- 1Faculty of Medicine, Jordan University of Science & Technology, Irbid, Jordan
- 2Faculty of Medicine, University of Aleppo, Aleppo, Syria
- 3Médical Oncology, Régional Hospital Center, Tangier, Morocco
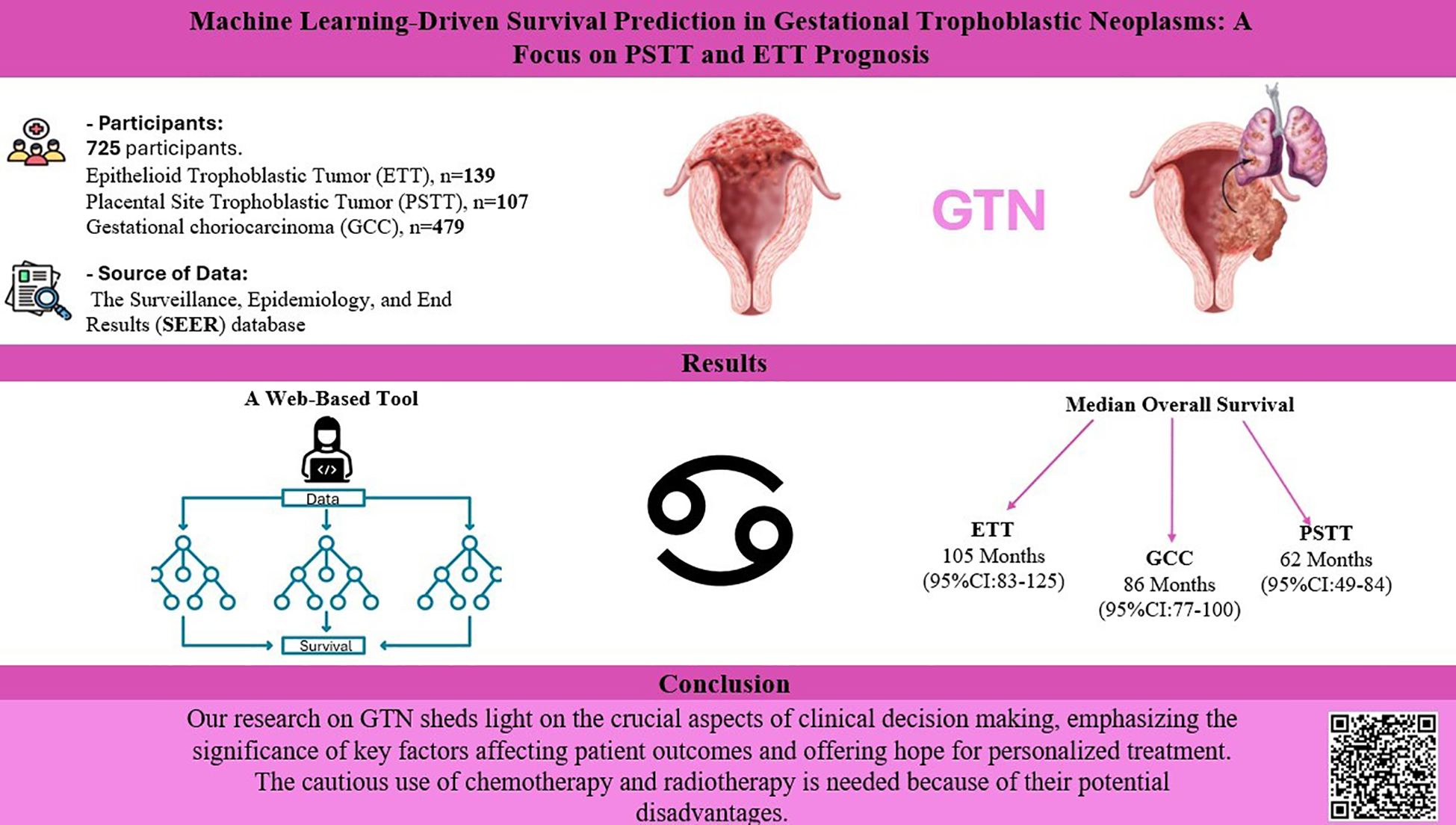
Introduction: The clinicopathological characteristics and prognosis of placental site trophoblastic tumor (PSTT) and epithelioid trophoblastic tumor (ETT) have not been well summarized. Consequently, we conducted the largest to date series of samples of both types and employed machine learning (ML) to assess treatment effectiveness and develop accurate prognostic models for patients with GTN. Gestational choriocarcinoma (GCC) was used as the control group to show the clinical features of PTSS and ETT.
Methods: The Surveillance, Epidemiology, and End Results (SEER) database provided the data used for this study’s analysis. To identify the prognostic variables, we conducted Cox regression analysis and constructed prognostic models using five ML algorithms to predict the 5-year survival. A validation method incorporating the area under the curve (AUC) of the receiver operating characteristic (ROC) curve was used to validate the accuracy and reliability of the ML models. We also investigated the role of multiple therapeutic options using the Kaplan-Meier survival analysis.
Results: The study population comprised 725 patients. Among them, 139 patients had ETT, 107 had PSTT, and 479 had GCC. There were no significant differences in survival between the different tumor groups. Multivariate Cox regression analysis revealed that metastasis was a significant prognostic factor for GCC, while older age and radiotherapy were significant prognostic factors for PTSS and ETT. ML models revealed that the Gradient Boosting classifier accurately predicted the outcomes, followed by the random forest classifier, K-Nearest Neighbors, Logistic Regression, and multilayer perceptron models. The most significant contributing factors were tumor size, year of diagnosis, age, and race.
Discussion: Our study provides a method for treatment and prognostic assessment of patients with GTN. The ML we developed can be used as a convenient individualized tool to facilitate clinical decision making.
1 Introduction
Gestational trophoblastic neoplasms (GTNs) are a group of malignant tumors originating from placental villous and extra-villous trophoblasts, consisting of invasive mole, choriocarcinoma, placental site trophoblastic tumor (PSTT), and epithelioid trophoblastic tumor (ETT) (1–3). While most patients are asymptomatic, vaginal bleeding is the most common presenting symptom (4).
PSTT is the least common form of GTN and originates from extravillous intermediate trophoblasts on the maternal side of the placental bed that invade the myometrium. The average age at the presentation is 31 years, ranging from 20 to 63 years (4, 5). Abnormal vaginal bleeding (31.3-79.4% of cases) is the most common symptom, followed by amenorrhea (5, 6).
ETT originates from chorionic laeve-type intermediate trophoblasts and can be found in the uterus (40%), uterine cervix (31%), or extrauterine sites, such as the lungs (19%), fallopian tubes, ovaries, and pelvic peritoneum (6, 7). The mean age of women affected by ETT is 36.1 years, 15–48 years) (5). Abnormal vaginal bleeding (57-67% of cases) is the most common symptom, followed by amenorrhea, abdominal pain, and abdominal bloating (6).
Contrary to widespread belief, hCG is not an accurate marker of PSTT/ETT disease because both tumors do not secrete hCG due to their origin from intermediate trophoblasts instead of syncytiotrophoblasts (3, 5, 8).
The primary treatment for PSTT and ETT is surgical intervention, including hysterectomy and resection of the metastatic disease. Hysterectomy has a good survival rate for stage I ETT and PSTT, with over 90% of patients surviving for ten years. Conservative options, such as uterine curettage and hysteroscopic resection, may be considered for fertility preservation. In some cases, chemotherapy may be administered in addition to surgery. However, ETT is generally resistant to conventional chemotherapies. Surgical resection and chemotherapy have led to 10-year overall survival rates of approximately 50% for stage II-IV disease (1, 9).
Artificial intelligence (AI) includes ML, which focuses on computer data analysis and learning algorithm refinement. ML has been successful in addressing complex problems, especially in medicine, where it has been applied to medical image recognition, treatment support, and biomedical research (10–12).
Owing to the scarcity of cases of PSTT and ETT, their clinicopathological characteristics and prognosis have not been well summarized. Additionally, it is crucial to develop reliable predictions for personalized care and improved management. In this study, we used ML techniques to predict survival and identify potential prognostic factors. Because GCC is the most common type among GTN, we set it as a control group to show the clinical features of PSTT and ETT.
2 Materials and methods
2.1 Data extraction and variables
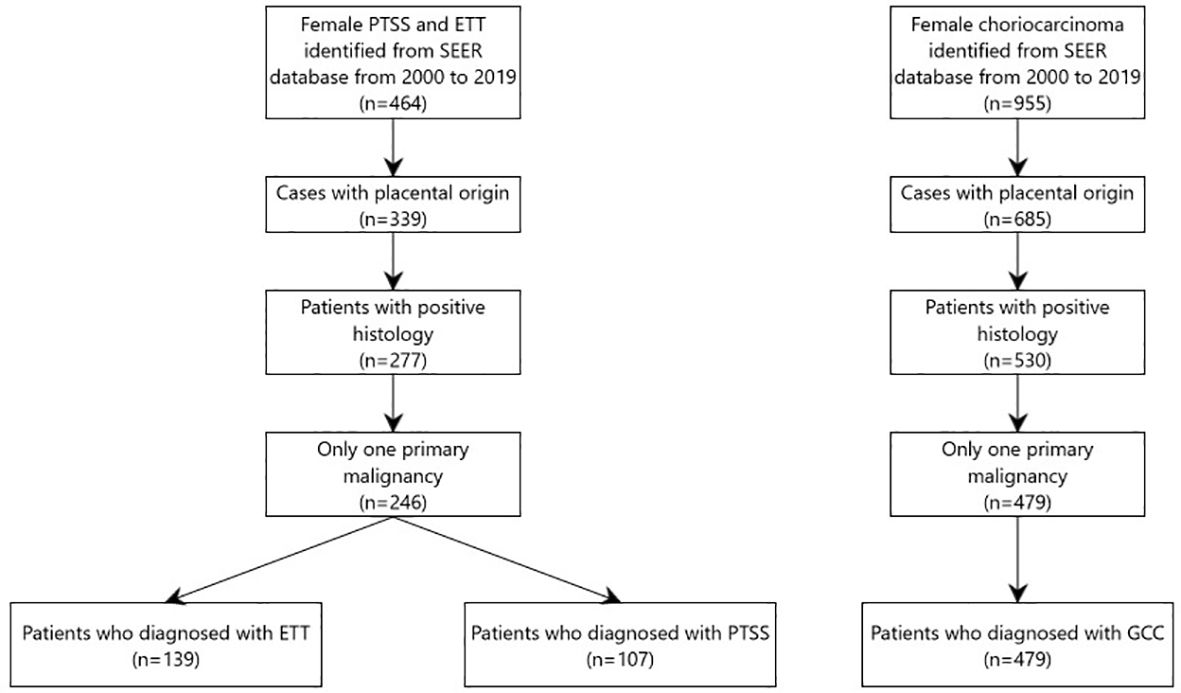
We extracted the data from “Incidence-SEER 18 Regs Custom Data (with additional treatment fields), Nov 2020 Sub (2000–2019 varying)” database. All patients diagnosed between 2000 to 2019 were identified using SEER*Stat software (version 8.4.0.1). Data of all patients diagnosed based on the third edition of the International Classification of Diseases for Oncology (ICD-O-3) were included in this study. Histology codes for disorders were as follows: (i) GCC included 9100/3 (choriocarcinoma); (ii) PTSS: 9104/3 (malignant placental site trophoblastic tumor); and (iii) ETT: 9105/3 (trophoblastic tumor, epithelioid). Patients who met any of the following criteria were excluded: diagnosis not confirmed by histology, not the first tumor or other malignancies in the body, and not arising from the placenta. Finally, 139 patients were eligible for ETT, 107 for PTSS, and 479 for GCC. The detailed screening process is shown in the flow diagram in (Figure 1).
The following SEER variables were selected for our study: Patient characteristics (including age, race, and marital status), tumor characteristics (including SEER stage, tumor size and metastasis), therapeutic methods (including surgery, chemotherapy, and radiotherapy), and survival information were obtained from the database. Age was divided into two groups: ≤30 and >30 years; SEER stages were classified into two groups: localized (within the organ), regional (extension to adjacent organs or regional lymph nodes) and distant (involvement of distant organs or distant metastasis). The primary survival outcomes were overall survival (OS) which was calculated from diagnosis to any cause of death and cancer-specific survival (CSS) which was calculated from diagnosis to death caused by GTN.
2.2 Statistical analysis
2.2.1 Data processing and statistical methods
The All data were analyzed using R software (v4.0.0) and the following packages: “readxl,” “tidyverse,” “Hmisc,” “data.table,” “Table 1,” “MatchIt,” “survminer,” “survival,” and “broom.” Chi-square analysis was used to assess categorical variables and evaluate the clinicopathological characteristics of patients with GTN. The K-M method was used to estimate survival rates, and the log-rank test was used to analyze differences between survival curves. Multivariate Cox proportional hazards models identified independent predictors of OS and CSS. A two-tailed P value less than 0.05 was considered statistically significant. Five ML ensembles were employed: random forest classifier (RFC), Gradient Boosting classifier (GBC), Logistic Regression (LR), K-Nearest Neighbors (KNN), and multilayer perceptron (MLP). These algorithms were selected because of their ability to handle complex multidimensional datasets. All ML implementations were processed using the scikit-learn 0.18 package in Python.
2.2.2 Model training
To ensure model robustness, standard scaling was applied uniformly across all features using the StandardScaler module from Scikit-learn. This normalized the data, thereby alleviating the potential impacts of different scales among the features. Subsequently, ML models were trained on a dataset with a binary classification output predicting the target “5-year survival.” The features included demographics, tumor characteristics, and management approaches. The dataset was randomly split into 7:3 for training (n =172) and testing (n =74) sets for the PSTT and ETT. In addition, training (n =335) and testing (n =144) sets were used for GCC.
2.2.3 Feature importance and model evaluation
The feature contribution in predicting “5-year survival” was calculated using the permutation importance method. ROC curves and AUC scores were used to evaluate the model discriminatory power. The roc_curve function from the sklearn.metrics module computes false positive rate (FPR) and true positive rate (TPR). The predicted probabilities of the positive class were obtained using the predict-proba method for each model. AUC scores were calculated using the roc_auc_score function. A custom plotting function, plot_roc_curve, and visualized ROC curves of the multiple models. Additional model evaluation included a mean bootstrap estimate with a 95% Confidence Interval, 10-fold cross-validation, and a classification report for precision, recall, and F1-score.
3 Results
3.1 Patient characteristics
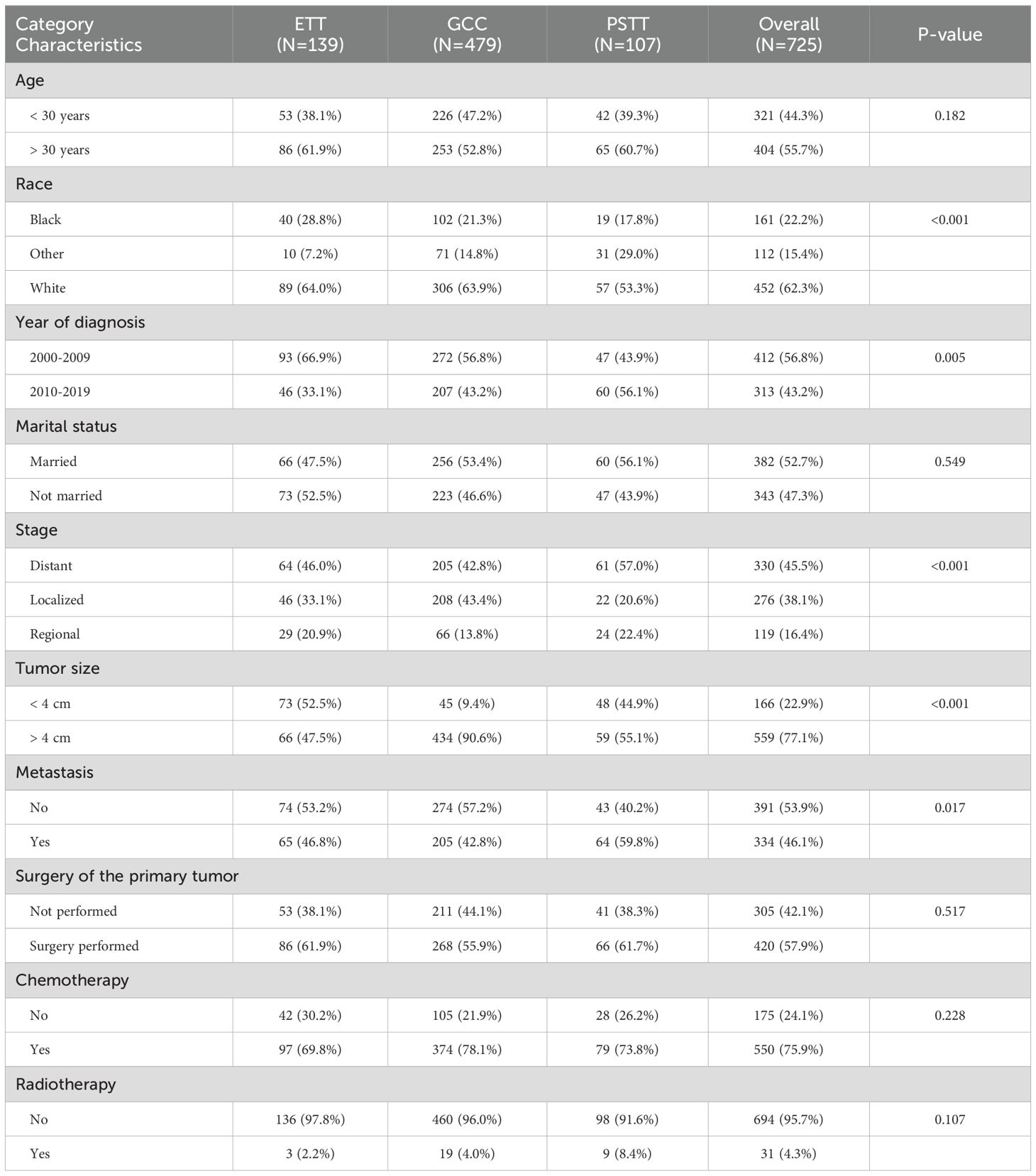
A total of 728 patients who met the inclusion criteria were included in the study (Table 1). The median age at diagnosis was 37 years. Most of the patients were of white race (62.3%) and more than half were married (52.7%). The most common stage of primary tumor histology was distant metastasis, which occurred in 330 patients. The average tumor size was 6.13 cm. Notably, 46.1% of the patients had a primary tumor that had already metastasized. More than two-thirds of the patients underwent chemotherapy (75.9%), 57.9% underwent surgery, and 4.3% underwent radiotherapy. Compared to the GCC group, the ETT and PSTT groups had significantly more patients with smaller primary tumor sizes. The highest rate of metastatic primary tumors was observed in the PSTT group (59.8% vs. < 47% in ETT/GCC). The rate of primary surgical resection was highest in the ETT patient group, followed by the PSTT and GCC groups, whereas there was no significant difference in the rates of receiving chemotherapy and radiotherapy.
3.2 Survival analysis between gestational trophoblastic tumors
Survival analysis was performed to investigate potential differences in OS and CSS among the three groups, as shown in (Figure 2). The results did not indicate statistically significant differences in OS or CSS between the groups (p=0.63, p=0.35, respectively). The median overall survival for the ETT group was 105 months (95%CI:83-125), 86 months (95%CI:77-100) for the GCC group, and 62 months(95%CI:49-84) for the PSTT group, and the difference was compared using the Kruskal-Wallis test(p=0.05005). Regarding the median CSS, the differences between the groups were not statistically significant (p=0.395). Although statistically insignificant, GCC presented the highest 1-, 3-, and 5-year overall survival rates among all GTN groups (95%, 93%, and 92%, respectively), whereas ETT showed the highest 1-, 3-, and 5-year cancer-specific survival rates, albeit statistically insignificant (Supplementary Table S1).
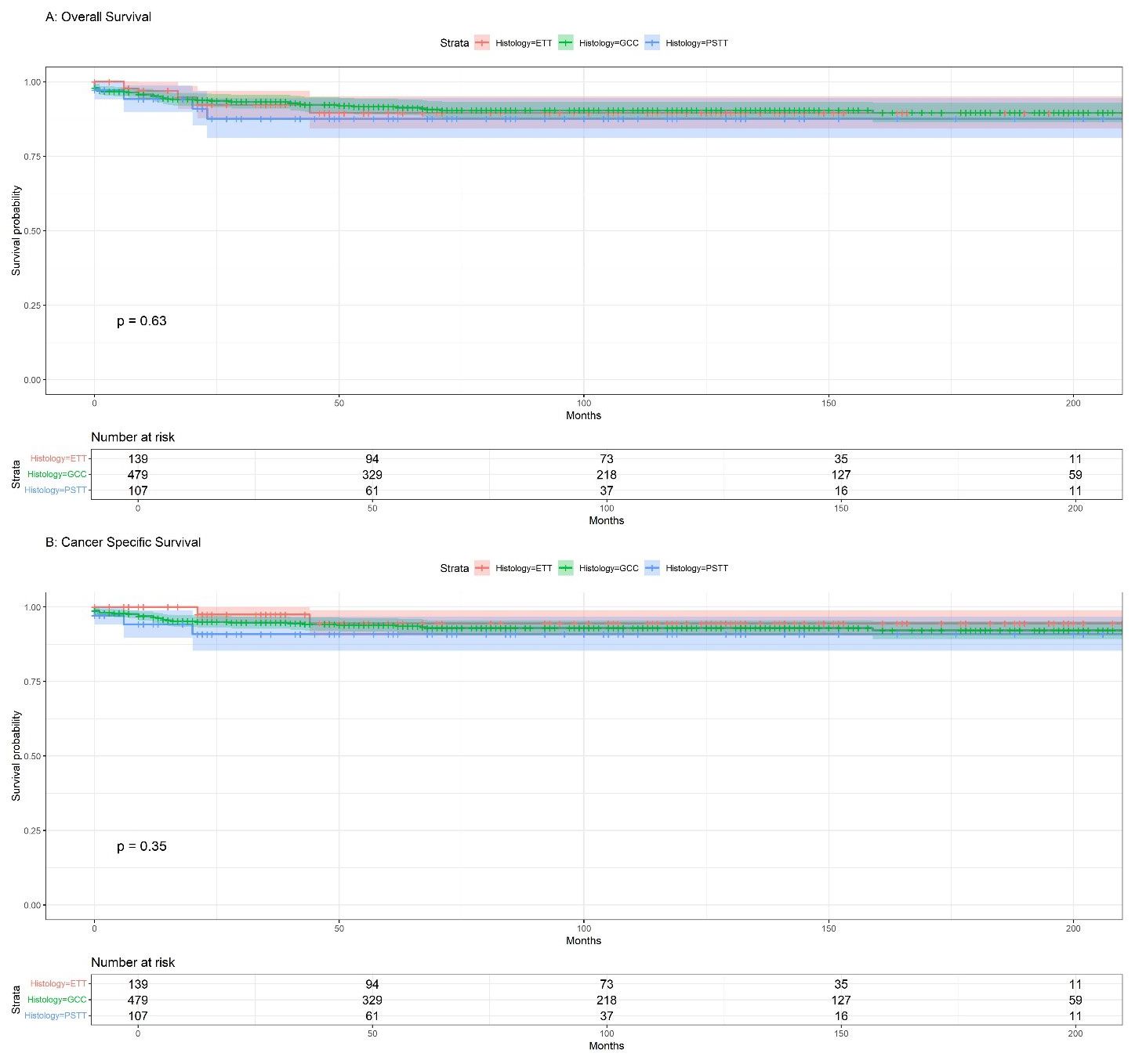
Figure 2. (A) Kaplan-Meier overall survival (OS) for GTN; (B) Kaplan-Meier cancer-specific survival (CSS) for GTN.
3.3 Prognostic factors
Univariate analysis was performed, and only significant variables were considered for the multivariate Cox regression. As shown in (Figure 3), our results indicated that metastasis had a significantly poor effect on OS and CSS in patients with GCC. Radiotherapy had a significant effect on OS and CSS, whereas older age only on OS in patients with PSTT and ETT (Figure 4).
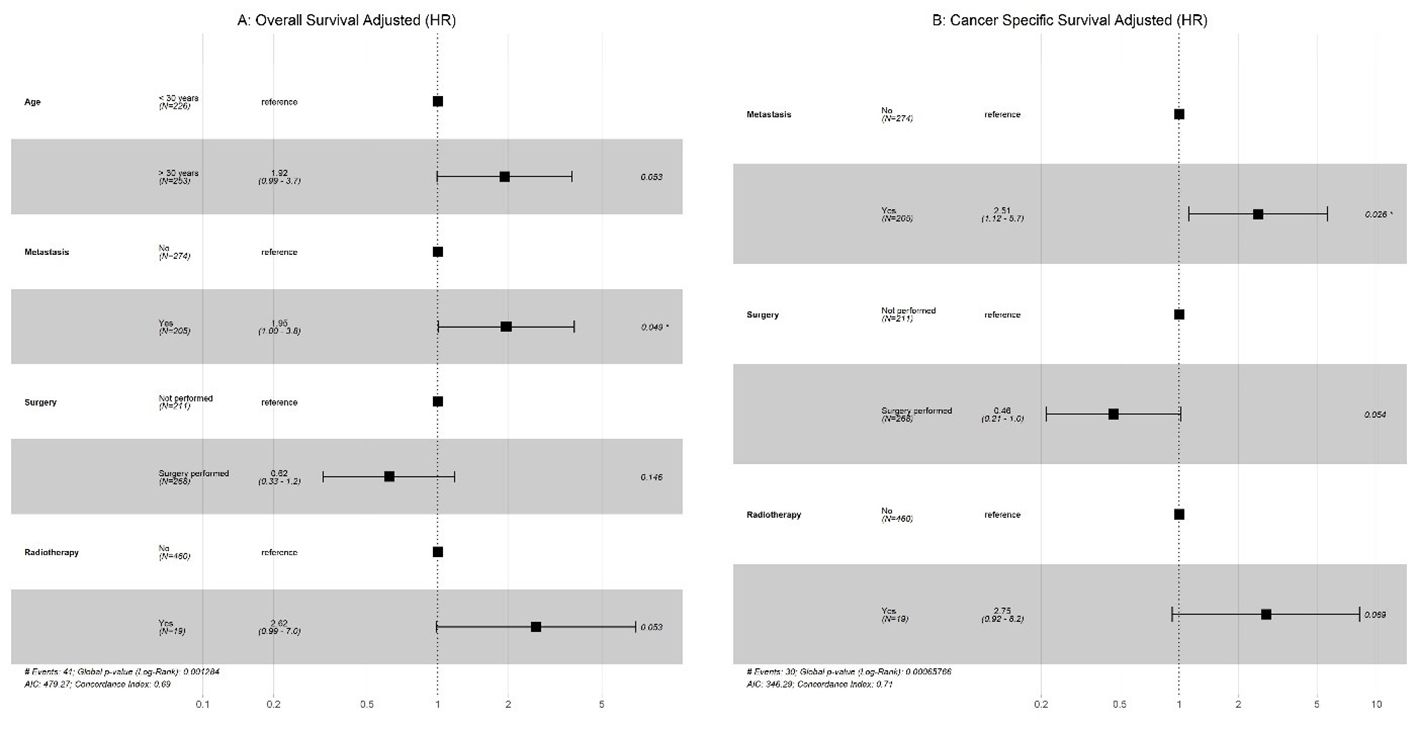
Figure 3. GCC: (A) Multivariate Cox regression for overall survival; (B) Multivariate Cox regression for cancer-specific survival (CSS).
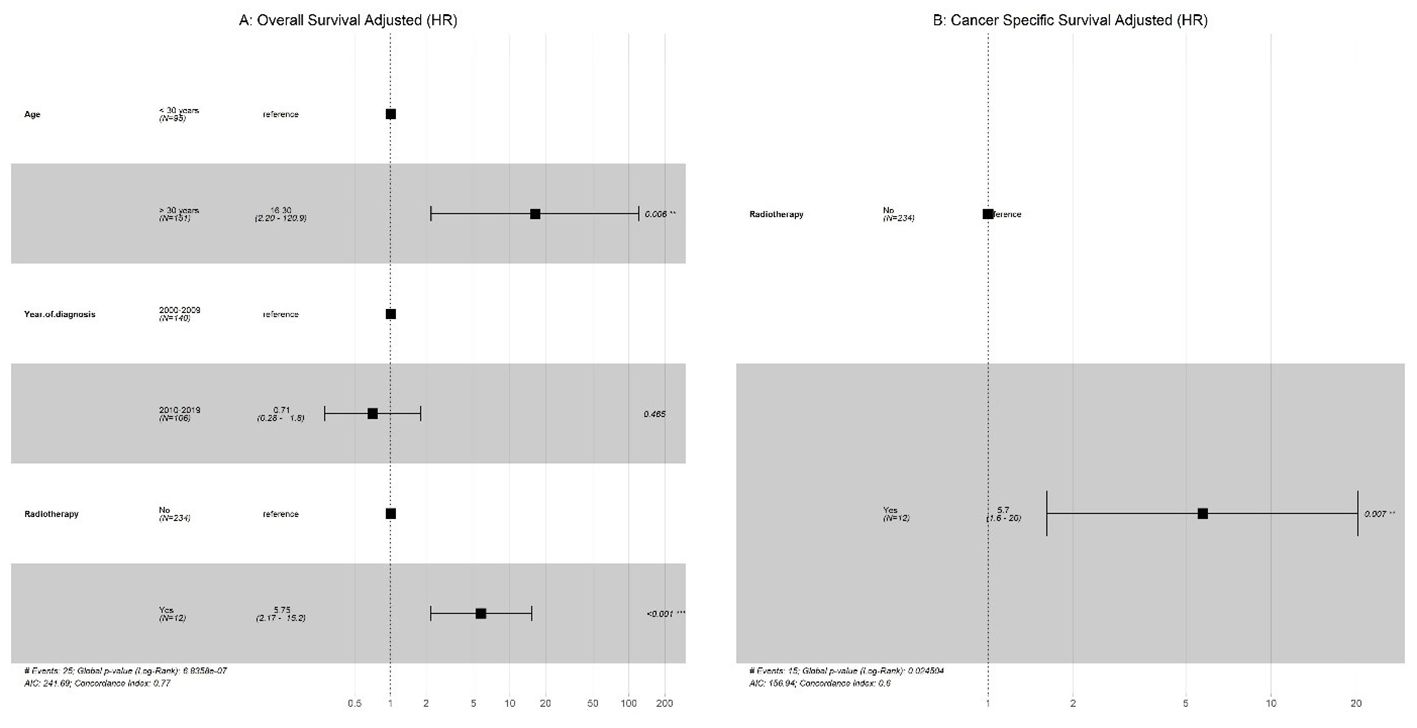
Figure 4. PTSS + EST: (A) Multivariate Cox regression for overall survival; (B) Multivariate Cox regression for cancer-specific survival (CSS).
3.4 Model performances and interpretability
The Detailed performance metrics for all machine learning algorithms (MLMs) are summarized in (Supplementary Table 3). ROC curves of all MLMs and the most contributing features in prediction in the RFC model are displayed in (Figure 5) for GCC and in (Figure 6) for PTSS and ETT. A web-based tool was developed using a random forest model that incorporated various clinical variables to predict the survival of patients diagnosed with GTN. Clinicians and researchers can input patient-specific data, such as age, sex, race, tumor stage, tumor size, treatment type, and histology, to estimate survival duration.
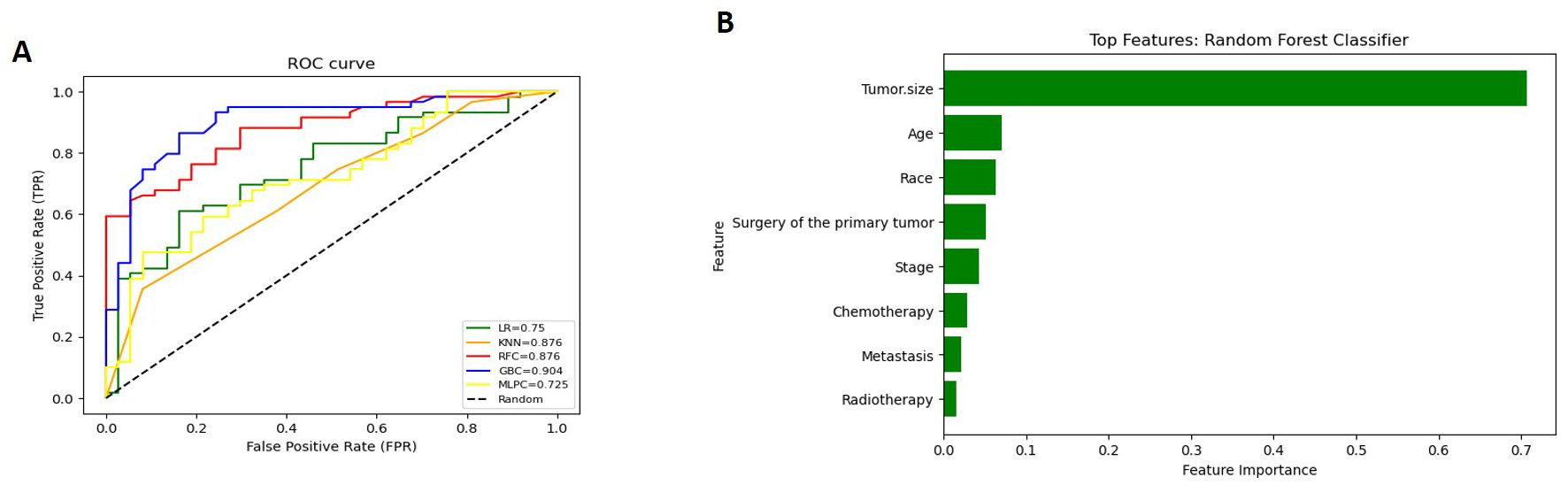
Figure 5. (for GCC): (A) Receiver Operating Characteristic (ROC) curves of all Machine Learning Models (MLMs); (B) Permutation Features Importance (Random Forest Classifier).
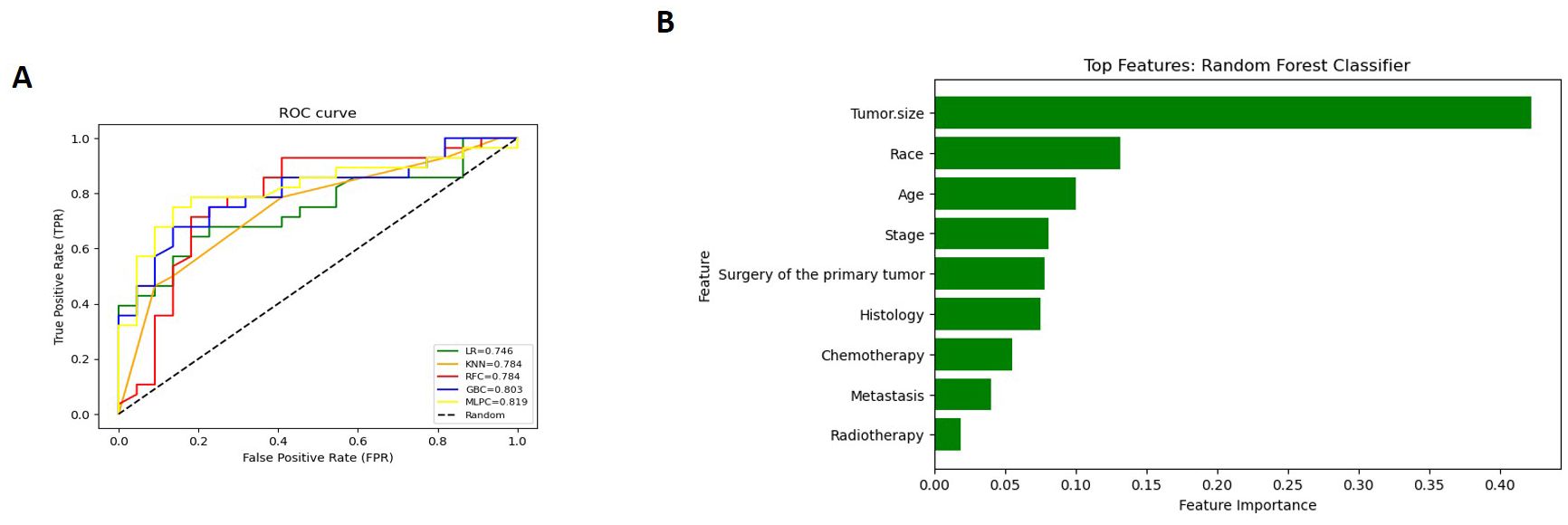
Figure 6. (for ETT and PTSS): (A) Receiver Operating Characteristic (ROC) curves of all Machine Learning Models (MLMs); (B) Permutation Features Importance (Random Forest Classifier).
The interactive tool, designed for ease of use and rapid calculation, is accessible through the following link: https://sakhrshwayyat.shinyapps.io/Gestational_Trophoblastic_Neoplasia/. For instance, upon entering patient data, the tool instantly predicts the expected survival months.
4 Discussion
PSTT and ETT are uncommon forms of GTN originating from intermediate trophoblast cells. Because their origin differs from other GTN types, it is expected that the clinical presentation of PSTT and ETT, their tumor marker profile, and their treatment approach also differ significantly (3). Our research examined various factors to determine their relationship with survival, and we were the first to develop AI prognostic models for GTN patients, which have proven to be highly accurate in predicting the survival of these patients.
Based on our research, being older (age 30 or above) is linked to a less favorable outcome in terms of both OS and CSS. This aligns with certain sources, indicating that advanced age is a risk factor for a poor prognosis in GTN (1, 13, 14). However, other studies have suggested that age does not significantly affect survival and does not necessarily result in poor outcomes (15, 16).
After reviewing the literature, a study conducted in the UK screened all PSTT/ETT diagnoses between 1973 and 2014. Its prevalence was 0.2%, with a mortality rate of 19% (14). In contrast, another study in China retrospectively analyzed 108 PSTT patients registered in two GTD centers or six tertiary hospitals between 1998 and 2013, revealing an incidence of 3% and a mortality rate of only 6.5% (1). The differences between these findings in China and the UK could be attributed to the Chinese study focusing solely on patients with PTSS, unlike the UK study, which included both PSTT and ETT cases. Additionally, differences in population genetics, potentially due to distinct racial backgrounds and variations in management approaches, might contribute to these differences (17). Although about 60% of the patients in our study were white, race did not significantly affect prognosis, as shown through our Cox regression analysis. However, ML models revealed that race was one of the significant factors affecting survival, indicating the need for further research.
In the literature, suggesting that tumor size is not a prognostic factor (6, 18). Nevertheless, conflicting viewpoints exist in a few studies that argue that tumor size is associated with a poorer prognosis (17, 19).
The Cox regression results of our study indicated that tumor size does not play a significant role in the OS and CSS of patients with ETT and PSTT. However, our ML models revealed that tumor size plays an important role in survival prediction, which may further reflect the superiority of ML. Specifically, unlike traditional linear regression analysis, which has the problem of overfitting, it can better eliminate unnecessary features. ML enables us to obtain more accurate predictive models by continuously improving operational efficiency and self-improvement.
Multiple studies have highlighted the significance of patient cancer staging (FIGO classification) as a crucial prognostic indicator (1, 13, 18, 20). One study conducted on patients with PSTT at a tertiary care center between 1996 and 2011 emphasized that stage IV disease stood out as a highly significant and clinically relevant predictor of poor prognosis (15). Another study, involving patients sourced from the International PSTT and ETT database, found that patients with FIGO stages II–IV exhibited worse outcomes than those with FIGO stage I disease, based on univariable analysis (18). However, our study lacked FIGO stage information but included SEER stage and metastasis data. In addition, our study results indicated that presence of metastasis disease significantly impacts survival months.
In our research, approximately 58% of the patients underwent surgery for the primary tumor. The usual indication for surgery in the early stages, often serving as the primary treatment in stage I, where the disease is less advanced and in its early phase. Numerous studies in the literature advocate surgery as the initial management for both ETT and PSTT (1, 18, 20). Several surgical procedures have been mentioned in the literature, including total abdominal hysterectomy, endometrial resection, excision of extrauterine lesions, pelvic lymph node dissections, curettage, and excision of uterine lesions (1, 20). While hysterectomy is commonly recommended, conservative surgery may be considered for young patients desiring fertility preservation (21). In premenopausal women, the standard practice involves preserving the ovaries unless the disease is evident or there is a family history of ovarian cancer (20). For other forms of GTN, surgical resection of residual masses does not offer any benefits (22). However, a study involving 62 patients with PTSS revealed the importance of resecting residual masses after detecting viable tumor cells in some patients undergoing mass resection. This was explained by the lower sensitivity of PSTT to chemotherapy (20).
PSTT and ETT have a slower cell growth rate than other types of GTN. Therefore, they do not respond as strongly to chemotherapy, distinguishing their treatment approach from other trophoblastic tumors (23). The results of our study indicated that chemotherapy did not significantly affect the prognosis or survival rates of patients diagnosed with PSTT and ETT. Similar studies reinforce this finding, suggesting that chemotherapy should only be considered for high-risk cases (such as advanced-stage or potential recurrence) (1, 13, 20, 24, 25). A specific study at the Women & Infants Hospital-Rhode Island Hospital revealed discouraging outcomes for five patients with PSTT who underwent chemotherapy. While some response was observed, it was short-lived, prompting the suggestion that chemotherapy should be reserved for recurrence after initial surgery, rather than as the first-line treatment (25). Another study showed poor clinical outcomes in patients treated with multiagent chemotherapy, with or without surgery, as 11 of them did not survive (18). However, for women diagnosed with metastatic PSTT/ETT, surgery alone is not curative, necessitating multiagent systemic chemotherapy (6). Initially thought to be unresponsive to chemotherapy, various reports have demonstrated the success of different multiagent chemotherapy approaches in treating metastatic disease (19, 26, 27). Since 75% of the patients included in our study took chemotherapy and the results showed that taking chemotherapy had no significant impact on survival, the idea of whether or not to give chemotherapy to future patients should be considered since chemotherapy treatment is related to many side effects and giving it can do more harm than good. One study outlined several adverse effects in gynecological cancer patients receiving chemotherapy, such as limb numbness, fatigue, hair loss, decreased appetite, taste alterations, muscle pain, nausea, vomiting, itching, or rash (28). Additionally, another study highlighted how cancer and chemotherapy can reduce a patient’s physical activity, alter their appearance, diminish their sense of attractiveness, and subsequently lower their self-esteem (29, 30). Factors such as multiple prior chemotherapy treatments and younger age were identified as predictors of reduced quality of life during chemotherapy in another study (31). Moreover, numerous studies have emphasized the impact of cancer treatment, particularly chemotherapy, on aspects like sexual desire, functioning, and emotional relationships in women (32, 33). Premature menopause resulting from these treatments has been associated with lower quality of life, decreased sexual functioning, distressing menopausal symptoms, psychological distress related to fertility concerns, and uncertainty about the long-term effects of premature menopause (33). Furthermore, a study involving breast cancer patients undergoing chemotherapy reported that 12.50% and 1.78% of participants experienced “moderate” and “severe” depression, respectively (34).
GCC is a rare but highly aggressive form of cancer originating in the trophoblastic cells of the placenta during pregnancy (35, 36). GCC is characterized by rapid growth and a high potential for metastasis, frequently spreading to organs such as the lungs (35, 37). Our study demonstrated that metastases significantly affect OS and CSS. Treatment for GCC typically involves multiagent chemotherapy (38, 39). Although surgery and radiotherapy are used less frequently, they may be necessary for cases of resistant or recurrent GCC (36–38). According to our univariate analysis, chemotherapy did not significantly affect the prognosis or survival rates of patients diagnosed with GCC. While surgery and radiotherapy were significant factors in OS and CSS in the univariate analysis, however, multivariate analysis indicated that surgery and radiotherapy did not significantly affect OS and CSS.
In our study, 31 patients underwent radiotherapy, revealing a negative impact on patient survival, indicating that radiotherapy is an unfavorable prognostic factor. This could be attributed to the physical and psychological side effects of radiation therapy, which substantially influence a patient’s daily functioning and diminish their overall quality of life (40). Yet, it’s important to highlight that one study indicated the potential effectiveness of radiotherapy when applied selectively (19), and another mentioned that two patients achieved complete remission due to pelvic radiotherapy (21).
This study had several limitations that should be considered. The absence of FIGO staging for GTN restricts the depth of the prognostic analysis. Additionally, the retrospective nature of this study introduces inherent limitations as it is not possible to establish causal relationships through retrospective analyses. The absence of specific chemotherapy regimens and surgical procedures limited the ability of this study to provide detailed insights into the efficacy of different therapeutic interventions. Additionally, the use of existing databases limited our ability to stratify indicators, such as the interval between surgery and diagnosis or the site of metastasis, and the rarity of GTN prevented model validation with actual hospital data.
However, this study had several strengths. The incorporation of ML techniques for predicting survival shows the growing potential of AI in healthcare and contributes to the development of personalized medicine. Moreover, this study adopted a comprehensive approach by incorporating a wide range of patient characteristics, tumor features, and treatment options, which provides a holistic understanding of the demographic and clinical aspects of GTN. Furthermore, a large sample size enhances the reliability of the analyses and comparisons, leading to more robust and generalizable results.
5 Conclusion
Our research on GTN sheds light on the crucial aspects of clinical decision making, emphasizing the significance of key factors affecting patient outcomes and offering hope for personalized treatment. The cautious use of chemotherapy and radiotherapy is needed because of their potential disadvantages. Further research should explore the molecular markers and validate the generalizability of our findings.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
Ethical approval was not required for the study involving humans in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and the institutional requirements.
Author contributions
SA: Conceptualization, Formal analysis, Writing – original draft, Writing – review & editing. ZH: Methodology, Writing – original draft, Writing – review & editing. MA: Formal analysis, Software, Writing – original draft, Writing – review & editing. TA: Methodology, Visualization, Writing – original draft, Writing – review & editing. RO: Visualization, Writing – original draft, Writing – review & editing. MA-K: Visualization, Writing – original draft, Writing – review & editing. SK: Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2024.1457531/full#supplementary-material
Abbreviations
PSTT, placental site trophoblastic tumor; ETT, epithelioid trophoblastic tumor; ML, machine learning; GCC, Gestational choriocarcinoma, The Surveillance, Epidemiology, and End Results (SEER); AUC, area under the curve; ROC, receiver operating characteristic; GBC, Gradient Boosting classifier; RFC, random forest classifier; KNN, K-Nearest Neighbors; LR, Logistic Regression; MLP, multilayer perceptron; GTNs, Gestational trophoblastic neoplasms; AI, Artificial intelligence; ICD-O, International Classification of Diseases for Oncology; OS, overall survival; CSS, cancer-specific survival.
References
1. Zhao J, Lv WG, Feng FZ, Wan XR, Liu JH, Yi XF, et al. Placental site trophoblastic tumor: A review of 108 cases and their implications for prognosis and treatment. Gynecol Oncol. (2016) 142:102–8. doi: 10.1016/j.ygyno.2016.05.006
2. Lee HJ, Shin W, Jang YJ, Choi CH, Lee JW, Bae DS, et al. Clinical characteristics and outcomes of placental site trophoblastic tumor: Experience of single institution in Korea. Obstet Gynecol Sci. (2018) 61:319–27. doi: 10.5468/ogs.2018.61.3.319
3. Horowitz NS, Goldstein DP, Berkowitz RS. Placental site trophoblastic tumors and epithelioid trophoblastic tumors: Biology, natural history, and treatment modalities. Gynecol Oncol. (2017) 144:208–14. doi: 10.1016/j.ygyno.2016.10.024
4. Lok C, Frijstein M, van Trommel N. Clinical presentation and diagnosis of Gestational Trophoblastic Disease. Best Pract Res Clin Obstet Gynaecol. (2021) 74:42–52. doi: 10.1016/j.bpobgyn.2020.12.001
5. Lucas R, Cunha TM, Santos FB. Placental site trophoblastic tumor: A case report and review of the literature. J Radiol Case Rep. (2015) 9:14–22. doi: 10.3941/jrcr.v9i4.2146
6. Gadducci A, Carinelli S, Guerrieri ME, Aletti GD. Placental site trophoblastic tumor and epithelioid trophoblastic tumor: Clinical and pathological features, prognostic variables and treatment strategy. Gynecol Oncol. (2019) 153:684–93. doi: 10.1016/j.ygyno.2019.03.011
7. McGregor SM, Furtado LV, Montag AG, Brooks R, Lastra RR. Epithelioid trophoblastic tumor: expanding the clinicopathologic spectrum of a rare Malignancy. Int J Gynecol Pathol. (2020) 39:8–18. doi: 10.1097/PGP.0000000000000563
8. Kurt S, Ilgen O, Ulukus EC, Yuzuguldu RI, Celiloglu M. Evaluation of epithelioid trophoblastic tumor in the light of literature. Arch Iran Med. (2021) 24:579–82. doi: 10.34172/aim.2021.81
9. Feng X, Wei Z, Zhang S, Du Y, Zhao H. A review on the pathogenesis and clinical management of placental site trophoblastic tumors. Front Oncol. (2019) 9:937. doi: 10.3389/fonc.2019.00937
10. Handelman GS, Kok HK, Chandra RV, Razavi AH, Lee MJ, Asadi H. eDoctor: machine learning and the future of medicine. J Intern Med. (2018) 284:603–19. doi: 10.1111/joim.12822
11. Deo RC. Machine learning in medicine. Circulation. (2015) 132:1920–30. doi: 10.1161/CIRCULATIONAHA.115.001593
12. Komura D, Ishikawa S. Machine learning approaches for pathologic diagnosis. Virchows Archiv. (2019) 475:131–8. doi: 10.1007/s00428-019-02594-w
13. Baergen RN, Rutgers JL, Young RH, Osann K, Scully RE. Placental site trophoblastic tumor: A study of 55 cases and review of the literature emphasizing factors of prognostic significance. Gynecol Oncol. (2006) 100:511–20. doi: 10.1016/j.ygyno.2005.08.058
14. Froeling FEM, Ramaswami R, Papanastasopoulos P, Kaur B, Sebire NJ, Short D, et al. Intensified therapies improve survival and identification of novel prognostic factors for placental-site and epithelioid trophoblastic tumours. Br J Cancer. (2019) 120:587–94. doi: 10.1038/s41416-019-0402-0
15. Hyman DM, Bakios L, Gualtiere G, Carr C, Grisham RN, Makker V, et al. Placental site trophoblastic tumor: analysis of presentation, treatment, and outcome. Gynecol Oncol. (2013) 129:58–62. doi: 10.1016/j.ygyno.2012.12.029
16. Chang YL, Chang TC, Hsueh S, Huang KG, Wang PN, Liu HP, et al. Prognostic factors and treatment for placental site trophoblastic tumor-report of 3 cases and analysis of 88 cases. Gynecol Oncol. (1999) 73:216–22. doi: 10.1006/gyno.1999.5344
17. Hancock BW, Tidy J. Placental site trophoblastic tumour and epithelioid trophoblastic tumour. Best Pract Res Clin Obstet Gynaecol. (2021) 74:131–48. doi: 10.1016/j.bpobgyn.2020.10.004
18. Frijstein MM, Lok CAR, van Trommel NE, ten Kate-Booij MJ, Massuger LFAG, van Werkhoven E, et al. Management and prognostic factors of epithelioid trophoblastic tumors: Results from the International Society for the Study of Trophoblastic Diseases database. Gynecol Oncol. (2019) 152:361–7. doi: 10.1016/j.ygyno.2018.11.015
19. Feltmate CM, Genest DR, Wise L, Bernstein MR, Goldstein DP, Berkowitz RS. Placental site trophoblastic tumor: A 17-year experience at the New England Trophoblastic Disease Center. Gynecol Oncol. (2001) 82:415–9. doi: 10.1006/gyno.2001.6265
20. Schmid P, Nagai Y, Agarwal R, Hancock B, Savage PM, Sebire NJ, et al. Prognostic markers and long-term outcome of placental-site trophoblastic tumours: a retrospective observational study. Lancet. (2009) 374:48–55. doi: 10.1016/S0140-6736(09)60618-8
21. How J, Scurry J, Grant P, Sapountzis K, Ostor A, Fortune D, et al. Placental site trophoblastic tumor. Report of three cases and review of the literature. Int J Gynecol Cancer. (1995) 5:241–9. doi: 10.1046/j.1525-1438.1995.05040241.x
22. Powles T, Savage P, Short D, Young A, Pappin C, Seckl MJ. Residual lung lesions after completion of chemotherapy for gestational trophoblastic neoplasia: should we operate? Br J Cancer. (2006) 94:51–4. doi: 10.1038/sj.bjc.6602899
23. Braga A, Paiva G, Cattai CJ, Elias KM, Horowitz NS, Berkowitz RS. Current chemotherapeutic options for the treatment of gestational trophoblastic disease. Expert Opin Pharmacother. (2023) 24:245–58. doi: 10.1080/14656566.2022.2150075
24. Gloor E, Dialdas J, Hurlimann J, Ribolzi J, Barrelet L. Placental site trophoblastic tumor (trophoblastic pseudotumor) of the uterus with metastases and fatal outcome. Clinical and autopsy observations of a case. Am J Surg Pathol. (1983) 7:483–6. doi: 10.1097/00000478-198307000-00011
25. Lathrop JC, Lauchlan S, Nayak R, Ambler M. Clinical characteristics of placental site trophoblastic tumor (PSTT). Gynecol Oncol. (1988) 31:32–42. doi: 10.1016/0090-8258(88)90266-1
26. Randall TC, Coukos G, Wheeler JE, Rubin SC. Prolonged remission of recurrent, metastatic placental site trophoblastic tumor after chemotherapy. Gynecol Oncol. (2000) 76:115–7. doi: 10.1006/gyno.1999.5654
27. King LA, Okagaki T, Twiggs LB. Resolution of pulmonary metastases with chemotherapy in a patient with placental site trophoblastic tumor. Int J Gynecol Cancer. (1992) 2:328–31. doi: 10.1046/j.1525-1438.1992.02060328.x
28. Hsu H-C, Tsai S-Y, Wu S-L, Jeang S-R, Ho M-Y, Liou W-S, et al. Longitudinal perceptions of the side effects of chemotherapy in patients with gynecological cancer. Support Care Cancer. (2017) 25:3457–64. doi: 10.1007/s00520-017-3768-7
29. Lewandowska A, Rudzki G, Lewandowski T, Próchnicki M, Rudzki S, Laskowska B, et al. Quality of life of cancer patients treated with chemotherapy. Int J Environ Res Public Health. (2020) 17. doi: 10.3390/ijerph17196938
30. Gujral S, Conroy T, Fleissner C, Sezer O, King PM, Avery KNL, et al. Assessing quality of life in patients with colorectal cancer: an update of the EORTC quality of life questionnaire. Eur J Cancer. (2007) 43:1564–73. doi: 10.1016/j.ejca.2007.04.005
31. Le T, Leis A, Pahwa P, Wright K, Ali K, Reeder B, et al. Quality of life evaluations in patients with ovarian cancer during chemotherapy treatment. Gynecol Oncol. (2004) 92:839–44. doi: 10.1016/j.ygyno.2003.11.034
32. Fobair P, Spiegel D. Concerns about sexuality after breast cancer. Cancer J. (2009) 15:19–26. doi: 10.1097/PPO.0b013e31819587bb
33. Knobf MT. The influence of endocrine effects of adjuvant therapy on quality of life outcomes in younger breast cancer survivors. Oncologist. (2006) 11:96–110. doi: 10.1634/theoncologist.11-2-96
34. de Souza BF, de Moraes JA, Inocenti A, dos Santos MA, de Camargo Silva AEB, Miasso AI. Women with breast cancer taking chemotherapy: depression symptoms and treatment adherence. Rev Lat Am Enfermagem. (2014) 22:866–73. doi: 10.1590/0104-1169.3564.2491
35. Bogani G, Ray-Coquard I, Mutch D, Vergote I, Ramirez PT, Prat J, et al. Gestational choriocarcinoma. Int J Gynecol Cancer. (2023) 33:1504–14. doi: 10.1136/ijgc-2023-004704
36. Joyce CM, Fitzgerald B, McCarthy TV, Coulter J, O’Donoghue K. Advances in the diagnosis and early management of gestational trophoblastic disease. BMJ Med. (2022) 1:e000321. doi: 10.1136/bmjmed-2022-000321
37. Ngan HYS, Seckl MJ, Berkowitz RS, Xiang Y, Golfier F, Sekharan PK, et al. Diagnosis and management of gestational trophoblastic disease: 2021 update. Int J Gynecol Obstet. (2021) 155:86–93. doi: 10.1002/ijgo.13877
38. Clark JJ, Slater S, Seckl MJ. Treatment of gestational trophoblastic disease in the 2020s. Curr Opin Obstet Gynecol. (2021) 33:7–12. doi: 10.1097/GCO.0000000000000674
39. Blagden SP, Foskett MA, Fisher RA, Short D, Fuller S, Newlands ES, et al. The effect of early pregnancy following chemotherapy on disease relapse and foetal outcome in women treated for gestational trophoblastic tumours. Br J Cancer. (2002) 86:26–30. doi: 10.1038/sj.bjc.6600041
40. Khurshid F, Razzaq AA, Khurshid H. Psychological side effects of radiation therapy on cancer patients(2023). Available online at: https://j.stmu.edu.pk (Accessed May 20, 2024).
Keywords: clinical decision making, machine learning, placenta, prognosis, survival analysis, treatment outcome, trophoblastic tumor
Citation: Alshwayyat S, Haddadin Z, Alshwayyat M, Alshwayyat TA, Odat RM, Al-kurdi MA-m and Kharmoum S (2024) Machine learning-driven survival prediction in gestational trophoblastic neoplasms: a focus on PSTT and ETT prognosis. Front. Oncol. 14:1457531. doi: 10.3389/fonc.2024.1457531
Received: 30 June 2024; Accepted: 09 September 2024;
Published: 30 September 2024.
Edited by:
Jian-Jun Wei, Northwestern University, United StatesReviewed by:
Komsun Suwannarurk, Thammasat University, ThailandShuzhen Wang, Capital Medical University, China
Copyright © 2024 Alshwayyat, Haddadin, Alshwayyat, Alshwayyat, Odat, Al-kurdi and Kharmoum. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mohammed Al-mahdi Al-kurdi, bW9oYW1tZWRtYWhkaWt1cmRpQGdtYWlsLmNvbQ==
 Sakhr Alshwayyat1
Sakhr Alshwayyat1 Ramez M. Odat
Ramez M. Odat Mohammed Al-mahdi Al-kurdi
Mohammed Al-mahdi Al-kurdi