- 1Hematology Oncology Department, the Southern Central Hospital of Yunnan Province, Honghe, Yunnan, China
- 2Geriatric Oncology Department, the Third Affiliated Hospital of Kunming Medical University, Kunming, Yunnan, China
- 3Department of Oncology, the Pingxiang People’s Hospital, Pingxiang, Jiangxi, China
- 4Department of Oncology, The First Hospital of Nanchang, Nangchang, Jiangxi, China
- 5Department of Spinal Surgery, Southern Central Hospital of Yunnan Province, Honghe, China
Background: Most epithelial ovarian cancer (EOC) eventually develops recurrence. Identification of high-risk patients can prompt earlier intervention and improve long-term outcomes. We used laboratory and clinical data to create models based on machine learning for EOC platinum resistance recurrence identification.
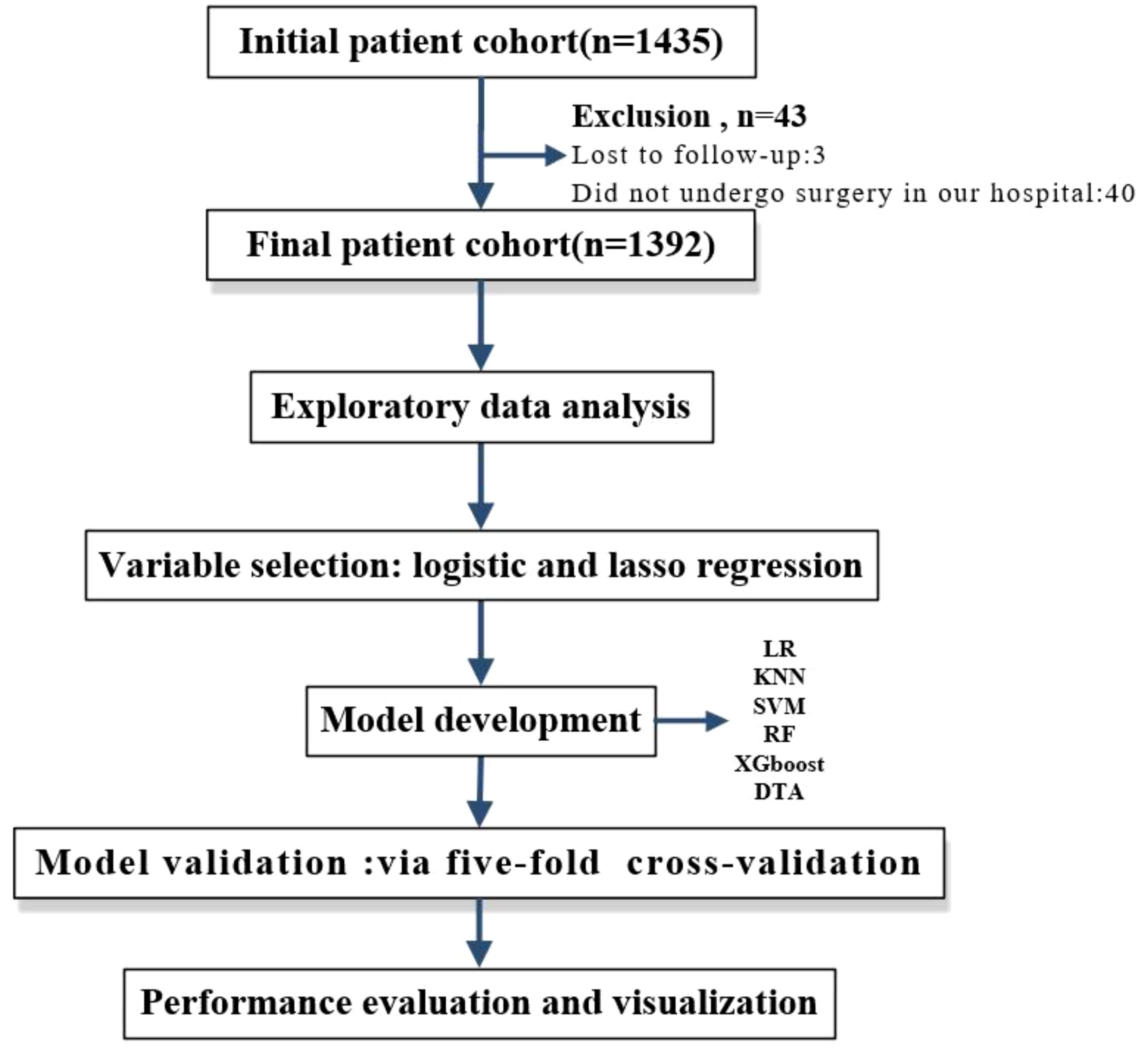
Methods: This study was designed as a retrospective cohort analysis. Initially, we identified 1,392 patients diagnosed with epithelial ovarian cancer who underwent platinum-based chemotherapy at Yunnan Cancer Hospital between January 1, 2012, and June 30, 2022. We collected data on the patients’ clinicopathologic characteristics, routine laboratory results, surgical information, details of chemotherapy regimens, and survival outcomes. Subsequently, to identify relevant variables influencing the recurrence of platinum resistance, we screened thirty potential factors using two distinct variable selection methods: Lasso regression and multiple logistic regression analysis. Following this screening process, five machine learning algorithms were employed to develop predictive models based on the selected variables. These included decision tree analysis (DTA), K-Nearest neighbor (KNN), support vector machine (SVM), random forest (RF), and eXtreme gradient boosting (XGBoost). The performance of these models was compared against that of traditional logistic regression. To ensure robust internal validation and facilitate comparison among model performance metrics, a five-fold cross-validation method was implemented. Key performance indicators for the models included the area under the receiver operating characteristic curve (AUC), sensitivity, specificity, and average accuracy. Finally, we will visualize these models through nomograms, decision tree diagrams, variable importance plots, etc., to assist clinicians in their practice.
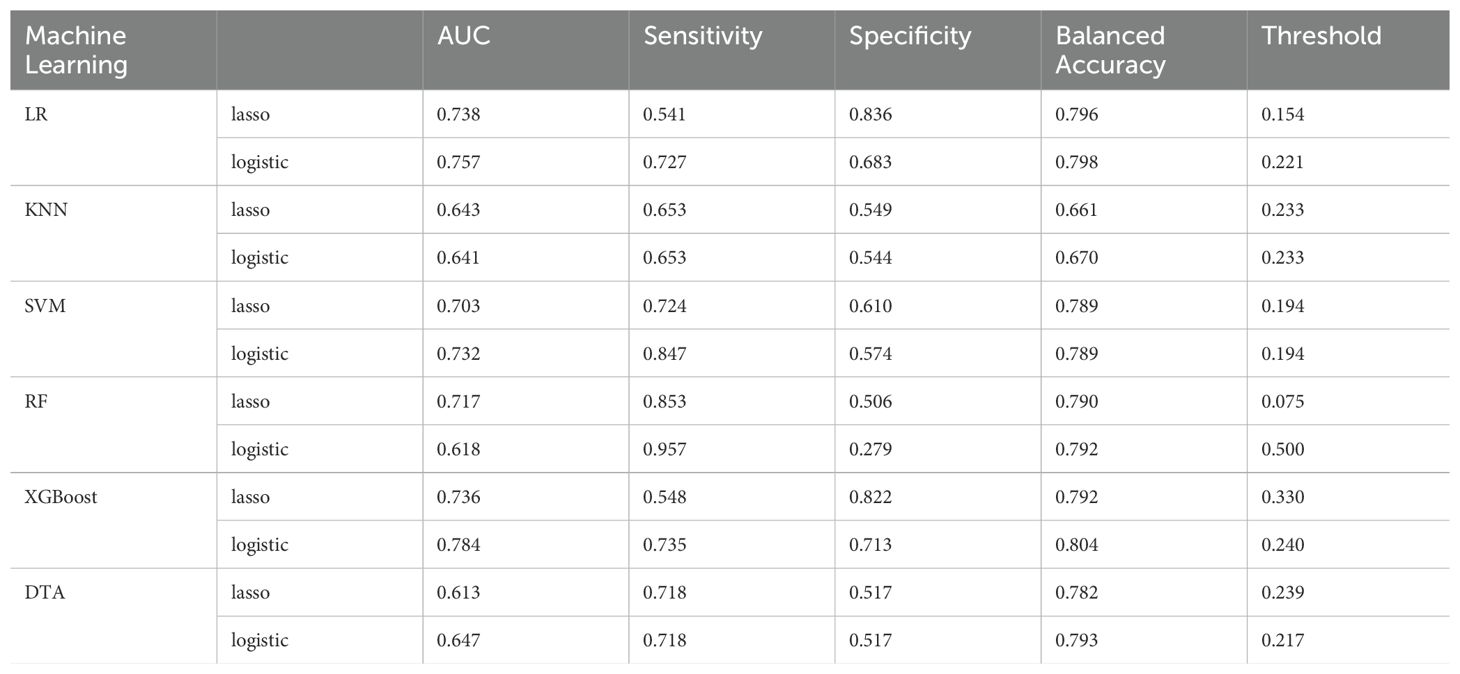
Results: Multiple logistic regression analysis identified eight variables associated with platinum resistance recurrence. In the lasso regression, seven variables were selected. Based on the findings from both Lasso regression and multiple logistic regression analysis, models were developed using these 7 and 8 factors. Among these, the XGBoost model derived from multiple logistic regression exhibited superior performance and demonstrated good discrimination during internal validation, achieving an AUC of 0.784, a sensitivity of 0.735, a specificity of 0.713, an average accuracy of 80.4%, with a cut-off value set at 0.240. Conversely, the LR model based on lasso regression yielded commendable results as well; it achieved an AUC of 0.738, a sensitivity of 0.541, a specificity of 0.836, with a cut-off value established at 0.154 and an accuracy rate of 79.6%. Finally, we visualized both models through nomograms to illustrate the significance of each variable involved in their development.
Conclusions: We have successfully developed predictive models for platinum-resistant recurrence of epithelial ovarian cancer, utilizing routine clinical and laboratory data. Among these models, the XGBoost model—derived from variables selected through multiple logistic regression—demonstrated the best performance. It exhibited high AUC values and average accuracy during internal validation, making it a recommended tool for clinical use. However, due to variations in time and context, influencing factors may change over time; thus, continuous evolution of the model is necessary. We propose a framework for this ongoing model adaptation.
1 Background
Seventy percent of patients diagnosed with epithelial ovarian cancer (EOC) present at an advanced stage (Federation of International of Gynecologists and Obstetricians (FIGO) stages III and IV) (1–3) The standard treatment approach involves primary debulking surgery (PDS), aimed a t achieving no visible residual tumor, followed by adjuvant chemotherapy based on platinum and paclitaxel (4, 5). A significant proportion of patients can attain complete remission. However, approximately 75% of those with advanced-stage disease will ultimately experience a relapse, resulting in poor survival outcomes (6, 7).
Following first-line treatment, around 15% of patients exhibit platinum-resistant recurrence; conversely, many remain platinum-sensitive at the time of their initial recurrence. Nevertheless, after undergoing multiple relapses, most cases of advanced ovarian cancer inevitably progress to a state that is resistant to platinum-based therapies.
Treatment options for patients experiencing platinum-resistant recurrence are currently quite limited. Existing guidelines primarily advocate for non-platinum monotherapy in the management of platinum-resistant ovarian cancer. Although recent guidelines have introduced new combinations—such as oral cyclophosphamide combined with pembrolizumab and bevacizumab, fam-trastuzumab deruxtecan-nxki, and Mirevtuxiamab Soratansine plus bevacizumab—the overall efficacy for treating platinum-resistant ovarian cancer remains suboptimal (8). Effective methods or drugs capable of truly reversing resistance are scarce. The prognosis for patients with platinum-resistant recurrence is poor, characterized by a progression-free survival (PFS) time of only 3 to 4 months and a response rate to chemotherapy of less than 15%. The median survival duration is reported to be under 12 months (9).
Platinum-resistant recurrence is currently defined as ovarian cancer that responds to initial chemotherapy but progresses or relapses within six months following the completion of treatment. Beyond this six-month period, it is unlikely that patients will exhibit significant symptoms or signs indicative of recurrence. Recurrence is primarily assessed through imaging examinations and monitoring serum levels of Carbohydrate Antigen 125 (CA125). This necessitates waiting for disease progression before determining whether a patient has experienced a platinum-resistant relapse. If we can identify platinum-resistant recurrences early on, patients likely to be resistant may consider undergoing platinum-based treatments either as monotherapy or in combination with other agents during first-line therapy. For instance, the antiangiogenic agent bevacizumab can be effectively combined with poly (ADP-ribose) polymerase (PARP) inhibitors such as Olaparib as part of maintenance therapy, alongside appropriate applications involving cell cycle regulators (10–12). The initial chemotherapy cycle may be appropriately intensified, or conventional intraperitoneal perfusion chemotherapy should be considered to extend the platinum-free interval (PFI) and convert potential platinum-resistant patients into those who are platinum-sensitive. Furthermore, clinicians can modify the follow-up plan for patients to enable more rigorous surveillance for recurrence. Early detection of platinum-resistant recurrence empowers clinicians to reassess treatment strategies and individualize follow-up plans, thereby enhancing the long-term prognosis of ovarian cancer.
However, accurately predicting the recurrence of platinum resistance remains a significant challenge. Most risk models have been developed primarily to forecast
PFS and overall survival (OS) in ovarian cancer (13–15). Previous models assessing platinum sensitivity were constructed using logistic regression (LR), a conventional statistical approach (16). The performance of these models tends to decline when applied to populations outside the original study cohort. With the advent of machine learning and its expanding applications, researchers are increasingly exploring the use of artificial intelligence within the medical domain (17, 18). To enhance long-term prognostic outcomes for patients with EOC, we aim to utilize routine clinical and laboratory data to develop machine learning models that predict the recurrence of platinum resistance in EOC patients.
2 Methods
2.1 Study population
Participants included patients with EOC who received first-line treatment at Yunnan Cancer Hospital between January 1, 2012, and June 30, 2022. The following inclusion and exclusion criteria were established:
2.1.1 Inclusion criteria
1. Surgical procedures were performed at our hospital, with a pathological diagnosis of EOC;
2. Administration of platinum-based first-line chemotherapy;
3. Availability of demographic information, clinical data, and follow-up records.
2.1.2 Exclusion criteria
1. Patients who did not receive platinum-based neoadjuvant chemotherapy or platinum-based first-line chemotherapy;
2. Presence of multiple primary malignant tumors;
3. Undergoing other treatments such as maintenance therapy involving bevacizumab or PARP inhibitors;
4. Loss to follow-up prior to six months post-treatment initiation;
5. Enrollment period less than six months without recurrence occurring;
6. Diagnosis of severe infectious diseases or mental disorders.
2.2 Data collection
After reviewing the existing literature and consulting with experts, we identified the variables to be collected (16, 19–22). In accordance with the Transparent Reporting of a Multivariable Prediction Model for Individual Prognosis or Diagnosis statement (23), it was established that the number of outcome events in the development cohort should be at least ten times greater than the number of variables. Furthermore, we stipulated that each variable must have an event count exceeding ten; otherwise, it would be excluded from analysis. Concurrently, any missing values were addressed by removing cases where a variable was absent in more than 30% of patients.
Ultimately, we compiled a total of 30 variables encompassing sociodemographic characteristics, surgical records, chemotherapy-related information, routine laboratory tests—including complete blood count (CBC) values—as well as renal and liver function indicators and other chemotherapy-related metrics (see Table 1). Laboratory data such as CA125 levels, CBC results, and lactate dehydrogenase (LDH) serum concentrations were obtained within one week prior to the commencement of surgery. All relevant data were extracted from pathology reports and medical records.
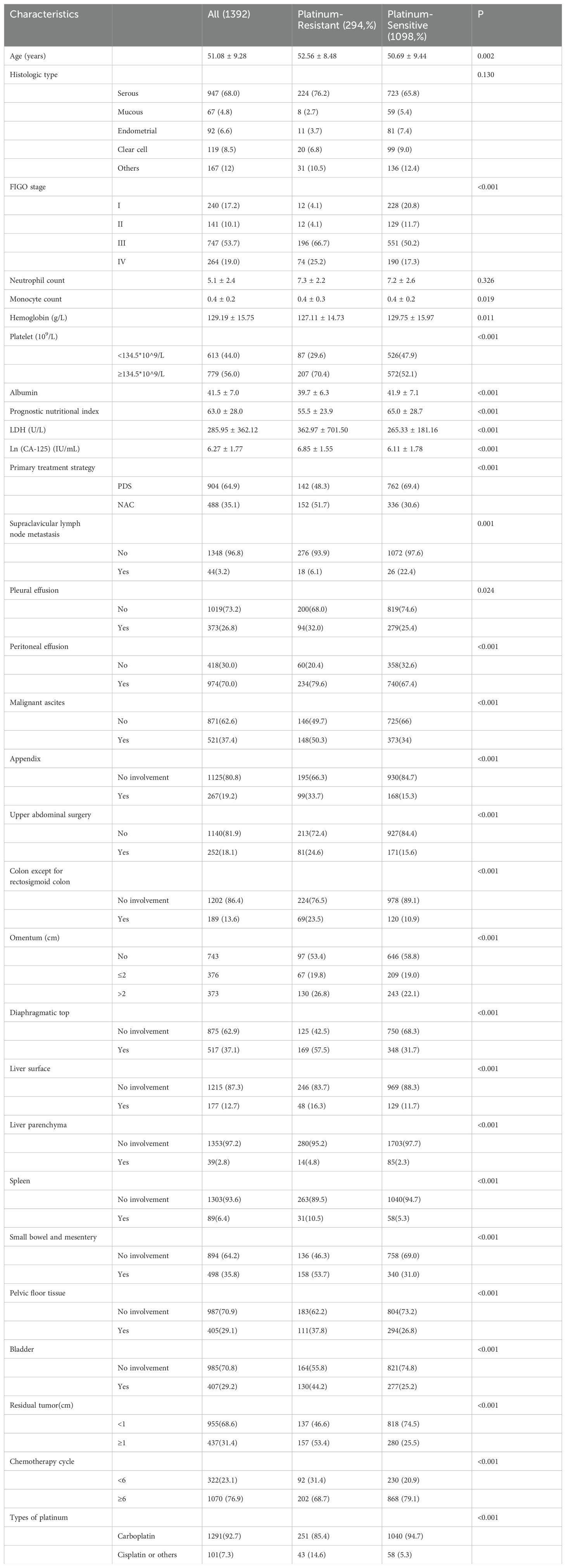
Table 1. Clinicopathologic characteristics between platinum-resistant group and platinum- sensitive group.
This study conducted follow-up visits every two cycles of chemotherapy through clinical assessments and radiological evaluations. The follow-up period spanned from April 2012 to December 2022.
Hydrothorax and ascites were defined as the presence of any pleural effusion or pelvic fluid detected via ultrasound. In patients with measurable tumors, recurrence was determined using the Response Evaluation Criteria in Solid Tumors (RECIST) based on CT scans. Recurrence was indicated by an increase of at least 20% in the sum of the maximum diameters of tumor lesions, along with an absolute increase of at least 5 mm, or by the emergence of new tumor lesions. For cases where cancer could not be measured, tumor recurrence was evaluated through serum CA125 levels: specifically, if a patient’s serum CA125 level exceeded the upper limit of the reference range on two separate occasions at least one week apart.
According to statements from the Gynecologic Cancer Oncology Group (CGOG), The time interval between completion of platinum-based chemotherapy and disease progression is referred to as the platinum-free interval (9). Platinum resistance was classified as occurring when PFI was less than six months. Conversely, patients with a PFI equal to or greater than six months—regardless of disease recurrence status—were categorized as part of a platinum-sensitive cohort (24).
This research received approval from the Ethics Committee of Yunnan Cancer Hospital.
2.3 Data analysis
An exploratory analysis was performed. Skewed distribution factors, such as serum CA-125 levels, were ln-transformed to address the skewness in the data. The distribution of continuous variables was presented using mean and standard deviation. The maximum Youden index was employed to identify the optimal cut-off point for continuous variables. For binary, continuous, and ranked data, we utilized the chi-square test, Student’s t-test, and Wilcoxon rank-sum test respectively.
2.4 Variable selection and model development
To select appropriate variables, we employed two methods for variable screening. Firstly, a univariate and multiple logistic regression analysis based on Akaike’s Information Criterion was utilized to identify variables predictive of platinum resistance. The second method involved lasso regression, which effectively eliminates factors with low contributions to the model’s predictive ability among highly collinear variables, thereby achieving the goal of reducing the number of variables.
In this study, five types of supervised machine learning classifiers were used to build models: decision tree analysis (DTA), support vector machine (SVM) (24, 25), K-Nearest neighbor (KNN), random forest (RF), and eXtreme gradient boosting (XGBoost). The traditional development method of LR served as the baseline for comparison. Performance indicators for the models included AUC, sensitivity, specificity, and average accuracy. Five-fold cross-validation was implemented to compare the performance across these six models (Figure 1). Exploratory data analysis was conducted using IBM SPSS Statistics software while all other statistical analyses were performed using R version 4.2.1. A p-value of less than 0.05 was considered statistically significant.
3 Results
3.1 Baseline information
A total of 1,435 patients were enrolled in the study; however, 40 patients did not undergo surgery at our hospital. The longest follow-up period was 118 months, while the shortest was 0 months, resulting in a median follow-up time of 13 months. Three cases were lost to follow-up (loss rate: 0.2%), leaving a final cohort of 1,392 patients for analysis. Among these, 294 patients (21.1%) experienced recurrence with platinum resistance (platinum-resistant group), whereas the remaining 1,098 patients were classified as platinum-sensitive.
Overall, significant differences between the two groups were observed concerning hemoglobin levels, platelet counts, and albumin concentrations (Table 1). In terms of primary treatment modalities, 904 patients (64.9%) underwent PDS, while 488 patients (35.1%) received interval debulking surgery following neoadjuvant chemotherapy (NAC). Notably, the platinum-sensitive group was younger on average compared to the platinum-resistant group (mean age: 50.69 vs. 52.56 years; p =0.002) and exhibited a higher likelihood of being in FIGO stages I-II.
Additionally, serum CA-125 levels were lower in the platinum-sensitive group compared to their resistant counterparts (mean: 2.65 vs. 2.97 IU/mL; p<0.001), as well as a reduced proportion receiving NAC treatment (30.6% vs.51.7%; p<0.001). Post-surgery outcomes indicated that complete cytoreduction rates in the platinum-sensitive group surpassed those seen in the platinum-resistant group significantly (74.5% vs.46.6%; P <0.001). While first-line chemotherapy regimens for both groups were comparable, the number of chemotherapy cycles administered to the platinum-resistant group was notably fewer than that given to their sensitive counterparts (p<0.001).
3.2 Model development
For patients whose pathological reports indicated adenocarcinoma without further specification to serous or mucinous types, the pathological classification was categorized as “other.” Consequently, the variable “histologic type” was excluded from the variable screening step.
Multivariate logistic regression analysis identified eight independent variables influencing platinum resistance recurrence: LDH levels, FIGO stage, platelet count, supraclavicular lymph node metastasis, primary treatment strategy, residual tumor size, type of platinum (carboplatin/cisplatin or others), and number of chemotherapy cycles (Table 2). Intuitively, involvement of the omentum and larger residual tumor size were associated with an increased risk of platinum-resistant recurrence; conversely, a greater number of chemotherapy cycles correlated with a reduced risk.
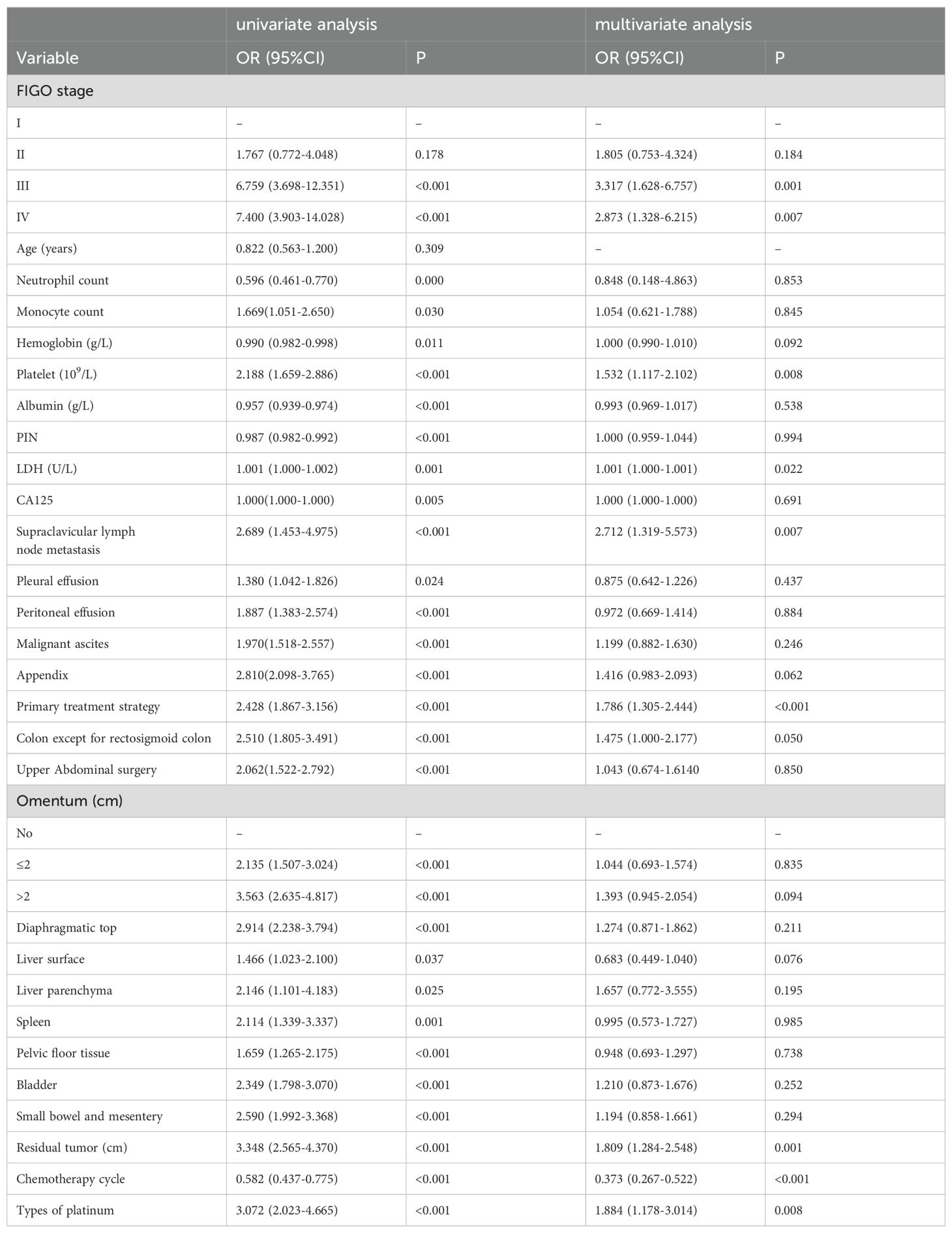
Table 2. Univariate and multivariate logistic regression analysis of independent risk factors affecting platinum resistance recurrence.
In the lasso regression analysis, when the parameter λ was set to 0.047430, the models achieved a balance between complexity and performance. A total of seven variables were selected: types of platinum, FIGO stage, primary treatment strategy, appendix involvement, diaphragmatic top status, residual tumor size, and omentum condition.
Subsequently, utilizing these two sets of variables, we developed prediction models for platinum-resistance recurrence employing five machine learning algorithms. These models were then compared with traditional model fitting methods such as LR. We utilized the area under the curve (AUC) and average accuracy obtained through cross-validation as comprehensive measures of model performance. Additionally, we calculated sensitivity and specificity metrics to further evaluate our models.
3.2.1 LR
3.2.1.1 Based on the results of multivariate Logistic regression
Based on the results of multivariate logistic regression, the LR model established from the multivariate analysis yielded an AUC of 0.757 and an average accuracy of 81.3% (Figure 2A). This model will henceforth be referred to as “Logistic-LR.” The formula for predicting the probability of platinum resistance recurrence using the Logistic-LR model is: P= -5.59721 + 0.27335*X1 + 0.49898*X2 + 0.35923*X3 + 0.67842*X4 + 0.60799*X5 + 0.94185*X6 -0.18319 *X7*+0.81598 X8, X1 to X8 represent LDH, platelet count, FIGO stage, primary treatment strategy, supraclavicular lymph node metastasis, residual tumor size, chemotherapy cycle, and types of platinum respectively.
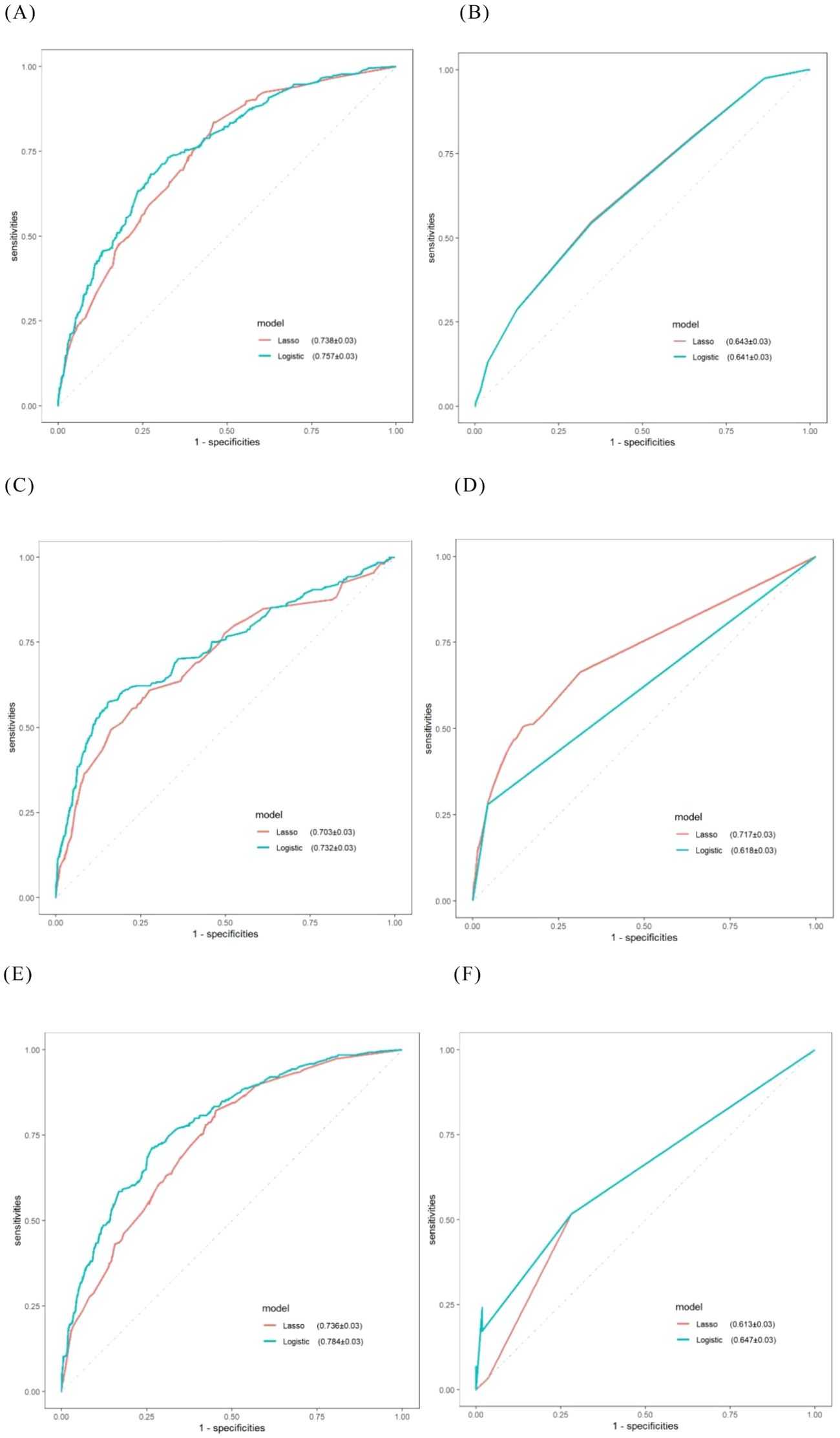
Figure 2. ROC curves for machine learning models obtained via internal validation. (A) LR; (B) KNN; (C) SVM; (D) RF; (E) XGBoost; (F) DTA.
3.2.1.2 Based on the results of lasso regression
The AUC for the LR model derived from lasso regression was found to be 0.738 (Figure 2A), with an average accuracy of 79.6%. This model will subsequently be referred to as “Lasso-LR.” Future models will follow this naming convention consistent with that used for LR models. The formula for predicting platinum resistance recurrence probability using the Lasso-LR model is: P= -4.8769 + 0.7401*X1 + 0.2255*X2 + 0.5815*X3 + 0.3846*X4 + 0.5730*X5 + 0.1573 *X6+ 0.5297 *X7, where X1 to X7 correspond to types of platinum, FIGO stage, primary treatment strategy, appendix, residual tumor size, diaphragmatic top and omentum respectively. Nomograms were constructed based on both LR models described above (Figure 3).
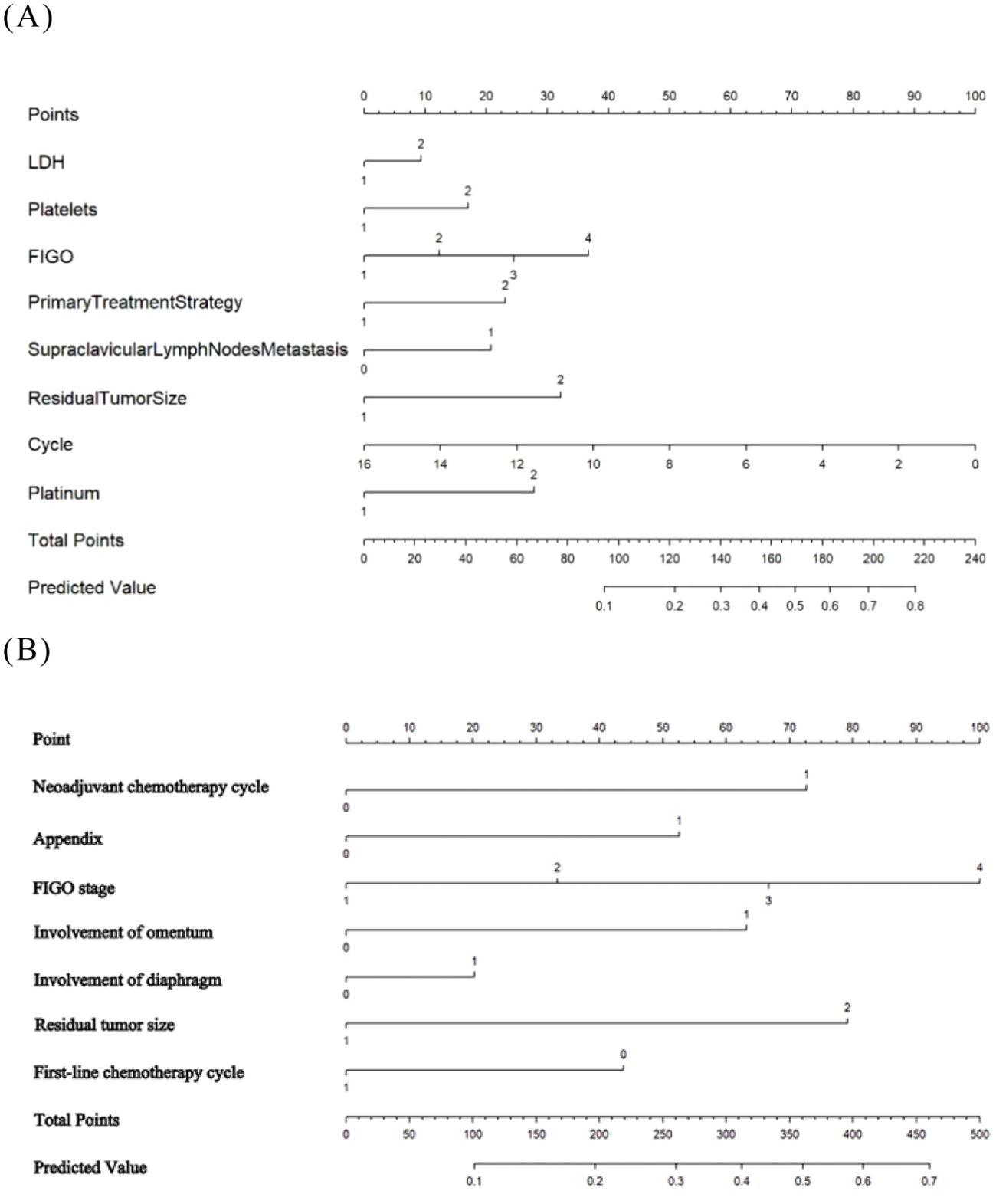
Figure 3. The developed nomogram predicting EOC platinum resistance recurrence. (A) The Logistic-LR model. (B) The Lasso- LR model.
3.2.2 KNN
3.2.2.1 Based on the results of multivariate Logistic regression
The AUC of the “Logistic-KNN” model was 0.641 (Figure 2B). The cut-off value was established at 0.233, yielding a sensitivity of 0.653, specificity of 0.544, and an average accuracy of 67.0%.
3.2.2.2 Based on the results of lasso regression
The AUC for the “Lasso-KNN” model was recorded at 0.643 (Figure 2B). At a cut-off value of 0.233, this model demonstrated a sensitivity of 0.653, specificity of 0.549, and an average accuracy of 66.1%.
3.2.3 SVM
When employing SVM to construct models, we explored various kernels including linear kernel, RBF kernel, polynomial kernel, and sigmoid kernel respectively. Our analysis revealed that the RBF kernel provided the best fit for the model and exhibited strong performance in validation; Thus we ultimately selected it for our modeling approach.
3.2.3.1 Based on the results of multivariate Logistic regression
The AUC for the “Logistic-SVM” model reached an impressive value of 0.732 (Figure 2C), with an average accuracy reported at 78.9%.
3.2.3.2 Based on the results of lasso regression
The AUC for the “Lasso-SVM” model was determined to be 0.703 (Figure 2C), also achieving an average accuracy of approximately 78.9%.
3.2.4 RF
3.2.4.1 Based on the results of multivariate Logistic regression
The AUC of the “Logistic-RF” model was 0.618 (Figure 2D). At a cut-off value of 0.225, the sensitivity was recorded at 0.957, while the specificity stood at 0.279, resulting in an average accuracy of 79.2%.
3.2.4.2 Based on the results of lasso regression
The AUC of the “Lasso-RF” model was 0.717 (Figure 2D), with an average accuracy of 79.0%. At a cut-off value of 0.075, the sensitivity was recorded at 0.853, while the specificity stood at 0.506.
To enhance the transparency of the model, we presented the ranking of variable importance (26). The “MeanDecreaseGini” metric indicated each variable’s contribution—whether positive or negative—to the risk of platinum resistance recurrence as defined by the model (Figures 4A, B).
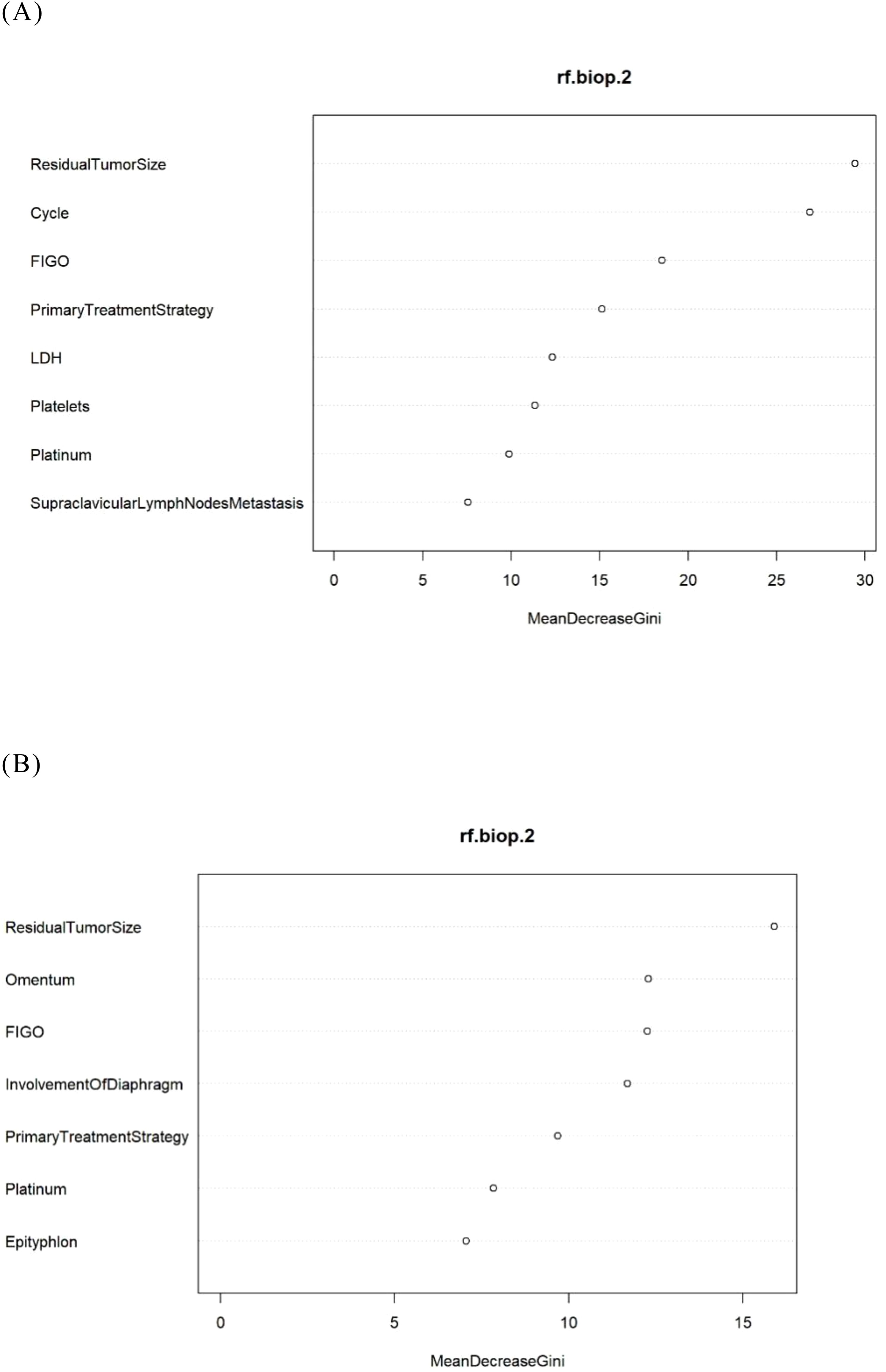
Figure 4. The feature importance in the RF model. (A) The Logistic-RF model. (B) The Lasso- RF model.
3.2.5 XGBoost
3.2.5.1 Based on the results of multivariate Logistic regression
The AUC for the “Logistic-XGBoost” model was 0.784 (see Figure 2E), with an average accuracy of 84.0%. At a cut-off value of 0.240, the sensitivity was recorded at 0.735 and the specificity at 0.713.
3.2.5.2 Based on the results of lasso regression
The AUC of the “Lasso-XGBoost” model was 0.736 (Figure 2E), with an average accuracy of 79.2%. At a cut-off value of 0.330, the sensitivity was recorded at 0.548 and the specificity at 0.822.
In addition, we visualized the importance ranking of variables within the XGBoost model (Figure 5). Notably, the three most significant variables for predicting platinum resistance recurrence in the Logistic-XGBoost model were identified as chemotherapy cycle, residual tumor size, and FIGO stage.
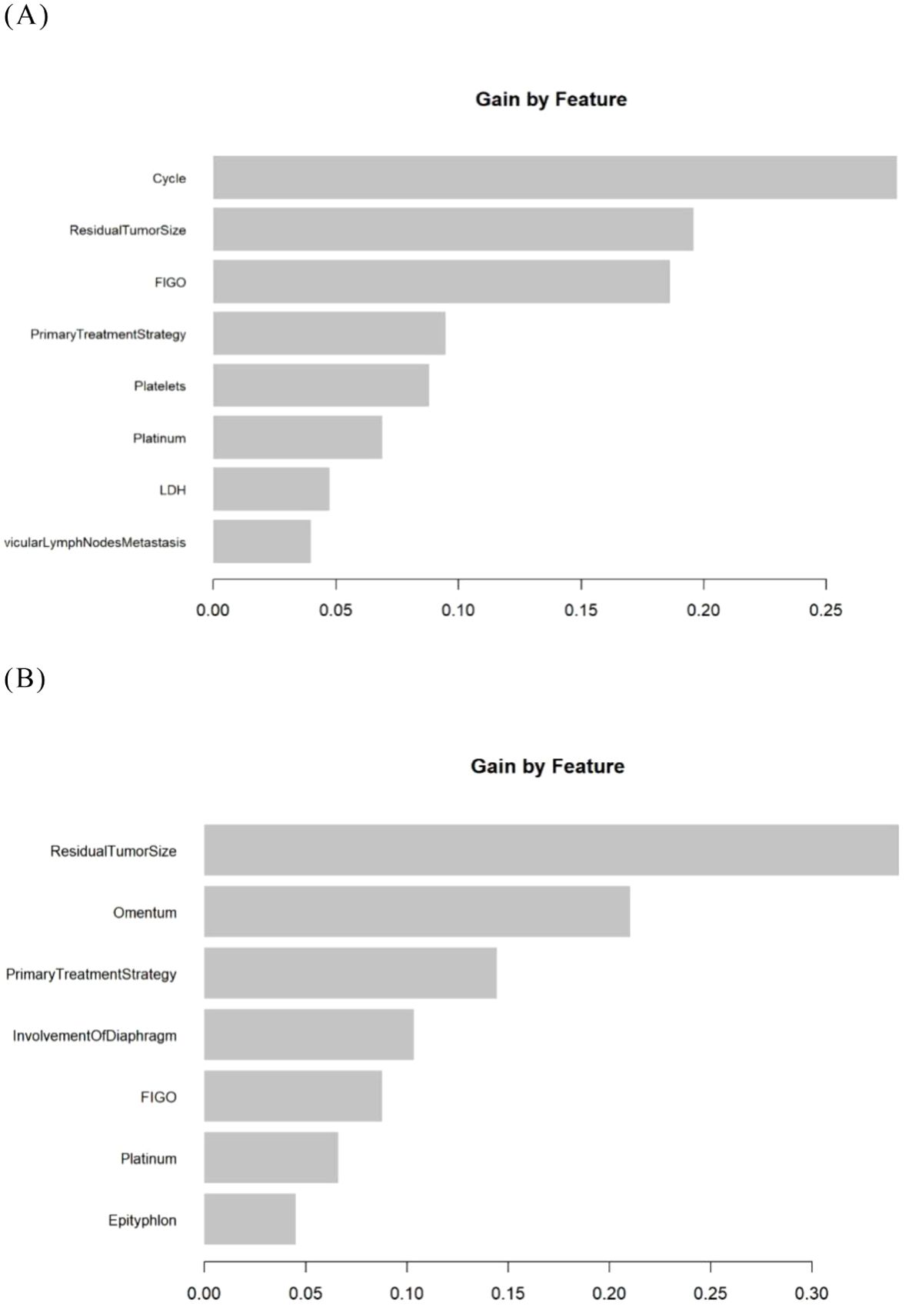
Figure 5. The importance ranking of variables in the XGBoost models. (A) The Logistic- XGBoost model. (B) The Lasso- XGBoost model.
3.2.6 DTA
3.2.6.1 Based on the results of multivariate Logistic regression
The goodness-of-fit plot indicates that the optimal complexity parameter (cp) value was 0.01 (Figure 6A). We set the cp value to 0.01 and optimized the model through pruning. Ultimately, the AUC of the “Logistic-DTA” model reached 0.647, with an average accuracy of 79.3%. At a cut-off value of 0.217, the sensitivity was found to be 0.718, while the specificity was recorded at 0.517 (Figure 2F).
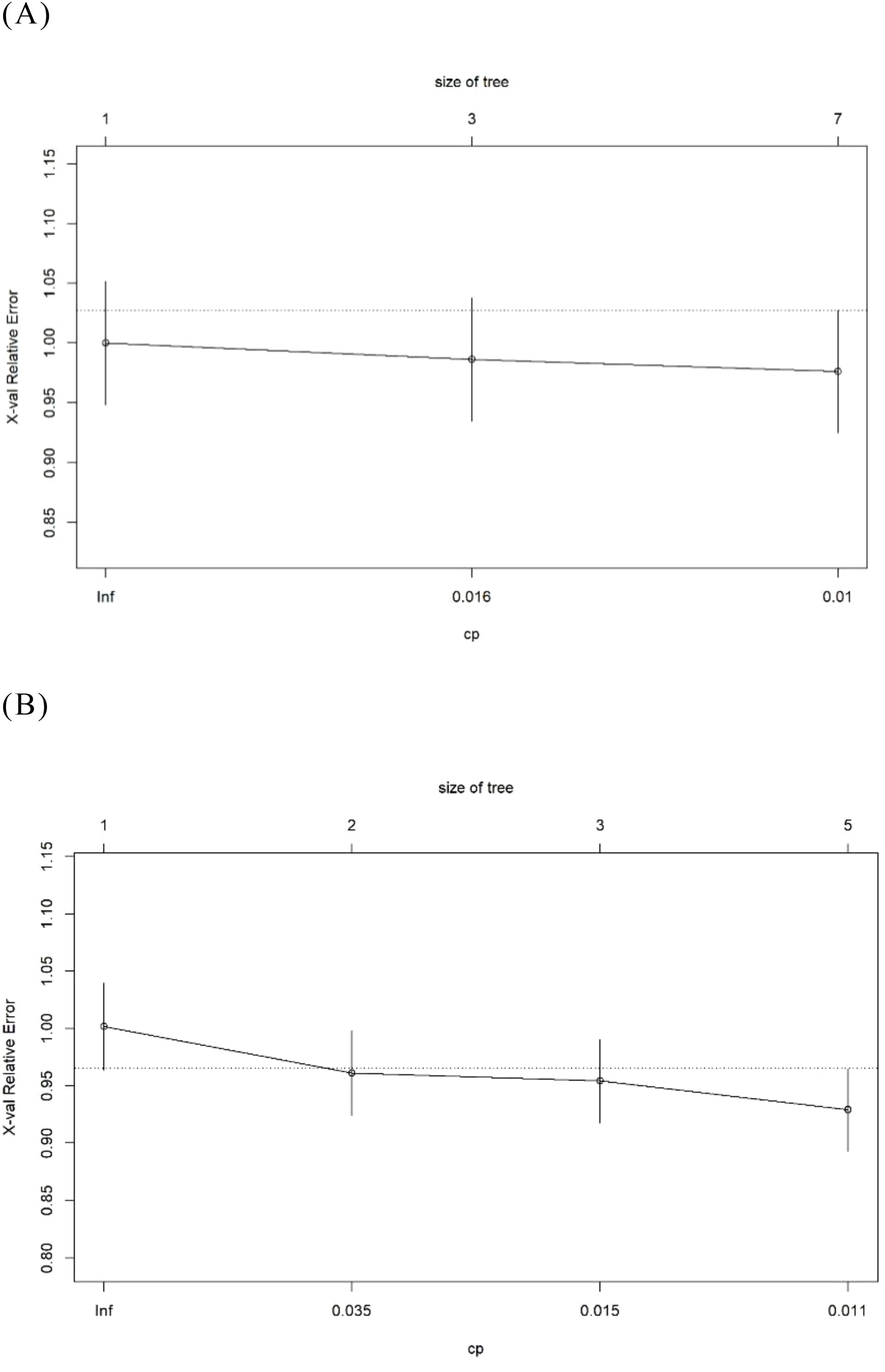
Figure 6. The goodness of fit graph in the DTA models. (A) The Logistic- DTA model. (B) The Lasso- DTA model.
3.2.6.2 Based on the results of lasso regression
The goodness-of-fit graph indicated that the optimal cp value was 0.011, which was subsequently set to this value (Figure 6B). Ultimately, the AUC of the “Lasso-DTA” model was determined to be 0.613, with an average accuracy of 78.2%. At a cut-off value of 0.239, the sensitivity reached 0.718 and specificity was recorded at 0.517.
We visualized the decision tree model (Figure 7). The terminal boxes in the classification tree represented leaf nodes, each corresponding to the final probability of platinum resistance recurrence as derived from the decision tree analysis.
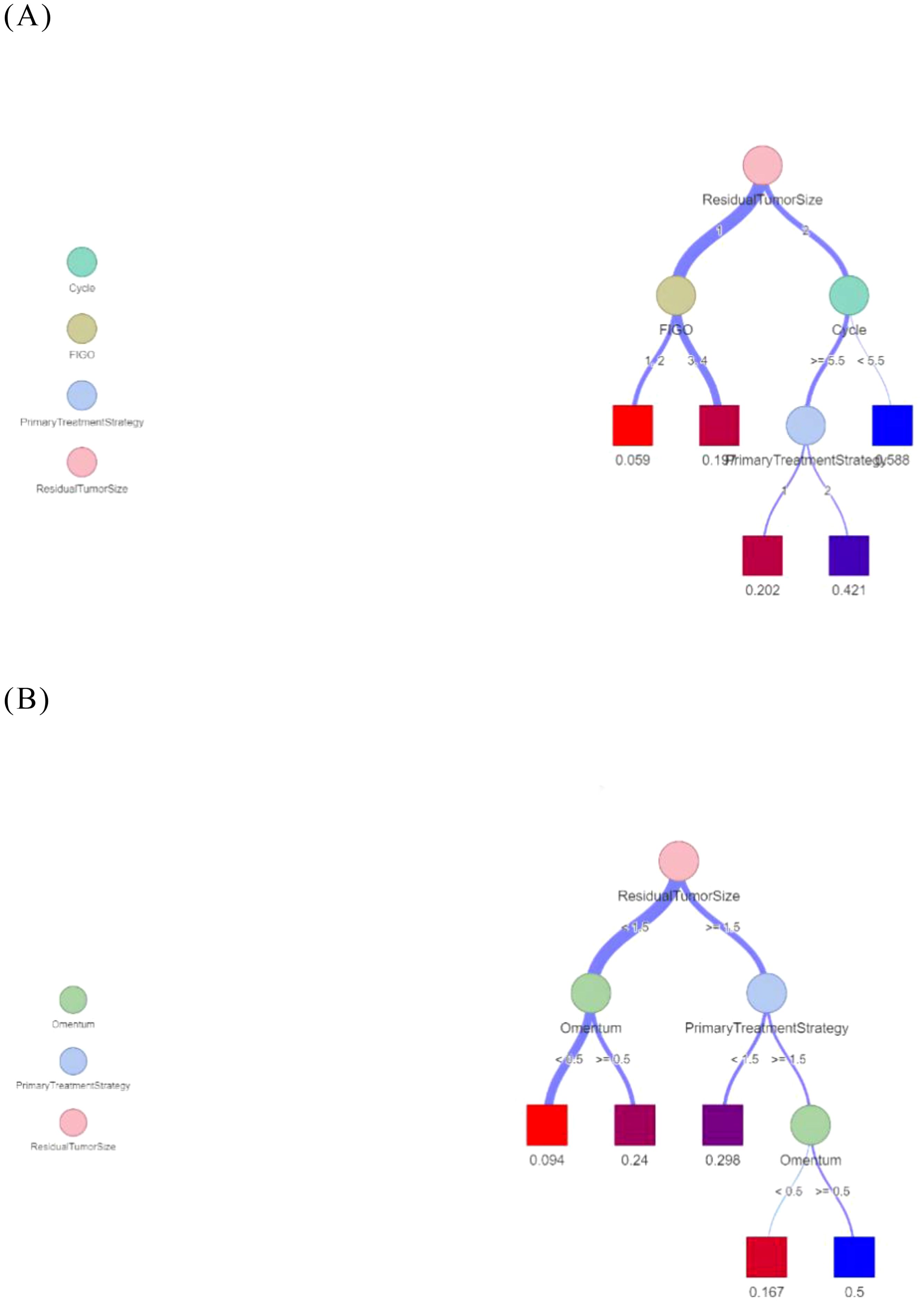
Figure 7. Decision trees for predicting recurrence of platinum resistance. (A) The Logistic- DTA model. (B) The Lasso- DTA model.
Table 3 illustrates the capability of each model to differentiate between platinum-sensitive and platinum-resistant cases by presenting specific metrics. The ROC curves for the six models, derived from five-fold cross-validation results, are displayed in Figure 8. The XGBoost model, which was developed based on eight variables identified through multivariate logistic regression analysis, demonstrated the highest performance among all models evaluated.
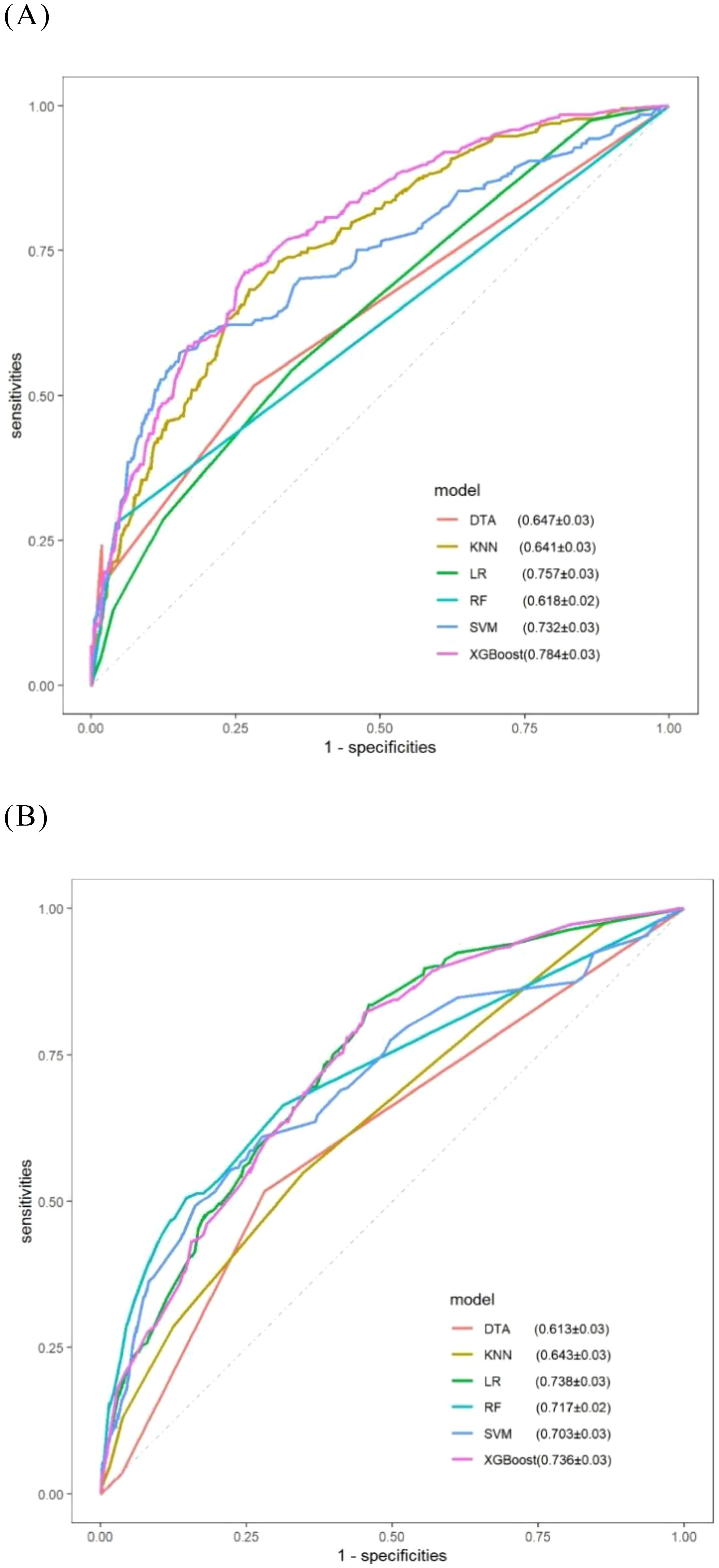
Figure 8. ROC curves for comparative models obtained via five-fold cross-validation. (A) Models consisting of eight selected variables; (B) Models consisting of seven selected variables.
4 Develop models based on previous literature and professional knowledge
In this study, the p-values for the appendix and omentum were found to be greater than 0.05 in the multivariate logistic regression analysis. However, several studies have identified the appendix and omentum as independent factors influencing platinum resistance recurrence. Additionally, results from lasso regression further indicated that both the appendix and omentum are significant independent predictors of platinum-resistant recurrence. Consequently, we adopted an exploratory approach to model development by integrating insights from existing literature with professional expertise. The appendix and omentum were incorporated either separately or simultaneously into eight variables selected through multivariate logistic regression to construct our model (Table 4). Notably, we observed a substantial enhancement in the performance of the RF model when both factors were included in its construction, with the AUC increasing from 0.627 to 0.887.
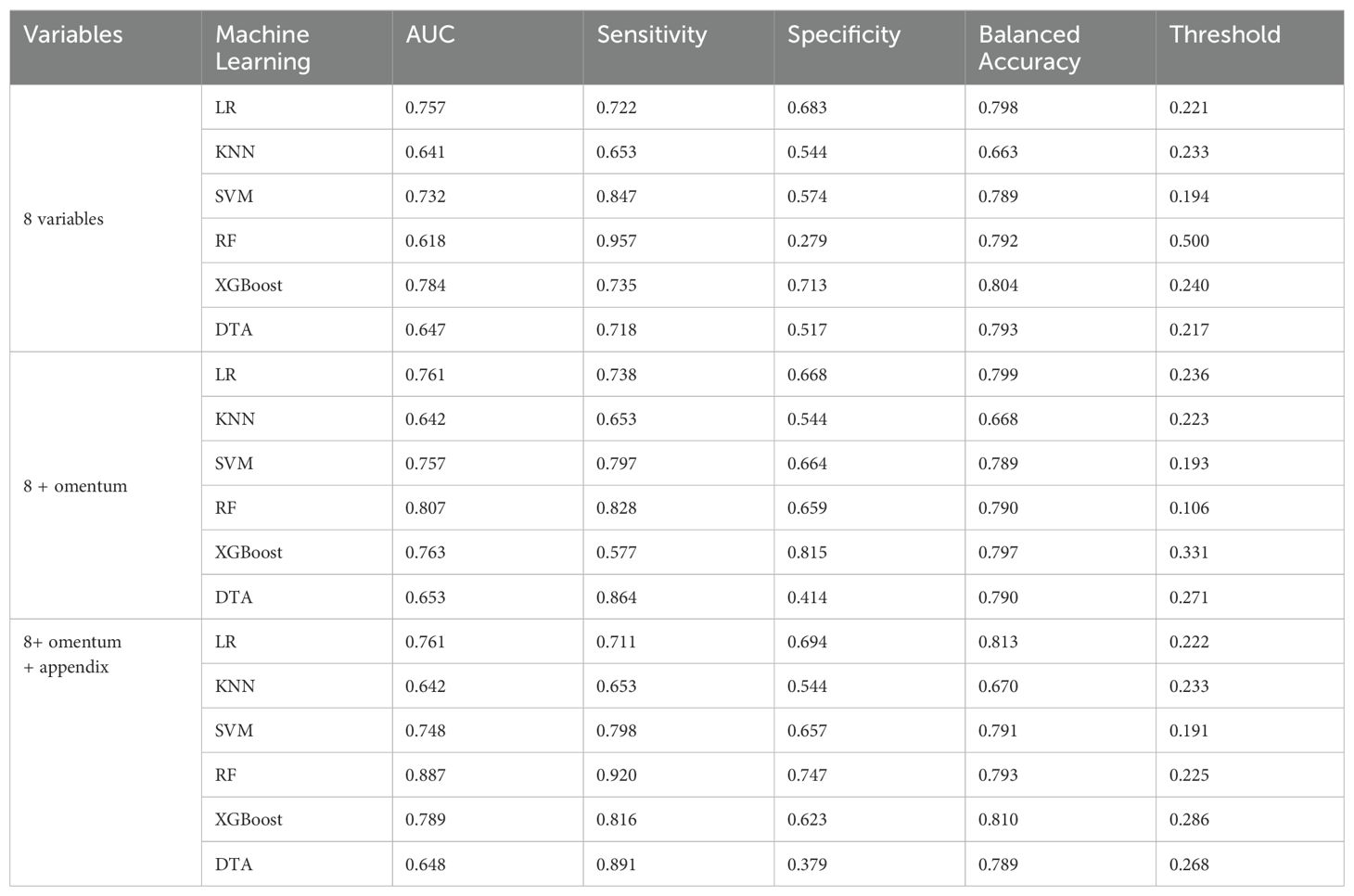
Table 4. Performance of machine learning models based on multivariate Logistic regression results and professional knowledge.
5 Discussion
The primary objective of this study is to develop a machine learning predictive model for assessing the risk of platinum-resistant recurrence in patients with EOC. Among the models evaluated, the Logistic-XGBoost exhibited superior performance (AUC = 0.784). We recommend utilizing the XGBoost model, which incorporates eight variables. This model can be implemented in clinical practice once pathological data are obtained following surgical treatment and is anticipated to contribute significantly to clinical trial design and future research endeavors.
The enhanced accuracy of the Logistic-XGBoost model underscores the significant contribution of hidden variables identified in previous studies, as well as the novel clinical relevance of these variables, including LDH (27) and surgery-related information. This highlights the potential utility of routine laboratory data and clinical indicators as biomarkers for platinum-resistant recurrence. Elevated serum LDH levels are observed in patients experiencing active tumor growth and tissue destruction. In recent years, extensive research has been conducted on the prognostic value of serum LDH across various cancers, including lymphoma, lung cancer, colorectal cancer, breast cancer, kidney cancer, and liver cancer (28).
Furthermore, combining serum LDH with other tumor markers such as alpha-fetoprotein, CA125, and human chorionic gonadotropin can enhance the accurate determination of histological types in ovarian cancer (29). This study presents a novel association between serum LDH levels and platinum-resistant recurrence in EOC, specifically indicating that higher LDH levels correlate with an increased likelihood of platinum-resistant recurrence. Future large-scale and rigorously designed prospective studies are essential to validate the clinical significance of these markers and establish precise cutoff values.
Previous studies have identified inflammatory factor indicators, such as white blood cell count, the neutrophil-to-lymphocyte ratio, and the platelet-to-lymphocyte ratio, as independent factors influencing platinum-resistant recurrence (30–32). However, during the preliminary data preprocessing of this study, it became evident that these ratios—including white blood cell count, absolute values of various white blood cell classifications, the neutrophil-to-lymphocyte ratio, and the platelet-to-lymphocyte ratio—do not serve as independent prognostic factors for platinum-resistant recurrence. It is crucial to acknowledge that systemic diseases unrelated to cancer (such as inflammatory conditions or infections) may affect peripheral blood complete counts and potentially lead to inaccuracies in analysis results.
In the model construction phase, this research utilized six distinct machine learning algorithms: LR, K-Nearest Neighbors KNN, RF, SVM, DTA, and XGBoost (33, 34). All of these are classified as supervised machine learning algorithms. Unlike unsupervised learning, which does not utilize labeled data, supervised learning provides the computer with labeled datasets for training and subsequently applies the acquired patterns to make predictions on unknown data. Each algorithm has its own unique strengths and weaknesses, leading to significantly varied performance when applied to the same dataset. Currently, no single algorithm can comprehensively solve all problems in this domain. Therefore, this study recommends that researchers conducting similar investigations consider employing all widely recognized algorithms in order to identify the most effective one for developing a clinical prediction model.
The potential advantages of machine learning include its capacity to detect complex patterns and greater flexibility in managing missing data, as well as accommodating nonlinear relationships among parameters. Notably, in this study, the model fitted using variable selection results from Lasso regression indicated that the traditional LR model performed optimally; at this stage, machine learning did not surpass traditional LR methods.
Furthermore, the black box nature of machine learning presents an additional limitation when employing these models. While traditional models can be articulated in a clear mathematical form such as f(x) = β0 + β1X1 + β2X2 + β3X3……, machine learning models resist such straightforward formulation, prioritizing predictive accuracy over interpretability. It is crucial to select an appropriate modeling approach based on the specific characteristics of the data and research objectives rather than adopting machine learning indiscriminately.
In constructing clinical prediction models, influencing factors may evolve due to temporal and spatial variations; thus, continuous adaptation of the model is necessary. This study provides a conceptual framework for model evolution.
This study acknowledges several limitations. Firstly, due to its retrospective design, this research may be subject to issues such as selection bias, information bias, confounding bias, and temporal bias. As a non-randomized observational study, it has the potential to either overestimate or underestimate the risk of platinum-resistant recurrence in ovarian cancer. Furthermore, the retrospective nature of the study makes missing or incomplete data in medical records an unavoidable challenge. For instance, there was a significant amount of missing data regarding pathological types (35), degrees of differentiation, and assessments of chemotherapy responses within this study. Some pathological types were classified merely as adenocarcinoma in the reports, which hindered further distinctions; consequently, histological type was not included in the variable selection. The rates of missing data for degree of differentiation and HE4 were 31.46% and 86.49%, respectively; thus, we excluded the analysis related to degree of differentiation. Given the extended time span involved in this research, time bias is inevitably present; future studies will require data from multi-center or large national databases to effectively evaluate the clinical applicability value of the model.
Secondly, while the model developed herein underwent internal validation only, it necessitates external validation across various timeframes and settings to enhance its applicability and generalizability further. Additionally, prospective studies are needed to more accurately assess its clinical utility and identify more precise variables for inclusion.
Lastly, currently only a subset of patients at our institution has undergone germline or somatic testing for breast cancer susceptibility genes (BRCA) 1/2; therefore, this study did not incorporate molecular characteristics such as gene mutations into variable considerations. As relevant genetic and molecular data accumulate at our institution, integrating results from BRCA1/2 gene testing with other molecular detection outcomes is anticipated to facilitate the development of a more accurate prediction model; furthermore, incorporating factors related to maintenance therapy should be considered in subsequent investigations.
Despite these limitations, this study has successfully established a prediction model for assessing platinum-resistant recurrence risk among EOC patients. With further validation and refinement, this model could enable early identification of patients at risk for platinum-resistant recurrence, ultimately improving prognosis for EOC patients.
6 Conclusions
1. Multiple logistic regression showed that LDH, FIGO stage, platelet count, supraclavicular lymph node metastasis, primary treatment strategy, residual tumor size, type of platinum (carboplatin/cisplatin or others), chemotherapy cycle were independent influencing factors of platinum resistance recurrence.
2. Among the constructed machine learning models, logistic-XGBoost model has the best performance, with an AUC of 0.784 and an average accuracy of 0.804. The model can be applied to clinical practice after obtaining pathological data in surgical treatment, and is expected to play a role in clinical trial design and future research.
With further refinement and external validation, the model can potentially improve the prognosis of EOC by early identification of platinum resistance recurrence.
3. When constructing the clinical prediction model, it is suggested that researchers try all the commonly used model fitting methods, and select the best fitting method that is most consistent with the data characteristics.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Ethics Committee of Yunnan Cancer Hospital. (19th Oct,2021/KYCS2021282). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
L-RY: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. MY: Conceptualization, Investigation, Methodology, Project administration, Software, Validation, Writing – review & editing. L-LC: Investigation, Methodology, Software, Writing – review & editing. L-HL: Methodology, Validation, Writing – original draft. YH: Data curation, Methodology, Writing – original draft. Z-TM: Software, Visualization, Writing – review & editing. W-QW: Conceptualization, Investigation, Writing – review & editing. FL: Data curation, Methodology, Writing – review & editing. Z-JL: Formal analysis, Methodology, Writing – original draft. Y-FW: Conceptualization, Funding acquisition, Methodology, Project administration, Writing – original draft, Writing – review & editing. Y-LS: Investigation, Methodology, Writing – review & editing. X-LL: Conceptualization, Investigation, Writing – original draft.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was funded by Kunming Medical University Postgraduate Innovation Fund (No.2022S317). The authors declare that no other grants or support were received during the preparation of this manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. CA Cancer J Clin. (2024) 74:12–49. doi: 10.3322/caac.21820
2. Momenimovahed Z, Tiznobaik A, Taheri S, Salehiniya H. Ovarian cancer in the world: epidemiology and risk factors. Int J Womens Health. (2019) 11:287–99. doi: 10.2147/ijwh.s197604
3. Smith LH, Morris CR, Yasmeen S, Parikh-Patel A, Cress RD, Romano PS, et al. Ovarian cancer: can we make the clinical diagnosis earlier? Cancer. (2005) 104:1398–407. doi: 10.1002/cncr.21310
4. Ledermann JA, Raja FA, Fotopoulou C, Gonzalez-Martin A, Colombo N, Sessa C, et al. Newly diagnosed and relapsed epithelial ovarian carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. (2013) 24 Suppl 6:vi24–32. doi: 10.1093/annonc/mdt333
5. Perelli F, Fusi G, Lonati L, Gargano T, Maffi M, Avanzini S, et al. Laparoscopic ovarian tissue collection for fertility preservation in children with Malignancies: a multicentric experience. Front Surg. (2024) 11:1352698. doi: 10.3389/fsurg.2024.1352698
6. Lheureux S, Gourley C, Vergote I, Oza AM. Epithelial ovarian cancer. Lancet. (2019) 393:1240–53. doi: 10.1016/s0140-6736(18)32552-2
8. Daly MB, Pal T, Maxwell KN, Churpek J, Kohlmann W, AlHilli Z, et al. NCCN guidelines® Insights: genetic/familial high-risk assessment: breast, ovarian, and pancreatic, version 2.2024. J Natl Compr Canc Netw. (2023) 21:1000–10. doi: 10.6004/jnccn.2023.0051
9. Colombo PE, Fabbro M, Theillet C, Bibeau F, Rouanet P, Ray-Coquard I, et al. Sensitivity and resistance to treatment in the primary management of epithelial ovarian cancer. Crit Rev Oncol Hematol. (2014) 89:207–16. doi: 10.1016/j.critrevonc.2013.08.017
10. Madariaga A, Rustin GJS, Buckanovich RJ, Trent JC, Oza AM. Wanna get away? Maintenance treatments and chemotherapy holidays in gynecologic cancers. Am Soc Clin Oncol Educ Book. (2019) 39:e152–66. doi: 10.1200/edbk_238755
11. Burger RA, Brady MF, Bookman MA, Fleming GF, Monk BJ, Huang H, et al. Incorporation of bevacizumab in the primary treatment of ovarian cancer. N Engl J Med. (2011) 365:2473–83. doi: 10.1056/NEJMoa1104390
12. McMullen M, Madariaga A, Lheureux S. New approaches for targeting platinum-resistant ovarian cancer. Semin Cancer Biol. (2021) 77:167–81. doi: 10.1016/j.semcancer.2020.08.013
13. Gerestein CG, Eijkemans MJ, de Jong D, van der Burg ME, Dykgraaf RH, Kooi GS, et al. The prediction of progression-free and overall survival in women with an advanced stage of epithelial ovarian carcinoma. Bjog. (2009) 116:372–80. doi: 10.1111/j.1471-0528.2008.02033.x
14. Chi DS, Palayekar MJ, Sonoda Y, Abu-Rustum NR, Awtrey CS, Huh J, et al. Nomogram for survival after primary surgery for bulky stage IIIC ovarian carcinoma. Gynecol Oncol. (2008) 108:191–4. doi: 10.1016/j.ygyno.2007.09.020
15. Barlin JN, Yu C, Hill EK, Zivanovic O, Kolev V, Levine DA, et al. Nomogram for predicting 5-year disease-specific mortality after primary surgery for epithelial ovarian cancer. Gynecol Oncol. (2012) 125:25–30. doi: 10.1016/j.ygyno.2011.12.423
16. Paik ES, Sohn I, Baek SY, Shim M, Choi HJ, Kim TJ, et al. Nomograms predicting platinum sensitivity, progression-free survival, and overall survival using pretreatment complete blood cell counts in epithelial ovarian cancer. Cancer Res Treat. (2017) 49:635–42. doi: 10.4143/crt.2016.282
17. Senders JT, Staples PC, Karhade AV, Zaki MM, Gormley WB, Broekman MLD, et al. Machine learning and neurosurgical outcome prediction: A systematic review. World Neurosurg. (2018) 109:476–486.e471. doi: 10.1016/j.wneu.2017.09.149
18. Langerhuizen DWG, Janssen SJ, Mallee WH, van den Bekerom MPJ, Ring D, Kerkhoffs GMMJ, et al. What are the applications and limitations of artificial intelligence for fracture detection and classification in orthopaedic trauma imaging? A systematic review. Clin Orthop Relat Res. (2019) 477:2482–91. doi: 10.1097/corr.0000000000000848
19. Kawakami E, Tabata J, Yanaihara N, Ishikawa T, Koseki K, Iida Y, et al. Application of artificial intelligence for preoperative diagnostic and prognostic prediction in epithelial ovarian cancer based on blood biomarkers. Clin Cancer Res. (2019) 25:3006–15. doi: 10.1158/1078-0432.ccr-18-3378
20. Martínez A, Pomel C, Filleron T, De Cuypere M, Mery E, Querleu D, et al. Prognostic relevance of celiac lymph node involvement in ovarian cancer. Int J Gynecol Cancer. (2014) 24:48–53. doi: 10.1097/igc.0000000000000041
21. Winter WE, Maxwell GL, Tian C, Sundborg MJ, Rose GS, Rose PG, et al. Tumor residual after surgical cytoreduction in prediction of clinical outcome in stage IV epithelial ovarian cancer: a Gynecologic Oncology Group Study. J Clin Oncol. (2008) 26:83–9. doi: 10.1200/jco.2007.13.1953
22. Makar AP, Baekelandt M, Tropé CG, Kristensen GB. The prognostic significance of residual disease, FIGO substage, tumor histology, and grade in patients with FIGO stage III ovarian cancer. Gynecol Oncol. (1995) 56:175–80. doi: 10.1006/gyno.1995.1027
23. Collins GS, Reitsma JB, Altman DG, Moons KG. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): the TRIPOD Statement. Eur J Clin Invest. (2015) 45:204–14. doi: 10.1111/eci.12376
24. Alkema NG, Wisman GB, van der Zee AG, van Vugt MA, de Jong S. Studying platinum sensitivity and resistance in high-grade serous ovarian cancer: Different models for different questions. Drug Resist Update. (2016) 24:55–69. doi: 10.1016/j.drup.2015.11.005
25. Huang S, Cai N, Pacheco PP, Narrandes S, Wang Y, Xu W, et al. Applications of support vector machine (SVM) learning in cancer genomics. Cancer Genomics Proteomics. (2018) 15:41–51. doi: 10.21873/cgp.20063
26. Handelman GS, Kok HK, Chandra RV, Razavi AH, Huang S, Brooks M, et al. Peering into the black box of artificial intelligence: evaluation metrics of machine learning methods. AJR Am J Roentgenol. (2019) 212:38–43. doi: 10.2214/ajr.18.20224
27. Ikeda A, Yamaguchi K, Yamakage H, Abiko K, Satoh-Asahara N, Takakura K, et al. Serum lactate dehydrogenase is a possible predictor of platinum resistance in ovarian cancer. Obstet Gynecol Sci. (2020) 63:709–18. doi: 10.5468/ogs.20117
28. Wulaningsih W, Holmberg L, Garmo H, Malmstrom H, Lambe M, Hammar N, et al. Serum lactate dehydrogenase and survival following cancer diagnosis. Br J Cancer. (2015) 113:1389–96. doi: 10.1038/bjc.2015.361
29. Boran N, Kayikçioğlu F, Yalvaç S, Tulunay G, Ekinci U, Köse MF, et al. Significance of serum and peritoneal fluid lactate dehydrogenase levels in ovarian cancer. Gynecol Obstet Invest. (2000) 49:272–4. doi: 10.1159/000010258
30. Miao Y, Yan Q, Li S, Li B, Feng Y. Neutrophil to lymphocyte ratio and platelet to lymphocyte ratio are predictive of chemotherapeutic response and prognosis in epithelial ovarian cancer patients treated with platinum-based chemotherapy. Cancer biomark. (2016) 17:33–40. doi: 10.3233/cbm-160614
31. Cozzi GD, Samuel JM, Fromal JT, Keene S, Crispens MA, Khabele D, et al. Thresholds and timing of pre-operative thrombocytosis and ovarian cancer survival: analysis of laboratory measures from electronic medical records. BMC Cancer. (2016) 16:612. doi: 10.1186/s12885-016-2660-z
32. Angeles MA, Ferron G, Cabarrou B, Balague G, Martínez-Gómez C, Gladieff L, et al. Prognostic impact of celiac lymph node involvement in patients after frontline treatment for advanced ovarian cancer. Eur J Surg Oncol. (2019) 45:1410–6. doi: 10.1016/j.ejso.2019.02.018
33. Raita Y, Goto T, Faridi MK, Brown DFM, Camargo CA, Hasegawa K, et al. Emergency department triage prediction of clinical outcomes using machine learning models. Crit Care. (2019) 23:64. doi: 10.1186/s13054-019-2351-7
34. Deo RC. Machine learning in medicine. Circulation. (2015) 132:1920–30. doi: 10.1161/circulationaha.115.001593
Keywords: platinum resistance, recurrence, model, nomogram, early detection of cancer
Citation: Yang L-R, Yang M, Chen L-L, Shen Y-L, He Y, Meng Z-T, Wang W-Q, Li F, Liu Z-J, Li L-H, Wang Y-F and Luo X-L (2024) Machine learning for epithelial ovarian cancer platinum resistance recurrence identification using routine clinical data. Front. Oncol. 14:1457294. doi: 10.3389/fonc.2024.1457294
Received: 30 June 2024; Accepted: 16 October 2024;
Published: 08 November 2024.
Edited by:
Lixin Wan, Moffitt Cancer Center, United StatesReviewed by:
Federica Perelli, Azienda USL Toscana Centro, ItalyNatale Calomino, University of Siena, Italy
Copyright © 2024 Yang, Yang, Chen, Shen, He, Meng, Wang, Li, Liu, Li, Wang and Luo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xin-Lei Luo, OTYzMTYwODcwQHFxLmNvbQ==; Yu-Feng Wang, MTM1NzcwMzc1ODVAMTYzLmNvbQ==; Lin-Hui Li, MzQ4NTgwMjcwOEBxcS5jb20=
†These authors have contributed equally to this work and share first authorship
 Li-Rong Yang
Li-Rong Yang Mei Yang2†
Mei Yang2† Zhi-Jin Liu
Zhi-Jin Liu