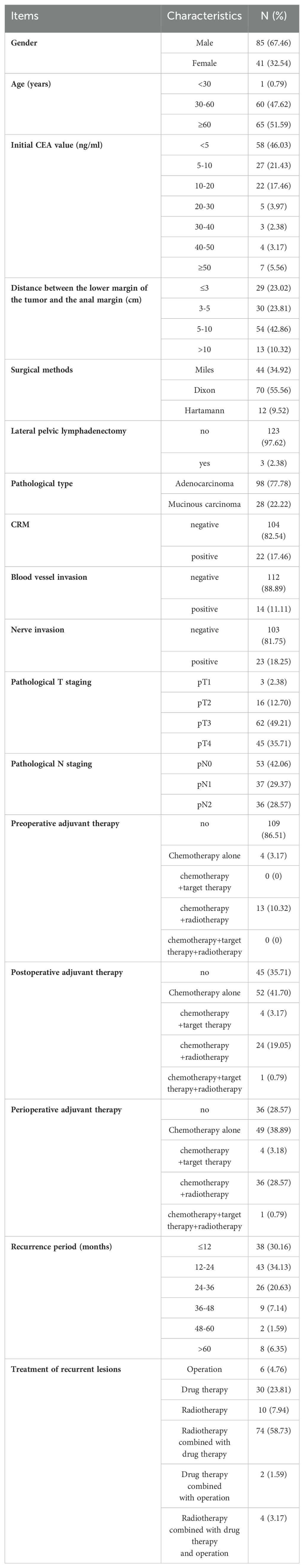
Results: Of the 126 patients who met our criteria, 186 lesions were analyzed. Within these, 75.40% of cases (95/126) and 83.33% of lesions (155/186) were located within the pelvic cavity. Conversely, 3.97% of cases (5/126) and 3.33% of lesions (6/186) occurred outside the pelvic cavity. Additionally, 20.63% of cases (26/126) and 13.44% of lesions (25/186) were found in both regions. Recurrences were predominantly observed in mesenteric regions (MR) (40.86%, 76/186) and presacral regions (PR) (32.26%, 60/186). In addition, 86.51% of patients (109/126) had recurrent lesions in HRA and the suspected lesions areas. Further analysis showed that initial CEA levels and adjuvant therapy types were identified as independent predictors for recurrence in MR/PR and initially suspected lesions. 86.51% of patients had recurrent lesions in HRA and the suspected lesions areas
Conclusion: The MR, PR, and areas of initial suspicious lesions are high-risk zones for post-surgical recurrence of LARC. Exploratory study of involved-field irradiation (IFI) can be carried out in the context of TNT in LARC.
Introduction
The approach to neoadjuvant therapy for locally advanced rectal cancer (LARC) has entered the era of total neoadjuvant treatment (TNT) after went through the stages of single-chemotherapy, conventional fractionated radiotherapy alone, short-course radiotherapy (SCRT) and long-course chemoradiotherapy (LCRT). From the preferred recommendation of TNT in the National Comprehensive Cancer Network (NCCN) guideline for LARC patients, we can find that the chemotherapy regimen of CAPEOX or FOLFOX, traditionally administered postoperatively, has been arranged before the total mesorectal excision (TME), while LCRT or SCRT regimen unchanged. This shift underscores an enhanced emphasis on chemotherapy in the neoadjuvant treatment for LARC.
Radiation therapy (RT) has been a fundamental component of neoadjuvant treatment for LARC, contributing significantly to the improvement of anal preservation rate and the reduction of local recurrence rate. The delineation of neoadjuvant radiotherapy targets, historically based on Roels et al.’s 2006 analysis of postoperative recurrence sites in rectal cancer patients, did not distinguish between pre- and postoperative radiotherapy settings (1). In 2012, Valentin and his colleagues put forth guidelines for preoperative radiotherapy targeting based in varying T and N stages, later solidified in expert consensus whether the irradiation extents for lateral lymph nodes and inclusion of the ischiorectal fossa should be defined as a target (2, 3). Up to now, in clinical practice, the radiotherapy target setting of both LCRT and SCRT adheres to this consensus.
In the context of TNT mode, neoadjuvant therapy for rectal cancer has greatly increased the weight of chemotherapy. This raises pertinent questions: Will the elective nodal irradiation (ENI) of pelvic field combined with involved-field irradiation (IFI) of increasing dose in high-risk areas lead to an over-treatment? Could the successful IFI strategies used in lung and esophageal cancers be replicated in radiotherapy for LARC? Although there are no definitive answers to these questions, there is no doubt that investigating these concerns holds substantial clinical significance.
In this study, we evaluated 126 rectal cancer patients who experienced local recurrence post-surgery and analyzed the recurrence site and patterns, aiming to inform the optimal preoperative radiotherapy target setting for LARC patients under TNT mode.
Materials and methods
Patients
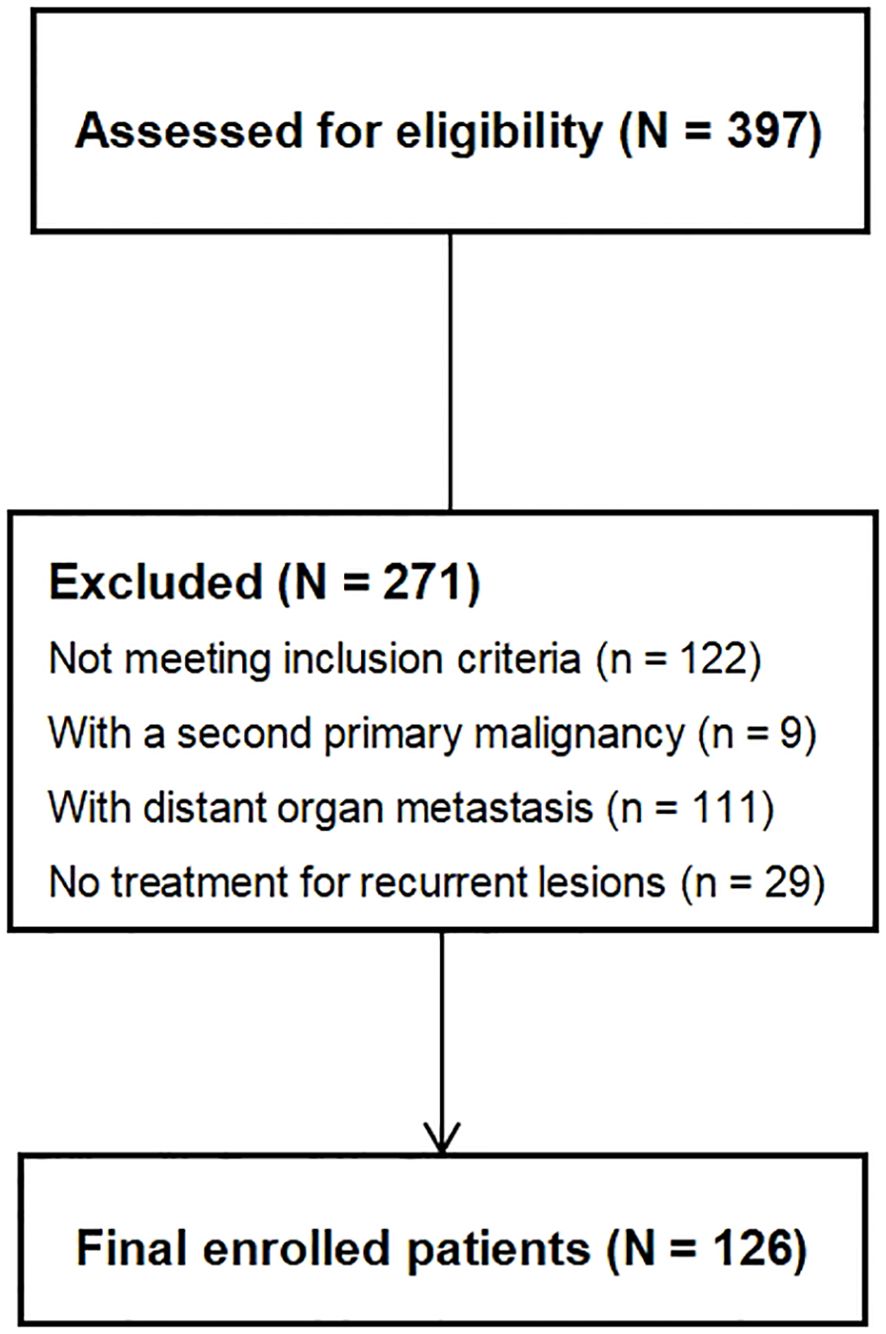
From January 2009 to July 2023, rectal cancer patients who underwent radical surgery in our hospital and were diagnosed with local recurrence during follow-up were included in this study. The main inclusion criteria were as follows: (1) Diagnosis of rectal cancer, irrespective of gender and age; (2) Complete diagnostic and treatment history in our hospital; (3) Underwent at least one abdominopelvic enhanced CT or MRI at the initial visit; (4) Underwent at least one abdominopelvic enhanced CT or MRI+DWI at the time of local recurrence diagnosis; (5) Pathological confirmation for patient who underwent surgery for local recurrent lesions; (6) Significant reduction in lesion volume and/or symptom relief after radiotherapy and/or chemotherapy in patient who did not undergo surgery. The exclusion criteria included: (1) Patients with a second primary malignancy at initial diagnosis or during follow-up; (2) Patients with both local recurrent lesions and distant organ metastasis; (3) Patients who refuse treatment for recurrent lesions; (4) Patients who do not meet the above inclusion criteria. All participants were identified in an institutional tumor registry through a protocol approved by the institutional review board with waiver of informed consent.
Assessment of local recurrence
Local recurrence was identified by imaging as invasive or asymmetric masses not attributable to postoperative structural changes, or masses visible initially but not meeting diagnostic criteria, which then exhibited growth during follow-up. Lesions confirmed by pathological biopsy after resection or shrunk after RT and/or drug therapy were clinically diagnosed as local recurrence. The above non-pathological assessments were independently performed by two senior radiologists.
Definition of local recurrence site
In this study, recurrence sites were classified into internal pelvic cavity (IPC) and external pelvic cavity (EPC). IPC encompassed the mesenteric regions (MR) (incl. anastomosis and rectal stump), presacral regions (PR) (Defined as: lesions located in front of the sacrum and the distance between the posterior margin of lesion and the anterior margin of the sacrum was within 1 cm), and the lateral lymphatic drainage region (LLDR) (incl. obturator, internal iliac, and external iliac). EPC included the perianal, the inguinal area (IA), and the paravascular area (PA) between the inferior mesenteric artery and the common iliac artery.
Relationship between recurrences and initial lesions
Here, we compared and analyzed the locations of recurrent and initial lesions (incl. confirmed and/or suspicious) to identify patterns between them. A multivariate analysis was conducted to determine factors influencing site consistency.
Statistical analysis
Statistical analysis was performed using SPSS software 25.0 (SPSS, Inc., Chicago, IL, USA) and MedCalc Version 32.0. Chi-square test was used to evaluate the distribution of recurrence sites. A forward stepwise logistic regression was used to analyze factors associated with recurrence site consistency with initial lesions. The Hosmer and Lemeshow test was used to assess the logistic regression model’s goodness of fit, and receiver operating characteristic (ROC) curve analysis was used to evaluate the model’s predictive performance. In this study, P<0.05 was considered statistically significant.
Results
Characteristics of enrolled patients
According to the inclusion and exclusion criteria, a total of 126 patients were selected for this study, as shown in Figure 1. Among them, 10 cases were confirmed by postoperative pathology, while 116 cases were validated based on the efficacy of nonoperative treatment. The baseline characteristics of patients are shown in Table 1.
Local recurrence sites
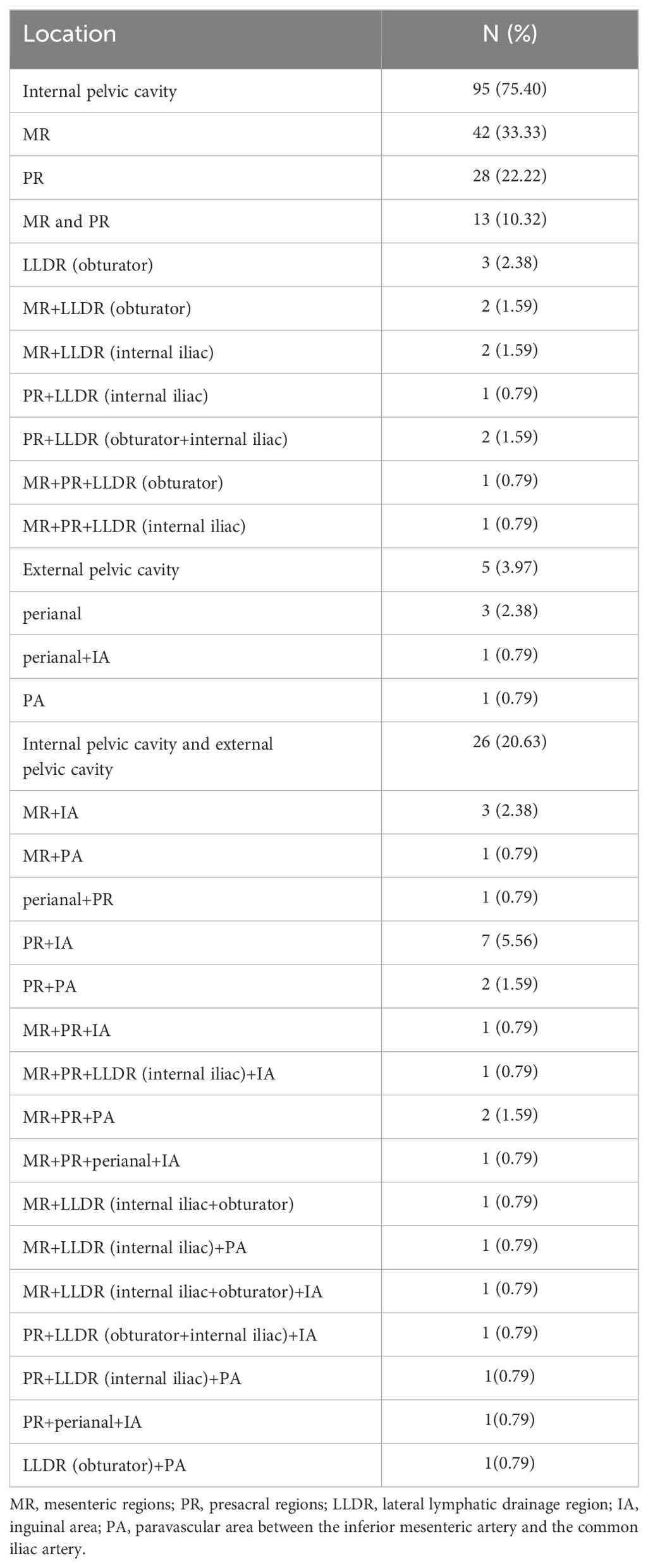
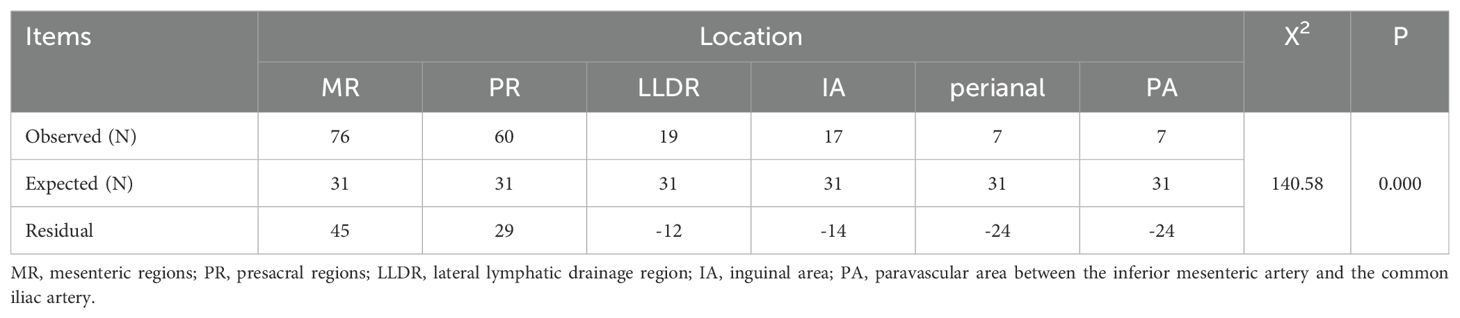
Among the 126 patients, 95 cases (75.40%) underwent IPC recurrence, with the breakdown as follows: MR (42, 33.33%), PR (28, 22.22%), MR+PR (13, 10.32%), LLDR (3, 2.38%), and combined LLDR with MR and/or PR (9, 7.14%). There were 5 cases (3.97%) of EPC recurred, including perianal (3, 2.38%), perianal and IA (1, 0.79%), PA (1, 0.79%). Additionally, 26 cases (20.63%) had both IPC and EPC recurrences, detailed in Table 2. In this study, all PR lesions were below the anterior inferior margin of the 2nd sacrum, and no external iliac lesions were observed in patients with LLDR recurrence. Of the 186 recurrent lesions in total, MR and PR were the most common recurrence sites, accounting for 40.86% (76/186) and 32.26% (60/186), respectively. In addition, the recurrence rates for LLDR, IA, perianal, and PA were 10.22%, 9.14%, 3.76%, and 3.76%, respectively, as shown in Table 3. Distribution of recurrence lesions was obviously unbalanced, which embodied that the occurrence frequency of lesions in MP and PR was much higher than that in other areas. Due to their anatomical proximity, MR and PR were collectively defined as high-frequency recurrence area (HRA) for this study.
Pattern analysis of local recurrence sites
Comparing the initial and recurrence imaging data, we found that in addition to 83 patients with the recurrent lesions located in HRA, 26 cases’ lesions located in areas suspected in initial imaging (Incl. 9 cases in LLDR, 8 in IA, 4 in PA, 2 in LLDR+IA, 1 in LLDR+PA, and 2 in IA+PA), as shown in Figure 2 and Supplementary Table 1. Beyond the 109 patients (86.51%) mentioned above, 17 (13.49%) patients had with new lesion locations not identified in initial imaging, with the breakdown as follows: 3 cases with simple perianal recurrence, 1 case with perianal+IA, 2 cases with MR+PR+perianal+IA, 2 cases with simple obturator, 1 case with MR+PR+obturator, 2 cases with MR+PR+obturator+internal iliac, 1 case with MR+PR+internal iliac, 1 case with simple IA, 1 case with IA+internal iliac, 1 case with simple PA, and 2 cases with PA+internal iliac recurrence.
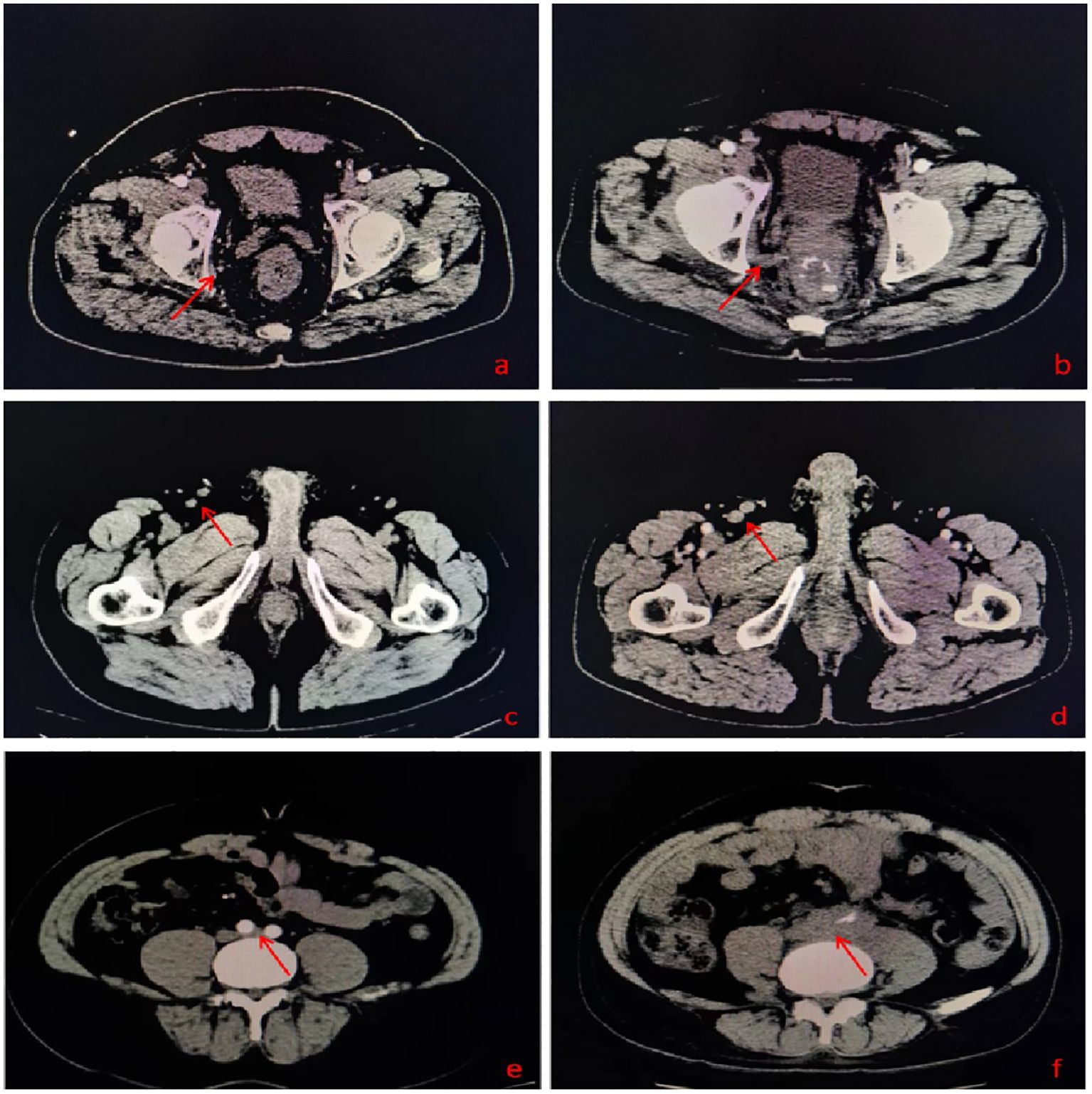
Figure 2. (A) A suspected lesion in the obturator lymphatic drainage region in initial imaging; (B) Recurrent lesion in the obturator lymphatic drainage region; (C) A suspected lesion in the inguinal lymphatic drainage region in initial imaging; (D) Recurrent lesion in the inguinal lymphatic drainage region; (E) A suspected lesion in the para-aortic lymphatic drainage region in initial imaging; (F) Recurrent lesion in the para-aortic lymphatic drainage region.
Among the 186 locally recurrent lesions, 136 lesions were located in HRA, and 31 lesions located in areas suspected in initial imaging (Incl. 12 in LLDR, 12 in IA, 7 in PA), as shown in Supplementary Table 2. In addition to the 167 (90.76%) lesions mentioned above, 19 (10.22%) new lesions were not identified in the initial imaging, including 7 in LLDR, 5 in IA, and 7 lesions in perianal region.
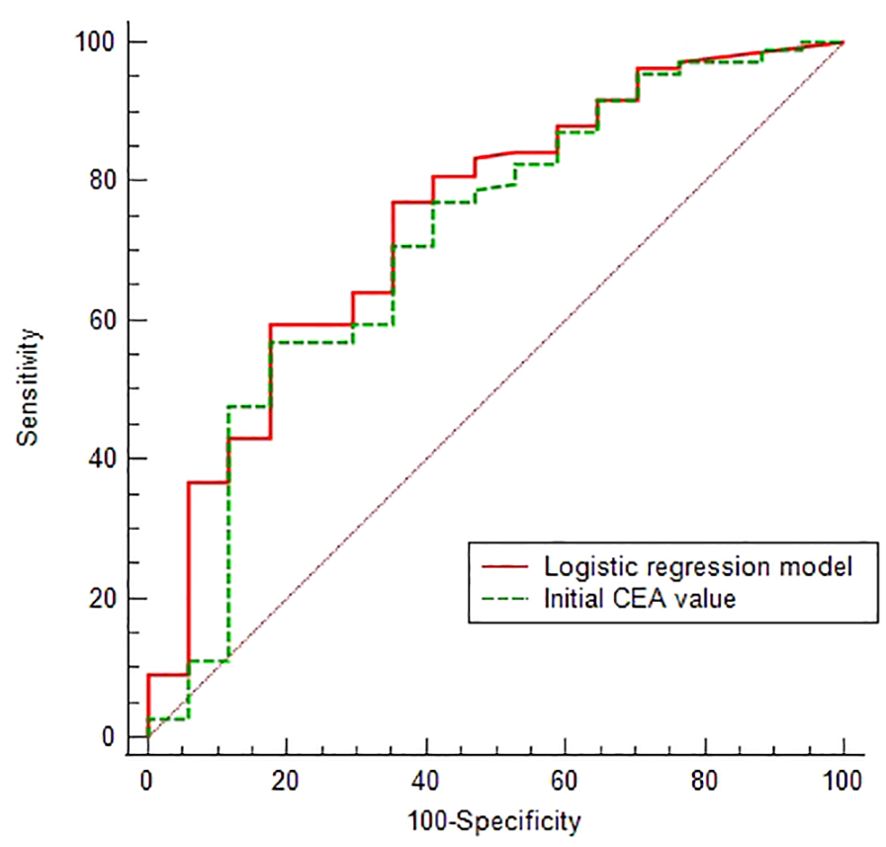
Given that 86.51% of patients had recurrent lesions in HRA and the suspected lesions areas, we conducted a Logistic analysis of the relationship between 16 clinical factors and this condition (the variable assignment were detailed in Supplementary Table 3). In order to reduce the distortion of model evaluation, we conducted collinearity statistics for all factors before performing the logistic analysis. A variance inflation factor (VIF) of predictors ≥10, including the postoperative and perioperative treatment methods, was thought to be highly correlated with at least one of the other predictors in the aforementioned model. When excluding the factor of postoperative treatment methods from the model, we observed that the VIF of all factors was <2. Then the logistic analysis identified initial CEA values and perioperative treatment methods as a negative and a positive predictor, respectively (Table 4), and the Logistic regression model was . The predictive performances of the CEA and Logistic regression model were obtained by ROC analysis, yielding the Area Under Curve (AUC) of 0.713 (95% CI 0.626-0.790) and 0.747 (95% CI 0.661-0.820), respectively. On the basis of the optimal cut-off values of 6.54 ng/ml and 0.534, the sensitivity, specificity, positive predictive value and negative predictive values were 59.63% and 56.88%, 82.35% and 82.35%, 95.59% and 95.38%, 24.13% and 22.95%, respectively (Figure 3).
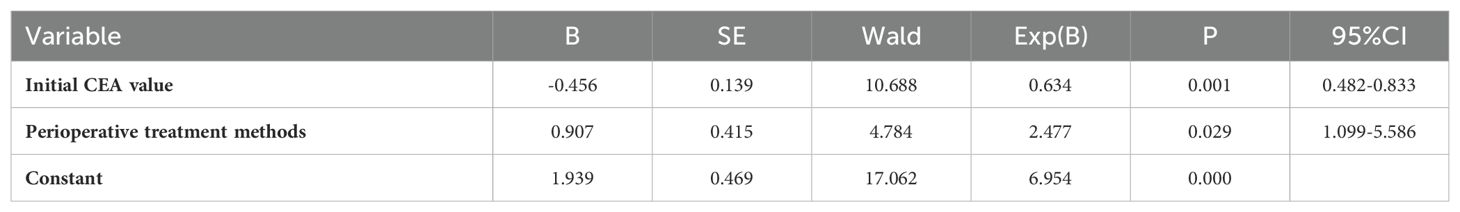
Table 4. Multivariate analysis of consistency between the location of recurrent lesions and the initial lesions.
Discussion
With the advancement of radiotherapy technology and the renewal of treatment concept, the radiotherapy of malignant tumors has entered an era of precision. On the premise of ensuring the curative effect of patients, reasonable minimize of irradiation field is of great value in reducing radiotherapy-related damage and ensuring smooth treatment execution. This approach is evident in the shift from ENI to IFI in treating esophageal and lung cancer (4–9). As a local treatment modality, RT plays an vital role in preventing local recurrence of tumors. Consequently, it is crucial to obtain the pattern and regularity of local recurrence sites for for setting accurate radiotherapy targets.
For the recurrence site of rectal cancer, Roels et al., in their comprehensive analysis of 17 studies, reported higher local recurrence rates in the mesorectal and presacral site, at 87% and 49%, respectively (1). Our study aligns with these findings: 95 of the 126 patients had recurrent lesions located in the pelvic cavity, of which 87.37% (83/95) occurred in MR and/or PR, and 73.12% of the 186 recurrent lesions occurred in these two areas. These results underscore the MR and PR as high-risk recurrence areas in rectal cancer, warranting their inclusion in neoadjuvant radiotherapy target areas.
In clinical practice, lymph node properties of rectal cancer are generally evaluated from three aspects: whether its short diameter is greater than 0.5cm, whether the margin is regular and whether the signal is uniform. Although the introduction of the new PET functional probe of Ga68 PSMA-11 and the application of magnetic resonance lymphography have significantly improved the diagnostic accuracy, widespread application of these new technologies still requires a time and experience (10, 11). Therefore, the study of determining the properties of lymph nodes by imaging technology is a research focus. In this study, 10.22%, 9.14%, and 3.76% of 186 recurrent lesions were found in the LLDR, IA and PA, respectively. Among them, 63.16% (12/19), 70.59% (12/17) and 100% (7/7) of the recurrent lesions showed suspicious lesions at the corresponding locations on the initial imaging, while new recurrent lesions in these areas only accounted for 6.45% (12/186). Therefore, we believe that strengthening the intensity of imaging examination in initial patients and incorporating suspicious lesions into the preoperative radiotherapy target could potentially reduce the local recurrence in rectal cancer patients.
As an important tumor biomarker, CEA has been widely recognized for its significant value in evaluating the disease progression and prognosis of colorectal cancer patients (12, 13). In this study, we further analyzed factors that may influence the high rate of recurrence in HRA and suspected lesion areas and found that the initial CEA value was a negative predictor of this status. Analysis of its predictive value indicated that when the cut-off value was less than or equal to 6.54 ng/ml, its positive predictive value and negative predictive value were 95.59% and 24.13%, respectively, which further indicated that low initial CEA (≤6.54 ng/ml) correlate with less aggressive tumor behavior. Additionally, we also observed that undergoing multiple perioperative treatment methods positively impacted the recurrence lesion location, emphasizing the important of intensive perioperative treatment in inhibiting tumor migration. However, the predictive model for the recurrent lesions location constructed in this study, despite its high positive predictive value (95.38%), exhibited a low negative prediction value (22.95%). Therefore, we thought that this model had limited efficacy in predicting whether new lesions would occur in the non-HRA and the sites outside the initial suspected lesion area.
At present, the TNT mode is the only preoperative regimen recommended by the NCCN guidelines for LARC patients with pMMR/MSS status (14). This regimen marks a significant shift from the traditional LCRT and SCRT, primarily by intensifying the chemotherapy component to a duration of 12-16 weeks. Previous studies have underscored that pivotal role of chemotherapy in the neoadjuvant setting for rectal cancer. For example, the EORTC 22921 trial involving 1011 patients demonstrated that adding chemotherapy to preoperative radiotherapy in cT3-4 resectable rectal cancer patients enhanced tumor downstaging and downsizing (15), with patients showing ypT0-2 status gaining benefits in disease-free survival and overall survival (16). Maas and colleagues analyzed 3313 patients from 13 datasets based on their response to neoadjuvant chemoradiotherapy (nCRT) and found that although adding chemotherapy during the interval between radiotherapy and surgery did not benefit the prognosis of patients who obtained pathological complete response (pCR), it was beneficial for patients with ypT1-2 and ypT3-4 (17). In the recent PROSPECT trial, researchers even mentioned the concept of omitting radiotherapy in neoadjuvant therapy of LARC patients, although they recruited patients with only cT1-3 and not anxious about preserving sphincter. This idea further underscores the growing importance of chemotherapy in treatment (18).
While the impact of pCR on long-term prognosis remains a subject of debate (19–22), it is clear that neoadjuvant chemoradiotherapy offers high-response patients the opportunity for anal preservation, low recurrence, and adoption of “Wait and Watch”. In the exploration of LCRT based TNT mode, Garcia-Aguilar and colleagues found that patients receiving sequential 6-cycle mFOLFOX6 following nCRT exhibited a pCR rate more than double that of those who received only nCRT (38% vs.18%) (23). Similarly, in the SCRT-based TNT model, the RAPIDO Trial reported significantly higher pCR rates with the SCRT sequential 6-cycle CapeOX or 9-cycle FOLFOX4 regimen compared to nCRT (28.37% vs. 14.32%) (24, 25). These results highlight that the increased weight of chemotherapy in neoadjuvant therapy significantly improves the responsiveness of LARC patients to neoadjuvant therapy.
According to the requirements of ICRU 83 (26), the target area of neoadjuvant radiotherapy for rectal cancer needs to include MR, PR, LLDR, and the ischiorectal fossa of patients with levator ani muscle invasion. Patients undergoing long-course nCRT also need to implement additional irradiation on the MR and the affected lymph drainage area, that is, a combination of ENI and IFI. In addition, previous research had demonstrated that intensified radiotherapy directed at the primary tumor (not on the lymph nodes) could also enhance tumor regression rates, attain higher pCR rate and reduce the local recurrence rate. Especially for patients with difficulty in preserving anus or with high-risk factors (MRF+, T4, etc.) (27–29).
At present, there are still few studies on IFI in the target setting of pelvic malignancies. YANG et al. analyzed the prognostic factors affecting patients with recurrent ovarian cancer and found that the use of ENI or IFI target setting mode did not lead to differences in survival (30). Li et al. compared elderly bladder cancer patients receiving IFI and ENI, noting no significant difference in overall survival and local progression-free survival between the two groups. However, the acute toxicity rate in the IFI group was significantly lower than that in the ENI group (45.23% vs 72.00%, P=0.008) (31). In our study, a substantial majority of patients (86.51%, 109/126) and relapses (90.86%, 167/186) occurred in HAR and regions with preoperatively suspicious positive lymph nodes. Therefore, we believe that exploring IFI for LARC patients in the context of TNT treatment has important clinical value.
Although we obtained complete data on local recurrence in patients with rectal cancer, our retrospective analysis spanning 14 years has limitations. Firstly, to ensure the completeness of the clinical data, this study only reviewed 126 patients in our center and did not conduct a multi-center review. Secondly, in the analysis of factors that affect the location of recurrent lesions in HRA and suspicious lesion locations, due to the complexity of patient treatment plans and courses, we only assigned orderly values to the treatment methods. While our analysis indicated a higher correlation of recurrence location with intensive treatment, further research is needed to validate these findings, particularly in the context of IFI. Thirdly, we did not perform internal or external validation on the predictive model due to a limited number of patients with new recurrent lesions outside HRA and suspicious lesions. In the future, we will continue to accumulate data or conduct multi-center reviews to complete this work.
In summary, this study classified the sites of recurrent lesions of rectal cancer and compared them with the initial images, and preliminarily discovered the patterns of recurrence sites. Based on this, we believe that exploring IFI for LARC patients is feasible, especially in the context of TNT mode. Should IFI demonstrate similar efficacy and prognosis to ENI, it could significantly reduce radiotherapy adverse events and treatment delay in LARC patients.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by The Fourth Hospital of Hebei Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because This study is a retrospective study.
Author contributions
LX: Conceptualization, Data curation, Funding acquisition, Investigation, Software, Writing – original draft. SZ: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing – original draft. YG: Formal analysis, Project administration, Validation, Writing – original draft. JS: Methodology, Supervision, Writing – original draft. YX: Investigation, Resources, Validation, Visualization, Writing – original draft. LW: Formal analysis, Methodology, Project administration, Validation, Writing – original draft. XW: Formal analysis, Investigation, Methodology, Project administration, Writing – review & editing. FQ: Conceptualization, Formal analysis, Project administration, Resources, Writing – original draft. ML: Data curation, Methodology, Supervision, Writing – original draft. YW: Formal analysis, Project administration, Validation, Writing – original draft. CG: Resources, Validation, Visualization, Writing – original draft. JW: Resources, Visualization, Writing – review & editing. FW: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Resources, Visualization, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was funded by Hebei Provincial Health Commission research fund project (20240091) and Natural Science Foundation of Hebei Province (H2022206584).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2024.1459024/full#supplementary-material
References
1. Roels S, Duthoy W, Haustermans K, Penninckx F, Vandecaveye V, Boterberg T, et al. Definition and delineation of the clinical target volume for rectal cancer. Int J Radiat Oncol Biol Phys. (2006) 65:1129–42. doi: 10.1016/j.ijrobp.2006.02.050
2. De Bari B, Bosset JF, Gérard JP, Maingon P, Valentini V. Multidisciplinary management of rectal cancer. Cancer Radiother. (2012) 16:711–20. doi: 10.1016/j.canrad.2012.10.007
3. Valentini V, Gambacorta MA, Barbaro B, Chiloiro G, Coco C, Das P, et al. International consensus guidelines on Clinical Target Volume delineation in rectal cancer. Radiother Oncol. (2016) 120:195–201. doi: 10.1016/j.radonc.2016.07.017
4. Cheng YJ, Jing SW, Zhu LL, Wang J, Wang L, Liu Q, et al. Comparison of elective nodal irradiation and involved-field irradiation in esophageal squamous cell carcinoma: a meta-analysis. J Radiat Res. (2018) 59:604–15. doi: 10.1093/jrr/rry055
5. Lyu J, Yisikandaer A, Li T, Zhang X, Wang X, Tian Z, et al. Comparison between the effects of elective nodal irradiation and involved-field irradiation on long-term survival in thoracic esophageal squamous cell carcinoma patients: A prospective, multicenter, randomized, controlled study in China. Cancer Med. (2020) 9:7460–8. doi: 10.1002/cam4.3409
6. Wang H, Song C, Zhao X, Deng W, Shen W. The role of involved field irradiation versus elective nodal irradiation in definitive radiotherapy or chemoradiotherapy for esophageal cancer- a systematic review and meta-analysis. Front Oncol. (2022) 12:1034656. doi: 10.3389/fonc.2022.1034656
7. Chen M, Bao Y, Ma HL, Hu X, Wang J, Wang Y, et al. Involved-field radiotherapy versus elective nodal irradiation in combination with concurrent chemotherapy for locally advanced non-small cell lung cancer: a prospective randomized study. BioMed Res Int. (2013) 2013:371819. doi: 10.1155/2013/371819
8. Schild SE, Fan W, Stinchcombe TE, Vokes EE, Ramalingam SS, Bradley JD, et al. Toxicity related to radiotherapy dose and targeting strategy: A pooled analysis of cooperative group trials of combined modality therapy for locally advanced non-small cell lung cancer. J Thorac Oncol. (2019) 14:298–303. doi: 10.1016/j.jtho.2018.09.021
9. Topkan E, Ozdemir Y, Guler OC, Kucuk A, Besen AA, Mertsoylu H, et al. Comparison of involved field radiotherapy versus elective nodal irradiation in stage IIIB/C non-small-cell lung carcinoma patients treated with concurrent chemoradiotherapy: A propensity score matching study. J Oncol. (2020) 2020:7083149. doi: 10.1155/2020/7083149
10. Koh DM, George C, Temple L, Collins DJ, Toomey P, Raja A, et al. Diagnostic accuracy of nodal enhancement pattern of rectal cancer at MRI enhanced with ultrasmall superparamagnetic iron oxide: findings in pathologically matched mesorectal lymph nodes. AJR Am J Roentgenol. (2010) 194:W505–13. doi: 10.2214/AJR.08.1819
11. Fortuin AS, Deserno WM, Meijer HJ, Jager GJ, Takahashi S, Debats OA, et al. Value of PET/CT and MR lymphography in treatment of prostate cancer patients with lymph node metastases. Int J Radiat Oncol Biol Phys. (2012) 84:712–8. doi: 10.1016/j.ijrobp.2011.12.093
12. Tsai PL, Su WJ, Leung WH, Lai CT, Liu CK. Neutrophil-lymphocyte ratio and CEA level as prognostic and predictive factors in colorectal cancer: A systematic review and meta-analysis. J Cancer Res Ther. (2016) 12:582–9. doi: 10.4103/0973-1482.144356
13. Egenvall M, Martling A, Veres K, Horváth-Puhó E, Wille-Jørgensen P, Høirup Petersen S, et al. No benefit of more intense follow-up after surgery for colorectal cancer in the risk group with elevated CEA levels - An analysis within the COLOFOL randomized clinical trial. Eur J Surg Oncol. (2021) 47:2053–9. doi: 10.1016/j.ejso.2021.03.235
14. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Rectal Cancer (v. 6. 2023) [DB/OL] . Available online at: https://www.nccn.org/guidelines/category_1 (accessed January 01, 2024).
15. Bosset JF, Calais G, Mineur L, Maingon P, Radosevic-Jelic L, Daban A, et al. Enhanced tumorocidal effect of chemotherapy with preoperative radiotherapy for rectal cancer: preliminary results—EORTC 22921. J Clin Oncol. (2005) 23:5620–7. doi: 10.1200/JCO.2005.02.113
16. Collette L, Bosset JF, den Dulk M, Nguyen F, Mineur L, Maingon P, et al. Patients with curative resection of cT3-4 rectal cancer after preoperative radiotherapy or radiochemotherapy: does anybody benefit from adjuvant fluorouracil-based chemotherapy? A trial of the European Organisation for Research and Treatment of Cancer Radiation Oncology Group. J Clin Oncol. (2007) 25:4379–86. doi: 10.1200/JCO.2007.11.9685
17. Maas M, Nelemans PJ, Valentini V, Crane CH, Capirci C, Rödel C, et al. Adjuvant chemotherapy in rectal cancer: defining subgroups who may benefit after neoadjuvant chemoradiation and resection: a pooled analysis of 3,313 patients. Int J Cancer. (2015) 137:212–20. doi: 10.1002/ijc.29355
18. Schrag D, Shi Q, Weiser MR, Gollub MJ, Saltz LB, Musher BL, et al. Preoperative treatment of locally advanced rectal cancer. N Engl J Med. (2023) 389:322–34. doi: 10.1056/NEJMoa2303269
19. Valentini V, van Stiphout RG, Lammering G, Gambacorta MA, Barba MC, Bebenek M, et al. Selection of appropriate end-points (pCR vs 2yDFS) for tailoring treatments with prediction models in locally advanced rectal cancer. Radiother Oncol. (2015) 114:302–9. doi: 10.1016/j.radonc.2015.02.001
20. Deng Y, Chi P, Lan P, Wang L, Chen W, Cui L, et al. Modified FOLFOX6 with or without radiation versus fluorouracil and leucovorin with radiation in neoadjuvant treatment of locally advanced rectal cancer: initial results of the chinese FOWARC multicenter, open-label, randomized three-arm phase III trial. J Clin Oncol. (2016) 34:3300–7. doi: 10.1200/JCO.2016.66.6198
21. Deng Y, Chi P, Lan P, Wang L, Chen W, Cui L, et al. Neoadjuvant modified FOLFOX6 with or without radiation versus fluorouracil plus radiation for locally advanced rectal cancer: final results of the chinese FOWARC trial. J Clin Oncol. (2019) 37:3223–33. doi: 10.1200/JCO.18.02309
22. Zhang JW, Chi P, Lan P, Cui L, Chen DD, Cao J, et al. Long-term outcome of neoajuvant mFOLFOX6 with or without Radiation versus Fluorouracil plus Radiation for locally advanced rectal cancer (FOWARC): A multicencter, radomized phase III trail. ASCO. (2023). doi: 10.1200/JCO.2023.41.16_suppl.3505
23. Garcia-Aguilar J, Chow OS, Smith DD, Marcet JE, Cataldo PA, Varma MG, et al. Effect of adding mFOLFOX6 after neoadjuvant chemoradiation in locally advanced rectal cancer: a multicentre, phase 2 trial. Lancet Oncol. (2015) 16:957–66. doi: 10.1016/S1470-2045(15)00004-2
24. van der Valk MJM, Marijnen CAM, van Etten B, Dijkstra EA, Hilling DE, Kranenbarg EM, et al. Compliance and tolerability of short-course radiotherapy followed by preoperative chemotherapy and surgery for high-risk rectal cancer - Results of the international randomized RAPIDO-trial. Radiother Oncol. (2020) 147:75–83. doi: 10.1016/j.radonc.2020.03.011
25. Bahadoer RR, Dijkstra EA, van Etten B, Marijnen CAM, Putter H, Kranenbarg EM, et al. Short-course radiotherapy followed by chemotherapy before total mesorectal excision (TME) versus preoperative chemoradiotherapy, TME, and optional adjuvant chemotherapy in locally advanced rectal cancer (RAPIDO): a randomised, open-label, phase 3 trial. Lancet Oncol. (2021) 22:29–42. doi: 10.1016/S1470-2045(20)30555-6
26. Prescribing ICRU. Recording, and Reporting Photon-Beam Intensity-Modulated Radiation Therapy (IMRT) Report 83. J ICRU (2010) 10:1–106.
27. Spatola C, Privitera G, Milazzotto R, Tocco A, Acquaviva G, Marletta F, et al. Trends in combined radio-chemotherapy for locally advanced rectal cancer: a survey among radiation oncology centers of Sicily region on behalf of AIRO. Radiol Med. (2019) 124:671–81. doi: 10.1007/s11547-019-01007-x
28. Lo Greco MC, La Rocca M, Marano G, Finocchiaro I, Liardo RLE, Milazzotto R, et al. Integrated intensified chemoradiation in the setting of total neoadjuvant therapy (TNT) in patients with locally advanced rectal cancer: A retrospective single-arm study on feasibility and efficacy. Cancers (Basel). (2023) 15:921. doi: 10.3390/cancers15030921
29. Caravatta L, Lupattelli M, Mantello G, Gambacorta MA, Chiloiro G, DI Tommaso M, et al. Treatment volume, dose prescription and delivery techniques for dose-intensification in rectal cancer: A national survey. Anticancer Res. (2021) 41:1985–95. doi: 10.21873/anticanres.14966
30. Yang H, Zhang K, Liu Z, Wang T, Shi F, Su J, et al. Clinical analysis of conformal and intensity-modulated radiotherapy in patients with recurrent ovarian cancer. Sci Rep. (2020) 10:17172. doi: 10.1038/s41598-020-74356-7
Keywords: locally advanced rectal cancer, recurrence location, radiotherapy target area, involved-field irradiation, total neoadjuvant therapy
Citation: Xiao L, Zhuo S, Gao Y, Sun J, Xiao Y, Wang L, Wang X, Qu F, Liu M, Wang Y, Gao C, Wang J and Wu F (2024) Could elective nodal irradiation for locally advanced rectal cancer be omitted in the context of total neoadjuvant therapy? An analysis of the recurrence sites of rectal cancer. Front. Oncol. 14:1459024. doi: 10.3389/fonc.2024.1459024
Received: 10 July 2024; Accepted: 06 November 2024;
Published: 27 November 2024.
Edited by:
Nadia Gisella Di Muzio, Vita-Salute San Raffaele University, ItalyCopyright © 2024 Xiao, Zhuo, Gao, Sun, Xiao, Wang, Wang, Qu, Liu, Wang, Gao, Wang and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fengpeng Wu, d2ZwemhqQDEyNi5jb20=
†These authors have contributed equally to this work
 Linlin Xiao
Linlin Xiao Shiyu Zhuo†
Shiyu Zhuo† Fuyin Qu
Fuyin Qu Ming Liu
Ming Liu Yi Wang
Yi Wang Chao Gao
Chao Gao Jun Wang
Jun Wang Fengpeng Wu
Fengpeng Wu