- 1Department of Medical Oncology, Catalan Institute of Oncology (ICO), L’Hospitalet de Llobregat, Barcelona, Spain
- 2Cancer Epidemiology Research Programme, Catalan Institute of Oncology (ICO), Institut d’Investigació Biomèdica de Bellvitge (IDIBELL), L’Hospitalet de Llobregat, Barcelona, Spain
- 3CIBER en Epidemiología y Salud Pública (CIBERESP), Madrid, Spain
- 4Department of Medical Oncology, Catalan Institute of Oncology (ICO), B-ARGO group, IGTP, Badalona, Spain
- 5Department of Radiation Oncology, Catalan Institute of Oncology (ICO), L’Hospitalet de Llobregat, Barcelona, Spain
- 6Clinical Nutrition Unit, Catalan Institute of Oncology (ICO), IDIBELL, L’Hospitalet de Llobregat, University of Barcelona, Barcelona, Spain
- 7Department of Oral and Maxillofacial Surgery. Hospital Universitari Bellvitge, Barcelona, Spain
- 8Department of Otorhinolaryngology, Head and Neck Surgery, Hospital Universitari Bellvitge, Barcelona, Spain
Objectives: Anti-PD-(L)1 agents changed the landscape of recurrent or metastatic head and neck squamous cell carcinoma (R/M HNSCC) treatment. Previous studies showed improved response rates to salvage chemotherapy (SCT) after progression to anti-PD-(L)1 agents. This study aims to evaluate the outcomes of SCT and to identify predictors of response and survival in patients with R/M HNSCC.
Materials and methods: Retrospective cohort analysis of 63 R/M patients treated with SCT after antiPD-(L1)-based therapy between January 2015 and August 2022. The overall response rate (ORR) was evaluated. Progression-free survival (PFS) and overall survival (OS) were estimated with Kaplan–Meier method. Progression-free survival 2 was calculated from anti-PD-(L)1-therapy start until progression to SCT (PFS2-I). Logistic regression and Cox regression analyses were performed to identify predictors of outcome.
Results: A total of 63 patients were included: 76% were men, and median age was 60 years. PD-L1 status was available in 68% (61% positive). Up to 71% received SCT as third line or beyond. ORR to SCT was 49% with higher rates in PD-L1 positive tumors, 71% vs. 18% (p=0.001), and cetuximab-containing regimens, 68% vs. 39% (p=0.026). PD-L1 status was the only predictor of ORR in the adjusted model (OR=8.6, 95% CI 1.7–43.0). OS and PFS were 9.3 months (95% CI, 6.5–12.3) and 4.1 months (95% CI, 3.0–5.8) respectively. PFS2-I was 8.6 months (95% CI, 6.6–10.5). In the multivariate analysis, PD-L1 was the only independent factor for OS (HR=0.3; 95% CI, 0.1–0.7), PFS (HR=0.2; 95% CI, 0.1–0.5; p<0.001), and PFS2-I (HR=0.2; 95% CI 0.1–0.5; p<0.001).
Conclusion: PDL1 status appeared as a strong predictor of response of efficacy for SCT after anti-PD-(L)1 agents. Patients receiving cetuximab-containing regimens trended towards greater benefit. This highlights the importance of treatment sequencing and personalized treatment strategies.
Introduction
The prognosis of patients with recurrent or metastatic head and neck squamous cell carcinoma (R/M HNSCC) remains poor, with a guarded median overall survival (OS) ranging from 10 months to 14 months (1). The combination of cetuximab, 5-fluorouracil, and platinum has been the standard of care in the first-line setting over the past decade (2). Upon progression, the only treatment option was single-agent chemotherapy, with limited response rates and a median survival of 6 months (3, 4).
Anti-PD-1 agents have emerged as the new standard of care in R/M HNSCC, as they showed an OS improvement in both first-line and platinum-refractory settings (5). The Checkmate 141 and Keynote 040 trials demonstrated increased OS in platinum-refractory R/M HNSCC, using nivolumab and pembrolizumab, respectively, although in the Keynote 040 the benefit was limited to patients with PD-L1-positive disease (6, 7). In the first-line setting, pembrolizumab alone or in combination with platinum-based chemotherapy improved OS compared to the EXTREME regimen in patients with PD-L1-positive disease (1). However, none of these trials demonstrated benefit in terms of PFS. Additionally, ORR to anti-PD-1 agents alone was modest. The OS improvement therefore may be partly explained by subsequent treatments following anti-PD-1, possibly due to enhanced sensitivity to salvage chemotherapy (SCT).
In this regard, the effect of first line on subsequent anticancer therapy was explored in the Keynote 048 study: longer PFS2-I [defined as time from anti-PD-(L)1-based therapy start until progression to SCT or death from any cause] was observed in the PD-L1 CPS ≥20 and CPS ≥1 patients treated with pembrolizumab alone, and in total population, regardless of PD-L1 expression, treated with the combination of chemotherapy and pembrolizumab when compared to cetuximab plus chemotherapy (8).
In other tumor types, such as non-small-cell lung cancer (NSCLC) and urothelial cancer, several trials have demonstrated enhanced responses to SCT following progression on immune checkpoint inhibitors (ICI), surpassing historical benchmarks (9, 10). In R/M HNSCC, a few retrospective series with limited number of patients have shown increased response rates to chemotherapy after anti-PD-1 treatment, compared to historical data (Table 1) (11–20).
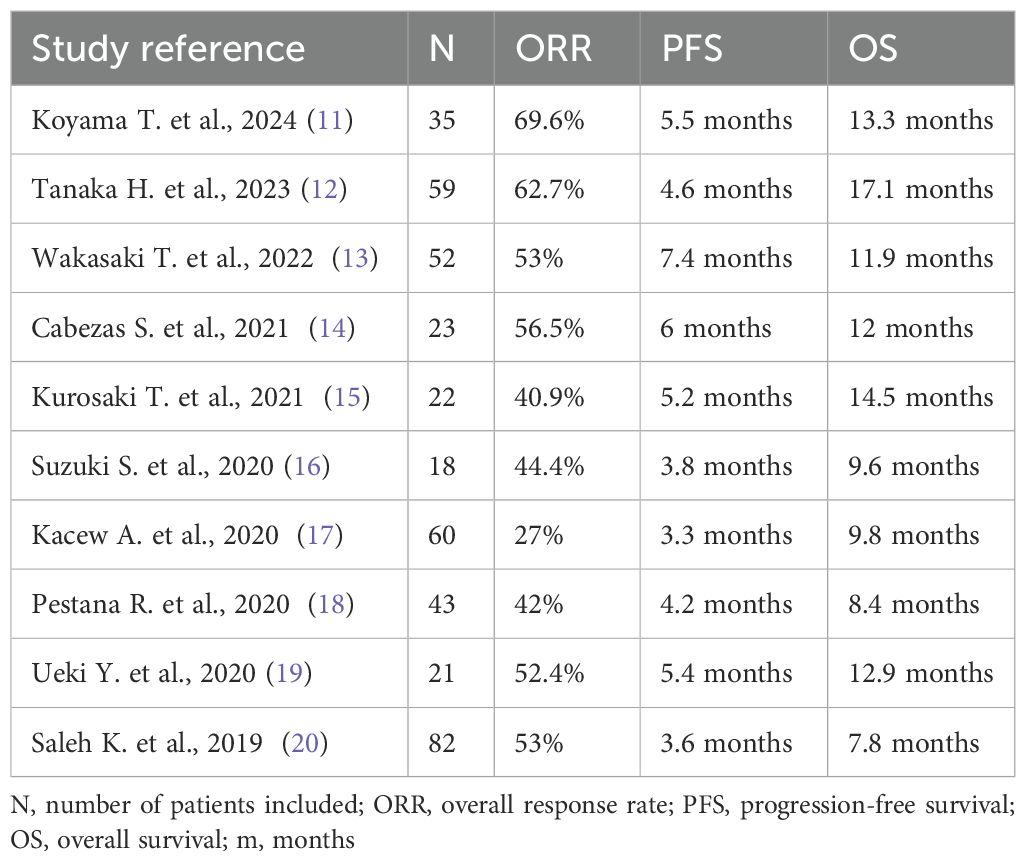
Table 1. Summary of retrospective studies evaluating the role of salvage chemotherapy after anti-PD-1 treatment in R/M head and neck cancer.
Understanding the response patterns and potential predictors of favorable SCT outcomes could have significant implications in tailoring the best treatment sequence for patients with R/M HNSCC. Moreover, it is unclear whether the addition of Cetuximab to SCT leads to improved responses and survival in this setting.
The aim of this study was to evaluate the efficacy of SCT with/without Cetuximab in patients with R/M HNSCC after progression on anti-PD-(L)1 alone or in combination with other immunotherapies and to identify predictors of treatment outcome in terms of ORR, PFS, PFS2-I, and OS.
Materials and methods
Study population and design
Retrospective multicenter cohort analysis of R/M HNSCC patients treated with SCT following disease progression on or after anti-PD-(L)1 agents was conducted between January 2015 and August 2022 in two monographic cancer centers [Institut Català d’Oncologia (ICO) Badalona and ICO-Hospitalet]. Selection criteria for inclusion were as follows: 1) recurrent/metastatic HNSCC, 2) progression on or after treatment with anti-PD-(L)1 agents used at any line, and 3) received SCT after anti-PD-(L)1 agents. Patient demographics, disease characteristics including PD-L1 status, treatment characteristics, and response were retrospectively reviewed by two independent investigators. A third rater reviewed 10% of the data to ensure accuracy.
PD-L1 expression was measured using an immunohistochemistry assay (PD-L1 IHC 22C3 pharmDx; Dako North America, Carpinteria, CA). PD-L1 was considered positive when ≥1% of cells showed partial membrane staining, according to the Combined Positive Score (CPS) or the Tumor Proportion Score (TPS) (21, 22).
Human papillomavirus (HPV) status was evaluated in oropharyngeal cancer, and tumors were considered HPV-related if HPV-DNA polymerase chain reaction and p16INK4a immunohistochemistry determination were both positive.
Platinum-refractory disease was defined as progression on platinum-based chemotherapy for advanced disease or relapsing within 6 months of platinum-based therapy with curative intent. Patients with primary resistance to immunotherapy (refractory disease) were defined as those progressing within 3 months since the start of immunotherapy for recurrent/metastatic disease, in concordance with SITC guidelines (23).
Tumor response to SCT was assessed according to the Response Evaluation Criteria in Solid Tumors (RECIST), version 1.1. The objective response rate (ORR) was defined as the proportion of patients who exhibited complete response (CR) or partial response (PR) as the best response. The disease control rate (DCR) was defined as the proportion of patients who exhibited CR, PR, or stable disease (SD) as the best response. Response evaluation was performed using computed tomography every 8–12 weeks as per institutional protocols.
Statistical analysis
Logistic regression analysis was performed to identify determinants for ORR. Crude and adjusted odds ratio (OR) and 95% confidence intervals (CIs) were calculated, and models were compared using log-likelihood ratio test.
PFS was defined as the time from the initiation of SCT until disease progression, death due to any cause, or the cutoff date. OS was defined as the time from the initiation of SCT until death due to any cause or the cutoff date. PFS2-I was defined as the time from the initiation of anti-PD-(L)1 agents until disease progression on subsequent SCT, death due to any cause, or the cutoff date. Adverse events (AEs) were recorded using the National Cancer Institute Common Terminology Criteria for Adverse Events, version 5.0.
Overall survival, PFS, and PFS2-I were estimated using the Kaplan–Meier method and log-rank test for comparisons between curves. Patients who were lost to follow-up or were still alive without progression by the end of the study were censored at the date of last follow-up. A multivariate Cox regression model was performed to identify predictors of efficacy to SCT in terms of PFS, PFS2-I, and OS. Crude and adjusted hazard ratios (HR) and its 95% CI were calculated. Models were compared using log-likelihood ratio test, and proportional hazards assumption was assessed both by the proportional hazard test and the scaled Shoenfeld residuals.
Variables considered as potential determinants of efficacy in terms of ORR, OS, PFS, and PFS2-I were age, PD-L1 status, baseline ECOG (at SCT initiation), number of previous lines, platinum resistance, and cetuximab-containing regimen, and were included in the univariate analysis. Those variables that showed a statistically significant impact in terms of efficacy were included in the multivariate model.
All analyses were performed using STATA (StataCorp, 2020, Stata Statistical Software: Release 16.1, College Station, TX: StataCorp LLC), and the statistical significance threshold was set at 0.05.
Results
Cohort characteristics
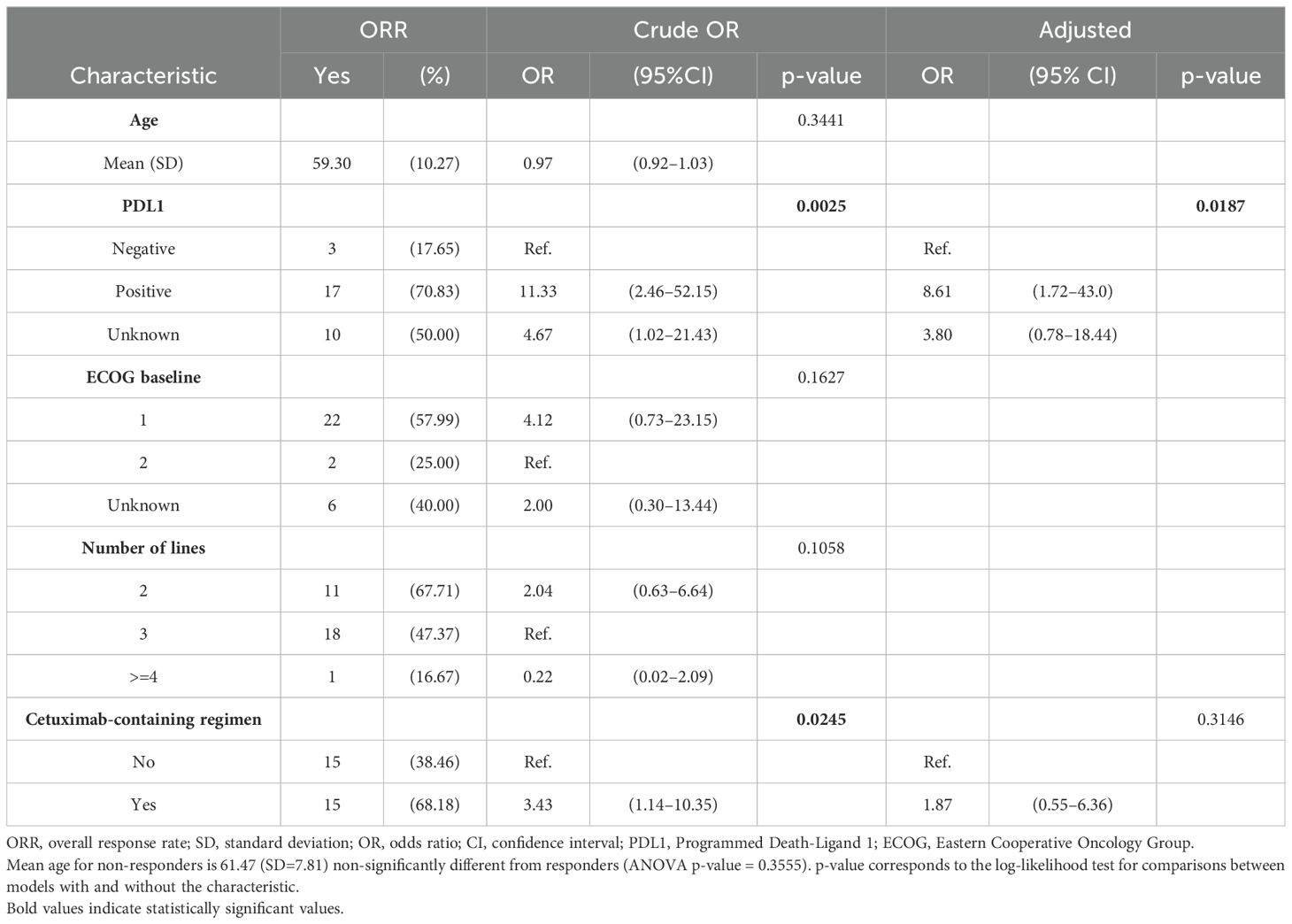
From January 2015 to August 2022, a total of 63 patients who received SCT met eligibility criteria and were included in the analysis. Baseline cohort characteristics are summarized in Table 2. Most patients were men (76%) and former/current smokers (86%). The most prevalent primary tumor location was the oral cavity (33%), followed by larynx (27%) and oropharynx (25%). Only 37.5% (N=6) of oropharyngeal cases were HPV-related. PD-L1 status was available in 68.3% cases, of which 26 (60.5%) were positive (≥1) either by CPS or TPS. A total of 10 (15.9%) patients presented with platinum-refractory disease. Two-thirds of patients had received two lines of therapy for R/M disease prior to SCT. Half of the patients received a PD-(L)1 inhibitor as monotherapy (49%), while the other half received a combination of PD-(L)1 inhibitor plus another immuno-oncology agent. The median PFS and OS to antiPD-(L)1 based therapy were 2.6 months (95% CI, 2.2–2.9) and 12.9 months (95% CI, 9.7–21.3), respectively. The ORR was 12.7%, and the DCR was 38.1%. No significant differences in ORR were observed by PD-L1 status (positive vs. negative): 17.7% vs. 7.7%, respectively (p = 0.369). There were 41 patients (65.1%) with immunotherapy-refractory disease.
Efficacy of salvage chemotherapy following antiPD-(L)1 treatment
Chemotherapy regimens used as SCT are described in Table 2. A total of 34 patients (54%) received paclitaxel monotherapy. Regimens including chemotherapy plus cetuximab were administered in 33 patients (36.6%).
Radiologic response evaluation was available in 61 patients (not available in two cases because of clinical deterioration before radiological evaluation of response related to infectious diseases). Of those, ORR to SCT was 49.2% with nine (14.8%) complete responses. ORR was significantly higher in the PD-L1-positive compared to PD-L1-negative tumors: 70.8% vs. 17.7% (p= 0.001), and for regimens containing cetuximab vs. no cetuximab: 68.2% vs. 38.5% (p= 0.026). DCR was 55.6% for the total cohort and was greater in the PD-L1-positive compared to PD-L1-negative tumors: 85.0% vs. 66.7% (p = 0.002).
Median duration of follow-up from initiation of SCT to data cutoff or death, whichever occurred first, was 7.6 months (0.3–71.1). PFS and OS were 4.1 months (95% CI, 3.0–5.8) and 9.3 months (95% CI, 6.5–12.3 months), respectively. PFS 2 was 8.6 months (95% CI, 6.6–10.5 months).
PD-L1-positive status was associated with better outcome to SCT in terms of PFS [6.1 months (95% CI, 4.9–9.9) vs. 2.0 months (95% CI, 1.0–3.3); p< 0.001], OS [16.7 months (95% CI, 5.6–NR) vs. 6.1 months (95% CI, 2.9–7.9); p 0.001], and PFS2-I [11.3 months (95% CI, 7.2–24.3) vs. 4.4 months (95% CI, 3.3–6.9); p< 0.001] (Figure 1). OS was significantly higher in patients with Eastern Cooperative Oncology Group (ECOG) performance status 1 vs. 2 [12.3 months (95% CI, 7.1–19.0) vs. 5.4 months (95% CI, 0.3–8.5); p 0.001], with no differences in PFS nor PFS2-I.
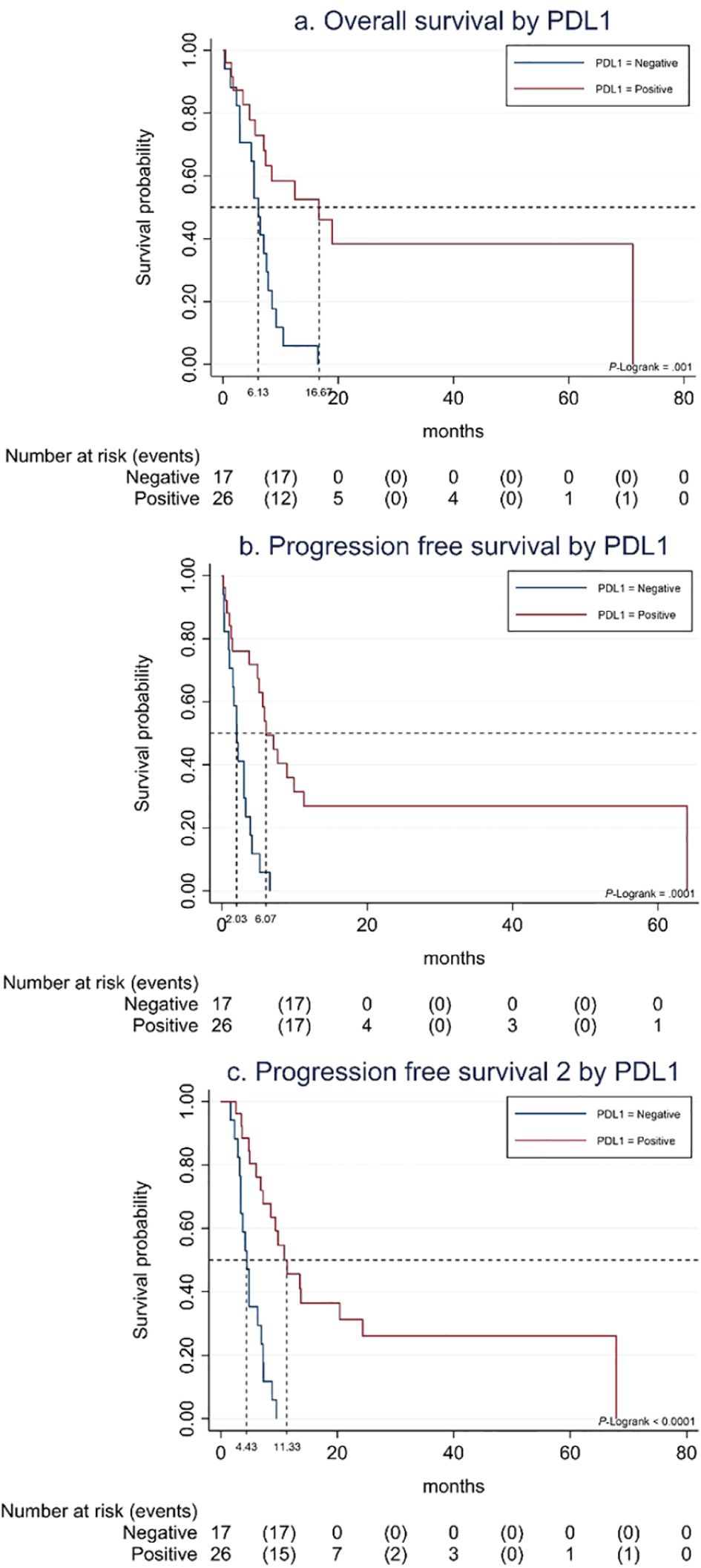
Figure 1. (A) Overall survival to SCT by PDL-1 status. (B) Progression-free survival to SCT by PDL-1 status. (C) Progression-free survival 2 to SCT by PDL-1 status.
There were no significant differences in SCT outcomes between platinum sensitive vs. refractory patients in terms of ORR [51.0% vs. 40.0%; p=0.525], PFS [4.13 months (95% CI, 3–5.8) vs. 2.4 months (95% CI, 0.7–NR); p=0.620], and OS [8.5 months (95% CI, 6.5–12.2) vs. 10.5 months (95% CI, 1.8–NR); p=0.685].
Patients receiving regimens containing cetuximab trended towards greater PFS [6.1 months (95% CI, 1.5–9.9) vs. 3.8 months (95% CI, 2.0–5.2); p=0.078] and PFS2-I [10.9 months (95% CI, 7.2–20.4) vs. 6.9 months (95% CI, 4.9–9.4), p=0.063] but not OS [12.5 months (95% CI, 5.6–NR) vs. 8.4 months (95% CI, 6.1–10.5); p=0.255] when compared to non-cetuximab regimens (Supplementary Figure S1). Taxanes-containing regimens did not show greater PFS, OS, or PFS2-I compared to those without taxanes (p=0.478, p=0.197, and p=0.548 respectively). A subgroup analysis was performed in patients who received Paclitaxel + Cetuximab vs. weekly Paclitaxel monotherapy as SCT regimen. Patients receiving Paclitaxel + Cetuximab showed increased ORR (72.7% vs. 36.4%, p = 0.036), PFS [9.9 months (95% CI, 0.7–NR) vs. 3.8 months (95% CI, 2–5.1), p=0.0340], and OS [12.5 months (95% CI, 1.8–NR) vs. 7.6 months (95% CI, 5.4–10.4), p=0.119] compared to those receiving Paclitaxel monotherapy.
In the subgroup of patients diagnosed with oropharyngeal cancer, HPV status positive vs. negative did not have an impact in SCT efficacy: ORR, 50.0% vs. 62.5% (p=0.960); PFS, 2.2 months (95% CI, 0.17–NR) vs. 5.1 (95% CI, 1.0–9.1) (p=0.698); and OS, 10.4 months (95% CI, 1.8–NR) vs. 12.0 months (95% CI, 1.3–NR) (p 0.999).
No differences in SCT outcomes were observed according to response to prior immunotherapy (responders vs. refractory) between immunotherapy responders vs. non-responders in terms of ORR (50.0% vs. 47.3%; p=0.859), PFS [3.9 months (95% CI, 0.17–8.9) vs. 4.9 months (95% CI, 2.2–5.8); p=0.892], and OS [12.2 months (95% CI, 6.4–NR) vs. 8.4 months (95% CI, 5.6–12.0); p=0.653]. A subgroup analysis was performed in patients with immunotherapy-refractory disease evaluable for response (N=40): ORR was 40.0%, with eight patients (20%) achieving CR. PFS was 3.8 months (95% CI, 1.8–5.1), OS was 7.4 months (95% CI, 5.4–9.3), and PFS2-I was 6.3 months (95% CI, 4.2–7.2).
Predictors of response and survival to SCT
Only PD-L1 status showed a significant association with improved ORR in the logistic regression analysis. Patients with PD-L1-positive disease exhibited higher ORR to SCT compared to PD-L1-negative patients (OR=8.61; 95% CI, 1.72–43.00), after adjusting for PD-L1 and cetuximab-containing regimen (Table 3).
In the multivariate Cox regression analysis for OS (Supplementary Table S1), only PD-L1 status was an independent prognostic factor for OS (HR=0.30; 95% CI, 0.13–0.70; p=0.006) after adjusting for ECOG. PD-L1 status also significantly impacted on PFS (HR=0.23; 95% CI, 0.11–0.48; p=0.001). In terms of PFS2-I, only PDL1 status was a significant predictor for improved PFS2-I (HR = 0.20; 95% CI, 0.08–0.46; p=0.001), after adjusting for ECOG and the cetuximab-containing regimen.
In the subgroup analysis for patients receiving Paclitaxel + Cetuximab vs. weekly Paclitaxel monotherapy, PD-L1status remained the only prognostic factor for OS (HR, 0.33; 95% CI, 0.12–0.90; p = 0.030), PFS (HR, 0.25; 95% CI, 0.09–0.70; p = 0.008), and PFS2-I (HR, 0.21; 95% CI, 0.07–0.58; p = 0.003), after adjusting for ECOG and cetuximab-containing regimen.
Discussion
In this retrospective analysis, we evaluated the efficacy of SCT plus/minus cetuximab following progression on or after anti-PD-(L)1 agents in patients with R/M HNSCC. The observed ORR was close to 50% and was higher in patients with PD-L1-positive disease and when using cetuximab-based regimens. In this pre-treated patient population, PFS and OS were 4.1 months (95% CI, 3.0–5.8) and 9.3 months (95% CI, 6.5–12.3), respectively. PFS2-I was 8.6 months (95% CI, 6.6–10.5). SCT efficacy remained notable in immunotherapy-refractory patients, with an ORR of 40% and up to eight patients (20%) achieving a CR. These results are of relevance when compared to historical data in the pre-immunotherapy era, particularly when more than two-thirds received SCT as a third line of treatment or beyond.
These data are in line with other retrospective series evaluating the role of SCT after anti-PD-(L)1 treatment (see Table 1). Notably, in a French retrospective cohort of 82 patients, Saleh et al. reported an ORR of 30% to SCT, which increased to 53% in cetuximab-based chemotherapy regimens. The PFS was 3.6 months in this cohort (20). Similarly, in a retrospective study conducted by Pestana et al., involving 43 patients with R/M HNSCC who received SCT after anti-PD-1 treatment, an ORR of 42% and a PFS of 4.2 months were observed. Interestingly, the ORR with single agent cetuximab was 37.5% without significant differences compared to chemo-containing regimens (18). In contrast, other series such as Koyama et al., Tanaka et al., and Cabezas et al. reported higher ORR, PFS, and OS measures. These differences could potentially be attributed to factors such as patients being less heavily pretreated (with a high percentage receiving immune checkpoint inhibitors in the first-line setting) and the consistent use of combined chemotherapy with cetuximab in all patients.
The role of PD-L1 as an independent prognostic factor and predictor of benefit to antiPD(L)-1 agents in R/M HNSCC is well-established. However, it is less clear whether it has role in predicting response to SCT. In our cohort, positive PD-L1 expression significantly correlated with improved ORR to SCT, which is in concordance with the results reported by Ueki et al. (19). Nonetheless, controversies exist in the literature regarding the prognostic significance of PD-L1 in this context, probably influenced by small sample sizes and heterogeneous populations in the published series. Immunotherapy-based treatments can induce immune-mediated changes in the tumor microenvironment, especially in PD-L1 positive tumors, creating an inflamed phenotype characterized by increased immune cell infiltration and subsequently enhanced PD-L1 expression. A hot tumor microenvironment has been associated with improved responses to chemotherapy and increased susceptibility to immune cell-mediated cytotoxicity (24). As a result, the combination of ICI-induced microenvironment changes and the subsequent cytotoxic chemotherapy effect might act synergistically and improve therapeutic outcomes with a greater effect in PD-L1-positive patients (25). Moreover, the phenomenon of induced immunogenic cell death might contribute to increased sensitivity to chemotherapy. Immunogenic cell death is known to release damage-associated molecular patterns and tumor-associated antigens (TAAs) from dying tumor cells, triggering an immune response against the tumor, enhancing antigen presentation and priming of cytotoxic T-cells, ultimately leading to immune-mediated tumor cells death. Therefore, when chemotherapy is subsequently administered, the activated T cells may respond to released tumor-associated antigens and kill tumor cells effectively (26).
On the other hand, chemotherapy agents have been shown to deplete regulatory T cells (T regs) and myeloid-derived suppressor cells (MDSCs), which contribute to immune suppression and promote tumor growth. By reducing these suppressive cell populations, chemotherapy might reduce immunosuppression in the tumor microenvironment allowing a more favorable immune landscape. Consequently, the activated cytotoxic T cells, unleashed by immunotherapy and primed to recognize TAAs, may encounter reduced immune inhibition, further promoting tumor cell killing (27).
We found that patients receiving cetuximab plus SCT showed greater ORR and a trend to better PFS and OS when compared to chemotherapy agents alone, as had been previously suggested by other small retrospective series (14).
Besides its direct EGFR inhibition, cetuximab has been shown to induce an antibody-dependent cellular cytotoxicity (ADCC) via natural killer (NK) activation (28, 29). Administration of cetuximab in combination with ICI enhances cetuximab-mediated ADCC in vitro (30) and the combination of pembrolizumab with cetuximab in a phase II trial involving R/M HNSCC patients demonstrated increased ORR when compared to historical responses of both agents when given as monotherapy, indicating an additive or potentially synergistic effect of both drugs combined (31). In this regard, several trials are currently investigating cetuximab in combination with different IO agents. The increased efficacy of SCT with cetuximab following antiPD-1 therapy could be due to a delayed overlapping effect of this treatment sequences.
In the multivariate analysis evaluation, no independent impact on ORR, OS, PFS, or PFS2-I was observed for age, ECOG, or number of previous lines. Although patients with ECOG 1 exhibited a trend towards improved OS, this trend did not reach statistical significance when adjusted for PD-L1 status. No differences in outcomes were found by chemotherapy regimens (taxanes vs. other including platinum) probably due to the small sample size and the fact that non-taxane regimens were more frequently used in earlier lines of treatment.
The authors acknowledge the inherent limitations of a retrospective study and the small sample size, which includes heavily pretreated patients. Furthermore, the heterogeneity of the SCT regimens used in the studied population might have impacted our results. Another limitation stems from the unknown PD-L1 status in up to a third of patients, and the heterogeneity in the evaluation of PD-L1 status by either TPS or CPS. Of note, CPS was not the standard at the time these patients were treated. The modest cohort size may have limited the ability to detect differences between platinum-sensitive and platinum-refractory patients. Furthermore, most patients receiving non-cetuximab regimens were treated with chemotherapy monotherapy, potentially affecting the higher perceived efficacy of cetuximab-containing regimens that incorporated chemotherapy combinations. Given the retrospective nature of this study, patient-centered efficacy measures, such as quality of life and patient-reported outcomes, are not available. These limitations underscore the need for prospective clinical trials with larger sample sizes and control arms. Moreover, future investigations should explore potential biomarkers beyond PD-L1 to identify patients who would most benefit from this sequential treatment strategy.
In conclusion, this study confirms the increased efficacy of SCT after anti-PD-(L)1 agents in patients with pre-treated R/M HNSCC, while it identifies potential predictors of benefit that may aid treatment decision-making in clinical practice. The observed increased ORR compared to pivotal trials, particularly in PD-L1-positive patients and cetuximab-based regimens, indicate a promising direction for personalized treatment sequence strategies. Larger and prospective studies are needed to further understand the underlying mechanisms driving these responses and guide therapeutic approaches.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
Ethical approval was not required for the study involving humans in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and the institutional requirements.
Author contributions
SL: Conceptualization, Data curation, Formal analysis, Methodology, Writing – original draft, Writing – review & editing. MP: Conceptualization, Data curation, Methodology, Writing – review & editing. ST: Formal analysis, Methodology, Writing – review & editing. AF-D: Data curation, Writing – review & editing. JB: Writing – review & editing. MJ: Writing – review & editing. ZV: Writing – review & editing. EV: Writing – review & editing. IL: Writing – review & editing. LA: Writing – review & editing. MD: Writing – review & editing. MF: Writing – review & editing. AB: Writing – review & editing. AL: Writing – review & editing. BC: Writing – review & editing. RM: Writing – review & editing. MO: Conceptualization, Data curation, Methodology, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
We would like to extend our sincere appreciation to all those who contributed to this retrospective study. We are grateful to the patients whose data made this research possible. We also thank the reviewers and editors for their valuable feedback in improving this manuscript. Thank you to everyone involved in the completion of this research.
Conflict of interest
SL has speaker’s Bureau/Travel accommodations expenses: MSD, Merck. MP has speaker’s Bureau/Travel accommodations expenses: Eisai and MSD. ST has sponsorship for grants from Merck, Roche, GSK, IDT, Hologic, Seegene. AF-D has speaker’s Bureau: MSD and Angelini Pharma; and travel accommodations expenses: MSD, Lilly, Roche, Merck, BMS, and Pfizer. JB has speaker’s Bureau/Travel accommodations expenses: MSD, Merck. MJ has speaker’s Bureau/Travel accommodations expenses: MSD, Merck. ZV has speaker’s Bureau/Travel accommodations expenses: MSD, Merck. EV has speaker’s Bureau/Travel accommodations expenses: MSD, Merck. IL has speaker’s Bureau/Travel accommodations expenses: Merck. LA has advisory arrangements with Pfizer, Nutricia; Speaker’s Bureau: MSD, Merck, Nestle; Travel accommodations expenses: Nestle, Vegenat. AL has speaker’s Bureau/Travel accommodations expenses: Merck. BC has advisory arrangements: BMS, Merck and MSD; Training grants: BMS, Merck and MSD; Speaker’s Bureau/Travel accommodations expenses: BMS, Merck and MSD. RM has consulting/advisory arrangements with Pfizer, MSD, Merck KgA, Boehringer, GSK; Speakers’ Bureau: Merck KgA, MSD, Roche. MO has consulting/advisory arrangements with Merck, MSD and Transgene. Research support from Merck and Roche. The institution receives clinical trial support from Abbvie, Ayala Pharmaceutical, MSD, ALX Oncology, Debiopharm International, Merck, ISA Pharmaceuticals, Roche Pharmaceuticals, Boehringer Ingelheim, Seagen, Gilead. Speaker’s Bureau/Travel accommodations expenses: BMS, MSD, Merck.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2024.1458479/full#supplementary-material
References
1. Burtness B, Harrington KJ, Greil R, Soulières D, Tahara M, de Castro G, et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet. (2019) 394:1915–28. doi: 10.1016/S0140-6736(19)32591-7
2. Vermorken JB, Mesia R, Rivera F, Remenar E, Kawecki A, Rottey S, et al. Platinum-based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med. (2008) 359:1116–27. doi: 10.1056/NEJMoa0802656
3. Grau JJ, Caballero M, Verger E, Monzó M, Blanch JL. Weekly paclitaxel for platin-resistant stage IV head and neck cancer patients. Acta Otolaryngol. (2009) 129:1294–9. doi: 10.3109/00016480802590451
4. Martinez-Trufero J, Isla D, Adansa JC, Irigoyen A, Hitt R, Gil-Arnaiz I, et al. Phase II study of capecitabine as palliative treatment for patients with recurrent and metastatic squamous head and neck cancer after previous platinum-based treatment. Br J Cancer. (2010) 102:1687–91. doi: 10.1038/sj.bjc.6605697
5. Machiels JP, René Leemans C, Golusinski W, Grau C, Licitra L, Gregoire V. Squamous cell carcinoma of the oral cavity, larynx, oropharynx and hypopharynx: EHNS–ESMO–ESTRO Clinical Practice Guidelines for diagnosis, treatment and follow-up†. Ann Oncol. (2020) 31:1462–75. doi: 10.1016/j.annonc.2020.07.011
6. Ferris RL, Blumenschein G, Fayette J, Guigay J, Colevas AD, Licitra L, et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med. (2016) 375:1856–67. doi: 10.1056/NEJMoa1602252
7. Cohen EEW, Soulières D, Le Tourneau C, Dinis J, Licitra L, Ahn MJ, et al. Pembrolizumab versus methotrexate, docetaxel, or cetuximab for recurrent or metastatic head-and-neck squamous cell carcinoma (KEYNOTE-040): a randomised, open-label, phase 3 study. Lancet. (2019) 393:156–67. doi: 10.1016/S0140-6736(18)31999-8
8. Harrington KJ, Rischin D, Greil R, Soulieres D, Tahara M, Castro G, et al. KEYNOTE-048: Progression after the next line of therapy following pembrolizumab (P) or P plus chemotherapy (P+C) vs EXTREME (E) as first-line (1L) therapy for recurrent/metastatic (R/M) head and neck squamous cell carcinoma (HNSCC). J Clin Oncol. (2020) 38:6505–6505. doi: 10.1200/JCO.2020.38.15_suppl.6505
9. Szabados B, van Dijk N, Tang YZ, van der Heijden MS, Wimalasingham A, Gomez de Liano A, et al. Response rate to chemotherapy after immune checkpoint inhibition in metastatic urothelial cancer. Eur Urol. (2018) 73:149–52. doi: 10.1016/j.eururo.2017.08.022
10. Park SE, Lee SH, Ahn JS, Ahn MJ, Park K, Sun JM. Increased response rates to salvage chemotherapy administered after PD-1/PD-L1 inhibitors in patients with non–small cell lung cancer. J Thorac Oncol. (2018) 13:106–11. doi: 10.1016/j.jtho.2017.10.011
11. Koyama T, Kiyota N, Imamura Y, Boku S, Shibata N, Satake H, et al. A phase II trial of paclitaxel plus biweekly cetuximab for patients with recurrent or metastatic head and neck cancer previously treated with both platinum-based chemotherapy and anti-PD-1 antibody. J Clin Oncol. (2023) 41:103476. doi: 10.1200/JCO.2023.41.16_suppl.6032
12. Tanaka H, Enokida T, Okano S, Fujisawa T, Tanaka N, Takeshita N, et al. Subsequent chemotherapy with paclitaxel plus cetuximab-based chemotherapy following immune checkpoint inhibitor in recurrent or metastatic squamous cell carcinoma of the head and neck. Front Oncol. (2023) 13:1221352. doi: 10.3389/fonc.2023.1221352
13. Wakasaki T, Yasumatsu R, Uchi R, Taura M, Matsuo M, Komune N, et al. Outcome of chemotherapy following nivolumab treatment for recurrent and/or metastatic head and neck squamous cell carcinoma. Auris Nasus Larynx. (2020) 47:116–22. doi: 10.1016/j.anl.2019.05.001
14. Cabezas-Camarero S, Cabrera-Martín MN, Merino-Menéndez S, Paz-Cabezas M, García-Barberán V, Sáiz-Pardo Sanz M, et al. Safety and efficacy of cetuximab-based salvage chemotherapy after checkpoint inhibitors in head and neck cancer. Oncologist. (2021) 26:e1018–35. doi: 10.1002/onco.13754
15. Kurosaki T, Mitani S, Tanaka K, Suzuki S, Kanemura H, Haratani K, et al. Safety and efficacy of cetuximab-containing chemotherapy after immune checkpoint inhibitors for patients with squamous cell carcinoma of the head and neck: a single-center retrospective study. Anticancer Drugs. (2021) 32:95–101. doi: 10.1097/CAD.0000000000001006
16. Suzuki S, Toyoma S, Kawasaki Y, Koizumi K, Iikawa N, Shiina K, et al. Clinical outcomes of cetuximab and paclitaxel after progression on immune checkpoint inhibitors in recurrent or metastatic head and neck squamous cell carcinoma. Medicina (Lithuania). (2021) 57:1151. doi: 10.3390/medicina57111151
17. Kacew AJ, Harris EJ, Lorch JH, Schoenfeld JD, Margalit DN, Kass JI, et al. Chemotherapy after immune checkpoint blockade in patients with recurrent, metastatic squamous cell carcinoma of the head and neck. Oral Oncol. (2020) 105:104676. doi: 10.1016/j.oraloncology.2020.104676
18. Pestana RC, Becnel M, Rubin ML, Torman DK, Crespo J, Phan J, et al. Response rates and survival to systemic therapy after immune checkpoint inhibitor failure in recurrent/metastatic head and neck squamous cell carcinoma. Oral Oncol. (2020) 101:104523. doi: 10.1016/j.oraloncology.2019.104523
19. Ueki Y, Takahashi T, Ota H, Shodo R, Yamazaki K, Horii A. Role of programmed death-ligand 1 in predicting the treatment outcome of salvage chemotherapy after nivolumab in recurrent/metastatic head and neck squamous cell carcinoma. Head Neck. (2020) 42:3275–81. doi: 10.1002/hed.v42.11
20. Saleh K, Daste A, Martin N, Pons-Tostivint E, Auperin A, Herrera-Gomez RG, et al. Response to salvage chemotherapy after progression on immune checkpoint inhibitors in patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck. Eur J Cancer. (2019) 121:123–9. doi: 10.1016/j.ejca.2019.08.026
21. Ito T, Okamoto I, Tokashiki K, Sato H, Okada T, Yamashita G, et al. PD-L1 expression and survival rates using TPS and CPS for nivolumab-treated head-and-neck cancer. Anticancer Res. (2022) 42:1547–54. doi: 10.21873/anticanres.15628
22. Emancipator K, Huang L, Aurora-Garg D, Bal T, Cohen EEW, Harrington K, et al. Comparing programmed death ligand 1 scores for predicting pembrolizumab efficacy in head and neck cancer. Modern Pathol. (2021) 34:532–41. doi: 10.1038/s41379-020-00710-9
23. Kluger HM, Tawbi H, Feltquate D, Lavallee T, Rizvi NA, Sharon E, et al. Society for Immunotherapy of Cancer (SITC) checkpoint inhibitor resistance definitions: Efforts to harmonize terminology and accelerate immuno-oncology drug development. J Immunother Cancer. (2023) 11:e007309. doi: 10.1136/jitc-2023-007309
24. Gettinger S, Choi J, Hastings K, Truini A, Datar I, Sowell R, et al. Impaired HLA class I antigen processing and presentation as a mechanism of acquired resistance to immune checkpoint inhibitors in lung cancer. Cancer Discov. (2017) 7:1420–35. doi: 10.1158/2159-8290.CD-17-0593
25. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. (2012) 12:252–64. doi: 10.1038/nrc3239
26. Galluzzi L, Buqué A, Kepp O, Zitvogel L, Kroemer G. Immunogenic cell death in cancer and infectious disease. Nat Rev Immunol. (2017) 17:97–111. doi: 10.1038/nri.2016.107
27. Yost KE, Satpathy AT, Wells DK, Qi Y, Wang C, Kageyama R, et al. Clonal replacement of tumor-specific T cells following PD-1 blockade. Nat Med. (2019) 25:1251–9. doi: 10.1038/s41591-019-0522-3
28. Lattanzio L, Denaro N, Vivenza D, Varamo C, Strola G, Fortunato M, et al. Elevated basal antibody-dependent cell-mediated cytotoxicity (ADCC) and high epidermal growth factor receptor (EGFR) expression predict favourable outcome in patients with locally advanced head and neck cancer treated with cetuximab and radiotherapy. Cancer Immunol Immunother. (2017) 66:573–9. doi: 10.1007/s00262-017-1960-8
29. Lu S, Concha-Benavente F, Shayan G, Srivastava RM, Gibson SP, Wang L, et al. STING activation enhances cetuximab-mediated NK cell activation and DC maturation and correlates with HPV+ status in head and neck cancer. Oral Oncol. (2018) 78:186–93. doi: 10.1016/j.oraloncology.2018.01.019
30. Concha-Benavente F, Kansy B, Moskovitz J, Moy J, Chandran U, Ferris RL. PD-L1 mediates dysfunction in activated PD-1 þ NK cells in head and neck cancer patients. Cancer Immunol Res. (2018) 6:1548–60. doi: 10.1158/2326-6066.CIR-18-0062
31. Sacco AG, Chen R, Worden FP, Wong DJL, Adkins D, Swiecicki P, et al. Pembrolizumab plus cetuximab in patients with recurrent or metastatic head and neck squamous cell carcinoma: an open-label, multi-arm, non-randomised, multicentre, phase 2 trial. Lancet Oncol. (2021) 22:883–92. doi: 10.1016/S1470-2045(21)00136-4
Keywords: head and neck, squamous cell carcinoma, HNSCC, immunotherapy, anti-PD-(L)1, salvage chemotherapy, SCT, treatment sequencing
Citation: Llop S, Plana M, Tous S, Ferrando-Díez A, Brenes J, Juarez M, Vidales Z, Vilajosana E, Linares I, Arribas L, Duch M, Fulla M, Brunet A, Lozano A, Cirauqui B, Mesía R and Oliva M (2024) Salvage chemotherapy after progression on immunotherapy in recurrent/metastatic squamous cell head and neck carcinoma. Front. Oncol. 14:1458479. doi: 10.3389/fonc.2024.1458479
Received: 02 July 2024; Accepted: 25 October 2024;
Published: 25 November 2024.
Edited by:
Carlo Resteghini, Humanitas University, ItalyReviewed by:
Santiago Cabezas-Camarero, San Carlos University Clinical Hospital, SpainLuigi Lorini, University of Brescia, Italy
Copyright © 2024 Llop, Plana, Tous, Ferrando-Díez, Brenes, Juarez, Vidales, Vilajosana, Linares, Arribas, Duch, Fulla, Brunet, Lozano, Cirauqui, Mesía and Oliva. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Marc Oliva, bW9saXZhQGljb25jb2xvZ2lhLm5ldA==
 Sandra Llop
Sandra Llop Maria Plana
Maria Plana Sara Tous
Sara Tous Angelica Ferrando-Díez4
Angelica Ferrando-Díez4 Jesús Brenes
Jesús Brenes Lorena Arribas
Lorena Arribas Alicia Lozano
Alicia Lozano Beatriz Cirauqui
Beatriz Cirauqui Ricard Mesía
Ricard Mesía Marc Oliva
Marc Oliva