
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Oncol. , 14 February 2025
Sec. Cancer Molecular Targets and Therapeutics
Volume 14 - 2024 | https://doi.org/10.3389/fonc.2024.1456959
This article is part of the Research Topic Therapeutic Mechanism of Osteosarcoma View all 10 articles
Osteosarcoma (OS) is the most common bone malignancy in children and adolescents, and although current neoadjuvant chemotherapy has shown efficacy against OS, the long-term survival rate for patients with OS remains low, highlighting the need to find more effective treatments. In cancer cells, abnormal activation of signaling pathways can widely affect cell activity from growth and proliferation to apoptosis, invasion and metastasis. Wnt/β-catenin is a complex and unique signaling pathway that is considered to be one of the most important carcinogenic pathways in human cancer. Research have confirmed that the Wnt/β-catenin signaling pathway is an important driving factor for the occurrence and development of osteosarcoma, and abnormal activation of this pathway can promote the pathological processes of cell proliferation, invasion, migration, tumor angiogenesis and chemical resistance of osteosarcoma. However, inhibition of Wnt/β-catenin signaling pathway can effectively inhibit or reverse the above pathological processes. Therefore, manipulating the expression or function of the Wnt/β-catenin pathway may be a potential targeted pathway for the treatment of OS. In this review, we describe the characteristics of the Wnt/β-catenin signaling pathway and summarize the role and mechanism of this pathway in OS. This paper discusses the therapeutic significance of inhibiting or targeting Wnt/β-catenin pathway in OS and the shortcomings of current studies on this pathway in OS and the problems to be solved. This review helps us to understand the role of Wnt/β-catenin on OS, and provides a theoretical basis and new ideas for targeting Wnt/β-catenin pathway as a therapeutic target for OS.
Osteosarcoma (OS) is the most common aggressive malignant bone tumor in children and adolescents worldwide (1). It originates from the original transformed cells of mesenchymal origin and mainly affects the differentiation of osteoblasts and produces immature bone (2, 3). Although the exact cause of OS is not fully understood, it is clear that the development and pathogenesis of OS is associated with multiple factors, including age, sex, ethnicity, genetics, and familial factors (4). OS is characterized by locally aggressive growth and high metastatic potential (5, 6), characterized by local invasion of bone and soft tissue, loss of function of the affected limbs, and distant metastasis, most often to the lungs (7). At present, the main treatments for OS are surgery, radiotherapy, neoadjuvant chemotherapy and postoperative adjuvant chemotherapy and other multi-scientific and multi-mode treatments, but the therapeutic effect is not satisfactory. Due to the early onset of bone and lung metastasis of OS, the 5-year overall survival rate of OS patients with metastasis at diagnosis is less than 30% (8, 9). More importantly, due to the complexity of the progression mechanism of osteosarcoma, the etiology and molecular mechanism of the pathogenesis are still vague or unknown. Therefore, there is an urgent need to further understand the physiological and pathological mechanism of OS, develop more effective anti-OS agents and new therapeutic strategies to improve the symptoms of OS from the molecular pathological level, so as to improve the prognosis and quality of life of osteosarcoma patients.
Although the cause of OS has not been fully elucidated, there is a large amount of evidence that the disease is related to the dysregulation of various intracellular signaling pathways, especially the Wnt/β-catenin pathway (10). Wnt/β-catenin signaling pathway has been reported to be one of the most important carcinogenic pathways in almost all human cancers (11) and seems to be a good candidate for molecular therapies in malignant tumors (12, 13). The activation of Wnt/β-catenin signaling pathway is also associated with the occurrence, development and pathological mechanism of a variety of diseases, such as cancer, Alzheimer’s disease, schizophrenia, diabetes and Parkinson’s disease (14–17). It has been found that overactivation of Wnt/β-catenin signaling pathway plays a carcinogenic role in various sarcomas by driving cell cycle progression and increasing cell proliferation (18–20). It has been confirmed that Wnt/β-catenin pathway is over-activated in osteosarcoma (21), and abnormal activation of this pathway can promote pathological processes such as cell cycle, migration, invasion, angiogenesis, chemotherapy resistance and accelerate the occurrence and development of OS, while inhibition of Wnt/β-catenin pathway activity can effectively inhibit the above processes (22). Therefore, targeting Wnt/β-catenin could provide a new perspective for designing more effective drugs for the treatment of OS. In this review, we reviewed the biological characteristics of Wnt/β-catenin, the role and mechanism of Wnt/β-catenin in the pathological process of OS. This paper aims to provide evidence for the effect of Wnt/β-catenin signaling pathway activation on OS and the treatment of OS, and to emphasize the role of inhibiting Wnt/β-catenin pathway in delaying OS by clarifying the mechanism, so as to provide theoretical basis and new ideas for targeting Wnt/β-catenin pathway as therapeutic targets for OS.
Wnt/β-catenin signaling is an evolutionarily conserved signal that regulates many important embryonic and somatic processes such as cell fate determination, organogenesis, tissue homeostasis, and various pathological states (23, 24). In addition, the abnormal regulation of this signal transduction is often closely related to many aspects of tumor occurrence and development, malignant transformation and recurrence (23, 25–27). Wnt signaling can be classified into classical or non-classical pathways, the classical pathway is involved in cell survival, proliferation, differentiation and migration, while the non-classical pathway regulates cell polarity and migration (28). However, most current studies on the Wnt pathway focus on the Wnt/β-catenin branch of the Wnt pathway, the disorder of which is associated with a variety of diseases (29).
Classic Wnt pathway is a signal cascade mediated by β-catenin. The classical Wnt pathway is marked by the accumulation and translocation of the adherence-linked protein β-catenin in the nucleus. β-catenin is an Integral protein in the Wnt signaling pathway that regulates gene transcription and intercellular adhesion. Mutations in beta-catenin lead to amino acid substitution, resulting in inappropriate phosphorylation of the protein. Subsequently, ubiquitin ligase E3 does not correctly recognize the phosphorylated protein. Thus, dysregulation of the Wnt pathway leads to β-catenin accumulation without degradation and then transfer to the nucleus, thus activating transcription of oncogenes (30). Wnt is a secretory glycoprotein that binds to the cell surface transmembrane frizzled serpentine receptors (FZD) and low-density lipoprotein receptor-associated protein 5/6 (LRP5/6) complex, resulting in the accumulation of β-catenin in the nucleus, which leads to cell cycle activation and transcriptional regulation (31). FZD is the main binding site of Wnt (32), which mainly contains 7 transmembrane and extracellular N-terminal cysteine-rich domains (CRDS) (33, 34). However, in the absence of Wnt signaling, cytoplasmic β-catenin is degraded by β-catenin-destroying complexes such as Axin, adenomatous polyposis (APC), protein phosphatase 2A (PP2A), glycogen synthase kinase 3 (GSK3), and casein kinase 1α (CK1α). This results in the failure of the classical Wnt pathway (35, 36) (Figure 1).
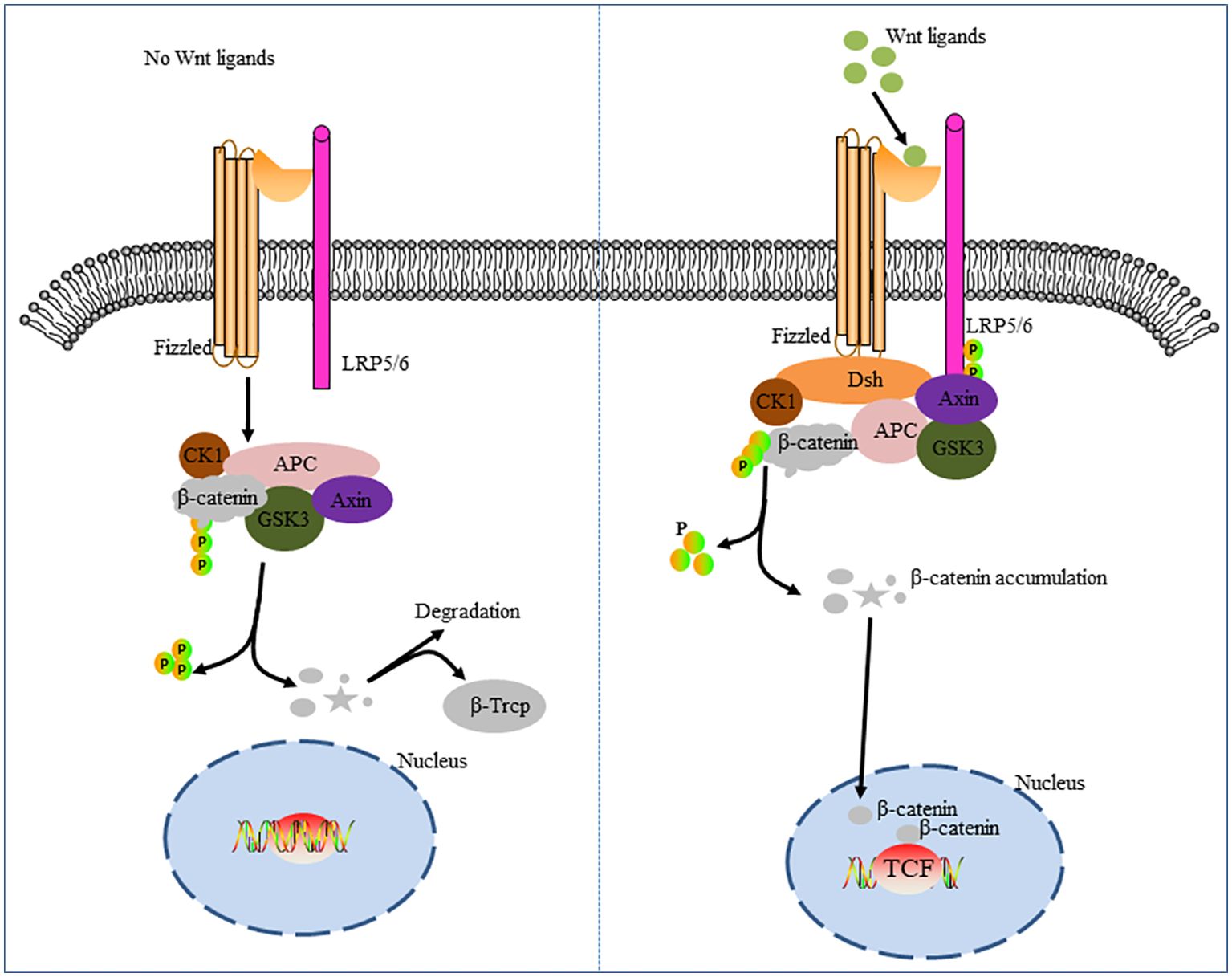
Figure 1. Classic Wnt/β-catenin pathway cascade diagram. The figure on the left shows the deactivation of the Wnt pathway. In the absence of Wnt signaling, β-catenin in the cytoplasm is recognized, folded, and phosphorylated by a destructive complex composed of scaffold proteins Axin, APC, GSK3β, and CK1, and targeted for degradation by a β-TRCP-mediated proteomic mechanism. The figure on the right shows activation of the Wnt pathway. The signal induces double phosphorylation of LRP6 by CK1 and GSK3-β via the Fizzled receptor and LRP5/6 co-receptor complex, which allows the axon-containing protein complex to transfer from the cytoplasm to the plasma membrane. Dsh is also recruited to the cell membrane and binds to Fizzied, and Axin binds to phosphorylated LRP5/6. The complex forms on the Fizzled/LRP5/6 membrane and induces stabilization of β-catenin by separating and/or degrading axons. β-catenin, which accumulates in the cytoplasm, translocates into the nucleus and, together with the transcription factor TCF, drives the expression of downstream target genes.
The non-classical pathway is often referred to as the beta-catenin-independent pathway, which can be further divided into the Wnt/Planar Cell polarity (PCP) pathway and the Wnt/Ca2+ pathway. In the Wnt/PCP pathway, after the Wnt molecule binds to the receptor FZD, it recruits Dvl and further activates the small GTPases Rho and Rac, triggering the recruitment of downstream RHO-associated kinases (ROCK) and c-Jun N-terminal kinase (JNK), thereby allowing cytoskeletal recombination (Figure 2). In the Wnt/Ca2+ pathway, Dvl is activated when Wnt binds to FZD, and the activated Dvl recruits phospholipase C (PLC). PLC converts phosphatidylinositol 4, 5-diphosphate (PIP2) to diacylglycerol (DAG) and inositol 1,4, 5-triphosphate (IP3). IP3 stimulates the release of Ca2+ from the endoplasmic reticulum, and DAG and Ca2+ together activate downstream protein kinase C (PKC), calcineurin (CaN), and Ca2+/calmodulin-dependent protein kinase II (CaMKII), thereby regulating intracellular calcium flux and downstream calcium-dependent cytoskeleton and/or transcriptional responses (Figure 3).
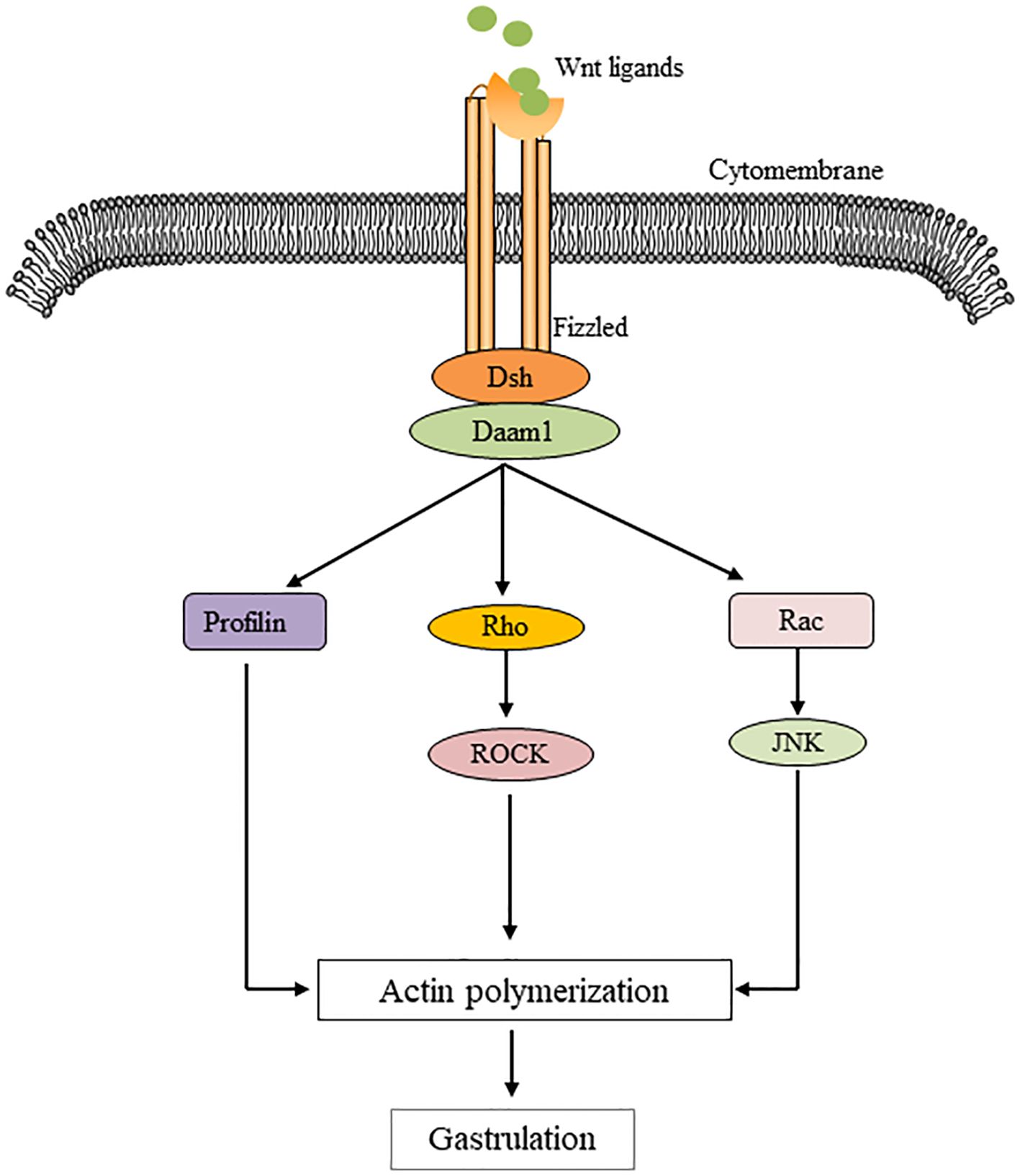
Figure 2. Illustration of a planar cell polarity transition cascade. Wnt signal transduction via Fizzled independent of LRP5/6 leads to Dsh activation. Dsh mediates the activation of Rho via Daam1, which activates Rho kinase (ROCK). Daam1 also mediates actin polymerization via the actin binding protein Profilin. Dsh also mediates the activation of Rac, which in turn activates JNK. Signals from Rock, JNK, and Profilin are integrated into cytoskeletal changes in cell polarization and movement during gastrum formation.
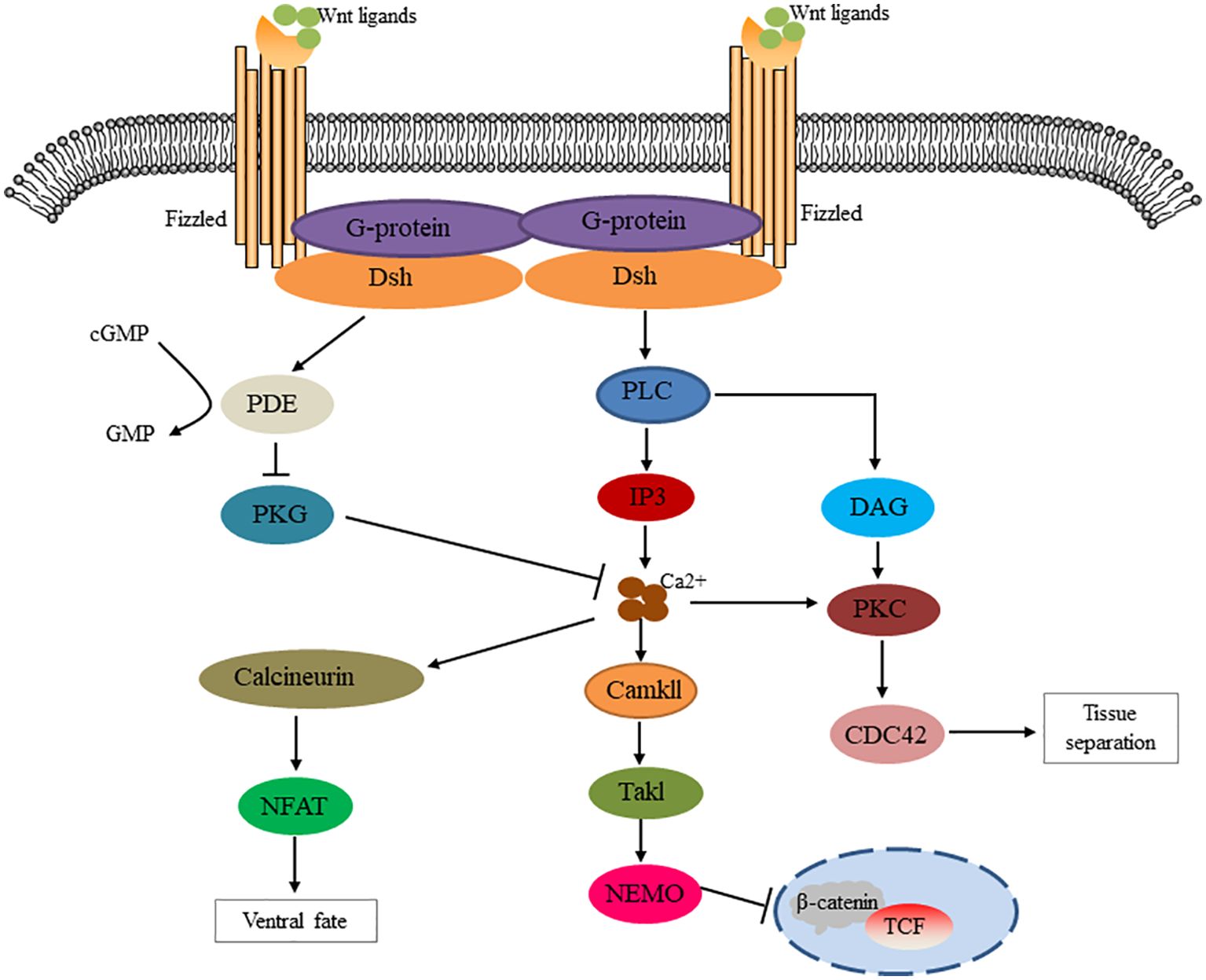
Figure 3. Schematic diagram of Wnt/Ca2+ signaling cascade. The Wnt signaling via Fizzled mediates the activation of Dsh through g protein activation. Dishevelled activates phosphodiesterase PDE, which inhibits PKG and in turn inhibits Ca2+ release. Dsh activates IP3 via PLC, resulting in the release of intracellular Ca2+, which activates CamK11 and calcineurin. NF-AT activation by calcineurin regulates the fate of ventral cells. CamK11 activates TAK and NLK, inhibits β-catenin/TCF function and negatively regulates dorsal axis formation. DAG mediates tissue separation and cell motility during gastrulation through PKC activation of CDC42.
Research have confirmed that the Wnt/β-catenin signaling pathway is involved in the development of osteosarcoma, and the Wnt signaling pathway is abnormally activated in OS and plays a crucial role in tumorigenic and metastatic transmission (37, 38). Wnt-β-catenin pathway has been reported to be significantly higher in human OS tissues and cell lines than in normal tissues or osteoid osteomas (39, 40). In addition, the analysis of relevant patient samples found that Wnt/β-catenin level in osteosarcoma tissues was significantly higher than that in neighboring healthy tissues, and was associated with poor prognosis and lung metastasis and diffusion (41, 42). Haydon et al. found that 33 out of 47 OS samples had increased Wnt/β-catenin expression level accumulation (43).
As an intracellular signaling pathway, Wnt/β-catenin pathway has been found in many types of cancer and plays an important regulatory role in the occurrence and development of tumors. Kinase analysis has identified active Wnt/β-catenin signaling in most OS cell lines (44). Wnt/β-catenin pathway is a complex signaling pathway, and its abnormal activation plays a key role in the pathogenesis of OS (45). A large amount of evidence has shown that dysregulation of this pathway is involved in multiple pathological processes of OS, including tumor cell proliferation, invasion and metastasis, and chemical resistance (46, 47) (Figure 4).
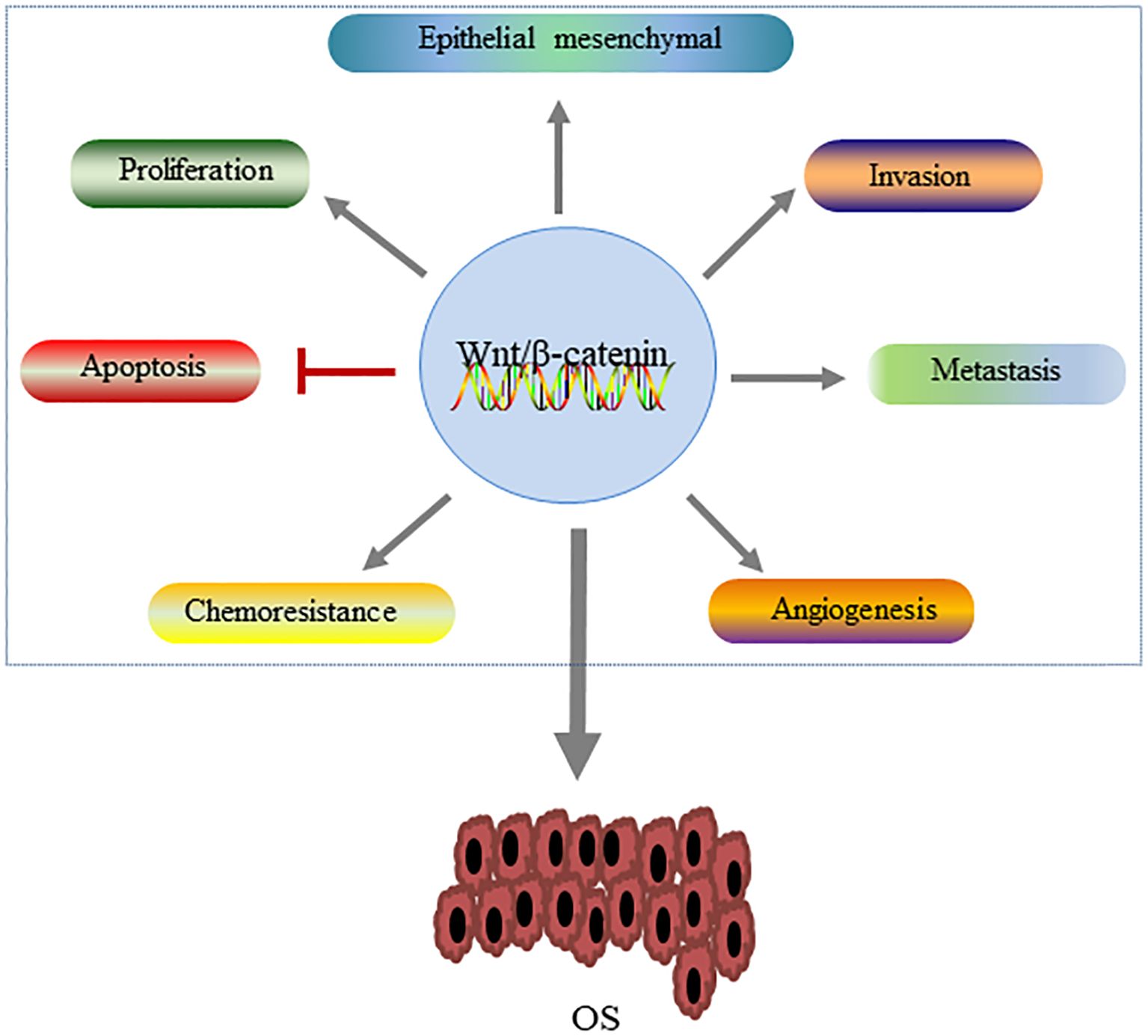
Figure 4. The role of the Wnt/β-catenin pathway in the development of OS. Activation of this signaling pathway regulates OS by promoting tumor cell proliferation, epithelial-mesenchymal transformation and tumor cell invasion and metastasis, angiogenesis and chemical resistance, and inhibiting apoptosis.
The autonomous growth of cancer cells is usually controlled by changes in the expression of growth factors or growth factor receptors, resulting in cell proliferation. Many studies have shown that abnormal activation of Wnt/β-catenin signaling pathway plays a crucial role in the occurrence and development of osteosarcoma (38, 48, 49). Cell proliferation is an important feature of tumorigenesis and development, and also an important factor leading to poor prognosis. A large number of studies have shown that abnormal activation of Wnt signaling and high expression of β-catenin in OS are associated with abnormal histological morphology and cell proliferation and differentiation of osteosarcoma, ultimately leading to the occurrence of osteosarcoma (45, 50). When the level of β-catenin in the cytoplasm reaches a certain concentration, it begins to transfer to the nucleus and bind specifically to the nuclear transcription factor TCF/LEF, resulting in the exposure of downstream target gene promoter, activation and expression of promoter, causing abnormal cell proliferation and anti-apoptosis, thus promoting the formation of tumors (51). Huang et al. found that the Wnt/β-catenin pathway was activated in tumor tissues of patients with OS, and the activated Wnt/β-catenin pathway induced cell cycle progression and promoted the proliferation of OS cells. While cinnamaldehyde can inhibit Wnt/β-catenin signaling activity by down-regulating the phosphorylation of β-catenin and GSK-β as well as downstream target proteins c-Myc and MMP7, thereby inhibiting cell proliferation and promoting tumor cell apoptosis (52). In another study, activation of the Wnt/β-catenin pathway accelerated the progression of osteosarcoma, while inhibition of the activity of this pathway significantly inhibited the proliferation, migration, and invasion of osteosarcoma cells (53). In addition, Chen et al. (37) demonstrated abnormal activation of Wnt/β-catenin signaling in osteosarcoma cells, involving the autocrine Wnt signaling cycle and the upregulation of specific Wnt ligands and receptors. Activation of Wnt/β-catenin signaling with Wnt3a or GSK-3β inhibitors drives proliferation of osteosarcoma cells, whereas downregulation of activated Wnt signaling with dnTCF4 or siLEF1 inhibits proliferation and induces cell cycle arrest. From these studies, it was determined that activation of the Wnt/β-catenin pathway can promote cell proliferation in OS, thereby accelerating OS progression. Inhibition of this pathway can be an effective target to inhibit the progression of OS.
Apoptosis is a kind of programmed cell death, which is an important process of normal development and tissue homeostasis. In many cancers, including osteosarcoma, apoptosis is the primary mechanism by which chemotherapy and radiotherapy induce cell death. The balance between cell proliferation and apoptosis in cancer cells is disturbed and leads to excessive proliferation of cancer cells through different molecular mechanisms (e.g. resistance to apoptosis). A large number of studies have shown that Wnt/β-catenin signaling pathway plays an important role in regulating the apoptosis process of tumor cells and is a key gene mediator involved in tumor cell apoptosis (12). Anti-apoptosis is a common feature of cancer cells, which is associated with increased expression of anti-apoptotic factors such as Bcl-2 or Bcl-xL or decreased expression, inactivation or mutation of pro-apoptotic factors such as Foxo3a or p53 (54). Interestingly, Wnt/β-catenin directly regulates an effective anti-apoptotic pathway and can also activate the expression of several anti-apoptotic genes, Bcl-2, Bad and Bcl-xl (55). Activation of the Wnt/β-catenin signaling pathway during cancer progression can promote the viability of osteosarcoma cells and inhibit apoptosis, and inhibition of this signaling can reverse this phenomenon (56). The mechanism may be through inhibiting Bcl-2 and promoting the expression levels of Bad and Bax (57). Studies have shown that miR-1-3p can deactivate Wnt/β-catenin signaling activity, thereby inhibiting the proliferation and cell cycle progression of osteosarcoma cells and promoting cell apoptosis (58). In addition, studies have also found that melittin is an anti-tumor Chinese medicine with few side effects, which can inhibit the activity of Wnt/β-catenin signaling pathway, up-regulate the ratio of Bax/Bcl-2 in osteosarcoma cells and inhibit the expression of proliferative protein, thus inducing apoptosis and inhibiting proliferation of tumor cells (59). These studies indicate that the abnormal expression of Wnt/β-catenin pathway plays an important role in the regulation of OS, and the activation of NF-κB pathway can inhibit the apoptosis of cancer cells. This allows targeting the Wnt/β-catenin pathway to eliminate the inhibition of OS cell apoptosis, so targeting Wnt/β-catenin may be a potential means to treat OS.
Epithelial mesenchymal transformation (EMT) is a process of migration of adherent epithelial cells to mesenchymal cells under certain conditions. It gives epithelial cells the characteristics of mesenchymal cells. In malignant tumors, EMT is closely related to the invasion and metastasis of tumor cells (60, 61). Proliferation and invasion play a crucial role in the progression of malignant tumors, including OS (7). There is growing evidence that the Wnt/β-catenin pathway promotes these aggressive behaviors (62, 63). On the other hand, osteosarcoma is an interstitial tumor, and patients with high malignancy tend to have distant metastases at an early stage and have a poor prognosis, in which EMT plays an important role (64). EMT is an important mechanism of embryogenesis, wound healing, fibrosis and other physiological processes (65–67), as well as an important process of distant metastasis and migration of tumor cells (68). Studies have shown that EMT plays an important role in the metastasis and migration progression of various tumor cells, including OS (69–71). It is well known that there are multiple interactions between molecular pathways and EMT mechanisms that promote cancer cell invasion. Many studies have shown that Wnt signaling pathway increases the invasion and malignancy of tumor cells through EMT induction (72–74). Wnt-induced EMT can enhance the proliferation of cancer cells and trigger their resistance to apoptosis (75, 76). The Wnt/β-catenin pathway is activated in OS, and the activated Wnt/β-catenin pathway promotes the migration and invasion of OS cells by enhancing the epithelial-mesenchymal transformation of OS (77). At the same time, activation of the Wnt/β-catenin pathway was associated with prognosis in patients with OS, and this study found that patients with over-activation of this pathway had significantly lower prognosis than patients with expression (78). Similar findings have been reported in another study of microRNAs on cancer occurrence and malignant progression in various tumors. This study found that inhibition of Wnt/β-catenin pathway activity could not only inhibit the EMT of OS, but also inhibit the progression and prognosis of OS (79). The invasion and metastasis of tumor cells are the main causes of death in patients with malignant tumors, and about 90% of patients with malignant tumors die from tumor metastasis (80). Studies on the prognosis of OS have found that the Wnt-β-catenin pathway can be used as a biological marker of metastasis (5). Lung metastasis is the most common site of osteosarcoma and the most common cause of death (81). Therefore, metastasis prediction is of great significance in the design of treatment strategies. Since the expression of β-catenin in the cytoplasm is significantly associated with the incidence of distant metastasis (82), β-catenin has been used as a biomarker for the potential of OS metastasis to the lung (81). β-catenin is a key component of the Wnt/β-catenin signaling pathway, relocating from the cytoplasm to the nucleus after Wnt ligand stimulation, thereby regulating gene expression (83). Wnt/β-catenin may also promote cancer cell migration and invasion by inducing the expression of transfer-related proteins such as intercellular adhesion molecule-1 (ICAM-1) and matrix metalloproteinases (MMPs) (84–88). It has been reported that inhibiting the Wnt/β-catenin pathway in OS cells MG-63 can inhibit the expression of MMP14, thus inhibiting the invasion and movement of MG-63 cells (89). In addition, Fu et al. (90) also found that the activation of Wnt/β-catenin in OS can promote the activity of MMP-9, and the enhancement of the activity of the latter can promote the invasion and metastasis of OS cells. In addition, KLF5 is a positive regulator of the Wnt/β-catenin signaling pathway, and KLF5 can increase β-catenin expression and interact directly with β-catenin to stabilize it and promote its nuclear transfer (91). ML264 is a small molecule inhibitor of KLF5, and ML264 can inhibit the activity of Wnt/β-catenin signaling pathway, thereby inducing G0/G1 cell cycle arrest and inhibiting the migration and invasion ability of osteosarcoma cells (92). Based on the above studies, it can be determined that the activation of Wnt/β-catenin can promote the invasion and migration of OS, and inhibiting the activation of Wnt/β-catenin signaling pathway may be a potential therapeutic target to prevent the deterioration of osteosarcoma.
Angiogenesis is a complex biological process that leads to the development of new blood vessels and plays a key role in the progression and metastasis potential of various malignant tumors (93). In the process of angiogenesis, VEGF is the most important factor of angiogenesis, which can increase capillary permeability and promote the migration of tip cells (94). MMPs accelerated the proliferation and differentiation of endothelial cells cultured on type IV and type I collagen in a dose-dependent manner (95). It has been confirmed that Wnt/β-catenin signaling pathway plays an important role in the regulation of tumor angiogenesis. Studies have shown that activation of Wnt/β-catenin pathway can promote cytoskeletal recombination and new blood vessel formation of endothelial cells, possibly through promoting the expression of VEGF and MMPs (96, 97). In addition, transcriptional regulation of VEGF by β-catenin/TCF complex involves TCF binding sites in VEGF gene promoters. Activation of Wnt/β-catenin can induce VEGF overexpression, thus promoting angiogenesis (98, 99). However, there are relatively few studies on the role of Wnt/β-catenin pathway in the angiogenesis of OS, and its mechanism has not been fully cleared. However, it can be preliminarily confirmed that the abnormal expression of NF-κB pathway can promote the angiogenesis of OS.
Neoadjuvant therapy and adjuvant chemotherapy in addition to radical surgery have been shown to significantly improve the prognosis of patients with osteosarcoma. At present, the treatment of OS patients is mainly based on radical surgical treatment with standard three-drug chemotherapy regimen (including doxorubicin, cisplatin and high-dose methotrexate) to improve the survival rate (100). However, chemotherapy can create chemotherapy resistance and even lead to disease recurrence or metastasis (101). There are several mechanisms of drug resistance in chemotherapy, including reduced intracellular drug accumulation, drug inactivation, increased DNA repair, and signal transduction pathway perturbation (102). Some studies have found that abnormal activation of the Wnt-β-catenin signaling pathway is involved in chemotherapy resistance of OS cells, especially resistance to standard three-drug chemotherapy. Doxorubicin is an anthracycline antibiotic that is widely used to treat a variety of cancers, including OS. Wnt-β-catenin signaling targeting T-cell factor represses syndecan-2, a key modulator of apoptosis and chemosensitivity in OS cells, contributing to the resistance of OS to doxorubicin (103, 104). Methotrexate (MTX) is another common component of chemotherapy regimens for osteosarcoma. MTX resistance is a problem in OS chemotherapy and one of the mechanisms underlying MTX resistance is associated with Wnt/β-catenin signaling. Ma and colleagues (105) found that knocking down β-catenin increased the sensitivity of Saos2 cells to MTX-induced cell death. Thus, Wnt/β-catenin signaling may contribute to MTX resistance. In addition, it has been found that activation of the Wnt/β-catenin pathway in human OS cells can induce cell resistance to cisplatin, and the use of Wnt/β-catenin pathway inhibitors can improve or reverse the resistance of OS cells to cisplatin (106, 107). In summary, the Wnt/β-catenin pathway is often abnormally expressed in OS and regulates the pathophysiological processes of osteosarcoma cell proliferation and apoptosis, invasion, migration, tumor angiogenesis, and chemical drug resistance.
In addition to the abnormal expression of Wnt/β-catenin pathway, abnormal activation of other signaling pathways, such as phosphoinositol 3-kinase/protein kinase B (PI3K/AKT) and NF-κB signaling pathways, also play an important role in the pathogenesis of OS. Signaling pathways may interact, and enhancement of one signaling pathway may enhance or inhibit the other. In the process of tumor formation and development, Wnt/β-catenin signaling pathway can directly or indirectly interact with other signaling pathways to regulate the pathophysiological processes of OS. This section describes crosstalk between Wnt/β-catenin and the PI3K/Akt and NF-κB pathways in OS.
Phosphatidylinositol 3-kinase/protein kinase B (PI3K/Akt) signaling pathway is an important signal transduction bridge connecting extracellular signals and cellular responses (108). Phosphatidylinositol 3-kinase (PI3K) is a large class of signaling lipases, a family of enzymes that phosphorylates the 3’-OH of phosphatidylinositol inositol rings (109). Protein kinase B (Akt), an evolutionarily conserved serine protein kinase of the serine/threonine kinase family, is a central mediator in PI3K signaling (110). The PI3K/Akt signaling pathway is a highly conserved signal transduction network in eukaryotic cells and can promote cell survival, cell growth and cell cycle progression (111, 112). It has been reported that the PI3K/Akt signaling pathway is a common activation pathway in human cancer, and it is believed that the dysfunction of this pathway will drive the occurrence and development of cancer and participate in the regulation of cancer pathological process (113–115).
The PI3K/Akt and Wnt/β-catenin signaling pathways are generally not abnormally activated in a variety of tumors, including osteosarcoma (116, 117). These two pathways play an important role in the occurrence and development of osteosarcoma by regulating cell cycle, inhibiting apoptosis, promoting angiogenesis, enhancing metastasis and inducing chemotherapy resistance (118, 119). GSK-3β is a key protein in the downstream PI3K/Akt pathway (120), and activation of PI3K/Akt pathway can significantly inhibit the expression of GSK-3β (121). GSK3-β phosphorylates serine/threonine residues in β-catenin proteins, thereby promoting ubiquitination and subsequent proteasome-mediated β-catenin degradation (41). In addition, it has been found that overexpression of Wnt5a in non-transforming Wnt family members can stimulate the migration of osteosarcoma MG63 cells by promoting the phosphorylation of PI3K and Akt (122). In addition, another transforming member of the Wnt family, Wnt7b, activates mTORC1 via PI3K-AKT signaling, thereby promoting bone formation (123). Therefore, we speculated that PI3K/Akt and Wnt/β-catenin signaling pathways in OS regulate each other, and jointly participate in the regulation of the pathological process of OS.
As an intracellular signaling pathway, NF-κB signaling pathway plays a key role in a variety of physiological and pathological processes, such as inducing immune and inflammatory responses and regulating apoptosis (124–126). The NF-κB signaling pathway has been reported to be an important carcinogenic pathway in human cancers. Dysregulation of this pathway has been found in many types of cancer, such as prostate cancer, colorectal cancer, bladder cancer, breast cancer, and osteosarcoma (127–131). It has been reported that the NF-κB pathway is over-activated in osteosarcoma, leading to excessive proliferation of tumor cells and accelerated development of OS (132). In addition, abnormal expression of this pathway is widely involved in cell processes such as proliferation, apoptosis, cycle, chemotherapy resistance, and metastasis of tumors, including osteosarcoma (133, 134).
Studies have shown that Wnt/β-catenin pathway components can regulate inflammation and immune responses through interaction with NF-κB (135). NF-κB has been shown to indirectly regulate Wnt/β-catenin by regulating target genes that affect β-catenin activity or stability (136). Several studies have shown that the NF-κB and Wnt signaling pathways collaborate at multiple levels in different physiological and pathological contexts. In breast cancer cells, beta-catenin binds to the p65-p50 complex and inhibits its nuclear translocation (137). β-TrCP1 simultaneously activates NF-κB and inhibits the Wnt pathway in vascular smooth muscle cells (138). IKKα and IKKβ are key activators of the NF-κB pathway, which regulate Wnt/β-catenin signaling activity in different ways (139). IKKα inhibitors block the expression of CCND1, a downstream Wnt gene, in mouse embryonic fibroblasts (136).
At present, the main treatment strategies for OS are surgery, radiotherapy and chemotherapy. Although current neoadjuvant chemotherapy has shown efficacy against OS, the long-term survival rate for patients with OS remains low, highlighting the need to find new treatments. As an important pathway regulating cell growth, metabolism, survival, and chemotherapy resistance, targeting the Wnt/β-catenin pathway may be a potential therapeutic approach for patients with OS. This section focuses on the therapeutic effects of non-coding RNAs and drugs on OS by regulating the Wnt/β-catenin signaling pathway.
Non-coding RNA plays an indispensable role in the growth and development of organisms through its influence on transcription and translation. Abnormal expression of NcRNAs has been shown to affect the development and evolution of OS disease. MiRNA s are highly conserved ncRNAs that can affect mRNA expression by binding to the 3′ untranslated region of mRNA (3′UTR) (140). It was found that multiple miRNAs were overexpressed in OS. For example, miR-21-5p (141) and miR-374a (142) are upregulated in OS cells and tissues and promote OS cell migration by activating Wnt/β-catenin signaling, while downregulation of these miRNAs can inhibit the activity of Wnt/β-catenin to inhibit the progression of osteosarcoma. In addition, some miRNAs are underexpressed in OS and participate in the regulation of the pathological process of OS by activating the Wnt/β-catenin signaling pathway. For example, decreased miR-22-3p and increased miR-22-3p in OS tissues and cells inhibit the Wnt/β-catenin pathway by targeting TCF7L2, thereby preventing the progression of osteosarcoma (143). In addition, the expression of miR-1-3p is decreased in osteosarcoma tissues and cells, and upregulation of miR-1-3p inhibits the proliferation and cell cycle process of osteosarcoma cells by targeting CDK14 and inactivating Wnt/β-catenin signaling, while promoting cell apoptosis (58). In addition to the above miRNAs, the overexpression of miR-199b-3p (144) and miR-377-3p (79) can also inhibit the progression of osteosarcoma by inhibiting the Wnt/β-catenin signaling pathway. In addition, overexpression of miR-140 may inhibit the proliferation of human OS cells and may enhance drug sensitivity by directly regulating Wnt/β-catenin signaling (145).
Circular RNAs (circRNAs) are a class of non-coding RNAs characterized by covalently closed loops that have been found in a variety of diseases, including cancer (146). It was found that the expression of various circRNAs in OS was up-regulated and involved in the regulation of its pathological process. For example, Circ_0003732 is up-regulated in osteosarcoma tissues and cells, and activates the Wnt/β-catenin signaling pathway by regulating the miR-377-3p/CPEB1 axis, promoting the proliferation, migration and invasion of osteosarcoma cells, and inhibiting apoptosis. Silencing circ_0003732 can reverse the effect on the progression of osteosarcoma cells (147). The expression of hsa_circ_0087302 is low in osteosarcoma cells, and overexpression of hsa_circ_0087302 can inhibit the proliferation, cell cycle, migration and invasion of osteosarcoma cells by inhibiting the Wnt/β-catenin signaling pathway (148). In addition, circUBAP2 expression is upregulated in osteosarcoma tissues and cells, and knock down circUBAP2 can act as a sponge of miR-506-3p to inhibit Wnt/β-catenin signaling pathway activity, thereby inhibiting cell proliferation, migration and invasion, and promoting apoptosis of cisplatin-resistant osteosarcoma cells (149).
Long non-coding RNAs (LncRNAs) are endogenous ncRNAs with a length > 200 nucleotide transcripts and do not have protein-coding capabilities (150). By acting as a miRNA molecular sponge and competitively binding to miRNA, it is involved in a variety of in vivo pathophysiological processes, including cancer proliferation and invasion (151, 152). UCA1 influences the invasion and migration of osteosarcoma cells by mediating the Wnt/β-catenin pathway through the miR-145/HMGA1 axis (153). lncRNA SNHG10 is overexpressed in OS and acts as a sponge of miR-182-5p to activate the Wnt/β-catenin signaling pathway, promoting the proliferation, migration and invasion of osteosarcoma cells, while downregulation of SNHG10 can inhibit the above results (154). MRPL23-AS1 competitively interacts with miR-30b to activate the Wnt/β-catenin pathway, thus promoting tumorigenesis and metastasis of OS (155). In addition, HOTAIR (156), LncRNA FLVCR1-AS1 (53), CASC15 (157), and LINC00665 (158) are all upregulated in OS cells and affect the cell cycle by activating the Wnt/β-catenin pathway, thereby promoting cell proliferation (Table 1).
At present, it has been found that many drugs can participate in the regulation of the pathological process of OS by inhibiting the activity of Wnt/β-catenin signaling pathway. For example, Resveratrol is a natural phenol (159). It has been reported that the treatment of resveratrol can arrest the cell cycle of various malignant tumors, promote cell apoptosis and inhibit the proliferation of cancer cells (160). In addition, resveratrol inhibits cell growth and induces senescence in OS cells by altering DNA metabolism (161). Zou et al. (30) found that resveratrol could inhibit the expression of β-catenin and c-Myc protein and mRNA, thereby inhibiting the proliferation of OS cells. Another study also came to a similar conclusion that resveratrol can inhibit the activity of Wnt/β-catenin signaling pathway and down-regulate the levels of c-myc, cyclin D1, MMP-2 and MMP-9, thus promoting apoptosis and inhibiting the proliferation and invasion of OS cells (162). Curcumin is a natural compound that comes from the roots of turmeric. Its anti-cancer properties have been demonstrated in many types of cancer, including OS (163, 164). Curcumin has been reported to delay the progression of osteosarcoma by regulating the Wnt/β-catenin pathway in osteosarcoma cells (165). Leow et al. found that curcumin analogs could inhibit the activity of Wnt/β-catenin pathway and prevent the invasion of osteosarcoma cells (90). Baicalein, a flavonoid extracted from the root of scutellaria baicalensis, has been proven to play an anti-tumor role by inhibiting the metastasis of various cancers and inducing apoptosis (166, 167). Studies have shown that baicalin can delay the progression of osteosarcoma by inhibiting the Wnt/β-catenin signaling pathway (168). Melatonin is a natural derivative of tryptophan, an amino acid with various biological activities (169), which plays a key inhibitory role in the pathogenesis of various types of tumors (170, 171). Li et al. (172) found that melatonin inhibited the expression of lncRNA JPX by regulating the Wnt/β-catenin pathway, thereby inhibiting the progression of OS. In addition, Oridonin (173), dihydroartemisinin (174), polyfolin I (175) and oleandrin (176) can also inhibit cell proliferation and induce apoptosis by inhibiting the activity of Wnt/β-catenin pathway, thus inhibiting the progression of OS (Table 2). Based on the above study, it is not difficult to find that targeting the Wnt/β-catenin pathway may be an innovative approach to treat osteosarcoma.
Due to the complexity of the tumor microenvironment, it is difficult to control the progression and recurrence of cancer with a single traditional treatment. Therefore, the combination treatment strategy has gradually become an inevitable trend in cancer treatment (177). Through combination therapy, Wnt/β-catenin signaling pathway inhibitors combined with other drugs can promote therapeutic efficacy and improve prognosis. Hattinger CM et al. used lithium carbonate in combination with neoadjuvant chemotherapy and 9-ING-41 in combination with doxorubicin to effectively inhibit GSK3-β, thereby enhancing the inhibitory effect on Wnt/β-catenin signaling pathway activity (178). In addition, preclinical studies by Leow et al. showed that inhibiting the Wnt/β-catenin pathway with curcumin and PKF118-310 reduced nuclear β-catenin levels, which in turn reduced intrinsic and activated β-catenin/TCF transcriptional activity and, consequently, the expression of β-catenin target genes. This resulted in the down-regulation of MMP-9, a reduction in the expression of cyclin-D, c-MYC, and survivin, and inhibition of the potential for migration. This had a suppressive effect on cell proliferation and increased cell mortality (165).
Wnt/β-catenin signaling pathway is an intracellular signaling pathway that is finely regulated, and abnormal expression of this pathway plays a crucial role in the occurrence and development of a variety of malignant tumors, including OS. Structural activation of Wnt/β-catenin is a novel hallmark of various tumor types, and many in vitro and animal models have shown that this pathway is involved in multiple steps of cell proliferation, apoptosis, epithelial-mesenchymal transformation, tumor angiogenesis, invasion, and metastasis through complex molecular mechanisms. At present, under the multi-science and multi-mode therapy, the research work to improve the effect of chemotherapy has led to the improvement of the survival rate of patients, but the prognosis of OS is not satisfactory, so molecular targeted therapy has gradually attracted widespread attention. Combined with the role of the Wnt/β-catenin pathway in the progression of OS, it can be seen that this pathway may be a potential target for OS therapy, and targeting the Wnt/β-catenin pathway may be an innovative approach for the treatment of osteosarcoma and a potential target for cancer drug development.
Most existing preclinical trial reports suggest that the Wnt/β-catenin pathway plays an important role in OS. Since Wnt/β-catenin signal plays a variety of functions in the pathological process of OS, it is generally difficult to verify the specific role of this signaling pathway in OS. Current epidemiological studies on Wnt/β-catenin and OS have some limitations, including lack of depth of study design, incomplete study subjects, and traditional targeted drug delivery. At a macro level, most of the current research designs on the Wnt/β-catenin pathway in the field of OS are still at the stage of cell or rodent research, and different experimental conditions and modeling will inevitably produce different experimental results. At the same time, there is a lack of clinical research in this field. At the micro level, current studies have focused on the protein expression of the Wnt/β-catenin pathway, rather than the gene and single-cell level. The development of OS is a complex process involving multiple molecular signaling pathways, so the interaction of the Wnt/β-catenin signaling pathway with other pathways in OS is also noteworthy. In terms of drug delivery, although some drugs are already in the preclinical research stage, the route of administration largely determines the therapeutic effect, efficacy and safety of drugs (179). Due to the first-pass effect of oral administration, only a small amount of the active ingredient can reach the designated site. Multiple adverse reactions may also be induced, thus limiting clinical application (180).
Wnt/β-catenin is expressed not only in cancer cells, but also in healthy cells, which may lead to unexpected effects of treatment. Therefore, one of the major challenges facing the use of Wnt/beta-catenin inhibitors in the treatment of osteosarcoma is safety and efficacy. Blocking or disabling Wnt/β-catenin signaling may impair immunity or induce toxic reactions. In addition to the development of cancer, many other factors, such as microbial infections or physical and chemical damage, can cause damage to the body (181). Therefore, systemic administration of Wnt/β-catenin inhibitors may worsen immune function or response. The degree of toxicity depends on the dose, time, and baseline health of the patient. Strategies to mitigate these toxicities and improve safety therefore include dose optimization and the use of modified or alternative Wnt/β-catenin-targeting drugs (182). Tumor heterogeneity is also a challenge for Wnt/β-catenin targeted therapies, as some cancer cells may have different levels of Wnt/β-catenin expression, which can lead to differences in the sensitivity of different cells to Wnt/β-catenin targeted therapies. In addition, due to the presence of immunosuppressive cells such as tumor-associated macrophages and tumor-associated neutrophils, cancer cells can upregulate immune checkpoint molecules to evade immune surveillance and escape immune cell destruction, which interferes with the effectiveness of Wnt/β-catenin targeted therapy (183). At presently, some challenges remain existed in the treatment accurately targeting the Wnt/β-catenin signaling pathway. The biggest obstacle is devoid of reliable predictive biomarkers that can identify patients who will be most likely to benefit from these types of therapy.
To overcome these challenges, a variety of strategies are being developed. One approach is to combine targeted therapies with other immune checkpoint inhibitors to overcome the immune escape mechanism adopted by tumor cells (184). Another approach is to develop bispecic antibodies that can simultaneously target Wnt/β-catenin and another immune checkpoint molecule. Finally, other advanced and efficient techniques and methods should be used to enhance the efficacy of Wnt/β-catenin targeted therapy.
The mechanism of action of the Wnt/β-catenin pathway in OS has been widely discussed, but there is still much to be improved. First, due to the lack of large-scale multi-center clinical trials, the specific molecular mechanisms leading to the activation or inhibition of the Wnt/β-catenin signaling pathway during OS have not been cleared. Therefore, future studies should focus on large-scale clinical studies and pharmacological studies to further explore the specific mechanism of action of Wnt/β-catenin signaling pathway in OS, and strive to provide reliable medical evidence for the development of clinical treatment for patients with OS. Second, current studies targeting the Wnt/β-catenin pathway for OS treatment have been too conventional. Future studies are needed to determine how to link targeted Wnt/β-catenin with corresponding front-line therapies for OS with improved drug delivery strategies leading to internalization to achieve in vivo drug delivery, targeted drug release, and biological activity to enhance drug administration efficiency, improve therapeutic effectiveness, and maximize targeting and reduce resistance. It is continuously released to enhance the efficiency of drug use, improve the therapeutic effect and reduce side effects (185). In particular, mesenchymal stem cells, nanoparticles and hydrogels are used. The main limitation of nanoparticles, however, is their toxic profile. Due to the wide variety of nanoparticles, the toxicity characteristics of nanoparticles are not well characterized, and the toxicity assessment of each class of these nanoparticles requires great efforts.
As an intracellular signaling pathway, abnormal activation of Wnt/β-catenin signaling pathway can promote the proliferation of osteosarcoma cells, epithelial-mesenchymal transformation, invasion and metastasis, tumor angiogenesis, and chemical resistance of tumor cells, and inhibit the activity of Wnt/β-catenin signaling pathway can delay the progression of osteosarcoma. Based on the role of the Wnt/β-catenin pathway in the development of OS, we suggest that targeting or manipulating the expression or function of the relevant Wnt/β-catenin signaling pathway may be an innovative approach to the treatment of OS and a potential target for cancer drug development.
YD: Writing – original draft, Writing – review & editing. QC: Writing – original draft, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work is supported by funding of Ganzhou City “Science and technology and National Regional Medical Center” joint project (No. 2022-YB1396), Ganzhou City guiding science and technology plan project (No. 20222ZDX7705) and Ganzhou Municipal Science and Technology Project (GZ2022ZSF096).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Yin F, Wang Z, Jiang Y, Zhang T, Wang Z, Hua Y, et al. Reduction-responsive polypeptide nanomedicines significantly inhibit progression of orthotopic osteosarcoma. Nanomedicine. (2020) 23:102085. doi: 10.1016/j.nano.2019.102085
2. Luetke A, Meyers PA, Lewis I, Juergens H. Osteosarcoma treatment - where do we stand? A state of the art review. Cancer Treat Rev. (2014) 40:523–32. doi: 10.1016/j.ctrv.2013.11.006
3. Bielack S, Carrle D, Casali PG. Osteosarcoma: ESMO clinical recommendations for diagnosis, treatment and follow-up. Ann Oncol. (2009) 20 Suppl 4:137–9. doi: 10.1093/annonc/mdp154
4. Ottaviani G, Jaffe N. The etiology of osteosarcoma. Cancer Treat Res. (2009) 152:15–32. doi: 10.1007/978-1-4419-0284-9_2
5. Mirabello L, Troisi RJ, Savage SA. Osteosarcoma incidence and survival rates from 1973 to 2004: data from the Surveillance, Epidemiology, and End Results Program. Cancer. (2009) 115:1531–43. doi: 10.1002/cncr.v115:7
6. Suehara Y, Alex D, Bowman A, Middha S, Zehir A, Chakravarty D, et al. Clinical genomic sequencing of pediatric and adult osteosarcoma reveals distinct molecular subsets with potentially targetable alterations. Clin Cancer Res. (2019) 25:6346–56. doi: 10.1158/1078-0432.CCR-18-4032
7. Zhang J, Yu X-H, Yan Y-G, Wang C, Wang W-J. PI3K/Akt signaling in osteosarcoma. Clin Chim Acta. (2015) 444:182–92. doi: 10.1016/j.cca.2014.12.041
8. Panez-Toro I, Muñoz-García J, Vargas-Franco JW, Renodon-Cornière A, Heymann M-F, Lézot F, et al. Advances in osteosarcoma. Curr osteoporosis Rep. (2023) 21:330–43. doi: 10.1007/s11914-023-00803-9
9. Self C, Macquarrie KL, Cost CR. Osteosarcoma/ewing sarcoma. Pediatr Rev. (2022) 43:256–65. doi: 10.1542/pir.2021-005065
10. Fuloria S, Yadav G, Menon SV, Ali H, Pant K, Kaur M, et al. Targeting the Wnt/β-catenin cascade in osteosarcoma: The potential of ncRNAs as biomarkers and therapeutics. Pathology Res Pract. (2024) 259:155346. doi: 10.1016/j.prp.2024.155346
11. Liu J, Xiao Q, Xiao J, Niu C, Li Y, Zhang X, et al. Wnt/β-catenin signalling: function, biological mechanisms, and therapeutic opportunities. Signal transduction targeted Ther. (2022) 7:3. doi: 10.1038/s41392-021-00762-6
12. Singla A, Wang J, Yang R, Geller DS, Loeb DM, Hoang BH. Wnt signaling in osteosarcoma. Adv Exp Med Biol. (2020) 1258:125–39. doi: 10.1007/978-3-030-43085-6_8
13. Ji H, Kong L, Wang Y, Hou Z, Kong W, Qi J, et al. CD44 expression is correlated with osteosarcoma cell progression and immune infiltration and affects the Wnt/β-catenin signaling pathway. J Bone Oncol. (2023) 41:100487. doi: 10.1016/j.jbo.2023.100487
14. Nusse R, Varmus H. Three decades of Wnts: a personal perspective on how a scientific field developed. EMBO J. (2012) 31:2670–84. doi: 10.1038/emboj.2012.146
15. Cisternas P, Henriquez JP, Brandan E, Inestrosa NC. Wnt signaling in skeletal muscle dynamics: myogenesis, neuromuscular synapse and fibrosis. Mol Neurobiol. (2014) 49:574–89. doi: 10.1007/s12035-013-8540-5
16. Varela-Nallar L, Inestrosa NC. Wnt signaling in the regulation of adult hippocampal neurogenesis. Front Cell Neurosci. (2013) 7:100. doi: 10.3389/fncel.2013.00100
17. Ríos JA, Cisternas P, Arrese M, Barja S, Inestrosa NC. Is Alzheimer’s disease related to metabolic syndrome? A Wnt signaling conundrum. Prog Neurobiol. (2014) 121:125–46. doi: 10.1016/j.pneurobio.2014.07.004
18. Fang F, Vancleave A, Helmuth R, Torres H, Rickel K, Wollenzien H, et al. Targeting the Wnt/β-catenin pathway in human osteosarcoma cells. Oncotarget. (2018) 9:36780–92. doi: 10.18632/oncotarget.26377
19. Trautmann M, Sievers E, Aretz S, Kindler D, Michels S, Friedrichs N, et al. SS18-SSX fusion protein-induced Wnt/β-catenin signaling is a therapeutic target in synovial sarcoma. Oncogene. (2014) 33:5006–16. doi: 10.1038/onc.2013.443
20. Vijayakumar S, Liu G, Rus IA, Yao S, Chen Y, Akiri G, et al. High-frequency canonical Wnt activation in multiple sarcoma subtypes drives proliferation through a TCF/β-catenin target gene, CDC25A. Cancer Cell. (2011) 19:601–12. doi: 10.1016/j.ccr.2011.03.010
21. Du Z, Li F, Wang L, Huang H, Xu S. Regulatory effects of microRNA−184 on osteosarcoma via the Wnt/β−catenin signaling pathway. Mol Med Rep. (2018) 18:1917–24. doi: 10.3892/mmr.2018.9184
22. Monroe DG, Mcgee-Lawrence ME, Oursler MJ, Westendorf JJ. Update on Wnt signaling in bone cell biology and bone disease. Gene. (2012) 492:1–18. doi: 10.1016/j.gene.2011.10.044
23. Duchartre Y, Kim YM, Kahn M. The Wnt signaling pathway in cancer. Crit Rev oncology/hematol. (2016) 99:141–9. doi: 10.1016/j.critrevonc.2015.12.005
24. Clevers HJC. Wnt/β-catenin signaling in development and disease. Cell. (2006) 127:469–80. doi: 10.1016/j.cell.2006.10.018
25. Polakis P. Wnt signaling in cancer. Cold Spring Harb Perspect Biol. (2012) 4:a008052. doi: 10.1101/cshperspect.a008052
26. Tai D, Wells K, Arcaroli J, Vanderbilt C, Aisner DL, Messersmith WA, et al. Targeting the WNT signaling pathway in cancer therapeutics. Oncologist. (2015) 20:1189–98. doi: 10.1634/theoncologist.2015-0057
27. Le PN, Keysar SB, Miller B, Eagles JR, Chimed T-S, Reisinger J, et al. Wnt signaling dynamics in head and neck squamous cell cancer tumor-stroma interactions. Mol carcinogenesis. (2019) 58:398–410. doi: 10.1002/mc.22937
28. Zhao H, Ming T, Tang S, Ren S, Yang H, Liu M, et al. Wnt signaling in colorectal cancer: pathogenic role and therapeutic target. Mol Cancer. (2022) 21:144. doi: 10.1186/s12943-022-01616-7
29. Nusse R, Clevers HJC. Wnt/β-catenin signaling, disease, and emerging therapeutic modalities. Cell. (2017) 169:985–99. doi: 10.1016/j.cell.2017.05.016
30. Zou Y, Yang J, Jiang D. Resveratrol inhibits canonical Wnt signaling in human MG-63 osteosarcoma cells. Mol Med Rep. (2015) 12:7221–6. doi: 10.3892/mmr.2015.4338
31. Komiya Y, Habas R. Wnt signal transduction pathways. Organogenesis. (2008) 4:68–75. doi: 10.4161/org.4.2.5851
32. Clark HF, Brentrup D, Schneitz K, Bieber A, Goodman C, Noll M. Dachsous encodes a member of the cadherin superfamily that controls imaginal disc morphogenesis in Drosophila. Genes Dev. (1995) 9:1530–42. doi: 10.1101/gad.9.12.1530
33. Hsieh JC, Rattner A, Smallwood PM, Nathans J. Biochemical characterization of Wnt-frizzled interactions using a soluble, biologically active vertebrate Wnt protein. Proc Natl Acad Sci U.S.A. (1999) 96:3546–51. doi: 10.1073/pnas.96.7.3546
34. Bhanot P, Brink M, Samos CH, Hsieh JC, Wang Y, Macke JP, et al. A new member of the frizzled family from Drosophila functions as a Wingless receptor. Nature. (1996) 382:225–30. doi: 10.1038/382225a0
35. He X, Semenov M, Tamai K, Zeng X. LDL receptor-related proteins 5 and 6 in Wnt/beta-catenin signaling: arrows point the way. Development. (2004) 131:1663–77. doi: 10.1242/dev.01117
36. Gordon MD, Nusse R. Wnt signaling: multiple pathways, multiple receptors, and multiple transcription factors. J Biol Chem. (2006) 281:22429–33. doi: 10.1074/jbc.R600015200
37. Chen C, Zhao M, Tian A, Zhang X, Yao Z, Ma X. Aberrant activation of Wnt/β-catenin signaling drives proliferation of bone sarcoma cells. Oncotarget. (2015) 6:17570–83. doi: 10.18632/oncotarget.v6i19
38. Iwaya K, Ogawa H, Kuroda M, Izumi M, Ishida T, Mukai K. Cytoplasmic and/or nuclear staining of beta-catenin is associated with lung metastasis. Clin Exp Metastasis. (2003) 20:525–9. doi: 10.1023/A:1025821229013
39. Zhou L, Park BH, Park JH, Jang KY, Park HS, Wagle S, et al. Overexpression of the prolyl isomerase PIN1 promotes cell growth in osteosarcoma cells. Oncol Rep. (2013) 29:193–8. doi: 10.3892/or.2012.2112
40. Yang JZ, Zhang XH, Liu JR, Ding Y, Gao F, Wang Y. Expression and significance of N-cadherin and β-catenin protein in osteosarcoma. Zhonghua Zhong Liu Za Zhi. (2010) 32:586–9.
41. Liu W, Zhao Z, Wang Y, Li W, Su Q, Jia Q, et al. Dioscin inhibits stem-cell-like properties and tumor growth of osteosarcoma through Akt/GSK3/β-catenin signaling pathway. Cell Death Dis. (2018) 9:343. doi: 10.1038/s41419-018-0363-x
42. Lu Y, Guan GF, Chen J, Hu B, Sun C, Ma Q, et al. Aberrant CXCR4 and β-catenin expression in osteosarcoma correlates with patient survival. Oncol Lett. (2015) 10:2123–9. doi: 10.3892/ol.2015.3535
43. Haydon RC, Deyrup A, Ishikawa A, Heck R, Jiang W, Zhou L, et al. Cytoplasmic and/or nuclear accumulation of the beta-catenin protein is a frequent event in human osteosarcoma. Int J Cancer. (2002) 102:338–42. doi: 10.1002/ijc.v102:4
44. Major MB, Roberts BS, Berndt JD, Marine S, Anastas J, Chung N, et al. New regulators of Wnt/beta-catenin signaling revealed by integrative molecular screening. Sci Signal. (2008) 1:ra12. doi: 10.1126/scisignal.2000037
45. Lin CH, Ji T, Chen CF, Hoang BH. Wnt signaling in osteosarcoma. Adv Exp Med Biol. (2014) 804:33–45. doi: 10.1007/978-3-319-04843-7_2
46. Rabbani SA, Arakelian A, Farookhi R. LRP5 knockdown: effect on prostate cancer invasion growth and skeletal metastasis in vitro and in vivo. Cancer Med. (2013) 2:625–35. doi: 10.1002/cam4.2013.2.issue-5
47. Chu T, Teng J, Jiang L, Zhong H, Han B. Lung cancer-derived Dickkopf1 is associated with bone metastasis and the mechanism involves the inhibition of osteoblast differentiation. Biochem Biophys Res Commun. (2014) 443:962–8. doi: 10.1016/j.bbrc.2013.12.076
48. Lin CH, Guo Y, Ghaffar S, McQueen P, Pourmorady J, Christ A, et al. Dkk-3, a secreted wnt antagonist, suppresses tumorigenic potential and pulmonary metastasis in osteosarcoma. Sarcoma. (2013) 2013:147541. doi: 10.1155/2013/147541
49. Cai Y, Cai T, Chen Y. Wnt pathway in osteosarcoma, from oncogenic to therapeutic. J Cell Biochem. (2014) 115:625–31. doi: 10.1002/jcb.24708
50. Di Fiore R, Guercio A, Puleio R, Di Marco P, Drago-Ferrante R, D’Anneo A, et al. Modeling human osteosarcoma in mice through 3AB-OS cancer stem cell xenografts. J Cell Biochem. (2012) 113:3380–92. doi: 10.1002/jcb.v113.11
51. Liu C, Li Y, Semenov M, Han C, Baeg GH, Tan Y, et al. Control of beta-catenin phosphorylation/degradation by a dual-kinase mechanism. Cell. (2002) 108:837–47. doi: 10.1016/S0092-8674(02)00685-2
52. Huang Y, Chen J, Yang S, et al. Cinnamaldehyde inhibits the function of osteosarcoma by suppressing the Wnt/β-catenin and PI3K/Akt signaling pathways. Drug Des Devel Ther. (2020) 14:4625–37. doi: 10.2147/DDDT.S277160
53. Jiang S, Kong P, Liu X, Yuan C, Peng K, Liang Y. LncRNA FLVCR1-AS1 accelerates osteosarcoma cells to proliferate, migrate and invade via activating wnt/β-catenin pathway. J Buon: Off J Balkan Union Oncol. (2020) 25:2078–85.
54. Chen Y, Chen S, Liang H, Yang H, Liu L, Zhou K, et al. Bcl-2 protects TK6 cells against hydroquinone-induced apoptosis through PARP-1 cytoplasm translocation and stabilizing mitochondrial membrane potential. Environ Mol mutagenesis. (2018) 59:49–59. doi: 10.1002/em.22126
55. Sha L, Ma D, Chen C. Exosome-mediated Hic-5 regulates proliferation and apoptosis of osteosarcoma via Wnt/β-catenin signal pathway. Aging. (2020) 12:23598–608. doi: 10.18632/aging.103546
56. Wei Z, Zheng D, Pi W, Qiu Y, Xia K, Guo W. Isoquercitrin restrains the proliferation and promotes apoptosis of human osteosarcoma cells by inhibiting the Wnt/β-catenin pathway. J Bone Oncol. (2023) 38:100468. doi: 10.1016/j.jbo.2023.100468
57. Yang C, Zhang L, Huang H, Yuan X, Zhang P, Ye C, et al. Alantolactone inhibits proliferation, metastasis and promotes apoptosis of human osteosarcoma cells by suppressing Wnt/β-catenin and MAPKs signaling pathways. Genes Dis. (2022) 9:466–78. doi: 10.1016/j.gendis.2020.07.014
58. Zhang G, Guan Q, Zhao Y, Wang S, Li H. miR-1-3p inhibits osteosarcoma cell proliferation and cell cycle progression while promoting cell apoptosis by targeting CDK14 to inactivate Wnt/beta-catenin signaling. Mol Biotechnol. (2023) 66:1704–17. doi: 10.1007/s12033-023-00811-1
59. Xie X, Li Y, Zhu H, Chen L, Chen D, Lin S, et al. Melittin Inhibits Growth of Human Osteosarcoma 143B Cells through Induction of Apoptosis via Suppressing the Wnt/β-catenin Signaling Pathway. Anti-cancer Agents medicinal Chem. (2022) 22:3172–81. doi: 10.2174/1871520622666220509121627
60. Dudas J, Ladanyi A, Ingruber J, Steinbichler TB, Riechelmann H. Epithelial to mesenchymal transition: A mechanism that fuels cancer radio/chemoresistance. Cells. (2020) 9:428. doi: 10.3390/cells9020428
61. Chong ZX, Yeap SK, Ho WY. Unraveling the roles of miRNAs in regulating epithelial-to-mesenchymal transition (EMT) in osteosarcoma. . Pharmacol Res. (2021) 172:105818. doi: 10.1016/j.phrs.2021.105818
62. Lu Q, Huang H, Wang X, Xia H, Zhang L, Xu J, et al. EChinatin inhibits the growth and metastasis of human osteosarcoma cells through Wnt/β-catenin and p38 signaling pathways. Pharmacol Res. (2023) 191:106760. doi: 10.1016/j.phrs.2023.106760
63. Ye C, Wei M, Huang H, Wang Y, Zhang L, Yang C, et al. Nitazoxanide inhibits osteosarcoma cells growth and metastasis by suppressing AKT/mTOR and Wnt/β-catenin signaling pathways. Biol Chem. (2022) 403:929–43. doi: 10.1515/hsz-2022-0148
64. Yu L, Liu S, Guo W, Zhang C, Zhang B, Yan H, et al. hTERT promoter activity identifies osteosarcoma cells with increased EMT characteristics. Oncol Lett. (2014) 7:239–44. doi: 10.3892/ol.2013.1692
65. Haensel D, Dai X. Epithelial-to-mesenchymal transition in cutaneous wound healing: Where we are and where we are heading. Dev Dyn. (2018) 247:473–80. doi: 10.1002/dvdy.v247.3
66. Kim DH, Xing T, Yang Z, Dudek R, Lu Q, Chen Y-H. Epithelial mesenchymal transition in embryonic development, tissue repair and cancer: A comprehensive overview. J Clin Med. (2017) 7:1. doi: 10.3390/jcm7010001
67. Rout-Pitt N, Farrow N, Parsons D, Donnelley M. Epithelial mesenchymal transition (EMT): a universal process in lung diseases with implications for cystic fibrosis pathophysiology. Respir Res. (2018) 19:136. doi: 10.1186/s12931-018-0834-8
68. Dai J, He H, Lin D, Wang C, Zhu Y, Xu D. Up-regulation of E-cadherin by saRNA inhibits the migration and invasion of renal carcinoma cells. Int J Clin Exp Pathol. (2018) 11:5792–800.
69. Liu W, Jiang D, Gong F, Huang Y, Luo Y, Rong Y, et al. miR-210-5p promotes epithelial-mesenchymal transition by inhibiting PIK3R5 thereby activating oncogenic autophagy in osteosarcoma cells. Cell Death Dis. (2020) 11:93. doi: 10.1038/s41419-020-2270-1
70. Hardy KM, Booth BW, Hendrix MJ, Salomon DS, Strizzi L. ErbB/EGF signaling and EMT in mammary development and breast cancer. J Mammary Gland Biol Neoplasia. (2010) 15:191–9. doi: 10.1007/s10911-010-9172-2
71. Al Moustafa AE, Achkhar A, Yasmeen A. EGF-receptor signaling and epithelial-mesenchymal transition in human carcinomas. Front Biosci (Schol Ed). (2012) 4:671–84. doi: 10.2741/s292
72. Yi Z, Pu Y, Gou R, Chen Y, Ren X, Liu W, et al. Silencing of RIPK4 inhibits epithelial−mesenchymal transition by inactivating the Wnt/β−catenin signaling pathway in osteosarcoma. Mol Med Rep. (2020) 21:1154–62. doi: 10.3892/mmr.2020.10939
73. Lu Y, Sun W, Zhang L, Li J. Silencing of MAGI1 promotes the proliferation and inhibits apoptosis of glioma cells via the Wnt/β-catenin and PTEN/AKT signaling pathways. Onco Targets Ther. (2019) 12:9639–50. doi: 10.2147/OTT.S215400
74. Cheng C, Huang Z, Zhou R, An H, Cao G, Ye J, et al. Numb negatively regulates the epithelial-to-mesenchymal transition in colorectal cancer through the Wnt signaling pathway. Am J Physiol Gastrointest Liver Physiol. (2020) 318:G841–g53. doi: 10.1152/ajpgi.00178.2019
75. Wu W, Guo L, Liang Z, Liu Y, Yao Z. Lnc-SNHG16/miR-128 axis modulates Malignant phenotype through WNT/β-catenin pathway in cervical cancer cells. J Cancer. (2020) 11:2201–12. doi: 10.7150/jca.40319
76. Qiao Y, Zhou Y, Song C, Zhang X, Zou Y. MID1 and MID2 regulate cell migration and epithelial-mesenchymal transition via modulating Wnt/β-catenin signaling. Ann Transl Med. (2020) 8:1021. doi: 10.21037/atm-20-5583
77. Zhang Y, Sheng Z, Su C, Xia Y, Chen X, Huang X, et al. Caudatin inhibits the proliferation, invasion, and glycolysis of osteosarcoma cells via the Wnt/β- catenin pathway. Evidence-Based complementary Altern medicine: eCAM. (2022) 2022:4026688. doi: 10.1155/2022/4026688
78. Ding Q, Mo F, Cai X, Zhang W, Wang J, Yang S, et al. LncRNA CRNDE is activated by SP1 and promotes osteosarcoma proliferation, invasion, and epithelial-mesenchymal transition via Wnt/β-catenin signaling pathway. J Cell Biochem. (2020) 121:3358–71. doi: 10.1002/jcb.v121.5-6
79. Liang K, Liao L, Liu Q, Ouyang Q, Jia L, Wu G. microRNA-377-3p inhibits osteosarcoma progression by targeting CUL1 and regulating Wnt/β-catenin signaling pathway. Clin Trans oncology: Off Publ Fed Spanish Oncol Societies Natl Cancer Institute Mexico. (2021) 23:2350–7. doi: 10.1007/s12094-021-02633-6
80. Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. (2009) 139:871–90. doi: 10.1016/j.cell.2009.11.007
82. Bongiovanni L, Mazzocchetti F, Malatesta D, Romanucci M, Ciccarelli A, Buracco P, et al. Immunohistochemical investigation of cell cycle and apoptosis regulators (survivin, β-catenin, p53, caspase 3) in canine appendicular osteosarcoma. BMC Vet Res. (2012) 8:78. doi: 10.1186/1746-6148-8-78
83. Mora-Blanco EL, Mishina Y, Tillman EJ, Cho Y-J, Thom CS, Pomeroy SL, et al. Activation of β-catenin/TCF targets following loss of the tumor suppressor SNF5. Oncogene. (2014) 33:933–8. doi: 10.1038/onc.2013.37
84. Ahmad A, Ahsan H. Ras-mediated activation of NF-κB and DNA damage response in carcinogenesis. Cancer Invest. (2020) 38:185–208. doi: 10.1080/07357907.2020.1721523
85. Iademarco MF, Mcquillan JJ, Rosen GD, Dean DC. Characterization of the promoter for vascular cell adhesion molecule-1 (VCAM-1). J Biol Chem. (1992) 267:16323–9. doi: 10.1016/S0021-9258(18)42004-2
86. Whelan J, Ghersa P, Hooft van Huijsduijnen R, Gray J, Chandra G, Talabot F, et al. An NF kappa B-like factor is essential but not sufficient for cytokine induction of endothelial leukocyte adhesion molecule 1 (ELAM-1) gene transcription. Nucleic Acids Res. (1991) 19:2645–53. doi: 10.1093/nar/19.10.2645
87. Borghaei RC, Rawlings PL Jr., Javadi M, Woloshin J. NF-kappaB binds to a polymorphic repressor element in the MMP-3 promoter. Biochem Biophys Res Commun. (2004) 316:182–8. doi: 10.1016/j.bbrc.2004.02.030
88. Bond M, Fabunmi RP, Baker AH, Newby AC. Synergistic upregulation of metalloproteinase-9 by growth factors and inflammatory cytokines: an absolute requirement for transcription factor NF-kappa B. FEBS Lett. (1998) 435:29–34. doi: 10.1016/S0014-5793(98)01034-5
89. Zhang F, Chen A, Chen J, Yu T, Guo F. SiRNA-mediated silencing of beta-catenin suppresses invasion and chemosensitivity to doxorubicin in MG-63 osteosarcoma cells. Asian Pac J Cancer Prev. (2011) 12:239–45.
90. Leow PC, Bahety P, Boon CP, Lee CY, Tan KL, Yang T, et al. Functionalized curcumin analogs as potent modulators of the Wnt/β-catenin signaling pathway. Eur J Med Chem. (2014) 71:67–80. doi: 10.1016/j.ejmech.2013.10.073
91. Mcconnell BB, Bialkowska AB, Nandan MO, Ghaleb AM, Gordon FJ, Yang VW. Haploinsufficiency of Krüppel-like factor 5 rescues the tumor-initiating effect of the Apc(Min) mutation in the intestine. Cancer Res. (2009) 69:4125–33. doi: 10.1158/0008-5472.CAN-08-4402
92. Huang H, Han Y, Chen Z, Pan X, Yuan P, Zhao X, et al. ML264 inhibits osteosarcoma growth and metastasis via inhibition of JAK2/STAT3 and WNT/β-catenin signalling pathways. J Cell Mol Med. (2020) 24:5652–64. doi: 10.1111/jcmm.v24.10
93. Olsen JJ, Pohl S, Deshmukh A, Visweswaran M, Ward NC, Arfuso F, et al. The role of wnt signalling in angiogenesis. Clin Biochem Rev. (2017) 38:131–42.
94. Chandolu V, Dass CR. Cell and molecular biology underpinning the effects of PEDF on cancers in general and osteosarcoma in particular. J biomedicine Biotechnol. (2012) 2012:740295. doi: 10.1155/2012/740295
95. Huo N, Ichikawa Y, Kamiyama M, Ishikawa T, Hamaguchi Y, Hasegawa S, et al. MMP-7 (matrilysin) accelerated growth of human umbilical vein endothelial cells. Cancer Lett. (2002) 177:95–100. doi: 10.1016/S0304-3835(01)00772-8
96. Dufourcq P, Leroux L, Ezan J, Descamps B, Lamazière J-MD, Costet P, et al. Regulation of endothelial cell cytoskeletal reorganization by a secreted frizzled-related protein-1 and frizzled 4- and frizzled 7-dependent pathway: role in neovessel formation. Am J Pathol. (2008) 172:37–49. doi: 10.2353/ajpath.2008.070130
97. Duplàa C, Jaspard B, Moreau C, D’Amore PA. Identification and cloning of a secreted protein related to the cysteine-rich domain of frizzled. Evidence role endothelial Cell Growth control. Circ Res. (1999) 84:1433–45. doi: 10.1161/01.RES.84.12.1433
98. Easwaran V, Lee SH, Inge L, Guo L, Goldbeck C, Garrett E, et al. beta-Catenin regulates vascular endothelial growth factor expression in colon cancer. Cancer Res. (2003) 63:3145–53.
99. Zhang X, Gaspard JP, Chung DC. Regulation of vascular endothelial growth factor by the Wnt and K-ras pathways in colonic neoplasia. Cancer Res. (2001) 61:6050–4.
100. Chou AJ, Gorlick R. Chemotherapy resistance in osteosarcoma: current challenges and future directions. Expert Rev Anticancer Ther. (2006) 6:1075–85. doi: 10.1586/14737140.6.7.1075
101. D’adamo DR. Appraising the current role of chemotherapy for the treatment of sarcoma. Semin Oncol. (2011) 38 Suppl 3:S19–29. doi: 10.1053/j.seminoncol.2011.09.004
102. He H, Ni J, Huang J. Molecular mechanisms of chemoresistance in osteosarcoma (Review). Oncol Lett. (2014) 7:1352–62. doi: 10.3892/ol.2014.1935
103. Dieudonné FX, Marion A, Haÿ E, Marie PJ, Modrowski D. High Wnt signaling represses the proapoptotic proteoglycan syndecan-2 in osteosarcoma cells. Cancer Res. (2010) 70:5399–408. doi: 10.1158/0008-5472.CAN-10-0090
104. Dieudonné FX, Marion A, Marie PJ, Modrowski D. Targeted inhibition of T-cell factor activity promotes syndecan-2 expression and sensitization to doxorubicin in osteosarcoma cells and bone tumors in mice. J Bone mineral research: Off J Am Soc Bone Mineral Res. (2012) 27:2118–29. doi: 10.1002/jbmr.1650
105. Ma Y, Ren Y, Han EQ, Li H, Chen D, Jacobs JJ, et al. Inhibition of the Wnt-β-catenin and Notch signaling pathways sensitizes osteosarcoma cells to chemotherapy. Biochem Biophys Res Commun. (2013) 431:274–9. doi: 10.1016/j.bbrc.2012.12.118
106. Li Z, Zhao L, Wang Q. Overexpression of long non-coding RNA HOTTIP increases chemoresistance of osteosarcoma cell by activating the Wnt/β-catenin pathway. Am J Transl Res. (2016) 8:2385–93.
107. Scholten DJ 2nd, Timmer CM, Peacock JD, Pelle DW, Williams BO, Steensma MR. Down regulation of Wnt signaling mitigates hypoxia-induced chemoresistance in human osteosarcoma cells. PloS One. (2014) 9:e111431. doi: 10.1371/journal.pone.0111431
108. Fukuchi M, Nakajima M, Fukai Y, Miyazaki T, Masuda N, Sohda M, et al. Increased expression of c-Ski as a co-repressor in transforming growth factor-beta signaling correlates with progression of esophageal squamous cell carcinoma. Int J Cancer. (2004) 108:818–24. doi: 10.1002/ijc.v108:6
109. Tang LA, Dixon BN, Maples KT, Poppiti KM, Peterson TJ. Current and investigational agents targeting the phosphoinositide 3-kinase pathway. Pharmacotherapy. (2018) 38:1058–67. doi: 10.1002/phar.2018.38.issue-10
110. Carmona FJ, Montemurro F, Kannan S, Rossi V, Verma C, Baselga J, et al. AKT signaling in ERBB2-amplified breast cancer. Pharmacol Ther. (2016) 158:63–70. doi: 10.1016/j.pharmthera.2015.11.013
111. Tian LY, Smit DJ, Jücker M. The role of PI3K/AKT/mTOR signaling in hepatocellular carcinoma metabolism. Int J Mol Sci. (2023) 24:2652. doi: 10.3390/ijms24032652
112. Ahmad I, Hoque M, Alam SSM, Zughaibi TA, Tabrez S. Curcumin and plumbagin synergistically target the PI3K/Akt/mTOR pathway: A prospective role in cancer treatment. Int J Mol Sci. (2023) 24:6651. doi: 10.3390/ijms24076651
113. Lee JH, Kim C, Um JY, Sethi G, Ahn KS. Casticin-induced inhibition of cell growth and survival are mediated through the dual modulation of Akt/mTOR signaling cascade. Cancers. (2019) 11:254. doi: 10.3390/cancers11020254
114. Ong PS, Wang LZ, Dai X, Tseng SH, Loo SJ, Sethi G. Judicious toggling of mTOR activity to combat insulin resistance and cancer: current evidence and perspectives. Front Pharmacol. (2016) 7:395. doi: 10.3389/fphar.2016.00395
115. Yuan Y, Long H, Zhou Z, Fu Y, Jiang B. PI3K-AKT-targeting breast cancer treatments: natural products and synthetic compounds. Biomolecules. (2023) 13:93. doi: 10.3390/biom13010093
116. Wang Y, Chen J, Huang Y, Yang S, Tan T, Wang N, et al. Schisandrin B suppresses osteosarcoma lung metastasis in vivo by inhibiting the activation of the Wnt/β−catenin and PI3K/Akt signaling pathways. Oncol Rep. (2022) 47:50. doi: 10.3892/or.2022.8261
117. Yuan X-H, Zhang P, Yu T-T, Huang H-K, Zhang L-L, Yang C-M, et al. Lycorine inhibits tumor growth of human osteosarcoma cells by blocking Wnt/β-catenin, ERK1/2/MAPK and PI3K/AKT signaling pathway. Am J Trans Res. (2020) 12:5381–98.
118. Keremu A, Maimaiti X, Aimaiti A, Yushan M, Alike Y, Yilihamu Y, et al. NRSN2 promotes osteosarcoma cell proliferation and growth through PI3K/Akt/MTOR and Wnt/β-catenin signaling. Am J Cancer Res. (2017) 7:565–73.
119. Huang H, Lu Q, Yuan X, Zhang P, Ye C, Wei M, et al. Andrographolide inhibits the growth of human osteosarcoma cells by suppressing Wnt/β-catenin, PI3K/AKT and NF-κB signaling pathways. Chemico-biological Interact. (2022) 365:110068. doi: 10.1016/j.cbi.2022.110068
120. Kim DE, Kim B, Shin HS, Kwon HJ, Park E-S. The protective effect of hispidin against hydrogen peroxide-induced apoptosis in H9c2 cardiomyoblast cells through Akt/GSK-3β and ERK1/2 signaling pathway. Exp Cell Res. (2014) 327:264–75. doi: 10.1016/j.yexcr.2014.07.037
121. Bai X, Guo X, Zhang F, Zheng L, Ding W, Yang S. Resveratrol combined with 17β-estradiol prevents IL-1β Induced apoptosis in human nucleus pulposus via the PI3K/AKT/Mtor and PI3K/AKT/GSK-3β Pathway. J Invest surgery: Off J Acad Surg Res. (2021) 34:904–11. doi: 10.1080/08941939.2019.1705941
122. Zhang A, He S, Sun X, Ding L, Bao X, Wang N. Wnt5a promotes migration of human osteosarcoma cells by triggering a phosphatidylinositol-3 kinase/Akt signals. Cancer Cell Int. (2014) 14:15. doi: 10.1186/1475-2867-14-15
123. Chen J, Tu X, Esen E, Joeng KS, Lin C, Arbeit JM, et al. WNT7B promotes bone formation in part through mTORC1. PLoS Genet. (2014) 10:e1004145. doi: 10.1371/journal.pgen.1004145
124. Barnes PJ, Karin M. Nuclear factor-kappaB: a pivotal transcription factor in chronic inflammatory diseases. N Engl J Med. (1997) 336:1066–71. doi: 10.1056/NEJM199704103361506
125. Foo SY, Nolan GP. NF-kappaB to the rescue: RELs, apoptosis and cellular transformation. Trends Genet. (1999) 15:229–35. doi: 10.1016/s0168-9525(99)01719-9
126. Bakkar N, Guttridge DC. NF-kappaB signaling: a tale of two pathways in skeletal myogenesis. Physiol Rev. (2010) 90:495–511. doi: 10.1152/physrev.00040.2009
127. Chang M, Zhu D, Chen Y, Zhang W, Liu X, Li X-L, et al. Total flavonoids of litchi seed attenuate prostate cancer progression via inhibiting AKT/mTOR and NF-kB signaling pathways. Front Pharmacol. (2021) 12:758219. doi: 10.3389/fphar.2021.758219
128. Othman MS, Al-Bagawi AH, Obeidat ST, Fareid MA, Habotta OA, Moneim AEA. Antitumor activity of zinc nanoparticles synthesized with berberine on human epithelial colorectal adenocarcinoma (Caco-2) cells through acting on Cox-2/NF-kB and p53 pathways. Anticancer Agents Med Chem. (2021) 22:2002–10. doi: 10.2174/1871520621666211004115839
129. Tao H, Liao Y, Yan Y, He Z, Zhou J, Wang X, et al. BRCC3 promotes tumorigenesis of bladder cancer by activating the NF-κB signaling pathway through targeting TRAF2. Front Cell Dev Biol. (2021) 9:720349. doi: 10.3389/fcell.2021.720349
130. Ren C, Han X, Lu C, Yang T, Qiao P, Sun Y, et al. Ubiquitination of NF-κB p65 by FBXW2 suppresses breast cancer stemness, tumorigenesis, and paclitaxel resistance. Cell Death Differ. (2022) 29:381–92. doi: 10.1038/s41418-021-00862-4
131. Chou C-H, Lu K-H, Yang J-S, Hsieh Y-H, Lin C-W, Yang S-F. Dihydromyricetin suppresses cell metastasis in human osteosarcoma through SP-1- and NF-κB-modulated urokinase plasminogen activator inhibition. Phytomedicine. (2021) 90:153642. doi: 10.1016/j.phymed.2021.153642
132. Dong S, Liu P-M, Du X-Y, Li F-Y, Cao Y, Lin D-Y, et al. Glycyrrhetinic acid inhibits proliferation of osteosarcoma cell line MG63 by inhibiting NF-κB signaling pathway. Zhongguo Ying Yong Sheng Li Xue Za Zhi. (2020) 36:399–401. doi: 10.12047/j.cjap.5966.2020.085
133. Javaid N, Choi S. Toll-like receptors from the perspective of cancer treatment. Cancers (Basel). (2020) 12:297. doi: 10.3390/cancers12020297
134. Zhou J, Liu Q, Qian R, Liu S, Hu W, Liu Z. Paeonol antagonizes oncogenesis of osteosarcoma by inhibiting the function of TLR4/MAPK/NF-κB pathway. Acta histochemica. (2020) 122:151455. doi: 10.1016/j.acthis.2019.151455
135. Nejak-Bowen K, Kikuchi A, Monga SP. Beta-catenin-NF-κB interactions in murine hepatocytes: a complex to die for. Hepatol (Baltimore Md). (2013) 57:763–74. doi: 10.1002/hep.26042
136. Albanese C, Wu K, D’amico M, Jarrett C, Joyce D, Hughes J, et al. IKKalpha regulates mitogenic signaling through transcriptional induction of cyclin D1 via Tcf. Mol Biol Cell. (2003) 14:585–99. doi: 10.1091/mbc.02-06-0101
137. Deng J, Miller SA, Wang HY, Xia W, Wen Y, Zhou BP, et al. beta-catenin interacts with and inhibits NF-kappa B in human colon and breast cancer. Cancer Cell. (2002) 2:323–34. doi: 10.1016/S1535-6108(02)00154-X
138. Wang CY, Mayo MW, Korneluk RG, Goeddel DV, Baldwin AS. NF-kappaB antiapoptosis: induction of TRAF1 and TRAF2 and c-IAP1 and c-IAP2 to suppress caspase-8 activation. Sci (New York NY). (1998) 281:1680–3. doi: 10.1126/science.281.5383.1680
139. Lamberti C, Lin KM, Yamamoto Y, Verma U, Verma IM, Byers S, et al. Regulation of beta-catenin function by the IkappaB kinases. J Biol Chem. (2001) 276:42276–86. doi: 10.1074/jbc.M104227200
140. Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. (2004) 116:281–97. doi: 10.1016/S0092-8674(04)00045-5
141. Wu Z, Zhou Z, Zhang W, Yu Y. MiR-21-5p inhibition attenuates Warburg effect and stemness maintenance in osteosarcoma cells via inactivation of Wnt/β-catenin signaling. Acta Biochim Polonica. (2021) 68:725–32. doi: 10.18388/abp.2020_5631
142. Li W, Meng Z, Zou T, Wang G, Su Y, Yao S, et al. MiR-374a activates Wnt/β-catenin signaling to promote osteosarcoma cell migration by targeting WIF-1. Pathol Oncol research: POR. (2020) 26:533–9. doi: 10.1007/s12253-018-0556-8
143. Xue Y, Guo Y, Liu N, Meng X. MicroRNA-22-3p targeted regulating transcription factor 7-like 2 (TCF7L2) constrains the Wnt/β-catenin pathway and Malignant behavior in osteosarcoma. Bioengineered. (2022) 13:9135–47. doi: 10.1080/21655979.2021.2003942
144. Zhu D, Qi H, Zhu H. hsa-miR-199b-3p suppresses osteosarcoma progression by targeting CCDC88A, inhibiting epithelial-to-mesenchymal transition, and Wnt/beta-catenin signaling pathway. Sci Rep. (2023) 13:12544. doi: 10.1038/s41598-023-39537-0
145. Zhi W, Feng Q, Mingzhu Z. MiR-140 targets Wnt1 to inhibit the proliferation and enhance drug sensitivity in osteosarcoma cells. Cell Mol Biol (Noisy-le-Grand France). (2022) 68:140–6. doi: 10.14715/cmb/2022.68.1.18
146. Kristensen LS, Andersen MS, Stagsted LVW, Ebbesen KK, Hansen TB, Kjems J. The biogenesis, biology and characterization of circular RNAs. Nat Rev Genet. (2019) 20:675–91. doi: 10.1038/s41576-019-0158-7
147. Zhou Z, Liu T, Li Z, Wang L. Circ_0003732 promotes osteosarcoma progression through regulating miR-377-3p/CPEB1 axis and Wnt/β-catenin signaling pathway. Anti-cancer Drugs. (2022) 33:e299–310. doi: 10.1097/CAD.0000000000001206
148. Peng L, Liu Q, Wu T, Li P, Cai Y, Wei X, et al. Hsa_circ_0087302, a circular RNA, affects the progression of osteosarcoma via the Wnt/β-catenin signaling pathway. Int J Med Sci. (2022) 19:1377–87. doi: 10.7150/ijms.69501
149. Dong L, Qu F. CircUBAP2 promotes SEMA6D expression to enhance the cisplatin resistance in osteosarcoma through sponging miR-506-3p by activating Wnt/β-catenin signaling pathway. J Mol Histol. (2020) 51:329–40. doi: 10.1007/s10735-020-09883-8
150. Spizzo R, Almeida MI, Colombatti A, Calin GA. Long non-coding RNAs and cancer: a new frontier of translational research? Oncogene. (2012) 31:4577–87. doi: 10.1038/onc.2011.621
151. Paraskevopoulou MD, Hatzigeorgiou AG. Analyzing MiRNA-LncRNA interactions. Methods Mol Biol (Clifton NJ). (2016) 1402:271–86. doi: 10.1007/978-1-4939-3378-5_21
152. Li J, Li Z, Zheng W, Li X, Wang Z, Cui Y, et al. LncRNA-ATB: An indispensable cancer-related long noncoding RNA. Cell proliferation. (2017) 50:e12381. doi: 10.1111/cpr.2017.50.issue-6
153. Guan J, He J, Liao S, Wu Z, Lin X, Liu B, et al. LncRNA UCA1 accelerates osteosarcoma progression via miR-145 and Wnt/β-catenin pathway. Am J Trans Res. (2022) 14:6029–42.
154. Zhu S, Liu Y, Wang X, Wang J, Xi G. lncRNA SNHG10 promotes the proliferation and invasion of osteosarcoma via Wnt/β-catenin signaling. Mol Ther Nucleic Acids. (2020) 22:957–70. doi: 10.1016/j.omtn.2020.10.010
155. Zhang H, Liu S, Tang L, Ge J, Lu X. Long non-coding RNA (LncRNA) MRPL23-AS1 promotes tumor progression and carcinogenesis in osteosarcoma by activating Wnt/β-catenin signaling via inhibiting microRNA miR-30b and upregulating myosin heavy chain 9 (MYH9). Bioengineered. (2021) 12:162–71. doi: 10.1080/21655979.2020.1863014
156. Li H, Fu S, Wang W. Long non-coding RNA HOTAIR promotes human osteosarcoma proliferation, migration through activation of the Wnt/b-catenin signaling pathway. J Oncol. (2023) 2023:9667920. doi: 10.1155/2023/9667920
157. Wang H, Zhang P. lncRNA−CASC15 promotes osteosarcoma proliferation and metastasis by regulating epithelial−mesenchymal transition via the Wnt/β−catenin signaling pathway. Oncol Rep. (2021) 45:76. doi: 10.3892/or.2021.8027
158. Bai J, Zhang X, Jiang F, Shan H, Gao X, Bo L, et al. A feedback loop of LINC00665 and the Wnt signaling pathway expedites osteosarcoma cell proliferation, invasion, and epithelial-mesenchymal transition. Orthopaedic Surg. (2023) 15:286–300. doi: 10.1111/os.13532
159. Ferrucci V, Boffa I, de Masi G, Zollo M. Natural compounds for pediatric cancer treatment. Naunyn Schmiedebergs Arch Pharmacol. (2016) 389:131–49. doi: 10.1007/s00210-015-1191-5
160. Varoni EM, Lo Faro AF, Sharifi-rad J, Sharifi-Rad J, Iriti M. Anticancer molecular mechanisms of resveratrol. Front Nutr. (2016) 3:8. doi: 10.3389/fnut.2016.00008
161. Rusin M, Zajkowicz A, Butkiewicz D. Resveratrol induces senescence-like growth inhibition of U-2 OS cells associated with the instability of telomeric DNA and upregulation of BRCA1. Mech Ageing Dev. (2009) 130:528–37. doi: 10.1016/j.mad.2009.06.005
162. Xie D, Zheng GZ, Xie P, Zhang Q-H, Lin F-X, Chang B, et al. Antitumor activity of resveratrol against human osteosarcoma cells: a key role of Cx43 and Wnt/β-catenin signaling pathway. Oncotarget. (2017) 8:111419–32. doi: 10.18632/oncotarget.22810
163. Aggarwal BB, Sundaram C, Malani N, Ichikawa H. Curcumin: the Indian solid gold. Adv Exp Med Biol. (2007) 595:1–75. doi: 10.1007/978-0-387-46401-5
164. Chang R, Sun L, Webster TJ. Short communication: selective cytotoxicity of curcumin on osteosarcoma cells compared to healthy osteoblasts. Int J Nanomedicine. (2014) 9:461–5. doi: 10.2147/IJN.S55505
165. Leow P-C, Tian Q, Ong Z-Y, Yang Z, Ee P-LR. Antitumor activity of natural compounds, curcumin and PKF118-310, as Wnt/β-catenin antagonists against human osteosarcoma cells. Invest New Drugs. (2010) 28:766–82. doi: 10.1007/s10637-009-9311-z
166. Ye F, Wang H, Zhang L, Zou Y, Han H, Huang J. Baicalein induces human osteosarcoma cell line MG-63 apoptosis via ROS-induced BNIP3 expression. Tumour biology: J Int Soc Oncodevelopmental Biol Med. (2015) 36:4731–40. doi: 10.1007/s13277-015-3122-y
167. Guo Z, Hu X, Xing Z, Xing R, Lv R, Cheng X, et al. Baicalein inhibits prostate cancer cell growth and metastasis via the caveolin-1/AKT/mTOR pathway. Mol Cell Biochem. (2015) 406:111–9. doi: 10.1007/s11010-015-2429-8
168. Örenlili Yaylagül E, Ülger C. The effect of baicalein on Wnt/β-catenin pathway and miR-25 expression in Saos-2 osteosarcoma cell line. Turkish J Med Sci. (2020) 50:1168–79. doi: 10.3906/sag-2001-161
169. Lin P-H, Tung Y-T, Chen H-Y, Chiang Y-F, Hong H-C, Huang K-C, et al. Melatonin activates cell death programs for the suppression of uterine leiomyoma cell proliferation. J Pineal Res. (2020) 68:e12620. doi: 10.1111/jpi.12620
170. Gil-Martín E, Egea J, Reiter RJ, Romero A. The emergence of melatonin in oncology: Focus on colorectal cancer. Medicinal Res Rev. (2019) 39:2239–85. doi: 10.1002/med.21582
171. Proietti S, Cucina A, Minini M, Bizzarri M. Melatonin, mitochondria, and the cancer cell. Cell Mol Life sciences: CMLS. (2017) 74:4015–25. doi: 10.1007/s00018-017-2612-z
172. Li Y, Zou J, Li B, Du J. Anticancer effects of melatonin via regulating lncRNA JPX-Wnt/β-catenin signalling pathway in human osteosarcoma cells. J Cell Mol Med. (2021) 25:9543–56. doi: 10.1111/jcmm.v25.20
173. Liu Y, Liu Y-Z, Zhang R-X, Wang X, Meng Z-J, Huang J, et al. Oridonin inhibits the proliferation of human osteosarcoma cells by suppressing Wnt/β-catenin signaling. Int J Oncol. (2014) 45:795–803. doi: 10.3892/ijo.2014.2456
174. Liu Y, Wang W, Xu J, Li L, Dong Q, Shi Q, et al. Dihydroartemisinin inhibits tumor growth of human osteosarcoma cells by suppressing Wnt/β-catenin signaling. Oncol Rep. (2013) 30:1723–30. doi: 10.3892/or.2013.2658
175. Chang J, Li Y, Wang X, Hu S, Wang H, Shi Q, et al. Polyphyllin I suppresses human osteosarcoma growth by inactivation of Wnt/β-catenin pathway in vitro and in vivo. Sci Rep. (2017) 7:7605. doi: 10.1038/s41598-017-07194-9
176. Ma Y, Zhu B, Liu X, Yu H, Yong L, Liu X, et al. Inhibition of oleandrin on the proliferation show and invasion of osteosarcoma cells in vitro by suppressing Wnt/β-catenin signaling pathway. J Exp Clin Cancer research: CR. (2015) 34:115. doi: 10.1186/s13046-015-0232-8
177. He Y, Sun MM, Zhang GG, Yang J, Chen KS, Xu WW, et al. Targeting PI3K/Akt signal transduction for cancer therapy. Signal transduction targeted Ther. (2021) 6:425. doi: 10.1038/s41392-021-00828-5
178. Hattinger CM, Patrizio MP, Magagnoli F, Luppi S, Serra M. An update on emerging drugs in osteosarcoma: towards tailored therapies? Expert Opin Emerg Drugs. (2019) 24:153–71. doi: 10.1080/14728214.2019.1654455
179. Fleischmann RM, Bliddal H, Blanco FJ, Schnitzer TJ, Peterfy C, Chen S, et al. A phase II trial of lutikizumab, an anti-interleukin-1α/β Dual variable domain immunoglobulin, in knee osteoarthritis patients with synovitis. Arthritis Rheumatol (Hoboken NJ). (2019) 71:1056–69. doi: 10.1002/art.2019.71.issue-7
180. He Z, Wang B, Hu C, Zhao J. An overview of hydrogel-based intra-articular drug delivery for the treatment of osteoarthritis. Colloids surfaces B Biointerfaces. (2017) 154:33–9. doi: 10.1016/j.colsurfb.2017.03.003
181. Vallabhapurapu S, Karin M. Regulation and function of NF-kappaB transcription factors in the immune system. Annu Rev Immunol. (2009) 27:693–733. doi: 10.1146/annurev.immunol.021908.132641
182. Baud V, Karin M. Is NF-kappaB a good target for cancer therapy? Hopes and pitfalls. Nat Rev Drug Discovery. (2009) 8:33–40. doi: 10.1038/nrd2781
183. Dutta S, Ganguly A, Chatterjee K, Spada S, Mukherjee S. Targets of immune escape mechanisms in cancer: basis for development and evolution of cancer immune checkpoint inhibitors. Nat Rev Drug Discov. (2023) 12:218. doi: 10.3390/biology12020218
184. Bian Y, Lin T, Jakos T, Xiao X, Zhu J. The generation of dual-targeting fusion protein Pd-L1/CD47 for the inhibition of triple-negative breast cancer. Biology. (2022) 10:1843. doi: 10.3390/biomedicines10081843
Keywords: osteosarcoma (OS), Wnt/β-catenin, targeted therapy, chemotherapy, mechanism
Citation: Ding Y and Chen Q (2025) Wnt/β-catenin signaling pathway: an attractive potential therapeutic target in osteosarcoma. Front. Oncol. 14:1456959. doi: 10.3389/fonc.2024.1456959
Received: 29 June 2024; Accepted: 24 December 2024;
Published: 14 February 2025.
Edited by:
Tong-Chuan He, University of Chicago Medicine, United StatesReviewed by:
Yang Yang, Affiliated Hospital of Nantong University, ChinaCopyright © 2025 Ding and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qin Chen, Y2hlbnExOTgxMDFAMTYzLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.